Abstract
Until recently, astrocyte processes were thought to be too small to contain mitochondria. However, it is now clear that mitochondria are found throughout fine astrocyte processes and are mobile with neuronal activity resulting in positioning near synapses. In this review, we discuss evidence that astrocytic mitochondria confer selective resiliency to astrocytes during ischemic insults and the functional significance of these mitochondria for normal brain function.
Introduction
Astrocytes are no longer just the glue that hold neurons together. Previously relegated as passive supportive substrate for neural networks, astrocytes are emerging into the limelight as central components of CNS functioning. They buffer extracellular potassium and other ions, clear amino acid neurotransmitters, limit excitotoxicity, release neurotransmitters (8, 64), and facilitate appropriate synapse development (79, 128, 147, 163, 187). They are vital to the integrity of the blood-brain barrier and in the control of cerebral blood flow bridging neuronal activity to vascular response (101, 102). Their processes form specialized essentially non-overlapping microdomains throughout the brain, contacting hundreds of thousands of synapses and extend specialized endfeet that surround arterioles (28, 63, 126). Increasing evidence is unequivocally establishing astroglia as active partners in neuronal functioning in both normal and pathological states. In the setting of brain injury, astrocytes undergo many rapid changes, ranging from alterations in morphology, shifts in metabolic state, and initiation of intracellular signaling cascades that can affect the entire neurovascular unit. The response of astrocytes may shape the extent of injury and promote or hinder repair.
The specific role of astrocytic mitochondria in astroglial functioning and response to brain injury is starting to be elucidated but currently remains underexplored. Mitochondria are simplistically viewed as the cellular energy source, generating ~85% of the glucose-derived ATP, but their role extends far beyond to involve key functions integral to cellular health. They match energy demands with ATP supply, regulate Ca2+ signals, coordinate local metabolism, and integrate survival/death cues (79, 145). Although mitochondria within neurons have been extensively studied, much less is known about these organelles within astrocytes, largely attributable to the long-held belief that astrocytic processes were too small to house mitochondria. However, several recent studies have clearly demonstrated the presence of mitochondria within the fine distal astrocytic processes both in situ and in vivo (1, 40, 56, 76, 125, 170), sparking new investigations into mitochondrial functioning in astroglia. Preliminary work has begun to demonstrate unique roles astrocytic mitochondria may play in response to ischemia, equipping astrocytes with a resiliency and adaptability to an environment deprived of oxygen and glucose. This review will first describe what happens at the subcellular level in terms of bioenergetic changes within astrocytes and their mitochondria during ischemia followed by effects on the intracellular mitochondrial network dynamics, which will convey the facile ability of these organelles to recover and survive (FIGURE 1). Subsequently, we will move to the intercellular domain, highlighting the functional significance of mitochondria in astrocyte-neuron and astrocyte-blood vessel partnerships, and in the support of neuronal survival in setting of ischemia. Last, we will review the new literature documenting the heterogeneity of astrocytes and discuss implications on mitochondrial heterogeneity, raising the possibility that select subpopulations of astroglial mitochondria may be tailored to withstand and counteract ischemia. Thus targeting astrocytic mitochondria may be a novel approach to interventions mitigating injury from stroke and improving clinical outcomes.
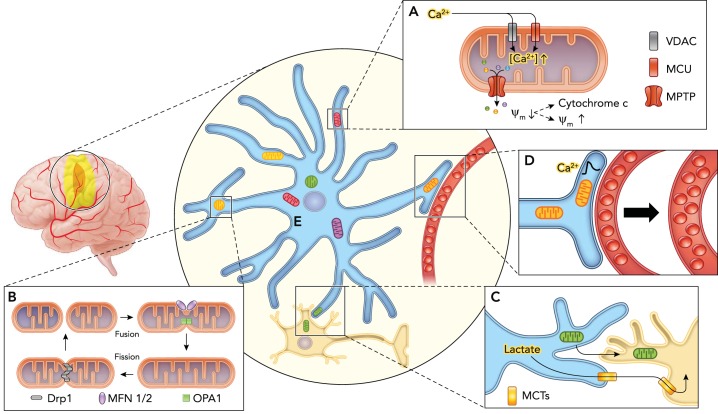
FIGURE 1.
Schematic illustrating the response of astrocytic mitochondria to ischemia
A: loss of oxygen and glucose leads to increased cytosolic Ca2+ concentrations that drives excessive accumulation of Ca2+ into the mitochondria via voltage-dependent anion channels (VDACs) and mitochondrial calcium uniporters (MCUs), triggering opening of the large mitochondrial permeability transition pore (MPTP). This allows the indiscriminate passage of small solutes out of the mitochondria causing dissipation of the mitochondrial membrane potential (ψm), which can culminate in release of cytochrome c and cellular apoptosis or membrane potential recovery with cell survival. B: mitochondria undergo morphological and network architectural changes in response to ischemia. They can adopt rounder discrete shape via fission mediated by dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1), or form elongated, tubular interconnected network via fusion mediated by the outer membrane GTPases mitofusin-1 and -2 (MFN1/MFN2) and the inner membrane protein optic atrophy 1 (OPA1). C: astrocytes can promote neuronal survival by providing lactate as an energy substrate via monocarboxylate transporters (MCT) to neurons in the setting of impaired oxidative phosphorylation, in addition to directly donating functional mitochondria via a CD38-dependent mechanism. D: astrocyte mitochondria are enriched within vascular endfeet and may play a central role in neurovascular coupling by regulating Ca2+ signals. E: mitochondria are heterogeneous in structure and function, which may contribute to astroglial diversity. A subpopulation of astrocytic mitochondria may also be selectively resilient to ischemia.
Bioenergetic Effects of Ischemia on Astrocytic Mitochondria
Ischemia provokes changes in mitochondrial membrane integrity, membrane potential, and enzymatic function, culminating in either mitochondrial loss or recovery. These pathways are mainly mediated by the effects of calcium, glutamate, and reactive oxygen species (ROS) during various phases of ischemia and reperfusion (6). Deprivation of oxygen and glucose leads to loss of oxidative phosphorylation energy substrates causing the failure of multiple membrane ion pumps that maintain the typical high extracellular Na+ concentration gradient. Subsequent Na+ influx into the cell stimulates the reverse action of the Na+/Ca2+ exchanger, causing rise in intracellular concentration of Ca2+ (19, 162). This increase in cytosolic Ca2+ results in an electro-chemical gradient that drives excessive accumulation of Ca2+ into mitochondria via voltage-dependent anion channels (VDACs) and the mitochondrial Ca2+ uniporter (MCU) (49, 119, 121, 196). Additionally, there is transfer of Ca2+ from the endoplasmic reticulum (ER) to mitochondria via direct communications between the two organelles, termed the mitochondrial-associated membrane (MAM), which encompasses the inositol 1,4,5-triphosphate receptor (IP3R) Ca2+-release channel (141). Intra-mitochondrial Ca2+ sequestration beyond buffering capacity triggers the opening of a large conductance pore in the inner mitochondrial membrane known as the mitochondrial permeability transition pore (MPTP) (12, 137). MPTP opening allows the indiscriminate passage of small solutes, undermining ionic gradients, and dissipates the mitochondrial membrane potential (ψm), leading to failure of anti-oxidant pathways and massive production of ROS (4, 48, 119, 121, 144). Consequently, there is osmotic swelling of the mitochondrial cristae and release of cytochrome c and NADH into the astrocyte cytoplasm (23, 37, 65, 152), which can initiate a cascade that leads to cellular apoptosis. Cyclosporine A, via binding to cyclophilin D, inhibits MPTP opening and limits ischemic cell death in vivo (52, 86, 87, 183, 184, 195). Elevated mitochondrial Ca2+ also activates several TCA-cycle dehydrogenases that generate ROS (24, 39). ROS oxidize mitochondrial lipids, sulfhydryl groups, and iron sulfur complexes required for respiratory enzyme function, causing impairment of mitochondrial oxidative phosphorylation (57, 97, 116, 191).
During reperfusion, there is a further increase in cytosolic Ca2+ secondary to excessive glutamate release (32, 88, 138). Excitotoxicity from high levels of glutamate is a major contributor to neuronal cell death during ischemia and uptake of glutamate by astrocytes via the glutamate transporters, GLAST and GLT-1, is a vital modulator of this process (143, 148, 180). Our laboratory found that astrocytic mitochondria are immobilized near glutamate transporters and synapses in response to glutamate uptake (76), a process that increases intracellular Ca2 through reversed operation of plasma membrane Na+/Ca2+ exchangers (77, 100, 146). Docking of mitochondria near sites of glutamate uptake may facilitate glutamate metabolism and ATP production to meet increased energetic demands, and buffer ionic changes caused by glutamate uptake (44).
