Abstract
Breathing occurs without thought but is controlled by a complex neural network with a final output of phrenic motor neurons activating diaphragm muscle fibers (i.e., motor units). This review considers diaphragm motor unit organization and how they are controlled during breathing as well as during expulsive behaviors.
Introduction
Several times in every minute of every day, we take a breath, typically without conscious effort and hopefully without major interruptions, even while we sleep. Indeed, breathing is essential for life. It seems so simple; yet taking a breath is a complex motor function that relies on the exquisite coordinated neural activation of a number of skeletal muscles that generate a negative intrathoracic pressure to draw air into our lungs—the inspiratory pump. The diaphragm is the major inspiratory pump muscle, and its neural activation generates a transdiaphragmatic pressure (Pdi) that drives air into the lungs. However, in addition to inspiration, activation of the diaphragm muscle is necessary for expulsive behaviors, including expectoration and sternutation, which are essential for clearing the airways and maintaining airway patency (38, 122, 163, 172, 173, 176, 182, 193). The diaphragm muscle also contributes to non-respiratory activities, such as swallowing and vocalization (4, 208, 222). In evolution, the diaphragm muscle is unique to mammals, and its physiological importance cannot be argued.
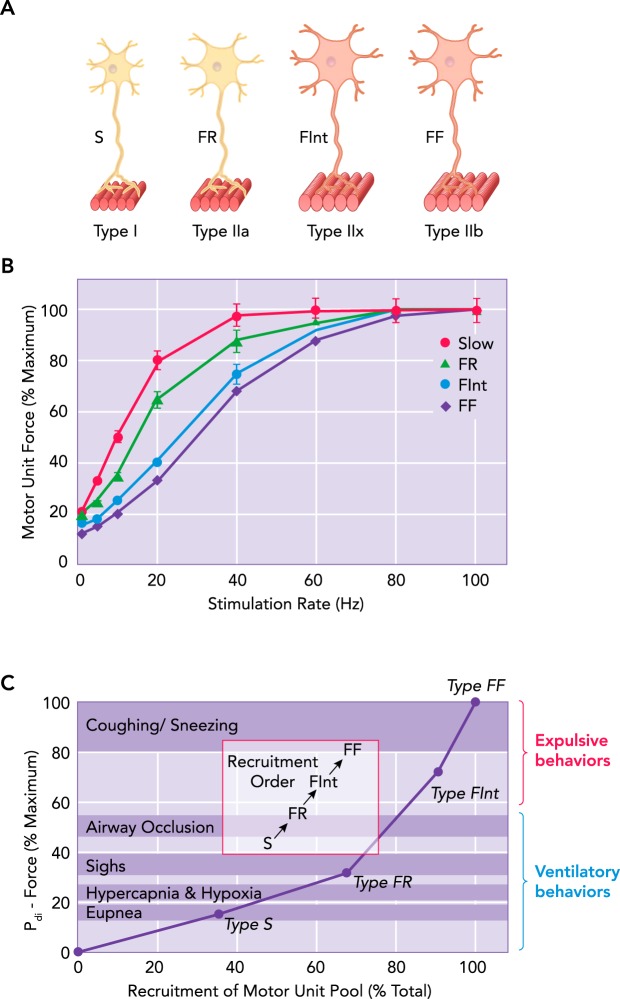
In all skeletal muscles, including the diaphragm, the final common output of neural control is the motor unit, consisting of a motor neuron and the group of muscle fibers it innervates (112). When the motor neuron is activated, the resulting action potential propagates along axonal branches to activate all muscle fibers of the motor unit in an all-or-none fashion. Importantly, all muscle fibers comprising a motor unit are homogeneous with respect to their mechanical, fatigue, and biochemical properties, which matches the properties of contractile protein expression that defines muscle fiber types (34, 147, 184, 195, 196, 199). The range of muscle-fiber mechanical and fatigue properties establishes the limits of the neural control of force generation and contraction of the muscle. Motor units and their muscle fibers can be classified into four types based on these mechanical and fatigue properties: 1) slow, fatigue-resistant (type S) motor units comprising type I muscle fibers; 2) fast, fatigue-resistant (type FR) motor units comprising type IIa muscle fibers; 3) fast, fatigue-intermediate (type FInt) motor units comprising type IIx muscle fibers; and 4) fast, fatigable (type FF) motor units comprising type IIx and/or IIb muscle fibers (FIGURE 1).
FIGURE 1.
Different DIAm motor unit types are distinguished by their intrinsic, mechanical, and fatigue properties
A: different DIAm motor unit types are distinguished by their intrinsic, mechanical, and fatigue properties, and are classified as type S, FR, FInt, and FF. Within a particular motor unit, all muscle fibers are homogeneous, as evidenced by myosin heavy chain (MyHC) expression. B: force (normalized to percent maximum tetanic force) generated by cat diaphragm motor units at different frequencies of stimulation. Results are for individual motor units classified by their contractile and fatigue properties. The steepest portion of the force-frequency curve occurs between 10 and 30 Hz for all types of motor units in the diaphragm muscle (52), consistent with onset and peak discharge frequencies of ~8 and ~25 Hz, respectively, for type S and FR units (top arrows) and ~15 and ~60 Hz, respectively, for type FInt and FF units (bottom arrows), reported in Ref. 198. C: diaphragm motor units are recruited to accomplish a range of motor behaviors. Ventilation (eupnea, hypercapnia, and hypoxia) is accomplished by recruitment type S and FR motor units, whereas higher-force airway clearance behaviors require recruitment of more fatigable DIAm motor units.
In 1957, Henneman showed that motor units recruited first have slower axonal conduction velocities reflecting smaller axonal diameters and motor neuron size (82). From these results, he hypothesized that motor unit recruitment depends on the intrinsic size-dependent electrophysiological properties of motor neurons. Smaller motor neurons with less surface area have smaller membrane capacitance and higher membrane input resistance. Thus, for a given level of synaptic current, smaller motor neurons have a greater change in membrane potential compared with larger motor neurons. The “size principle” of motor unit recruitment was subsequently shown to predict the orderly recruitment of motor units across a variety of skeletal muscles including the diaphragm.
This review compiles our current understanding of neuromotor control of the diaphragm pump muscle in three parts. First, we outline the basis for classification of diaphragm muscle fiber and motor unit types and how their heterogeneous properties provide for a range of neural control of force generation during different motor behaviors. Second, we examine the pathways in the central nervous system that provide synaptic input to phrenic motor neurons. These circuits are responsible for rhythmic respiratory-related activation of the diaphragm muscle during breathing or recruitment of diaphragm motor units during more forceful expulsive motor behaviors. Third, we explore how neuromotor control of the diaphragm muscle changes during our lifespan and how it is impacted by disease and other clinical conditions.
Diaphragm Motor Units
Cross-Bridge Cycling and Mechanical Properties
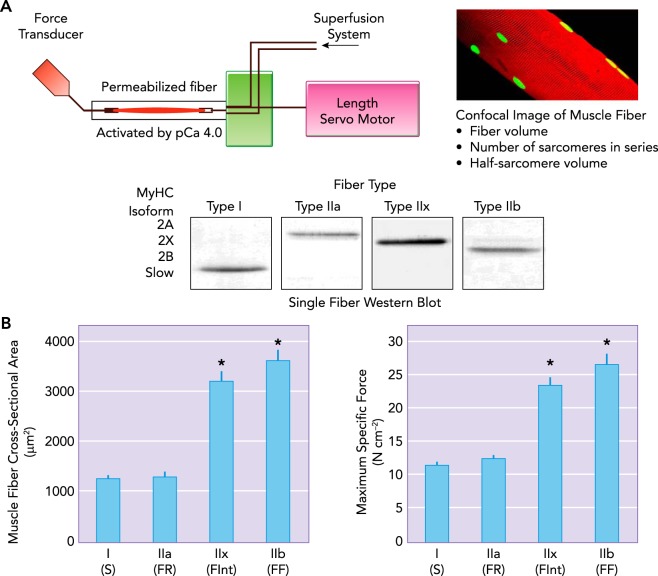
In skeletal muscle fibers, the basic functional unit underlying force generation and contraction is the cross bridge, formed by the binding of the myosin heavy chain (MyHC) head to the actin filament. Within a sarcomere, six actin filaments surround each myosin filament, and the position of MyHC heads spiral around the thick filament to align with the position of the actin filaments (179). The absolute number of parallel cross bridges formed contributes directly to the force produced. If the cross-sectional area of a fiber increases, there is also an increase in the number of MyHC heads in parallel that can form cross bridges and contribute to force generation (187). Muscle force is often normalized to fiber or muscle cross-sectional area (specific force) to account for this difference. During force generation and contraction, cross bridges pull actin filaments toward the midline of the sarcomere. Within the sarcomere, the force vectors on z lines are in opposite directions toward the midline of the sarcomere. In skeletal muscle fibers, with a fixed origin and moveable insertions, force generation depends on the number of strongly bound force-generating cross bridges in parallel per half-sarcomere. The actual number of cross bridges that can form depends on the MyHC content per half-sarcomere (n) and the proportion of these MyHC heads that are actually forming strongly bound force-generating cross bridges (αfs). This proportion of MyHC heads binding to actin depends on the overlap of thick and thin filaments (underlying the force-length relationship of skeletal muscle fibers) and the myoplasmic Ca2+ concentration (underlying the force-Ca2+ relationship of muscle) (179). Finally, each strongly bound cross bridge contributes an average unit of force (f). Thus the total force generated by a muscle fiber is determined by the following equation: (57–59). At maximal myoplasmic Ca2+ activation, differences in specific force between type I and II diaphragm muscle fibers are attributed to differences in MyHC content per half-sarcomere (n) and the unitary force generated by MyHC isoforms (f) (57–59) (FIGURE 2).
