Abstract
Precision-cut liver slices (PCLSs) provide a novel model for studies of alcoholic liver disease (ALD). This is relevant, as in vivo ethanol exposure does not appear to generate significant liver damage in ethanol-fed mice, except in the National Institute on Alcohol Abuse and Alcoholism binge model of ALD. Previous studies have shown that the two metabolites of ethanol consumption, malondialdhyde (MDA) and acetaldehyde (AA), combine to form MDA-AA (MAA) adducts, which have been correlated with the development and progression of ALD. In this study, murine PCLSs were incubated with ethanol and examined for the production of MAA adducts. PCLSs were homogenized, and homogenates were injected into C57BL/6 mice. PCLSs from control-, pair-, and ethanol-fed animals served as targets in in situ cytotoxic assays using primed T cells from mice hyperimmunized with control or ethanol-exposed PCLS homogenates. A CD45.1/CD45.2 passive-transfer model was used to determine whether T cells from the spleens of mice hyperimmunized with PCLS ethanol-exposed homogenates trafficked to the liver. PCLSs incubated with ethanol generated MAA-modified proteins in situ. Cytotoxic (CD8+) T cells from immunized mice killed naïve PCLSs from control- and pair-fed mice in vitro, a response that was blunted in PCLSs from ethanol-fed mice. Furthermore, CD45.1 CD8+ T cells from hyperimmunized mice trafficked to the liver but did not initiate liver damage. This study demonstrates that exposure to liver tissue damaged by ethanol mediates robust immune responses to well-characterized alcohol metabolites and native liver proteins in vitro. Moreover, although these proinflammatory T cells traffic to the liver, these responses appear to be dampened in vivo by locally acting pathways.
NEW & NOTEWORTHY This study shows that the metabolites of ethanol and lipid breakdown produce malondialdehyde-acetaldehyde adducts in the precision-cut liver slice model system. Additionally, precision-cut liver slices exposed to ethanol and harboring malondialdehyde-acetaldehyde adducts generate liver-specific antibody and T cell responses in the spleens of naïve mice that could traffic to the liver.
Keywords: alcoholic liver disease, antibody, in vitro inflammation, liver, malondialdehyde-acetaldehyde adducts, precision-cut liver slices, protein adducts, T cell transfer
INTRODUCTION
One consequence of repeated heavy drinking is alcoholic liver disease (ALD), which results in >80,000 deaths annually in the United States alone (44). Several studies have shown that the onset of ALD is, in part, attributed to immune mechanisms, as evidenced by detection of circulating antibodies and lymphocytes with specificity to various hepatic antigens (2, 41, 57). Animal models have been useful in detailed mechanistic studies of ALD (27, 50). However, while in vitro culture models have helped advance understanding of the underlying pathophysiology, they have severe limitations that hinder understanding of the pathophysiology of ALD (6, 34). Precision-cut liver slices (PCLSs) have been shown to be useful in the study of hepatic cells in response to various metabolites, toxicological agents, and fibrogenesis (14, 22, 31, 47). This is highly relevant, given the fact that ethanol-fed mice appear to be protected from the in vivo liver damage that characterizes ALD. PCLSs provide a model that maintains the normal lobular hepatic architecture with cell-cell and cell-matrix interactions by mimicking an in vivo model while allowing for the more detailed perspective provided by in vitro studies. Thus this in situ/ex vivo model also allows for broader investigations into the mechanism(s) of ALD, as PCLSs contain all the cell types and matrices found in the liver (19).
Early studies showed that ethanol exposure alone does not initiate immune responses and may actually be immunosuppressive, depending on the amount consumed (37). Chronic alcohol-feeding models have shown little or no damage to the livers of rodents in the absence of a second “hit.” Rather, many investigators have shown that it is the metabolites of ethanol that are capable of binding to proteins, rendering them immunogenic (28, 49, 52, 53). The majority of these studies were performed primarily with foreign proteins modified in vitro followed by immunization of animals in the context of an adjuvant (16, 25, 55, 56). However, the detection and potential role of ethanol metabolites binding (or adducting) to liver proteins have been inconclusive with respect to the pathogenesis of ALD (28, 30, 45, 55).
The formation of protein adducts has been well characterized in ALD and involves several pathways integral to the metabolism of ethanol, such as acetaldehyde (AA), malondialdehyde (MDA), and 4-hydroxy-2-nonenal (HNE). These adducts interfere with protein function, particularly the function of proteins containing lysine residues (12). MDA and HNE have been of particular interest, as they have been detected in ALD patients, and there is evidence suggesting that elevated levels of MDA and HNE may correlate with an increase in disease severity (48, 49).
Recent studies have shown that aldehydes formed from both of these pathways, MDA and AA, react with lysine on proteins in a synergistic way, ultimately resulting in separate hybrid adducts (46). One of these hybrid adducts, the MDA-AA (MAA) adduct, was detected (75 pmol MAA/mg of rat liver cytosol) when rats or mice were maintained for 5 wk on the Lieber-DeCarli alcohol-feeding model (54). While these rats developed fatty liver, little, if any, liver disease ensued. Interestingly, mice fed ethanol and exposed to MAA-modified liver self-proteins (MAA-induced autoimmune-like hepatitis) produced robust serum antibody responses and splenic T cell proliferative responses measured in vitro against liver self-proteins but developed only mild or no ALD (40). Importantly, however, increased antibody levels to the MAA adduct, which have been shown to be correlated with the severity of the liver disease, are found in the serum of ALD patients (33).
Immune responses to the MAA adduct have been the focus of a number of previously reported studies showing that 1) MAA-protein adducts injected in the absence of adjuvants into animals cause a significant antibody response to the adduct, carrier protein, and protein-carrier conjugate (42), 2) MAA adducts initiate immune responses by binding to scavenger receptors, upregulating adhesion molecules, inducing proinflammatory cytokines, producing antibody and T cell responses, and increasing the profibrotic response (41), and 3) daily injections of MAA-modified liver self-cytosols caused reactivity to self-protein, resulting in production of antibodies, T cells, or both against MAA adducts and liver self-antigens, as measured in vitro (40). Although these data suggest that MAA adduct modification of liver self-proteins may lead to loss of tolerance and relevant immune responses detected in animal studies, anti-MAA adduct immune responses do not appear to directly initiate liver damage in vivo. These observations suggest that other factors are necessary for the development and/or progression of ALD (56). As reported by Yüksel et al. (56), it appears that artificially breaking tolerance in the liver is relatively “easy.” In contrast, maintaining this state of tolerance breakdown, to yield chronic hepatitis, is much more difficult; liver immune homeostasis has been recognized to be strongly regulated by several immune response inhibitory mechanisms [e.g., regulatory T (Treg) cells, IL-6, NK1.1-positive T cells, regulatory B cells, and follicular T cells] (56). Since MAA-modified proteins are produced at very low levels and adducts are formed on multiple proteins in vivo following alcohol consumption, not all proteins are highly modified. Even when protein adducts are present, it has been shown that they are rapidly cleared by scavenger receptors on lymphatic endothelial cells and Kupffer cells, decreasing their immunogenicity (20). In contrast, when they are immunized with in vitro-prepared antigen, animals receive a bolus of MAA-modified proteins wherein virtually every protein has been modified. Exposure to the antigen occurs at relatively high doses and at sites outside the liver. Thus the clearance and immunosuppressive nature of the lymphatic endothelial cells and Kupffer cells are bypassed, resulting in a strong immune response due to the route and dose of the antigen.
In this study, syngeneic precision-cut liver slices (PCLSs) were exposed to ethanol, which is metabolized to AA, resulting in oxidative stress and, thereby, formation of MDA. Since there is no place for these aldehydes to go (no vasculature or dilution by fluid exchange), these molecules can combine to form MAA adducts on liver proteins at levels that are higher than normal but less than if MDA and AA were added in an in vitro setting. These PCLSs were then used to make a cytosol extract and employed as immunogens in naïve mice.
The purpose of this study was to determine if the metabolites of ethanol and lipid breakdown are capable of producing MAA adducts in the PCLS in situ model system. Additionally, we evaluated whether PCLSs exposed to ethanol and harboring MAA adducts generated liver-specific antibody and T cell responses in vitro after immunization in the absence of adjuvant. Finally, we explored whether these in vitro-measured immune responses could traffic to the liver and initiate ALD in vivo when transferred to naïve mice.
MATERIALS AND METHODS
Immunohistochemical staining for MAA adducts in human liver tissue.
Liver tissue from deidentified patients was obtained from the University of New Mexico Medical Center Pathology Department as part of routine diagnostic testing; all patients provided written informed consent under an Institutional Review Board-approved protocol. Paraffin-embedded liver sections from five patients, each with normal liver taken at autopsy, steatohepatitis, and ALD, were subjected to immunohistochemical staining. A polyclonal rabbit anti-MAA and a monoclonal mouse anti-MAA antibody (developed and reported previously by our laboratories) were used to stain sections for the presence of MAA adduct (39, 54). Primary antibodies were detected using Cy2 F(ab) fragment-goat anti-rabbit IgG and Cy5 F(ab) fragment-goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). Rabbit IgG and mouse IgG (Sigma Chemical, St. Louis, MO) were used as isotype controls. Tissue was mounted in Fluormount-G and visualized using a confocal laser-scanning microscope (Zeiss 710 Meta). Images were analyzed using Zen 2.1 Black software (Zeiss) and quantified using ImageJ (35).
