Abstract
Multiple metabolic pathways exhibit time-of-day-dependent rhythms that are controlled by the molecular circadian clock. We have shown that chronic alcohol is capable of altering the molecular clock and diurnal oscillations in several elements of hepatic glycogen metabolism (19, 44). Herein, we sought to determine whether genetic disruption of the hepatocyte clock differentially impacts hepatic glycogen content in chronic alcohol-fed mice. Male hepatocyte-specific BMAL1 knockout (HBK) and littermate controls were fed control or alcohol-containing diets for 5 wk to alter hepatic glycogen content. Glycogen displayed a significant diurnal rhythm in livers of control genotype mice fed the control diet. While rhythmic, alcohol significantly altered the diurnal oscillation of glycogen in livers of control genotype mice. The glycogen rhythm was mildly altered in livers of control-fed HBK mice. Importantly, glycogen content was arrhythmic in livers of alcohol-fed HBK mice. Consistent with these changes in hepatic glycogen content, we observed that some glycogen and glucose metabolism genes were differentially altered by chronic alcohol consumption in livers of HBK and littermate control mice. Diurnal rhythms in glycogen synthase (mRNA and protein) were significantly altered by alcohol feeding and clock disruption. Alcohol consumption significantly altered Gck, Glut2, and Ppp1r3g rhythms in livers of control genotype mice, with diurnal rhythms of Pklr, Glut2, Ppp1r3c, and Ppp1r3g further disrupted (dampened or arrhythmic) in livers of HBK mice. Taken together, these findings show that chronic alcohol consumption and hepatocyte clock disruption differentially influence the diurnal rhythm of glycogen and various key glycogen metabolism-related genes in the liver.
NEW & NOTEWORTHY We report that circadian clock disruption exacerbates alcohol-mediated alterations in hepatic glycogen. We observed differential responsiveness in diurnal rhythms of glycogen and glycogen metabolism genes and proteins in livers of hepatocyte-specific BMAL1 knockout and littermate control mice fed alcohol. Our findings provide new insights into potential mechanisms by which alcohol alters glycogen, an important energy source for liver and other organs.
Keywords: alcohol/ethanol, BMAL1, circadian clock, glycogen, liver
INTRODUCTION
Alcohol use can cause pathophysiological changes in the liver, including the early steatosis or fatty liver stage, which can progress to the more serious conditions of alcoholic steatohepatitis (severe fatty liver with lobular inflammation), fibrosis, and cirrhosis with prolonged heavy alcohol consumption. Numerous cellular and metabolic disturbances, including redox imbalance, oxidative stress, lipid peroxidation, and dysregulated cytokine networks, are proposed to underpin alcohol-induced hepatotoxicity (11, 26, 27). Chronic alcohol consumption also impairs energy production in the liver. Seminal work by Cunningham and colleagues (3, 45, 49) demonstrated that chronic alcohol consumption interferes with glycolytic ATP production as hepatocytes exposed to alcohol chronically lack sufficient substrate, i.e., glucose. Glucose is imported into hepatocytes by plasma membrane glucose transporters and can be increased in hepatocytes by gluconeogenesis and breakdown of glycogen, the intracellular storage form of glucose. Importantly, alcohol consumption lowers hepatic glycogen content (45, 46), which is predicted to impair ATP production and energy-dependent cellular repair mechanisms in the liver (12). Indeed, hepatocytes from alcohol-fed animals are more sensitive to secondary insults, like hypoxia and oxidants (38, 51). Thus additional study on how alcohol disrupts hepatic glycogen metabolism, an important energy store, will improve understanding of the early events contributing to alcohol toxicity.
Hepatic glycogen plays a central role in the regulation of blood glucose homeostasis, in addition to serving as an important fuel reservoir in both muscle and liver. Acute and chronic alcohol consumption result in lower glycogen levels in the liver (45, 46), and patients with alcoholic liver cirrhosis have reduced hepatic glycogen stores (22); however, the mechanisms responsible for these effects are poorly understood. Building on these previous studies, we found that chronic alcohol consumption alters time-of-day-dependent oscillations in glycogen metabolism at the gene, protein, enzyme activity, and metabolite level (44). Previous studies show that some components of glycogen metabolism might be regulated by the molecular circadian clock (14, 23, 50). Moreover, our laboratory and others (19, 41, 52) have shown that chronic alcohol consumption alters clock gene rhythms in the liver. Taken together, these results suggest that perturbation of the liver circadian clock by chronic alcohol consumption may disrupt time-of-day-dependent variations in various aspects of glycogen metabolism, including glycogen content and related glycogen metabolism genes and proteins.
An ever growing body of scientific evidence suggests that cellular circadian clocks control autonomous and self-sustaining 24-h rhythms in numerous behavioral and metabolic processes (2, 6). The circadian clock, located in all tissues, consists of interconnected transcriptional-translational positive and negative feedback loops that generate 24-h rhythms in metabolism (21). The positive arm of the molecular clock consists of the transcription factors, circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1), which dimerize and drive transcription of other clock genes; e.g., Period (Per) and Cryptochrome (Cry). Conversely, PER and CRY proteins form the negative arm of the clock and function by inhibiting CLOCK-BMAL1 binding to DNA. The CLOCK-BMAL1 heterodimer also promotes transcription of other noncore clock and many metabolic genes. These rhythms are believed to be highly beneficial as clocks function to separate and compartmentalize metabolic processes to the appropriate time of day; e.g., sleep or awake and feeding or fasting periods.
Disruption in circadian clocks by environmental means (shift work, high-fat diets) or genetic variants (SNPs, gene deletions, epigenetic alterations) is proposed to increase risk for disease (17, 20, 32). For example, shift workers are at higher risk for dyslipidemia, obesity, type 2 diabetes mellitus, and hypertension (1, 29, 31). Recent studies also show that night-shift workers are at higher risk for alcohol-induced liver injury due to increased alcohol-induced gut leakiness and inflammation (42). Consistent with this observation, whole body ClockΔ19 mutant mice have increased sensitivity to alcohol toxicity (48). Studies also show that chronic alcohol alters time-of-day rhythms in lipid and alcohol metabolism genes (19, 52). However, the impact circadian disruption, chronic alcohol, or both have on hepatic glycogen, an important source of energy for cells, is lacking.
Therefore, the purpose of this study was to fill this gap in knowledge and test the hypothesis that circadian disruption (by genetic means) exacerbates alcohol-mediated alterations in hepatic glycogen content. To achieve these goals, we generated a hepatocyte-specific BMAL1 knockout (HBK) mouse model. Male mice, both HBK and their control genotype littermates, were fed either a control or alcohol (ethanol)-containing liquid diet for 5 wk (19, 44). We observed that the diurnal rhythm in hepatic glycogen was significantly altered by chronic alcohol consumption. Alcohol feeding significantly decreased the mesor (mean content) and phase-shifted the acrophase (peak) of the glycogen rhythm in livers of control genotype mice, with a complete loss of the glycogen rhythm in livers of alcohol-fed HBK mice. Chronic alcohol feeding also disrupted the diurnal expression patterns of several glycogen and glucose metabolism genes in the liver, including Gck, Glut2, Gys2, Pklr, Ppp1r3b, Ppp1r3c, and Ppp1r3g. Moreover, we found that several of these genes are indeed regulated by the hepatocyte circadian clock. Similar findings were observed for protein levels of glycogen synthase (GS), the rate-limiting enzyme of glycogen synthesis. Collectively, these observations provide new insights into the role the circadian clock transcription factor BMAL1 plays in regulating hepatic glycogen metabolism following chronic alcohol consumption.
MATERIALS AND METHODS
Mice and liquid diet feeding regimen.
Eight-week-old male HBK mice [Alb-Cre(+/−)/BMAL1(flox/flox)] and their control genotype littermates [Alb-Cre(−/−)/BMAL1(flox/flox)] were weight matched and pair fed nutritionally adequate diets based on the original Lieber-DeCarli control and alcohol (ethanol)-containing liquid diets (24), as described previously (19, 44). The iso-caloric liquid diets (control: F1259SP and alcohol: F1258SP) were purchased from Bio-Serv (Frenchtown, NJ), and the alcohol-containing diet was prepared such that mice received 22% of total daily calories as ethanol (3% ethanol, wt/vol). Diets were replaced daily between Zeitgeber time (ZT) 10–12 (ZT 0 = lights on and ZT 12 = lights off) to minimize disruptions in feeding patterns. Food consumption was thus measured for the previous 24-h day. Mice were single-housed under a 12:12-h light-dark cycle in a temperature- (22–23°C) and humidity-controlled environment and maintained on the liquid diets for 5 wk. At the end of the feeding protocol, mice were euthanized by decapitation, and livers were collected at 4-h intervals at ZT 3, 7, 11, 15, 19, and 23. Tissues were immediately frozen in liquid nitrogen and stored at −80°C. A portion of liver was also placed in RNAlater (Life Technologies) to protect RNA from degradation and stored at −20°C. A small piece of liver was also placed in 10% formalin for histology. Trunk blood was collected in EDTA-coated 1.5-ml tubes, placed on ice, and used to prepare plasma. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and were in compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., National Academy of Sciences, 2011).
Biochemical measurements on plasma and histology.
Plasma samples were assayed for alcohol concentration, alanine aminotransferase (ALT) activity, and glucose content using commercially available reagent sets from Pointe Scientific (Canton, MI). Formalin-fixed liver tissue was embedded in paraffin, sectioned (5 µm), and stained with hematoxylin and eosin for visualization of steatosis. Images were obtained by using a Carl Zeiss Axiovert 10 inverted microscope (Carl Zeiss Microscopy) and Q-Capture Pro7 software (Q Imaging).
Liver glycogen measurement.
Homogenates (20 mg/ml in 1.0 M KOH) were prepared from frozen livers and heated for 20 min at 70°C to solubilize tissues. Samples (200 µl) of the digested liver tissue were treated with 34 µl glacial acetic acid to adjust pH and incubated overnight with amyloglucosidase (Roche) at 37°C to hydrolyze glycogen (13). The concentration of free glucose generated in samples was determined using the hexokinase and glucose-6-phosphate (G6P) dehydrogenase method (7, 15). Bovine glycogen standards (Sigma Aldrich) were treated in the same manner as the liver samples. Results are expressed as milligrams of glycogen per gram liver weight.
Western blot analyses.
Samples for BMAL1 protein analysis were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific), supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Western blot for BMAL1 was performed using an anti-BMAL1 antibody produced in rabbit (cat. no. SAB4300614, Sigma Aldrich). Equal amounts of nuclear protein were separated on 8% polyacrylamide gels, transferred to nitrocellulose membranes (43), and incubated with primary antibody overnight (1:1,000) at 4°C. Protein detection was achieved by using the appropriate secondary antibody, followed by enhanced chemiluminescence detection. Membranes were stained with Ponceau S to confirm equal protein loading for BMAL1 blots. Liver homogenates used for GS analyses (total and phosphorylated) were prepared in 0.25 M sucrose buffer, pH 7.4, supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Western blots were performed using anti-GS antibody (cat. no. 3886) and an anti-phospho-GS antibody (Ser640/641, cat. no. 3891) from Cell Signaling Technologies. An anti-β-actin antibody (cat. no. A5441) was used as a loading control (1:5,000). Equal amounts of cytosolic protein were separated on 7% Criterion TGX Precast Gels (Bio-Rad Laboratories), transferred to nitrocellulose membranes, and incubated with primary antibodies overnight (1:2,000) at 4°C. Immunoreactive proteins were visualized using appropriate secondary antibodies from Li-Cor (Lincoln, NE). The secondary antibody used for total and phosphorylated GS was IRDye 680RD (rabbit, cat. no. 926–68071), and the secondary antibody used for β-actin was 800CW (mouse, cat. no. 926–32210). Fluorescence was detected and quantified using a Li-Cor Odyssey imaging system. Protein abundances are expressed as the fold change set relative to control genotype mice fed control diet at ZT 3.
