Abstract
Highly inbred C57BL/6 mice show wide variation in their degree of insulin resistance in response to diet-induced obesity even though they are almost genetically identical. Here we employed transcriptional profiling by RNA sequencing (RNA-Seq) of visceral adipose tissue (VAT) and liver in young mice to determine how gene expression patterns correlate with the later development of high-fat diet (HFD)-induced insulin resistance in adulthood. To accomplish this goal, we partially removed and banked tissues from pubertal mice. Mice subsequently received HFD followed by metabolic phenotyping to identify two well-defined groups of mice with either severe or mild insulin resistance. The remaining tissues were collected at study termination. We then applied RNA-Seq to generate transcriptome profiles associated with worsened insulin resistance before and after the initiation of HFD. We found 244 up- and 109 downregulated genes in VAT of the most insulin-resistant mice even before HFD exposure. Downregulated genes included serine protease inhibitor, major urinary protein, and complement genes; upregulated genes represented mostly muscle constituents. These gene families were also differentially expressed in VAT of mice with high or low insulin resistance after HFD. Inflammatory genes predicted insulin resistance in liver, but not in VAT. In contrast, when we compared VAT of all mice before and after HFD, differentially expressed genes were predominantly composed of immune response genes. These data show a distinct set of gene transcripts in young mice correlates with the severity of insulin resistance in adulthood, providing insight into the pathogenesis of insulin resistance in early life.
Keywords: gene expression, obesity, insulin resistance, transcriptome, visceral adipose tissue
INTRODUCTION
Insulin resistance and obesity are major risk factors for the development of Type 2 diabetes, cardiovascular disease, and premature death. Historically, most individuals affected by Type 2 diabetes have been adults. However, there has been an alarming rise in the prevalence of obesity and Type 2 diabetes in youth in the past three decades (5), and recent data from The National Health and Nutrition Examination Survey confirm that 17% of all children and adolescents age 2–19 yr in the U.S. are now obese (21). Childhood obesity is strongly associated with obesity in adulthood (28). Despite this pediatric obesity epidemic, there has been only limited study of adipose tissue characteristics in childhood or adolescence.
Highly inbred rodent strains such as C57BL/6 mice are nearly identical genetically yet show substantial variation in longevity, adiposity, hepatic steatosis, and insulin resistance, even when reared in highly standardized laboratory conditions (2, 3, 7, 23, 34). In chronic obesity and nutrient excess, an inflammatory program is activated early in adipose expansion. Proinflammatory changes in adipose, liver, and other tissues are thought to play a causal role in disrupted energy homeostasis, insulin resistance, and defective insulin secretion (24). Many studies have investigated differential gene expression in adipose tissue of mice fed either a low-fat or high-fat diet (HFD). However, only a few studies have addressed transcriptomic signatures associated with variation in the severity of insulin resistance when all mice are placed on an HFD. Moreover, these gene expression studies have generally employed microarray analyses rather than nonbiased, sequencing approaches (11, 12, 32).
Here, we exploited the wide phenotypic variation among highly inbred male C57BL/6 mice in response to diet-induced obesity as a means to identify genetic signatures associated with insulin resistance (11). Using young mice as a model for childhood obesity, we aimed in this investigation to determine differences in gene expression of visceral adipose tissue (VAT) and liver of lean, adolescent C57BL/6 mice that predict the severity of insulin resistance induced by HFD in adulthood. We hypothesized that differential gene signatures in VAT could potentially predict which mice become more severely obese.
Both adipose and liver are classical insulin target tissues that regulate glucose homeostasis. We therefore partially removed VAT and liver tissue from 7 wk old male mice and banked them at −80°C. Mice subsequently received HFD followed by metabolic phenotyping to generate two distinct, well-defined groups of mice with either severe or mild insulin resistance. RNA sequencing (RNA-Seq) was employed to generate transcriptome profiles that predicted or correlated with worsened insulin resistance before and after the initiation of HFD. By using an unbiased approach, we sought to identify novel transcripts that regulate the development of obesity-related insulin resistance. We then performed bioinformatics analyses including gene set enrichment analyses to identify functional pathways and networks that differ between mice with the highest and lowest insulin resistance and to provide hypotheses that may explain the transcriptomic signature associated with the development of severe insulin resistance.
We provide evidence in this paper that visceral adipose gene expression differs between mice that later develop mild vs. severe diet-induced insulin resistance and that genes associated with serine protease inhibitors, major urinary proteins, and muscle constituents appear to play a role in regulating adipose tissue expansion and metabolism in obesity. This pattern differs from that observed in liver where inflammatory genes predict the later development of higher insulin resistance. Interestingly, when measured after HFD exposure, immune response genes were upregulated in subcutaneous adipose tissue (SAT), but not VAT, of more highly insulin-resistant mice, suggesting that these adipose depots have differing roles in the regulation of diet-induced insulin resistance.
MATERIALS AND METHODS
Animals.
C57/BL6N mice were purchased from Harlan Laboratories. Only male mice were used for this study. All animal procedures adhered to University of California, San Diego institutional guidelines for the ethical treatment of animals. Surgery was performed on the mice at 7 wk of age to obtain a small sample of the right epididymal white adipose tissue and left lateral liver lobe (19). There were 69 mice in total. Mice were allowed to recover for 1 wk and then started on HFD at 8 wk of age, continuing on the diet for 14 wk. Mice were fed 60% HFD chow (60% kcal from fat, D12492; Research Diets, New Brunswick, NJ), and maintained on a 12 h light-dark cycle with free access to food and water. Body weights measurements were obtained weekly. An insulin tolerance test (ITT) was performed after 12 wk of HFD, and a glucose tolerance test (GTT) was performed after 13 wk of HFD. Visceral adipose, subcutaneous adipose, and liver tissues were collected from mice at 14 wk of age (Fig. 1A).
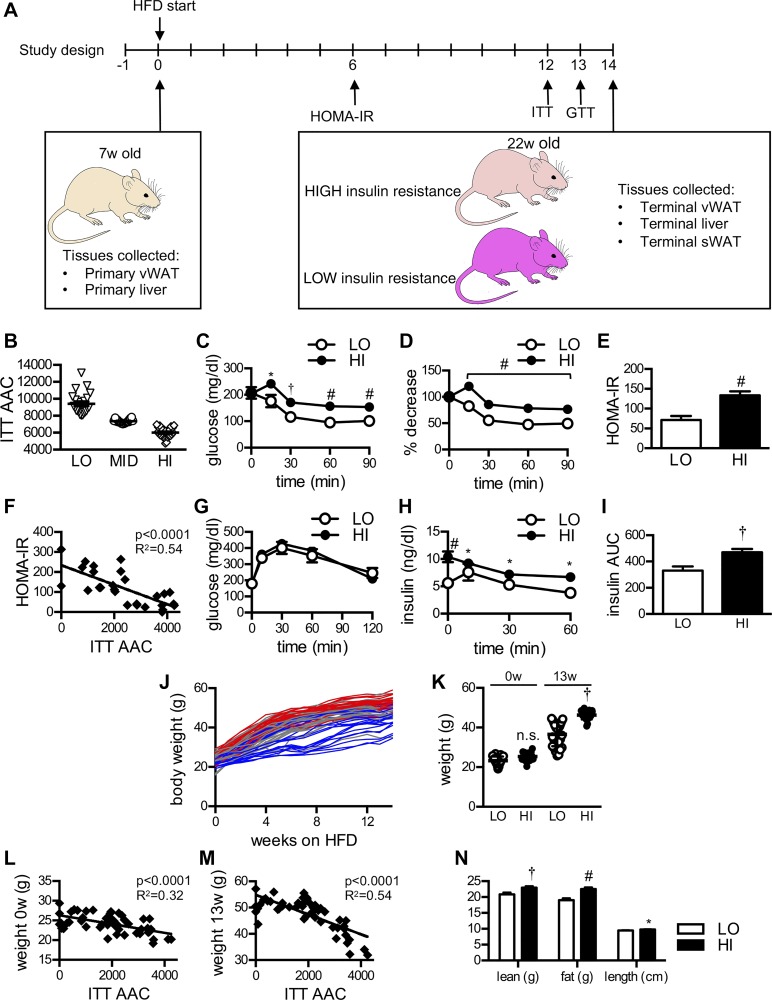
Fig. 1.