Animals.
Six-week-old female C57BL/6 (CD45.2 or CD45.1) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and maintained on a Purina chow diet. C57BL/6 (CD45.2) mice were allowed access to food and water up to 1 h before they were euthanized. In a separate experiment, mice were divided into chow-, pair-, and ethanol-fed (Lieber-DeCarli diet, Bethlehem, PA) groups and maintained on the assigned diet for 6 wk. For these studies, each group consisted of four mice, which were paired by weight into pair-, control-, and ethanol-fed groups. The concentration of ethanol was increased from 1% to 5% over a 1-wk period. The mice were then pair-fed as follows: the control mice were fed the isocaloric amount of the ethanol diet from the previous day. At euthanasia, all livers were removed and assessed histologically, and serum was obtained for determination of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center and the Omaha Veterans Affairs Medical Center in accordance with National Institutes of Health guidelines on the use of laboratory animals.
Mouse PCLSs.
C57BL/6 (CD45.2) mice weighing 20–25 g were anesthetized using isoflurane. PCLSs were prepared as previously described (19). Briefly, 8-mm liver cores were harvested and cut into 250-µm-thick sections using a tissue slicer (Vitron, Tucson, AZ). Slices were incubated in Williams E (WE) medium (Sigma Chemical) containing d-glucose and gentamicin with 95% O2-5% CO2 (carbogen) at 37°C for 30 min as a washout period. PCLSs were then loaded onto titanium-screen rollers (Vitron), inserted into sterile 20-ml glass vials containing 1.7 ml of WE medium, and placed into a roller incubator at 37°C in the presence of carbogen for 72 h. The medium was replaced daily.
Determination of MAA antigen in PCLSs incubated with ethanol.
PCLS lysates from control- and ethanol-fed mice were subjected to immunoprecipitation using a polyclonal rabbit anti-MAA antibody. Lysates were resolved using SDS-PAGE on 10% gels under reducing conditions and transferred to polyvinylidene difluoride membranes (Immun-Blot, Bio-Rad, Hercules, CA). Blots were blocked with Odyssey blocking buffer (LI-COR, Lincoln, NE), washed, and incubated with a monoclonal mouse anti-MAA antibody. Detection was accomplished using an IRDye-conjugated anti-mouse secondary antibody (LI-COR) followed by analysis on an Odyssey infrared scanner (LI-COR). Controls including phosphate-buffered saline and rabbit IgG were immunoprecipitated with the rabbit anti-MAA antibody. Unique MAA-modified proteins identified in the Western blot were subjected to SDS-PAGE, silver-stained, and cut out for analysis by mass spectroscopy using Applied Biomics (Hayward, CA) protein identification services.
Confocal imaging of MAA-modified proteins in PCLSs.
PCLSs subjected to control and ethanol media for 0, 24, 48, and 72 h were fixed in formalin and embedded in paraffin for immunohistochemical analysis. Sections were rehydrated and subjected to antigen retrieval techniques. Sections were blocked and incubated with an IgG rabbit polyclonal antibody to MAA or an Alexa Fluor-IgG2b rat monoclonal antibody to F4/80 (Nova Biologicals, Littleton, CO). Primary antibodies that were not directly labeled were detected using Cy2 F(ab) fragment-goat anti-rabbit IgG (H + L, Jackson ImmunoResearch). Rabbit IgG (Sigma Chemical) and Alexa Fluor-rat IgG2b (Nova Biologicals) were used as the isotype controls. Tissue was mounted in Fluormount-G and visualized using a confocal laser-scanning microscope (Zeiss 710 Meta). Images were analyzed using Zen 2.1 Black software (Zeiss) and quantified as described above.
Preparation and immunization of PCLS antigen.
PCLSs were incubated in WE control medium or 25 mM ethanol in WE medium. PCLSs were incubated for 3 days in the roller incubator at 37°C in the presence of carbogen. Whole cell lysates were prepared by homogenization of the PCLSs in ice-cold phosphate-buffered saline. Protein concentrations were determined, and antigens were separated into aliquots and stored at −70°C until use. Control and ethanol-exposed PCLS antigen (100 µg/mouse weekly for 5 wk) was used to hyperimmunize mice. In some cases, PCLS antigen was incubated with equal amounts of rabbit anti-MAA antibody to immunoprecipitate any MAA-modified antigens and then used as an antigen for hyperimmunization of the mice.
Serum antibody levels to liver proteins.
At week 6, 1 wk following the last immunization, serum was collected and tested for the presence of anti-liver and anti-MAA antibodies (40, 54). Briefly, 96-well Immulon IV (Nunc, Thermo Fisher Scientific, Waltham, MA) ELISA plates were coated with 2 µg/well of control- or ethanol-PCLS antigen, human serum albumin, or MAA-modified human serum albumin (MAA-Alb). Sera from animals were incubated with the coated antigens and then detected using a horseradish peroxidase-goat anti-mouse IgG Fc fragment-specific secondary antibody (Jackson ImmunoResearch). Absorbance of the samples was measured at 450 nm using a MRX II plate reader with Revelation software (Dynex Technologies, Chantilly, VA). Background antibodies directed against albumin were subtracted from the MAA-Alb reactivity, providing only antibody to the MAA epitope. Data are presented as arbitrary units (AUs) of mouse IgG detected in the assay relative to the standard curve. To show the specificity of the PCLS antigen following in vitro ethanol incubation, both control and ethanol PCLS antigens were immunoprecipitated with a polyclonal rabbit anti-MAA antibody to pull down any MAA-modified antigens. These antigens were washed, protein concentrations were determined, and the antigens were injected into mice at a concentration of 100 µg/wk for 5 wk in the absence of adjuvant, as previously described (19). Antibodies were detected by ELISA using the above-described conditions and control PCLS/MAA-Alb as the coating antigen.
Proliferation assays.
T cell proliferation assays were first performed to determine reactivity to liver homogenates and MAA antigens using CD3+ T cells. CD3+ T cells (5 × 105/well) from sham control PCLSs with and without MAA immunoprecipitate from immunized mice and ethanol-exposed PCLSs with and without MAA immunoprecipitate from immunized mice were incubated with liver homogenate and MAA-Alb antigens in 96-well flat-bottom plates at 0.3125–10 ng/ml. Irradiated (2,000 rad) splenocytes were added to the culture to serve as antigen-presenting cells. T cells from uninjected naïve mice were used as background and subtracted from the test samples. In some cases, T cells stimulated with albumin only were used as controls and subtracted from MAA-Alb-stimulated T cells. Cultures were incubated for 48 h at 37°C in 5% CO2, pulsed with 1.0 µCi/well [3H]thymidine (GE Healthcare, Piscataway, NJ) for 16 h, and harvested on a 96-well harvester (Tomtec, Orange, CT). Filter paper containing the incorporated thymidine was placed in scintillation fluid and counted on a scintillation counter (1450 Microbeta, Perkin Elmer Life Sciences, Waltham, MA). Data are expressed as stimulation index (SI) according to the following formula: SI = (mean cpm/ml of target – BKG)·(mean cpm/ml of control – BKG), where BKG is background standard.
To determine which T cell populations were involved in the response to liver homogenate and MAA-modified proteins, CD8+ and CD4+ cells were isolated. CD4+ or CD8+ T cells were extracted using CD4+ or CD8+ T Cell Isolation Kit II (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol. Both cell types were isolated from control and experimental mice and incubated with liver homogenate and MAA-Alb in proliferation assays, as described above.
T cell cytotoxicity on PCLSs.
After mice were injected with control and ethanol PCLS antigen, spleens were excised, and CD8+ T cells were isolated using Pan T Cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer’s protocol. Mice were fed the chow diet, pair-fed, or fed the ethanol diet for 6 wk, livers were extracted, and PCLSs were prepared as described above and incubated with CD8+ T cells from control- or ethanol-fed PCLS antigen-injected mice. Briefly, PCLSs from chow-, pair-, and ethanol-fed mice were incubated in WE medium for a 30-min washout period. PCLSs were placed into the incubation vial with 1.7 ml of WE medium, and 1 × 106 purified T cells were added directly onto the slice. The slices and T cells were incubated for 5 h at 37°C, placed into 2% Triton + WE medium, and solubilized for use in lactate dehydrogenase (LDH) assays. PCLSs with no T cells and T cells with no slices were used as controls in this experiment. LDH was measured to assess tissue damage using a cytotoxicity detection kit (LDH) (Roche Applied Science, Penzberg, Germany), as previously described (19).
IL-6 ELISA.
Naïve PCLSs were isolated and incubated with CD8+ T cells from chow-, pair-, and ethanol-fed mice injected with control- or ethanol-PCLS antigen for 24 h in titanium insert vials. Supernatants were collected and assayed for the presence of IL-6 using an ELISA kit (eBioscience, San Diego, CA). Absorbance of the samples was measured at 450 nm using an MRX II plate reader with Revelation software (Dynex Technologies).
Liver T cell phenotyping.