Although the prevailing thought had been that the collapse of mitochondrial membrane potential irreversibly leads to astrocyte cell death (45, 82), recent studies have demonstrated a resiliency of astrocytes despite experiencing profound mitochondrial depolarization and impairment of oxidative metabolism. Voloboueva et al. showed ongoing maintenance of mitochondrial membrane potential in cultured astrocytes treated with the astrocyte selective mitochondrial inhibitor fluorocitrate (FC) for up to 2 h, with a decline only seen after 3 h of FC treatment (189). The effects of FC were faster and larger in cocultures of neurons and astrocytes or when FC was combined with aspartate as a proxy for glutamate, both of which caused increased energetic demands. However, such profound loss of mitochondrial membrane potential was not accompanied by significant astrocytic cell death. Likewise Reichert et al. found astrocytes cultured with neurons experienced profound and prolonged mitochondrial depolarization beginning 45 min after the onset of oxygen and glucose deprivation (OGD) with maximal depolarization by 60 min of OGD, but this was also not associated with subsequent cell death (144). Furthermore, cultured astrocytes were able to recover mitochondrial membrane potential with reintroduction of oxygen and glucose, albeit in a longer timeframe (>1 h) than anticipated, suggesting there may be ultrastructural changes not amenable to rapid reversal. When evaluated in vivo using a model of transient middle cerebral artery occlusion (MCAO) in rats, astrocytic oxidative metabolism as measured by incorporation of injected radiolabel into glutamine was impaired following 2–3 h of ischemia; however, this likewise did not predict tissue infarction or astrocyte death (181).
Thus it is generally thought that astrocytes demonstrate enhanced resistance to ischemia compared with neurons, 100% of which are irreversibly injured with subsequent cell death by 60–70 min of OGD in culture compared with the 4 h of sustained OGD exposure required to provoke death of astrocytes (26, 59). The specific cellular and molecular mechanisms underlying this phenomenon are yet to be elucidated; however, multiple studies indicate there is bidirectional enhanced vulnerability to ischemia when astrocytes and neurons are cocultured. Astrocytic mitochondrial dysfunction induced by OGD increases neuronal death due to glutamate excitotoxicity (47, 147). Conversely, astrocytes cocultured with neurons undergo more rapid mitochondrial depolarization and exhibit increased cell death (144, 189). It has been postulated that astrocytic mitochondria undergo early depolarization during OGD to allow a shift away from aerobic metabolism to glycolysis to supply energetically impaired neurons with lactate (144), thereby preventing neuronal death. However, this would be a short-lived process dependent on astrocyte glycogen stores. Therefore, it would require quick restoration of respiratory function to prevent irreversible CNS injury. It is important to note that astrocytes in culture are quite disparate from in vivo, with cultured astrocytes adopting a monolayer morphology and lacking the multitude of synaptic and vascular contacts (90). Culturing conditions may also alter gene and receptor expression, limiting the ability to extrapolate to the in vivo situation (29, 107). Thus confirmation of these results in an in vivo system is necessary.
Mitochondrial Structure and Network Dynamics in Response to Ischemia
Mitochondria are highly dynamic organelles with an ability to alter their structure in response to metabolic demands. These organelles exist on an ever-changing spectrum from single small structures of varying morphologies to complex interconnected meshwork spanning multiple astrocytic processes (1, 78, 114, 131). It is thought that smaller mitochondria with higher surface-to-volume ratios are more efficient at generating ATP (15, 16, 182). The relative rates of fission and fusion define the shape, distribution, and network architecture within a cell. Mitochondrial fusion involves the coalescence of two or more mitochondria into one parent organelle, with the merger of both the inner and outer mitochondrial membranes. Fusion is mediated by the outer membrane GTPases mitofusin-1 and -2 (MFN1 and MFN2) and by the inner membrane protein optic atrophy 1 (OPA1) (34, 71, 104, 151, 166). Mitochondrial fragmentation into discrete daughter organelles via fission is controlled by dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1) (33, 80, 194). Fusion and fission events allow for the interchange of mitochondrial components, including mitochondrial DNA (mtDNA), lipids, and proteins (5, 14, 74, 109, 133), which may facilitate adaptable energetic responses to changing metabolic demands (41). Fission also facilitates entry into physically constrained sites of high activity. In fact, neuronal activity increases mitochondrial fission and subsequent recruitment of mitochondria to neuronal spines and filopodia. Deletion or expression of dominant-negative inhibitors of Drp1 reduces the number of mitochondria found in dendrites and synaptic terminals (94, 188), highlighting the fundamental roles fission and fusion play in facilitating mitochondrial presence at sites of need. Such events can also contribute to pathology when the normal balance is perturbed, since extensive fission leads to mitochondrial depolarization, cytochrome c release, and increased free radical production and apoptosis (84, 171, 179).
Several recent studies have documented the presence of mitochondria throughout astroglial cells from cell body out to the finest branches and terminal endfeet, which were once believed to be of too small caliber to accommodate these organelles (1, 56, 76, 114, 170). Using genetically encoded fluorescent proteins fused to peptides that target them for mitochondrial import, such as mito-GFP, which were delivered using viral transduction or expressed using inducible reporter mice, these studies have revealed a heterogeneous population of interconnected mitochondria occupying a substantial volume of individual astrocytes (up to ~50% of the length of a process can be occupied by mitochondria). A dense meshwork of elongated mitochondria is typically found in the soma and within main branches, whereas thinner and shorter mitochondria, ranging from 0.2 to 6 µm in length, mostly populate peripheral processes, including perineuronal protrusions (40, 56, 76, 124, 170).
The creation and maintenance of the complex mitochondrial network is presumably dependent on motor and trafficking machinery similar to those found in neurons, namely the adaptor proteins Miro and TRAKs that mediate reversible binding of mitochondria to the motor proteins kinesin and dynein (77, 78, 99, 170, 185, 198) (FIGURE 2). In cultured or acutely prepared hippocampal brain slices, ~15–30% of mitochondria in astrocyte processes are mobile during a 15-min imaging session (76, 170). Blocking neuronal activity with tetrodotoxin increases mitochondrial mobility, whereas enhancing neuronal activity with glutamate or electric stimulation arrests mitochondria at locations within the astrocyte process that are enriched in glutamate transporters and oppose neuronal glutamate receptors/synapses. Therefore, the astrocytic mitochondrial network seems to reorganize in response to network excitation, likely to remain in register with metabolic needs. The precise mechanisms by which this is performed in vivo and evidence demonstrating translation of mitochondrial network restructuring into changes in synaptic efficacy are currently lacking. Using two-photon imaging to monitor mitochondrial mobility in adult mice, we found that only ~10% of mitochondria are mobile during a 15-min imaging session (78). Whether this is a reflection of artificially provoked mitochondrial mobility in in vitro/in situ systems vs. developmental disparities as astrocyte cultures and slices are prepared from neonatal animals is unknown. Thus further direct investigation of astrocytic mitochondria mobility in vivo throughout brain development is warranted.
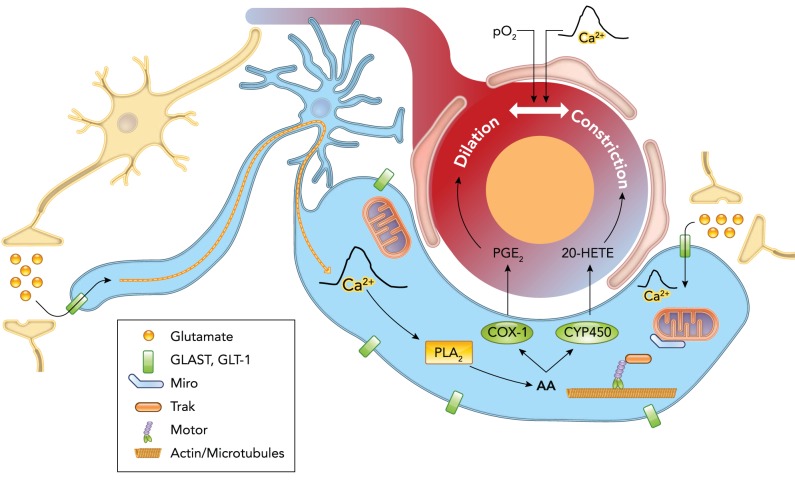
FIGURE 2.
