Abstract
In addition to regulating the ingestion and digestion of food, sensory feedback from gut to brain modifies emotional state and motivated behavior by subconsciously shaping cognitive and affective responses to events that bias behavioral choice. This focused review highlights evidence that gut-derived signals impact motivated behavior by engaging vagal afferents and central neural circuits that generally serve to limit or terminate goal-directed approach behaviors, and to initiate or maintain behavioral avoidance.
Interoception and Biofeedback to the Brain
The viscera and brain are engaged in constant conversation. A key facilitator of this conversation is the gut-brain axis, comprising top-down neural pathways through which the central nervous system (CNS) controls gastrointestinal (GI) digestive functions, and bottom-up pathways through which the CNS receives moment-to-moment interoceptive feedback about visceral state (208). In humans and other mammals, the luminal surface of the GI tract is more than 100 times larger than the surface area of the skin, and represents the principal body surface interface for host-environment interactions. Indeed, the scope and complexity of gut-brain signaling pathways far exceed those related to the skin and other organ systems (155). The impact of GI processes on higher-level executive functions, affective state, and motivated behavior has received ample experimental attention within the fields of physiology, neuroscience, and psychology (21, 50), including a growing interest in gut microbial influences on brain and behavior (36, 88, 140, 151, 156, 171). In addition to fine-tuning ingestive and digestive processes, GI sensory feedback to the brain provides the neural basis for so-called “gut feelings,” which shape the subconscious emotional responses of humans and other animals to the daily threats and opportunities that drive motivated behavior (85, 155). Such threats and opportunities can originate internally (e.g., gut pathogens or nutrients) or within the environment (e.g., predator or food cues), and elicit a constellation of behavioral, physiological, and affective responses that are organized by complex CNS circuits. These responses can be innate or conditioned through learning, and typically are accompanied by endocrine and autonomic adjustments that alter GI and other visceral functions and interoceptive feedback to the brain.
The powerful influence of gut-brain signaling on behavioral state permits animals to anticipate or flexibly respond to contingencies by biasing the emotional and cognitive processes that guide motivated behavior. William James and Carl Lange were the first to formally propose that emotional feelings represent the perceptual consequences of sensory feedback from the body, providing the conceptual basis for the highly influential James-Lange theory of emotion (136). In James' words, “If our hypothesis be true, it makes us realize more deeply than ever how much our mental life is knit up with our corporeal frame, in the strictest sense of the term” (115). The core of this theory persists today amid mounting evidence that interoceptive feedback about GI and other visceral functions strongly impacts the emotional and cognitive processes that shape motivated behavior (37, 48, 50, 53, 56, 155). Accordingly, the body's physical state is proposed to serve as the basis for mood, affect, and other cognitive components of motivated behavior in both reductionist and complex systems accounts of emotion. The pivotal role of interoceptive feedback on motivated behavior arises in large part from the vital homeostatic control functions of spinal and hindbrain visceral circuits and their interconnections with higher limbic and cortical regions, offering a means through which goal-directed approach and avoidance behaviors are generated in accordance with emergent physiological and cognitive challenges and anticipated outcomes (208). Although many moment-to-moment decisions and choices appear to be under voluntary control, a constant undercurrent of subconscious influence over motivated behavior arises from within the body (273, 277). This article will review key aspects of interoceptive signaling pathways from the GI tract to the CNS, and describe how approach and avoidance behaviors are impacted by vagal sensory feedback from the gut in experimental rodent models. We also highlight evidence pointing to specific neurons in the caudal brain stem that transmit vagal sensory signals from the gut to brain regions that organize motivated behaviors. We propose that vagally mediated recruitment of these central neural pathways serves to limit or terminate positively reinforced behaviors, while increasing avoidance behaviors.
Interoceptive Signaling from Gut to Brain
Sensory signaling from the GI tract to the CNS is critical not only for regulating food seeking, intake, and digestion, but also for biasing emotional state and prioritizing motivated behaviors (25, 56, 267). Gut sensory information is encoded by intrinsic and extrinsic primary afferent neurons, by immune cells, and by enteroendocrine cells within the GI tissue (155) (FIGURE 1). GI endocrine and immune cells release gut hormones, cytokines, and other signaling factors that act peripherally on visceral sensory nerves (73, 123, 176, 194, 215), or may directly access the CNS at circumventricular brain regions lacking a blood-brain vascular barrier (79, 113), via active transport across the barrier (18) or through passive diffusion in cases of barrier disruption, as occurs in inflammation, neurodegenerative disease, or autoimmune disorders (17, 109, 114, 163).
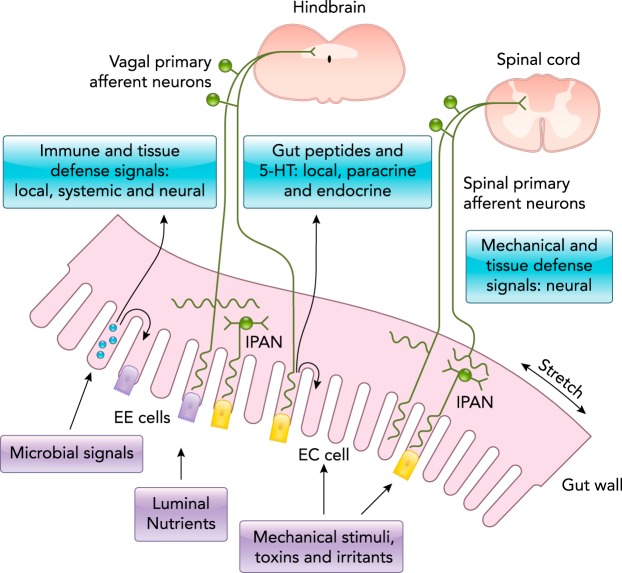
FIGURE 1.
Encoding sensory signals in the gut
Gut signals are carried by vagal and spinal primary afferent neurons projecting into the hindbrain and spinal cord, respectively. Mechanical stimuli (stretch, pressure, distortion) can activate spinal, vagal, and intrinsic primary afferent neurons (IPANs) directly, without intermediary enteroendocrine (EE) or enterochromaffin (EC) cells. Although no synaptic connections have been found between IPANs and extrinsic afferents, the latter form networks around myenteric ganglia neurons that receive synaptic input from IPANs. Serotonin (5-HT), peptides, cytokines, and other molecules produced by gut endocrine and immune cells in response to microbial signals, luminal nutrients, mechanical stimuli, and other factors can signal to the brain through the circulation, through EE and EC cells, and also via receptors expressed by primary vagal and spinal afferent neurons. Figure is adapted from Ref. 155, with permission.
Circulating Factors
An abundance of pharmacological evidence supports the role of pancreatic-, adipose tissue-, and GI-derived signaling factors in shaping motivated behavior by accessing the brain via the systemic circulation. The principle sources of caloric/nutritional feedback to the brain are hormones such as leptin, insulin, cholecystokinin (CCK), glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and other regulatory molecules signaling current and future availability of nutrients and stored energy (22, 46, 73, 194). At least one gut hormone (ghrelin) is released from gastric tissue when the stomach is empty, signaling an absence of nutrient absorption that facilitates appetitive food-seeking behavior (60, 131, 179). Leptin, CCK, GLP-1, ghrelin, and other peripherally derived signaling factors are released into the circulation and appear to affect neural activity within a variety of CNS regions, including those involved in motivation and approach behavior (179, 232, 245, 277). Although it is not yet clear whether or how CNS circuit activity is modified by these circulating factors under physiological (as opposed to experimental) conditions, food intake and the motivation to work for food is reduced after intraparenchymal administration of “anorexigenic” factors such as leptin, amylin, GLP-1, or insulin within specific CNS nuclei implicated in the appetitive and consummatory phases of ingestion (discussed further, below) (81, 109, 142, 162, 245, 266, 277), whereas central administration of ghrelin increases palatable food intake and reinforcement (179, 233). Stronger evidence for physiologically relevant, direct central actions comes from studies in which central receptors are disabled to interrupt the actions of endogenous (as opposed to administered) factors. For example, operant responding for palatable food is increased in rodents after virally mediated knockdown of leptin receptor expression within the midbrain ventral tegmental area (VTA) (61), whereas administration of a ghrelin receptor antagonist into the VTA or other brain regions not only suppresses food intake and reward but also interferes with the reinforcing properties of drugs such as cocaine, alcohol, and amphetamine (1, 69). Thus circulating GI-related factors that signal metabolic states of caloric surfeit or deficiency may directly access CNS circuits to influence reward circuit activity and approach behaviors in general, thereby linking motivated behavior to current physiological state (110, 277).
Extrinsic Neural Pathways
Despite the existence of hormonal signaling pathways from the GI tract to the CNS, the linkage between gut functions and emotional/cognitive processes is afforded primarily through extrinsic afferent neural signaling pathways to the spinal cord and caudal brain stem. It is important to note that neural activity within these afferent pathways is both sensitized and supplemented by gut microbial, immune, and endocrine signaling (97, 123, 124, 140, 157, 176, 219), generating an impressive degree of plasticity and versatility in the quality and magnitude of neural signals reaching the CNS (73, 74).
Neural signals from the gut (FIGURE 1) reach the CNS via spinal and vagal primary sensory neurons whose pseudo-unipolar cell bodies occupy the dorsal root or nodose ganglia, respectively, and whose central axon terminals synapse within the spinal cord or the caudal nucleus tractus solitarius (cNTS) in the lower brain stem (FIGURE 2, A AND B) (4, 5, 99, 164, 230, 237, 259). Spinal afferents from the GI tract enter lamina I of the dorsal horn and converge centrally with vagal sensory pathways by virtue of collateralized spinal inputs to the cNTS, the pontine parabrachial nucleus (PBN), and other brain stem regions (160). Despite this convergence, spinal and vagal sensory signals from the GI tract are qualitatively different, as revealed by direct electrophysiological recordings of afferent traffic en route to the CNS. Spinal afferent neurons innervating the upper GI tract are specialized to detect and protect against tissue damage, and are critical for nociceptive signaling (103, 155). On the other hand, GI vagal afferents appear to lack the properties of true nociceptors; instead, activation of vagal sensory inputs to the CNS has been implicated in antinociception through engagement of central opioid signaling (72, 270). Spinal pain signaling pathways from gut to brain clearly impact emotional state and motivated behavior, and peripheral factors that chronically sensitize and activate these pathways likely contribute to the high comorbidity of GI disorders with dysphoric motivational states of anxiety and depression, as thoroughly reviewed elsewhere (83, 143, 155). The remainder of this review will focus not on spinal afferents or pain signaling from the GI tract but instead on interoceptive signals conveyed from the gut to the brain via vagal afferents that terminate within the cNTS.