Livers from mice immunized with control- or ethanol-PCLS antigen were perfused with ice-cold saline to remove blood products and extracted for mechanical disruption. Livers were minced with a scalpel and scissors and pushed through a stainless steel mesh with the end of a syringe plunger. Cells were suspended in RPMI 1640 medium containing 10% FCS and centrifuged at 50 g for 5 min to remove hepatocytes. The supernatant was centrifuged at 480 g for 10 min, resuspended in 5 ml of medium, and layered onto mouse Lympholyte. Tubes were then centrifuged at 1,500 g for 10 min, and cells were collected at the interface and washed three times with ice-cold medium. Cells were then counted and subjected to flow cytometry. Liver nonparenchymal cells were phenotyped using a multicolor basic T cell panel that included the following antibodies; allophycocyanin (APC)-Cy7-rat anti-mouse CD3, APC-rat anti-mouse CD4, Brilliant Violet 650-rat anti-mouse CD8, and Brilliant Violet 605-rat anti-mouse CD45R (BD Biosciences, San Diego, CA), Alexa Fluor 488-rat anti-mouse CD183 (Novus Biologicals), and phycoerythrin (PE)-Cy7-Armenian hamster anti-mouse CD194 (Sony Biotechnology, San Jose, CA). A Treg cell/Th17 panel was also performed to determine the role of these cells in this process. Antibodies used for this panel were as follows: peridinin-chlorophyll-protein complex (PerCP)-Cy5.5-rat anti-mouse CD4, FITC-rat anti-mouse CD25, PE-rat anti-mouse lymphocyte activation gene 3 (LAG-3), APC-rat anti-mouse folate receptor 4 (FOLR4), Brilliant Violet 650-rat anti-mouse glucocorticoid-induced tumor necrosis factor receptor-related gene (GITR) ligand, and V450-rat anti-mouse IL-17A (BD Biosciences). Compensation beads were used to correct for spectral overlap. Cells were stained with a LIVE/DEAD cell vitality kit (Invitrogen, Carlsbad, CA), and dead cells were gated out of the analysis. Data are expressed as percent positive compared with the antibody controls.
CD45.1/CD45.2 T cell transfer studies.
PCLSs were isolated from CD45.1-expressing mice and incubated with control and ethanol media for 3 days. Control- and ethanol-PCLS antigens were prepared as described above and injected into syngeneic CD45.1 mice weekly for 5 wk. At week 6, CD3+ T cells were isolated as described above and injected into naïve CD45.2-expressing mice via the tail vein at a concentration of 1 × 106 cells/mouse and allowed to traffic within the mouse for 4 days. After this incubation period, CD3+ T cells were isolated from the liver and tested by flow cytometry for expression of CD45.1 using a PE-labeled anti-mouse CD45.1 antibody (eBioscience). An eFluor 450 antibody against mouse CD3 was used, first, to gate-out positive cells and, then, to gate-on positive CD45.1 expression. PE-labeled mouse IgG2aκ (eBioscience) was used as the isotype control. Data are expressed as percent positive among the selected population of cells.
To further confirm that CD45.1 cells migrated into the liver, paraffin-embedded sections of the liver tissues were visualized using confocal microscopy, as outlined above. Antibodies from the above-described flow cytometry experiment were used in addition to the polyclonal rabbit anti-MAA antibody, and stained tissue was viewed using a confocal laser-scanning microscope (Zeiss 710 Meta) and analyzed using Zen 2.1 Black software (Zeiss).
Statistical analysis.
Values are means ± SE. Statistical significance was achieved at P < 0.05. All statistical analysis was performed using Sigma Plot 10.0 with SigmaStat (Jandel Scientific, 2006) and one-way or multiple ANOVA where appropriate.
RESULTS
Detection of MAA-modified proteins in human liver tissue by immunohistochemistry.
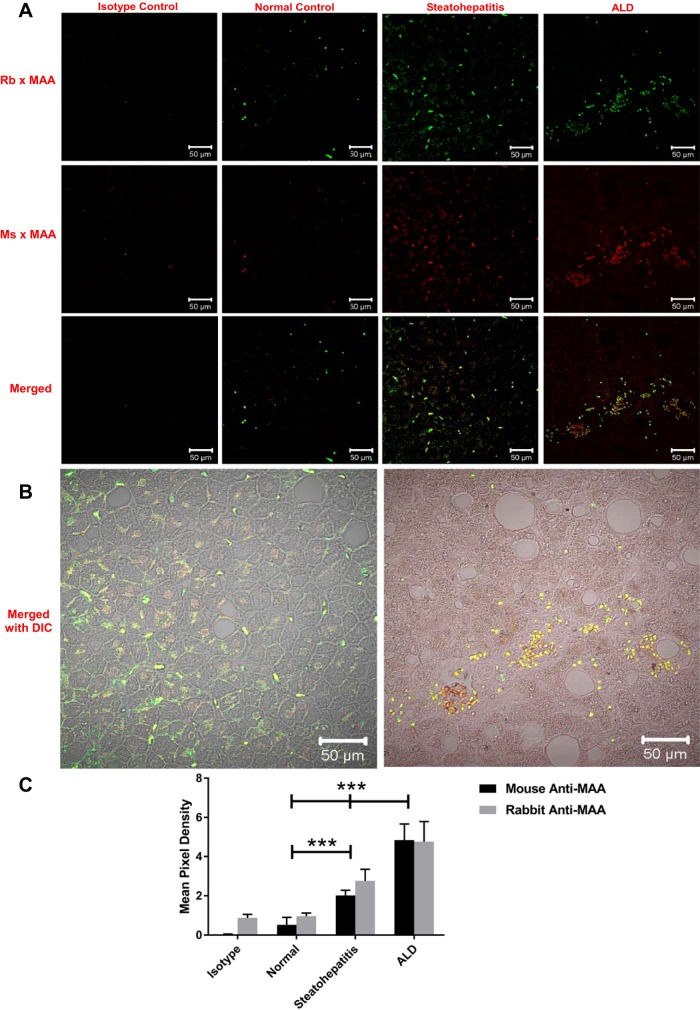
MAA-modified proteins have been suggested to play a role in development and/or progression of ALD. Therefore, the initial studies were performed to evaluate whether MAA-modified proteins are found in normal liver tissues at autopsy, livers of patients with steatohepatitis, and livers of patients with ALD. As shown in Fig. 1, A and C, only minimal reactivity was seen with the rabbit polyclonal [green fluorescence, 0.96 mean pixel density (MPD); Fig. 1A, top] and the mouse monoclonal (red fluorescence, 0.52 MPD; Fig. 1A, middle) anti-MAA antibodies in the normal liver tissue. However, a dramatic increase (P < 0.001) in reactivity to MAA adduct was seen with the rabbit polyclonal anti-MAA antibody (green fluorescence, 2.77 MPD) and mouse monoclonal antibody (red fluorescence, 2.02 MPD) in patients with steatohepatitis (Fig. 1A, bottom). Interestingly, a significant increase (P < 0.001) in MAA adduct was detected with polyclonal (green fluorescence, 4.76 MPD) and monoclonal (red fluorescence, 4.845 MPD) anti-MAA antibodies in the livers from patients with ALD compared with steatohepatitis patients (Fig. 1A, bottom). In addition, the MPD difference in staining between the two antibodies on the various tissues shows that the two antibodies have the same specificity. Interestingly, the amount of staining was significantly increased in tissue from patients with ALD compared with tissue from patients with steatohepatitis and normal liver tissue (Fig. 1C). Additionally, the amount of staining differed significantly between the steatohepatitis group and the normal control and isotype control groups. Thus more MAA adducts appear to be present in the livers from patients with ALD than any of the other groups, but a significant amount of staining for MAA adduct was observed in the steatohepatitis group.
Fig. 1.
Immunohistochemical staining for malondialdehyde-acetaldehyde (MAA) adducts in human liver tissue. Paraffin-embedded liver sections from patients with normal liver, steatohepatitis, and alcoholic liver disease (ALD) were subjected to immunohistochemical staining using a rabbit (Rb) and a mouse (Ms) anti-MAA antibody. A: staining with rabbit anti-MAA antibody (green, top) and mouse anti-MAA antibody (red, middle) and merged images (yellow, bottom). B: merged differential interference contrast (DIC) images of steatohepatitis and ALD samples stained with both anti-MAA antibodies. C: quantification of mean pixel density. Sections were mounted in Fluoromount-G and viewed using a confocal laser-scanning microscope (Zeiss 710 Meta). Images were analyzed using Zen 2.1 Black (Zeiss) and ImageJ software. Values are means ± SE (n = 5). ***P < 0.001 vs. normal and steatohepatitis.
Detection of MAA-modified proteins in PCLSs from C57BL/6 mice by immunoblotting.
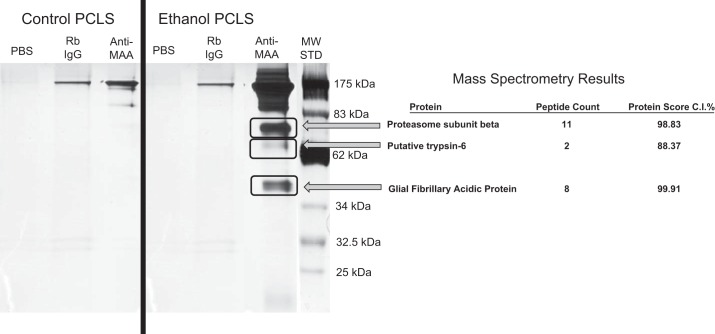
To determine if MAA modification of proteins occurs in situ, PCLSs were incubated with control medium or 25 mM ethanol-containing medium for 72 h, as previously reported (19). PCLS lysates were subjected to immunoprecipitation using an antibody directed against MAA adduct (54). As shown in Fig. 2, PCLSs incubated with ethanol produced multiple MAA-modified proteins at 180, 175, 90, 78, 40, and 15 kDa (lane 6; anti-MAA). Immunoprecipitation of control PCLS antigen with anti-MAA produced a ∼175-kDa band, which was most likely the IgG control band. Immunoprecipitated proteins from the PCLSs exposed to ethanol for 72 h were cut out and sent for mass spectroscopy and protein identification. Results with high confidence scores indicated the presence of three unique peptides, proteasome subunit β, putative trypsin-6, and glial fibrillary acidic protein.