Schematic illustrating astrocytes as central mediators of neurovascular coupling
Neuronal activity causes release of glutamate, which is taken up by astrocytes, triggering a Ca2+ signal that may be propagated down to or separately occurs within the vascular endfeet. This endfoot Ca2+ signal stimulates release of vasoactive factors, namely arachidonic acid (AA) and its metabolites—prostaglandins (PGs), epoxyeicosatrienoic acids (EETs), 20-hydroxyeicosatetraenoic acid (20-HETE)—onto cerebral blood vessels, evoking dilatation or constriction. The direction of blood vessel caliber change may be modulated by the partial pressure of oxygen in the blood (Po2) or magnitude of the Ca2+ signal. The uptake of glutamate by astrocytes causes the immobilization of mitochondria near glutamate transporters in the processes or endfeet via changes in the binding of the transport proteins Miro and Trak. Astrocytic mitochondria are important in the generation and shaping of Ca2+ signals and thus likely play a key role in the control of blood flow in response to neuronal activity.
Mitochondrial network remodeling occurs in astrocytes in response to ischemic conditions in vitro and in vivo. Transient OGD (30 min) causes a delayed increase in the number and decreased length of mitochondria followed by a nearly 50% loss of mitochondria from astrocyte processes in organotypic hippocampal slice cultures (124). This process is dependent on the pathological activation of glutamate transport and increased astrocytic Ca2+. Similarly, in a separate study of astrocyte-neuronal cocultures subject to OGD, astrocytic mitochondria were noted to adopt rounder morphology with less filament-like structures and fewer intramitochondrial connections even 1 h after reintroduction of oxygen and glucose (144). Whether mitochondrial network architecture modifications are compensatory and restorative to ongoing cellular functioning in the face of energy deficit vs. merely reactive is not known, although one can speculate there may be a gradient with initial changes being adaptive to the ischemic environment but ultimately unable to compensate for ongoing ischemia. In a study by Owens et al., morphological changes in astrocytic and neuronal mitochondria were assessed following transient global cerebral ischemia induced by bilateral common carotid artery occlusion and hypotension using a transgenic mouse model that fluorescently labeled cell-type-specific mitochondria (130). In neurons, mitochondria became smaller and more spherical in shape early after exposure to ischemia, suggesting increased fission, since there was comparable reduction in tubular mitochondrial structures from baseline, although decreased fusion may have also played a role. Subsequently, a portion of these spherical mitochondria appeared swollen and presumably sustained irreversible membrane rupture visualized as fluorescent dye leakage into cytosol in days thereafter. Astrocytic mitochondria similarly showed an increase in spherical subpopulation, with a corresponding marked decrease in tubular mitochondria at 2 h following reinstatement of oxygen and glucose. However, in contrast to neurons, mitochondria in astrocytes demonstrated increased fusion at 4 h of recovery, indicated by an increase in tubular population. Furthermore, at 24 h post-ischemic insult, the astrocytic mitochondria demonstrated normal baseline morphology without evident fragmentation. The percentage of spherical and rod-shaped organelles as well as tubular mitochondria were not significantly different compared with the control group. These results suggest increased ability of astrocytic compared with neuronal mitochondria to recover from ischemic stress and prevent cell death. Whether this is due to intrinsic astrocyte or astrocytic mitochondrial properties or to differences in nature of ischemic load on astrocytes vs. neurons is not clear. Nevertheless, the dynamic remodeling of the mitochondrial network likely provides an adaptive mechanism to maintain mitochondrial and ultimately cellular functionality in setting of ischemia. Glutamate may play a pivotal role in this process, since work from our laboratory has demonstrated that activation of glutamate transport by astrocytes is necessary and sufficient to cause mitochondrial arrest within astrocyte processes (76). Thus glutamate released by neurons in response to ischemic injury may be a master regulator of astrocytic mitochondrial network dynamics, which may facilitate astroglial survival and thereby promote neighboring neuronal survival limiting the extent of irreversible injury.
Astrocytic Mitochondrial Rescue of Neurons in Response to Ischemia
Restoring neuronal ion gradients after excitation imposes the greatest energy demand, yet astrocytes are the main metabolizers of glucose and contain the largest stores of glycogen in the brain (50). Thus astrocytes are tasked with providing bioenergetic support to surrounding neurons. The high energy needs of neurons are met by mitochondrial oxidative phosphorylation (66, 120), necessitating the availability or delivery of appropriate substrates. The primary mechanism by which this is performed is the transfer of lactate from astrocytes to neurons, known as the astrocyte-neuronal lactate shuttle (ANLS) (25, 134). Neuronal activity stimulates glutamate release, prompting increased glutamate uptake by astrocytes. Subsequent elevation in astrocytic intracellular Na+ concentration activates the Na+-K+-ATPase to reestablish the Na+ gradient and stimulates glucose uptake as well as glycolysis (21, 134, 135, 176). With this comes increased production of lactate, which is released into the extracellular space and is taken up by neurons via monocarboxylate transporters (MCT) and used as an energy source (11, 21, 68, 139, 140). This has been demonstrated in culture conditions of glucose deprivation, where the consequent reduction in neuronal synaptic transmission is restored with exogenous supply of lactate to astrocytes (50, 156–158). Furthermore, this effect is blocked by MCT inhibitors (50, 53). Lactate can also be generated by partial oxidation of glutamate (44, 103, 167). Interestingly, when both glucose and lactate are readily available at physiological concentrations, lactate is the preferred oxidative substrate in cultured neurons, as revealed by a study using NMR spectroscopy to identify differentially radiolabeled lactate and glucose products (22).
The ability of astrocytes to generate ATP via glycolysis affords a resiliency to these cells in face of hypoxemia and hypoglycemia, unlike neurons (91, 144). Respiration-deficient astrocytes can survive long-term and be phenotypically and functionally normal, as evidenced by studies using a mouse mutant induced to have mitochondrial complex IV dysfunction by tamoxifen-Cre-mediated targeting of the nuclear Cox10 gene. This gene is necessary for appropriate COX assembly and mitochondrial respiratory chain function. In these GlastCreERT2;Cox10flox/flox mice, a large percentage of adult astrocytes and nearly all cerebellar Bergmann glia lack mitochondrial respiration upon tamoxifen-induced deletion of Cox10 (43, 113). Not only do these astrocytes survive, they do so for >1 yr after gene targeting, without observable phenotypic change and maintenance of normal regional histology and functioning (174). Interestingly, despite loss of respiratory chain function, mitochondrial number and morphology were not altered compared with normal astrocytes, but brain lactate was increased. Beyond demonstrating that astrocytes can survive for prolonged periods on glycolysis alone, these results suggest that astrocytes could be a continuous source of energy substrate (i.e., lactate) to neighboring neurons in the setting of impaired oxygen delivery, such as following stroke.
In addition to provision of energy substrates via the lactate shuttle, astrocytes may also rescue neurons from ischemic injury by donation of functional mitochondria. Hayakawa et al. recently reported the transfer of functional mitochondria from astrocytes to neurons via a CD38-dependent mechanism following OGD in culture or focal stroke in a mouse model (67). Cultured cortical neurons subjected to OGD demonstrated restored ATP levels and increased viability when astrocyte-conditioned medium (ACM) was added. Fluorescently labeled mitochondria with MitoTracker Red CMXRos in extracellular vesicles present in ACM were seen within treated neurons. The neuroprotective effect was eliminated by removing extracellular mitochondria from the ACM and was not seen with application of ATP-only containing liposomes, suggesting a role for astrocytic mitochondria beyond provision of ATP. siRNA-mediated knockdown of CD38, an ADP-ribose cyclase enzyme involved in calcium mobilization, significantly decreased mitochondrial transfer to injured neurons, as visualized by reduced fluorescently labeled mitochondrial density associated with neuronal soma. These effects were also shown in vivo using a mouse model of focal cerebral ischemia. Fluorescently labeled extracellular mitochondrial particles collected from ACM were injected into the peri-infarct cortex and, 24 h later, visualized to be present within adjacent neurons. These neurons demonstrated general upregulation of cell-survival related signals, as well as increase in a mitochondrial marker. Knockdown of CD38 in vivo did not alter total infarct size but did lead to an overall reduction in neuronal mitochondria number, suggesting possible impairment of astrocyte-neuronal transfer. Although there are caveats to this study, including possible leakage of the fluorescent dye into the extracellular space due to mitochondrial membrane damage and mere surface association of these fluorescent particles with neurons rather than true intracellular presence (13), it raises the possibility of a novel mechanism by which astrocytic mitochondria can directly provide bioenergetic and metabolic support to injured neurons, thereby providing a potential new therapeutic target in stroke. The specific cell signaling pathways directing mitochondrial release from astrocytes and entry into neurons as well as mechanism of transfer—via extracellular microvesicles vs. tunneling nanotubes (150)—have yet to be elucidated. Furthermore, direct evidence of transferred astrocytic mitochondria being truly functional within neurons is currently lacking and will need further exploration.