Mice on the same high-fat diet (HFD) demonstrate high and low levels of insulin resistance. A: study design. Mice underwent surgery at 7 wk old and were allowed to recover. They were then started on HFD for 14 wk. Homeostasis model assessment of insulin resistance (HOMA-IR) was determined after 6 wk of HFD, and insulin tolerance test (ITT) and glucose tolerance test (GTT) were performed after 12 and 13 wk of HFD, respectively. B: ITT area above curve (AAC) was calculated to determine the severity of insulin resistance. The highest 1/3 (23 mice) was used as the high insulin resistance group (HI) and the lowest 1/3 (23 mice) was used as the low insulin resistance group (LO). There were 69 mice total. C, D: during ITT, blood glucose levels were higher and the percent decrease in blood glucose levels were lower in the HI group than the LO group, as expected. E: HOMA-IR values were significantly higher in the HI compared with the LO group. F: HOMA-IR correlated well with ITT AAC. G: blood glucose levels measured during GTT were not significantly different between the HI and LO groups. H, I: serum insulin levels were significantly higher in the HI group when measured at baseline and during the GTT. Insulin area-under-the-curve (iAUC) values were also higher in HI vs. LO mice, consistent with increased compensatory hyperinsulinemia in these more highly insulin-resistant mice. Serum insulin was measured using an insulin ELISA assay. J, K: weekly measurements of mouse body weights while on HFD. Red lines represent 23 mice that were included in the high insulin resistance group. Blue lines represent 23 mice that were included in the low insulin resistance group. Gray lines represent mice that were in the middle group. Body weights were not significantly different between groups before HFD. From the 1st week of HFD to the end of HFD, mice in the HI group had significantly greater weight gain than mice in the LO group. Body weights of high vs. low insulin resistance mice became distinctly separated by the 13th week of HFD. Regression plots of body weights against ITT AAC showed that body weight correlated well with the severity of their insulin resistance both before (L) and after 13 wk of HFD exposure (M). N: lean mass, fat mass, and body length were all significantly higher in the HI group compared with the LO group. Mice underwent live imaging DEXA scanning at 14 wk of age. Data are represented as means ± SE *P < 0.05, †P < 0.01, #P < 0.005.
Measurement of body composition by dual-energy X-ray absorptiometry scanning.
Body composition was assessed in mice by dual-energy X-ray absorptiometry (DEXA) scan (Lunar PIXImus Densitometer, GE Medical Systems). Each mouse was anesthetized via intraperitoneal injection of ketamine-xylazine during the procedure. The mice were placed on the scanner bed in the prone position with limbs stretched away from the body.
Metabolic studies.
For glucose tolerance (GTT) or insulin tolerance (ITT) testing, mice were allowed to feed overnight and then fasted for 7 h (GTT) or 4 h (ITT). After collection of basal blood work, animals were given intraperitoneal dextrose (1 g/kg; Hospira, San Diego, CA) for the GTT, or intraperitoneal insulin (0.6 U/kg, Novolin R, Novo-Nordisk) for the ITT. Blood samples were drawn by tail nick at basal and indicated times, and glucose was measured with a One-Touch glucose meter (Lifescan, Milpitias, CA). Plasma insulin was quantified using the Ultra Sensitive Mouse Insulin ELISA kit (ALPCO, Salem, NH). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from glucose and insulin concentrations obtained after 6 h of food withdrawal, by the following formula: [fasting blood glucose (mg/dl) × fasting insulin (µU/ml)] ÷ 405.
RNA-Seq library preparation and sequencing.
The adipose and liver tissue samples were immediately preserved in RNAlater stabilization solution (Qiagen, Valencia, CA) and banked at −80°C. After the mice in the high and low insulin resistance tertiles were identified, total RNA was then extracted with the RNeasy kit (Qiagen). RNA libraries were prepared using the TruSeq RNA Library Preparation Kit (Illumina, San Diego, CA). Multiple RNA samples were pooled to create one RNA library. Reads generated by the sequencer, typically 100 base pair paired ended, were first mapped to the mouse transcriptome (RefSeq), which at present contains 35,751 sequences of protein-coding and nonprotein-coding genes, by an efficient alignment program, Bowtie (13). Alignment of one read to several transcript variants of the same genetic locus counted as one alignment to that locus. The resulting table of numbers of alignments per gene per biological replicate per experimental condition was then analyzed by the statistical algorithm DESeq (1) in which gene counts were modeled as results of negative binomial process. A sorted list of genes was subjected to gene ontology (GO) and pathway analyses using a nonparametric variant of the Gene Set Enrichment Analysis algorithm, referred to here as GOrank (30). We also used the ToppGene Suite (https://toppgene.cchmc.org/) for further GO analysis and identification of putative transcription factor binding sites by inputting differentially expressed (DE) genes with a q value <0.05 into the ToppFun program. FDR (false discovery rate) correction was set at <0.05.
RNA isolation and quantitative RT-PCR.
Total RNA was extracted from liver and adipose tissue using the RNeasy kit (Qiagen). First-strand cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad) and random hexamers. RT quantitative (q)PCR was carried out by using iTaq SYBR Green Supermix (Bio-Rad) and StepOnePlus System (Applied Biosystems). Relative gene expression levels were calculated after normalization to the standard housekeeping gene Gapdh by the ΔΔCT method. Primer sequences are available in Supplemental Table S1. (The online version of this article contains supplemental material.)
Reactome mapping.
We extracted known pathways that may be, at least partially, constituted by the genes showing significant changes in their expression levels. For this purpose, we used the ReactomeFIViz app (33), which works on Cytoscape (27), a software platform for molecular network analyses and visualizations. The lists of genes showing significant changes (q < 0.05) were collected and fed into the app. Reactome FI Network version 2014 was used to map the genes onto the known pathways. The app generated a molecular network connecting subset of the given genes on Cytoscape, where each edge corresponded to one part of a known pathway. Using the functionalities of the app, we split the network into modules (subnetworks), gave different colors to different modules, and added pathway information to each module, based on the annotations of the genes participating in each module. The visualized network on Cytoscape was exported as a PDF file and was labeled using Adobe Illustrator.
Functional enrichment analyses using BiNGO.
We used BiNGO (17), another Cytoscape app, to analyze gene functions that were enriched in the genes showing significant changes. Although the app does not show possible pathway connections between the genes, it performs statistical assessment of the functional enrichments. The set of genes showing significant changes in their expression levels (q < 0.05) was imported into the app. The app then searched for the enriched GO terms, i.e., biological processes and cellular components, associated with the gene set. The hierarchical structure of GO terms was graphically displayed on Cytoscape, and the GO terms that were significantly enriched in the given gene set were highlighted. All genes with their expression levels measured were used as a background gene set for the statistical assessment of the enrichments. The output graphic was labeled with Adobe Illustrator.
Statistical analysis.
For metabolic parameters of mice, all values are expressed as means ± SE unless otherwise noted. Using GraphPad Prism 6, we applied two-way analysis of variance (ANOVA) to determine differences between groups, and repeated-measures ANOVA testing for comparisons over time, using the Tukey’s test for post hoc analysis. Linear regression was used to examine the interrelationship between body weight and insulin resistance as measured by the ITT area above the curve. P values of <0.05 were considered significant. Genes identified from the RNA-Seq data were sorted by their significance in a typical two-way comparison (highest vs. lowest insulin resistance groups). Significance was calculated as the q value, which is the largest FDR at which a gene is deemed differentially expressed. A corrected P cut-off or q value of 0.05 was used to selected the regulated genes with the lowest FDR.
RESULTS
Young C57BL/6 mice placed on HFD show significant variation in the severity of their insulin resistance.