RNA isolation and gene expression.
RNA was isolated from RNAlater preserved (liver) or frozen (muscle, heart, and adipose) tissues using TRI-Reagent (Sigma-Aldrich). Isolated RNA was DNase treated using a DNase Treatment Kit (Life Technologies) and converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Gene expression analysis was performed by qPCR using Taqman Gene Expression assays containing specific primers and probes (Applied Biosystems). The relative levels of target gene PCR products were normalized to those of the housekeeping gene Gapdh, except for liver Bmal1, which was normalized to Ppia. Relative gene expression was determined using the ΔΔCt method (25), and gene expression data are expressed as the fold change set relative to the trough of control genotype mice fed control diet.
Statistical analysis.
Two-factor and three-factor ANOVA were performed to determine statistical significance of the main effects of genotype, diet, and time, as well as interactions for genotype × diet, genotype × time, diet × time, and genotype × diet × time on experimental measurements. Repeated-measures ANOVA was used to determine statistical significance of body weight measurements (weeks 2–5). Cosinor analysis was performed to determine whether experimental measures where rhythmic (fit a cosine wave) during the course of the day, as well as to obtain the key chronobiological parameters of mesor, amplitude, and acrophase (peak or trough) of the rhythm. Data were fitted to a cosine wave equation, f(t) = mesor + amplitude × cos[(2πt/T) + acrophase], in SPSS (IBM) using a nonlinear regression module (33). The mesor (midline estimating statistic of rhythm) = mean of the oscillation; amplitude = 1/2 the distance between the peak and the trough; t = time-point (ZT 3, 7, 11, 15, 19, or 23); T = the period (fixed to 24 h); and acrophase = the ZT time of the cosine maximum. Rhythmicity was determined using a linear regression model, f(t) = M + cos (2πt/T) + sin (2πt/T), and data were considered rhythmic if the P value of the R2 was ≤ 0.05. Student’s t-test was used to compare the parameter estimates among the four experimental treatment groups. Data are presented as means ± SE. Data that significantly fit a cosine function are represented in graphs by solid lines, whereas nonsignificant data are represented by dashed lines (see Figs. 3–7). Sample sizes are included in Figs. 1–7 legends, and statistical significance was set at P ≤ 0.05.
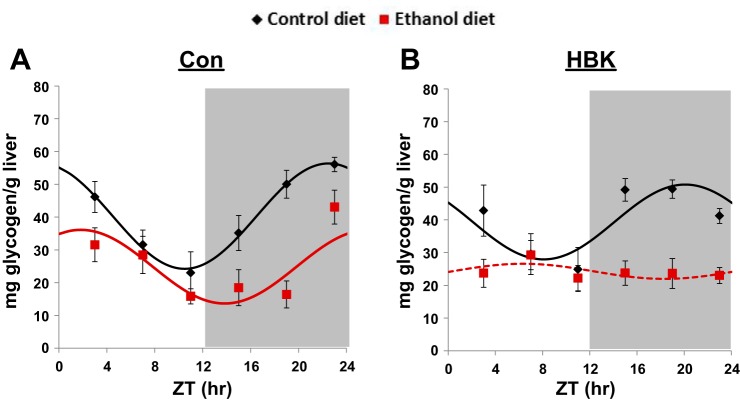
Fig. 3.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on hepatic glycogen content. Male hepatocyte-specific BMAL1 knockout (HBK) and control genotype (Con) littermates, 8 wk of age, were fed a control or alcohol-containing liquid diet for 5 wk. Livers were collected every 4 h at ZT 3, 7, 11, 15, 19, and 23 (ZT 0: lights on; ZT 12: lights off, gray shading). A and B: hepatic glycogen content for Con and HBK mice, respectively. Data were fitted to a cosine function and are expressed as means ± SE for n = 5–8 mice/genotype/diet/time point. Solid lines indicate a significant cosine fit, whereas dashed lines indicate a nonsignificant fit. Results for ANOVA and Cosinor analyses are provided in Tables 3 and 4, respectively.
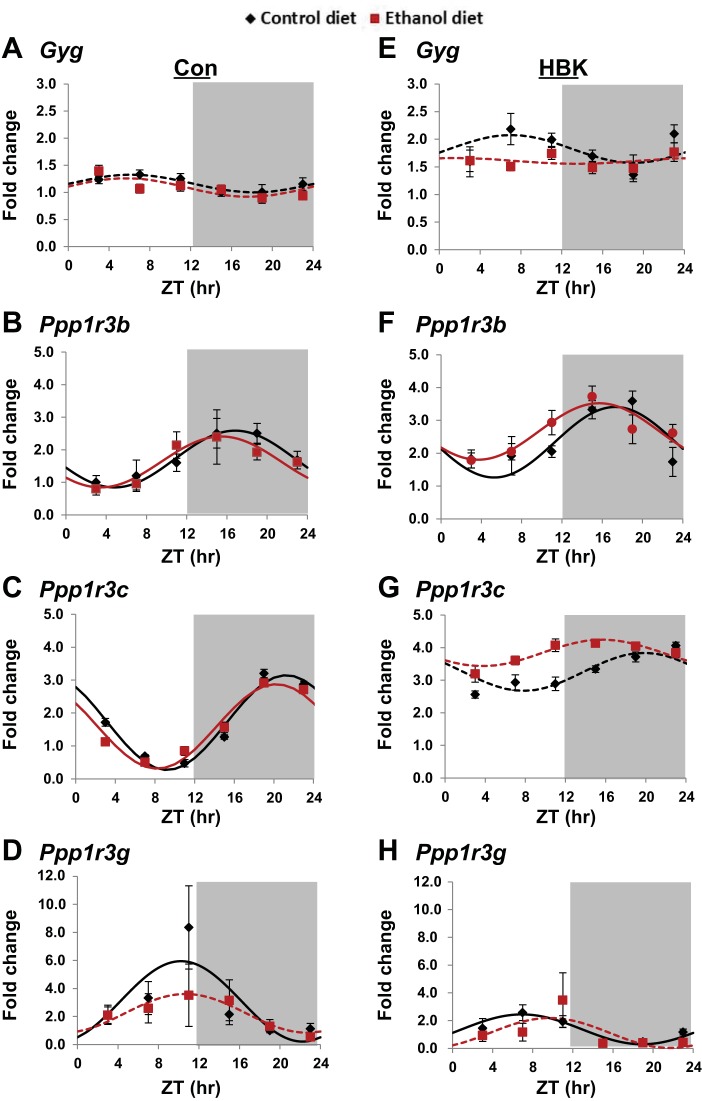
Fig. 7.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on glycogenin and glycogen particle targeting proteins. Diurnal gene expression profiles of glycogenin (Gyg; A and E), protein phosphatase regulatory subunit 3B (Ppp1r3b; B and F), protein phosphatase regulatory subunit 3C (Ppp1r3c; C and G), and protein phosphatase regulatory subunit 3G (Ppp1r3g; D and H) were determined in livers collected from control and alcohol-fed hepatocyte-specific BMAL1 knockout (HBK; E–H) and control genotype (Con; A–D) mice at ZT 3, 7, 11, 15, 19, and 23 h (ZT 0: lights on; ZT 12: lights off, gray shading). mRNA levels were normalized to Gapdh and are displayed as a fold-change from the trough in Con mice fed the control diet. Data were fitted to a cosine function and expressed as means ± SE for n = 5–8 mice/genotype/diet/time point. Solid lines indicate a significant cosine curve fit, whereas dashed lines indicate a nonsignificant fit. Results for Cosinor and ANOVA analyses are provided in Tables 5 and 6, respectively.
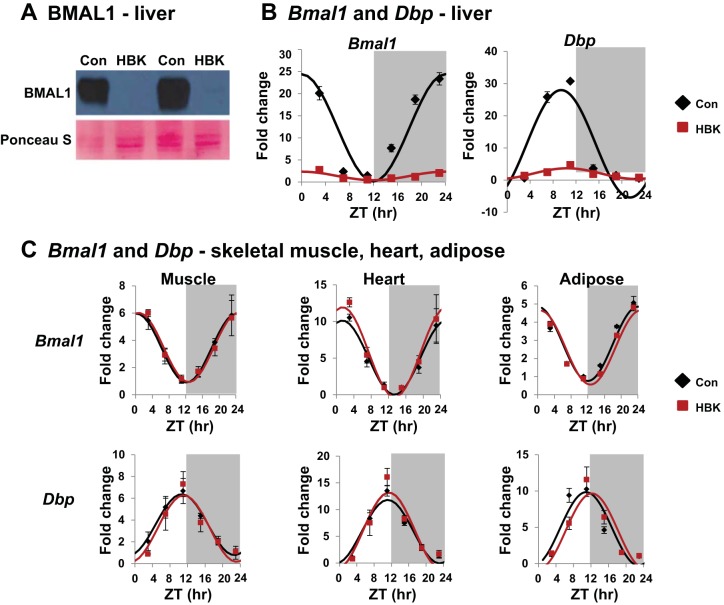
Fig. 1.
Validating the hepatocyte-specific BMAL1 knockout (HBK) mouse model. Livers from male mice [HBK and control genotype (Con) littermates, 12–14 wk of age] fed a standard laboratory chow diet and maintained under a 12:12-h light-dark schedule were collected every 4 h at ZT 3, 7, 11, 15, 19, and 23 (ZT 0: lights on; ZT 12: lights off, gray shading). A: BMAL1 protein abundance in liver. B: diurnal gene expression profiles of Bmal1 and Dbp in liver. C: diurnal gene expression profiles of Bmal1 and Dbp in skeletal muscle, heart, and adipose. mRNA levels were normalized to Gapdh, except for Bmal1 in liver, which was normalized to Ppia and displayed as fold change from Con trough. Data were fitted to a cosine function and are expressed as means ± SE for n = 3–4 mice/genotype/time point for gene expression. Results for Cosinor and ANOVA analyses are provided in Tables 1 and 2, respectively.
RESULTS
Hepatocyte-specific deletion of Bmal1 impacts the circadian clock in the liver.
To test our hypothesis, we generated a HBK mouse model. To validate the model, we measured the levels of BMAL1 protein in livers of HBK mice and their littermate controls. As predicted, livers from HBK mice show an absence of BMAL1 protein compared with livers of control mice (Fig. 1A). HBK mice have a significant attenuation in the content and diurnal oscillation of mRNAs encoding for Bmal1 and Dbp (a classic circadian clock target gene; Fig. 1B). Residual Bmal1 mRNA in livers of HBK mice is likely from hepatic nonparenchymal cells that do not express Cre recombinase. In contrast to the liver, circadian clock function in extrahepatic tissues remains intact, as shown by identical high-amplitude rhythms of Bmal1 and Dbp in skeletal muscle, heart, and adipose (epididymal fat) from HBK and littermate control mice (Fig. 1C). Moreover, normal circadian rhythms in locomoter and feeding activity have been reported for HBK mice, demonstrating an intact and functional central circadian clock (23). Together, these results confirm that deletion of Bmal1 is specific to the liver in HBK mice. Cosinor and ANOVA analyses for these data are included in Tables 1 and 2, respectively.
Table 1.