Regulation of Cerebral Blood Flow by Astrocytic Mitochondria
At the other end of the astrocyte-neuronal connection are arterioles that are encapsulated by astrocytic endfeet, giving rise to the neurovascular unit. Given their anatomic positioning between neurons and blood vessels, astrocytes are strategically positioned to mediate neurovascular coupling (83, 101, 102, 163). In fact, based on this observation alone, Ramon y Cajal postulated that astrocytes may regulate blood flow via effects on vessel diameter (30). Although numerous studies over the past several decades have advocated that astrocytes translate electrical neuronal activity into increased cerebral blood flow (CBF), the precise signaling mechanisms by which this occurs have yet to be defined (3, 73), and alternative postulates placing astrocytes on the sidelines have been proposed (69, 108). Whether astrocytes are key mediators or mere bystanders in neurovascular coupling has yet to be established; however, if astrocytes are at the hub of this process, there is accumulating evidence that astrocytic Ca2+ signaling may be required. Since mitochondria are primary modulators of Ca2+ homeostasis, astrocytic mitochondria may play a heretofore under-recognized role in the functional hyperemia response.
On a simplified level, the model that astrocytes mediate the neurovascular response depicts neuronal activity, causing the release of neurotransmitters, most notably glutamate, leading to receptor activation on astrocyte processes as well as transporter-mediated uptake, which initiates an intracellular Ca2+ signal that is propagated to the astrocytic endfeet. This endfoot Ca2+ signal likely stimulates release of vasoactive factors onto the cerebral blood vessels, evoking dilatation and consequent increased blood flow to meet the increased metabolic demands imposed by neuronal activation (85, 173, 177, 199). There have been contrasting results in terms of the dynamics of the Ca2+ signal, which have led many to question the role of calcium signaling in the functional hyperemic response. Temporally, studies have shown that astrocytic Ca2+ oscillations both precede (51, 172, 173) as well as follow vasodilation of cerebral arterioles (123). Additionally, there has been note of several seconds of time delay between signal and response, suggesting kinetics that may be too slow to drive the very rapid blood flow changes seen in vivo (115, 123, 154, 155). Although yet to be fully resolved, these disparities may reflect methodological differences in recording small, fast Ca2+ transients in addition to the highly complex subcellular Ca2+ dynamics within astrocytic microdomains causing varied results based on location of measurement (42, 117, 132, 160, 161). Given there are multiple independent sources of Ca2+ signals throughout the astrocyte that may simultaneously occur to encode different activities, some of the recorded signals may not be involved in the control of blood flow (1, 77, 160). More recent studies have confirmed the presence of stimulus-evoked rapid Ca2+ signals specifically within astrocyte processes and vascular endfeet that are fast enough to cause blood vessel diameter changes in vivo (96, 129).
Vasoactive factors that may be released in response to astrocytic endfeet Ca2+ signals to mediate the functional hyperemic response include nitric oxide (NO), prostaglandins, arachidonic acid metabolites, and adenosine (FIGURE 2). Studies employing two-photon Ca2+ uncaging in astrocyte endfeet in cortical brain slices have shown both dilatation and constriction of adjacent arterioles (115, 199). These arteriole caliber changes were inhibited by blocking phospholipase A2, which is an enzyme that causes the release of arachidonic acid (AA) from membrane lipids, and by blocking the conversion of AA into its metabolite 20-hydroxyeicosatetraenoic acid (20-HETE), which is a potent vasoconstrictor. Experiments in vivo have confirmed cortical arteriole dilatation in response to Ca2+ transients in astrocyte endfeet via a mechanism that likewise involves AA (177). This study found that inhibition of cyclooxygenase-1 (COX-1), which synthesizes prostaglandin E2 (PGE2) from AA and is expressed in astrocytic endfeet, blocked Ca2+-mediated arteriole dilatation. Work in retinal explants has shown Ca2+ uncaging in astrocytes and Muller cells causes both arteriole constrictions mediated by 20-HETE and arteriole dilatations via synthesis of other vasoactive agents and AA metabolites (105). Whether blood vessels undergo dilatation vs. constriction in response to astrocyte Ca2+ oscillations has been postulated to be due to the Po2) (60). Whereas high oxygen content (95% O2, 5% CO2) led to blood vessel constrictions evoked by Ca2+ uncaging in astrocytes from hippocampal and neocortical slices, low Po2 (20% O2, 5% CO2) reversed caliber change to dilatations. This modulatory effect of blood oxygen content may be mediated by extracellular lactate concentrations that can alter specific AA metabolite levels. However, oxygen modulation of neurovascular coupling has yet to be observed in vivo. The magnitude of the Ca2+ signal has also been implicated as a determinant of blood vessel response polarity where moderate increases in astrocyte Ca2+ induce vasodilation, whereas large increases provoke vasoconstriction (58). This effect has been hypothesized to be mediated through changes in the extracellular potassium concentration surrounding vascular smooth muscle cells. In sum, accumulating data by many different laboratories have found that Ca2+ transients in astrocyte endfeet provoke adjacent arteriole diameter dilatation or constriction via formation and release of vasoactive lipids. Further direct observation of such Ca2+ elevations occurring in astrocytic endfeet in vivo following neuronal synaptic activity evoked by physiological stimulus would strengthen this model.
An alternative means of modulating polarity of blood vessel caliber response may stem from astrocytic mitochondrial regulation of Ca2+ signals. Evidence exists that astrocytic mitochondria play a role in neurovascular coupling—selective inhibition of astrocyte mitochondrial aconitase by fluorocitrate causes loss of tone of rat retinal arterioles in vivo (118). Mitochondria are a source as well as a buffer of Ca2+, in addition to modulators of the spatial restriction and kinetics of Ca2+ transients. Numerous groups have observed spatially restricted Ca2+ signals within microdomains of astrocytic processes (42, 61, 77, 136, 160, 169). When mitochondrial dysfunction is induced via dissipation of its membrane potential by FCCP, Ca2+ transients in cultured and organotypic slice astrocytic processes demonstrate slower rate of decay, increased amplitude, and increased spatial spread (18, 77). When the Ca2+-sensitive positioning of mitochondria in astrocytic processes is inhibited by expressing Ca2+-insensitive mutant variants of Miro, spontaneous Ca2+ signals are increased in frequency, amplitude, and half-life (77). When mitochondria are lost from the astrocyte processes following OGD, spontaneous Ca2+ signals in distal astrocytic processes are dramatically increased (124). Photoablation of individual mitochondria with KillerRed-mito causes a similar large transient increase in cytoplasmic Ca2+ associated with increased spatial spread (77). These data support the active role mitochondria play in shaping the Ca2+ signals in astrocytes. Ca2+ transients are also generated by mitochondria. Astrocytic mitochondria co-localize with spontaneous Ca2+ signals in astrocyte processes, suggesting mitochondria to be a potential source of Ca2+ increase (77, 124). Likewise, two-photon in vivo imaging of Ca2+ signals within astrocyte processes of awake mice revealed up to 85% of spontaneous Ca2+ elevations within microdomains were co-localized with mitochondria (1). Moreover, inhibition of the MPTP opening caused an ~35% reduction in these Ca2+ transients, and application of neuronal activator increased their number and frequency. These results strongly suggest that efflux of Ca2+ from mitochondria may contribute to generation of microdomain Ca2+ signals in astrocyte processes.