We and others have observed highly variable weight gain and insulin resistance in C57BL/6 when they are fed HFD despite being nearly genetically identical (2, 9, 34). A previous study that performed microarray analysis of epididymal fat collected from C57BL/6 mice before HFD indicated that differential gene signatures could potentially predict which mice would become more severely obese (11). To further explore how differences in gene expression result in variable metabolic phenotypes, we collected visceral fat (right epididymal white adipose tissue) and liver tissues (20) (left lateral liver lobe) from 69 young, peripubertal male C57BL/6N mice at 7 wk of age (6) before the initiation of HFD. These tissues were surgically removed from each mouse following daytime fasting and stored at −80°C. These banked tissues are referred to as “primary tissue” for this study.
The mice were started on a 60% HFD 1 wk after their surgery. Fasting glucose and insulin values were measured after 6 wk of HFD at the study midpoint to calculate a HOMA-IR. We then performed an ITT and GTT after 12 and 13 wk of HFD, respectively (Fig. 1A). After 14 wk of HFD, VAT (right epididymal fat and perirenal fat) and the remaining liver tissues were collected from the mice and referred to as “terminal tissues.”
The HFD-fed mice with the highest (HI) and lowest (LO) levels of insulin resistance were determined by area-above-the-curve (AAC) measurements during the ITT. The tertiles with the highest and lowest ITT AAC measurements were defined as the HI and LO groups, respectively (Fig. 1B). Given this definition, it was expected that the LO insulin resistance group would have lower blood glucose levels (Fig. 1C) and a greater percent glucose decrease during the ITT than the HI group (Fig. 1D). HOMA-IR levels measured at the study midpoint were also higher in the HI group compared with the LO group (Fig. 1E) and correlated well with the ITT AAC (Fig. 1F).
During the GTT, there was no significant difference between the blood glucose curves of the LO and HI groups (Fig. 1G). However, concurrently measured serum insulin levels were higher in the HI group compared with the LO group (Fig. 1, H and I), confirming that the HI group indeed had greater insulin resistance.
Highly insulin-resistant mice have higher body weights reflecting increased fat mass, increased lean mass, and increased body length.
Mouse body weights were measured weekly throughout the study period. Although there was some overlap, the HI and LO groups became more distinctly separated in terms of body weight by the 13th week of HFD (Fig. 1, J and K). By the end of the study period, the HI group had significantly higher body weights compared with the LO group (HI 46.2 ± 0.5 g vs. LO 36.6 ± 1.1 g, P < 0.0001). Interestingly, even though prepubertal weights did not statistically differ between groups before the start of HFD, a regression plot of body weights at study week 0 against the severity of insulin resistance as measured by ITT AAC at study week 13 showed a strong correlation between these two variables (Fig. 1L). As expected, the body weight after HFD and degree of weight gain by study week 13 also correlated well with the degree of insulin resistance (Fig. 1M). Subsequent body composition analysis by DEXA scan confirmed that the HI insulin-resistant mice had increased fat mass and increased lean mass (Fig. 1N). Their body lengths were also longer in comparison to the LO mice, likely resulting from increased linear growth (HI 9.49 ± 0.07 cm vs. LO 9.75 ± 0.07 cm, P = 0.02). The overall body fat percentage did not significantly differ between groups (HI 49.6 ± 0.45% vs. LO 47.8 ± 1.0%, P = 0.13).
Serpin, Mup, and muscle-related genes are DE in primary visceral adipose of pubertal-aged mice that develop high or low HFD-induced insulin resistance even before HFD exposure.
To investigate differential gene expression that might account for the differing HI and LO insulin-resistant phenotypes after HFD, we performed RNA-Seq analysis of both primary and terminal tissues for the HI and LO groups. Read counts were mapped for 15,198 genes in primary visceral adipose tissues of both HI and LO groups (Fig. 2A). The 191 most highly expressed primary visceral adipose genes, arbitrarily defined as genes with an average read count of >10,000 reads per gene, represented commonly known white adipose genes such as Scd1, Adipoq, Cidec, Retn, Ccdc80, and Dgat2 (29) and were generally expressed at similar levels in both groups (Supplemental Table S2A). However, there was a notable exception in ribosomal RNA species with significantly reduced 45S preribosomal, 28S ribosomal, and 18S ribosomal RNA transcripts in mice with higher insulin resistance (Fig. 2B, Supplemental Table S2A).
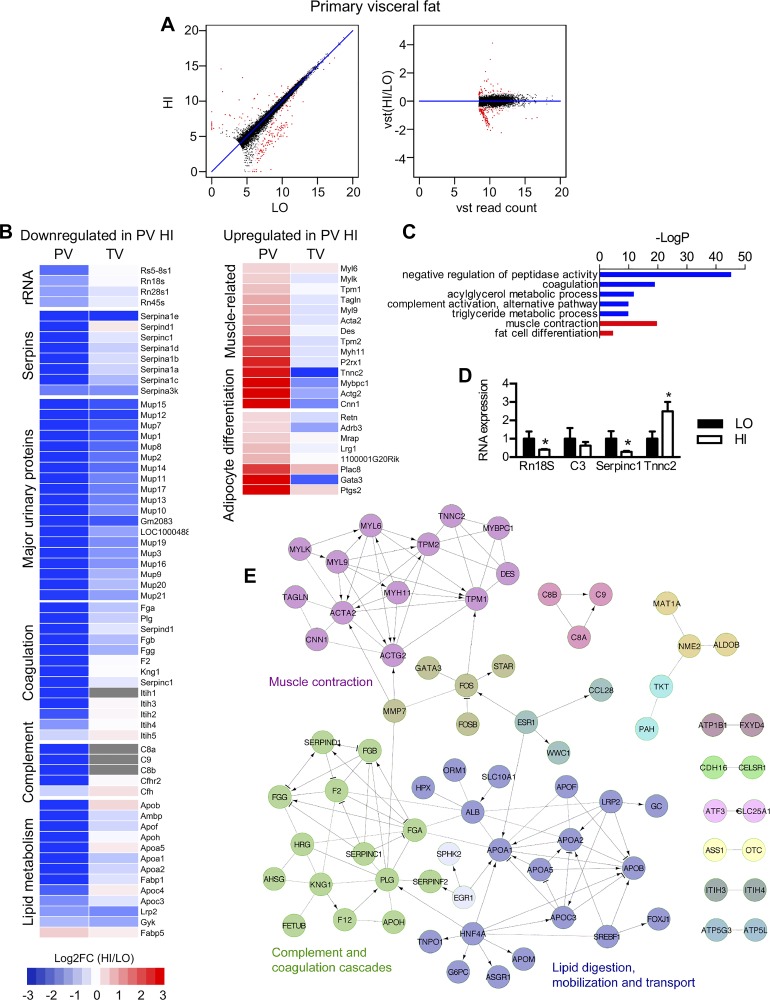
Fig. 2.
Serpin, Mup, and muscle-related genes are differentially expressed (DE) before high-fat feeding in primary visceral (PV) adipose of mice who later develop high or low insulin resistance A: XY and variance stabilizing transformation (VST) scatterplots show 353 genes (red dots) that are DE in primary epididymal fat between high and low insulin resistance mice, meeting a 5% FDR cut-off. B: heat maps on the left show decreased expression of rRNA, Serpin, major urinary protein, lipid metabolism, and complement genes in PV adipose tissue of high insulin resistance (HI) mice compared with low insulin resistance (LO) mice. Relative expression is expressed as the log2-transformed fold change of HI vs. LO mice [or log2FC (HI/LO)]. Blue boxes indicate genes that were underexpressed in HI mice; red boxes indicate genes that were overexpressed in HI mice. These PV genes were compared with terminal visceral (TV) adipose genes from the same mice, represented in the adjacent column. If the gene was not identified in the TV data set, it is represented as a gray box in the heat map. The heat map on the right shows increased expression muscle-related and adipocyte differentiation genes in PV but not TV adipose tissues of HI mice. C: biological pathways that differ in PV fat between HI and LO groups by GOrank gene ontology (GO) analysis. Downregulated pathways in highly insulin-resistant mice are shown in blue, and upregulated pathways are shown in red. D: to validate expression of some gene targets identified by RNA-Seq, we performed quantitative (q)PCR analysis of selected gene targets from PV adipose of individual mice from HI and LO groups (n = 6 per group). E: network analyses using the Reactome database and Cytoscape platform that confirmed that some of the 353 DE genes represent known pathways for muscle contraction, lipid metabolism, and complement and coagulation cascades.