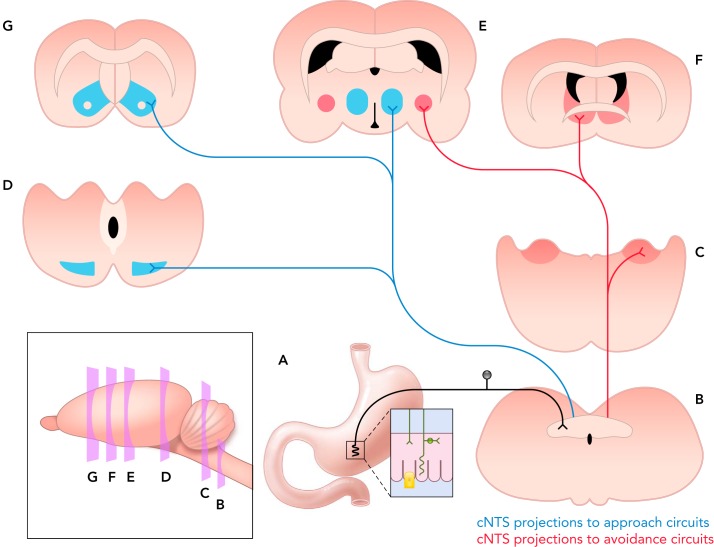
FIGURE 2.
cNTS neural projections to key CNS regions that shape motivated behavior
The peripheral axons of GI vagal afferent neurons, whose cell bodies occupy the nodose ganglia, ramify throughout the upper GI tract (A) and are specialized to detect distension, smooth muscle tone, locally released gut hormones, and other signaling factors, including microbial products and immune mediators (see FIGURE 1). The central axons of vagal afferents enter the dorsal region of the caudal medulla, terminating densely within the cNTS (B). Of the many chemically distinct neural populations residing within the cNTS, three are responsive to interoceptive vagal sensory input and also project to higher brain regions: GLP-1 neurons, NA neurons of the A2 group, and CCK-expressing neurons. These interoceptive-responsive cNTS neurons modulate motivated behavior via direct projections to CNS regions implicated in approach (blue) and avoidance behaviors (red). Inputs to approach behavior circuits (blue): GLP-1 and A2 NA neurons directly innervate the MDS, targeting both the VTA (D) and NAc (G). cNTS projections to the LHA (E) and other central sites (e.g., medial hypothalamus, thalamus, lateral septum) provide additional routes for ascending interoceptive neural pathways to influence brain regions involved in behavioral reinforcement and reward. Inputs to avoidance behavior circuits (red): GLP-1 and A2 neurons also form direct and indirect projections to the BST (F) and CeA (E). The ventrolateral (vl) BST receives particularly dense input from medullary NA neurons that co-express PrRP. GLP1, A2, and CCK-expressing cNTS neurons provide dense input to the pontine PBN (C), which densely innervates both the CeA and BST. cNTS, caudal nucleus tractus solitarius; GLP-1, glucagon-like peptide-1; NA, noradrenergic; CCK, cholecystokinin; MDS, mesolimbic dopamine system; VTA, ventral tegmental area; NAc, nucleus accumbens; LHA, lateral hypothalamic area; BST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; PrRP, prolactin-releasing peptide.
Vagal Sensory Pathways from Gut to Brain
Vagal sensory afferents monitoring pre- and post-absorptive sites within the GI tract comprise the large majority of fibers within the vagus nerve (23). Vagal afferents are tonically active, providing a steady stream of glutamatergic input to postsynaptic cNTS neurons that is tuned on a moment-to-moment basis by gut distension, smooth muscle tone, locally released gut peptides, and immune mediators. The peripheral axons of some vagal afferents ramify within the mesentery and in intramuscular arrays throughout the smooth muscle layers of the esophagus, the gastric fundus and antrum, the pylorus, and the small intestine (26, 27, 189, 195, 224), where they are poised to detect muscle tension and contraction. Some vagal sensory axons terminate in close proximity to interstitial cells of Cajal within the smooth muscle layers (190) or within the myenteric and submucosal plexuses of the enteric nervous system, where they interact with several classes of intrinsic primary afferent neurons (155). Other vagal afferent fibers permeate the mucosal lining to terminate within a single epithelial-cell width of the luminal gut surface along the basal lamina of the epithelium (23, 24, 191), where their firing activity is regulated in a receptor-mediated manner by signaling factors released from mucosal immune cells, enteroendocrine cells, and other intermediate cells in the lamina propria (194). It is important to note that, although individual vagal afferents can be classified as mechano- or chemoreceptive, the majority appear to be polymodal and respond to a wide range of both mechanical and chemical stimuli (97). Vagal sensory neurons express a diverse array of receptors specialized for detecting locally released and circulating gut hormones, cytokines, and microbial products that vary according to diet, feeding status, and stress exposure (73, 74, 97, 123, 176, 194, 215), significantly expanding the potential for physiological plasticity of vagal sensory signaling from gut to brain. In this manner, vagal afferent neuronal plasticity effectively fine-tunes the major neural route through which information about the gut environment and ingested nutrients reaches the CNS to influence behavior.
The central axons of GI vagal sensory neurons enter the dorsal region of the caudal brain stem via multiple rootlets that converge in the solitary tract and terminate within the cNTS (FIGURE 2, A AND B) (4, 120). The cNTS is considered the “visceral” NTS, distinct from the more rostral “gustatory” NTS (141), and is a key component of the dorsal vagal complex (DVC), which also includes the area postrema (AP) and dorsal motor nucleus of the vagus. The DVC is a critical central node for interoceptive feedback from body to brain (100, 101, 200, 202, 205, 273, 275). Vagal sensory inputs to the cNTS are glutamatergic (181, 193, 241), and their activation generates tightly synced, large-amplitude excitatory postsynaptic receptor-mediated currents in cNTS neurons to provide high-fidelity transmission of sensory nerve activity (9). Glutamatergic vagal afferents also express a large, heterogeneous array of neuropeptides (97) whose synaptic signaling and/or neuromodulatory properties within the cNTS are largely unknown. In addition to afferent neural inputs, the AP and a significant portion of the subjacent cNTS contain fenestrated capillaries (51, 119, 229), providing local parenchymal access to blood-borne factors arising from the gut (e.g., toxins, cytokines, hormones, and transported glucose and other nutrients) that can affect local neural activity (31, 111, 187, 194, 199, 269). Thus cNTS neurons receive direct and relayed synaptic input from vagal GI and other sensory neurons surveying the entire visceral landscape, and also are privy to circulating factors that provide additional interoceptive feedback signals.
Since vagal sensory neurons express receptors for CCK, ghrelin, GLP-1, and many other gut hormones, receptor-mediated mechanisms can efficiently modify vagal afferent signaling to the cNTS in response to hormone stimulation within peripheral tissues or via the circulation (22, 59, 73, 97). Gut hormones released in response to intestinal nutrients sensitize (i.e., lower the activation threshold) of vagal afferents that deliver distension-related negative feedback signals to the cNTS, which generate satiety and the cessation of feeding-related approach and consummatory behavior (78, 154, 166, 224, 234, 268). The firing activity of distension-sensitive vagal afferents also is increased by factors such as circulating leptin (182, 183). Leptin levels increase or decrease on a slow time scale in proportion to adipose tissue mass, but also increase acutely after release from the gastric mucosa in response to mechanical stimulation (13, 38). Thus factors that increase GI vagal afferent signaling to the cNTS can be envisioned primarily as activating a vagal sensory “brake” that attenuates feeding-related approach and consummatory behavior. To extend the analogy, increased ghrelin signaling from an empty stomach reduces the firing activity of GI vagal afferents (59), effectively releasing the vagal afferent brake and facilitating ingestive approach and consummatory behavior.
In the remainder of this focused review, we will highlight evidence that gut-derived signals influence motivated behavior by altering GI vagal afferent activity and/or the activity of cNTS neurons, which innervate a central neural network implicated in emotionality and goal-directed behavior. Research findings in rats and mice indicate that sensory feedback from the gut attenuates not only food intake but a range of other positively reinforced approach and consummatory behaviors, while also increasing avoidance behaviors.
Motivated Behaviors and Vagally Mediated Influences
An animal's survival depends critically on its ability to obtain the resources necessary for health and well-being, and to avoid harmful and potentially life-threatening situations. The adaptive behavioral processes that facilitate these critical abilities are called motivated behaviors because they energize, activate, and direct behavior toward a motive or goal. Motivated (i.e., goal-directed) behaviors are closely linked to emotional/hedonic state, and can be broadly divided into two categories: approach and avoidance. Motivated approach and avoidance behaviors can be driven by innate factors (e.g., the rewarding properties of sweet taste and nutrient absorption, or the aversive properties of hunger, pain, and nausea), or by learned associations (e.g., cue-potentiated feeding, drug self-administration, or conditioned avoidance of flavors previously paired with gastric malaise). The complex CNS circuits that control initiation, maintenance, and termination of motivated behaviors are highly conserved across species, guiding humans and other animals toward stimuli and situations that may satisfy critical homeostatic needs, and away from those that may threaten homeostasis. These complex, multifaceted neural systems include key circuit nodes located at every level of the neuraxis, with each node receiving interoceptive feedback signals relayed directly or indirectly from the cNTS (FIGURE 2).