Mitochondria are enriched in astrocytic endfeet ensheathing blood vessels. This was demonstrated by sparse labeling of astrocytic mitochondria in vivo using genetically encoded mitochondrially localized fluorescent proteins and in EM studies (10, 78, 101). Thus mitochondria are present at the precise sites of astrocyte-vascular interface and functionally have the capacity to contribute to and regulate Ca2+ signals that are critical to neurovascular coupling, yet the role of astrocytic mitochondria in this response remains unknown. These perivascular mitochondria may actively shape the microdomain Ca2+ signals that determine release of either a vasoconstrictor or vasodilator. One could postulate that, in scenarios of ischemia where mitochondria experience transient dysfunction or undergo loss, the altered spatial restriction, kinetics, and buffering of Ca2+ transients may result in abnormal constriction or dilatation of cerebral blood vessels exacerbating injury.
Selective Resiliency of Subpopulations of Astrocytic Mitochondria
The morphological diversity of astroglial cells was first detailed by Ramon y Cajal over a century but only recently have molecular and functional subtypes of astrocytes begun to be defined (7, 27, 31, 168, 197). Multiple recent studies have documented the heterogeneity of astrocytes in the mature healthy brain, manifested as different morphologies, expression profiles, as well as physiological and functional properties. There are protoplasmic and fibrous astrocytes as well as specialized forms such as Bergmann glia and Muller cells tailored to their neuroanatomical region, and stem cell progenitors found in the subventricular and subgranular zones (106, 111, 175). Transcriptome analyses on microdissected regions of the rodent brain have found interregional differences in gene expression and molecular profiles of astrocytes that appear, in part, to be intrinsic rather than driven by external environmental cues (46, 110, 125, 193). Varied expression levels of ion channels, such as the Kir4.1 channel, causing differences in inward current density, rates of potassium buffering, and consequent thresholds for synaptic transmission (127, 159), plus variations in spontaneous Ca2+ activity and extent of astroglial coupling all give rise to distinct electrophysiological properties (17, 72, 92, 122, 149, 178). Different subpopulations may also be active at different phases of CNS maturation (7, 95). Data on astrocyte functional diversity with brain region-dependent specializations has begun to emerge, with several studies demonstrating subgroup differences in ability to support synapse formation, neuronal differentiation, neurite outgrowth, or neuronal migration (54, 55, 95, 165). However, further work is needed to correlate function with distinct genetic, molecular signatures and morphological phenotypes, and to elucidate intra-regional functional differences. The latter is made difficult by technical challenges of isolating or targeting subgroups of astrocytes intermixed within a single brain region.
Astrocyte heterogeneity extends to pathological states of CNS disease and injury with variability in reactive astrogliosis and vulnerability to metabolic stress. Hypertrophic morphology, aggregation at site of injury vs. in the periphery, proliferation, formation of glial scar, injury, or disease-specific changes in gene expression that may either facilitate recovery or propagate ongoing injury have all been reported in the literature (2, 70). Astrocyte reactivity is a context-dependent tailored response that integrates myriad intracellular and extracellular cues. It is not a one-size-fits-all phenomenon. Likewise, astrocyte susceptibility to injury is not a homogeneous all-or-none event. Subsets of neurons are selectively vulnerable to ischemic insults with both inter- and intra-regional differences reported (62, 75, 81, 142, 153, 164, 169). Although the literature on neuronal selective vulnerability is wide and deep, the concept of selective vulnerability in astroglia is just emerging. Like neurons, there is astrocytic variation in vulnerability to ischemic insults both in vitro and in vivo—protoplasmic cortical astrocytes are more sensitive than fibrous astrocytes, and astrocytes from striatum are more vulnerable compared with those from cortex or hippocampus (98, 192). Regional variability in sensitivity to ischemic insults have largely been attributed to intrinsic differences between populations of neurons, often focused on the energetic demands of the neurons in the local circuit. Higher energetic demands have correlated with increased earlier susceptibility. However, as more evidence is coming to light elucidating the inherent diversity of astroglia independent of neurons, differential vulnerability of specific brain regions to ischemia may stem from variability in astrocyte resiliency to ischemic stress. Moreover, broadening the view to the astrocyte-neuron unit from a cell-centric perspective, the complex and dynamic interactions within the unit also likely play a role in defining response and vulnerability to injury. Indeed, as discussed above, the combination of neurons and astrocytes together during transient ischemia and reperfusion augments the injury sustained by both cell types, with the injury occurring earlier and being more severe.
A facet of astrocyte heterogeneity and ischemic resiliency that has yet to be explored is the contribution of mitochondrial diversity. Like the cells in which they reside, mitochondria are not homogeneous in structure or function. Subpopulations of mitochondria have been described, albeit on a simpler diversity scale. The first evidence for mitochondrial heterogeneity came from experiments performed in the 1970s in which so-called “synaptic” mitochondria presumably from neurons and “non-synaptic” forms presumably from glial cells were differentially isolated from brain homogenates (20, 35, 89). Respiratory enzyme activity and rates of oxidative phosphorylation differed between the two types of mitochondria (89, 93). Moreover, synaptic mitochondria isolated from hippocampal or neocortical brain regions more quickly lost respiratory chain function post-ischemia compared with the nonsynaptic population from these same cells. This was measured by degree of inhibition of complex I required to cause loss of oxidative phosphorylation, which was 25% from baseline function in synaptic mitochondria vs. 60% for nonsynaptic mitochondria (38). Such a difference could predispose neurons to increased vulnerability in settings of ischemia. Since these early experiments, ultrastructural analyses have established mitochondrial morphology, content, localization, and distribution to be highly variable across tissue type as well as between cells of same tissue (9, 186). A proteomic analysis of mitochondria from brain, kidney, liver, and heart found that only 57% of mitochondrial proteins consistently overlapped in their expression between the examined tissues (112), highlighting a significant degree of cell-specific differences in mitochondrial composition. Functional heterogeneity of mitochondria within single cells, including cortical astrocytes, has been observed with respect to membrane potential, cytosolic calcium sequestration, timing of MPTP opening in response to pro-apoptotic factors, and α-ketoglutarate dehydrogenase levels (36, 190). Since these are all key pathways involved in the subcellular response to ischemia, inherent mitochondrial robustness may fundamentally shape astrocyte resistance to ischemia. Specific subclasses of astrocytic mitochondria may exist that are adept in recovering from excessive Ca2+ sequestration and loss of membrane potential, thereby attaining functional recovery. Focusing on those brain regions and specific cells that are least prone to undergo irreversible cell death in the face of cerebral ischemia may provide key insight into the existence of such subpopulations and the mechanisms by which they withstand injury.
Mitochondrial network architecture varies according to the astrocyte cell morphology in which the organelles reside. Given the multitude of astrocyte subpopulations with very different morphologies, there is likewise comparable heterogeneity of astrocytic mitochondrial organization. The specific mitochondrial network architecture has been attributed to the morphological constraints imposed by the parent astrocyte geometry as well as to reorganization following metabolic activity. However, it may be the case that mitochondria are not simply malleable to imposed external constraints. Given the key role mitochondria play in all the various astrocytic functions, especially in the face of ischemia, perhaps their network modifications actively drive the shape of the astrocyte and directly contribute to their diversity.
Conclusions and Future Directions
Considering the myriad of roles astrocytes play separate from sustaining, regulating, and responding to neuronal activity, one could consider astrocytes to be the cardinal cell type in the brain. At the heart of astrocyte function is the dynamic astrocytic mitochondrial network that likely determines neurovascular unit response and resiliency of the network to ischemia. Although astroglial mitochondria have emerged as distinct functional entities, our current understanding of these structures is rudimentary. Future investigations to be considered are:
The characterization of mitochondrial dynamics and network architecture in different astroglial subpopulations in normal, healthy brain.
The characterization of mitochondrial dynamics and network architecture in the setting of ischemia comparing infarct core to surrounding penumbra or normal unaffected tissue in various regions of the brain.
Studies have demonstrated that ischemic pre-conditioning lessens injury experienced during subsequent exposures to more severe ischemia. Could plasticity of astroglial mitochondria play a role in greater adaptability to subsequent ischemia?
The characterization of effects on calcium signals and neurovascular coupling with the selective ablation of mitochondria in astrocytic perivascular endfeet.
Acknowledgments
E.K.S. was supported by a National Institute of Neurological Disorders and Stroke-funded training program (T32 NS-007413); her stipend was supplement by the Clinical Neuroscience Supplement program that is funded by the Children’s Hospital of Philadelphia Research Institute. M.B.R. was supported by the National Institute of Neurological Disorders and Stroke-funded R56 NS-077773.
No conflicts of interest, financial or otherwise, are declared by the authors.