We identified 353 genes that were considered to be DE between groups, meeting an FDR value of < 5% (q value < 0.05) (Fig. 2A). Of these DE primary visceral adipose genes, 244 were downregulated and 109 were upregulated in mice with higher insulin resistance. To better understand their biological relationship, we performed gene ontology analysis using the GOrank program and found that downregulated genes primarily reflected four gene families: Serpin (serine protease inhibitor) genes, Mup (major urinary protein) genes, lipid metabolism-related genes (primarily apolipoproteins), and complement genes (Fig. 2, B and C; Supplemental Table S2B). In contrast, upregulated genes were mostly related to structural constituents of muscle and muscle contraction as well as fat cell differentiation. Differential gene expression of a few selected targets (Rn18s, Serpinc1, and Tnnc2) was validated by qPCR from RNA samples of individual mice (Fig. 2D). We next performed additional network analyses using the Reactome database and Cytoscape platform that confirmed that some of the 353 DE genes represented known pathways for muscle contraction, lipid metabolism, and complement and coagulation cascades (Fig. 2E).
Using ToppGene for further GO analysis and identification of putative transcription factor binding sites, we again identified endopeptidase inhibitor and lipid transport gene families among the genes upregulated in primary VAT of highly insulin-resistant mice and muscle constituent genes among the downregulated adipose genes. By this method, 14 downregulated genes were found to have HNF1 (hepatic nuclear factor 1) transcription factor binding sites, and 13 upregulated, muscle-related genes had SRF (serum response factor) transcription factor binding sites (Supplemental Table S5), suggesting that these transcription factors regulate adipose gene expression in this context. It should be noted that these putative HNF-1 target genes represent gene targets previously identified in the liver and are not known targets of HNF-1 in adipose tissue.
Mup, Serpin-related, complement, and muscle-related gene families are also DE in terminal visceral adipose of mice with high or low HFD-induced insulin resistance.
To determine whether these gene expression differences persisted after HFD, we next analyzed terminal VAT of mice with high and low insulin resistance. When we evaluated the most highly expressed genes, unlike primary visceral fat, ribosomal RNA species were not found to be differentially expressed (Supplemental Table S3A). We found 519 genes in these terminal tissues to be DE between groups, again meeting a 5% FDR (Fig. 3A). Of these, 469 genes were downregulated in the HI insulin-resistant mice, but only 50 were upregulated. The Mup and serine endopeptidase inhibitor gene families were again highly represented in DE genes of terminal VAT, and again strongly downregulated in mice with the highest insulin resistance, similar to the pattern in primary VAT (Fig. 3, B and D, and Supplemental Table S3B). In contrast, muscle-related genes representing components of the actin cytoskeleton and possibly vascular smooth muscle were also DE, but downregulated in terminal VAT of highly insulin-resistant mice, instead of upregulated as seen in primary VAT. In addition, complement genes were DE, but upregulated in terminal VAT, instead of downregulated as seen in primary VAT. Differential gene expression of a few selected targets (C3, Serpinc1, and Tnnc2) was validated by qPCR from RNA samples of individual mice (Fig. 3C). Using ToppGene to identify possible transcriptional regulators, we again identified putative binding sites for HNF1 in downregulated genes of terminal visceral adipose (Supplemental Table S5). Moreover, the expression of HNF1b was itself reduced in terminal VAT in mice with the highest insulin resistance.
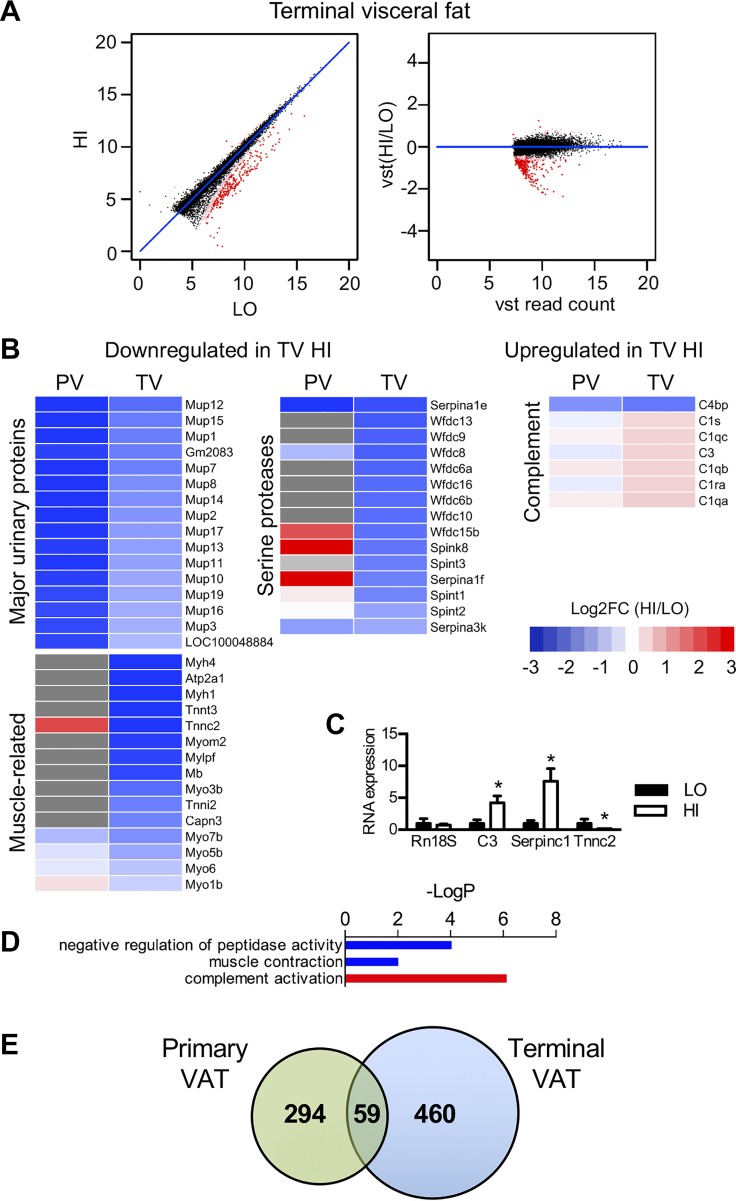
Fig. 3.
Mup, Serpin-related, complement, and muscle-related gene families are also differentially expressed in terminal visceral (TV) adipose of mice with high or low HFD-induced insulin resistance. A: XY and VST scatterplots show 519 genes (red dots) that are differentially expressed in TV fat between high and low insulin resistance mice. B: heat maps again show reduced expression of Mup and Serpin-related genes and now also decreased expression of muscle-related genes in high insulin resistance mice. The heat map on the right shows increased expression complement genes in high insulin resistance mice. These TV genes were compared with primary visceral (PV) adipose genes from the same mice, represented in the adjacent column. Genes not identified in the TV data set are represented by gray boxes in the heat map. C: qPCR analysis of selected gene targets from TV adipose of individual mice from HI and LO groups (n = 6 per group). D: biological pathways that differ in TV fat between HI and LO groups by GOrank analysis. Downregulated pathways in high insulin resistant mice are shown in blue, and upregulated pathways are shown in red. E: Venn diagram indicating overlapping DE genes in primary and terminal visceral fat. There were 294 DE genes unique to PV adipose and 460 DE genes unique to TV adipose. There were 59 DE genes found in both groups, including 14 Mup genes and 5 Serpin-related genes. Gene lists can be found in Supplemental Table S4.
Although several gene families were represented in the DE genes between HI and LO insulin-resistant mice both before and after HFD, only 59 specific genes were found in common between the DE gene sets of primary and terminal VAT (Fig. 3E). Mup genes were again notably featured in this group, represented by 17 Mup family members (listed in Supplementary Table S3A). Regarding Serpin-related genes, only Serpina1e, Serpina1f, Serpina3k, Spink8, and Wfdc15b were found in both PV and TV DE genes. Interestingly, Serpina1f, Wfdc15b, and Mup19 have been recently reported as secreted murine proteins (35), providing a potential mechanism by which adipose tissue could regulate insulin sensitivity in other organs, such as muscle or liver.