Approach Behaviors
Goal-directed approach behaviors are guided by the positive reinforcing properties of current or anticipated appetitive stimuli and associated cues (e.g., food, sexual partners, pain relief, social interaction) that generate hedonic pleasure and/or diminish a negative hedonic state. The reinforcing properties that motivate goal-directed appetitive behaviors such as feeding, sexual activity, and drug self-administration largely depend on recruitment of the mesolimbic dopamine system (MDS), comprising dopaminergic (DA) projections from the midbrain VTA to the ventral striatum [i.e., nucleus accumbens (NAc)] (FIGURE 2, D AND G) and other corticolimbic regions that access the motor systems that generate goal-directed behavior (211). In experimental animals and in humans, increased DA signaling within the NAc follows exposure to innate stimuli or learned cues with emotional significance and incentive salience, and DA transients are closely linked to reinforcement and reward-based learning (105, 177, 221, 238). For example, extracellular DA levels within the NAc increase in response to the rewarding taste of sucrose or after intestinal glucose transport, but fall in response to the aversive taste of quinine or after systemic administration of the nauseogenic agent lithium chloride, perhaps “marking” these innately rewarding or aversive stimuli as worthy of attention and learning about associated cues (35, 86, 212, 226). Interestingly, extracellular DA levels within the rat striatum closely reflect the caloric density and nutritive features of GI contents (64, 65, 226), suggesting moment-to-moment “tuning” of MDS reward circuits based on postabsorptive nutrient signaling from the GI tract. Indeed, intestinal transport/absorption of glucose, protein, fat, and other nutrients is sufficient to increase DA signaling within the MDS, which is necessary for the reinforcement that initiates appetitive food seeking and maintains consumption, as well as for feeding-related Pavlovian and instrumental learning (58, 211, 226, 236).
Ascending neural projections from the cNTS reach the VTA and NAc directly (200, 242, 260) and also indirectly via relays within the PBN (47, 89) and lateral hypothalamic area (LHA) (276). LHA inputs to the VTA heavily influence DA signaling within the MDS (12, 108, 122, 192, 248), and the LHA receives substantial synaptic inputs from the cNTS (116, 210, 242, 272) that include inputs originating from gastric vagal afferents (210). Classic studies demonstrated that LHA stimulation elicits a robust feeding response that can be blocked not only by antagonism of DA receptors (184) but also by subdiaphragmatic vagal nerve transection (14, 188), evidence that vagal sensory and/or motor loops are a critical component of the LHA-MDS-mediated effect on feeding-related approach behavior. Thus, by influencing neurophysiological signaling within the MDS and “extra-MDS” circuits, GI sensory signals relayed through the cNTS can modulate the motivational properties of environmental stimuli and thereby shape approach behavior.
Despite this evidence, results from other experiments support neither the necessity nor the sufficiency of GI vagal afferent signaling in driving CNS reward circuits that facilitate nutrient-associated positive reinforcement. A refined surgical approach called “subdiaphragmatic afferent vagotomy” (SDA) (172), in which half of the vagal motor innervation of the GI tract is preserved despite complete interruption of GI vagal afferent feedback to the CNS, has been used to more selectively examine the role of vagal afferents in GI-based nutrient reward signaling and reinforcement. Results from SDA experiments in mice and rats argue against any necessary role for vagal afferent signaling in the primary reinforcing properties of intestinal nutrients (227). Although the inability of SDA to eliminate flavor conditioning reinforced by intestinal glucose, lipid, and other nutrients challenges the idea that vagal afferents are required, it must be acknowledged that half of the GI vagal afferent cell bodies and their central axonal inputs to the cNTS persist after SDA, despite transection of the subdiaphragmatic portion of their peripheral axons that physically contact GI tissue. Thus leptin, insulin, GLP-1, and other factors released into the circulation in response to intestinal nutrients may be detected by semi-intact vagal afferents and conveyed to the cNTS in a manner that is sufficient for nutrient reward.
The available evidence indicates that central neural pathways for vagally mediated influences on MDS reward systems appear to be utilized primarily to deliver negative feedback signals from the GI tract that reduce the rewarding properties of nutrients and other reinforcing stimuli, in part by inducing competing motivational states associated with satiety, malaise, and nausea (see below; FIGURE 2). Indeed, most gut hormones released by intestinal absorption/transport of glucose, fat, and protein suppress rather than stimulate feeding (277), similar to other natural and experimental stimuli that activate vagal afferent inputs to the cNTS and reduce food intake and other motivated approach behaviors (201, 204). Importantly, vagally mediated, feeding-related cNTS stimulation appears to promote normal meal-induced satiety, whereas threat-induced stimulation of the cNTS (e.g., due to excessive GI distension, inflammation, or toxin exposure) recruits higher numbers or different populations of cNTS neurons that contribute to aversion, malaise, and avoidance behavior (134, 147, 213, 214).
Avoidance Behaviors
Avoidance behaviors are geared toward reducing exposure to negative, dysphoric emotional states such as fear, anxiety, pain, and nausea. In contrast to positively reinforced approach behaviors, avoidance behaviors are often characterized by behavioral suppression or withdrawal coupled with defensive posturing and increased vigilance/arousal, during which attention is focused on the aversive stimulus. Indeed, “anxiety-like behavior” in rodents is characterized by behavioral suppression and avoidance (e.g., reduced food intake, social interaction, locomotion, and exploration) accompanied by increased sympathetic activity, muscle tone, and startle responses (139, 178). Avoidance behaviors are generally guided by the negative reinforcing properties of successfully reducing interaction with aversive stimuli and associated cues. Similar to positive reinforcement, DA signaling within the MDS is critical to maintain and strengthen avoidance behaviors that prevent or minimize aversive experiences or outcomes (174).
The coordinated physiological and behavioral signs of avoidance behavior depend on the output of widely distributed neural circuits, including the bed nucleus of the stria terminalis (BST) and the central nucleus of the amygdala (CeA), often referred to as the “central extended amygdala” (29, 43, 62, 66, 257, 263). Research in rodents supports the view that the CeA is critical for eliciting short-lasting, stimulus-specific fear responses, including conditioned fear and avoidance behavior, whereas the BST is necessary for more sustained and generalized anxiety-like responses to innate or conditioned threat stimuli and contexts (256, 258). The highly interconnected CeA-BST receive robust direct and relayed interoceptive input from GI-related regions of the cNTS and PBN that are important for conditioned avoidance behavior (29, 39, 42, 222, 223), together with direct input from the cortex, hippocampus, and other amygdalar subnuclei that process stimuli associated with environmental threats. Projections from the CeA and BST target widespread areas of the CNS that coordinate the physiological and behavioral components of fear, anxiety, and other avoidance behaviors (75, 218, 240, 252, 258). Thus GI sensory feedback to the brain can initiate and modify avoidance behaviors associated with nausea, fear, and anxiety by modulating neural activity within the CeA-BST (30, 39, 42).
Studies in rodents have implicated vagal afferent signaling to the cNTS and higher brain regions in innate anxiety, learned fear, and the effect of food intake and physiological arousal on emotional learning and memory (80, 125, 158, 165, 175, 216, 264). In rats, interruption of vagal afferent signaling from the GI tract to the CNS via SDA reduces anxiety-like behavior, attenuates conditioned fear extinction, promotes an imbalance of noradrenergic and GABAergic systems within the prefrontal cortex and NAc (127), and impacts cognitive flexibility (128), clearly supporting a role for vagally mediated conveyance of “gut feelings” to the brain. The vagus nerve also is proposed as the most important neural pathway for communication between gut microbes and the brain (28, 84, 140, 144), and vagal nerve integrity is necessary for anxiolysis and certain other CNS-mediated effects induced by intestinal probiotics in rodents (33, 88, 180). Considered together, the anxiolytic effects of SDA and the necessity of an intact vagus for the anxiolytic effects of GI probiotic treatment suggest that vagal afferent signaling normally promotes an avoidance-dominated motivational state that can be initiated by gut inflammatory signaling and can evolve into full-blown “sickness behavior,” as discussed below.
Behavioral Reorganization During Inflammation and Illness
The importance of interoceptive feedback signals for assigning priority to motivated behavior is particularly apparent during times of visceral challenge related to GI infection, toxin exposure, and inflammation. Such challenges elicit a myriad of defensive and protective physiological and behavioral responses associated with a motivational state of avoidance; when these responses escalate and converge, they are collectively referred to as “sickness behavior.” Sickness behavior in humans and other animals is characterized by self-reported or observed symptoms that can include lethargy, dysphoria, nausea, anxiety, social isolation, malaise, anhedonia, loss of appetite, sleepiness, hyperalgesia, reduced grooming (self-care), and loss of libido. These symptoms and responses represent an adaptive reorganization of motivated behavioral priorities that generally support survival and recovery after exposure to toxins and pathogens, including microbial pathogens in the gut. Bacterial translocation from gut lumen to mucosa can be triggered not only by injury but also by stress and high-fat diet, both of which promote local release of proinflammatory cytokines from gut immune cells (6, 88, 171, 249, 274). In this regard, the critical role of gut microbial populations in emotionality is supported by evidence that transplantation of gut microbes harvested from high-fat-diet-fed donor mice into chow-fed recipient mice promotes increased anxiety-like behaviors and other cognitive alterations in the recipients (36). Importantly, recipient mice in that study also displayed disrupted intestinal barrier function, leading to increased circulating endotoxin and signs of central neuroinflammation.
