Abstract
Early gut mucosal restitution is a process by which intestinal epithelial cells (IECs) migrate over the wounded area, and its defective regulation occurs commonly in various critical pathological conditions. This rapid reepithelialization is mediated by different activating small GTP-binding proteins, but the exact mechanism underlying this process remains largely unknown. Recently, it has been reported that interaction between p21-activated kinase-interacting exchange factor (β-PIX) and G protein-coupled receptor kinase-interacting protein 1 (GIT1) activates small GTPases and plays an important role in the regulation of cell motility. Here, we show that induced association of β-PIX with GIT1 is essential for the stimulation of IEC migration after wounding by activating Rac1. Levels of β-PIX and GIT1 proteins and their association in differentiated IECs (line of IEC-Cdx2L1) were much higher than those observed in undifferentiated IECs (line of IEC-6), which was associated with an increase in IEC migration after wounding. Decreased levels of endogenous β-PIX by its gene-silencing destabilized β-PIX/GIT1 complexes, repressed Rac1 activity and inhibited cell migration over the wounded area. In contrast, ectopic overexpression of β-PIX increased the levels of β-PIX/GIT1 complexes, stimulated Rac1 activity, and enhanced intestinal epithelial restitution. Increased levels of cellular polyamines also stimulated β-PIX/GIT1 association, increased Rac1 activity, and promoted the epithelial restitution. Moreover, polyamine depletion decreased cellular abundances of β-PIX/GIT1 complex and repressed IEC migration after wounding, which was rescued by ectopic overexpression of β-PIX or GIT1. These results indicate that β-PIX/GIT1/Rac1 association is necessary for stimulation of IEC migration after wounding and that this signaling pathway is tightly regulated by cellular polyamines.
NEW & NOTEWORTHY Our current study demonstrates that induced association of β-PIX with GIT1 is essential for the stimulation of intestinal epithelial restitution by activating Rac1, and this signaling pathway is tightly regulated by cellular polyamines.
Keywords: cell migration, cellular polyamines, early rapid mucosal repair, gut mucosal injury, intestinal epithelial cells
INTRODUCTION
The successful repair of damaged mucosa and ulcers in the gastrointestinal (GI) tract requires epithelial cell decisions that regulate signaling networks controlling expression of different genes involved in cell survival, migration, and proliferation. Early mucosal restitution is an important and primary repair modality in the GI tract, and its defective regulation underlies various critical pathological states such as mucosal bleeding and ulcers, disruption of epithelium integrity, and gut barrier dysfunction (13, 26, 47). Epithelial restitution occurs as a consequence of intestinal epithelial cell (IEC) migration to reseal superficial wounds, and this process is independent of cell proliferation (7, 26, 43, 45). This rapid IEC migration after wounding is tightly regulated by numerous extracellular and intracellular factors at multiple levels, but the exact mechanism underlying this process remains to be fully investigated. Our previous studies demonstrate that an activating small GTP-binding protein, Rac1, is essential for stimulation of IEC migration after wounding, whereas Rac1 silencing inhibits intestinal epithelial restitution (36).
The small GTPases, particularly members of the Rho family, regulate a diverse array of intracellular signaling pathways and affect actin-myosin cytoskeletal reorganization, thus modulating cell spreading, adhesion turnover, and migration (6, 11, 19, 28, 42). A large family of guanine nucleotide exchange factors (GEFs) functions as the upstream effectors and controls activation and inactivation of various small GTPases (10, 44). The p21-activated kinase-interacting exchange factor (β-PIX) is an important member of the GEF family, and it specifically modulates the activities of Rac1 and Cdc42 (1, 2, 9, 23). It has been reported that specific deletion of β-PIX or its defective mutant inhibits cell motility by altering focal adhesion turnover (8, 20, 27). Recently, β-PIX has been shown to reduce focal adhesion disassembly and promote cell motility by regulating myosin light chain activation in keratinocytes (15). β-PIX and Rac1 are also implicated in the regulation of p21-activated kinase-mediated phosphorylation of myosin light chain, events that are required for stimulation of cell migration (54). In another study, Kuo et al. (20) document that β-PIX plays an important role in the negative regulation of focal adhesion maturation and affects the promotion of lamellipodial protrusion and turnover to drive cell migration in human foreskin fibroblasts (HFF1). β-PIX is shown to be a substrate for focal adhesion kinase, and focal adhesion kinase-induced β-PIX phosphorylation enhances its binding to Rac1 and induces the recruitment of β-PIX to the focal complex (3, 5, 46). β-PIX also interacts with G protein-coupled receptor kinase interactor 1 (GIT1) via the GIT-binding site in the carboxy-terminal portion of β-PIX, and this complex acts as a scaffold for multiple signaling proteins in response to various stressful environments (10, 17, 25, 56).