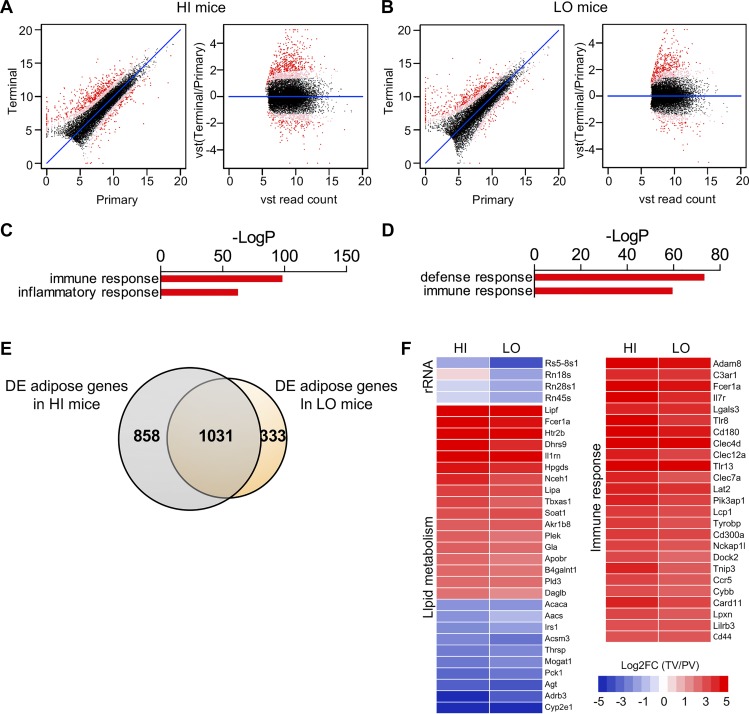
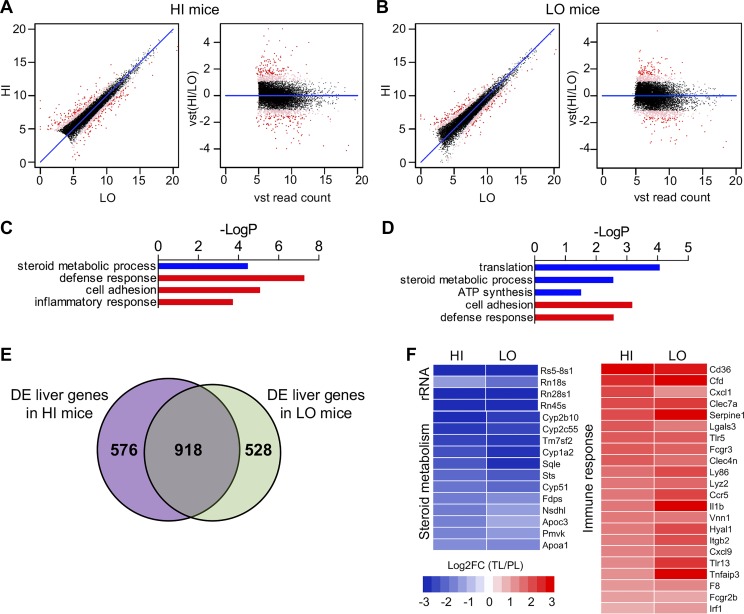
To investigate the effect of HFD-induced obesity on gene expression in visceral adipose, we next compared changes in gene expression between primary and terminal VAT. In contrast to the previous comparisons, there were many more genes that were DE when we compared primary VAT vs. terminal VAT, with 1,888 DE genes in HI group (1,248 genes that increased and 640 genes that decreased in terminal VAT) and 1,363 DE genes in the LO group (944 genes that increased and 419 that decreased in terminal VAT). There was a high degree of similarity between the DE genes of the HI and LO groups with 1,031 genes shared by both, predominantly showing that immune and inflammatory response pathways were induced with the development of HFD-induced obesity (Fig. 4), similar to findings previously reported by others (24).
Fig. 4.
Immune genes are induced in visceral fat with the development of diet-induced obesity. XY and VST scatterplots show >1,300 DE genes (red dots) when we compared expression levels between primary and terminal visceral (TV) fat in the HI group (A) and the LO group (B). C, D: GOrank analysis shows the immune response genes are highly upregulated in both HI and LO mice following high-fat feeding. E: there was a high degree of similarity between the DE genes of the HI and LO groups with 1,031 genes shared by both, predominantly reflecting immune and inflammatory response pathways. F: heat maps show decreased expression of rRNA, increased expression of immune response genes, and mixed changes in lipid metabolism genes when we compared visceral adipose of mice before and after HFD. These patterns were consistent in both HI and LO groups. Please note that relative expression is expressed as the log2-transformed fold change of TV vs. primary liver (PV) mice [or log2FC (TV/PV)].
Immune and muscle-related genes are DE in terminal subcutaneous adipose of mice with high or low HFD-induced insulin resistance.
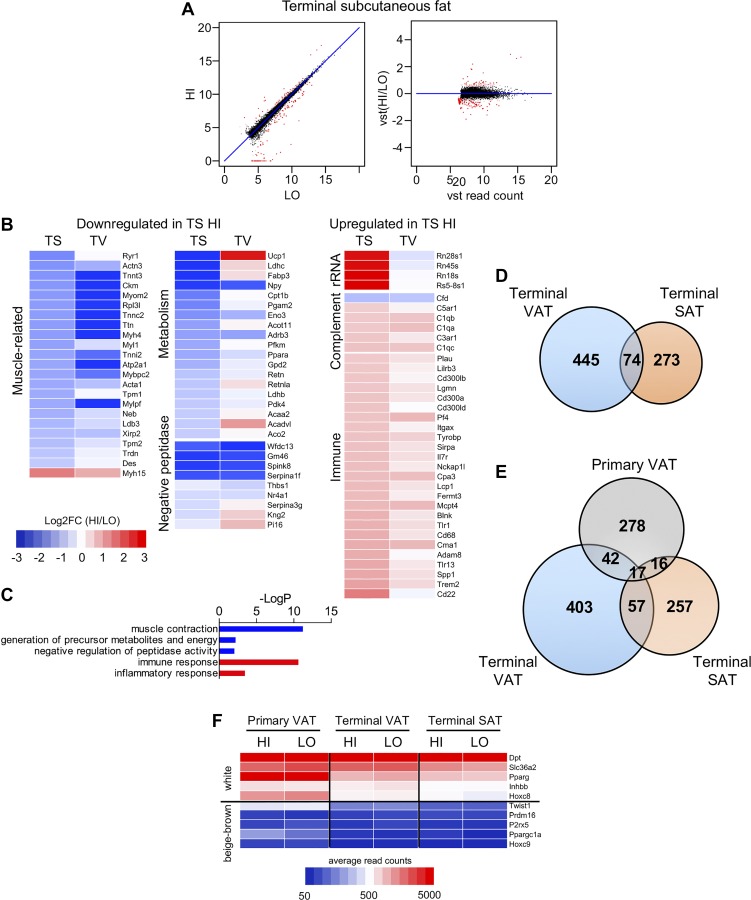
We next examined the transcriptome in terminal SAT of mice following HFD. Of the 13,250 genes identified by RNA-Seq in this analysis, we identified 347 genes to be DE between HI and LO groups, meeting a 5% FDR, with 172 downregulated genes and 175 upregulated genes in the mice with the highest insulin resistance (Fig. 5A). Ribosomal RNA species were DE, as they were in primary VAT, but increased in terminal SAT of highly insulin-resistant mice, rather than decreased as seen in primary VAT (Fig. 5, B and C, and Supplemental Table S6A). Complement and muscle-related genes were also DE in terminal SAT of HI insulin-resistant mice with an expression pattern similar to terminal VAT but opposite to that seen in primary VAT. Chronic inflammation and genes of the innate immune system are known to be increased in obese, insulin-resistant states. Interestingly, terminal SAT showed robust upregulation of immune response genes in HI insulin-resistant mice, a pattern not seen in primary or terminal VAT (Fig. 5, B and C, and Supplemental Table S6E). Of note, serpin, major urinary protein, and lipid metabolism genes were not DE in this tissue type. Using ToppGene, we identified putative transcription factor binding sites for SRF in several muscle-related genes, similar to the finding in primary VAT (Supplemental Table S5).