GI bacterial translocation can be mimicked experimentally by systemic administration of endotoxin, which stimulates cytokine production and reliably reduces food intake, willingness to work for food, sexual behavior, and social interaction in rats and mice (34, 49, 185, 271). Instead, avoidance behaviors predominate, as evidenced by increased alertness but decreased locomotion and exploration (32, 144, 170). Whereas systemic cytokines can influence the CNS via numerous routes, vagal afferents express receptors for proinflammatory cytokines and are activated in response to peripheral endotoxin or cytokine administration (118, 133, 146). Subdiaphragmatic vagotomy attenuates many of the behavioral and physiological consequences of systemic endotoxin or cytokine administration, including activation of the neuroendocrine stress response, hyperthermia, conditioned taste aversion, reduced willingness to work for food, and decreased social interaction (32, 34, 57, 82, 106, 132, 146, 261). Conversely, other studies utilizing total subdiaphragmatic vagotomy or the more refined SDA surgical approach (discussed above) demonstrated little or no effect of vagal interruption on the ability of endotoxin or cytokine treatment to induce sickness behavior (106, 118, 186, 225). It is possible that vagal afferents are critical for signaling immune challenge during early stages of GI bacterial infection, when low levels of cytokines produce local effects on vagal nerve endings but are too “dilute” to signal systemically. Indeed, subdiaphragmatic vagotomy was reported to block hyperthermic responses to low but not high peripheral doses of cytokine (107). Consistent with this idea, gut bacterial infections that are subthreshold to increase systemic proinflammatory cytokines can still promote anxiety-like behavior in rodents (145), concurrent with activation of cFos expression by vagal afferent neurons, cNTS neurons, and neurons within the BST and CeA (96).
Although the experimental evidence cited above was obtained in laboratory rats and mice, the impact of gut-to-brain and vagal afferent signaling in mood, emotion, and motivated behavior also has been demonstrated in humans. For example, an increased prevalence of emotional dysregulation, depression, and mood disorders has been reported in patients with inflammatory bowel diseases—which produce a chronic pro-inflammatory GI state and systemic cytokine imbalances (98, 173) known to affect vagal sensory neurons—suggesting that inflammation-related disruption of normal vagal signaling contributes to clinical mood disorders. Indeed, vagal nerve stimulation (VNS) is an approved and effective treatment for severe clinical depression (40, 95, 102, 150, 217) with efficacy for improving mood and reducing anxiety in clinical populations (40). VNS has been reported to attenuate encoding of affectively negative information, potentially due to engagement of the ventromedial prefrontal cortex, insula, and dorsal pons (40, 50). Given the proposed role of gut vagal afferent signaling in supporting innate anxiety-like avoidance behaviors in rodents (127), it seems paradoxical that VNS is anxiolytic in humans. However, electrical nerve stimulation is clearly quite different than natural recruitment of vagal afferents by GI stimuli, and it is unknown how “psychically effective” levels of VNS actually impact neural signaling within the cNTS and limbic forebrain.
Central Neural Pathways for Vagal Afferent Modulation of Motivated Behavior
As reviewed in the preceding sections, experimental and clinical research strongly supports an important role for vagal afferent signaling in gut-to-brain regulation of motivated behavior and emotional state. On the whole, this evidence suggests that recruitment of vagal afferents contributes to suppression of motivated approach behaviors and augmentation of avoidance behaviors. These contributions must be actualized through the cNTS, whose component neurons innervate CNS approach and avoidance circuits (FIGURE 2). It is important to note that cNTS neurons also receive synaptic input from spinal, brain stem, hypothalamic, limbic forebrain, and cortical regions, and are sensitive (via the area postrema) to blood-borne signals, including those signaling the availability of glucose and the presence of toxins and cytokines. Vagal afferent input from the GI tract modulates cNTS neural responses to these other inputs, providing “interoceptive tuning” of cNTS neurons that innervate central circuits implicated in motivated approach and avoidance behavior. For this reason, it is not surprising that activation of cNTS neurons tends to reduce both ingestive and non-ingestive motivated approach behaviors, while increasing avoidance behaviors.
Chemically Identified cNTS Signaling Pathways That Impact Motivated Approach and Avoidance Behaviors
Projection neurons within the rodent cNTS that carry GI interoceptive signals to higher brain regions include two non-overlapping neural populations [noradrenergic (NA) neurons comprising the A2 cell group, and GLP-1-expressing neurons] (FIGURE 3), plus a third population of CCK-expressing neurons that partially overlaps with the GLP-1 population, at least in mice (213). Together, these neural populations account for the majority of cNTS projections to other CNS regions that shape motivated behavior, including regions that modulate DA signaling within the MDS (11, 68, 110, 159, 169, 196, 200, 202, 232).
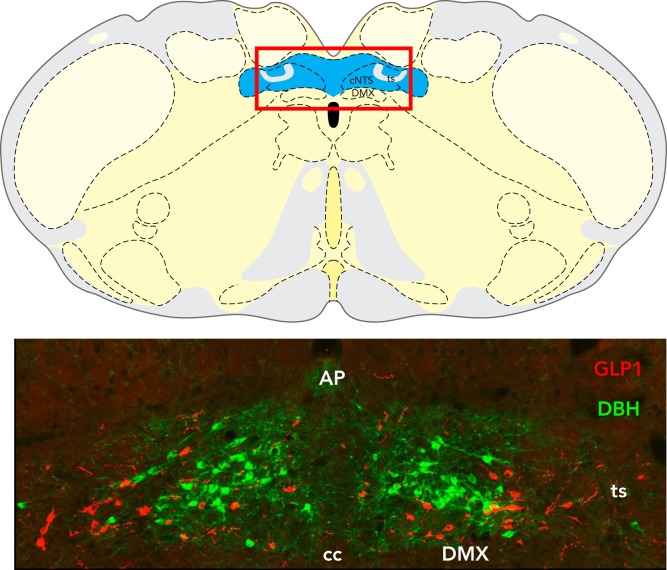
FIGURE 3.
Location of A2 and GLP-1 neurons within the hindbrain cNTS
Top: schematic coronal section through the rat caudal medulla illustrating the location of the cNTS (highlighted in blue). The red-lined box indicates the area shown at higher magnification in the photomicrograph on the bottom. In this image, dopamine beta hydroxylase (DBH)-positive NA neurons of the A2 cell group are green, whereas GLP-1-positive neurons are red. AP, area postrema; ts, tractus solitarius; cc, central canal; DMX, dorsal motor nucleus of the vagus. Figure is adapted from Ref. 147, with permission from Frontiers in Neuroscience.
In rats, cFos activation of A2 and GLP-1 neurons is markedly increased after exposure to stimuli that are perceived to threaten homeostasis, including restraint, systemic administration of supraphysiological doses of the gut peptide CCK, exposure to an illuminated elevated platform, exposure to predator odor, systemic administration of endotoxin or lithium chloride, or rapid ingestion of an extremely large meal (134, 147, 149, 168, 201, 204). Consistent with the ability of these “threat” stimuli to recruit cNTS projection neurons, each stimulus also suppresses subsequent food intake and increases avoidance behaviors, including anxiety-like behavior (147, 200). Indeed, as discussed further below, a growing body of evidence implicates signaling from A2, GLP-1, and CCK-positive neural populations to higher brain regions in mediating the powerful influence of interoceptive state on motivated behavior (147, 149, 214).
The A2 cell group.
The A2 cell group comprises NA neurons that are located entirely within the cNTS. Signaling from this NA population is poised to elicit avoidance behavior via receptor activation within numerous midbrain and forebrain regions, including the BST and CeA (16, 169, 202, 278) (FIGURE 2). A2 neurons appear to be critical for activating these limbic forebrain regions in response to vagally mediated interoceptive threats that increase avoidance behavior in rats, including large systemic doses of CCK (203), lithium chloride (207), and endotoxin. Regarding the latter, endotoxin-induced sickness behavior is associated with robust activation of A2 noradrenergic neurons that innervate the BST and CeA (30, 93, 94, 169). Moreover, the integrity of the A2 neurons is critical not only for BST-CeA neural recruitment following endotoxin but also for endotoxin-induced social withdrawal and reductions in exploratory behavior (91, 152).
Exteroceptive threats also recruit CNS circuits with important relays through the cNTS, supporting a role for vagal sensory modulation of exteroceptive stimulus-induced behavioral responses. In rats, immobilization or restraint stress, which elicits robust avoidance behavior, activates the large majority of A2 neurons and substantially increases extracellular levels of NE within the BST and CeA (63), whereas antagonism of NA receptors within the BST-CeA attenuates the ability of restraint stress to suppress social interaction and increase avoidance (44). Furthermore, removal or blockade of NA signaling in the BST blocks the anxiogenic effect of systemic yohimbine (which increases both peripheral and central NA signaling, including signaling from A2 neurons) (167, 278), and also attenuates the aversive properties of precipitated opiate withdrawal (11, 67, 68). In this regard, NA signaling within the BST (which originates primarily from the cNTS) has been proposed to drive the dysphoric, avoidance-dominated state of drug withdrawal by modulating inputs to the VTA that are implicated in anxiety, motivation, and affect (117, 235, 254).
Neurons synthesizing preproglucagon, the protein precursor for GLP-1.
Neurons synthesizing preproglucagon, the protein precursor for GLP-1, reside within the cNTS and subjacent reticular formation. GLP-1 released from intestinal endocrine cells can cross the BBB (45, 121), but its rapid degradation makes it unlikely to reach the brain in sufficient quantities to engage central GLP-1 receptors (112). Instead, the cNTS and medullary reticular formation provide the sole source of synaptic GLP-1 signaling within the brain, at least in rats (104, 137, 161, 204, 245). GLP-1 neurons are located adjacent to A2 neurons within the cNTS, but these are two distinct neural populations (147, 200).
Despite relatively sparse GLP-1 axonal inputs to the rat VTA and NAc (104, 200), GLP-1 signaling plays a significant and physiologically relevant role in suppressing reinforcement and approach behavior toward food, drugs, and other rewards via receptor signaling within the MDS and other brain regions (70, 110, 196–198, 231, 232). For example, selective pharmacological antagonism of GLP-1 receptors (GLP-1R) within the VTA, NAc, or LHA increases food intake (3, 76) and motivation for sucrose reward (243), whereas GLP-1R agonists reduce food palatability and motivation (70). Moreover, central administration of GLP-1R agonist attenuates the ability of cocaine to elicit phasic DA signaling within the NAc (87), whereas genetic knockdown of GLP-1R expression within the VTA increases cocaine self-administration (220).