FIGURE 2.
Technique for estimating maximum specific force of different diaphragm muscle fibers
A: technique for estimating maximum specific force of different diaphragm muscle fibers, distinguished by MyHC expression. Single permeabilized diaphragm skeletal muscle fibers are mounted between a force transducer and a servo motor (length control). The chamber is superfused with Ca2+-activating solution, and the rate and magnitude of force produced by a single fiber assessed. Differences in specific force between different fiber types is related to the different MyHC content per half sarcomere and differing unitary forces produced by different MyHC isoforms. B: in the diaphragm muscle of most species, type I and IIa diaphragm muscle fibers have smaller cross-sectional areas than those of type IIx and IIb fibers. There are also differences in maximum specific force produced across diaphragm muscle fiber types, with type IIb producing greater force than type IIx > type IIa > type I (59).
When forces are exerted on an external load, muscle fibers can also contract. The force (or load)-velocity relationship of muscle is an essential mechanical property of muscle fibers. The velocity of muscle fiber shortening depends inversely on the external load imposed. Thus the force-velocity relationship is anchored at zero load, where maximum unloaded shortening velocity occurs, and at a maximum load where the intrinsic force generated by the muscle fiber is offset by the external load and no shortening occurs. Cross bridges cycle between a bound state, where they can generate force, and a detached state. This duty cycle depends on external load and thus determines the rate of cross-bridge cycling. Thus, with increasing velocity of shortening, the number of attached cross bridges decreases. The inverse is also true so that when velocity decreases, more cross bridges are attached and generate force.
As cross bridges cycle, ATP is hydrolyzed, and total ATP consumption in a skeletal muscle fiber can be determined by , where n is the number of MyHC heads, αfs is the fraction of MyHC heads that are strongly bound to actin, b is the total number of sarcomeres in series, and gapp is the rate constant for cross-bridge detachment. With increasing cross-bridge cycling, ATP consumption increases.
In 1923, Wallace O. Fenn observed that heat production during muscle contraction increases as work (or power) increases (termed the Fenn effect) (46). Power in the context of muscle fibers is the product of the force generated and the velocity of shortening. Work is the power produced by muscle fibers over a period of time. As power or work of a muscle fiber increases, energy requirements (ATP consumption) also increase. Peak power or work of a muscle fiber occurs at ~33% of maximum velocity and maximum force. In diaphragm muscle fibers, the maximum ATP consumption corresponds to peak power output (188, 192).
Classification of Motor Unit and Muscle Fiber Types
In the diaphragm muscle, as in other skeletal muscles, motor units are commonly classified into four types (type S, FR, FInt and FF; see FIGURE 1), according to the mechanical and fatigue properties of their constituent muscle fibers (52, 58, 59, 172, 179, 180, 182–184, 186) (FIGURE 2). Importantly, all of the muscle fibers within an individual motor unit display homogeneous contractile protein expression and biochemical properties (52, 75, 132, 133). This homogeneity of muscle fiber type within motor units has been confirmed in the adult cat diaphragm (41, 96, 184, 196).
In modern classification schemes, different muscle fiber types are identified by the expression of different MyHC isoforms that vary in their ATPase activities and cross-bridge cycling rates. Type I muscle fibers in type S motor units express the MyHCSlow isoform that displays a lower rate of ATP hydrolysis, leading to slower cross-bridge cycling rate and slower velocity of shortening. These type I fibers generally have smaller cross-sectional areas and generate less force per cross-sectional area (specific force) (FIGURE 2); thus their contribution to overall force generation is proportionately lower (56–59). However, type I fibers also have a high mitochondrial volume density and oxidative capacity that allows greater energy efficiency and contributes to their fatigue resistance during repeated activation (41, 186).
Type IIa muscle fibers in type FR diaphragm motor units express the MyHC2A isoform that displays a higher ATP hydrolysis rate compared with MyHCSlow, and thus a faster cross-bridge cycling rate and velocity of shortening. The cross-sectional areas of type IIa fibers are also smaller, and they generate approximately the specific force compared with type I fibers; thus their contribution to overall force generation is proportionately smaller than other type II fibers (FIGURE 2). Similar to type I fibers, type IIa also have a high mitochondrial volume density and oxidative capacity that contributes to their greater fatigue resistance during repeated activation (56–59).
Type IIx and IIb fibers in type FInt and FR diaphragm motor units express MyHC2X and/or MyHC2B isoforms. In fact, relatively few diaphragm fibers express the MyHC2B isoform alone. Co-expression of expression of MyHC2X and MyHC2B isoforms in diaphragm muscle fibers is fairly common, and it appears that differences in mechanical, energetic, and fatigue properties depend on the extent of co-expression (186). Fibers comprising MyHC2X and MyHC2B display the highest ATP hydrolysis rates and thus the fastest cross-bridge cycling rates and velocities of shortening (56–59). Type IIx and/or IIb muscle fibers have the largest cross-sectional areas (68, 111, 128, 142, 169, 189, 219) and generate the highest muscle-specific forces (56–59) (FIGURE 2); thus their relative contribution to diaphragm force generation is greatest of all fiber types.
Diaphragm Force and Transdiaphragmatic Pressure Generation
As the diaphragm muscle contracts, the downward movement increases abdominal pressure while decreasing intrathoracic pressure. The pressure gradient across the diaphragm or transdiaphragmatic pressure (Pdi) is a reflection of the diaphragm muscle force generated during different ventilatory and higher force expulsive behaviors in a variety of species (61, 70, 72, 100, 120, 122, 170, 173, 175, 178, 182). To provide information regarding the range of forces or Pdi that can be generated by the diaphragm muscle, bilateral phrenic nerve stimulation is used to obtain the maximum Pdi (Pdimax). In humans, Pdimax can also be estimated using maximum voluntary efforts such as the “sniff” test (139). In humans, Pdimax is ~11.0 kPa, and the Pdi generated during eupnea is ~8% of Pdimax (175). In cats, Pdimax is ~9.0 kPa and the Pdi generated during eupnea is ~10% of Pdimax (182). In the rat and mouse, Pdimax is ~8.0 kPa, and the Pdi generated during eupnea is ~20% of Pdimax (62, 72, 100). When ventilation is stimulated by hypoxia (10% O2) and/or hypercapnia (5% CO2), the forces generated by the diaphragm muscle increase to ~20–30% Pdimax in cats and rats (120, 122, 170, 173, 174, 176–178, 182, 193). Exposure to hypoxia and hypercapnia represents a robust ventilatory stimulus, but diaphragm forces increase even further during sustained airway occlusion, e.g., as occurs during obstructive sleep apnea. Across species, the Pdi generated during sustained airway occlusion is ~50–60% of Pdimax (120, 122, 170, 173, 174, 176–178, 182, 193). Thus the forces generated during even these extreme ventilatory behaviors do not approach the maximal force-generating capacity of the diaphragm muscle, so there is considerable force reserve (FIGURE 1).
Diaphragm forces generated during expulsive behaviors are much higher, and thus recruitment of a greater fraction of the motor unit pool is necessary (FIGURE 1). Thus about half of the force-generating capacity of the diaphragm muscle is used only during brief, high-force, expulsive behaviors (e.g., coughing, sneezing) (120, 122, 170, 173, 174, 176–178, 182, 193). These results indicate that, to fully evaluate diaphragm muscle weakness, it is necessary to measure diaphragm muscle activity across a range of motor behaviors (175, 191, 203).
Diaphragm Motor Unit Recruitment and Pdi Generation
Lower force ventilatory behaviors of the diaphragm muscle are accomplished by the maximal recruitment of only fatigue-resistant type S and FR motor units, even during hypoxic/hypercapnic conditions. However, during more extreme ventilatory challenges such as sustained airway occlusion, the additional recruitment of at least some type of FInt motor units is required. The Pdi generated during sneezing and coughing requires near full recruitment of all diaphragm motor units, particularly the more fatigable type FInt and FF motor units (FIGURE 1C) (61, 70, 72, 100, 120, 122, 170, 173, 175, 178, 182). Although considerable force reserve exists for the diaphragm muscle to generate these higher-force expulsive behaviors, this comes at the expense of fatigue; so these higher forces cannot be sustained.
Neural Control of Diaphragm Muscle Activation
There are five main components involved in neural control of diaphragm muscle activation: 1) phrenic motor neurons as the neuronal component of a motor unit and the final common output for force generation; 2) central pattern generator responsible for timing and pattern of the motor behavior; 3) pre-motor neurons responsible for transmitting the output of the central pattern generator; 4) interneurons responsible for modulating or coordinating premotor neuron and/or phrenic motor neuron excitability—these interneurons also serve to integrate sensory feedback (e.g., chemoreceptive, lung stretch, propriospinal or other afferent inputs); and 5) direct cortical premotor input to motor neurons via the corticospinal pathway.
Phrenic Motor Neurons
Diaphragm motor units are innervated via the phrenic nerve by phrenic motor neurons (collectively, the phrenic motor pool) located within the ventral horn (lamina IX) of the cervical spinal cord, segments C3–C5 in rats (3, 123, 142, 151, 202), C3–C6 in mice (150), C4–C6 in cats (216), and C3–C5 in humans (99). In the rat, there are ~230 phrenic motor neurons on each side of the cervical spinal cord (3, 123, 142, 151), bilaterally innervating the diaphragm muscle (providing a total of ~460 motor units). Innervation of the diaphragm muscle displays a somatotopic organization. The rostral cervical segments of the phrenic motor neuron pool innervates the ventral region of the costal and crural areas of the diaphragm muscle (196). More caudal segments of the phrenic motor pool innervate the more dorsal portions of both the costal and crural regions (196).