Fig. 2.
MAA antigen in precision-cut liver slices (PCLSs) incubated with ethanol. PCLSs were incubated with control and ethanol-containing media for 3 days, and homogenates [PCLS antigens (PCLSA)] were prepared and subjected to immunoprecipitation using a polyclonal rabbit (Rb) anti-MAA antibody. Western blot using the monoclonal mouse anti-MAA antibody shows unique bands in the PCLSs incubated with ethanol. Bands (black rectangle) from control and ethanol-treated PCLSs were cut out and sent for mass spectrometry to Applied Biomics. Ethanol-treated PCLSs had a positive hit for proteasome subunit β, putative trypsin-6, and glial fibrillary acidic protein. Gels are representative of 5 separate samples, one of which was used for mass spectrometry. Proteins were identified as significant if an 88–99% confidence ID [confidence interval (CI)] score was obtained. MW STD, molecular weight standard.
Detection of MAA-modified proteins in PCLSs from C57BL/6 mice by immunohistochemisty.
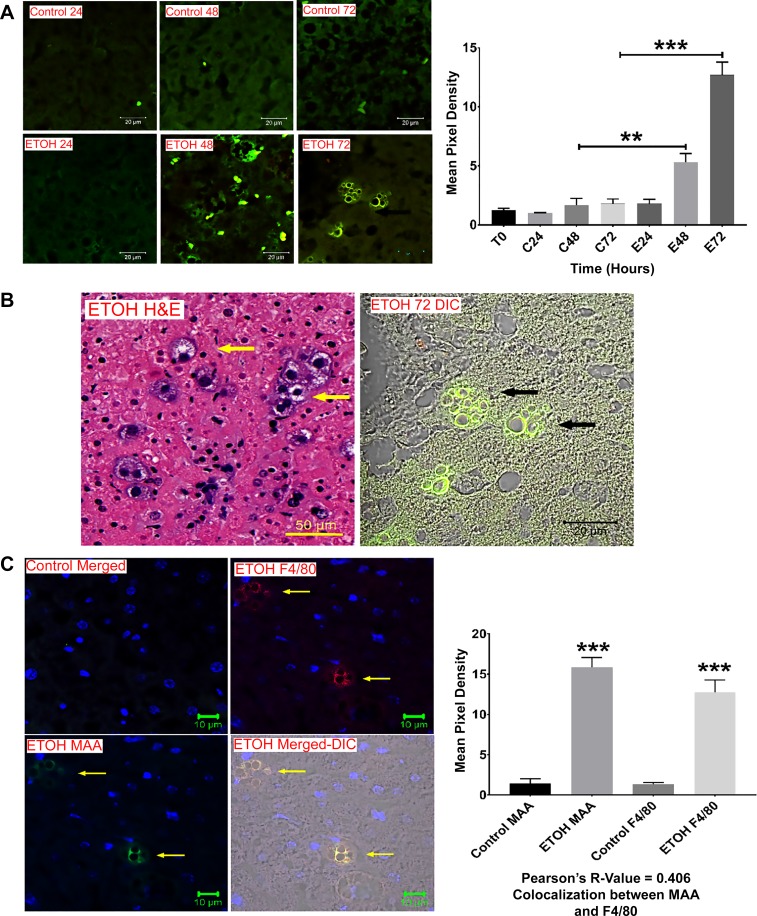
To further confirm that MAA adducts were being produced in PCLSs following incubation with ethanol, paraffin-embedded slices were stained with a rabbit anti-MAA antibody. As shown in Fig. 3A, anti-MAA antibody showed no reactivity to slices incubated alone or with control medium for up to 72 h. However, starting at 48 h, antibody to MAA (green fluorescence) was present in ethanol-exposed tissues, and the amount of staining was significantly (P < 0.001) increased in the 72-h sample compared with all others. During the staining process, aggregates resembling macrophages that were positive for MAA-modified proteins were observed (Fig. 3B). Therefore, slides were stained for F4/80 (red fluorescence; Fig. 3C), and colocalization indicated the presence of MAA-modified proteins in liver macrophages, as demonstrated by the correlation (R = 0.406) between anti-MAA and anti-F4/80 antibodies.
Fig. 3.
Confocal microscopy of MAA antigen in PCLSs. Paraffin-embedded sections from control and ethanol-incubated PCLSs were subjected to immunohistochemical staining using a rabbit anti-MAA antibody. A: increasing reactivity to MAA antigen (green, left) over time in the ethanol (EtOH)-treated PCLSs (E24, E48, and E72) compared with minimal reactivity to control (C24, C48, and C72) samples and quantification of mean pixel density (right). **Significantly increased compared with controls and E24 (P < 0.01). ***Significantly increased compared with all other treatments (P < 0.001). B: hematoxylin-eosin (H&E)-stained giant cells (arrows) in the liver slice, which was stained with MAA. C: slides were stained with the marker F4/80 (red) to confirm the presence of MAA-modified proteins (arrows) in liver macrophages (left). Sections were mounted in Fluoromount-G and viewed using a confocal laser-scanning microscope (Zeiss 710 Meta). Images were analyzed using Zen 2.1 Black (Zeiss) and ImageJ software. MAA and F4/80 were colocalized together as demonstrated by Pearson’s correlation (right). Values are means ± SE (n = 5). ***P < 0.001 vs. controls.
Antibody responses to ethanol-PCLS antigen.
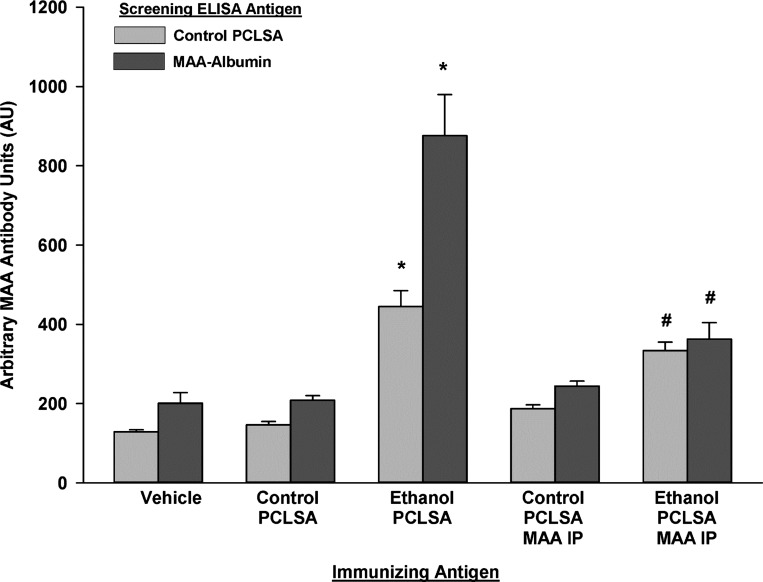
Since it is known that MAA modification of proteins renders the protein immunogenic, the next set of experiments were performed to evaluate whether PCLSs incubated in the presence of ethanol and, thus, containing MAA-modified proteins, would initiate antibody and T cell responses. PCLSs incubated with control or ethanol-containing medium were homogenized and injected into naïve syngeneic C57BL/6 mice. With control PCLS used as the ELISA antigen, mice immunized with ethanol-PCLS antigen demonstrated significantly higher serum IgG antibody concentrations (444.72 ± 80.06 AU) than mice immunized with control-PCLS antigen (146.68 ± 16.15 AU) or sham-immunized mice (128.83 ± 10.32 AU) (P < 0.001; Fig. 4). In addition, serum antibodies from these mice that reacted to MAA-Alb were detected following immunization with ethanol-PCLS antigen (876.09 ± 206.81 AU) compared with serum antibodies from mice immunized with control-PCLS antigen (208.24 ± 23.49 AU) and the vehicle control (200.93 ± 53.97 AU) (P < 0.001; Fig. 4). To show that these antibody responses were specific to the MAA adduct, control and ethanol-exposed PCLS homogenized antigens were immunoprecipitated with a rabbit antibody to MAA adduct to remove the MAA-modified antigens before the mice were immunized. Serum antibodies to proteins and MAA-Alb were significantly decreased to almost background levels. These antibodies were significantly (P < 0.001) reduced compared with antibodies from ethanol-PCLS antigen-injected animals (Fig. 4).
Fig. 4.
Serum antibody to MAA-modified liver proteins in C57BL/6 mice. Mice were injected with control and ethanol PCLSA weekly for 5 wk. At week 6, serum was collected and tested against control PCLSA and MAA-modified human serum albumin. A separate group of mice was injected with control or ethanol PCLSA that had been immunoprecipitated (PCLSA MAA IP) with the rabbit anti-MAA antibody to remove the MAA antigen. Values are means ± SE (n = 5 mice per group). *Significantly increased compared with vehicle or control PCLSA (P < 0.01). #Significantly decreased compared with ethanol PCLSA (P < 0.01).
T cell responses following hyperimmunization with PCLS antigens.