Author contributions: E.K.S. and M.B.R. jointly discussed potential topics for this review. E.K.S. drafted the manuscript and prepared figures. E.K.S. and M.B.R. edited and revised the manuscript. E.K.S. and M.B.R. approved the final version of this manuscript
References
- 1.Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93: 587–605.e7, 2017. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett 565: 23–29, 2014. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res 29: 601–608, 2004. doi: 10.1023/B:NERE.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- 5.Ban-Ishihara R, Ishihara T, Sasaki N, Mihara K, Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc Natl Acad Sci USA 110: 11863–11868, 2013. doi: 10.1073/pnas.1301951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bano D, Ankarcrona M. Beyond the critical point: an overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci Lett S0304-3940(17)30695-X. In press. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7: a020362, 2014. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazargani N, Attwell D. Amines, astrocytes, and arousal. Neuron 94: 228–231, 2017. doi: 10.1016/j.neuron.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci 120: 838–848, 2007. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin Kacerovsky J, Murai KK. Stargazing: monitoring subcellular dynamics of brain astrocytes. Neuroscience 323: 84–95, 2016. doi: 10.1016/j.neuroscience.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Bergersen L, Waerhaug O, Helm J, Thomas M, Laake P, Davies AJ, Wilson MC, Halestrap AP, Ottersen OP. A novel postsynaptic density protein: the monocarboxylate transporter MCT2 is co-localized with delta-glutamate receptors in postsynaptic densities of parallel fiber-Purkinje cell synapses. Exp Brain Res 136: 523–534, 2001. doi: 10.1007/s002210000600. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front Physiol 4: 95, 2013. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MV, Schneider RT, McConnell MJ. Mitochondrial transfer from astrocytes to neurons following ischemic insult: guilt by association? Cell Metab 24: 376–378, 2016. doi: 10.1016/j.cmet.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Bertholet AM, Delerue T, Millet AM, Moulis MF, David C, Daloyau M, Arnauné-Pelloquin L, Davezac N, Mils V, Miquel MC, Rojo M, Belenguer P. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis 90: 3–19, 2016. doi: 10.1016/j.nbd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni-Freddari C, Fattoretti P, Giorgetti B, Spazzafumo L, Solazzi M, Balietti M. Age-related decline in metabolic competence of small and medium-sized synaptic mitochondria. Naturwissenschaften 92: 82–85, 2005. doi: 10.1007/s00114-004-0591-z. [DOI] [PubMed] [Google Scholar]
- 16.Bertoni-Freddari C, Fattoretti P, Paoloni R, Caselli U, Giorgetti B, Solazzi M. Inverse correlation between mitochondrial size and metabolic competence: a quantitative cytochemical study of cytochrome oxidase activity. Naturwissenschaften 90: 68–71, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Rönnbäck L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience 92: 255–265, 1999. doi: 10.1016/S0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 18.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol 145: 795–808, 1999. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bondarenko A, Chesler M. Calcium dependence of rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia 34: 143–149, 2001. doi: 10.1002/glia.1049. [DOI] [PubMed] [Google Scholar]
- 20.Booth RF, Clark JB. A method for the rapid separation of soluble and particulate components of rat brain synaptosomes. FEBS Lett 107: 387–392, 1979. doi: 10.1016/0014-5793(79)80414-7. [DOI] [PubMed] [Google Scholar]
- 21.Bouzier-Sore AK, Merle M, Magistretti PJ, Pellerin L. Feeding active neurons: (re)emergence of a nursing role for astrocytes. J Physiol Paris 96: 273–282, 2002. doi: 10.1016/S0928-4257(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 22.Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 24: 1687–1694, 2006. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 23.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol 286: H39–H46, 2004. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- 24.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 25.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol 587: 5591–5600, 2009. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno VM, Goldberg MP, Dugan LL, Giffard RG, Choi DW. Neuroprotective effect of hypothermia in cortical cultures exposed to oxygen-glucose deprivation or excitatory amino acids. J Neurochem 63: 1398–1406, 1994. doi: 10.1046/j.1471-4159.1994.63041398.x. [DOI] [PubMed] [Google Scholar]
- 27.Buosi AS, Matias I, Araujo AP, Batista C, Gomes FC. Heterogeneity in synaptogenic profile of astrocytes from different brain regions. Mol Neurobiol. In press. [DOI] [PubMed] [Google Scholar]
- 28.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cajal RYS. Algunas conjeturas sobre el mecanismo anatómico de la ideación, asociación y atención Rev Med Cir Pract 19: 497–508, 1895. [Google Scholar]
- 31.Chaboub LS, Deneen B. Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci 34: 379–388, 2012. doi: 10.1159/000343723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan PH. Role of oxidants in ischemic brain damage. Stroke 27: 1124–1129, 1996. doi: 10.1161/01.STR.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 33.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci 1201: 34–39, 2010. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Chan DC. Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18, R2: R169–R176, 2009. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark JB, Nicklas WJ. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem 245: 4724–4731, 1970. [PubMed] [Google Scholar]
- 36.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21: 1616–1627, 2002. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999. doi: 10.1042/bj3410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davey GP, Canevari L, Clark JB. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. J Neurochem 69: 2564–2570, 1997. doi: 10.1046/j.1471-4159.1997.69062564.x. [DOI] [PubMed] [Google Scholar]
- 39.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 128: 161–163, 1972. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derouiche A, Haseleu J, Korf HW. Fine astrocyte processes contain very small mitochondria: glial oxidative capability may fuel transmitter metabolism. Neurochem Res 40: 2402–2413, 2015. doi: 10.1007/s11064-015-1563-8. [DOI] [PubMed] [Google Scholar]
- 41.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 42.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 14: 1276–1284, 2011. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 43.Diaz F, Thomas CK, Garcia S, Hernandez D, Moraes CT. Mice lacking COX10 in skeletal muscle recapitulate the phenotype of progressive mitochondrial myopathies associated with cytochrome c oxidase deficiency. Hum Mol Genet 14: 2737–2748, 2005. doi: 10.1093/hmg/ddi307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int 63: 244–258, 2013. doi: 10.1016/j.neuint.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia 50: 362–388, 2005. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- 46.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135: 749–762, 2008. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci 15: 4545–4555, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugan LL, Kim-Han JS. Astrocyte mitochondria in in vitro models of ischemia. J Bioenerg Biomembr 36: 317–321, 2004. doi: 10.1023/B:JOBB.0000041761.61554.44. [DOI] [PubMed] [Google Scholar]
- 49.Dux E, Mies G, Hossmann KA, Siklós L. Calcium in the mitochondria following brief ischemia of gerbil brain. Neurosci Lett 78: 295–300, 1987. doi: 10.1016/0304-3940(87)90376-4. [DOI] [PubMed] [Google Scholar]
- 50.Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci 16: 25959–25981, 2015. doi: 10.3390/ijms161125939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol 305: H609–H619, 2013. doi: 10.1152/ajpheart.00359.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci 18: 5151–5159, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao C, Zhou L, Zhu W, Wang H, Wang R, He Y, Li Z. Monocarboxylate transporter-dependent mechanism confers resistance to oxygen- and glucose-deprivation injury in astrocyte-neuron co-cultures. Neurosci Lett 594: 99–104, 2015. doi: 10.1016/j.neulet.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Abreu J, Moura Neto V, Carvalho SL, Cavalcante LA. Regionally specific properties of midbrain glia: I. Interactions with midbrain neurons. J Neurosci Res 40: 471–477, 1995. doi: 10.1002/jnr.490400406. [DOI] [PubMed] [Google Scholar]
- 55.García-Marqués J, De Carlos JA, Greer CA, López-Mascaraque L. Different astroglia permissivity controls the migration of olfactory bulb interneuron precursors. Glia 58: 218–230, 2010. doi: 10.1002/glia.20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 31: 18275–18288, 2011. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilboe DD, Kintner D, Fitzpatrick JH, Emoto SE, Esanu A, Braquet PG, Bazan NG. Recovery of postischemic brain metabolism and function following treatment with a free radical scavenger and platelet-activating factor antagonists. J Neurochem 56: 311–319, 1991. doi: 10.1111/j.1471-4159.1991.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 58.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci 13: 3510–3524, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grosche J, Matyash V, Möller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 2: 139–143, 1999. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 62.Guzzetta F, Deodato F, Randò T. Brain ischemic lesions of the newborn. Childs Nerv Syst 16: 633–637, 2000. doi: 10.1007/s003810000318. [DOI] [PubMed] [Google Scholar]
- 63.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27: 6473–6477, 2007. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72: 335–355, 2010. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halestrap AP, Doran E, Gillespie JP, O’Toole A. Mitochondria and cell death. Biochem Soc Trans 28: 170–177, 2000. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 66.Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32: 8940–8951, 2012. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535: 551–555, 2016. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res 79: 11–18, 2005. doi: 10.1002/jnr.20294. [DOI] [PubMed] [Google Scholar]