Fig. 5.
Immune and muscle-related genes are differentially expressed in terminal subcutaneous (TS) adipose of DIO mice with high or low insulin resistance. A: XY and VST scatterplots show 172 genes (red dots) that are differentially expressed in TS fat between high and low insulin resistance mice. B: heat maps show reduced expression of muscle-related genes and increased expression of rRNA, complement, and immune genes in high insulin resistance mice. C: biological pathways that differ in TS fat between HI and LO groups by GOrank analysis. Downregulated pathways in high insulin resistance mice are again shown in blue, and upregulated pathways are shown in red. D: Venn diagram indicating overlapping DE genes in terminal visceral and terminal subcutaneous fat. There were 445 DE genes unique to terminal visceral fat and 273 DE genes unique to terminal visceral fat. There were only 74 genes that were found in both groups. E: Venn diagram indicating overlapping DE genes in primary visceral, terminal visceral, and terminal subcutaneous fat. Gene lists can be found in Supplemental Table S4. F: heat map shows high expression of markers associated with white adipocytes and low expression of beige/brown adipocyte markers in all adipose depots studied (primary visceral, terminal visceral, and terminal subcutaneous fat). Expression levels of each marker were similar between mice with high and low insulin resistance. Blue boxes indicate genes with lower read counts; red boxes indicate genes with higher read counts.
Only 74 genes were found in common between DE sets of terminal VAT and terminal SAT. Of these, 14 muscle-related genes and four complement genes were identified; muscle-related genes were decreased and complement genes were increased in both terminal visceral and subcutaneous fat. In contrast to the comparison between primary and terminal VAT, no Mup genes were present in this subset (Fig. 5D, Supplemental Table S4B). Additional subset analysis revealed only 17 DE genes in common between primary VAT, terminal VAT, and terminal SAT (Fig. 5E, Supplemental Table S4C) with no predominant gene family identified. As expected, known markers for differentiated white adipocytes (Pparg, Dpt, Slc36a2, Inhbb, Hoxc8) were found to be robustly expressed in all three fat depots (primary VAT, terminal VAT, and terminal SAT), and other markers for brown or beige adipocytes (Ucp1, Cidea, Prdm16, Ppargc1a, P2rx5, Hoxc9, Twist1, Cox8b, Car4) were undetected or expressed only at low levels (8, 16, 29) (Fig. 5F). None of these markers were DE between HI and LO groups.
Immune response genes are DE in primary liver of mice with high or low HFD-induced insulin resistance.
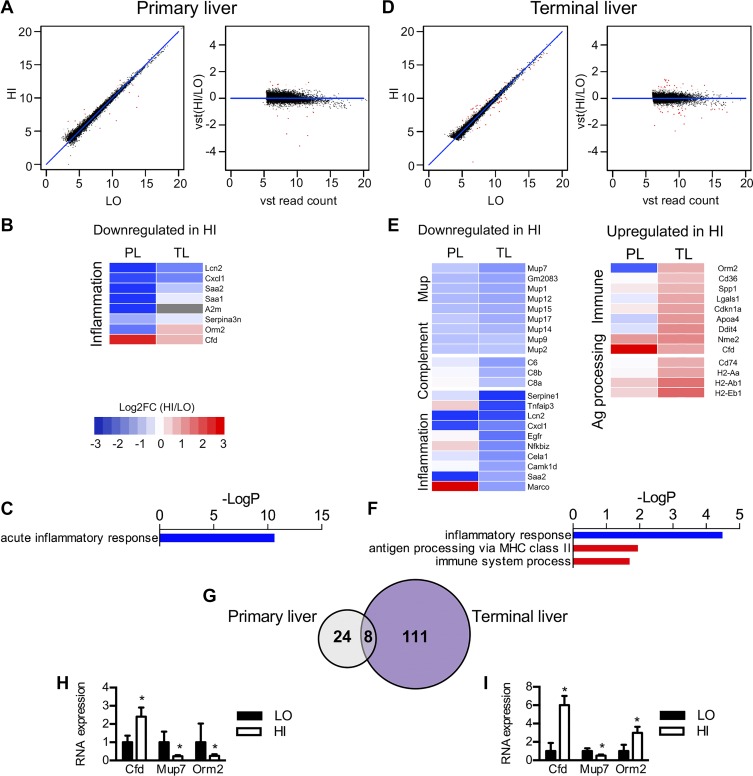
To further investigate differential gene expression that might account for the differing metabolic phenotypes after HFD, we also performed RNA-Seq analysis of both primary and terminal liver tissues for the HI and LO insulin resistance groups. Read counts were mapped for 11,538 genes in primary liver tissues (Fig. 6A). The 148 most highly expressed primary liver genes, again defined as genes with an average read count of >10,000 reads per gene, were representative of liver tissue and generally expressed at similar levels in both groups. Mup, Serpin, and ribosomal RNA genes were represented in the most highly expressed genes but were not differentially expressed between HI and LO groups, in contrast to the findings in primary VAT (Supplemental Table S7A). Only 32 DE genes were found to meet a 5% FDR. Of these, 15 genes were downregulated and 17 were upregulated in highly insulin-resistant mice. Using GOrank again for GO analysis, we found the expression of acute inflammatory response genes to be reduced in primary liver tissues of the mice with the highest insulin resistance (Fig. 6, B and C, and Supplemental Table S7D). No other pathways were identified with this method.
Fig. 6.
Inflammatory response genes are DE in liver of mice who develop either high or low HFD-induced insulin resistance both before and after HFD exposure. A: scatterplots show only 32 genes (red dots) that are DE in primary liver (PL) between high and low insulin resistance mice. B: the heat map shows reduced expression of inflammation genes in primary liver of high insulin resistance mice. C: GOrank analysis shows the acute inflammatory pathway to be downregulated in primary liver of highly insulin resistant mice. D: scatterplots show 116 genes that are DE in terminal liver (TL) between high and low insulin resistance mice. E: heat maps show reduced expression of Mup and inflammatory genes in TL of high insulin resistance mice, but also increased expression of other immune genes related to antigen processing via MHC class II molecules. F: GOrank analysis in TL tissues showing biological pathways that differ between low and high insulin resistance mice. G: Venn diagrams shows only 8 DE genes in common to both PL and TL. qPCR analysis of selected gene targets from primary (H) and terminal (I) liver tissues of individual mice from HI and LO groups (n = 6 per group).
In terminal liver, 116 DE genes were identified between HI and LO groups (Fig. 6D). Of these 61 were upregulated and 55 were downregulated. Several Mup family member genes were highly expressed in terminal liver in addition to primary liver. However, their expression was significantly reduced in the HI insulin resistance group, similar to primary visceral adipose, but differing from primary liver where they were not DE (Fig. 6E, Supplemental Table S8A). Acute inflammatory genes again were downregulated in HI insulin-resistant mice, similar to primary liver tissues. However, several genes associated with antigen presentation (Cd74, H2-Aa, H2-Ab1, H2-Eb1) and the immune response were upregulated in the same mice (Fig. 6, E and F, Supplemental Table S8E). Differential expression of Cfd, Mup7, and Orm2 was confirmed by qPCR from liver samples of individual mice, as shown in Fig. 6, H and I.
To examine the effect of high-fat feeding on hepatic gene expression, we compared primary and terminal liver tissues in the same mice before and after HFD and found over 1,400 genes to be DE. The expression of genes related to ribosomal RNA, lipid biosynthesis, and mitochondrial oxidative phosphorylation was reduced in the terminal liver after HFD. In contrast, genes related to inflammation were significantly increased in their expression in terminal liver vs. primary liver in the same mice (Fig. 7).
Fig. 7.