Beyond reducing the reinforcing properties of otherwise rewarding stimuli, GLP-1 signaling also is implicated in innate and conditioned avoidance behavior (135, 209, 228, 244, 251). In rats, central antagonism of GLP-1R is anxiolytic (149), whereas GLP-1R agonists are anxiogenic (7, 126). GLP-1 neurons within the cNTS express cytokine receptors and display increased calcium permeability in response to cytokines (8), suggesting that GI or systemic inflammation may sensitize GLP-1 neurons to shift the animal toward behavioral states of avoidance. Together, these studies provide compelling evidence that central GLP-1 signaling pathways from the cNTS play a constitutive role in limiting approach and supporting avoidance behaviors.
The neuropeptide CCK.
The neuropeptide CCK is co-expressed by a subset of GLP-1 neurons in mice (90), whereas other CCK neurons appear to lack GLP-1 expression, and vice-versa. Similar to A2 neurons, CCK-expressing neurons within the cNTS are responsive to nutritional state in mice (213), and their activation reduces appetite (52). Interestingly, separate populations of cNTS CCK neurons innervate the PBN and paraventricular nucleus of the hypothalamus (PVH) (52, 214), and optogenetic activation of the CCK pathway from cNTS to PBN inhibits food intake and produces behavioral signs of aversion, including conditioned taste avoidance, whereas activation of the CCK pathway to the PVH inhibits food intake but has reinforcing effects in fasted mice (214). The reinforcing effect of CCK signaling from the cNTS to the PVH may reflect the satisfaction of satiety, which “puts the brake” on motivated approach behavior related to further food seeking and intake (52). Conversely, CCK inputs to the PBN may be recruited by excessive or pathological stimulation of vagal GI or other inputs to the cNTS that increase avoidance behavior (213, 214).
Interoceptive tuning of A2 and GLP-1 signaling from the cNTS.
The accumulated evidence reviewed here suggests that both interoceptive and exteroceptive signals can redirect motivated behavior from approach to avoidance via recruitment of cNTS neurons that directly or indirectly suppress activity within MDS-related reward circuits and increase activity within BST/CeA-related avoidance circuits. Motivated behaviors are profoundly affected by physiological (i.e., interoceptive) state (246), as exemplified by the strong influence of food restriction/deprivation on drug self-administration and other motivated approach and avoidance behaviors in rats (20, 41, 149). Recently, we discovered that overnight food deprivation eliminates or significantly reduces the ability of interoceptive and exteroceptive stressors to activate cNTS A2 and GLP-1 neurons in rats (148, 149), offering a potential mechanism for interoceptive tuning of motivated behavior. Overnight fasting also reduces anxiety-like behavior and attenuates BST neural activation in rats after acute stress (149). Thus overnight fasting-induced alterations in sensory signaling from the GI tract, likely due to altered nodose sensory signaling to the cNTS, may desensitize A2 and GLP-1 neurons to stimuli that normally activate these neurons in the fed state, thereby attenuating anxiety and other avoidance behaviors. The anxiolytic properties of caloric deficit may increase approach behaviors such as exploration and foraging, even in potentially dangerous environments, to ensure survival by replenishing energy stores (2, 54, 55, 149).
Conclusions and Future Directions
More than 130 years ago, William James and Carl Lange placed interoceptive signaling at the center of emotional regulation and motivated behavior. In this review, we have highlighted research demonstrating that neural, hormonal, cytokine, and microbial signals originating in the gut strongly influence goal-directed approach and avoidance behaviors, primarily by reducing approach and increasing avoidance. GI vagal afferents are well-positioned to carry gut-derived signals to the brain via synaptic inputs to the cNTS, with A2, GLP-1-, and CCK-positive neurons implicated in signaling to pontine, midbrain, hypothalamic, and forebrain regions that shape approach and avoidance behaviors.
Although substantial evidence supports a role for vagal afferents in the gut-brain connection, many questions remain. For example, although diet, stress, and other factors clearly interact to alter gut microbial populations, and although the gut microbiome can powerfully influence motivated behavior, focused research approaches are needed to determine the underlying mechanisms and precise role of vagal afferents in carrying microbial-related signals to the CNS. What is the role of vagal sensory signaling in the ability of probiotics or dietary changes to impact centrally mediated stress responses and affective behavior? What are the specific effects of microbial signals on plasticity of gene expression by vagal sensory neurons within the nodose ganglia, and on neurons within the cNTS that carry gut-derived signals to higher brain regions? Furthermore, given the powerful impact of early life events on the developmental assembly and life-long function of central visceral sensory and motor circuits, stress responsiveness, and emotionality (15, 88, 129, 130, 206, 208, 255, 262), it is critical to determine how pre- and postnatal stress exposure, maternal diet during pregnancy and lactation, offspring diet from infancy through adolescence, and other environmental factors affect gene expression and signal transmission in vagal sensory pathways from gut to brain.
It also is unclear whether gut-brain signaling pathways that impact motivated behavior differ substantially in male and female animals, including humans. The vast majority of the animal research reviewed in this article was conducted using male rats and mice, typically to avoid the potential “complications” of estrus cyclicity and/or the added time and expense necessary to conduct sufficiently powered experiments that include both sexes (19). However, gonadal hormone status clearly affects GI vagal afferent signaling to the cNTS and behavioral responses to GI signals in rodents (10, 77, 153). Future research on gut-brain signaling pathways must incorporate female subjects, especially considering the important role of vagal afferents in innate anxiety-like behavior in rodents (33, 127). In this regard, many anorexia nervosa sufferers report that their anxiety is alleviated by voluntary fasting/caloric restriction (which alters vagal afferent signaling to the cNTS), and many experience a resurgence of anxiety when food is reintroduced therapeutically (250). Anxiety is implicated in etiological and maintenance models of anorexia nervosa, bulimia nervosa, and other clinical eating disorders that occur predominantly in females (138, 253). For animal research to provide insights into these and other anxiety-related disorders that affect food intake and other motivated behaviors in humans, the research must include well-designed, adequately powered comparative analyses of both sexes.
To reveal the impact of GI vagal afferent signaling pathways on motivated behavior, it also is imperative that future studies utilize the most precise methodologies as they continue to evolve. The SDA technique (172) has been the “gold standard” for eliminating GI vagal afferent inputs to the brain, while preserving GI vagal motor functionality. However, SDA is technically challenging, disrupts half of the vagal motor signaling to the gut, and does not selectively target phenotypically unique subsets of GI vagal afferents. A promising new technique involves bilateral, toxin-induced destruction of GI vagal afferent cell bodies that express CCK receptors (71), representing a new, highly selective approach that preserves vagal motor innervation of the GI tract. Another laboratory is using powerful Cre-based technologies to identify and characterize the functional role of genetically specified subsets of vagal afferent neurons (265), opening up new opportunities for manipulating vagal afferents via optogenetics or DREADDs (designer receptors exclusively activated by designer drugs), approaches that already have been used to dissect the role of specific subtypes of cNTS neurons and their downstream targets in regulating motivated behavior (e.g., Refs. 52, 92, 214, 247). Considering that both the A2 and GLP-1 neural populations co-express glutamate and other neuropeptides (239, 279), selective opto- or chemostimulation techniques offer an advantage over the use of pharmacological approaches that target only a subset of neurotransmitter receptors, although these stimulation techniques are unlikely to closely model natural stimulation parameters. Nevertheless, these and other promising developments offer novel opportunities for animal researchers to revisit the role of GI vagal afferent signaling in the physiological and emotional components of motivated behavior. Such studies can only improve our relatively limited understanding of how interoceptive gut-to-brain signaling pathways affect human physiology, emotion, and behavior under conditions of health and disease.
Acknowledgments
This work was funded by National Institutes of Health Grants DK-100685 and MH-059911 (to L.R.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: J.W.M. and L.R. conceived and designed research; J.W.M. performed experiments; J.W.M. and L.R. analyzed data; J.W.M. and L.R. interpreted results of experiments; J.W.M. and L.R. prepared figures; J.W.M. and L.R. drafted manuscript; J.W.M. and L.R. edited and revised manuscript; L.R. approved final version of manuscript.