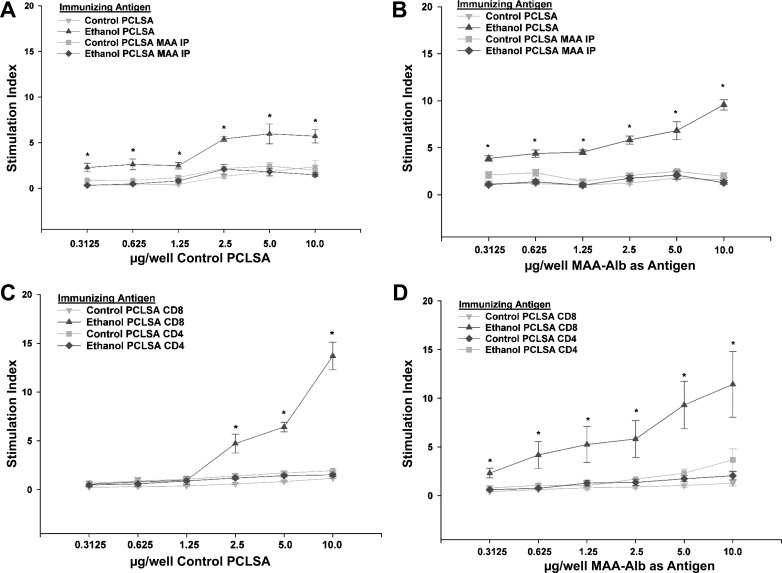
Antibody responses do not generally occur to a great extent in the absence of T cell responses. Thus, to evaluate the T cell responses to MAA adduct and normal liver antigens, CD3+ T cells were isolated from mice immunized with control- and ethanol-PCLS antigen homogenates. As shown in Fig. 5A, T cells from mice immunized with ethanol-PCLS antigen showed a significantly elevated SI against normal liver proteins compared with mice immunized with control-PCLS antigen or vehicle (P < 0.01). Importantly, responses of the vehicle control and control-PCLS antigen groups were similar and, for simplicity, are not shown. Differences were evident at all concentrations but were amplified at higher concentrations. As with antibody responses discussed above, immunoprecipitation using anti-MAA antibodies of the homogenates before immunization reduced the T cell responses to almost background levels. When MAA-Alb was used as the stimulating antigen in the proliferation assay, T cells from ethanol-exposed PCLS antigen-immunized mice showed a dose response to MAA-Alb (Fig. 5B). This proliferation was significantly increased (P < 0.001) compared with the vehicle- and control-PCLS antigen-immunized mouse. As anticipated, preincubation and immunoprecipitation of the antigen before immunization with anti-MAA antibody reduced the SI to background levels, showing that these responses were MAA-dependent.
Fig. 5.
T cell proliferation to MAA-modified liver proteins. CD3+, CD4+, and CD8+ T cells from spleens of hyperimmunized mice were collected and evaluated for proliferation responses against control PCLSA and MAA-modified human serum albumin (MAA-Alb). A: proliferation of CD3+ T cells against control PCLSA from mice immunized with control PCLSA, ethanol PCLSA, control PCLSA MAA IP, and ethanol PCLSA MAA IP. *Significantly increased compared with control PCLSA, control PCLSA MAA IP, and ethanol PCLSA MAA IP (P < 0.001). B: proliferation of CD3+ T cells against MAA-Alb from mice immunized with control PCLSA, ethanol PCLSA, control PCLSA MAA IP, and ethanol PCLSA MAA IP. *Significantly increased compared with control PCLSA, control PCLSA MAA IP, and ethanol PCLSA MAA IP (P < 0.001). C: proliferation of CD4+ or CD8+ T cells against control PCLSA from mice immunized with control PCLSA or ethanol PCLSA. *Significantly increased compared with control PCLSA CD4 and ethanol PCLSA CD4 and control PCLSA CD8 (P < 0.001). D: proliferation of CD4+ or CD8+ T cells against MAA-Alb from mice immunized with control PCLSA or ethanol PCLSA. *Significantly increased compared with control PCLSA CD4 and ethanol PCLSA CD4 and control PCLSA CD8 (P < 0.01). Values are means ± SE (n = 5 animals for each experiment).
Studies were performed to determine if the above-described responses were meditated by CD4+ or CD8+ T cells. As demonstrated in Fig. 5C, CD8+ T cells from ethanol-PCLS antigen-immunized mice proliferated at a SI of 5–15 against the control liver homogenate compared with <1 T for cells from vehicle- and control-PCLS antigen-injected mice. A proliferative response to MAA-Alb by CD8+ T cells was also present (Fig. 5D), but only by T cells from the ethanol-PCLS antigen-immunized mice. In contrast, none of the immunized mice showed significant CD4+ T cell responses to control-PCLS or MAA-Alb antigen.
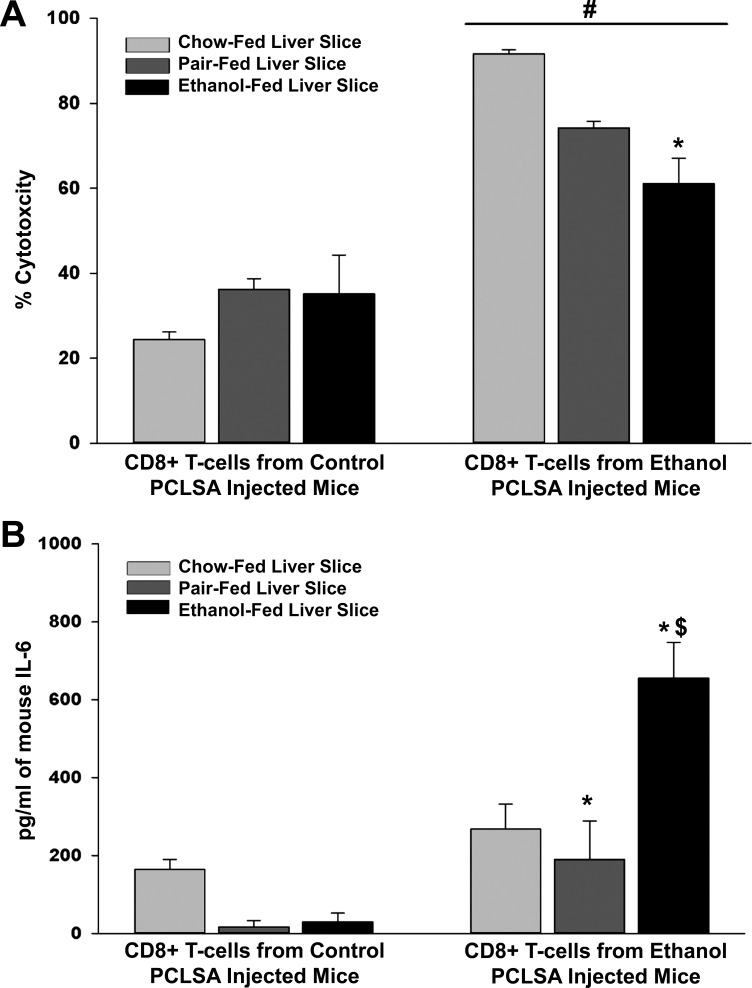
Cytotoxicity responses following hyperimmunization with PCLS antigens.
As the primary T cell proliferative responses to PCLS antigens were of the CD8+ phenotype with a primary function of cytotoxicity, we used cytotoxicity assays that were developed using PCLSs as targets to determine if CD8+ T cells from ethanol-PCLS antigen-immunized mice could kill naïve liver tissue. Slices from chow-, pair-, and ethanol-fed mice were incubated with CD8+ T cells from control- or ethanol-PCLS antigen-immunized mice. CD8+ T cells isolated from ethanol-PCLS antigen-immunized chow-fed mice resulted in 90% cytotoxicity in PCLSs from chow-fed mice compared with 60% in PCLSs from ethanol-fed mice (P < 0.01; Fig. 6A). Cytotoxic responses were decreased in PCLSs from pair-fed mice, although this difference did not achieve statistical significance. CD8+ T cells from control-PCLS antigen-immunized mice demonstrated <40% cytotoxicity against PCLSs from chow-, pair-, and ethanol-fed mice.
Fig. 6.
CD8+ T cell cytotoxicity and IL-6 levels in PCLS cocultures. CD8+ T cells were isolated from mice immunized with control or ethanol PCLSA and incubated on liver slices from chow-, pair-, or ethanol-fed mice. Samples were collected and tested for percent cytotoxicity and IL-6 levels. A: cytotoxicity generated against liver slices from chow-, pair-, and ethanol-fed mice initiated by CD8+ T cells from mice immunized with control or ethanol PCLSA. #Significantly increased in all 3 groups compared with CD8+ T cells from control PCLSA-injected mice (P < 0.001). *Significantly decreased compared with liver slices from chow-fed liver slices (P < 0.01). B: IL-6 generated in liver slice cultures from chow-, pair-, and ethanol-fed mice cocultured with CD8+ T cells from mice immunized with control or ethanol PCLSA. *Significantly increased compared with chow- or ethanol-fed liver slices. $Significantly increased compared with chow- or pair-fed liver slices incubated with CD8+ T cells from ethanol PCLSA-injected mice (P < 0.001). Values are means ± SE (n = 4 animals per group).
IL-6 release by cytotoxic T cells upon exposure to ethanol-fed liver slices.