The GIT has two isoforms: GIT1 and GIT2; both are GTPase-activating proteins (GAPs) that share a conserved domain architecture and associate with several protein-binding partners (16, 56). Upon binding to Rac1, GIT1 and GIT2 regulate cytoskeletal dynamics, affect membrane trafficking, and maintain the directional cell migration (10, 22). GIT1 has also been identified as a direct interactor with G protein-coupled receptor kinase (GRK), thereby regulating ADP ribosylation factor (Arf)-dependent membrane trafficking (29, 30, 56). The β-PIX/GIT1 complex can interact with PLC-γ1, and this interaction is required for activation of Rac1 and Cdc42 (16, 17). The present study tests the hypothesis that the interaction of β-PIX with GIT1 regulates IEC migration over the wounded area by altering Rac1 activity. First, we determined the expression pattern of β-PIX and GIT1 and their interactions during epithelial restitution after wounding in IECs. Second, we examined whether manipulating the cellular levels of β-PIX affects IEC migration by altering β-PIX/GIT1/Rac1 association. Finally, we determined whether expression of β-PIX and GIT1 and their interaction are regulated by cellular polyamines. Our findings indicate that β-PIX promotes cell migration after wounding by interacting with GIT1 and Rac1 and that polyamines are required for expression of β-PIX and GIT1 in IECs.
MATERIALS AND METHODS
Chemicals and cell culture.
Disposable culture ware was purchased from Corning Glass Works (Corning, NY). Tissue culture media, LipofectAMINE 2000, and dialyzed fetal bovine serum (dFBS) were obtained from Invitrogen (Carlsbad, CA), and chemicals were obtained from Sigma (St. Louis, MO). The affinity-purified rabbit polyclonal antibodies against β-PIX (cat. no. 4515) and GIT1 (cat. no. 2919) were purchased from Cell Signaling Technologies (Danvers, MA), and the antibodies against Rac1 (cat. no. SC-217) and GAPDH (cat. no. SC-25778) were from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibody conjugated to horseradish peroxidase was purchased from Sigma. l-α-Difluoromethylornithine (DFMO) was from Genzyme (Cambridge, MA).
The IEC-6 cell line was purchased from the American Type Culture Collection (ATCC) at passage 13. IEC-6 cells were derived from normal rat intestinal crypt cells and were developed and characterized by Quaroni et al. (31). Stock cells were maintained in T-150 flasks in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate. Flasks were incubated at 37○C in a humidified atmosphere of 90% air and 10% CO2, and passages 15–20 were used in the experiments. Stable Cdx2-transfected IEC cells (IEC-Cdx2L1) were developed from IEC-6 cells and maintained as described previously (32, 35, 41, 53). Before experiments, IEC-Cdx2L1 cells were grown in DMEM containing 4 mM IPTG for 16 days to induce cell differentiation, as described earlier (32, 35, 36). Stable line of IECs overexpressing ornithine decarboxylase (ODC) (ODC-IEC) was developed from IEC-6 cells as described (38, 57). ODC-IECs highly expressed a more stable ODC variant with full enzyme activity and contained high levels of cellular polyamines including putrescine, spermidine, and spermine (12, 13, 57). Caco-2 cells were purchased from ATCC and maintained in standard culture conditions as described (36, 40, 41).
IEC-6 cells originated from intestinal crypts; they are nontumorigenic and retain undifferentiated status of intestinal crypt cells, whereas IEC-Cdx2L1 cells represented differentiated IECs. As reported in our previous studies, induced expression of the Cdx2 gene in IEC-Cdx2L1 cells by treatment with 4 mM IPTG for 16 days resulted in a differentiated phenotype. These differentiated IEC-Cdx2L1 cells were polarized and exhibited multiple morphological and molecular characteristics of intestinal epithelial differentiation. This line of differentiated IEC-Cdx2L1 cells has been extensively used and is widely accepted as the best in vitro model system for cell division-independent stage of epithelial restitution (35, 36, 41). Our previous studies have shown that differentiated IEC-Cdx2L1 cells exhibit increased cell migration compared with that observed in undifferentiated IEC-6 cells after wounding (32). For this reason, we used IEC-Cdx2L1 cells as model in the study of loss-of-function of β-PIX. The reason for choosing Caco-2 cells as a model for study of gain-of-function is that this line of cells represents polarized human colonic epithelial cells and provides extended findings in human cells.
RNA interference and plasmid construction.