References
- 1.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229–3239, 2006. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology 135: 1125–1134, 1994. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- 3.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283: 248–268, 1989. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 5.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104: 502–509, 1993. doi: 10.1016/0016-5085(93)90419-D. [DOI] [PubMed] [Google Scholar]
- 6.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3: 559–572, 2011. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP. GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65: 54–66, 2016. doi: 10.1016/j.psyneuen.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Anesten F, Holt MK, Schéle E, Pálsdóttir V, Reimann F, Gribble FM, Safari C, Skibicka KP, Trapp S, Jansson JO. Preproglucagon neurons in the hindbrain have IL-6 receptor-α and show Ca2+ influx in response to IL-6. Am J Physiol Regul Integr Comp Physiol 311: R115–R123, 2016. doi: 10.1152/ajpregu.00383.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305: R1215–R1267, 2013. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci 877: 486–498, 1999. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 12.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res 1314: 74–90, 2010. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature 394: 790–793, 1998. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 14.Ball GG. Vagotomy: effect on electrically elicited eating and self-stimulation in the lateral hypothalamus. Science 184: 484–485, 1974. doi: 10.1126/science.184.4135.484. [DOI] [PubMed] [Google Scholar]
- 15.Banihashemi L, O’Neill EJ, Rinaman L. Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience 192: 413–428, 2011. doi: 10.1016/j.neuroscience.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci 26: 11442–11453, 2006. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y, Cook DG, Reed MJ. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 12: 223, 2015. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302: 822–827, 2002. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 19.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 20.Bell SM, Thiele TE, Seeley RJ, Bernstein IL, Woods SC. Effects of food deprivation on conditioned taste aversions in rats. Pharmacol Biochem Behav 60: 459–466, 1998. doi: 10.1016/S0091-3057(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 21.Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. Eur J Neurosci 18: 2103–2109, 2003. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud H-R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berthoud H-R, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 24.Berthoud H-R, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 156: 123–131, 1996. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Münzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152: 1728–1738, 2017. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 195: 183–191, 1997. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319: 261–276, 1992. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 28.Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev 73, Suppl 1: 28–31, 2015. doi: 10.1093/nutrit/nuv019. [DOI] [PubMed] [Google Scholar]
- 29.Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct 218: 187–208, 2013. doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience 156: 1093–1102, 2008. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blouet C, Schwartz GJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab 16: 579–587, 2012. doi: 10.1016/j.cmet.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III 317: 499–503, 1994. [PubMed] [Google Scholar]
- 33.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055, 2011. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bret-Dibat JL, Bluthé RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun 9: 242–246, 1995. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 35.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815–834, 2010. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E IV, Taylor CM, Welsh DA, Berthoud HR. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607–615, 2015. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron OG. Visceral brain-body information transfer. Neuroimage 47: 787–794, 2009. doi: 10.1016/j.neuroimage.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol 45: 1–16, 2012. doi: 10.5115/acb.2012.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos CA, Bowen AJ, Han S, Wisse BE, Palmiter RD, Schwartz MW. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat Neurosci 20: 934–942, 2017. doi: 10.1038/nn.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14: 716–727, 2017. doi: 10.1007/s13311-017-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll ME. Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr 169: 6–25, 1998. [PubMed] [Google Scholar]
- 42.Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci 35: 4582–4586, 2015. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877: 217–241, 1999. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 44.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience 112: 13–21, 2002. doi: 10.1016/S0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci 361: 1187–1209, 2006. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clemmensen C, Müller TD, Woods SC, Berthoud HR, Seeley RJ, Tschöp MH. Gut-brain cross-talk in metabolic control. Cell 168: 758–774, 2017. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience 168: 263–272, 2010. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666, 2002. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 49.Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res 542: 330–335, 1991. doi: 10.1016/0006-8993(91)91587-Q. [DOI] [PubMed] [Google Scholar]
- 50.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron 77: 624–638, 2013. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham ET Jr, Miselis RR, Sawchenko PE; Cunningham . The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience 58: 635–648, 1994. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 52.D’Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, Heisler LK. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife 5: e12225, 2016. doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalgleish T. The emotional brain. Nat Rev Neurosci 5: 583–589, 2004. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 54.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology 140: 4015–4023, 1999. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- 55.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol 14: 303–347, 1993. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 56.Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace, 1999. [Google Scholar]
- 57.Dantzer R, Bluthé RM, Layé S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci 840: 586–590, 1998. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 58.Darvas M, Wunsch AM, Gibbs JT, Palmiter RD. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc Natl Acad Sci USA 111: 2764–2769, 2014. doi: 10.1073/pnas.1400332111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Date Y. Ghrelin and the vagus nerve. Methods Enzymol 514: 261–269, 2012. doi: 10.1016/B978-0-12-381272-8.00016-7. [DOI] [PubMed] [Google Scholar]
- 60.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 61.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, Figlewicz DP, Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 69: 668–674, 2011. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35: 105–135, 2010. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. J Comp Neurol 478: 22–34, 2004. doi: 10.1002/cne.20259. [DOI] [PubMed] [Google Scholar]
- 64.de Araujo IE, Ferreira JG, Tellez LA, Ren X, Yeckel CW. The gut-brain dopamine axis: a regulatory system for caloric intake. Physiol Behav 106: 394–399, 2012. doi: 10.1016/j.physbeh.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron 57: 930–941, 2008. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 66.de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci 877: 1–32, 1999. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 67.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403: 430–434, 2000. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 68.Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res 806: 127–140, 1998. doi: 10.1016/S0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- 69.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 340: 80–87, 2011. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 70.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diepenbroek C, Quinn D, Stephens R, Zollinger B, Anderson S, Pan A, de Lartigue G. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol 313: G342–G352, 2017. doi: 10.1152/ajpgi.00095.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diop L, Rivière PJ, Pascaud X, Dassaud M, Junien JL. Role of vagal afferents in the antinociception produced by morphine and U-50,488H in the colonic pain reflex in rats. Eur J Pharmacol 257: 181–187, 1994. doi: 10.1016/0014-2999(94)90710-2. [DOI] [PubMed] [Google Scholar]
- 73.Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 592: 2927–2941, 2014. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dockray GJ. The versatility of the vagus. Physiol Behav 97: 531–536, 2009. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436: 430–455, 2001. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 76.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 283: R1378–R1385, 2002. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- 78.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol 276: R1545–R1549, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson AV. Circumventricular organs: integrators of circulating signals controlling hydration, energy balance, and immune function. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA Jr, Menani JV, Johnson AK. Boca Raton, FL: CRC Press, 2014. [PubMed] [Google Scholar]
- 80.Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry 46: 1140–1152, 1999. doi: 10.1016/S0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 81.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 296: R9–R19, 2009. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fleshner M, Goehler LE, Schwartz BA, McGorry M, Martin D, Maier SF, Watkins LR. Thermogenic and corticosterone responses to intravenous cytokines (IL-1beta and TNF-alpha) are attenuated by subdiaphragmatic vagotomy. J Neuroimmunol 86: 134–141, 1998. doi: 10.1016/S0165-5728(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 83.Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 264: 651–660, 2014. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- 84.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817: 115–133, 2014. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 85.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun 24: 9–16, 2010. doi: 10.1016/j.bbi.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 86.Fortin SM, Chartoff EH, Roitman MF. The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology 41: 906–915, 2016. doi: 10.1038/npp.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fortin SM, Roitman MF. Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol Behav 176: 17–25, 2017. doi: 10.1016/j.physbeh.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress 7: 124–136, 2017. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319: 229–259, 1984. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 90.Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology 153: 4600–4607, 2012. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaykema RP, Goehler LE. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue? Brain Behav Immun 25: 443–460, 2011. doi: 10.1016/j.bbi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, Harris TE, Patel MK, Williams KW, Scott MM. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 127: 1031–1045, 2017. doi: 10.1172/JCI81335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaykema RPA, Chen C-C, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res 1130: 130–145, 2007. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 94.Gaykema RPA, Daniels TE, Shapiro NJ, Thacker GC, Park S-M, Goehler LE. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res 1294: 61–79, 2009. doi: 10.1016/j.brainres.2009.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 47: 287–295, 2000. doi: 10.1016/S0006-3223(99)00308-X. [DOI] [PubMed] [Google Scholar]
- 96.Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun 19: 334–344, 2005. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Grabauskas G, Owyang C. Plasticity of vagal afferent signaling in the gut. Medicina (Kaunas) 53: 73–84, 2017. doi: 10.1016/j.medici.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 15: 1105–1118, 2009. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 99.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neurosci Lett 76: 151–156, 1987. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 100.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes 33, Suppl 1: S11–S15, 2009. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 102.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 29: 493–500, 2005. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Grundy D. What activates visceral afferents? Gut 53, Suppl 2: ii5–ii8, 2004. doi: 10.1136/gut.2003.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol 521: 2235–2261, 2013. doi: 10.1002/cne.23282. [DOI] [PubMed] [Google Scholar]