As outlined above, cytotoxicity was significantly decreased in PCLSs from ethanol-fed mice immunized with ethanol-PCLS antigen (Fig. 6A). IL-6, which acts as a proinflammatory and an anti-inflammatory cytokine responsible for downregulating certain immune responses, was measured using culture fluid from the cytotoxicity studies after addition of CD8+ T cells. As shown in Fig. 6B, among mice immunized with ethanol-PCLS antigen, IL-6 was nearly four- to sixfold higher in the culture fluid of PCLSs from ethanol- than chow- and pair-fed mice. Culture fluid from control-PCLS antigen-immunized mice expressed minimal amounts of the cytokine, regardless of diet. These data indicate that ethanol exposure increases IL-6, thereby potentially decreasing the ability of CD8+ T cells to kill PCLSs in this in situ culture system.
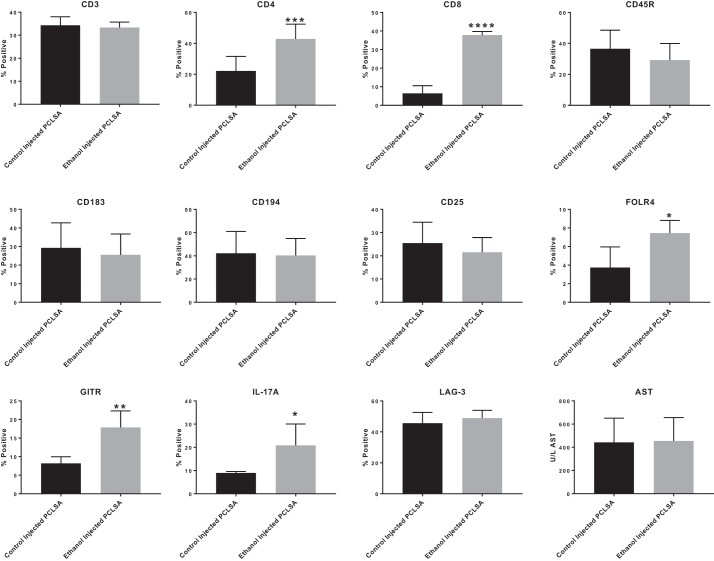
T cell phenotype in hyperimmunized mice.
To study T cell involvement in these responses, liver nonparenchymal cells from mice immunized with control- and ethanol-PCLS antigen were phenotyped for cell surface markers. As demonstrated in Fig. 7, ethanol-PCLS antigen had no effect on CD3. However, CD4+ T cells (P < 0.001) and CD8+ T cells (P < 0.0001) were increased in the ethanol-PCLS antigen-immunized compared with control-immunized mice. No changes were observed in the CD25 and Th1 (CD183) or Th2 (CD194) markers. However, there was a significant increase in FOLR4 (P < 0.01) and GITR (P < 0.001), both markers of Treg cells, in the ethanol-PCLS antigen- compared with control antigen-immunized mice. The most unique observation was the significant increase in IL-17A (P < 0.01) following injection with ethanol-PCLS antigen. There was no change in LAG3 expression for either of the groups. Also, serum collected from these mice was tested for AST, and no increase in liver damage was found.
Fig. 7.
T cell and regulatory T cell phenotyping. Nonparenchymal cells were isolated from control PCLSA- and ethanol PCLSA-immunized mice, and cell surface markers were tested by flow cytometry. There was no change in CD3, CD45R, CD183, or CD194 between control PCLSA- and ethanol PCLSA-injected mouse livers. However, there was a significant shift in CD4+ cells (***P < 0.001) and CD8+ cells (****P < 0.0001) in livers from ethanol PCLSA-injected animals compared with controls. Regulatory T cells in the liver showed no change in CD25 or lymphocyte activation gene 3 (LAG-3). There was a significant increase in folate receptor 4 (FOLR4) (*P < 0.05), glucocorticoid-induced tumor necrosis factor receptor-related gene (GITR) (**P < 0.01), and IL-17A (*P < 0.05) in ethanol PCLSA-injected mice compared with controls. No response was seen in the serum aspartate aminotransferase (AST) when comparing control PCLSA- and ethanol PCLSA-injected animals. Values are means ± SE (n = 5 separate animals per group).
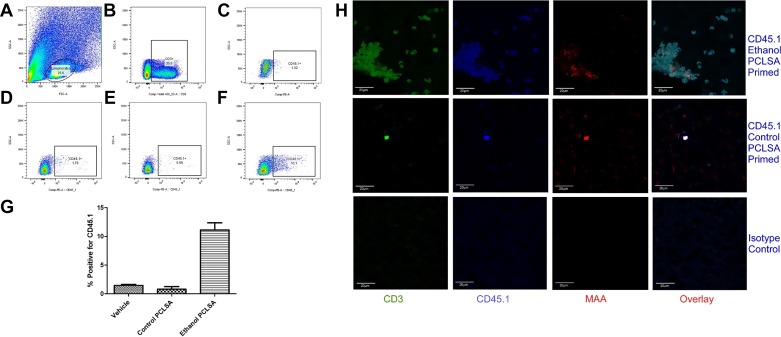
Transfer of T cells from hyperimmunized CD45.1 mice to naïve CD45.2 mice.
One of the major findings of the current study is the ability of ethanol-exposed PCLS homogenate-immunized mice to produce anti-liver tissue antibody and CD8+ T cells that can be measured in vitro. Yet, when these mice were evaluated for AST/ALT serum levels and liver histology, no damage was reported, which is in direct contradiction to the in vitro studies (data not shown). To evaluate whether ethanol-PCLS antigen-specific T cells are recruited to the liver, we employed the CD45.1/CD45.2 mouse model system to track cell migration (4, 36).
Briefly, PCLSs from CD45.1 mice were incubated with control and ethanol media, and PCLS homogenates from these cultures were injected into syngeneic CD45.1 mice (see materials and methods). T (CD3+) cells from these mice were isolated and injected into naïve CD45.2 mice and allowed to circulate. T cells were isolated from the liver and evaluated for surface markers by flow cytometry. As depicted in Fig. 8A, first, lymphocytes and, then, CD3+ T cells were gated-out of the population (Fig. 8B). Cells were further gated for CD45.1 on the isotype control (Fig. 8C), T cells from saline-injected mice (Fig. 8D), T cells from control-PCLS-injected mice (Fig. 8E), and T cells from ethanol-PCLS antigen-injected mice (Fig. 8F). As shown in Fig. 8G, there was a significant increase in percent positivity of the CD45.1 T cells from the ethanol-exposed PCLS-immunized CD45.2 mice. The presence of CD45.1-positive T cells in the livers of these mice was further confirmed by confocal microscopy (Fig. 8H). Interestingly, MAA-modified protein adducts were found in close proximity to the CD45.1 cells in the livers (Fig. 8H), strongly suggesting that the passively transferred CD45.1+ cells were responding to ethanol-modified proteins in the liver, but no damage was observed on histological sections, as identified by no increase in ALT/AST levels (data not shown).
Fig. 8.
Identification of CD45.1/CD45.2 T cells in livers of naïve mice. CD45.1 T cells from control- and ethanol-PCLSA-immunized CD45.1 mice were isolated and transferred into naïve CD45.2-expressing mice at a concentration of 1 × 106 cells/mouse for 4 days. CD3+ T cells were isolated from the liver and analyzed by flow cytometry. A: gating of lymphocytes from total liver cell population. B: gating of CD3+ T cells. C: gating of CD45.1 cells in isotype control. D: gating of CD45.1 cells from saline-injected mice. E: gating of CD45.1 cells from control-PCLSA-immunized mice. F: gating of CD45.1 cells from ethanol-PCLSA-immunized mice. G: CD45.1 transferred cells from ethanol-PCLSA-immunized mice were significantly increased (P < 0.001) compared with CD45.1 T cells from saline-injected or control-PCLSA-immunized mice. Values are means ± SE (n = 5 animals per group). H: livers were stained for CD3, CD45.1, and MAA and then overlaid to demonstrate colocalization. Sections were mounted in Fluoromount-G and viewed using a confocal laser-scanning microscope (Zeiss 710 Meta). Images were analyzed using Zen 2.1 Black software (Zeiss). Photomicrographs are representative of 5 separate immunohistochemical images.
DISCUSSION
Human ALD is characterized by inflammatory infiltrates, including CD4+/CD8+ T cells (5, 15, 18). The detection of circulating antibodies and lymphocytes with specificity to hepatic antigens in patients with ALD strongly supports the hypothesis that there is an immune component in the development and/or progression of ALD (7, 9, 21). In support of this hypothesis, a number of studies have reported development of immune responses in the spleen and/or lymph nodes of mice that were immunized at extrahepatic sites with modified syngeneic liver proteins (17, 23, 29, 38). Yet the damage to the liver in these model systems is negligible.
Previous studies have demonstrated the presence of circulating antibodies to MAA-modified proteins in the serum of ALD patients (33). Using immunohistochemical techniques, we showed, for the first time, MAA-modified proteins in the livers of both steatohepatitis and ALD patients. Interestingly, the polyclonal antibody to MAA, and possibly some conformation of the protein, reacted with tissues from both the steatohepatitis and ALD patients. However, the monoclonal antibody (which recognizes a MAA epitope unique to ALD) reacted more in the ALD patient, indicating more deposition of MAA adduct, most likely due to the increased concentration of AA reacting with MDA from membrane lipid peroxidation.