Cosinor analysis of Bmal1 and Dbp gene expression in tissues from chow-fed HBK and control genotype mice
| Rhythmicity |
Cosinor Output Parameters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Gene | Genotype | r2 | P value | Mesor | P value | Amplitude | P value | Acrophase | P value |
| Liver | Bmal1 | Con | 0.89 | <0.001 | 12.27 | <0.001 | 12.15 | <0.001 | 10.64 | <0.001 |
| HBK | 0.46 | <0.001 | 1.37 | 0.97 | 12.83 | |||||
| Dbp | Con | 0.74 | <0.001 | 23.19 | <0.001 | 34.37 | <0.001 | 9.41 | 0.194 | |
| HBK | 0.67 | <0.001 | 4.10 | 3.41 | 10.46 | |||||
| Muscle | Bmal1 | Con | 0.67 | <0.001 | 3.44 | 0.909 | 2.53 | 0.975 | 12.21 | 0.519 |
| HBK | 0.77 | <0.001 | 3.48 | 2.55 | 12.70 | |||||
| Dbp | Con | 0.69 | <0.001 | 1.74 | 0.475 | 1.36 | 0.719 | 10.68 | 0.618 | |
| HBK | 0.61 | <0.001 | 1.57 | 1.49 | 11.13 | |||||
| Heart | Bmal1 | Con | 0.76 | <0.001 | 5.06 | 0.263 | 5.06 | 0.264 | 13.35 | 0.923 |
| HBK | 0.80 | <0.001 | 5.81 | 6.13 | 13.41 | |||||
| Dbp | Con | 0.85 | <0.001 | 5.86 | 0.793 | 5.94 | 0.297 | 11.13 | 0.633 | |
| HBK | 0.74 | <0.001 | 6.06 | 7.11 | 11.43 | |||||
| Adipose | Bmal1 | Con | 0.78 | <0.001 | 2.81 | 0.447 | 2.04 | 0.988 | 11.02 | 0.390 |
| HBK | 0.71 | <0.001 | 2.60 | 2.04 | 11.63 | |||||
| Dbp | Con | 0.79 | <0.001 | 4.79 | 0.684 | 5.05 | 0.918 | 9.88 | 0.078 | |
| HBK | 0.69 | <0.001 | 4.51 | 5.15 | 11.19 | |||||
Table 2.
Two-factor ANOVA of Bmal1 and Dbp gene expression in tissues from chow-fed HBK and control genotype mice
| Genotype |
Time |
Interaction |
|||||
|---|---|---|---|---|---|---|---|
| Tissue | Gene | F | P value | F | P value | F | P value |
| Liver | Bmal1 | 1389.76 | <0.001 | 207.51 | <0.001 | 155.81 | <0.001 |
| Dbp | 182.46 | <0.001 | 102.51 | <0.001 | 71.65 | <0.001 | |
| Muscle | Bmal1 | 0.01 | 0.939 | 17.44 | <0.001 | 0.13 | 0.985 |
| Dbp | 0.40 | 0.531 | 14.59 | <0.001 | 0.28 | 0.920 | |
| Heart | Bmal1 | 1.10 | 0.302 | 28.25 | <0.001 | 0.27 | 0.929 |
| Dbp | 0.40 | 0.530 | 50.77 | <0.001 | 0.67 | 0.647 | |
| Adipose | Bmal1 | 0.45 | 0.506 | 20.36 | <0.001 | 0.16 | 0.977 |
| Dbp | 0.03 | 0.856 | 30.09 | <0.001 | 1.89 | 0.122 | |
Responsiveness to chronic alcohol consumption at the level of select body, liver, and plasma parameters.
HBK and littermate control mice were fed the Lieber-DeCarli control or alcohol-containing liquid diets for 5 wk using a protocol known to disrupt hepatic glycogen metabolism (44). There was no difference in amount of the alcohol diet consumed between HBK and littermate control mice for each 24-h day (Table 3). Ethanol consumption was maintained at 22–23 g ethanol consumed·kg body wt−1·day−1 in both genotypes when the dietary ethanol concentration was held constant at 22% total daily calories during weeks 2–5 of the feeding protocol (data not shown). Blood alcohol concentration was equivalent between alcohol-fed control genotype and HBK mice at two different times of the day (Fig. 2A), with higher levels in the active (ZT 19) vs. inactive (ZT 3) period of the day (Table 3). There was no difference in body weights between HBK and littermate control mice before the feeding study was begun (day 0: control genotype: 23.04 ± 0.23 g, and HBK genotype: 23.01 ± 0.22 g, P = 0.92). Previous studies show that body weights of HBK mice do not differ from that of littermate control mice (23). Body weight was recorded weekly and increased over the course of the study for all four experimental groups following an initial small decrease in body weight during the first week when mice were acclimating to increasing alcohol content in the diet (Fig. 2B). Three-factor ANOVA showed significant main effects of diet and time for body weight during the alcohol feeding period (Table 3).
Table 3.
ANOVA for various body, liver, and serum parameters
| Genotype |
Diet |
Time |
Genotype × Diet |
Genotype × Time |
Diet × Time |
Genotype × Diet × Time |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P value | F | P value | F | P value | F | P value | F | P value | F | P value | F | P value | |
| Ethanol intake, g/kg | 0.35 | 0.554 | n.a. | 6.75 | <0.001 | n.a. | 0.60 | 0.614 | n.a. | n.a. | ||||
| Blood alcohol, mg/dl | 0.01 | 0.921 | n.a. | 9.39 | 0.007 | n.a. | 0.05 | 0.827 | n.a. | n.a. | ||||
| Body weight, g | 2.81 | 0.096 | 42.36 | <0.001 | 92.13 | <0.001 | 0.52 | 0.471 | 0.89 | 0.466 | 6.43 | <0.001 | 0.01 | 1.00 |
| Liver weight, g | 0.03 | 0.859 | 34.45 | <0.001 | n.a. | 0.39 | 0.535 | n.a. | n.a. | n.a. | ||||
| Liver/body weight | 1.55 | 0.216 | 13.92 | <0.001 | n.a. | 1.31 | 0.255 | n.a. | n.a. | n.a. | ||||
| ALT, IU/l | 8.14 | 0.016 | 0.30 | 0.600 | n.a. | 0.01 | 0.908 | n.a. | n.a. | n.a. | ||||
| Glucose, mg/dl | 5.55 | 0.024 | 6.08 | 0.018 | 9.11 | 0.005 | 0.76 | 0.388 | 0.01 | 0.92 | 1.15 | 0.290 | 0.76 | 0.390 |
| Liver glycogen, mg/g | 0.31 | 0.579 | 56.95 | <0.001 | 7.74 | <0.001 | 0.02 | 0.890 | 3.81 | 0.003 | 4.45 | 0.001 | 0.44 | 0.821 |
n.a., Not applicable for this measure. Time = weeks on diets for body weight and ethanol intake, and time of day for blood alcohol and plasma glucose. Body weight, weeks 1–5, was analyzed by repeated-measures ANOVA.
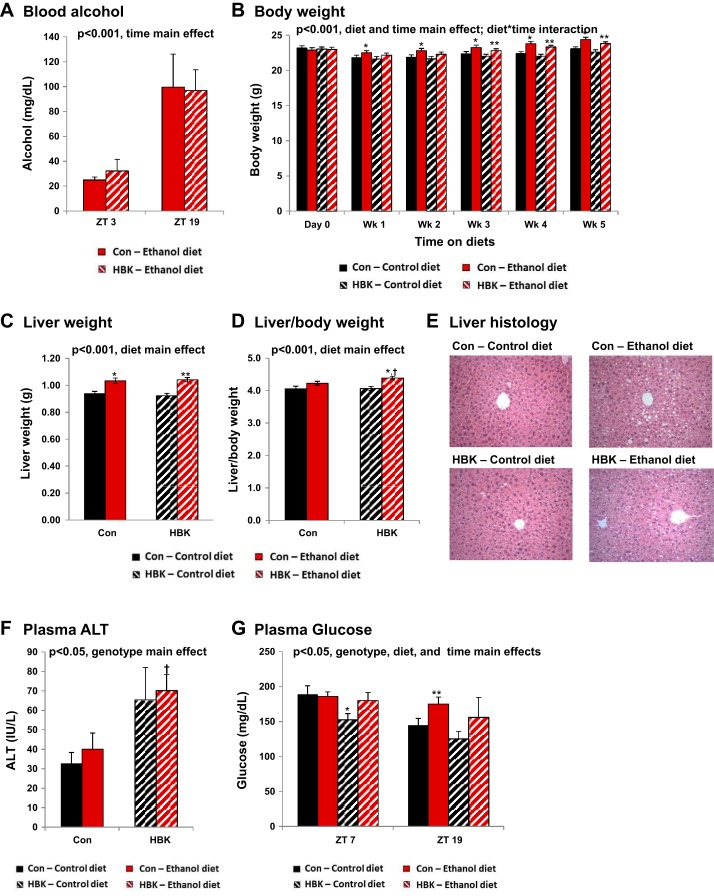
Fig. 2.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on body, liver, and plasma measurements. Male hepatocyte-specific BMAL1 knockout (HBK) and control genotype (Con) littermates, 8 wk of age, were fed a control or alcohol-containing liquid diet for 5 wk. A: blood alcohol concentration (ZT 3 and ZT 19). B: initial and weekly body weight measurements. C: liver weight. D: liver-to-body weight ratio at the end of the feeding study. E: representative liver sections (ZT 11) stained with hematoxylin-eosin for steatosis (×200 magnification). Plasma ALT (ZT 11; F) and glucose levels (ZT 7 and 19; G) were determined at the end of the feeding study. Values are means ± SE. The sample size for body weight, liver weight, liver-to-body weight ratio, and ethanol consumption ranged from n = 30–39 mice/genotype/diet group. Note the large sample size due to the diurnal nature of this study. The sample size for blood alcohol, ALT, and glucose range from n = 3–8 mice/genotype/diet/time point. Solid black bars, Con mice fed control diet; solid red bars, Con mice fed the alcohol diet; striped black bars, HBK mice fed control diet; striped red bars, HBK mice fed the alcohol diet. Results for ANOVA analyses are provided in Table 3. *P < 0.05, compared with Con-control diet. **P < 0.05, compared with HBK-control diet. †P < 0.05, compared with Con-ethanol diet.
Alcohol consumption significantly increased liver weight in both HBK and control genotype mice compared with their control diet counterparts (Fig. 2C). The liver-to-body weight ratio was statistically elevated in livers of HBK mice fed the alcohol diet compared with control littermates fed alcohol (Fig. 2D). Increased liver weight in response to alcohol consumption is largely accounted for by triglyceride accumulation and increased protein content in hepatocytes (4, 5). Two-factor ANOVA showed a significant effect of diet, whereas there was no significant effect of genotype in either of these liver parameters (Table 3). Steatosis (presence of lipid droplets) was observed in livers of both alcohol-fed HBK and control genotype mice, whereas steatosis was not observed in livers of mice fed the control diet (Fig. 2E). ALT activity was mildly elevated in plasma from both control and alcohol diet-fed HBK mice compared with littermate controls (Fig. 2F). Two-factor ANOVA showed a significant main effect of genotype, but not diet, for ALT (Table 3). The reason for increased plasma ALT levels in HBK mice is not known; however, it is important to note that plasma ALT may not be increased by steatosis alone, as normal ALT values are found in patients with significant fatty liver disease (47). Moreover, the ethanol concentration in the diet was lower (3% wt/vol) than that typically used (≥5% wt/vol) in studies aimed at examining mechanisms of toxicity, injury, and inflammation; thus the lack of elevated ALT levels in the present study is not unexpected.