Adult phrenic motor neuron size is heterogeneous, matching the range of diaphragm motor unit types. In the rat, the somal surface areas of phrenic motor neurons range from 1,000 to 8,000 µm2 (median ~4,500 µm2) (123, 142, 145, 151). In ~80% of cases, somal surface areas of phrenic motor neurons display a significant biomodal distribution, perhaps reflecting differences between phrenic motor neurons innervating type S and FR motor units, and larger phrenic motor neurons innervating type FInt and FF motor units. Other than size, it is difficult to identify phrenic motor neurons innervating different motor unit types. It has been reported that larger motor neurons (FInt and FF motor units) do not express the synaptic vesicle protein SV2A (18), and we have recent evidence to support the absence of SV2A mRNA in larger phrenic motor neurons.
Size Principle and Diaphragm Motor Unit Recruitment
Although much is still to be defined in regard to phrenic motor neuron inputs, it remains immutable that the individual phrenic motor neuron is the final integrator of signals from pattern generators and other neural circuits. The diaphragm motor unit remains the final executor of neuromotor control and produces motor force output across a range of ventilatory and higher force, non-ventilatory behaviors. It still remains unclear whether phrenic motor neurons constituting the different types of diaphragm motor units receive diverse premotor inputs. Regardless, neuromotor control of the diaphragm muscle during different motor behaviors requires production of graded levels of force generation, a property dependent on recruitment and rate coding of motor units themselves (FIGURES 1 AND 3).
FIGURE 3.
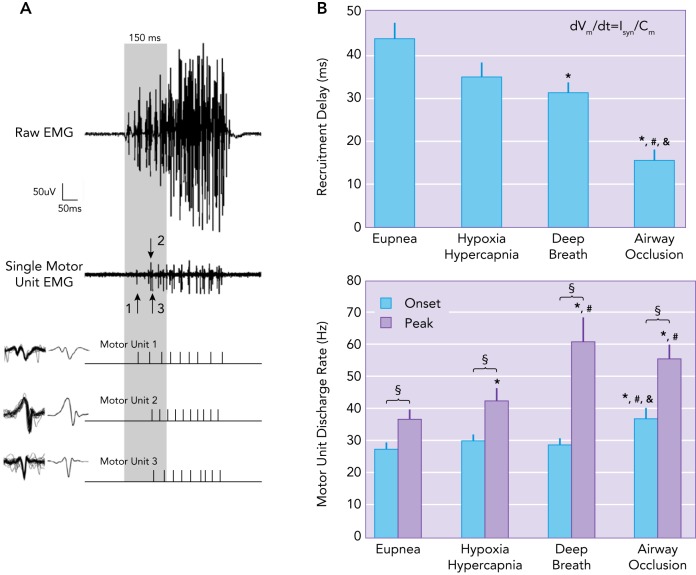
Example of single motor unit recordings with compound EMG activity during eupnea
A: example of single motor unit recordings with compound EMG activity during eupnea. The motor unit action potentials, represented with their averaged waveforms, are recruited at the initial 150 ms of DIAm EMG activity (165). B: recruitment order was maintained with increasing neural drive (Isyn), whereas recruitment delay decreases and discharge rate increases. Mean recruitment delay (ms) is reduced compared with eupnea for deep breaths and airway occlusions. The peak discharge rate for motor units is increased compared with onset discharge rate for all behaviors assessed. Peak discharge rates for hypoxia-hypercapnia are greater than those of eupnea, with deep breaths and airway occlusion greater than both eupnea and hypoxia-hypercapnia behaviors (163).
Motor units are recruited in an orderly fashion based on the intrinsic electrophysiological properties of motor neurons (14, 98, 182). These intrinsic properties are largely size-dependent, since, for a given magnitude of synaptic input, smaller, more excitable motor neurons are recruited before larger motor neurons. Smaller motor neurons have smaller membrane capacitance (Cm) and higher membrane input resistance. The excitability of a motor neuron reflects the rate of change of membrane potential (dVm/dt) for a given amount of current input, in this case synaptic current (Isyn) (FIGURES 1 AND 3). Mathematically, this may be expressed as the following equation . Thus, per arbitrary unit of synaptic current (Isyn), smaller motor neurons (with a low Cm) have a greater change in membrane potential (dVm/dt) compared with larger motor neurons (FIGURE 3) (48, 204, 217, 218). Motor unit type (that generally matches their mechanical and fatigue properties) also appears to be an important determinant of motor unit recruitment order, with type S and FR motor units recruited first followed by type FInt and FF motor units (FIGURE 1) (13, 126, 204). In agreement, it has been shown that the force developed by a motor unit is highly indicative of its recruitment order (217). In the diaphragm muscle, it is very likely that type S and FR motor units are recruited first, especially to accomplish the lower-force, high duty cycle (active vs. inactive) behavior of breathing. During rhythmic breathing, inspiration typically has a duty cycle of 30–40%, which would cause marked fatigue if type FInt and FF motor units were recruited. Higher-force, expulsive sneezing and coughing behaviors of the diaphragm muscle have very short durations, allowing sufficient time for recovery from any fatigue that might occur.
Based on these observations, a model of diaphragm motor unit recruitment was formulated based on an orderly recruitment of type S, FR, FInt, and FF motor units (52, 120, 122, 163, 173, 174, 178, 182). In the initial model in the cat diaphragm, the force contribution of each motor unit type was based on direct measurements of motor unit mechanical properties (52, 171, 172, 174, 181, 182). Subsequently, in the rat diaphragm muscle, the force contributed by different motor unit types was estimated based on measurements of specific force generated by single muscle fibers of different types (56–59), the cross-sectional area of different muscle fiber types (111, 128, 142, 189, 219), the proportion of different fiber types in the diaphragm muscle (41, 52, 172, 184, 186), and the assumption of comparable innervation ratios across motor unit types (53, 173, 174) (FIGURE 2). In each of these models, ventilatory behaviors (i.e., eupnea and response to hypoxia/hypercapnea) are accomplished by the recruitment of type S and FR motor units (120, 122, 170, 173, 174, 176, 182). The number of phrenic motor neurons recruited during breathing in cats is ~23% of the total pool (95), corresponding to the relative proportions of type S and FR motor units in the diaphragm muscle (52, 182, 184). To perform higher-force, expulsive behaviors (i.e., coughing and sneezing), the recruitment of additional FInt and FF motor units is required (120, 122, 170, 173, 174, 176, 182). The progressions in force generated by diaphragm motor units (type FF > FInt > FR > S) (52, 56–59, 181, 182) results in various slopes of force development during the sequential recruitment of motor units (FIGURE 1).
Skeletal muscle force production increases with increased stimulus frequency, then reaches a plateau that corresponds to maximum tetanic force. This relationship is sigmoidal in shape, and tetanic fusion of force occurs at lower stimulation frequencies for type S and FR motor units compared with type FInt and FF (FIGURE 1) (52, 198). The model for motor unit recruitment assumes different activation rates for different motor unit types. Motor unit discharge frequencies change during inspiration (14, 103, 198), with onset discharges being lower compared with the peak activity discharges occurring later within the inspiratory burst (89, 198). The steepest portion of the force-frequency curve occurs between 10 and 30 Hz for all types of motor units in the diaphragm muscle (52), consistent with onset and peak discharge frequencies of ~8 and ~25 Hz, respectively, for type S and FR units and ~15 and ~60 Hz, respectively, for type FInt and FF units (198).
Motor units are also recruited systematically depending on motor unit type to develop a range of forces required to accomplish different motor behaviors (120, 122, 164, 165, 170, 173, 174, 176, 178). Recruitment order is maintained with increasing neural drive (Isyn), whereas recruitment delay decreases and discharge rate increases (165) (FIGURE 3). Onset discharge rates are comparable across ventilator behaviors, including eupnea, hypoxia-hypercapnia, and deep breaths (163, 165) (FIGURE 3). Accordingly, type S and FR motor units, which develop lower forces but are fatigue resistant, are recruited first, especially during sustained motor behaviors (103, 119, 120, 163). Higher-force, shorter-duration motor behaviors are achieved by the additional recruitment of type FInt and FF motor units. The size principle for motor unit recruitment in the diaphragm muscle has been confirmed (26, 95, 120, 163, 170, 174, 176, 178, 182, 193) and underlies neuromotor control of force generation across a range of motor behaviors.
Central Pattern Generator
Motor behaviors requiring the diaphragm muscle, such as ventilation, sneeezing, coughing, swallowing, and vocalization are likely to be governed by several distinct central pattern generators. In particular, the neuronal circuitry responsible for the rhythmic behavior of breathing is the best characterized central pattern generator affecting phrenic motor neurons and diaphragm muscle activation. For ventilatory behaviors, this central pattern generator plays an indispensable role in determining the timing, duration, and pattern of the different phases of the respiratory cycle. Other central pattern generators affecting diaphragm muscle neuromotor control may also exist in the brain stem and the spinal cord, e.g., for swallowing (27), coughing, and sneezing. To avoid deleterious combinations of rhythmic behaviors, all pattern generators must be integrated with that of the pattern generator for respiration. For example, swallowing interrupts the central pattern generator for respiration.