- 105.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci 19: 117–126, 2016. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol 278: R331–R336, 2000. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- 107.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol 280: R929–R934, 2001. doi: 10.1152/ajpregu.2001.280.4.R929. [DOI] [PubMed] [Google Scholar]
- 108.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559, 2005. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 109.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr 34: 237–260, 2014. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci 9: 66–70, 2016. doi: 10.1016/j.cobeha.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 113.Hoyda TD, Smith PM, Ferguson AV. Gastrointestinal hormone actions in the central regulation of energy metabolism: potential sensory roles for the circumventricular organs. Int J Obes 33, Suppl 1: S16–S21, 2009. doi: 10.1038/ijo.2009.11. [DOI] [PubMed] [Google Scholar]
- 114.Hsu TM, Kanoski SE. Blood-brain barrier disruption: mechanistic links between Western diet consumption and dementia. Front Aging Neurosci 6: 88, 2014. doi: 10.3389/fnagi.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.James W. What is an emotion? Mind IX: 188–205, 1884. doi: 10.1093/mind/os-IX.34.188. [DOI] [Google Scholar]
- 116.Jeanningros R. Modulation of lateral hypothalamic single unit activity by gastric and intestinal distension. J Auton Nerv Syst 11: 1–11, 1984. doi: 10.1016/0165-1838(84)90003-1. [DOI] [PubMed] [Google Scholar]
- 117.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228, 2013. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnston GR, Webster NR. Cytokines and the immunomodulatory function of the vagus nerve. Br J Anaesth 102: 453–462, 2009. doi: 10.1093/bja/aep037. [DOI] [PubMed] [Google Scholar]
- 119.Kachidian P, Pickel VM. Localization of tyrosine hydroxylase in neuronal targets and efferents of the area postrema in the nucleus tractus solitarii of the rat. J Comp Neurol 329: 337–353, 1993. doi: 10.1002/cne.903290305. [DOI] [PubMed] [Google Scholar]
- 120.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211: 248–265, 1982. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 121.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18: 7–14, 2002. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 122.Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci 33: 7618–7626, 2013. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL, Wittert GA, Blackshaw LA, Page AJ. Diet-induced adaptation of vagal afferent function. J Physiol 590: 209–221, 2012. doi: 10.1113/jphysiol.2011.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kentish SJ, Page AJ. The role of gastrointestinal vagal afferent fibres in obesity. J Physiol 593: 775–786, 2015. doi: 10.1113/jphysiol.2014.278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kerfoot EC, Chattillion EA, Williams CL. Functional interactions between the nucleus tractus solitarius (NTS) and nucleus accumbens shell in modulating memory for arousing experiences. Neurobiol Learn Mem 89: 47–60, 2008. doi: 10.1016/j.nlm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci 23: 6163–6170, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klarer M, Arnold M, Günther L, Winter C, Langhans W, Meyer U. Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 34: 7067–7076, 2014. doi: 10.1523/JNEUROSCI.0252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klarer M, Weber-Stadlbauer U, Arnold M, Langhans W, Meyer U. Cognitive effects of subdiaphragmatic vagal deafferentation in rats. Neurobiol Learn Mem 142, Pt B: 190–199, 2017. doi: 10.1016/j.nlm.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 129.Koehnle TJ, Rinaman L. Early experience alters limbic forebrain Fos responses to a stressful interoceptive stimulus in young adult rats. Physiol Behav 100: 105–115, 2010. doi: 10.1016/j.physbeh.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koehnle TJ, Rinaman L. Progressive postnatal increases in Fos immunoreactivity in the forebrain and brain stem of rats after viscerosensory stimulation with lithium chloride. Am J Physiol Regul Integr Comp Physiol 292: R1212–R1223, 2007. doi: 10.1152/ajpregu.00666.2006. [DOI] [PubMed] [Google Scholar]
- 131.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 132.Konsman JP, Luheshi GN, Bluthé R-M, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci 12: 4434–4446, 2000. doi: 10.1046/j.0953-816X.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 133.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25: 154–159, 2002. doi: 10.1016/S0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 134.Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav 136: 47–54, 2014. doi: 10.1016/j.physbeh.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lachey JL, D’Alessio DA, Rinaman L, Elmquist JK, Drucker DJ, Seeley RJ. The role of central GLP-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology 146: 458–462, 2004. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 136.Lange CG, James W. The Emotions. New York: Hafner Pub. Co, 1967. [Google Scholar]
- 137.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. doi: 10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 138.Lavender JM, De Young KP, Wonderlich SA, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Peterson CB, Le Grange D. Daily patterns of anxiety in anorexia nervosa: associations with eating disorder behaviors in the natural environment. J Abnorm Psychol 122: 672–683, 2013. doi: 10.1037/a0031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184, 2000. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 140.Liu RT. The microbiome as a novel paradigm in studying stress and mental health. Am Psychol 72: 655–667, 2017. doi: 10.1037/amp0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lundy RF Jr, Norgren R. Gustatory system. In: The Rat Nervous System (3rd ed.), edited by Paxinos G. San Diego: Elsevier Academic Press, 2004, p. 890–921. [Google Scholar]
- 142.Lutz TA. Amylinergic control of food intake. Physiol Behav 89: 465–471, 2006. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 143.Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry 62, Suppl 8: 38–45, 2001. [PubMed] [Google Scholar]
- 144.Lyte M, Li W, Opitz N, Gaykema RPA, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89: 350–357, 2006. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 145.Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav 65: 63–68, 1998. doi: 10.1016/S0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- 146.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci 840: 289–300, 1998. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 147.Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Front Neurosci 6: 199, 2013. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav 121: 35–42, 2013. doi: 10.1016/j.physbeh.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci 35: 10701–10714, 2015. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, Nahas Z, Lisanby SH. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry 51: 280–287, 2002. doi: 10.1016/S0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- 151.Martin CR, Mayer EA. Gut-brain axis and behavior. Nestle Nutr Inst Workshop Ser 88: 45–53, 2017. doi: 10.1159/000461732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun 18: 123–134, 2004. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 153.Maske CB, Jackson CM, Terrill SJ, Eckel LA, Williams DL. Estradiol modulates the anorexic response to central glucagon-like peptide 1. Horm Behav 93: 109–117, 2017. doi: 10.1016/j.yhbeh.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mathis C, Moran TH, Schwartz GJ. Load-sensitive rat gastric vagal afferents encode volume but not gastric nutrients. Am J Physiol Regul Integr Comp Physiol 274: R280–R286, 1998. [DOI] [PubMed] [Google Scholar]
- 155.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12: 453–466, 2011. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34: 15490–15496, 2014. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 146: 1500–1512, 2014. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 12: 205–210, 2002. doi: 10.1016/S0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 159.Mejías-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci 29: 3613–3626, 2009. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol 255: 439–450, 1987. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 161.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 162.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305: E1367–E1374, 2013. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9: 540–549, 2003. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 164.Miselis RR, Rinaman L, Altschuler SM, Bao X, Lynn RB. Medullary viscerotopic representation of the alimentary canal innervation in rat. In: Brain-Gut Interactions, edited by Tache Y, Wingate D. Boca Raton, FL: CRC Press, 1991, p. 3–17. [Google Scholar]
- 165.Miyashita T, Williams CL. Enhancement of noradrenergic neurotransmission in the nucleus of the solitary tract modulates memory storage processes. Brain Res 987: 164–175, 2003. doi: 10.1016/S0006-8993(03)03323-7. [DOI] [PubMed] [Google Scholar]
- 166.Moran TH, Wirth JB, Schwartz GJ, McHugh PR. Interactions between gastric volume and duodenal nutrients in the control of liquid gastric emptying. Am J Physiol Regul Integr Comp Physiol 276: R997–R1002, 1999. [DOI] [PubMed] [Google Scholar]
- 167.Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J Comp Neurol 492: 426–441, 2005. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- 168.Myers EA, Rinaman L. Trimethylthiazoline supports conditioned flavor avoidance and activates viscerosensory, hypothalamic, and limbic circuits in rats. Am J Physiol Regul Integr Comp Physiol 288: R1716–R1726, 2005. doi: 10.1152/ajpregu.00479.2004. [DOI] [PubMed] [Google Scholar]
- 169.Myers EA, Rinaman L. Viscerosensory activation of noradrenergic inputs to the amygdala in rats. Physiol Behav 77: 723–729, 2002. doi: 10.1016/S0031-9384(02)00925-3. [DOI] [PubMed] [Google Scholar]
- 170.Nava F, Caputi AP. Central effects of cromoglycate sodium salt in rats treated with lipopolysaccharide. Eur J Pharmacol 367: 351–359, 1999. doi: 10.1016/S0014-2999(98)00986-8. [DOI] [PubMed] [Google Scholar]
- 171.Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci 11: 9, 2017. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994. [DOI] [PubMed] [Google Scholar]
- 173.Nowakowski J, Chrobak AA, Dudek D. Psychiatric illnesses in inflammatory bowel diseases - psychiatric comorbidity and biological underpinnings. Psychiatr Pol 50: 1157–1166, 2016. doi: 10.12740/PP/62382. [DOI] [PubMed] [Google Scholar]
- 174.Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci 32: 14804–14808, 2012. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Packard MG, Williams CL, Cahill L, McGaugh JL. The anatomy of a memory modulatory system: from periphery to brain. In: Neurobehavioral Plasticity - Learning, Development, and Response to Brain Insults, edited by Spear NE, Spear LP, Woodruff ML. Hillsdale, NJ: Lawrence Erlbaum Associates, 1995, p. 149–184. [Google Scholar]
- 176.Page AJ, Kentish SJ. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol Motil 29: e12973, 2017. doi: 10.1111/nmo.12973. [DOI] [PubMed] [Google Scholar]
- 177.Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten IB. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat Neurosci 19: 845–854, 2016. doi: 10.1038/nn.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct 214: 451–463, 2010. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 27: 424–434, 2015. doi: 10.1111/jne.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse”. FASEB J 28: 3064–3074, 2014. doi: 10.1096/fj.13-245282. [DOI] [PubMed] [Google Scholar]
- 181.Perrone MH. Biochemical evidence that L-glutamate is a neurotransmitter of primary vagal afferent nerve fibers. Brain Res 230: 283–293, 1981. doi: 10.1016/0006-8993(81)90407-8. [DOI] [PubMed] [Google Scholar]
- 182.Peters JH, Ritter RC, Simasko SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 290: C427–C432, 2006. doi: 10.1152/ajpcell.00439.2005. [DOI] [PubMed] [Google Scholar]
- 183.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 89: 477–485, 2006. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 184.Phillips AG, Nikaido RS. Disruption of brain stimulation-induced feeding by dopamine receptor blockade. Nature 258: 750–751, 1975. doi: 10.1038/258750a0. [DOI] [PubMed] [Google Scholar]
- 185.Porter MH, Hrupka BJ, Altreuther G, Arnold M, Langhans W. Inhibition of TNF-alpha production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am J Physiol Regul Integr Comp Physiol 279: R2113–R2120, 2000. doi: 10.1152/ajpregu.2000.279.6.R2113. [DOI] [PubMed] [Google Scholar]
- 186.Porter MH, Hrupka BJ, Langhans W, Schwartz GJ. Vagal and splanchnic afferents are not necessary for the anorexia produced by peripheral IL-1beta, LPS, and MDP. Am J Physiol Regul Integr Comp Physiol 275: R384–R389, 1998. [DOI] [PubMed] [Google Scholar]
- 187.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol Behav 100: 511–518, 2010. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 188.Powley TL, MacFarlane BA, Markell MS, Opsahl CR. Different effects of vagotomy and atropine on hypothalamic stimulation-induced feeding. Behav Biol 23: 306–325, 1978. doi: 10.1016/S0091-6773(78)91337-8. [DOI] [PubMed] [Google Scholar]