The small interfering (si)RNA that was designed to specifically cleave β-PIX mRNAs (siβ-PIX) was synthesized and purchased from Dharmacon (Lafayette, CO; cat. no. L098943-02-0020). Scrambled control siRNA (C-siRNA), without the sequence homology to any known genes, was used as the control. For each 60-mm cell culture dish, 20 µl of the 5 µM stock siβ-PIX or C-siRNA was mixed with 500 µl of Opti-MEM medium (Invitrogen). This mixture was gently added to a solution containing 6 µl of LipofectAMINE 2000 in 500 µl of Opti-MEM. The solution was incubated for 15 min at room temperature and gently overlaid onto monolayers of cells in 3 ml of medium, and cells were harvested for various assays after 48-h incubation.
The β-PIX or GIT1 expression vector that contains the full-length wild-type β-PIX was purchased from Origene Technologies (Rockville, MD; cat. no. SC318985), or GIT1 cDNA was from Addgene (Cambridge, MA; cat. no. 15225), in which β-PIX or GIT1 expression was directed by the pCMV promoter. Cells were transfected with the β-PIX or GIT1 expression vectors by using the LipofectAMINE 2000 and performed as recommended by the manufacturer (Invitrogen).
Immunoprecipitation and immunoblotting analysis.
Cell samples, dissolved in ice-cold RIPA-buffer, were sonicated and centrifuged at 4°C, and then the supernatants were collected for immunoprecipitation (IP). Equal amounts of proteins (400 μg) for each sample were incubated with the specific antibody against β-PIX or GIT1 (4 μg) at 4°C for 3 h, and protein A/G-PLUS-Agarose was added and incubated overnight at 4°C. The precipitates were washed five times with ice-cold Tris-buffered saline (TBS), and the beads were resuspended in SDS sample buffer. For immunoblotting, samples were subjected to electrophoresis on PAGE gels described previously (32–35, 53). Briefly, after the transfer of protein onto nitrocellulose membranes, the membranes were incubated for 1 h in 5% nonfat dry milk in 1× TBS-T buffer (0.1% Tween 20). Immunological evaluation was then performed overnight at 4°C in 5% nonfat dry milk-TBS-T buffer containing a specific antibody against β-PIX, GIT1, or Rac1. The membranes were subsequently washed with 1× TBS-T and incubated with the secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. The immunocomplexes on the membranes were reacted for 1 min with chemiluminescence reagent (NEL-100, DuPont-NEN).
Analysis of newly translated protein.
Levels of nascent synthesized β-PIX or GIT1 proteins were detected with a Click-iT protein analysis detection kit (Life technologies, Grand Island, NY) and performed following the company’s manual with minor modification (55). Briefly, cells were incubated in methionine-free medium and then exposed to l-azidohomoalanine (AHA). Cell lysates were mixed with reaction buffer containing biotin-alkyne reagent and CuSO4 for 20 min, and the biotin-alkyne-azide-modified protein complex was pulled down using paramagnetic streptavidin-conjugated Dynabeads. The pull-down material was resolved by 10% SDS-PAGE and analyzed by Western immunoblotting analysis using antibody against β-PIX, GIT1, or GAPDH.
Measurement of cell migration.
Migration assays were carried out as described in our earlier publications (6, 32, 40, 41, 53). Cells were plated at 6.25 × 104/cm2 in DMEM containing FBS on 60-mm dishes thinly coated with Matrigel according to the manufacturer’s instructions (BD Biosciences, Bedford, MA) and were incubated as described for stock cultures. Cells were fed on day 2, and cell migration was assayed on day 4. To initiate migration, the cell layer was scratched with a single-edge razor blade cut to ~27 mm in length. The scratch was made over the diameter of the dish and extended over an area 7–10 mm wide. The migrating cells in six contiguous 0.1-mm squares were counted at ×100 magnification beginning at the scratch line and extending as far out as the cells had migrated. All experiments were carried out in triplicates, and the results were reported as number of migrating cells per millimeter of scratch.
Statistical analysis.
All data for migration experiments are expressed as means ± SE from six dishes in one experiment and repeated three times (n = 3). IP and immunoblotting results were repeated three times. The significance of the difference between means was determined by ANOVA. The level of significance was determined using Duncan’s multiple range test (14), and values of P < 0.05 were considered significant.
RESULTS
Expression of β-PIX and GIT1 proteins and their interactions in IECs.