To test the hypothesis that MAA-modified proteins are generated by liver tissues, we used a murine model of PCLSs. For these studies, PCLSs were incubated with ethanol and tested for the presence of MAA-modified proteins. Western blot and immunohistochemical analysis revealed the presence of modified proteins that localized to the macrophage, as evidenced by colocalization with the F4/80 macrophage marker, which may be due to uptake by scavenger receptors (8, 10). These results would also support the role of MAA-modified proteins in antigen processing and presentation to initiate immune responses. Unique bands following immunoprecipitation with anti-MAA were cut out and sent for protein analysis by mass spectroscopy, which revealed the presence of proteasome subunit β, putative trypsin-6, and glial fibrillary acidic protein. This is interesting, because modification of the proteasome would prevent protein degradation, possibly amplifying immune responses to clear these proteins by other immunological mechanisms. In fact, proteasome activity has been shown to be downregulated following chronic ethanol administration (43). Consistent with these observations is our previous report showing that in vitro stimulation of multiple cell types with MAA-modified proteins increased uptake but decreased degradation of the adducted proteins (8, 51). However, further studies are needed to identify other MAA-modified proteins/macromolecules and the function of these altered materials.
MAA-modified liver proteins from PCLSs were further evaluated to determine whether they induced immune responses. After immunization with ethanol-PCLS antigen, circulating antibodies to both MAA and liver proteins were increased. Antibody production is driven by the presence of T helper cells. Prevention and onset of autoimmune liver disease by the induction of tolerance to normal liver proteins can be accomplished by Treg cells (24). T cell involvement was examined first by proliferation assays, which showed expansion of CD8+ T cells against both control-PCLS antigen and MAA-Alb. While the CD8+ T cell recall proliferative response was brisk, isolated primed CD4+ T cells responded with limited proliferation to liver protein homogenates. Other investigators have found that although CD4+ memory cells can proliferate in response to restimulation, they divide for a shorter period of time than primary responding cells. The reduced proliferation of CD4+ T cells was most probably a consequence of the reactivated memory cells producing a different cytokine response (increased IFN-γ and decreased IL-2). Thus CD4+ memory cells do not expand as exuberantly as memory CD8+ T cells in vitro (3, 26, 32).
Proliferation against normal liver PCLSs strongly suggests that the damage to PCLSs was induced by CD8+ T cell-mediated cells. Therefore, CD8+ T cells were isolated from these animals and incubated with control PCLSs as target tissues. Interestingly, cytotoxic responses to the ethanol liver slice were actually lower in T cells from animals immunized with ethanol-PCLS antigen than pair- or chow-fed animals, suggesting that other mechanisms are also involved. Thus we examined cytokine involvement in this suppression and found that IL-6 was significantly increased in the T cell cultures from the ethanol-fed mice on PCLSs from the livers of ethanol-fed mice. The ability of IL-6 to increase tolerance in the liver and prevent damage has been well documented and most likely is the reason less cytotoxicity was observed (11, 13). For a closer look at the T cell involvement, nonparenchymal cells were isolated from the livers of control- and ethanol-PCLS antigen-injected mice and phenotyped for cell surface markers. Liver T cell profiles showed an increase in both CD4+ and CD8+ cells in the ethanol-PCLS-injected compared with control-injected mice, demonstrating the involvement of these cells in this response. There was no indication of a shift in Th1 or Th2 responses, possibly due to the cytokines involved, resulting in a decreased immune response. Most interesting was the Treg cell profile, which revealed a shift toward a Th17 response, suggesting initiation of autoimmunity. However, there was no damage to the liver as determined by AST data. Future studies will be done to more closely examine cytokine production in the liver, production of chemokine and adhesion molecules, and manipulation cytokine, chemokine, and adhesion molecule production to drive the response using a second-hit model by initiating damage.
Specific T cells from the spleens of mice immunized with ethanol-PCLS antigen were evaluated for their ability to traffic back to the livers of naïve mice using a CD45.1/CD45.2 model. T cells from the spleens of CD45.1 mice immunized with ethanol-exposed CD45.1 PCLSs indeed trafficked back to the livers of CD45.2 mice, as detected by both flow cytometry and immunohistochemistry techniques. Important to the observations in this study, no damage was observed, leaving many unanswered questions as to how cells that are shown to be cytotoxic in vitro do not destroy the liver in vivo. Possible explanations include the following. 1) No stimulation occurs, as no danger signal is initiated in vivo. The danger signal is upregulated when the tissue is cut, which makes the in vitro assays work, but there is no damage in the naïve mouse liver to which these cells can bind. 2) T cells may be changing their phenotype from an inflammatory to a suppressive response. These cells are possibly changing to suppressor (Treg) cells by receiving a signal to remain quiescent. An explanation for lack of damage could be that T cells are shifted into CD25+Foxp3+ (Treg) cells and provide signals to suppress immune responses (1). More specific T cell experiments are warranted to determine the possible role of Treg cells in this response. 3) There may exist some undescribed mechanism(s) that we may be able to delineate using this model system in future studies.
In this study we found that PCLS metabolism of ethanol produces MAA adducts in situ. PCLSs containing these in situ-produced MAA-modified liver adducts were determined to be immunogenic following injection into naïve animals. T cells from these immunized mice were clearly able to damage liver tissues in situ/in vitro, but not in vivo, indicating involvement of other pathways in protecting the liver from injury. Increased IL-6 production in response to ethanol could be responsible for this shift from cytotoxic to suppressor cell phenotype or a decrease in costimulatory molecules; this idea is supported by the finding of Th17 cells in the liver following injections with ethanol-PCLS antigen. Trafficking studies show that liver-reactive T cells are attracted to the liver, yet they remain unable to elicit a cytotoxic response. More work is necessary to examine whether the phenotype of these T cells is altered in the livers of normal mice, resulting in suppression of cytotoxic T cells in the liver. Since products of alcohol metabolism can initiate an immune response to liver self-proteins (initiate the breaking of immune tolerance), a more mechanistic approach can be used to develop new strategies of prevention and treatment of a variety of immune-based liver diseases.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA-10435, R37 AA-07818, and R21 AA-15505-01A2, the Department of Veterans Affairs National Merit Review Program, and the Department of Internal Medicine at the UNMC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.D., L.W.K., D.R.A., T.R.M., and G.M.T. conceived and designed research; M.J.D., J.R.B., J.D.V., G.E.T., and C.D.H. performed experiments; M.J.D., B.M.W., J.R.B., J.D.V., C.D.H., and G.M.T. analyzed data; M.J.D., B.M.W., J.R.B., L.W.K., C.D.H., D.R.A., T.R.M., and G.M.T. interpreted results of experiments; M.J.D. prepared figures; M.J.D. and B.M.W. drafted manuscript; M.J.D., B.M.W., J.R.B., J.D.V., L.W.K., C.D.H., D.R.A., T.R.M., and G.M.T. edited and revised manuscript; M.J.D., B.M.W., J.R.B., J.D.V., L.W.K., G.E.T., C.D.H., D.R.A., T.R.M., and G.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Janice A. Taylor and James R. Talaska [Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center (UNMC)] for assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for support of the Core Facility. We also thank the team members of the Experimental Immunology Laboratory at the UNMC.