Respiratory pattern consists of three main phases: inspiration, post-inspiration, and expiration (154, 155). It is generally agreed that the Pre-Bötzinger Complex (PreBötC) in the medulla provides the spontaneously active “kernel” of neurons for the metronomic drive for the inspiratory phase, via interactions of various membrane channels (7). Experiments show that the PreBötC is essential for inspiration (125) and is the prime source of inspiratory excitatory drive to respiratory pre-motor neurons (201) via a core subpopulation of glutamatergic (Glu) pacemaker cells that project bilaterally (102). This pattern generation of the PreBötC is modulated by inhibitory neurotransmission at pre-motor neurons (10, 62). The Bötzinger Complex located rostral to the PreBötC provides for switching from inspiration to expiration (154, 155), possibly via inhibitory inputs to the PreBötC (156). Normally, expiration is passive; however, active expiration appears to have a distinct central pattern generator located in the region of the retrotrapezoid nucleus (RTN) (93).
Pre-Motor Neurons
Pre-motor neurons located primarily in the ventrolateral medulla [ventral respiratory group (VRG)] and dorsomedial medulla [dorsal respiratory group (DRG)] provide monosynaptic drive to phrenic motor neurons during inspiration (94, 134). These descending excitatory (Glu) premotor inputs are predominantly ipsilateral (28, 37, 45, 115), transmitted via bulbospinal pathways located in the ventrolateral and ventromedial funiculi (44, 45, 201), and generally thought to be widely distributed (19, 23), although the actual distribution of premotor input to phrenic motor neurons has not been fully characterized. In this regard, it should be noted that ventilatory behaviors do not require the recruitment of larger phrenic motor neurons innervating more fatigable FInt and FF motor units. It is entirely possible that synaptic input from pre-motor neurons mediating inspiratory drive from the respiratory central pattern generator for ventilatory behaviors is distributed primarily to smaller phrenic motor neurons. Larger phrenic motor neurons may receive synaptic input from other pre-motor neurons other more forceful expulsive motor behaviors. Unfortunately, far less is known about the location and function of other central pattern generators that mediate neuromotor control of the diaphragm muscle during swallowing, coughing, sneezing, etc. It is possible that these central pattern generators share common pre-motor neurons to those of inspiration or have distinct pre-motor neurons. It is likely that the central pattern generators involved in breathing and expulsive motor behaviors of the diaphragm muscle will have a certain degree of overlap in premotor components of neuromotor control (159, 201). For ventilatory behaviors, it is well recognized that phrenic motor neurons receive descending premotor input that is predominantly ipsilateral. However, retrograde tracing studies that examine the connectivity of phrenic motor neurons have indicated a variety of premotor inputs from both the brain stem and the spinal cord (26, 36, 37, 87, 95, 114, 161). Perhaps these connections reflect premotor inputs mediating the output of different central pattern generators other than that involved in rhythmic breathing behavior. Unfortuantely, retrograde tracing studies do not provide information regarding premotor inputs to phrenic motor neurons of different size/motor unit types.
Sensory (e.g., chemoreceptor and pulmonary stretch receptor) and behavioral (e.g., sleep-wake state) modulations must also be integrated with the repiratory pattern generator inputs at the level of the pre-motor neurons. Modulation of the respiratory pattern and/or of phrenic motor neuron activity may occur directly or indirectly in response to afferent inputs to phrenic motor neurons, from signaling initiated by mechanoreceptors in the lung and airway, peripheral and central chemoreceptors, to behavioral state influence mediated by serotonergic projections emanating from the raphe. Mechanoreceptors in the lung respond to lung inflation and are sensitive to the mechanical loading of breathing. Their activity peaks at the end of inspiration, thus preventing airway over-inflation (17, 130). These afferent inputs exert effects on ventilatory phrenic motor neuron discharge indirectly, via vagal nerve inputs in the nucleus tractus solitarius (NTS) (11, 30). Laryngeal mechanoreceptors also exert an indirect effect on phrenic motor neurons, decreasing inspiratory drive during upper airway collapse (162). Peripheral chemoreceptors, primarily in the carotid bodies, respond to hypoxia and hypercapnea by increasing ventilation (1, 104, 105, 213) via signaling to brain stem respiratory centers indirectly via the carotid sinus nerve (221). Central chemoreceptors are found in many brain stem areas (131) and are exquisitely sensitive to hypercapnea (137), and in response act to increase ventilation (74). Together, chemoreceptors act in an indirect modulatory fashion on the overall drive to phrenic motor neurons. The interactivity and the relative contributions to respiratory behaviors of peripheral and central chemoreceptors is subject to intense scrutiny and debate in the field. Populations of serotinergic neurons project to brain stem respiratory regions and the phrenic motor pool (85). These neurons may therefore influence phrenic activity indirectly via the rhythmic central pattern generator and premotor outputs (106, 107), as well as directly by excitatory actions on phrenic motor neurons (113, 129). Catecholamine modulation of respiration occurs via activation of the α1 or α2 adrenoreceptors, enhancing or inhibiting, respectively, the respiratory rhythmic central pattern generation (84, 207, 210, 211).
Interneurons
Phrenic motor neurons receive inputs from spinal cord interneurons, including those involved in proprioception. However, by stark contrast to muscles involved in locomotor behaviors (148), the diaphragm muscle is scarcely populated with muscle spindles (33). Thus direct muscle spindle proprioceptive feedback from the diaphragm does not contribute substantially to modulation of phrenic motor neuron excitability (20, 92). However, muscle spindles in intercostal muscles do feedback to phrenic motor neurons. This input has a primarily inhibitory effect on phrenic motor neuron excitability (35, 153). In particular, the intercostal to phrenic reflex suppresses phrenic nerve activity following strain on the chest wall (25, 152), an effect that appears to involve both disfacilitation of VRG premotor input (12, 152, 166) and interneuronal inhibition of phrenic motor neurons (8). Additional, local inhibition provided to phrenic motor neurons from interneurons within the spinal cord have been well characterized (8, 9, 31, 32, 115) and may provide some of the substrate (increased respiratory drive) required for the recovery of ventilatory and non-ventilatory behaviors following spinal cord injury (109, 223).
Corticospinal Input to Phrenic Motor Neurons
Direct corticospinal inputs onto phrenic motor neurons allow for voluntary control of breathing (55, 117) and the interplay between ventilation and complex human behaviors, such as speech (167). Phrenic motor neuron integration of the milieu of rhythmic pattern inputs, modulatory inputs, and cortical inputs is illustrated in the differential drive to breathe that occurs during the waking state compared with the sleeping state (47), whereby the awake state provides a resilience to apnea in hypercapnic conditions (22, 47). In contrast, sleep predisposes to episodes of apnea in the hypercapnic condition (22, 200). It remains unknown exactly how influential cortical arousal states are in the maintenance of eupnea.
Impairment of Diaphragm Neuromotor Control
Diaphragm Muscle Fiber Atrophy and Muscle Weakness
Impairment of diaphragm neuromotor control can result from changes at the level of diaphragm motor units. For example, diaphragm muscle fiber atrophy or weakening will affect their contribution to force generation. A number of studies have shown that the mechanical properties of diaphragm muscle fibers are differentially affected by lifespan (including development and aging) (39, 57, 63, 65, 66, 68, 70, 71, 138) and a variety of clinical conditions, including diabetes (15, 83), heart disease (118), chronic obstructive pulmonary disease (135), and neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) (157). These studies generally report that type IIx and/or IIb fibers are more vulnerable, whereas type I and IIa fibers are more resilient.
Diaphragm motor unit properties change dramatically across our lifespan. During early postnatal development, expression of MyHC2x and MyHC2b gradually emerge with the development and disproportionate growth of type IIx and IIb diaphragm fibers (142). Thus maximum diaphragm force (Pdimax) is substantially lower early in postnatal development (215), although the Pdi generated during breathing is approximately the same as in the adult. Thus the functional reserve capacity of diaphragm neuromotor control is markedly lower.
Sarcopenia is an age-related loss of muscle mass (atrophy of type IIx and/or IIb muscle fibers) and a decrease in specific force (weakness) (21, 29, 38). In rodents, sarcopenia has profound effects on the diaphragm muscle with a selective atrophy of type IIx and/or IIb muscle fibers that constitute type FInt and FF motor units and a reduction in maximum specific force (39, 63, 66, 68, 70). This coincides with a marked fragmentation of neuromuscular junction innervating type IIx and/or IIb muscle fibers (144). Functionally, diaphragm muscle sarcopenia impairs the high force, expulsive motor behaviors necessary to clear the airways (39, 63, 66, 68, 70). This inability to perform high-force, expulsive motor behaviors may underlie respiratory complications that are particularly common in older humans (42, 140, 205). Similarly, in a number of chronic diseases, selective wasting and weakening of type IIx and IIb diaphragm muscle fibers occurs (e.g., cachexia), impacts force reserve, and differentially impairs higher force expulsive motor behaviors. In extreme conditions, the involvement of type I and IIa diaphragm muscle fiber may place patients at increased risk of respiratory failure (190).
In ALS, resting eupneic breathing is not typically compromised in many patients (5, 6, 43, 212), and in patients without bulbar compromise, arterial blood gas remains normoxic (>80 Torr) during awake breathing (43, 146, 212). However, insufficient expulsive maneuvers are tightly correlated with disease progression (141). These deficits in higher-force behaviors are likely due to impairments in type IIx and/or IIb muscle fibers, consistent with studies showing a reduction of maximum specific force of the diaphragm muscle in rodent models of ALS (88, 157) and loss of more fatigable type FInt and FF motor units (54, 81, 149).