- 69.Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37: 161–181, 2014. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höke A, Silver J. Heterogeneity among astrocytes in reactive gliosis. Perspect Dev Neurobiol 2: 269–274, 1994. [PubMed] [Google Scholar]
- 71.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem 76: 751–780, 2007. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 72.Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28: 5207–5217, 2008. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 74.Ishihara T, Kohno H, Ishihara N. Physiological roles of mitochondrial fission in cultured cells and mouse development. Ann N Y Acad Sci 1350: 77–81, 2015. doi: 10.1111/nyas.12848. [DOI] [PubMed] [Google Scholar]
- 75.Ito U, Spatz M, Walker JT Jr, Klatzo I. Experimental cerebral ischemia in mongolian gerbils. I. Light microscopic observations. Acta Neuropathol 32: 209–223, 1975. doi: 10.1007/BF00696570. [DOI] [PubMed] [Google Scholar]
- 76.Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci 34: 1613–1624, 2014. doi: 10.1523/JNEUROSCI.3510-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson JG, Robinson MB. Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. J Neurosci 35: 15199–15213, 2015. doi: 10.1523/JNEUROSCI.2049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson JG, Robinson MB. Regulation of mitochondrial dynamics in astrocytes: Mechanisms, consequences, and unknowns. Glia. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobson J, Duchen MR. Interplay between mitochondria and cellular calcium signalling. Mol Cell Biochem 256-257: 209–218, 2004. doi: 10.1023/B:MCBI.0000009869.29827.df. [DOI] [PubMed] [Google Scholar]
- 80.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278: 36373–36379, 2003. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 81.Johnston MV, Trescher WH, Ishida A, Nakajima W, Zipursky A. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res 49: 735–741, 2001. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Juurlink BH, Hertz L. Ischemia-induced death of astrocytes and neurons in primary culture: pitfalls in quantifying neuronal cell death. Brain Res Dev Brain Res 71: 239–246, 1993. doi: 10.1016/0165-3806(93)90175-A. [DOI] [PubMed] [Google Scholar]
- 83.Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 23: 1–10, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 84.Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci 1147: 283–292, 2008. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 100: 307–317, 2006. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kristal BS, Dubinsky JM. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J Neurochem 69: 524–538, 1997. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- 87.Kristián T, Gertsch J, Bates TE, Siesjö BK. Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O. J Neurochem 74: 1999–2009, 2000. doi: 10.1046/j.1471-4159.2000.0741999.x. [DOI] [PubMed] [Google Scholar]
- 88.Kuroda S, Siesjö BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci 4: 199–212, 1997. [PubMed] [Google Scholar]
- 89.Lai JC, Clark JB. Preparation and properties of mitochondria derived from synaptosomes. Biochem J 154: 423–432, 1976. doi: 10.1042/bj1540423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD. Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37: 2569–2588, 2012. doi: 10.1007/s11064-012-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee DR, Helps SC, Gibbins IL, Nilsson M, Sims NR. Losses of NG2 and NeuN immunoreactivity but not astrocytic markers during early reperfusion following severe focal cerebral ischemia. Brain Res 989: 221–230, 2003. doi: 10.1016/S0006-8993(03)03373-0. [DOI] [PubMed] [Google Scholar]
- 92.Lee SH, Kim WT, Cornell-Bell AH, Sontheimer H. Astrocytes exhibit regional specificity in gap-junction coupling. Glia 11: 315–325, 1994. doi: 10.1002/glia.440110404. [DOI] [PubMed] [Google Scholar]
- 93.Leong SF, Lai JC, Lim L, Clark JB. The activities of some energy-metabolising enzymes in nonsynaptic (free) and synaptic mitochondria derived from selected brain regions. J Neurochem 42: 1306–1312, 1984. doi: 10.1111/j.1471-4159.1984.tb02788.x. [DOI] [PubMed] [Google Scholar]
- 94.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119: 873–887, 2004. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 95.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20: 396–405, 2017. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA 110: E4678–E4687, 2013. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Rosenthal RE, Starke-Reed P, Fiskum G. Inhibition of postcardiac arrest brain protein oxidation by acetyl-L-carnitine. Free Radic Biol Med 15: 667–670, 1993. doi: 10.1016/0891-5849(93)90171-P. [DOI] [PubMed] [Google Scholar]
- 98.Lukaszevicz AC, Sampaïo N, Guégan C, Benchoua A, Couriaud C, Chevalier E, Sola B, Lacombe P, Onténiente B. High sensitivity of protoplasmic cortical astroglia to focal ischemia. J Cereb Blood Flow Metab 22: 289–298, 2002. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 99.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol 20: 102–112, 2010. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 100.Magi S, Arcangeli S, Castaldo P, Nasti AA, Berrino L, Piegari E, Bernardini R, Amoroso S, Lariccia V. Glutamate-induced ATP synthesis: relationship between plasma membrane Na+/Ca2+ exchanger and excitatory amino acid transporters in brain and heart cell models. Mol Pharmacol 84: 603–614, 2013. doi: 10.1124/mol.113.087775. [DOI] [PubMed] [Google Scholar]
- 101.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58: 1094–1103, 2010. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 102.McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab 31: 795–806, 2011. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKenna MC, Stridh MH, McNair LF, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamate oxidation in astrocytes: roles of glutamate dehydrogenase and aminotransferases. J Neurosci Res 94: 1561–1571, 2016. doi: 10.1002/jnr.23908. [DOI] [PubMed] [Google Scholar]
- 104.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 127: 383–395, 2006. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 105.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol 92: 635–640, 2007. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4: 585–592, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller S, Romano C, Cotman CW. Growth factor upregulation of a phosphoinositide-coupled metabotropic glutamate receptor in cortical astrocytes. J Neurosci 15: 6103–6109, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 19: 1619–1627, 2016. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19: 630–641, 2014. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509: 189–194, 2014. doi: 10.1038/nature13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26: 891–907, 2012. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115: 629–640, 2003. doi: 10.1016/S0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 113.Mori T, Tanaka K, Buffo A, Wurst W, Kühn R, Götz M. Inducible gene deletion in astroglia and radial glia–a valuable tool for functional and lineage analysis. Glia 54: 21–34, 2006. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- 114.Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Götz M, Winklhofer KF, Hrelia S, Bergami M. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab 18: 844–859, 2013. doi: 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 115.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 116.Nakahara I, Kikuchi H, Taki W, Nishi S, Kito M, Yonekawa Y, Goto Y, Ogata N. Changes in major phospholipids of mitochondria during postischemic reperfusion in rat brain. J Neurosurg 76: 244–250, 1992. doi: 10.3171/jns.1992.76.2.0244. [DOI] [PubMed] [Google Scholar]
- 117.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol 87: 528–537, 2002. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 118.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci 370: 20140195, 2015. doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med 4: 149–177, 2004. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 120.Nicholls DG. Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci 1147: 53–60, 2008. doi: 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- 121.Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N. Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J Neurosci Res 85: 3206–3212, 2007. doi: 10.1002/jnr.21290. [DOI] [PubMed] [Google Scholar]
- 122.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron 62: 400–412, 2009. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzić S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci 33: 8411–8422, 2013. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O’Donnell JC, Jackson JG, Robinson MB. Transient oxygen/glucose deprivation causes a delayed loss of mitochondria and increases spontaneous calcium signaling in astrocytic processes. J Neurosci 36: 7109–7127, 2016. doi: 10.1523/JNEUROSCI.4518-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol 814: 23–45, 2012. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113: 221–233, 2002. doi: 10.1016/S0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 127.Olsen ML, Campbell SL, Sontheimer H. Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis. J Neurophysiol 98: 786–793, 2007. doi: 10.1152/jn.00340.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci 35: 13827–13835, 2015. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 18: 210–218, 2015. doi: 10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Owens K, Park JH, Gourley S, Jones H, Kristian T. Mitochondrial dynamics: cell-type and hippocampal region specific changes following global cerebral ischemia. J Bioenerg Biomembr 47: 13–31, 2015. doi: 10.1007/s10863-014-9575-7. [DOI] [PubMed] [Google Scholar]