- 189.Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol 283: G1217–G1225, 2002. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- 190.Powley TL, Phillips RJ. Vagal intramuscular array afferents form complexes with interstitial cells of Cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186: 188–200, 2011. doi: 10.1016/j.neuroscience.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519: 644–660, 2011. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud HR, Morrison CD, Münzberg H. Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J Neurosci 37: 6053–6065, 2017. doi: 10.1523/JNEUROSCI.0155-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Raab M, Neuhuber WL. Glutamatergic functions of primary afferent neurons with special emphasis on vagal afferents. Int Rev Cytol 256: 223–275, 2007. doi: 10.1016/S0074-7696(07)56007-9. [DOI] [PubMed] [Google Scholar]
- 194.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci 153: 41–46, 2010. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raybould HE. Vagal afferent innervation and the regulation of gastric motor function. In: Neuroanatomy and Physiology of Abdominal Vagal Afferents, edited by Ritter S, Ritter RC, Barnes CD. Boca Raton, FL: CRC Press, 1992, p. 195–219. [Google Scholar]
- 196.Reddy IA, Stanwood GD, Galli A. Moving beyond energy homeostasis: new roles for glucagon-like peptide-1 in food and drug reward. Neurochem Int 73: 49–55, 2014. doi: 10.1016/j.neuint.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One 10: e0119034, 2015. doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson JO, Liposits Z, Skibicka KP. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155: 4356–4367, 2014. doi: 10.1210/en.2014-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Riediger T, Schmid HA, Lutz TA, Simon E. Amylin and glucose co-activate area postrema neurons of the rat. Neurosci Lett 328: 121–124, 2002. doi: 10.1016/S0304-3940(02)00482-2. [DOI] [PubMed] [Google Scholar]
- 200.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rinaman L. Hindbrain contributions to anorexia. Am J Physiol Regul Integr Comp Physiol 287: R1035–R1036, 2004. doi: 10.1152/ajpregu.00437.2004. [DOI] [PubMed] [Google Scholar]
- 202.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23: 10084–10092, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol Regul Integr Comp Physiol 277: R582–R590, 1999. [DOI] [PubMed] [Google Scholar]
- 205.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28: 50–60, 2007. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav 104: 632–640, 2011. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol 293: R1495–R1503, 2007. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 208.Rinaman L, Koehnle TJ. Development of central visceral circuits. In: Oxford Handbook of Developmental Behavioral Neuroscience, edited by Blumberg MS, Freeman JH, Robinson SR. New York: Oxford University Press, 2010, p. 298–321. [Google Scholar]
- 209.Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol 283: R99–R106, 2002. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- 210.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 24: 2782–2786, 2004. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24: 1265–1271, 2004. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11: 1376–1377, 2008. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 7: 11905, 2016. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roman CW, Sloat SR, Palmiter RD. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience 358: 316–324, 2017. doi: 10.1016/j.neuroscience.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ronveaux CC, Tomé D, Raybould HE. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J Nutr 145: 672–680, 2015. doi: 10.3945/jn.114.206029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur J Neurosci 11: 1317–1323, 1999. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 217.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RK Jr, Goodman R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry 47: 276–286, 2000. doi: 10.1016/S0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 218.Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol 22: 633–641, 2008. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 179: 223–244, 2017. doi: 10.1016/j.trsl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 220.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology 41: 1917–1928, 2016. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27, 1998. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 222.Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci 2: 1424–1438, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 270: 416–426, 1988. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- 224.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition 16: 866–873, 2000. doi: 10.1016/S0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 225.Schwartz GJ, Plata-Salamán CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Am J Physiol Regul Integr Comp Physiol 273: R1193–R1198, 1997. [DOI] [PubMed] [Google Scholar]
- 226.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol 302: R1119–R1133, 2012. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003. doi: 10.1016/S0031-9384(02)00968-X. [DOI] [PubMed] [Google Scholar]
- 228.Seeley RJ, Dijk GV, Blake J, Thiele TH. The role of glucagon-like-peptide-1-(7–36) amide (GLP-1) in the control of food intake and mediating visceral illness in the CNS. In: Brainstem Mechanisms of Ingestion and Gastrointestinal Physiology. County Durham, UK: Lumley Castle, 1997, p. 34. [Google Scholar]
- 229.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol 234: 344–364, 1985. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 230.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238: 473–488, 1985. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 231.Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One 8: e61965, 2013. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181, 2013. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology 73: 274–283, 2013. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 234.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41–46, 1996. doi: 10.1016/0149-7634(95)00038-G. [DOI] [PubMed] [Google Scholar]
- 235.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct 213: 43–61, 2008. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20: 7737–7742, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Spencer NJ, Kyloh M, Beckett EA, Brookes S, Hibberd T. Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J Comp Neurol 524: 3064–3083, 2016. doi: 10.1002/cne.24006. [DOI] [PubMed] [Google Scholar]
- 238.Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One 9: e94771, 2014. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol 444: 191–206, 2002. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- 240.Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128: 7–14, 2004. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 241.Sykes RM, Spyer KM, Izzo PN. Demonstration of glutamate immunoreactivity in vagal sensory afferents in the nucleus tractus solitarius of the rat. Brain Res 762: 1–11, 1997. doi: 10.1016/S0006-8993(97)00368-5. [DOI] [PubMed] [Google Scholar]
- 242.TerHorst GJ, Streefland C. Ascending projections of the solitary tract nucleus. In: Nucleus of the Solitary Tract, edited by Barraco IRA. Boca Raton, FL: CRC Press, 1994, p. 93–103. [Google Scholar]
- 243.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am J Physiol Regul Integr Comp Physiol 311: R124–R132, 2016. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 245.Trapp S, Cork SC. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am J Physiol Regul Integr Comp Physiol 309: R795–R804, 2015. doi: 10.1152/ajpregu.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tsakiris M, Critchley H. Interoception beyond homeostasis: affect, cognition and mental health. Philos Trans R Soc Lond B Biol Sci 371: 20160002, 2016. doi: 10.1098/rstb.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu XA, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci 20: 708–716, 2017. doi: 10.1038/nn.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tyree SM, de Lecea L. Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front Syst Neurosci 11: 50, 2017. doi: 10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress 18: 381–399, 2015. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Van Wymelbeke V, Brondel L, Marcel Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am J Clin Nutr 80: 1469–1477, 2004. [DOI] [PubMed] [Google Scholar]
- 251.van Dijk G, Thiele TE. Glucagon-like peptide-1 (7-36) amide: a central regulator of satiety and interoceptive stress. Neuropeptides 33: 406–414, 1999. doi: 10.1054/npep.1999.0053. [DOI] [PubMed] [Google Scholar]
- 252.Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pêgo JM. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci 7: 32, 2013. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vitousek K, Manke F. Personality variables and disorders in anorexia nervosa and bulimia nervosa. J Abnorm Psychol 103: 137–147, 1994. doi: 10.1037/0021-843X.103.1.137. [DOI] [PubMed] [Google Scholar]
- 254.Vranjkovic O, Pina M, Kash TL, Winder DG. The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology 122: 100–106, 2017. doi: 10.1016/j.neuropharm.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Walker CD, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y, Baram TZ. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress 20: 421–448, 2017. doi: 10.1080/10253890.2017.1343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17: 9375–9383, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct 213: 29–42, 2008. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 258.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216, 2003. doi: 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 259.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol 421: 302–324, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 260.Wang ZJ, Rao ZR, Shi JW. Tyrosine hydroxylase-, neurotensin-, or cholecystokinin-containing neurons in the nucleus tractus solitarii send projection fibers to the nucleus accumbens in the rat. Brain Res 578: 347–350, 1992. doi: 10.1016/0006-8993(92)90269-F. [DOI] [PubMed] [Google Scholar]
- 261.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett 183: 27–31, 1995. doi: 10.1016/0304-3940(94)11105-R. [DOI] [PubMed] [Google Scholar]
- 262.Weber BC, Manfredo HN, Rinaman L. A potential gastrointestinal link between enhanced postnatal maternal care and reduced anxiety-like behavior in adolescent rats. Behav Neurosci 123: 1178–1184, 2009. doi: 10.1037/a0017659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 12387–12396, 2006. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav Neurosci 112: 1414–1422, 1998. doi: 10.1037/0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- 265.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Woods SC, Chavez M, Park CR, Riedy C, Kaiyala K, Richardson RD, Figlewicz DP, Schwartz MW, Porte D Jr, Seeley RJ. The evaluation of insulin as a metabolic signal influencing behavior via the brain. Neurosci Biobehav Rev 20: 139–144, 1996. doi: 10.1016/0149-7634(95)00044-F. [DOI] [PubMed] [Google Scholar]
- 267.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab 93, Suppl 1: S37–S50, 2008. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 269.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23: 2939–2946, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yan XJ, Feng CC, Liu Q, Zhang LY, Dong X, Liu ZL, Cao ZJ, Mo JZ, Li Y, Fang JY, Chen SL. Vagal afferents mediate antinociception of estrogen in a rat model of visceral pain: the involvement of intestinal mucosal mast cells and 5-hydroxytryptamine 3 signaling. J Pain 15: 204–217, 2014. doi: 10.1016/j.jpain.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 271.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmächer T. Illness, cytokines, and depression. Ann N Y Acad Sci 917: 478–487, 2000. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 272.Yuan CS, Barber WD. Interactions of gastric vagal and peripheral nerves on single neurons of lateral hypothalamus in the cat. Am J Physiol Gastrointest Liver Physiol 271: G858–G865, 1996. [DOI] [PubMed] [Google Scholar]
- 273.Zagon A. Does the vagus nerve mediate the sixth sense? Trends Neurosci 24: 671–673, 2001. doi: 10.1016/S0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 274.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55: 1553–1560, 2006. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci 30: 14907–14914, 2010. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhang YP, Ma C, Wen YQ, Wang JJ. Convergence of gastric vagal and cerebellar fastigial nuclear inputs on glycemia-sensitive neurons of lateral hypothalamic area in the rat. Neurosci Res 45: 9–16, 2003. doi: 10.1016/S0168-0102(02)00192-X. [DOI] [PubMed] [Google Scholar]
- 277.Zheng H, Berthoud H-R. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 23: 75–83, 2008. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- 278.Zheng H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze requires hindbrain noradrenergic neurons that target the anterior ventrolateral bed nucleus of the stria terminalis. Eur J Neurosci 37: 1340–1349, 2013. doi: 10.1111/ejn.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct 220: 3011–3022, 2015. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]