Phrenic Motor Neurons
The number of phrenic motor neurons is not constant but can change during our lifespan as well as in disease conditions. During late embryonic and early postnatal development, ~50% of motor neurons within the mammalian spinal cord undergo pruning or programmed cell death (108). Within the phrenic motor pool of the rat, this motor neuron loss occurs days before birth and numbers remain stable during late embryogenesis and early postnatal life within the mammalian spinal cord (2, 77). Importantly, phrenic motor neurons during embryogenesis and early postnatal development are smaller and relatively homogeneous in size (142). The full gamut of size-based heterogeneity of phrenic motor neurons is established only after weaning, a period of rapid growth of phrenic motor neurons and diaphragm muscle fibers (16, 121, 142). This growth appears to be exclusively of the larger phrenic motor neurons that innervate type IIx and/or IIb muscle fibers (142). In older animals, there is an age-related loss of spinal cord motor neurons in spinal cords of humans (97, 206) and rodent models (78–80, 90, 91), including in the cervical segments supplying the phrenic nerve (220). Ongoing investigations within our laboratory suggest that there is an age-related loss of phrenic motor neurons—primarily larger motor neurons. Similarly, rodent models of ALS show frank loss of C4 phrenic motor neurons (110), although the entirety of the phrenic motor pool was not assessed, and morphometric and temporal alterations remain to be clarified.
Neural Control of Phrenic Motor Neurons
In ALS, degeneration and abnormal activity of the cortical motor control centers occurs in patients (60, 76, 214) and rodent models (49, 51, 136, 160). There is growing evidence that similar dysfunctions of the central control of breathing may pose additional difficulties in generating adequate ventilator and expulsive behaviors of the respiratory pump (86). Nocturnal hypoventilation and sleep-disordered breathing is common in ALS (5, 6, 24, 86), with nocturnal hypoxia observed in patients with preserved diaphragm and phrenic nerve functions (5, 6, 24). The neuronal populations that govern diaphragm muscle pump activity may degenerate together or be asymmetrically affected. Evidence for degeneration of the respiratory central pattern generator has not been directly observed, although rat models of PreBötC dysfunction display a similar phenotype to ALS patients, namely, nocturnal disordered breathing progressing to central apnoea (125).
In addition to the possible degeneration of the pattern generator in ALS, the pre-motor neurons, interneuronal and corticospinal inputs to the phrenic motor neurons, may also be compromised. Although loss of the pre-motor neuron populations has not been studied in ALS, degenerative pathology and dysphagia are observed in motor neurons of the hypoglossal motor nuclei within the medulla of patients and rodent models (50, 73, 101, 209). Respiratory interneurons within the spinal cord have not been extensively studied in ALS, although the compensatory mechanisms of increased interneuronal activity may underlie the slower decline of respiratory function compared with locomotor function in rodent models (158). The deterioration of voluntary breathing in ALS implicates the decline in respiratory corticospinal neurons (127, 168).
Conclusions and Future Directions
Breathing is a complex motor function, and neuromotor control of the diaphragm muscle is essential. Phrenic motor neurons integrate synaptic inputs from pre-motor neurons and interneurons, and are activated in accordance with the discrete biochemical and contractile properties of muscle fibers they innervate that constitute motor units. Recruitment of motor units accomplishes the wide range of forces required for breathing as well as other diaphragm motor behaviors. These motor behaviors include ventilatory pump actions requiring low levels of force generation, deep inspirations (sighs) that require higher force and serve to mitigate atelectasis of the lung, and expulsive airway clearance behaviors, such as coughing and sneezing, requiring the generation of near maximal diaphragm forces. Intriguingly, conditions that impair motor unit function such as aging and ALS seems to specifically affect type FInt and FF motor units and thus the generation of larger forces. How and why the sparing of type S and FR motor units occurs in these and similar maladies should be of intense interest in the immediate future.
Acknowledgments
This review was supported by a National Institutes of Health Grants AG-044615 (G.C.S. and C.B.M.), AG-057052 (G.C.S. and C.B.M.), and HL-96750 (G.C.S. and C.B.M.), and an Australian National Health & Medical Research Council CJ Martin Early Career Fellowship (M.J.F.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: M.F. and G.C.S. prepared figures; M.F., C.B.M., and G.C.S. drafted manuscript; M.F., C.B.M., and G.C.S. edited and revised manuscript; M.F., C.B.M., and G.C.S. approved final version of manuscript.
References
- 1.Aaron EA, Powell FL. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol (1985) 74: 1635–1640, 1993. doi: 10.1152/jappl.1993.74.4.1635. [DOI] [PubMed] [Google Scholar]
- 2.Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J Comp Neurol 382: 459–468, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Argote S, Gransee HM, Mora JC, Stowe JM, Jorgenson AJ, Sieck GC, Mantilla CB. The impact of midcervical contusion injury on diaphragm muscle function. J Neurotrauma 33: 500–509, 2016. doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson CA, Dick TE, Orem J. Swallowing in sleep and wakefulness in adult cats. Sleep 18: 325–329, 1995. doi: 10.1093/sleep/18.5.325. [DOI] [PubMed] [Google Scholar]
- 5.Arnulf I, Similowski T, Salachas F, Garma L, Mehiri S, Attali V, Behin-Bellhesen V, Meininger V, Derenne JP. Sleep disorders and diaphragmatic function in patients with amyotrophic lateral sclerosis. Am J Respir Crit Care Med 161: 849–856, 2000. doi: 10.1164/ajrccm.161.3.9805008. [DOI] [PubMed] [Google Scholar]
- 6.Atalaia A, De Carvalho M, Evangelista T, Pinto A. Sleep characteristics of amyotrophic lateral sclerosis in patients with preserved diaphragmatic function. Amyotroph Lateral Scler 8: 101–105, 2007. doi: 10.1080/17482960601029883. [DOI] [PubMed] [Google Scholar]
- 7.Bellingham MC. Driving respiration: the respiratory central pattern generator. Clin Exp Pharmacol Physiol 25: 847–856, 1998. doi: 10.1111/j.1440-1681.1998.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 8.Bellingham MC. Synaptic inhibition of cat phrenic motor neurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232, 1999. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- 9.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. doi: 10.1016/0006-8993(90)91807-S. [DOI] [PubMed] [Google Scholar]
- 10.Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90, 1979. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- 11.Berger AJ, Averill DB. Projection of single pulmonary stretch receptors to solitary tract region. J Neurophysiol 49: 819–830, 1983. doi: 10.1152/jn.1983.49.3.819. [DOI] [PubMed] [Google Scholar]
- 12.Bolser DC, Lindsey BG, Shannon R. Medullary inspiratory activity: influence of intercostal tendon organs and muscle spindle endings. J Appl Physiol (1985) 62: 1046–1056, 1987. doi: 10.1152/jappl.1987.62.3.1046. [DOI] [PubMed] [Google Scholar]
- 13.Burke RE, Levine DN, Tsairis P, Zajac FE III. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234: 723–748, 1973. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol 518: 907–920, 1999. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan LA, Supinski GS. Hyperglycemia-induced diaphragm weakness is mediated by oxidative stress. Crit Care 18: R88, 2014. doi: 10.1186/cc13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron WE, Brozanski BS, Guthrie RD. Postnatal development of phrenic motoneurons in the cat. Brain Res Dev Brain Res 51: 142–145, 1990. doi: 10.1016/0165-3806(90)90269-5. [DOI] [PubMed] [Google Scholar]
- 17.Canning BJ, Chang AB, Bolser DC, Smith JA, Mazzone SB, McGarvey L, Adams TM, Altman KW, Barker AF, Birring SS, Blackhall F, Bolser DC, Boulet L-P, Braman SS, Brightling C, Callahan-Lyon P, Canning B, Chang AB, Coeytaux R, Cowley T, Davenport P, Diekemper RL, Ebihara S, El Solh AA, Escalante P, Feinstein A, Field SK, Fisher D, French CT, Gibson P, Gold P, Grant C, Harding SM, Harnden A, Hill AT, Irwin RS, Kahrilas PJ, Keogh KA, Lane AP, Lewis SZ, Lim K, Malesker MA, Mazzone P, Mazzone S, McGarvey L, Molasiotis A, Murad MH, Newcombe P, Nguyen HQ, Oppenheimer J, Prezant D, Pringsheim T, Restrepo MI, Rosen M, Rubin B, Ryu JH, Smith J, Tarlo SM, Turner RB, Vertigan A, Wang G, Weir K; CHEST Expert Cough Panel . Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 146: 1633–1648, 2014. doi: 10.1378/chest.14-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakkalakal JV, Nishimune H, Ruas JL, Spiegelman BM, Sanes JR. Retrograde influence of muscle fibers on their innervation revealed by a novel marker for slow motoneurons. Development 137: 3489–3499, 2010. doi: 10.1242/dev.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MI, Piercey MF, Gootman PM, Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res 81: 319–324, 1974. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- 20.Corda M, Voneuler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. J Physiol 178: 161–177, 1965. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta AK, Shea SA, Horner RL, Guz A. The influence of induced hypocapnia and sleep on the endogenous respiratory rhythm in humans. J Physiol 440: 17–33, 1991. doi: 10.1113/jphysiol.1991.sp018693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol 368: 63–87, 1985. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Carvalho M, Costa J, Pinto S, Pinto A. Percutaneous nocturnal oximetry in amyotrophic lateral sclerosis: periodic desaturation. Amyotroph Lateral Scler 10: 154–161, 2009. doi: 10.1080/17482960802382305. [DOI] [PubMed] [Google Scholar]
- 25.Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand 75: 568–579, 1969. doi: 10.1111/j.1748-1716.1969.tb04412.x. [DOI] [PubMed] [Google Scholar]