Finally, plasma glucose levels were also measured in a set of mice fed control and alcohol-containing diets. There were significant main effects of genotype, diet, and time for plasma glucose when data were analyzed by three-factor ANOVA (Table 3). At ZT 7, glucose was lower in plasma from HBK mice compared with littermate controls fed the control diet (Fig. 2G). This finding is consistent with the results reported in Lamia et al. (23), in which blood glucose levels were lower in HBK mice compared with control littermates during the inactive phase of the day. Furthermore, plasma glucose levels were higher in both genotypes of alcohol-fed mice compared with control diet-fed mice at ZT 19; however, statistical significance is only reached in the control genotype group (Fig. 2G). These findings suggest there are genotype and alcohol-mediated alterations in glucose handling and metabolism.
Differential responsiveness of hepatic glycogen content to chronic alcohol consumption in livers of HBK and littermate control mice.
To determine whether disruption of the hepatocyte clock elicits a differential effect on hepatic glycogen content following chronic alcohol consumption, we measured glycogen content in livers from HBK and control genotype mice. Livers were collected at 4-h intervals during one 24-h day. ANOVA and Cosinor analyses for these data are included in Tables 3 and 4, respectively.
Table 4.
Cosinor analysis of hepatic glycogen content in livers of control and alcohol-fed HBK and control genotype mice
| Rhythmicity |
Cosinor Parameters |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Diet | r2 | P value | Mesor | P value | Amplitude | P value | Acrophase | P value |
| Con | Con | 0.510 | <0.001 | 40.30 | <0.001 | 16.08 | 0.224 | 22.49 | <0.001 |
| ETOH | 0.310 | 0.002 | 24.87 | 11.23 | 1.83 | ||||
| HBK | Con | 0.270 | 0.007 | 39.36 | 11.43 | 20.08 | |||
| ETOH | 0.030 | 0.596 | |||||||
| Con | Con | 0.510 | <0.001 | 40.30 | 0.758 | 16.08 | 0.282 | 22.49 | 0.058 |
| HBK | Con | 0.270 | 0.007 | 39.36 | 11.43 | 20.08 | |||
ETOH, ethanol.
Hepatic glycogen levels displayed a significant diurnal rhythm in livers from control genotype mice fed the control diet, with the peak occurring at ZT 22.5 (Fig. 3A and Table 4). The glycogen rhythm was phase shifted in livers of alcohol-fed control genotype mice, peaking at ZT 1.8. Additionally, chronic alcohol feeding significantly decreased the mean level (mesor) of glycogen by 40% in livers of control genotype mice, but had no statistically significant effect on the amplitude of the rhythm (Fig. 3A and Table 4). Glycogen content remained rhythmic in livers of control-fed HBK mice peaking at ZT 20 (Fig. 3B and Table 4). The mesor of the glycogen rhythm in control-fed HBK mice is comparable to that measured in their control genotype counterparts, although differences in amplitude and phase were noted. The amplitude was decreased by 30%, and the peak of the glycogen rhythm was advanced by 2 h in livers of control-fed HBK mice (ZT 20) compared with control genotype mice (ZT 22); however, these differences did not reach statistical significance (Table 4). Importantly, the glycogen rhythm was completely lost in livers of HBK mice fed the alcohol-containing diet (Fig. 3B and Table 4). Finally, three-factor ANOVA revealed significant main effects of diet and time, as well as significant interactions for genotype × time and diet × time (Table 3). These results show differential responsiveness of HBK mice to alcohol feeding, in terms of the liver glycogen diurnal rhythm.
Chronic alcohol and Bmal1 deletion alter diurnal expression of Gys2 and other select hepatic glycogen metabolism genes.
To investigate whether differences in glycogen metabolism components may be responsible for the differential responsiveness of hepatic glycogen levels to alcohol, mRNA levels of glycogen metabolism genes were measured in livers of HBK and littermate control mice. We examined the diurnal gene expression patterns for glycogen synthase 2 (Gys2), glycogen phosphorylase (Pygl), glycogen synthase kinase 3β (Gskb), and protein phosphatase 1 (PP1) catalytic subunit-γ (Ppp1cc). Cosinor and ANOVA analyses for these data (Fig. 4) are included in Tables 5 and 6, respectively.
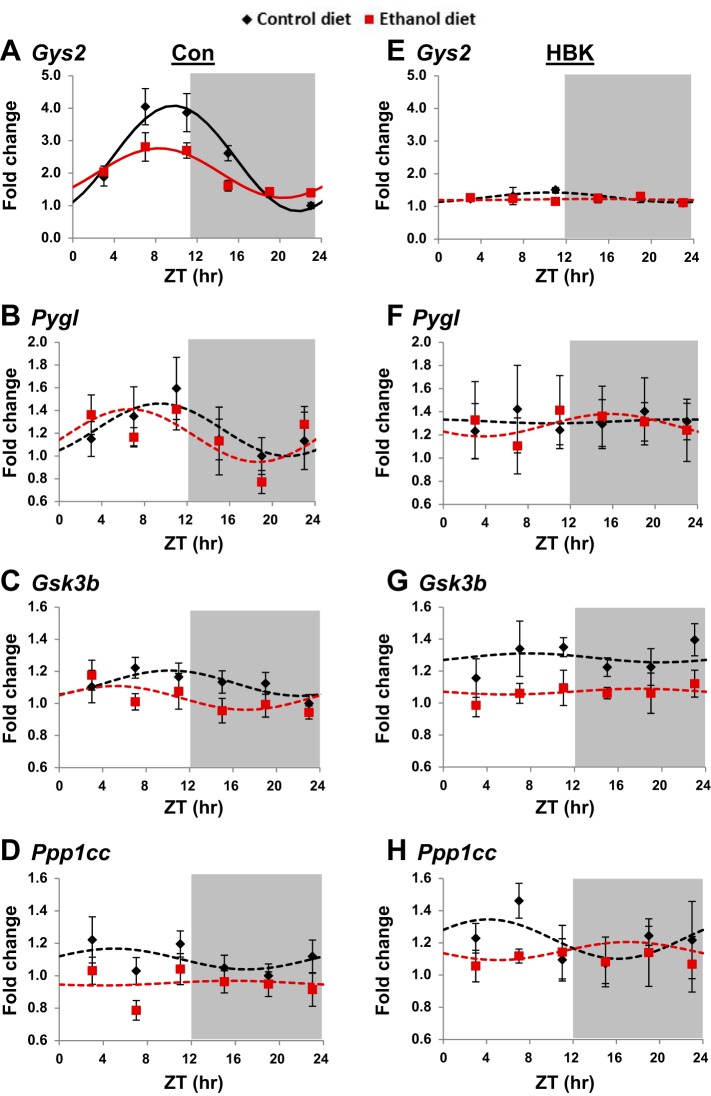
Fig. 4.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on glycogen metabolism genes. Diurnal gene expression profiles of glycogen synthase 2 (Gys2; A and E), glycogen phosphorylase (Pygl; B and F), glycogen synthase kinase 3β (Gsk3b; C and G), and protein phosphatase 1 catalytic subunit (Ppp1cc; D and H) were determined in livers collected from control and alcohol-fed hepatocyte-specific BMAL1 knockout (HBK; E–H) and control genotype (Con; A–D) mice at ZT 3, 7, 11, 15, 19, and 23 h (ZT 0: lights on; ZT 12: lights off, gray shading). mRNA levels were normalized to Gapdh and are displayed as a fold-change from the trough of Con mice fed the control diet. Data are fitted to a cosine function and expressed as means ± SE for n = 5–8 mice/genotype/diet/time point. Solid lines indicate a significant cosine curve fit, whereas dashed lines indicate a nonsignificant fit. Results for Cosinor and ANOVA analyses are provided in Tables 5 and 6, respectively.
Table 5.
Cosinor analysis of gene expression in livers of control and alcohol-fed HBK and control genotype mice
| Rhythmicity |
Cosinor Parameters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Genotype | Diet | r2 | P value | Mesor | P value | Amplitude | P value | Acrophase | P value |
| Gck | Con | Con | 0.276 | 0.006 | 1.45 | 0.012 | 0.53 | 0.269 | 17.61 | 0.125 |
| ETOH | 0.217 | 0.018 | 1.10 | 0.33 | 14.92 | |||||
| HBK | Con | 0.272 | 0.006 | 1.72 | 0.018 | 0.59 | 0.447 | 17.39 | 0.011 | |
| ETOH | 0.257 | 0.012 | 1.33 | 0.43 | 12.92 | |||||
| Glut1 | Con | Con | 0.010 | 0.827 | ||||||
| ETOH | 0.051 | 0.411 | ||||||||
| HBK | Con | 0.209 | 0.024 | 1.22 | 0.003 | 0.18 | 0.861 | 18.43 | 0.833 | |
| ETOH | 0.243 | 0.013 | 0.89 | 0.21 | 17.80 | |||||
| Glut2 | Con | Con | 0.474 | <0.001 | 1.51 | 0.50 | 9.74 | |||
| ETOH | 0.086 | 0.215 | ||||||||
| HBK | Con | 0.319 | 0.002 | 1.17 | 0.22 | 8.17 | ||||
| ETOH | 0.128 | 0.119 | ||||||||
| Con | Con | 0.474 | <0.001 | 1.51 | <0.001 | 0.50 | 0.012 | 9.74 | 0.189 | |
| HBK | Con | 0.319 | 0.002 | 1.17 | 0.22 | 8.17 | ||||
| Gsk3b | Con | Con | 0.080 | 0.223 | ||||||
| ETOH | 0.067 | 0.307 | ||||||||
| HBK | Con | 0.006 | 0.907 | |||||||
| ETOH | 0.004 | 0.934 | ||||||||
| Gyg | Con | Con | 0.131 | 0.080 | ||||||
| ETOH | 0.152 | 0.060 | ||||||||
| HBK | Con | 0.094 | 0.206 | |||||||
| ETOH | 0.009 | 0.876 | ||||||||
| Gys2 | Con | Con | 0.651 | <0.001 | 2.45 | 0.010 | 1.63 | <0.001 | 9.73 | 0.067 |
| ETOH | 0.536 | <0.001 | 2.00 | 0.77 | 8.30 | |||||
| HBK | Con | 0.090 | 0.220 | |||||||
| ETOH | 0.002 | 0.964 | ||||||||
| Pklr | Con | Con | 0.503 | <0.001 | 1.78 | 0.061 | 0.82 | 0.027 | 13.51 | 0.678 |
| ETOH | 0.265 | 0.005 | 1.54 | 0.41 | 14.04 | |||||
| HBK | Con | 0.009 | 0.863 | |||||||
| ETOH | 0.100 | 0.857 | ||||||||
| Ppp1cc | Con | Con | 0.033 | 0.553 | ||||||
| ETOH | 0.003 | 0.957 | ||||||||
| HBK | Con | 0.075 | 0.301 | |||||||
| ETOH | 0.021 | 0.723 | ||||||||
| Ppp1r3b | Con | Con | 0.397 | <0.001 | 1.71 | 0.671 | 0.87 | 0.760 | 16.82 | 0.329 |
| ETOH | 0.220 | <0.001 | 1.63 | 0.78 | 15.44 | |||||
| HBK | Con | 0.512 | <0.001 | 2.34 | 0.077 | 1.07 | 0.403 | 17.27 | 0.130 | |
| ETOH | 0.389 | <0.001 | 2.66 | 0.86 | 15.68 | |||||
| Con | Con | 0.397 | <0.001 | 1.71 | 0.002 | 0.87 | 0.442 | 16.82 | 0.660 | |
| HBK | Con | 0.512 | <0.001 | 2.34 | 1.07 | 17.27 | ||||
| Con | ETOH | 0.220 | <0.001 | 1.63 | <0.001 | 0.78 | 0.786 | 15.44 | 0.862 | |
| HBK | ETOH | 0.389 | <0.001 | 2.66 | 0.86 | 15.68 | ||||
| Ppp1r3c | Con | Con | 0.598 | <0.001 | 1.71 | 0.541 | 1.44 | 0.567 | 21.23 | 0.174 |
| ETOH | 0.631 | <0.001 | 1.59 | 1.28 | 20.21 | |||||
| HBK | Con | 0.129 | 0.118 | |||||||
| ETOH | 0.052 | 0.447 | ||||||||
| Ppp1r3g | Con | Con | 0.374 | 0.004 | 3.08 | 2.87 | 10.22 | |||
| ETOH | 0.112 | 0.135 | ||||||||
| HBK | Con | 0.376 | 0.001 | 1.37 | 1.08 | 6.91 | ||||
| ETOH | 0.116 | 0.131 | ||||||||
| Con | Con | 0.374 | 0.004 | 3.08 | 0.006 | 2.87 | 0.036 | 10.22 | 0.023 | |
| HBK | Con | 0.376 | 0.001 | 1.37 | 1.08 | 6.91 | ||||
| Pygl | Con | Con | 0.092 | 0.117 | ||||||
| ETOH | 0.128 | 0.098 | ||||||||
| HBK | Con | 0.000 | 0.993 | |||||||
| ETOH | 0.012 | 0.828 | ||||||||
Table 6.