REFERENCES
- 1.An Haack I, Derkow K, Riehn M, Rentinck MN, Kühl AA, Lehnardt S, Schott E. The role of regulatory CD4 T cells in maintaining tolerance in a mouse model of autoimmune hepatitis. PLoS One 10: e0143715, 2015. doi: 10.1371/journal.pone.0143715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RJ, Krasner N, Eddleston AL, Williams R, Tee DE, Doniach D, Kennedy LA, Batchelor JR. Histocompatibility antigens, autoantibodies, and immunoglobulins in alcoholic liver disease. BMJ 2: 727–729, 1976. doi: 10.1136/bmj.2.6038.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berard M, Tough DF. Qualitative differences between naïve and memory T cells. Immunology 106: 127–138, 2002. doi: 10.1046/j.1365-2567.2002.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charbonneau H, Tonks NK, Walsh KA, Fischer EH. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci USA 85: 7182–7186, 1988. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chedid A, Mendenhall CL, Moritz TE, French SW, Chen TS, Morgan TR, Roselle GA, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI, Samanta A, Weesner RE, Henderson WG. Cell-mediated hepatic injury in alcoholic liver disease. Veterans Affairs Cooperative Study Group 275. Gastroenterology 105: 254–266, 1993. doi: 10.1016/0016-5085(93)90034-A. [DOI] [PubMed] [Google Scholar]
- 6.Clemens DL, Calisto LE, Sorrell MF, Tuma DJ. Ethanol metabolism results in a G2/M cell-cycle arrest in recombinant Hep G2 cells. Hepatology 38: 385–393, 2003. doi: 10.1053/jhep.2003.50332. [DOI] [PubMed] [Google Scholar]
- 7.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res 22: 1927–1942, 1998. [PubMed] [Google Scholar]
- 8.Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC 3rd, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol 68: 1423–1430, 2005. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- 9.Duryee MJ, Klassen LW, Thiele GM. Immunological response in alcoholic liver disease. World J Gastroenterol 13: 4938–4946, 2007. doi: 10.3748/wjg.v13.i37.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, Thiele GM. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Biosci 9: 3145–3155, 2004. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- 11.El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol 1: 205–211, 2004. [PubMed] [Google Scholar]
- 12.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 13.Grant CR, Liberal R. Liver immunology: how to reconcile tolerance with autoimmunity. Clin Res Hepatol Gastroenterol 41: 6–16, 2016. doi: 10.1016/j.clinre.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Guyot C, Combe C, Balabaud C, Bioulac-Sage P, Desmoulière A. Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J Hepatol 46: 142–150, 2007. doi: 10.1016/j.jhep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease. III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol 287: G310–G314, 2004. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 16.Howell CD, Yoder TD. Murine experimental autoimmune hepatitis: nonspecific inflammation due to adjuvant oil. Clin Immunol Immunopathol 72: 76–82, 1994. doi: 10.1006/clin.1994.1109. [DOI] [PubMed] [Google Scholar]
- 17.Israel Y, Hurwitz E, Niemelä O, Arnon R. Monoclonal and polyclonal antibodies against acetaldehyde-containing epitopes in acetaldehyde-protein adducts. Proc Natl Acad Sci USA 83: 7923–7927, 1986. doi: 10.1073/pnas.83.20.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol 27: 23–27, 2002. doi: 10.1016/S0741-8329(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 19.Klassen LW, Thiele GM, Duryee MJ, Schaffert CS, DeVeney AL, Hunter CD, Olinga P, Tuma DJ. An in vitro method of alcoholic liver injury using precision-cut liver slices from rats. Biochem Pharmacol 76: 426–436, 2008. doi: 10.1016/j.bcp.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 174: 21–34, 2000. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 21.Laskin CA, Vidins E, Blendis LM, Soloninka CA. Autoantibodies in alcoholic liver disease. Am J Med 89: 129–133, 1990. doi: 10.1016/0002-9343(90)90288-O. [DOI] [PubMed] [Google Scholar]
- 22.Lerche-Langrand C, Toutain HJ. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology 153: 221–253, 2000. doi: 10.1016/S0300-483X(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 23.Li CJ, Nanji AA, Siakotos AN, Lin RC. Acetaldehyde-modified and 4-hydroxynonenal-modified proteins in the livers of rats with alcoholic liver disease. Hepatology 26: 650–657, 1997. doi: 10.1002/hep.510260317. [DOI] [PubMed] [Google Scholar]
- 24.Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Regulatory T cells: mechanisms of suppression and impairment in autoimmune liver disease. IUBMB Life 67: 88–97, 2015. doi: 10.1002/iub.1349. [DOI] [PubMed] [Google Scholar]
- 25.Lohse AW, Manns M, Dienes HP, Meyer zum Büschenfelde KH, Cohen IR. Experimental autoimmune hepatitis: disease induction, time course and T-cell reactivity. Hepatology 11: 24–30, 1990. doi: 10.1002/hep.1840110106. [DOI] [PubMed] [Google Scholar]
- 26.MacLeod MK, McKee A, Crawford F, White J, Kappler J, Marrack P. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci USA 105: 14521–14526, 2008. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews S, Xu M, Wang H, Bertola A, Gao B. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 306: G819–G823, 2014. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niemelä O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med 31: 1533–1538, 2001. doi: 10.1016/S0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 29.Niemelä O, Juvonen T, Parkkila S. Immunohistochemical demonstration of acetaldehyde-modified epitopes in human liver after alcohol consumption. J Clin Invest 87: 1367–1374, 1991. doi: 10.1172/JCI115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niemelä O, Parkkila S, Pasanen M, Iimuro Y, Bradford B, Thurman RG. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res 22: 2118–2124, 1998. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 31.Olinga P, Hof IH, Merema MT, Smit M, de Jager MH, Swart PJ, Slooff MJ, Meijer DK, Groothuis GM. The applicability of rat and human liver slices to the study of mechanisms of hepatic drug uptake. J Pharmacol Toxicol Methods 45: 55–63, 2001. doi: 10.1016/S1056-8719(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 32.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol 183: 2382–2389, 2009. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Aricó S, Sartori M, Bellomo G, Klassen LW, Thiele GM, Tuma DJ, Albano E. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology 31: 878–884, 2000. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 34.Schaffert CS, Todero SL, McVicker BL, Tuma PL, Sorrell MF, Tuma DJ. WIF-B cells as a model for alcohol-induced hepatocyte injury. Biochem Pharmacol 67: 2167–2174, 2004. doi: 10.1016/j.bcp.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82: 518–529, 2015. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol 168: 4827–4831, 2002. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 37.Szabo G, Saha B. Alcohol’s effect on host defense. Alcohol Res 37: 159–170, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terabayashi H, Kolber MA. The generation of cytotoxic T lymphocytes against acetaldehyde-modified syngeneic cells. Alcohol Clin Exp Res 14: 893–899, 1990. doi: 10.1111/j.1530-0277.1990.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 39.Thiele GM, Duryee MJ, Anderson DR, Klassen LW, Mohring SM, Young KA, Benissan-Messan D, Sayles H, Dusad A, Hunter CD, Sokolove J, Robinson WH, O’Dell JR, Nicholas AP, Tuma DJ, Mikuls TR. Malondialdehyde-acetaldehyde adducts and anti-malondialdehyde-acetaldehyde antibodies in rheumatoid arthritis. Arthritis Rheumatol 67: 645–655, 2015. doi: 10.1002/art.38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiele GM, Duryee MJ, Willis MS, Tuma DJ, Radio SJ, Hunter CD, Schaffert CS, Klassen LW. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res 34: 2126–2136, 2010. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis 24: 273–287, 2004. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 42.Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, Klassen LW. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res 22: 1731–1739, 1998. doi: 10.1111/j.1530-0277.1998.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomes PG, Trambly CS, Thiele GM, Duryee MJ, Fox HS, Haorah J, Donohue TM Jr. Proteasome activity and autophagosome content in liver are reciprocally regulated by ethanol treatment. Biochem Biophys Res Commun 417: 262–267, 2012. doi: 10.1016/j.bbrc.2011.11.097. [DOI] [PubMed] [Google Scholar]
- 44.Trimble G, Zheng L, Mishra A, Kalwaney S, Mir HM, Younossi ZM. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther 38: 596–602, 2013. doi: 10.1111/apt.12432. [DOI] [PubMed] [Google Scholar]
- 45.Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med 32: 303–308, 2002. doi: 10.1016/S0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 46.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology 23: 872–880, 1996. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 47.van de Bovenkamp M, Groothuis GM, Draaisma AL, Merema MT, Bezuijen JI, van Gils MJ, Meijer DK, Friedman SL, Olinga P. Precision-cut liver slices as a new model to study toxicity-induced hepatic stellate cell activation in a physiologic milieu. Toxicol Sci 85: 632–638, 2005. doi: 10.1093/toxsci/kfi127. [DOI] [PubMed] [Google Scholar]
- 48.Viitala K, Israel Y, Blake JE, Niemelä O. Serum IgA, IgG, and IgM antibodies directed against acetaldehyde-derived epitopes: relationship to liver disease severity and alcohol consumption. Hepatology 25: 1418–1424, 1997. doi: 10.1002/hep.510250619. [DOI] [PubMed] [Google Scholar]
- 49.Viitala K, Makkonen K, Israel Y, Lehtimäki T, Jaakkola O, Koivula T, Blake JE, Niemelä O. Autoimmune responses against oxidant stress and acetaldehyde-derived epitopes in human alcohol consumers. Alcohol Clin Exp Res 24: 1103–1109, 2000. doi: 10.1111/j.1530-0277.2000.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 50.Wilkin RJ, Lalor PF, Parker R, Newsome PN. Murine models of acute alcoholic hepatitis and their relevance to human disease. Am J Pathol 186: 748–760, 2016. doi: 10.1016/j.ajpath.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Willis MS, Klassen LW, Tuma DJ, Thiele GM. Malondialdehyde-acetaldehyde-haptenated protein induces cell death by induction of necrosis and apoptosis in immune cells. Int Immunopharmacol 2: 519–535, 2002. doi: 10.1016/S1567-5769(01)00195-3. [DOI] [PubMed] [Google Scholar]
- 52.Worrall S, de Jersey J, Wilce PA, Seppä K, Hurme L, Sillanaukee P. Relationship between alcohol intake and immunoglobulin A immunoreactivity with acetaldehyde-modified bovine serum albumin. Alcohol Clin Exp Res 20: 836–840, 1996. doi: 10.1111/j.1530-0277.1996.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 53.Worrall S, Thiele GM. Protein modification in ethanol toxicity. Adverse Drug React Toxicol Rev 20: 133–159, 2001. [PubMed] [Google Scholar]
- 54.Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology 115: 686–692, 1998. doi: 10.1016/S0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama H, Ishii H, Nagata S, Kato S, Kamegaya K, Tsuchiya M. Experimental hepatitis induced by ethanol after immunization with acetaldehyde adducts. Hepatology 17: 14–19, 1993. doi: 10.1002/hep.1840170105. [DOI] [PubMed] [Google Scholar]
- 56.Yüksel M, Laukens D, Heindryckx F, Van Vlierberghe H, Geerts A, Wong FS, Wen L, Colle I. Hepatitis mouse models: from acute-to-chronic autoimmune hepatitis. Int J Exp Pathol 95: 309–320, 2014. doi: 10.1111/iep.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zetterman RK, Sorrell MF. Immunologic aspects of alcoholic liver disease. Gastroenterology 81: 616–624, 1981. [PubMed] [Google Scholar]