- 26.Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol 57: 245–259, 1987. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- 27.Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol 465: 715–730, 1993. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 29.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol (1985) 95: 1717–1727, 2003. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 30.Donoghue S, Garcia M, Jordan D, Spyer KM. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol 322: 353–363, 1982. doi: 10.1113/jphysiol.1982.sp014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffin J, Douse MA. Bötzinger expiratory neurones inhibit propriobulbar decrementing inspiratory neurones. Neuroreport 4: 1215–1218, 1993. doi: 10.1097/00001756-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res 112: 35–40, 1996. doi: 10.1007/BF00227175. [DOI] [PubMed] [Google Scholar]
- 33.Duron B, Jung-Caillol MC, Marlot D. Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 152: 171–192, 1978. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- 34.Edgerton VR. Mammalian muscle fiber types and their adaptability. Am Zool 18: 113–125, 1978. doi: 10.1093/icb/18.1.113. [DOI] [Google Scholar]
- 35.Eldridge FL, Gill-Kumar P, Millhorn DE, Waldrop TG. Spinal inhibition of phrenic motoneurones by stimulation of afferents from peripheral muscles. J Physiol 311: 67–79, 1981. doi: 10.1113/jphysiol.1981.sp013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res 513: 35–42, 1990. doi: 10.1016/0006-8993(90)91086-V. [DOI] [PubMed] [Google Scholar]
- 37.Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269: 47–57, 1988. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- 38.Elliott JE, Greising SM, Mantilla CB, Sieck GC. Functional impact of sarcopenia in respiratory muscles. Respir Physiol Neurobiol 226: 137–146, 2016. doi: 10.1016/j.resp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott JE, Omar TS, Mantilla CB, Sieck GC. Diaphragm muscle sarcopenia in Fischer 344 and Brown Norway rats. Exp Physiol 101: 883–894, 2016. doi: 10.1113/EP085703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J Appl Physiol (1985) 67: 620–627, 1989. doi: 10.1152/jappl.1989.67.2.620. [DOI] [PubMed] [Google Scholar]
- 42.Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE; Cardiovascular Health Study Research Group . Respiratory muscle strength in the elderly. Correlates and reference values. Am J Respir Crit Care Med 149: 430–438, 1994. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 43.Fallat RJ, Jewitt B, Bass M, Kamm B, Norris FH Jr. Spirometry in amyotrophic lateral sclerosis. Arch Neurol 36: 74–80, 1979. doi: 10.1001/archneur.1979.00500380044004. [DOI] [PubMed] [Google Scholar]
- 44.Feldman JL, Cohen MI. Relation between expiratory duration and rostral medullary expiratory neuronal discharge. Brain Res 141: 172–178, 1978. doi: 10.1016/0006-8993(78)90627-3. [DOI] [PubMed] [Google Scholar]
- 45.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenn WO. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol 58: 175–203, 1923. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol 16: 15–20, 1961. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 48.Fleshman JW, Munson JB, Sypert GW, Friedman WA. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol 46: 1326–1338, 1981. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- 49.Fogarty MJ, Klenowski PM, Lee JD, Drieberg-Thompson JR, Bartlett SE, Ngo ST, Hilliard MA, Bellingham MC, Noakes PG. Cortical synaptic and dendritic spine abnormalities in a presymptomatic TDP-43 model of amyotrophic lateral sclerosis. Sci Rep 6: 37968, 2016. doi: 10.1038/srep37968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fogarty MJ, Mu EWH, Lavidis NA, Noakes PG, Bellingham MC. Motor areas show altered dendritic structure in an amyotrophic lateral sclerosis mouse model. Front Neurosci 11: 609, 2017. doi: 10.3389/fnins.2017.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fogarty MJ, Noakes PG, Bellingham MC. Motor cortex layer V pyramidal neurons exhibit dendritic regression, spine loss, and increased synaptic excitation in the presymptomatic hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. J Neurosci 35: 643–647, 2015. doi: 10.1523/JNEUROSCI.3483-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- 53.Fournier M, Sieck GC. Topographical projections of phrenic motoneurons and motor unit territories in the cat diaphragm. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Alan R. Liss, Inc, 1987, p. 215–226. [Google Scholar]
- 54.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 20: 2534–2542, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol 384: 109–118, 1987. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol (1985) 92: 1506–1514, 2002. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- 57.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Fang YH, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol (1985) 90: 380–388, 2001. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- 58.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 89: 695–703, 2000. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 59.Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol (1985) 87: 1894–1900, 1999. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- 60.Genç B, Jara JH, Lagrimas AK, Pytel P, Roos RP, Mesulam MM, Geula C, Bigio EH, Özdinler PH. Apical dendrite degeneration, a novel cellular pathology for Betz cells in ALS. Sci Rep 7: 41765, 2017. doi: 10.1038/srep41765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gill LC, Mantilla CB, Sieck GC. Impact of unilateral denervation on transdiaphragmatic pressure. Respir Physiol Neurobiol 210: 14–21, 2015. doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill PK, Kuno M. Excitatory and inhibitory actions on phrenic motoneurones. J Physiol 168: 274–289, 1963. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med 150: 174–178, 1994. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- 65.Greising SM, Call JA, Lund TC, Blazar BR, Tolar J, Lowe DA. Skeletal muscle contractile function and neuromuscular performance in Zmpste24 −/− mice, a murine model of human progeria. Age (Dordr) 34: 805–819, 2012. doi: 10.1007/s11357-011-9281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol 593: 431–440, 2015. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greising SM, Mantilla CB, Medina-Martínez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol 309: L46–L52, 2015. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greising SM, Mantilla CB, Sieck DC, Sieck GC. Transdiaphragmatic pressure measurements reveal age-related diaphragm muscle dysfunction during non-ventilatory behaviors. FASEB J 27, Suppl 1: 719.7, 2013. [Google Scholar]
- 72.Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol 188: 56–59, 2013. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haenggeli C, Kato AC. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci Lett 335: 39–43, 2002. doi: 10.1016/S0304-3940(02)01140-0. [DOI] [PubMed] [Google Scholar]
- 74.Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol 32: 225–266, 1905. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamm TM, Nemeth PM, Solanki L, Gordon DA, Reinking RM, Stuart DG. Association between biochemical and physiological properties in single motor units. Muscle Nerve 11: 245–254, 1988. doi: 10.1002/mus.880110309. [DOI] [PubMed] [Google Scholar]
- 76.Hammer RP Jr, Tomiyasu U, Scheibel AB. Degeneration of the human Betz cell due to amyotrophic lateral sclerosis. Exp Neurol 63: 336–346, 1979. doi: 10.1016/0014-4886(79)90129-8. [DOI] [PubMed] [Google Scholar]
- 77.Harris AJ, McCaig CD. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci 4: 13–24, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashizume K, Kanda K. Differential effects of aging on motoneurons and peripheral nerves innervating the hindlimb and forelimb muscles of rats. Neurosci Res 22: 189–196, 1995. doi: 10.1016/0168-0102(95)00889-3. [DOI] [PubMed] [Google Scholar]
- 79.Hashizume K, Kanda K. Neuronal dropout is greater in hindlimb motor nuclei than in forelimb motor nuclei in aged rats. Neurosci Lett 113: 267–269, 1990. doi: 10.1016/0304-3940(90)90595-Z. [DOI] [PubMed] [Google Scholar]
- 80.Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol 269: 425–430, 1988. doi: 10.1002/cne.902690309. [DOI] [PubMed] [Google Scholar]
- 81.Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 28: 154–164, 2007. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 83.Hida W, Shindoh C, Satoh J, Sagara M, Kikuchi Y, Toyota T, Shirato K. N-acetylcysteine inhibits loss of diaphragm function in streptozotocin-treated rats. Am J Respir Crit Care Med 153: 1875–1879, 1996. doi: 10.1164/ajrccm.153.6.8665049. [DOI] [PubMed] [Google Scholar]
- 84.Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol 143: 187–197, 2004. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 85.Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174: 76–88, 2010. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Howell BN, Newman DS. Dysfunction of central control of breathing in amyotrophic lateral sclerosis. Muscle Nerve 56: 197–201, 2017. doi: 10.1002/mus.25564. [DOI] [PubMed] [Google Scholar]
- 87.Hudson AL, Gandevia SC, Butler JE. Control of human inspiratory motoneurones during voluntary and involuntary contractions. Respir Physiol Neurobiol 179: 23–33, 2011. doi: 10.1016/j.resp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 88.Hwee DT, Kennedy A, Ryans J, Russell AJ, Jia Z, Hinken AC, Morgans DJ, Malik FI, Jasper JR. Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model. PLoS One 9: e96921, 2014. doi: 10.1371/journal.pone.0096921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol 26: 113–128, 1976. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- 90.Ishihara A, Naitoh H, Katsuta S. Effects of ageing on the total number of muscle fibers and motoneurons of the tibialis anterior and soleus muscles in the rat. Brain Res 435: 355–358, 1987. doi: 10.1016/0006-8993(87)91624-6. [DOI] [PubMed] [Google Scholar]
- 91.Jacob JM. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev 106: 205–216, 1998. doi: 10.1016/S0047-6374(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 92.Jammes Y, Arbogast S, De Troyer A. Response of the rabbit diaphragm to tendon vibration. Neurosci Lett 290: 85–88, 2000. doi: 10.1016/S0304-3940(00)01301-X. [DOI] [PubMed] [Google Scholar]
- 93.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res 81: 639–648, 1990. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 95.Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol 58: 105–124, 1987. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- 96.Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J Appl Physiol (1985) 77: 481–487, 1994. doi: 10.1152/jappl.1994.77.1.481. [DOI] [PubMed] [Google Scholar]