Three-factor ANOVA for gene expression
| Genotype |
Diet |
Time |
Genotype × Diet |
Genotype × Time |
Diet × Time |
Genotype × Diet × Time |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | F | P value | F | P value | F | P value | F | P value | F | P value | F | P value | F | P value |
| Gck | 4.74 | 0.032 | 13.53 | <0.001 | 6.84 | <0.001 | 0.17 | 0.678 | 0.31 | 0.907 | 2.59 | 0.029 | 0.42 | 0.836 |
| Glut1 | 14.89 | <0.001 | 0.02 | 0.901 | 1.72 | 0.134 | 0.32 | 0.576 | 1.61 | 0.163 | 0.54 | 0.745 | 0.42 | 0.837 |
| Glut2 | 10.70 | 0.001 | 0.28 | 0.598 | 9.59 | <0.001 | 5.76 | 0.018 | 1.28 | 0.276 | 1.33 | 0.254 | 0.69 | 0.631 |
| Gsk3b | 7.05 | 0.009 | 18.43 | <0.001 | 0.53 | 0.757 | 2.55 | 0.113 | 1.54 | 0.183 | 0.48 | 0.792 | 0.34 | 0.890 |
| Gyg | 74.62 | <0.001 | 5.03 | 0.027 | 2.75 | 0.022 | 1.00 | 0.320 | 1.48 | 0.202 | 1.46 | 0.207 | 0.39 | 0.854 |
| Gys2 | 101.35 | <0.001 | 7.13 | 0.009 | 17.47 | <0.001 | 4.29 | 0.040 | 14.12 | <0.001 | 3.27 | 0.008 | 1.93 | 0.094 |
| Pklr | 3.74 | 0.055 | 0.34 | 0.563 | 6.08 | <0.001 | 12.63 | 0.001 | 4.63 | 0.001 | 1.47 | 0.205 | 0.74 | 0.596 |
| Ppp1cc | 12.64 | 0.001 | 6.71 | 0.011 | 0.19 | 0.968 | 0.73 | 0.396 | 1.63 | 0.158 | 0.99 | 0.428 | 0.40 | 0.851 |
| Ppp1r3b | 32.61 | <0.001 | 0.74 | 0.391 | 15.69 | <0.001 | 1.96 | 0.164 | 0.47 | 0.801 | 1.95 | 0.091 | 0.49 | 0.785 |
| Ppp1r3c | 112.21 | <0.001 | 1.78 | 0.184 | 9.41 | <0.001 | 3.46 | 0.065 | 3.65 | 0.004 | 0.73 | 0.600 | 0.26 | 0.935 |
| Ppp1r3g | 13.75 | <0.001 | 4.86 | 0.029 | 9.68 | <0.001 | 2.60 | 0.110 | 1.89 | 0.101 | 0.92 | 0.472 | 2.74 | 0.022 |
| Pygl | 1.06 | 0.306 | 0.12 | 0.728 | 0.66 | 0.656 | 0.01 | 0.928 | 0.87 | 0.507 | 0.39 | 0.855 | 0.22 | 0.954 |
Consistent with our laboratory’s previous findings (44), we observed a significant diurnal rhythm in Gys2, the rate-limiting enzyme in glycogen synthesis, in livers of control genotype mice fed the control diet (Fig. 4A and Table 5). Alcohol feeding significantly decreased the mesor and amplitude of the Gys2 rhythm in livers of control genotype mice and phase advanced the peak of Gys2 expression by almost 2 h; however, this phase difference just missed statistical significance (Fig. 4A and Table 5, P = 0.067). Disruption of the hepatocyte clock completely abolished the Gys2 rhythm in livers of control and alcohol-fed HBK mice (Fig. 4E and Table 5). Three-factor ANOVA showed significant main effects of genotype, diet, and time for Gys2, as well as significant interactions for genotype × diet, genotype × time, and diet × time (Table 6).
We also examined the gene expression profile of Pygl, the rate-limiting enzyme in glycogen breakdown (glycogenolysis). While Pygl expression is arrhythmic in livers of littermate control mice (Table 5), mRNA levels were, on average, higher during the inactive and lower during the active period of the day (Fig. 4B). In contrast, Pygl expression remained constant throughout the day in livers of both HBK control and alcohol-fed mice (Fig. 4F and Table 5). No significant main effects or interactions were found for Pygl when data were analyzed by three-factor ANOVA (Table 6).
Gsk3b, a kinase implicated in regulating GS activity (16), was arrhythmic in livers of HBK and littermate control mice (Fig. 4, C and G, and Table 5). However, three-factor ANOVA revealed significant main effects of genotype and diet for Gsk3b (Table 6). Similar findings were observed for Ppp1cc, the catalytic subunit for PP1, which plays a key role in regulating glycogen metabolism through dephosphorylation of both GS and glycogen phosphorylase. Dephosphorylation of GS accelerates glycogen synthesis, whereas dephosphorylation of glycogen phosphorylase decreases glycogenolysis (reviewed in Refs. 8, 36). Ppp1cc was arrhythmic in livers of HBK and control genotype mice (Fig. 4, D and H, and Table 5). However, significant main effects of genotype and diet were found when results were analyzed by three-factor ANOVA (Table 6). Collectively, these results show that Gys2, the rate-limiting enzyme for glycogen synthesis, is regulated by the circadian clock, and that the diurnal expression of Gys2 and other glycogen metabolism genes in the liver are differentially altered by chronic alcohol consumption in HBK and littermate control mice.
Chronic alcohol and Bmal1 deletion alter diurnal expression patterns of GS protein.
We next determined whether the alterations we observed in Gys2 mRNA levels following chronic alcohol consumption in HBK and littermate control mice were linked to similar changes in protein levels. For this, we examined GS protein abundance and phosphorylation status over the course of the 24-h day (Fig. 5). The antibodies used for these measurements detect both the liver-specific isoform (GS2) encoded by the Gys2 gene and the more ubiquitously expressed muscle isoform (GS1) encoded by the Gys1 gene (see Fig. 5, E and J). Note that we did not measure Gys1 mRNA levels in this study. Cosinor and ANOVA analyses for these data are included in Tables 7 and 8, respectively.
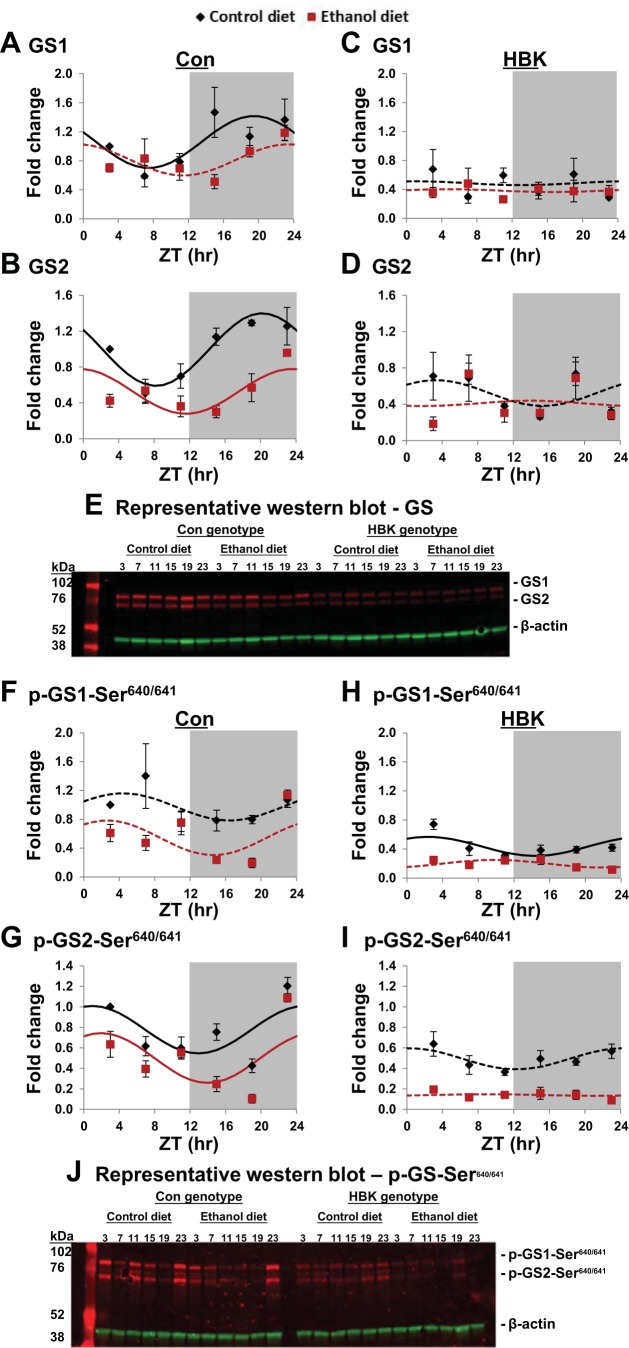
Fig. 5.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on protein abundance and phosphorylation status of glycogen synthase. Diurnal profiles of total glycogen synthase 1 (GS1; A and C) and glycogen synthase 2 (GS2; B and D) protein and phosphorylated glycogen synthase 1 (p-GS1; F and H) and phosphorylated glycogen synthase 2 (p-GS2; G and I) protein were determined in livers collected from control and alcohol-fed hepatocyte-specific BMAL1 knockout (HBK) and control genotype (Con) mice at ZT 3, 7, 11, 15, 19, and 23 h (ZT 0: lights on; ZT 12: lights off, gray shading). Protein levels are displayed as a fold-change from the Con mice fed the control diet at ZT 3. Data were fitted to a cosine function and are expressed as means ± SE for n = 3–4 mice/genotype/diet/time point. Solid lines indicate a significant cosine curve fit, whereas dashed lines indicate a nonsignificant fit. E and J: representative Western blots of data presented in A–D and F–I, respectively. Blots show protein bands for both isoforms of total and phosphorylated glycogen synthase (red bands); i.e., GS1 (muscle isoform) and GS2 (liver isoform). Blots for β-actin (green) are provided as a loading control. Results for Cosinor and ANOVA analyses are provided in Tables 7 and 8, respectively.