Inflammatory genes are induced and ribosomal RNA genes are repressed in liver with the development of diet-induced obesity. XY and VST scatterplots show over 1,400 DE genes (red dots) in a comparison of expression levels between primary (PL) and terminal liver (TL) in the HI group (A) and the LO group (B). C, D: GOrank analysis shows inflammatory or defense response genes were highly upregulated in both HI and LO mice following high-fat feeding. In contrast, the expression of genes related to ribosomal RNA, lipid biosynthesis, and mitochondrial oxidative phosphorylation was reduced in the TL after HFD. E: there was a high degree of similarity between the DE genes of the HI and LO groups with 918 genes shared by both, again predominantly reflecting inflammatory response pathways. F: heat maps show decreased expression of rRNA and steroid metabolism genes and increased expression of immune response genes in liver tissue of both high and low resistance mice following HFD. Please note that relative expression is expressed as the log2-transformed fold change of TL vs. PL mice [or log2FC (TL/PL)].
DISCUSSION
In this study, we investigated, in young mice, transcriptomic signatures in adipose and liver tissues that correlate with the later development of HFD-induced insulin results in adulthood. As these C57BL/6N mice are genetically identical, their high degree of variability in gene expression and metabolic phenotypes are independent of inherited genomic mutations and instead are presumed to reflect epigenetic changes.
Many investigators have studied differential gene expression in adipose tissue of mice fed either a low-fat or high-fat diet. However, relatively few have examined the variability of obesity phenotypes when all mice have been exposed to HFD (11, 12, 32). Koza et al. (11) used this approach to identify epigenetic determinants of diet-induced obesity in B6 mice by conducting microarray analysis of epididymal fat before HFD exposure to determine differential gene signatures that could potentially predict which mice became more severely obese. Our study builds upon their previous work by using RNA-Seq rather than microarray to provide a nonbiased, more comprehensive method to measure novel transcripts and by measuring insulin resistance rather than body weight to couple transcriptomic profiles to a metabolic outcome.
With this approach, we were able to identify visceral adipose genes in of young, pubertal-aged mice before HFD exposure that were predictive of the development of more severe insulin resistance in adulthood. Of the 15,198 genes mapped in our analysis, only 353 genes were DE in epididymal fat when we compared mice with the highest and lowest insulin resistance. Although chronic inflammation is associated with obesity-induced insulin resistance, inflammatory response genes were not represented in this set. Instead, these DE genes primarily reflected Serpin (serine protease inhibitor) genes, Mup (major urinary protein) genes, and genes related to structural constituents of muscle and muscle contraction. Lipid metabolism and complement genes were also significantly enriched in this set.
Over 20 peptidase inhibitor genes were downregulated in primary visceral fat of the most highly insulin-resistant mice. Many of these were from the Serpin family, predominantly representing Serpina1 (Serpin family A member 1). Serpina1, also known as alpha-1 antitrypsin (AAT), is an inhibitor of neutrophil elastase. In humans, reduced circulating AAT has been associated with an increased risk of Type 2 diabetes (25). Although we do not have direct evidence, we postulate that in mice with the highest insulin resistance, reduced expression of Serpina1 could result in increased neutrophil elastase activity and increased cellular insulin resistance (18, 31). This pattern was also observed in VAT of HI and LO mice after the development of obesity-related insulin resistance with reduced expression of serine protease genes.
Among the genes upregulated in primary VAT of HI mice, GO analysis revealed that genes related to muscle constituents and muscle contraction were highly represented. Increased expression of muscle-related genes in primary VAT could represent increased vasculature smooth muscle associated with angiogenesis and adipose tissue expansion in mice with the highest insulin resistance. This hypothesis is further supported by the increase in fat cell differentiation genes in the highly insulin-resistant mice. Interestingly, this pattern appeared to switch after HFD exposure with reduced expression of muscle-related genes in terminal adipose from both visceral and subcutaneous depots of HI mice.
Mup genes were among the most abundantly expressed genes in visceral fat and downregulated in highly insulin-resistant mice both before and after high fat feeding. They were also highly expressed in liver but not DE in this tissue. Mups are low-molecular-weight binding proteins that comprise part of the lipocalin family (15). Their function is not well defined, but Mup1 has been shown to increase energy expenditure by improving mitochondrial function in skeletal muscle of db/db mice (10). However, Mup genes are not expressed in humans, existing only as a unitary pseudogene disabled during primate evolution (37). Although they appear to play a role in murine glucose metabolism, they do not appear to be relevant to metabolic regulation in humans.
Ribosomal RNA species were also among the most abundantly expressed genes in all adipose and liver tissues that we examined and were significantly downregulated in VAT of young mice before HFD that later developed the most severe insulin resistance. In contrast, rRNA genes were not differentially expressed in VAT of HI and LO mice following HFD and were, in fact, upregulated in terminal subcutaneous fat of HI mice. Therefore, it is difficult to speculate on the metabolic contribution of rRNA to adipose tissue here. However, epigenetic repression of hepatic rRNA transcription following HFD has been previously shown in mice through recruitment of a nucleolar protein, nucleomethylin, to rRNA gene loci to limit intracellular energy consumption (22). We observed a significant decrease in rRNA expression in both terminal VAT and liver when compared with primary tissues, suggesting that HFD exposure was associated with rRNA repression in both visceral adipose and liver in this study.
As expected, the degree of weight gain was strongly associated with the severity of insulin resistance. Interestingly, increased body weight reflected not just adiposity but also an increase in lean mass. This increase in lean mass has been previously reported by others (11, 34, 36). We also observed increased body length in mice with the highest insulin resistance, perhaps an anabolic sequela of hyperinsulinemia due to high-fat feeding during a period when the mice were still growing. Interestingly, the prepubertal weight appeared to strongly predict of the degree of weight gain and insulin resistance after HFD. This finding is consistent with other reports that have linked initial body weight with weight gain on HFD (12, 34, 36).
To compare our results to the previously mentioned study by Koza et al. (11), we interrogated their data set listed in the Gene Expression Omnibus repository, which includes microarray expression values from inguinal fat of high and low weight gainers. In our analysis, there were 1,267 DE genes between high vs. low weight gainers that met a FDR value of < 5% (q value <0.05), compared with the 353 DE adipose genes in mice with high vs low insulin resistance in our study. Between their study and ours, there are 56 DE genes in common, representing several gene families also identified in our study, including major urinary protein, Serpin, lipid metabolism, and muscle-related gene families (Supplemental Table S9). Therefore, there appears to be some overlap in the gene signatures.
Chronic inflammation in adipose tissue has been well characterized in the pathogenesis of obesity-related insulin resistance, and we observed a significant induction of inflammatory genes in visceral adipose when compared before and after HFD. However, we did not observe gene expression changes reflecting this mechanism in VAT when comparing high and low insulin resistance groups, either before or after HFD. In contrast, we found that immune and complement genes were significantly upregulated in subcutaneous fat of the most highly insulin-resistant mice after high fat feeding. This finding highlights distinct differences in the gene signatures between visceral and subcutaneous adipose depots in our comparative model and suggests that inflammation in SAT may exacerbate the severity of insulin resistance in response to high-fat feeding. In contrast to adipose tissue, we did observe decreased inflammatory gene expression in the whole liver when comparing mice with high and low insulin resistance before HFD, which indicates that inflammatory gene changes in the liver rather than fat may be more predictive for the development of severe insulin resistance.
Our RNA-Seq data interpretation is bolstered by the fact that genes known to be abundantly expressed in adipose and liver tissues, as well as adipocyte markers, were appropriately represented in our analyses. However, our study only includes limited PCR validation of our target genes. It will be important in future work to expand these data to not only confirm their expression but also explore their potential function in mediating insulin resistance. In addition, we examined whole adipose and liver rather than fractioned cells as the surgically removed tissues from pubertal-age mice were of very small volumes. We analyzed pooled samples in each group for the same reason. Therefore, we could not differentiate gene expression by a specific cell type and instead inferred cellular function by referencing the known literature.