- 97.Kawamura N, Gotoda Y, Yoshikura H. The rise in medical care expenditures in Japan, trends in disease patterns: 1977-81. Am J Public Health 75: 1442–1444, 1985. doi: 10.2105/AJPH.75.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kernell D. The Motoneurone and Its Muscle Fibres. New York: Oxford University Press Inc, 2006. doi: 10.1093/acprof:oso/9780198526551.001.0001 [DOI] [Google Scholar]
- 99.Keswani NH, Hollinshead WH. The phrenic nucleus. III. Organization of the phrenic nucleus in the spinal cord of the cat and man. Proc Staff Meet Mayo Clin 30: 566–577, 1955. [PubMed] [Google Scholar]
- 100.Khurram OU, Sieck GC, Mantilla CB. Compensatory effects following unilateral diaphragm paralysis. Respir Physiol Neurobiol 246: 39–46, 2017. doi: 10.1016/j.resp.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kiernan JA, Hudson AJ. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain 114: 843–853, 1991. doi: 10.1093/brain/114.2.843. [DOI] [PubMed] [Google Scholar]
- 102.Koizumi H, Koshiya N, Chia JX, Cao F, Nugent J, Zhang R, Smith JC. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Botzinger complex respiratory microcircuits. J Neurosci 33: 2994–3009, 2013. doi: 10.1523/JNEUROSCI.4427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol (1985) 61: 1999–2004, 1986. doi: 10.1152/jappl.1986.61.6.1999. [DOI] [PubMed] [Google Scholar]
- 104.Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 24: 267–286, 1975. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- 105.Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol 24: 249–266, 1975. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- 106.Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol 476: 117–130, 1994. [PMC free article] [PubMed] [Google Scholar]
- 107.Lalley PM, Bischoff AM, Schwarzacher SW, Richter DW. 5-HT2 receptor-controlled modulation of medullary respiratory neurones in the cat. J Physiol 487: 653–661, 1995. doi: 10.1113/jphysiol.1995.sp020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Brain Res 256: 473–479, 1982. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- 109.Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 11: 1294–1301, 2008. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis MI, Sieck GC. Effect of acute nutritional deprivation on diaphragm structure and function. J Appl Physiol (1985) 68: 1938–1944, 1990. doi: 10.1152/jappl.1990.68.5.1938. [DOI] [PubMed] [Google Scholar]
- 112.Liddell EGT, Sherrington CS. Recruitment and some other Features of Reflex Inhibition. Proc R Soc Lond, B 97: 488–518, 1925. doi: 10.1098/rspb.1925.0016. [DOI] [Google Scholar]
- 113.Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461: 213–233, 1993. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184, 1994. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- 115.Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol (1985) 106: 138–152, 2009. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol 439: 545–558, 1991. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manders E, Bonta PI, Kloek JJ, Symersky P, Bogaard HJ, Hooijman PE, Jasper JR, Malik FI, Stienen GJ, Vonk-Noordegraaf A, de Man FS, Ottenheijm CA. Reduced force of diaphragm muscle fibers in patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L20–L28, 2016. doi: 10.1152/ajplung.00113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mantilla CB, Seven YB, Sieck GC. Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment. Prog Brain Res 209: 309–329, 2014. doi: 10.1016/B978-0-444-63274-6.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985) 104: 1818–1827, 2008. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249, 2009. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci 8: 1142–1144, 2005. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J Neurophysiol 93: 3024–3026, 2005. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- 127.Miscio G, Gukov B, Pisano F, Mazzini L, Baudo S, Salvadori A, Mauro A. The cortico-diaphragmatic pathway involvement in amyotrophic lateral sclerosis: neurophysiological, respiratory and clinical considerations. J Neurol Sci 251: 10–16, 2006. doi: 10.1016/j.jns.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 128.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol (1985) 79: 1640–1649, 1995. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- 129.Morin D, Monteau R, Hilaire G. Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study in the newborn rat. J Physiol 451: 605–629, 1992. doi: 10.1113/jphysiol.1992.sp019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Narula M, McGovern AE, Yang SK, Farrell MJ, Mazzone SB. Afferent neural pathways mediating cough in animals and humans. J Thorac Dis 6, Suppl 7: S712–S719, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol (1985) 106: 1464–1466, 2009. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nemeth PM, Hamm TM, Gordon DA, Reinking RM, Stuart DG. Application of cross-sectional single-fiber microchemistry to the study of motor-unit fatigability. In: Motor Control, edited by Gantchev GN, Dimitrov B, Gatev P. New York: Plenum, 1986. [Google Scholar]
- 133.Nemeth PM, Solanki L, Gordon DA, Hamm TM, Reinking RM, Stuart DG. Uniformity of metabolic enzymes within individual motor units. J Neurosci 6: 892–898, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Otake K, Sasaki H, Ezure K, Manabe M. Axonal projections from Bötzinger expiratory neurons to contralateral ventral and dorsal respiratory groups in the cat. Exp Brain Res 72: 167–177, 1988. doi: 10.1007/BF00248512. [DOI] [PubMed] [Google Scholar]
- 135.Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 200–205, 2005. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ozdinler PH, Benn S, Yamamoto TH, Güzel M, Brown RH Jr, Macklis JD. Corticospinal motor neurons and related subcerebral projection neurons undergo early and specific neurodegeneration in hSOD1G93A transgenic ALS mice. J Neurosci 31: 4166–4177, 2011. doi: 10.1523/JNEUROSCI.4184-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol 208: 436–450, 1965. [DOI] [PubMed] [Google Scholar]
- 138.Pette D, Henriksson J, Emmerich M. Myofibrillar protein patterns of single fibres from human muscle. FEBS Lett 103: 152–155, 1979. doi: 10.1016/0014-5793(79)81270-3. [DOI] [PubMed] [Google Scholar]
- 139.Polkey MI, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax 50: 1131–1135, 1995. doi: 10.1136/thx.50.11.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polkey MI, Harris ML, Hughes PD, Hamnegärd CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med 155: 1560–1564, 1997. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- 141.Polkey MI, Lyall RA, Yang K, Johnson E, Leigh PN, Moxham J. Respiratory muscle strength as a predictive biomarker for survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med 195: 86–95, 2017. doi: 10.1164/rccm.201604-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol (1985) 89: 563–572, 2000. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- 144.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 21: 887–895, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 145.Prakash YS, Sieck GC. Morphometric analysis of phrenic motoneuron pools using confocal microscopy. In: Kemp Station Symposium on Regulation of Respiration. Madison, WI: Kemp Natural Resources Station, 1992. [Google Scholar]
- 146.Prell T, Ringer TM, Wullenkord K, Garrison P, Gunkel A, Stubendorff B, Witte OW, Grosskreutz J. Assessment of pulmonary function in amyotrophic lateral sclerosis: when can polygraphy help evaluate the need for non-invasive ventilation? J Neurol Neurosurg Psychiatry 87: 1022–1026, 2016. doi: 10.1136/jnnp-2015-312185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PWR. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78: 2033–2038, 1995. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- 148.Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92: 1651–1697, 2012. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- 149.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9: 408–419, 2006. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 150.Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp Neurol 226: 254–258, 2010. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rana S, Sieck GC, Mantilla CB. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol 117: 545–555, 2017. doi: 10.1152/jn.00727.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Remmers JE. Extra-segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. J Physiol 233: 45–62, 1973. doi: 10.1113/jphysiol.1973.sp010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. Respir Physiol 10: 358–383, 1970. doi: 10.1016/0034-5687(70)90055-1. [DOI] [PubMed] [Google Scholar]
- 154.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982. [DOI] [PubMed] [Google Scholar]
- 155.Richter DW, Jordan D, Ballantyne D, Meesmann M, Spyer KM. Presynaptic depolarization in myelinated vagal afferent fibres terminating in the nucleus of the tractus solitarius in the cat. Pflugers Arch 406: 12–19, 1986. doi: 10.1007/BF00582946. [DOI] [PubMed] [Google Scholar]
- 156.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rizzuto E, Pisu S, Musarò A, Del Prete Z. Measuring neuromuscular junction functionality in the SOD1(G93A) animal model of amyotrophic lateral sclerosis. Ann Biomed Eng 43: 2196–2206, 2015. doi: 10.1007/s10439-015-1259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol 287: 192–204, 2017. doi: 10.1016/j.expneurol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 159.Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saba L, Viscomi MT, Caioli S, Pignataro A, Bisicchia E, Pieri M, Molinari M, Ammassari-Teule M, Zona C. Altered functionality, morphology, and vesicular glutamate transporter expression of cortical motor neurons from a presymptomatic mouse model of amyotrophic lateral sclerosis. Cereb Cortex 26: 1512–1528, 2016. doi: 10.1093/cercor/bhu317. [DOI] [PubMed] [Google Scholar]
- 161.Saji M, Miura M. Evidence that glutamate is the transmitter mediating respiratory drive from medullary premotor neurons to phrenic motoneurons: a double labeling study in the rat. Neurosci Lett 115: 177–182, 1990. doi: 10.1016/0304-3940(90)90451-E. [DOI] [PubMed] [Google Scholar]