Table 7.
Cosinor analysis of total and phosphorylated glycogen synthase protein in livers of control and alcohol-fed HBK and control genotype mice
| Rhythmicity |
Cosinor Parameters |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Genotype | Diet | r2 | P value | Mesor | P value | Amplitude | P value | Acrophase | P value |
| GS1 | Con | Con | 0.361 | 0.022 | 1.06 | 0.358 | 19.48 | |||
| ETOH | 0.259 | 0.091 | ||||||||
| HBK | Con | 0.003 | 0.970 | |||||||
| ETOH | 0.004 | 0.965 | ||||||||
| GS2 | Con | Con | 0.245 | <0.001 | 0.995 | <0.001 | 0.404 | 0.138 | 20.19 | 0.011 |
| ETOH | 0.398 | 0.010 | 0.529 | 0.250 | 23.57 | |||||
| HBK | Con | 0.081 | 0.489 | |||||||
| ETOH | 0.006 | 0.955 | ||||||||
| p-GS1 | Con | Con | 0.144 | 0.267 | ||||||
| ETOH | 0.219 | 0.123 | ||||||||
| HBK | Con | 0.323 | 0.025 | 0.436 | 0.131 | 3.180 | ||||
| ETOH | 0.189 | 0.136 | ||||||||
| p-GS2 | Con | Con | 0.360 | 0.023 | 0.777 | 0.003 | 0.232 | 0.925 | 22.92 | 0.824 |
| ETOH | 0.283 | 0.050 | 0.501 | 0.243 | 22.49 | |||||
| HBK | Con | 0.202 | 0.094 | |||||||
| ETOH | 0.005 | 0.945 | ||||||||
| β-actin (GS) | Con | Con | 0.074 | 0.562 | ||||||
| ETOH | 0.010 | 0.930 | ||||||||
| HBK | Con | 0.041 | 0.729 | |||||||
| ETOH | 0.104 | 0.437 | ||||||||
| β-actin (p-GS) | Con | Con | 0.010 | 0.929 | ||||||
| ETOH | 0.029 | 0.800 | ||||||||
| HBK | Con | 0.137 | 0.331 | |||||||
| ETOH | 0.134 | 0.341 | ||||||||
Table 8.
Three-factor ANOVA for total and phosphorylated glycogen synthase protein
| Genotype |
Diet |
Time |
Genotype × Diet |
Genotype × Time |
Diet × Time |
Genotype × Diet × Time |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | F | P value | F | P value | F | P value | F | P value | F | P value | F | P value | F | P value |
| GS1 | 57.34 | <0.001 | 6.60 | 0.013 | 1.37 | 0.251 | 1.24 | 0.271 | 2.15 | 0.072 | 1.97 | 0.097 | 1.87 | 0.114 |
| GS2 | 25.01 | <0.001 | 23.34 | <0.001 | 3.72 | 0.006 | 9.68 | 0.003 | 5.41 | <0.001 | 2.26 | 0.061 | 1.55 | 0.191 |
| p-GS1 | 110.85 | <0.001 | 58.53 | <0.001 | 5.14 | <0.001 | 4.29 | 0.043 | 5.15 | <0.001 | 3.83 | 0.004 | 3.73 | 0.005 |
| p-GS2 | 125.27 | <0.001 | 117.57 | <0.001 | 22.41 | <0.001 | 2.54 | 0.116 | 16.09 | <0.001 | 2.30 | 0.055 | 1.59 | 0.174 |
| β-actin (GS) | 0.10 | 0.755 | 0.24 | 0.623 | 0.11 | 0.990 | 3.74 | 0.060 | 0.12 | 0.987 | 0.30 | 0.909 | 0.53 | 0.749 |
| β-actin (p-GS) | 2.95 | 0.093 | 0.10 | 0.754 | 0.15 | 0.979 | 0.05 | 0.817 | 0.25 | 0.938 | 0.22 | 0.953 | 0.56 | 0.729 |
Both GS1 and GS2 proteins displayed significant diurnal rhythms peaking at ZT 19.5 and ZT 20.2, respectively, in livers from control genotype mice fed the control diet (Fig. 5, A and B, and Table 7). Chronic alcohol feeding significantly altered the diurnal rhythm in GS protein in control genotype mice. The mesor of the GS2 rhythm was significantly decreased, and the peak of GS2 was phase delayed almost 3.5 h by chronic alcohol feeding (Fig. 5B and Table 7). While time-of-day differences in GS1 protein were observed in livers of alcohol-fed littermate controls (Fig. 5A), these data did not fit a cosine function (Table 7). GS1 and GS2 proteins were arrhythmic in livers of control and alcohol-fed HBK mice (Fig. 5, C and D, and Table 7). There were significant main effects of genotype and diet for GS1 and GS2, significant main effect of time for GS2, and significant genotype × diet and genotype × time interactions for GS2 when data were analyzed by three-factor ANOVA (Table 8).
Extending our analyses, we measured the diurnal phosphorylation status of GS at Ser640/641, which is the inhibitory GSK-3β target site. Phosphorylated-GS2 (p-GS2) displayed diurnal rhythms with peaks near ZT 22 in livers of both control and alcohol-fed control genotype mice (Fig. 5G and Table 7). Alcohol feeding significantly decreased the mesor of p-GS2, but had no effect on the amplitude and acrophase of the rhythm (Table 7). p-GS1 was arrhythmic in livers of littermate controls (Fig. 5F); however, a low-amplitude rhythm of p-GS1 was observed in livers of control-fed, but not alcohol-fed, HBK mice (Fig. 5H). p-GS2 was also arrhythmic in livers of control and alcohol-fed HBK mice (Fig. 5I and Table 7). Three-factor ANOVA revealed significant differences in genotype, diet, and time for both p-GS1 and p-GS2 (Table 8). Significance for p-GS1 was observed for all interactions, whereas a significant interaction of genotype × time was found for p-GS2 (Table 8). Representative Western blots for total and phospho-GS1 and -GS2 and β-actin are provided in Fig. 5, E and J. There were no statistically significant differences in main effects and interactions for β-actin (Table 8).
Chronic alcohol and Bmal1 deletion alter diurnal expression of select glucose metabolism genes.
To complement our measures on glycogen metabolism components, we also examined the diurnal expression patterns of several genes involved in hepatic glucose metabolism and uptake. We determined the diurnal gene expression patterns for glucokinase (Gck), pyruvate kinase (Pklr), and glucose transporters 1 and 2 (Glut1/Slc2a1 and Glut2/Slc2a2) in livers of control and alcohol-fed HBK and littermate control mice (Fig. 6). Cosinor and ANOVA analyses for these data are included in Tables 5 and 6, respectively.
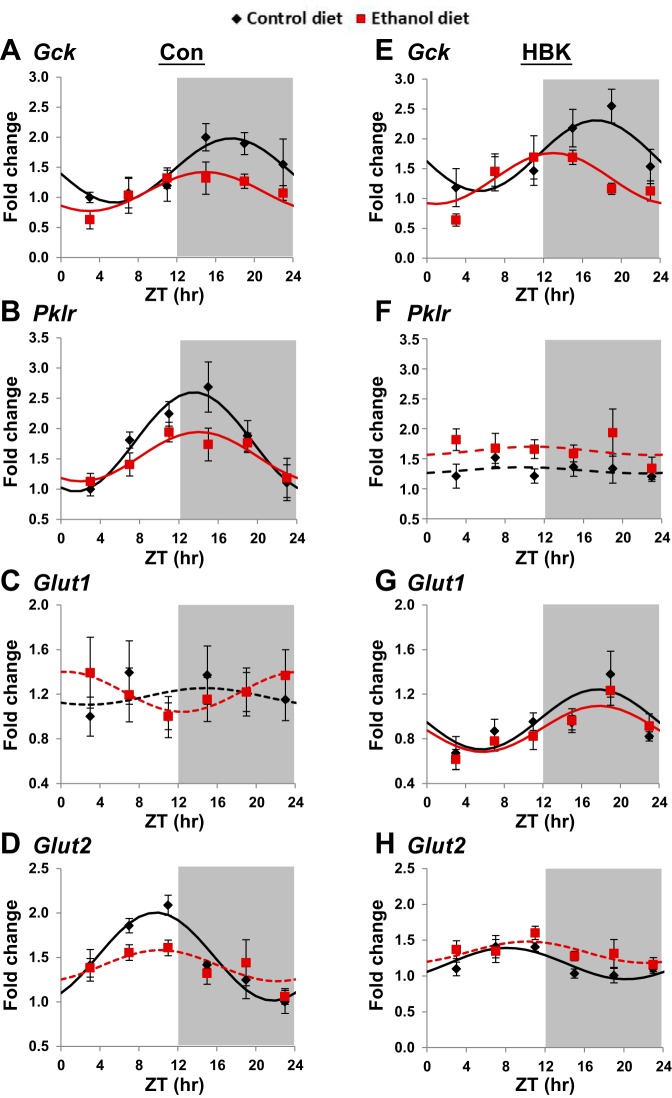
Fig. 6.
Effect of chronic alcohol consumption and hepatocyte-specific Bmal1 gene deletion on glucose metabolism genes. Diurnal gene expression profiles of glucokinase (Gck; A and E), pyruvate kinase, liver (Pklr; B and F), glucose transporter type 1 (Slc2a1/Glut1; C and G), and glucose transporter type 2, liver (Slc2a2/Glut2; D and H) were determined in livers collected from control and alcohol-fed hepatocyte-specific BMAL1 knockout (HBK; E–H) and control genotype (Con; A–D) mice at ZT 3, 7, 11, 15, 19, and 23 h (ZT 0: lights on; ZT 12: lights off, gray shading). mRNA levels were normalized to Gapdh and displayed as a fold-change from the trough of Con mice fed the control diet. Data were fitted to a cosine function and are expressed as means ± SE for n = 5–8 mice/genotype/diet/time point. Solid lines indicate a significant cosine curve fit, whereas dashed lines indicate a nonsignificant fit. Results for Cosinor and ANOVA analyses are provided in Tables 5 and 6, respectively.
Significant diurnal oscillations of Gck, Pklr, and Glut2 were found in the livers of control genotype mice fed the control diet with peak expression observed at ZT 17.6, 13.5, and 9.7, respectively (Fig. 6, A, B, and D). Alcohol feeding decreased the amplitude of Gck and Pklr and abolished the rhythm of Glut2 in livers of control genotype mice (Fig. 6, A, B, and D, and Table 5). Gck gene expression remained rhythmic with slightly higher mRNA levels in livers of control and alcohol-fed HBK mice compared with littermate control mice (Fig. 6E). Time-of-day-dependent oscillations in Pklr were completely lost in livers of control and alcohol-fed HBK mice (Fig. 6F and Table 5). Glut2 gene expression was rhythmic in livers from control-fed HBK mice peaking at ZT 8.1, but Glut2 was arrhythmic in livers of alcohol-fed HBK mice (Fig. 6H and Table 5). Importantly, the mesor and amplitude of Glut2 were significantly reduced by 29 and 56%, respectively, in livers of control-fed HBK mice compared with control genotype mice (Table 5). Glut1 expression was arrhythmic in livers of control and alcohol-fed control genotype mice (Fig. 6C). Interestingly, Glut1 was rhythmic in livers of control and alcohol-fed HBK mice, peaking during the middle of the active phase at approximately ZT 18 (Fig. 6G and Table 5).