This study would have benefited from the addition of a control group on standard chow to provide context for the gene expression changes observed in our HFD-fed mice. This group was unfortunately not included due to logistical considerations. Another limitation of this study is the lack of metabolic data such as fasting glucose and insulin levels before HFD exposure to assess whether baseline metabolic changes might account for the differences in insulin resistance observed later. Other confounders include differences in food intake or energy expenditure that were not measured here but could drive either differences in the body weight or severity of insulin resistance observed in our cohort. Previous studies have shown that these factors [increased food intake, increased metabolic efficiency (11) and reduced activity (36)] are associated with differential weight gain in high fat-fed C57BL/6 mice.
In summary, we provide novel data regarding early transcriptomic alterations in VAT in young mice that may account for later variation in their metabolic response to HFD-induced obesity. Adipose gene expression profiles can vary with age, and one cannot necessarily extrapolate data obtained from adults to youth (4, 14). Our study underscores the importance of using models of childhood as well as adult obesity as their conclusions regarding metabolic responses may differ. A significant finding from our GO analyses is that classically recognized gene pathways associated with adipose tissue dysfunction in obese adults including inflammation and hypoxia were not altered when we compared mice that later developed either high or low levels of insulin resistance. Less well recognized targets such as Serpin genes were represented instead in our childhood model, including Serpina1 (AAT). Many studies have explored the clinical applications of AAT including Type 1 diabetes mellitus. More recent literature has shown an association between AAT and insulin resistance phenotypes in mice (18) and humans (25, 26). Our study provides further impetus for its study as a novel therapeutic strategy for insulin resistance and Type 2 diabetes.
GRANTS
This work was supported by grants to J. J. Kim from the National Institute of Diabetes and Digestive and Kidney Diseases DK-075479 and P30 DK-063491 (UCSD/UCLA/Cedars-Sinai/Salk Diabetes Research Center) as well as a UCSD Pediatric Genome Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.C., A.J., O.O., S.T.K., and J.J.K. performed experiments; K.C., W.F., R. Sasik, R. Saito, and J.J.K. analyzed data; K.C., W.F., R. Saito, and J.J.K. interpreted results of experiments; K.C. and J.J.K. prepared figures; K.C. and J.J.K. drafted manuscript; K.C. and J.J.K. edited and revised manuscript; K.C. and J.J.K. approved final version of manuscript; J.J.K. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank the staff at the University of California, San Diego (UCSD) Institute of Genomic Medicine who carried out the sequencing for this project. We also thank Dr. Gautam Bandyopadhyay for assistance with the qPCR experiments.
REFERENCES
- 1.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 11: R106, 2010. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol 23: 570–582, 1995. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 3.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 282: E834–E842, 2002. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 4.Chu DT, Malinowska E, Gawronska-Kozak B, Kozak LP. Expression of adipocyte biomarkers in a primary cell culture models reflects preweaning adipobiology. J Biol Chem 289: 18478–18488, 2014. doi: 10.1074/jbc.M114.555821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311: 1778–1786, 2014. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconer DS. Weight and age at puberty in female and male mice of strains selected for large and small body size. Genet Res 44: 47–72, 1984. doi: 10.1017/S0016672300026240. [DOI] [PubMed] [Google Scholar]
- 7.Fengler VH, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Müller R, Kiemer AK, Magnes C, Haybaeck J, Lackner C, Sargsyan K. Susceptibility of different mouse wild type strains to develop diet-induced NAFLD/AFLD-associated liver disease. PLoS One 11: e0155163, 2016. doi: 10.1371/journal.pone.0155163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia RA, Roemmich JN, Claycombe KJ. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr Metab (Lond) 13: 24, 2016. doi: 10.1186/s12986-016-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr 117: 376–382, 1987. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- 10.Hui X, Zhu W, Wang Y, Lam KS, Zhang J, Wu D, Kraegen EW, Li Y, Xu A. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J Biol Chem 284: 14050–14057, 2009. doi: 10.1074/jbc.M109.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2: e81, 2006. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak LP, Newman S, Chao PM, Mendoza T, Koza RA. The early nutritional environment of mice determines the capacity for adipose tissue expansion by modulating genes of caveolae structure. PLoS One 5: e11015, 2010. doi: 10.1371/journal.pone.0011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu LF, Shen WJ, Ueno M, Patel S, Azhar S, Kraemer FB. Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PLoS One 8: e72367, 2013. doi: 10.1371/journal.pone.0072367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan DW, Marton TF, Stowers L. Species specificity in major urinary proteins by parallel evolution. PLoS One 3: e3280, 2008. doi: 10.1371/journal.pone.0003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X, Lee P, Chisholm DJ, James DE. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol (Lausanne) 6: 1, 2015. doi: 10.3389/fendo.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449, 2005. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 18.Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, Aubert G, Candelaria K, Thomas S, Shin DJ, Booth S, Baig SM, Bilal A, Hwang D, Zhang H, Lovell-Badge R, Smith SR, Awan FR, Jiang ZY. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab 17: 534–548, 2013. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int 28: 3–11, 2008. doi: 10.1111/j.1478-3231.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 3: 1167–1170, 2008. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief 219: 1–8, 2015. [PubMed] [Google Scholar]
- 22.Oie S, Matsuzaki K, Yokoyama W, Tokunaga S, Waku T, Han SI, Iwasaki N, Mikogai A, Yasuzawa-Tanaka K, Kishimoto H, Hiyoshi H, Nakajima Y, Araki T, Kimura K, Yanagisawa J, Murayama A. Hepatic rRNA transcription regulates high-fat-diet-induced obesity. Cell Reports 7: 807–820, 2014. doi: 10.1016/j.celrep.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes–related traits in mouse strains susceptible to diet-induced obesity. Diabetes 52: 1958–1966, 2003. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 24.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127: 1–4, 2017. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandström CS, Ohlsson B, Melander O, Westin U, Mahadeva R, Janciauskiene S. An association between Type 2 diabetes and alpha-antitrypsin deficiency. Diabet Med 25: 1370–1373, 2008. doi: 10.1111/j.1464-5491.2008.02584.x. [DOI] [PubMed] [Google Scholar]
- 26.Setoh K, Terao C, Muro S, Kawaguchi T, Tabara Y, Takahashi M, Nakayama T, Kosugi S, Sekine A, Yamada R, Mishima M, Matsuda F. Three missense variants of metabolic syndrome-related genes are associated with alpha-1 antitrypsin levels. Nat Commun 6: 7754, 2015. doi: 10.1038/ncomms8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 9: 474–488, 2008. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Ahn J, Suh Y, Davis ME, Lee K. Identification of novel tissue-specific genes by analysis of microarray databases: a human and mouse model. PLoS One 8: e64483, 2013. doi: 10.1371/journal.pone.0064483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 18: 1407–1412, 2012. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voigt A, Agnew K, van Schothorst EM, Keijer J, Klaus S. Short-term, high fat feeding-induced changes in white adipose tissue gene expression are highly predictive for long-term changes. Mol Nutr Food Res 57: 1423–1434, 2013. doi: 10.1002/mnfr.201200671. [DOI] [PubMed] [Google Scholar]
- 33.Wu G, Feng X, Stein L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol 11: R53, 2010. doi: 10.1186/gb-2010-11-5-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Smith DL Jr, Keating KD, Allison DB, Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 22: 2147–2155, 2014. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Ahn J, Suh Y, Hwang S, Davis ME, Lee K. Identification of CTLA2A, DEFB29, WFDC15B, SERPINA1F and MUP19 as novel tissue-specific secretory factors in mouse. PLoS One 10: e0124962, 2015. doi: 10.1371/journal.pone.0124962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang LN, Morgan DG, Clapham JC, Speakman JR. Factors predicting nongenetic variability in body weight gain induced by a high-fat diet in inbred C57BL/6J mice. Obesity (Silver Spring) 20: 1179–1188, 2012. doi: 10.1038/oby.2011.151. [DOI] [PubMed] [Google Scholar]
- 37.Zhang ZD, Frankish A, Hunt T, Harrow J, Gerstein M. Identification and analysis of unitary pseudogenes: historic and contemporary gene losses in humans and other primates. Genome Biol 11: R26, 2010. doi: 10.1186/gb-2010-11-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]