- 131.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 23: 1534–1545, 2011. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 132.Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146: 785–798, 2011. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 133.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One 3: e3257, 2008. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91: 10625–10629, 1994. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem 69: 2132–2137, 1997. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- 136.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25: 2192–2203, 2005. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petronilli V, Cola C, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. II. The minimal requirements for pore induction underscore a key role for transmembrane electrical potential, matrix pH, and matrix Ca2+. J Biol Chem 268: 1011–1016, 1993. [PubMed] [Google Scholar]
- 138.Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27: 327–331, 1996. doi: 10.1161/01.STR.27.2.327. [DOI] [PubMed] [Google Scholar]
- 139.Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cereb Blood Flow Metab 22: 586–595, 2002. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100: 617–627, 2000. doi: 10.1016/S0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- 141.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27: 6407–6418, 2008. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11: 491–498, 1982. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 143.Rao VL, Dogan A, Bowen KK, Todd KG, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur J Neurosci 13: 119–128, 2001. [PubMed] [Google Scholar]
- 144.Reichert SA, Kim-Han JS, Dugan LL. The mitochondrial permeability transition pore and nitric oxide synthase mediate early mitochondrial depolarization in astrocytes during oxygen-glucose deprivation. J Neurosci 21: 6608–6616, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578, 2012. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 146.Rojas H, Colina C, Ramos M, Benaim G, Jaffe E, Caputo C, Di Polo R. Sodium-calcium exchanger modulates the L-glutamate Ca(i) (2+) signalling in type-1 cerebellar astrocytes. Adv Exp Med Biol 961: 267–274, 2013. doi: 10.1007/978-1-4614-4756-6_22. [DOI] [PubMed] [Google Scholar]
- 147.Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett 103: 162–168, 1989. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 148.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 149.Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C. Plasticity of astroglial networks in olfactory glomeruli. Proc Natl Acad Sci USA 108: 18442–18446, 2011. doi: 10.1073/pnas.1107386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 303: 1007–1010, 2004. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 151.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874, 2001. [DOI] [PubMed] [Google Scholar]
- 152.Schild L, Huppelsberg J, Kahlert S, Keilhoff G, Reiser G. Brain mitochondria are primed by moderate Ca2+ rise upon hypoxia/reoxygenation for functional breakdown and morphological disintegration. J Biol Chem 278: 25454–25460, 2003. doi: 10.1074/jbc.M302743200. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 40: 599–636, 1991. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 154.Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schröter A, Rudin M, Helmchen F. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods 9: 597–602, 2012. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- 155.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320: 1638–1643, 2008. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 156.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res 744: 105–111, 1997. doi: 10.1016/S0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- 157.Schurr A, Payne RS, Miller JJ, Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res 774: 221–224, 1997. doi: 10.1016/S0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- 158.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240: 1326–1328, 1988. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 159.Seifert G, Hüttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhäuser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29: 7474–7488, 2009. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 13: 759–766, 2010. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15: 70–80, 2011. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Silver IA, Erecińska M. Ion homeostasis in rat brain in vivo: intra- and extracellular [Ca2+] and [H+] in the hippocampus during recovery from short-term, transient ischemia. J Cereb Blood Flow Metab 12: 759–772, 1992. doi: 10.1038/jcbfm.1992.107. [DOI] [PubMed] [Google Scholar]
- 163.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129: 877–896, 2004. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 164.Smith ML, Auer RN, Siesjö BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol 64: 319–332, 1984. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- 165.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature 417: 39–44, 2002. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 166.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532, 2009. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sonnewald U. Glutamate synthesis has to be matched by its degradation - where do all the carbons go? J Neurochem 131: 399–406, 2014. doi: 10.1111/jnc.12812. [DOI] [PubMed] [Google Scholar]
- 168.Sosunov AA, Wu X, Tsankova NM, Guilfoyle E, McKhann GM II, Goldman JE. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci 34: 2285–2298, 2014. doi: 10.1523/JNEUROSCI.4037-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci 18: 708–717, 2015. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stephen TL, Higgs NF, Sheehan DF, Al Awabdh S, López-Doménech G, Arancibia-Carcamo IL, Kittler JT. Miro1 regulates activity-driven positioning of mitochondria within astrocytic processes apposed to synapses to regulate intracellular calcium signaling. J Neurosci 35: 15996–16011, 2015. doi: 10.1523/JNEUROSCI.2068-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stetler RA, Leak RK, Gao Y, Chen J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J Cereb Blood Flow Metab 33: 22–32, 2013. doi: 10.1038/jcbfm.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol 128: 659–669, 2006. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med 17: 183–190, 2007. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Supplie LM, Düking T, Campbell G, Diaz F, Moraes CT, Götz M, Hamprecht B, Boretius S, Mahad D, Nave KA. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J Neurosci 37: 4231–4242, 2017. doi: 10.1523/JNEUROSCI.0756-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tabata H. Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci 9: 114, 2015. doi: 10.3389/fnins.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci USA 92: 4616–4620, 1995. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 178.Takata N, Hirase H. Cortical layer 1 and layer 2/3 astrocytes exhibit distinct calcium dynamics in vivo. PLoS One 3: e2525, 2008. doi: 10.1371/journal.pone.0002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tanaka A, Youle RJ. A chemical inhibitor of DRP1 uncouples mitochondrial fission and apoptosis. Mol Cell 29: 409–410, 2008. doi: 10.1016/j.molcel.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 180.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276: 1699–1702, 1997. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 181.Thoren AE, Helps SC, Nilsson M, Sims NR. Astrocytic function assessed from 1-14C-acetate metabolism after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 25: 440–450, 2005. doi: 10.1038/sj.jcbfm.9600035. [DOI] [PubMed] [Google Scholar]
- 182.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28: 1589–1600, 2009. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Uchino H, Elmér E, Uchino K, Li PA, He QP, Smith ML, Siesjö BK. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res 812: 216–226, 1998. doi: 10.1016/S0006-8993(98)00902-0. [DOI] [PubMed] [Google Scholar]
- 184.Uchino H, Elmér E, Uchino K, Lindvall O, Siesjö BK. Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol Scand 155: 469–471, 1995. doi: 10.1111/j.1748-1716.1995.tb09999.x. [DOI] [PubMed] [Google Scholar]
- 185.Ugbode CI, Hirst WD, Rattray M. Neuronal influences are necessary to produce mitochondrial co-localization with glutamate transporters in astrocytes. J Neurochem 130: 668–677, 2014. doi: 10.1111/jnc.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vafai SB, Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature 491: 374–383, 2012. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 187.Verkhratsky A, Nedergaard M, Hertz L. Why are astrocytes important? Neurochem Res 40: 389–401, 2015. doi: 10.1007/s11064-014-1403-2. [DOI] [PubMed] [Google Scholar]
- 188.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47: 365–378, 2005. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 189.Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem 102: 1383–1394, 2007. doi: 10.1111/j.1471-4159.2007.04634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Waagepetersen HS, Hansen GH, Fenger K, Lindsay JG, Gibson G, Schousboe A. Cellular mitochondrial heterogeneity in cultured astrocytes as demonstrated by immunogold labeling of alpha-ketoglutarate dehydrogenase. Glia 53: 225–231, 2006. doi: 10.1002/glia.20276. [DOI] [PubMed] [Google Scholar]
- 191.Wagner KR, Kleinholz M, Myers RE. Delayed decreases in specific brain mitochondrial electron transfer complex activities and cytochrome concentrations following anoxia/ischemia. J Neurol Sci 100: 142–151, 1990. doi: 10.1016/0022-510X(90)90025-I. [DOI] [PubMed] [Google Scholar]
- 192.Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol 169: 416–424, 2001. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 193.Yeh TH, Lee DY, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57: 1239–1249, 2009. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420, 2003. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yoshimoto T, Siesjö BK. Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res 839: 283–291, 1999. doi: 10.1016/S0006-8993(99)01733-3. [DOI] [PubMed] [Google Scholar]
- 196.Zaidan E, Sims NR. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem 63: 1812–1819, 1994. doi: 10.1046/j.1471-4159.1994.63051812.x. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20: 588–594, 2010. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]