Three-factor ANOVA showed significant main effects of genotype, diet, and time, as well as a significant diet × time interaction for Gck (Table 6). Significant main effects of genotype and time, as well as a genotype × diet interaction, were also found for Glut2. Significant main effects of time and interactions for genotype × diet and genotype × time were seen for Pklr, with the main effect of genotype just missing statistical significance (P = 0.055, Table 6). Glut1 gene expression displayed a significant main effect of genotype only. Together, these results highlight different responses in several glucose metabolism genes in livers of HBK and control genotype mice alone or when exposed to alcohol chronically, with significant genotype × diet interactions observed for Glut2 and Pklr.
Chronic alcohol and Bmal1 deletion alter diurnal rhythms in glycogenin and glycogen particle targeting proteins.
Lastly, we examined diurnal changes in mRNA levels for glycogenin (Gyg), the glycogen primer, and several scaffold proteins that regulate glycogen metabolism. The scaffold proteins we assessed influence glycogen metabolism by targeting PP1 to the glycogen particle, allowing it to dephosphorylate glycogen phosphorylase and GS (reviewed in Ref. 34). Specifically, we measured mRNA levels for PP1 regulatory subunit 3B (Ppp1r3b), Ppp1r3c, and Ppp1r3g. Cosinor and ANOVA analyses for these data (Fig. 7) are included in Tables 5 and 6, respectively.
The diurnal gene expression profile of Gyg was arrhythmic in all four experimental groups (Fig. 7, A and E, and Table 5). However, Gyg mRNA levels were significantly increased (57% increase) in livers of control-fed HBK mice compared with levels measured in livers of littermate control mice (P < 0.001). Significant main effects of genotype, diet, and time were found for Gyg (Table 6).
With regards to the three glycogen-targeting proteins, Ppp1r3b, Ppp1r3c, and Ppp1r3g, all three exhibited significant mRNA rhythms in livers of control genotype mice fed the control diet (Fig. 7, B–D, and Table 5). Interestingly, the peak (acrophase) of these rhythms was different for all three genes. The acrophase for Ppp1r3g, Ppp1r3b, and Ppp1r3c was ZT 10.2, 16.8, and 21.2, respectively, in livers from control-fed littermate control mice (Table 5). Rhythms of Ppp1r3b and Ppp1r3c were maintained in livers of alcohol-fed control genotype mice, and alcohol had no effect on the mesor, amplitude, and acrophase for these two genes (Fig. 7, B and C, and Table 5). In contrast, rhythmic expression of Ppp1r3g was significantly dampened in livers of alcohol-fed littermate control mice compared with their corresponding controls (Fig. 7D and Table 5). Ppp1r3b was also rhythmic in livers of control and alcohol-fed HBK mice (Fig. 7F), whereas Ppp1r3c was arrhythmic in livers of HBK mice (Fig. 7G). While attenuated, the diurnal mRNA expression of Ppp1r3g was rhythmic in livers of control-fed HBK mice, but not in livers of alcohol-fed HBK (Fig. 7H and Table 5). Comparisons also showed significant differences in rhythms between genotypes. For example, statistically significant differences in the mesor of hepatic Ppp1r3b was observed between control and alcohol-fed HBK mice and their corresponding littermate controls (Table 5). Significant differences in mesor, amplitude, and acrophase of Ppp1r3g were also found between control-fed HBK and littermate control mice (Table 5). Specifically, the mesor and amplitude of Ppp1r3g were significantly decreased in livers of control-fed HBK mice compared with control-fed littermate mice. Additionally, the peak of Ppp1r3g was phase advanced to ZT 6.9 in livers of control-fed HBK mice compared with a peak of ZT 10.2 in livers of control-fed littermate control mice (Table 5).
Three-factor ANOVA showed significant main effects of genotype and time for all three glycogen-targeting proteins, as well as a significant main effect of diet for Ppp1r3g (Table 6). A significant genotype × time interaction was found for Ppp1r3c, and a significant genotype × time × diet interaction was observed for Ppp1r3g (Table 6). Taken together, these results highlight the previously unknown role of the circadian clock in differentially regulating glycogen-targeting proteins in livers from both alcohol naive (control diet) and alcohol-fed mice.
DISCUSSION
Here, we report for the first time the combined effects of chronic alcohol and clock disruption on hepatic glycogen. Using mice with a disrupted hepatocyte circadian clock, we found that clock disruption exacerbates alcohol-mediated alterations in hepatic glycogen. We also found that alcohol and clock disruption alters the diurnal rhythms of several glycogen and glucose metabolism genes. Moreover, some glycogen metabolism genes were differentially altered by chronic alcohol in HBK and littermate control mice. Diurnal rhythms of Gys2 and GS2 were significantly altered by alcohol and clock disruption. Alcohol also significantly altered Gck and Glut2, as well as the Ppp1r3g, rhythm. Diurnal rhythms of Pklr, Glut2, Ppp1r3c, and Ppp1r3g were significantly dampened or were arrhythmic in livers of HBK mice. Collectively, these observations demonstrate that alcohol and clock disruption disrupt coordinated time-of-day oscillations in key components of the glycogen metabolism pathway in the liver.
We also observed time-of-day differences in plasma glucose between HBK and littermate control mice. HBK mice fed the control diet had lower plasma glucose compared with all other groups (Fig. 2G). This finding may be related to the observed decrease in amplitude of the bidirectional GLUT-2 transporter in livers of HBK mice (Fig. 6H). Lamia et al. (23) also observed lower levels of GLUT-2 mRNA and protein in livers of HBK mice. Plasma glucose was, however, slightly elevated by alcohol feeding (Fig. 2G). Previous studies showed elevated blood glucose in mice fed alcohol, indicating glucose intolerance (10). There is, however, a large degree of inconsistency in the literature regarding the impact alcohol has on glucose handling in both animal and humans studies (39); thus more work is needed in this area. We propose that the modest elevating effect alcohol had on plasma glucose was overridden by the small plasma glucose-lowering response observed in HBK mice.
Given our longstanding interest in disease-induced changes in hepatic energy metabolism, we examined the effects of alcohol, clock disruption, or both on hepatic glycogen content. The two rate-limiting enzymes in glycogen metabolism are GS (synthesis arm) and glycogen phosphorylase (breakdown arm). We measured diurnal rhythms of hepatic glycogen content alongside mRNA for glycogen phosphorylase (Pygl) and GS (Gys2), as well as for GS protein. Similar to our laboratory’s prior work (44), glycogen exhibited a significant diurnal rhythm in livers of control-fed control genotype mice that peaked in the latter part of the active phase of the day (Fig. 3A). In contrast, alcohol feeding decreased the mesor (mean content) and induced a phase-shift in the diurnal glycogen rhythm in livers of control genotype mice, such that peak glycogen content occurred during the early part of the inactive phase of the day (Fig. 3A). Evaluation of Pygl mRNA revealed arrhythmic expression with no effect of alcohol feeding (Fig. 4B), which also agrees with our laboratory’s earlier work (44). Based on this, we propose that increased glycogenolysis is most likely not responsible for lower glycogen in livers of alcohol-fed mice. As such, we focused our analyses on GS and other related proteins and enzymes.
Rhythmic expression of GS at both gene (Gys2) and protein (GS1/2) levels was observed in livers of control genotype mice, with alcohol feeding significantly changing these rhythms. We found that the high-amplitude rhythm of Gys2 peaked during the latter half of the inactive period in livers from control-fed littermate control mice. In contrast, chronic alcohol feeding phase advanced the Gys2 peak, as well as decreased the mesor and amplitude of the rhythm (Fig. 4A). Alcohol-dependent changes in GS protein content were also observed. Both GS isoforms (GS1 and GS2) exhibited significant diurnal rhythms in livers of control-fed littermate control mice (Fig. 5, A and B). Again, the rhythm of GS2 protein was significantly altered in livers of alcohol-fed littermate control mice with reductions in amplitude and mesor, and a significant phase delay in the peak of GS2 (Fig. 5B). A comparison of the acrophase (peak) of Gys2, GS2, and glycogen content between control-fed (ZT 9.7, 20.2, and 22.49 h, respectively) and alcohol-fed (ZT 8.3, 23.6, and 1.83 h, respectively) littermate control mice reveals significant differences between these two groups. Taken together, these data strongly suggest that temporal dysregulation in glycogen synthesis at multiple levels (gene and protein) likely mediates, in part, alcohol-induced disruption in hepatic glycogen. Specifically, the alcohol-mediated phase delay in GS2 protein may underpin the phase shift of the glycogen rhythm peak into the inactive phase of the day.
The activity of GS is strongly regulated by G6P. Multiple mechanisms exist for G6P to stimulate glycogen synthesis [see review by Ferrer et al. (18)]. G6P functions as a reversible allosteric activator of GS, it promotes a conformation change in GS favoring dephosphorylation (and activation) by PP1, and it mediates cellular redistribution of GS. Within this context, we observed an alcohol-mediated decrease in the mesor of Gck (Fig. 6A), the enzyme responsible for phosphorylation of glucose to G6P. Patients with alcoholic or biliary cirrhosis have decreased hepatic glycogen stores with significant reductions in Gck mRNA levels and glucokinase activities compared with control patients undergoing liver surgery for other causes (22). These findings, coupled with the observation that alcohol decreased Glut2 (Fig. 6D), leads us to speculate that alcohol feeding decreases the availability of substrate (glucose) and allosteric activator (G6P) in vivo for GS, thus contributing to lower glycogen levels in livers of alcohol-fed mice.
Especially intriguing are the differential responses to alcohol we observed between HBK and littermate control mice for some glycogen metabolism components. First, Gys2 appears to be clock regulated, as the diurnal oscillation of Gys2 was absent in livers of HBK mice (Fig. 4E). Previous studies suggested that Gys2 is regulated by the BMAL1 (23), CLOCK (14), and PER2 (50). Second, GS protein was arrhythmic in livers of HBK mice (Fig. 5). Collectively, these data support our observation of a complete loss in the glycogen rhythm in livers of alcohol-fed HBK mice (Fig. 3B). With this said, however, glycogen content remained rhythmic in livers of control-fed HBK mice (Fig. 3B), despite GS mRNA and protein being arrhythmic (Figs. 4 and 5). This interesting result supports the fact that extrahepatic factors, like hormones (insulin and glucocorticoids) or neural signals (hypothalamic) help maintain the glycogen rhythm (8, 30, 35), even when the liver clock has been genetically disrupted. Thus these novel findings suggest a “two-hit” mechanism for loss in the glycogen rhythm in alcohol-fed HBK mice, as alcohol alone or clock disruption alone was insufficient to abolish the glycogen rhythm. It is only the combination of these two stresses (alcohol and clock disruption) that causes a complete loss in the rhythmic nature of this important energy store in the liver. Future studies will be aimed at examining the impact alcohol has on extrahepatic factors that regulate hepatic glycogen metabolism, such as insulin, glucagon, vasopressin, and/or glucocorticoids in both HBK and control genotype mice. Conversely, the hepatic sensitivity of HBK and control genotype mice to these hormones should also be assessed following chronic alcohol feeding.