- 162.Sant’Ambrogio G, Tsubone H, Sant’Ambrogio FB. Sensory information from the upper airway: role in the control of breathing. Respir Physiol 102: 1–16, 1995. doi: 10.1016/0034-5687(95)00048-I. [DOI] [PubMed] [Google Scholar]
- 163.Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316, 2014. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Motor unit recruitment order in diaphragm muscle following spinal cord injury. FASEB J 24: 1064.1015, 2010. [Google Scholar]
- 165.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shannon R, Bolser DC, Lindsey BG. Medullary expiratory activity: influence of intercostal tendon organs and muscle spindle endings. J Appl Physiol (1985) 62: 1057–1062, 1987. doi: 10.1152/jappl.1987.62.3.1057. [DOI] [PubMed] [Google Scholar]
- 167.Shea SA. Behavioural and arousal-related influences on breathing in humans. Exp Physiol 81: 1–26, 1996. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- 168.Shimizu T, Komori T, Kugio Y, Fujimaki Y, Oyanagi K, Hayashi H. Electrophysiological assessment of corticorespiratory pathway function in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11: 57–62, 2010. doi: 10.3109/17482960903207385. [DOI] [PubMed] [Google Scholar]
- 169.Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol 180: 88–96, 2012. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sieck GC. Conceptual model of ventilatory muscle recruitment and diaphragmatic fatigue. In: Modeling and Parameter Estimation in Respiratory Control, edited by Khoo MCK. New York: Plenum Press, 1990, p. 113–123. [Google Scholar]
- 171.Sieck GC. Diaphragm motor units and their response to altered use. Semin Respir Crit Care Med 12: 258–269, 1991. doi: 10.1055/s-2007-1006245. [DOI] [Google Scholar]
- 172.Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988. [PubMed] [Google Scholar]
- 173.Sieck GC. Neural control of the inspiratory pump. NIPS 6: 260–264, 1991. [Google Scholar]
- 174.Sieck GC. Organization and recruitment of diaphragm motor units. In: The Thorax (2nd ed.), edited by Roussos C. New York, NY: Marcel Dekker, 1995, p. 783–820. [Google Scholar]
- 175.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- 176.Sieck GC. Recruitment and frequency coding of diaphragm motor units during ventilatory and non-ventilatory behaviors, in Respiratory Control (Swanson GD, Grodins FS, Hughson RL, editors). New York: Plenum Press, 1989, p. 441–450. doi: 10.1007/978-1-4613-0529-3_48. [DOI] [Google Scholar]
- 178.Sieck GC. Recruitment of diaphragm motor units during ventilatory and non-ventilatory behaviors. In: Motor Control VII. Proceedings of the VIIth International Symposium on Motor Control, Borovets, Bulgaria, edited by Stuart DG, Gantchev GN, Gurfinkel VS, Wiesendanger M. Tucson, AZ: Motor Control Press, 1996, p. 39–42. [Google Scholar]
- 179.Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Compr Physiol 3: 1553–1567, 2013. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sieck GC, Fournier M. Changes in diaphragm motor unit EMG during fatigue. J Appl Physiol (1985) 68: 1917–1926, 1990. doi: 10.1152/jappl.1990.68.5.1917. [DOI] [PubMed] [Google Scholar]
- 181.Sieck GC, Fournier M. Contractile and fatigue properties of diaphragm motor units. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Alan R. Liss, Inc, 1987, p. 227–237. [Google Scholar]
- 182.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 66: 2539–2545, 1989. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 183.Sieck GC, Fournier M, Belman MJ. Physiological properties of motor units in the diaphragm. In: Neurogenesis of Central Respiratory Rhythm, edited by Bianchi AL, Denavit-Saubie M. Hingham, MA: MTP, 1985, p. 227–229. [Google Scholar]
- 184.Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett 97: 29–34, 1989. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- 186.Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol (1985) 80: 2179–2189, 1996. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- 187.Sieck GC, Gransee HM. Respiratory Muscles: Structure, Function & Regulation. Williston, VT: Morgan & Claypool, 2012. [Google Scholar]
- 188.Sieck GC, Han YS, Prakash YS, Jones KA. Cross-bridge cycling kinetics, actomyosin ATPase activity and myosin heavy chain isoforms in skeletal and smooth respiratory muscles. Comp Biochem Physiol B Biochem Mol Biol 119: 435–450, 1998. doi: 10.1016/S0305-0491(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 189.Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol (1985) 66: 2196–2205, 1989. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- 190.Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. N Engl J Med 358: 1392–1394, 2008. doi: 10.1056/NEJMe0801226. [DOI] [PubMed] [Google Scholar]
- 191.Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol 169: 218–225, 2009. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sieck GC, Prakash YS. Cross-bridge kinetics in respiratory muscles. Eur Respir J 10: 2147–2158, 1997. doi: 10.1183/09031936.97.10092147. [DOI] [PubMed] [Google Scholar]
- 193.Sieck GC, Prakash YS. The diaphragm muscle. In: Neural Control of the Respitatory Muscles, edited by Miller AD, Bianchi AL, Bishop BP. Boca Raton, FL: CRC Press, 1997, p. 7–20. [Google Scholar]
- 195.Sieck GC, Roy RR, Edgerton VR, Harper RM. Distribution of muscle fiber types in the diaphragm In: Second Oxford Meeting Symposium: Modelling and Control of Breathing. Lake Arrowhead, CA: Oxford Meeting Symposium, 1982. [Google Scholar]
- 196.Sieck GC, Roy RR, Powell P, Blanco C, Edgerton VR, Harper RM. Muscle fiber type distribution and architecture of the cat diaphragm. J Appl Physiol Respir Environ Exerc Physiol 55: 1386–1392, 1983. [DOI] [PubMed] [Google Scholar]
- 198.Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- 199.Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF. SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol (1985) 79: 1629–1639, 1995. doi: 10.1152/jappl.1995.79.5.1629. [DOI] [PubMed] [Google Scholar]
- 200.Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol Respir Environ Exerc Physiol 55: 813–822, 1983. [DOI] [PubMed] [Google Scholar]
- 201.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Song A, Ashwell KW, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol (Berl) 202: 159–177, 2000. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- 203.Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, Luo YM, Roughton M, Polkey MI, Moxham J. The value of multiple tests of respiratory muscle strength. Thorax 62: 975–980, 2007. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sypert GW, Munson JB. Basis of segmental motor control: motoneuron size or motor unit type? Neurosurgery 8: 608–621, 1981. doi: 10.1227/00006123-198105000-00020. [DOI] [PubMed] [Google Scholar]
- 205.Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med 152: 677–682, 1995. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- 206.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219, 1977. doi: 10.1016/0022-510X(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 207.Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol 99: 2114–2125, 2008. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Umezaki T, Shiba K, Zheng Y, Miller AD. Upper airway motor outputs during vomiting versus swallowing in the decerebrate cat. Brain Res 781: 25–36, 1998. doi: 10.1016/S0006-8993(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 209.van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH Jr, Constantine-Paton M, Bellingham MC. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci 28: 10864–10874, 2008. doi: 10.1523/JNEUROSCI.1340-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Viemari JC, Hilaire G. Monoamine oxidase A-deficiency and noradrenergic respiratory regulations in neonatal mice. Neurosci Lett 340: 221–224, 2003. doi: 10.1016/S0304-3940(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 211.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95: 2070–2082, 2006. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- 212.Vitacca M, Clini E, Facchetti D, Pagani M, Poloni M, Porta R, Ambrosino N. Breathing pattern and respiratory mechanics in patients with amyotrophic lateral sclerosis. Eur Respir J 10: 1614–1621, 1997. doi: 10.1183/09031936.97.10071614. [DOI] [PubMed] [Google Scholar]
- 213.Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol (1985) 63: 2403–2410, 1987. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- 214.Vucic S, Kiernan MC. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain 129: 2436–2446, 2006. doi: 10.1093/brain/awl172. [DOI] [PubMed] [Google Scholar]
- 215.Watchko JF, Mayock DE, Standaert TA, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res 20: 658–661, 1986. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]
- 216.Webber CL Jr, Wurster RD, Chung JM. Cat phrenic nucleus architecture as revealed by horseradish peroxidase mapping. Exp Brain Res 35: 395–406, 1979. doi: 10.1007/BF00236759. [DOI] [PubMed] [Google Scholar]
- 217.Zajac FE, Faden JS. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J Neurophysiol 53: 1303–1322, 1985. doi: 10.1152/jn.1985.53.5.1303. [DOI] [PubMed] [Google Scholar]
- 218.Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53: 1323–1344, 1985. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- 219.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol (1985) 82: 1145–1153, 1997. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]
- 220.Zhang C, Goto N, Suzuki M, Ke M. Age-related reductions in number and size of anterior horn cells at C6 level of the human spinal cord. Okajimas Folia Anat Jpn 73: 171–177, 1996. doi: 10.2535/ofaj1936.73.4_171. [DOI] [PubMed] [Google Scholar]
- 221.Zhang W, Carreño FR, Cunningham JT, Mifflin SW. Chronic sustained hypoxia enhances both evoked EPSCs and norepinephrine inhibition of glutamatergic afferent inputs in the nucleus of the solitary tract. J Neurosci 29: 3093–3102, 2009. doi: 10.1523/JNEUROSCI.2648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zheng Y, Umezaki T, Nakazawa K, Miller AD. Role of pre-inspiratory neurons in vestibular and laryngeal reflexes and in swallowing and vomiting. Neurosci Lett 225: 161–164, 1997. doi: 10.1016/S0304-3940(97)00208-5. [DOI] [PubMed] [Google Scholar]
- 223.Zholudeva LV, Karliner JS, Dougherty KJ, Lane MA. Anatomical Recruitment of Spinal V2a Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury. J Neurotrauma 34: 3058–3065, 2017. doi: 10.1089/neu.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]