Other important targets that have not been studied within the context of alcohol-induced glycogen depletion are the glycogen particle targeting proteins. These proteins act as “scaffold” proteins by targeting the phosphatase PP1 to the glycogen particle (34). They influence glycogen content, as deletion and overexpression have been shown to decrease and increase hepatic glycogen content, respectively, in both mouse and cell culture models (28, 37). Herein, we discovered that three glycogen-targeting proteins exhibit significant diurnal rhythms in livers of control-fed littermate control mice with peaks at different times of the day (Fig. 7, B–D). Even more intriguing, Ppp1r3b and Ppp1r3g retained rhythmicity in livers of control-fed HBK mice, whereas Ppp1r3c was arrhythmic (Fig. 7, F–H), suggesting differential regulation by the clock. Diurnal rhythms were, however, dramatically different in livers from HBK vs. littermate control-fed mice, and we observed disparate responses to alcohol (Table 5). For example, alcohol had no effect on Ppp1r3b and Ppp1r3c rhythms in livers of control genotype mice, whereas Ppp1r3g was arrhythmic (Fig. 7D). While more work is needed to understand the complex role these proteins play in glycogen metabolism under normal and disease conditions, these initial studies suggest Ppp1r3g may participate in maintaining the normal diurnal glycogen rhythm in the liver. Similarly, the double “hit” of arrhythmic Ppp1r3c and Ppp1r3g in livers of HBK mice may partly explain the loss in the glycogen rhythm following alcohol feeding.
One limitation of this study is that we were unable to monitor time-of-day feeding patterns. Due to the liquid nature of this diet and the necessity to provide fresh diets daily, this regimen is not amenable to automated hands-free feeding systems; e.g., comprehensive laboratory animal monitoring system, that require solid diets (9). It is well documented that clock genes and downstream targets in liver can be entrained to food intake (40). Thus it is possible that some of the effects observed herein could be attributed to alcohol-mediated changes in feeding patterns. With this said, blood alcohol levels were elevated during the lights-off active period of the day (Fig. 2D), suggesting alcohol-fed mice consumed most of their food during the active period. Further, we (unpublished data) and others (23) have not observed disrupted feeding behaviors in HBK mice fed a normal chow diet. And we observed no genotype differences in the amount of diets consumed (Table 3). Together, we propose that the liver-specific alterations in hepatic glycogen metabolism we observed are independent of feeding patterns.
In summary, we have shown that chronic alcohol significantly disrupts diurnal rhythms of multiple glycogen metabolism components in the liver. We found that hepatocyte clock disruption differentially influences glycogen in response to alcohol. We propose that disruptions in Gck, Glut2, Gys2/GS, and Ppp1r3g most likely contribute to perturbations in the glycogen rhythm and content in the liver. Interestingly, glycogen-targeting proteins are known to mediate metabolic cross talk between glycogen and lipid metabolism (28). Thus it will be vital to extend work into this area, as alcohol induces opposing actions on these two energy stores. We propose that glycogen-targeting proteins could be new, exciting targets for treating steatosis. Finally, this study provides new information linking circadian disruption and alcohol to detrimental effects on hepatic energy metabolism and presumably liver disease in susceptible populations, including rotating shift workers and heavy alcohol consumers.
GRANTS
This work was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA-018841, R21 AA-020199, and R21 AA-024543 to S. M. Bailey.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.S.U., K.L.G., M.E.Y., and S.M.B. conceived and designed research; U.S.U., J.A.V., T.M.S., and A.N.F. performed experiments; U.S.U., J.A.V., T.M.S., K.L.G., and S.M.B. analyzed data; U.S.U., J.A.V., K.L.G., M.E.Y., and S.M.B. interpreted results of experiments; U.S.U., J.A.V., and S.M.B. prepared figures; U.S.U., J.A.V., and S.M.B. drafted manuscript; U.S.U., J.A.V., K.L.G., M.E.Y., and S.M.B. edited and revised manuscript; U.S.U., J.A.V., T.M.S., A.N.F., K.L.G., M.E.Y., and S.M.B. approved final version of manuscript.
REFERENCES
- 1.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev 23: 155–168, 2010. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SM, Udoh US, Young ME. Circadian regulation of metabolism. J Endocrinol 222: R75–R96, 2014. doi: 10.1530/JOE-14-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baio DL, Czyz CN, Van Horn CG, Ivester P, Cunningham CC. Effect of chronic ethanol consumption on respiratory and glycolytic activities of rat periportal and perivenous hepatocytes. Arch Biochem Biophys 350: 193–200, 1998. doi: 10.1006/abbi.1997.0514. [DOI] [PubMed] [Google Scholar]
- 4.Baraona E, Lieber CS. Alcohol and lipids. Recent Dev Alcohol 14: 97–134, 1998. doi: 10.1007/0-306-47148-5_5. [DOI] [PubMed] [Google Scholar]
- 5.Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res 20: 289–315, 1979. [PubMed] [Google Scholar]
- 6.Bass J. Circadian topology of metabolism. Nature 491: 348–356, 2012. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- 7.Bergmeyer HU, Bernt E, Schmidt F, Stork H. d-Glucose: determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Methods of Enzymatic Analysis (Bergmeyer HU, editor). New York: Academic, 1974, p. 1196–1201. [Google Scholar]
- 8.Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J 336: 19–31, 1998. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes 37: 843–852, 2013. doi: 10.1038/ijo.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr RM, Dhir R, Yin X, Agarwal B, Ahima RS. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res 37: 1091–1099, 2013. doi: 10.1111/acer.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis 12: 3–9, 2011. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health 27: 291–299, 2003. [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich KD. Glycogen determination with amyloglucosidase. In: Methods of Enzymatic Analysis (Bergmeyer HU, editor). New York: Academic, 1974, p. 1127–1131. [Google Scholar]
- 14.Doi R, Oishi K, Ishida N. CLOCK regulates circadian rhythms of hepatic glycogen synthesis through transcriptional activation of Gys2. J Biol Chem 285: 22114–22121, 2010. doi: 10.1074/jbc.M110.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durgan DJ, Moore MW, Ha NP, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, Hickson-Bick DL, Heird WC, Dyck JR, Chandler MP, Young ME. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol 293: H2385–H2393, 2007. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
- 16.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107: 519–527, 1980. doi: 10.1111/j.1432-1033.1980.tb06059.x. [DOI] [PubMed] [Google Scholar]
- 17.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci 119: 283–323, 2013. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer JC, Favre C, Gomis RR, Fernández-Novell JM, García-Rocha M, de la Iglesia N, Cid E, Guinovart JJ. Control of glycogen deposition. FEBS Lett 546: 127–132, 2003. doi: 10.1016/S0014-5793(03)00565-9. [DOI] [PubMed] [Google Scholar]
- 19.Filiano AN, Millender-Swain T, Johnson R Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS One 8: e71684, 2013. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kettner NM, Katchy CA, Fu L. Circadian gene variants in cancer. Ann Med 46: 208–220, 2014. doi: 10.3109/07853890.2014.914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15, Suppl 2: R271–R277, 2006. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 22.Krähenbühl L, Lang C, Lüdes S, Seiler C, Schäfer M, Zimmermann A, Krähenbühl S. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int 23: 101–109, 2003. doi: 10.1034/j.1600-0676.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177, 2008. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res 6: 523–531, 1982. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Lívero FA, Acco A. Molecular basis of alcoholic fatty liver disease: From incidence to treatment. Hepatol Res 46: 111–123, 2016. doi: 10.1111/hepr.12594. [DOI] [PubMed] [Google Scholar]
- 27.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 12: 231–242, 2015. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, Bridges D, Yang Y, Fisher K, Cheng A, Chang L, Meng ZX, Lin JD, Downes M, Yu RT, Liddle C, Evans RM, Saltiel AR. Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes 63: 2935–2948, 2014. doi: 10.2337/db13-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molzof HE, Wirth MD, Burch JB, Shivappa N, Hebert JR, Johnson RL, Gamble KL. The impact of meal timing on cardiometabolic syndrome indicators in shift workers. Chronobiol Int 34: 337–348, 2017. doi: 10.1080/07420528.2016.1259242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr 3: 286–294, 2012. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer FAJL. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms 32: 154–164, 2017. doi: 10.1177/0748730417697537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 199: 337–358, 2012. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 6: 305–323, 1979. [PubMed] [Google Scholar]
- 34.Newgard CB, Brady MJ, O’Doherty RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49: 1967–1977, 2000. doi: 10.2337/diabetes.49.12.1967. [DOI] [PubMed] [Google Scholar]
- 35.O’Hare JD, Zsombok A. Brain-liver connections: role of the preautonomic PVN neurons. Am J Physiol Endocrinol Metab 310: E183–E189, 2016. doi: 10.1152/ajpendo.00302.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J 441: 763–787, 2012. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruchti E, Roach PJ, DePaoli-Roach AA, Magistretti PJ, Allaman I. Protein targeting to glycogen is a master regulator of glycogen synthesis in astrocytes. IBRO Rep 1: 46–53, 2016. doi: 10.1016/j.ibror.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spach PI, Herbert JS, Cunningham CC. The interaction between chronic ethanol consumption and oxygen tension in influencing the energy state of rat liver. Biochim Biophys Acta 1056: 40–46, 1991. doi: 10.1016/S0005-2728(05)80070-2. [DOI] [PubMed] [Google Scholar]
- 39.Steiner JL, Crowell KT, Lang CH. Impact of alcohol on glycemic control and insulin action. Biomolecules 5: 2223–2246, 2015. doi: 10.3390/biom5042223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 41.Summa KC, Jiang P, Fitzpatrick K, Voigt RM, Bowers SJ, Forsyth CB, Vitaterna MH, Keshavarzian A, Turek FW. Chronic alcohol exposure and the circadian clock mutation exert tissue-specific effects on gene expression in mouse hippocampus, liver, and proximal colon. Alcohol Clin Exp Res 39: 1917–1929, 2015. doi: 10.1111/acer.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, Murphy T, Raeisi S, Fogg L, Vitaterna MH, Forsyth C, Turek F, Burgess HJ, Keshavarzian A. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol Gastrointest Liver Physiol 311: G192–G201, 2016. doi: 10.1152/ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Udoh US, Swain TM, Filiano AN, Gamble KL, Young ME, Bailey SM. Chronic ethanol consumption disrupts diurnal rhythms of hepatic glycogen metabolism in mice. Am J Physiol Gastrointest Liver Physiol 308: G964–G974, 2015. doi: 10.1152/ajpgi.00081.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Horn CG, Cunningham CC. Contributions of dietary carbohydrate and ethanol to alterations in liver glycogen levels and glycolytic activity. Alcohol 19: 139–144, 1999. doi: 10.1016/S0741-8329(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 46.Van Horn CG, Ivester P, Cunningham CC. Chronic ethanol consumption and liver glycogen synthesis. Arch Biochem Biophys 392: 145–152, 2001. doi: 10.1006/abbi.2001.2433. [DOI] [PubMed] [Google Scholar]
- 47.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274–285, 2011. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 48.Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, Vitaterna MH, Turek FW, Keshavarzian A. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res 40: 335–347, 2016. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young TA, Bailey SM, Van Horn CG, Cunningham CC. Chronic ethanol consumption decreases mitochondrial and glycolytic production of ATP in liver. Alcohol Alcohol 41: 254–260, 2006. doi: 10.1093/alcalc/agl017. [DOI] [PubMed] [Google Scholar]
- 50.Zani F, Breasson L, Becattini B, Vukolic A, Montani JP, Albrecht U, Provenzani A, Ripperger JA, Solinas G. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and G L expression. Mol Metab 2: 292–305, 2013. doi: 10.1016/j.molmet.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochim Biophys Acta 1807: 1573–1582, 2011. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Rep 4: 3725, 2014. doi: 10.1038/srep03725. [DOI] [PMC free article] [PubMed] [Google Scholar]