To determine whether the interaction of β-PIX with GIT1 plays a role in the regulation of intestinal epithelial restitution, basal expression of β-PIX, Rac1, and GIT1 proteins and their interaction were examined in three different lines of IECs, including IEC-6 (nontumorigenic and undifferentiated crypt cells), differentiated IEC-Cdx2L1 (exhibiting multiple morphological and molecular characteristics of intestinal villous cells), and Caco-2 cells (human colonic epithelial cells exhibiting polarization). All three lines of IECs have been extensively used and widely accepted as the in vitro model system for the cell division-independent stage of epithelial restitution (32–41). As shown in Fig. 1A, all three lines of IECs expressed β-PIX, Rac1, and GIT1 proteins, but the differentiated IEC-Cdx2L1 and Caco-2 cells contained higher basal levels of β-PIX, Rac1, and GIT1 than those observed in the undifferentiated IEC-6 cells. To examine β-PIX/GIT1 association, whole cell lysates were incubated with the antibody specifically against β-PIX for IP; the levels of β-PIX and GIT1 in the pull-down materials were examined by using anti-GIT1 or β-PIX antibody. As shown, β-PIX associated with GIT1 in all three cell lines, although the levels of β-PIX/GIT1 complexes in differentiated IEC-Cdx2L1 and Caco-2 cells increased compared with those observed in IEC-6 cells (Fig. 1B). We also used IgG as a negative control in IP assays and found that the incubation of cell lysates with IgG in the same condition did not pull down either β-PIX or GIT1 protein (data not shown). These results indicate that β-PIX physically interacts with GIT1 and forms β-PIX/GIT1 complexes in IECs.
Fig. 1.
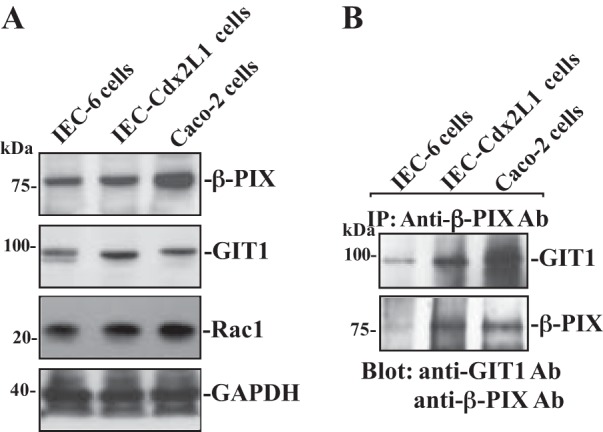
β-PIX (p21-activated kinase-interacting exchange factor) and GIT1 (G-protein-coupled receptor kinase-interacting protein 1) protein expression and their interactions in different lines of intestinal epithelial cells (IECs). A: representative immunoblots of β-PIX, GIT1, and Rac1 in IEC-6 (undifferentiated IECs), IEC-Cdx2L1 [differentiated IECs (line of IEC-Cdx2L1)], and Caco-2 cells. Levels of total β-PIX and GIT1 were examined by Western blot analysis, and GAPDH immunoblotting was performed as an internal control for equal loading. B: levels of GIT1 and β-PIX in materials immunoprecipitated (IP) by the anti-β-PIX antibody (Ab) in cells described in A. After whole cell lysates (400 µg) were IP by the specific antibody against β-PIX, precipitates were separated by performing SDS-PAGE gels. Levels of GIT1 and β-PIX were measured using Western blot analysis with specific antibodies. Three separate experiments were performed that showed similar results.
Levels of β-PIX, Rac1, and GIT1 increase in migrating cells after wounding.
To determine the involvement of β-PIX, Rac1, and GIT1 in the regulation of intestinal epithelial restitution, we examine changes in expression levels of β-PIX, Rac1, and GIT1 after wounding in differentiated IEC-Cdx2L1 cells. As shown in Fig. 2A, protein levels of β-PIX, Rac1, and GIT1 increased dramatically after wounding. The increase in β-PIX, Rac1, and GIT1 proteins occurred at 30 min after wounding, peaked at 60 min, and then gradually declined. Maximum increases in the levels of β-PIX and GIT1 after wounding were approximately three- to fivefold the prewounding control level. The β-PIX, Rac1, and GIT1 content was returned to near-normal level at 240 min after wounding. GAPDH protein served as a loading control in this study and exhibited no changes in its levels after wounding. Consistent with the immunoblotting results, β-PIX/GIT1/Rac1 association was also increased after wounding (Fig. 2B). The immunocomplexes of β-PIX/GIT1/Rac1 occurred rapidly after wounding (~30 min), and remained elevated for >120 min. Furthermore, the newly synthesized β-PIX and GIT1 protein levels were also significantly increased at ~30 min after wounding and returned to near-normal levels within ~120 min (Fig. 2C). These findings clearly indicate that β-PIX and GIT1 proteins rapidly increase after wounding, which is paralleled by an increase in cell migration, as was shown in our previous studies (37, 39).
Fig. 2.
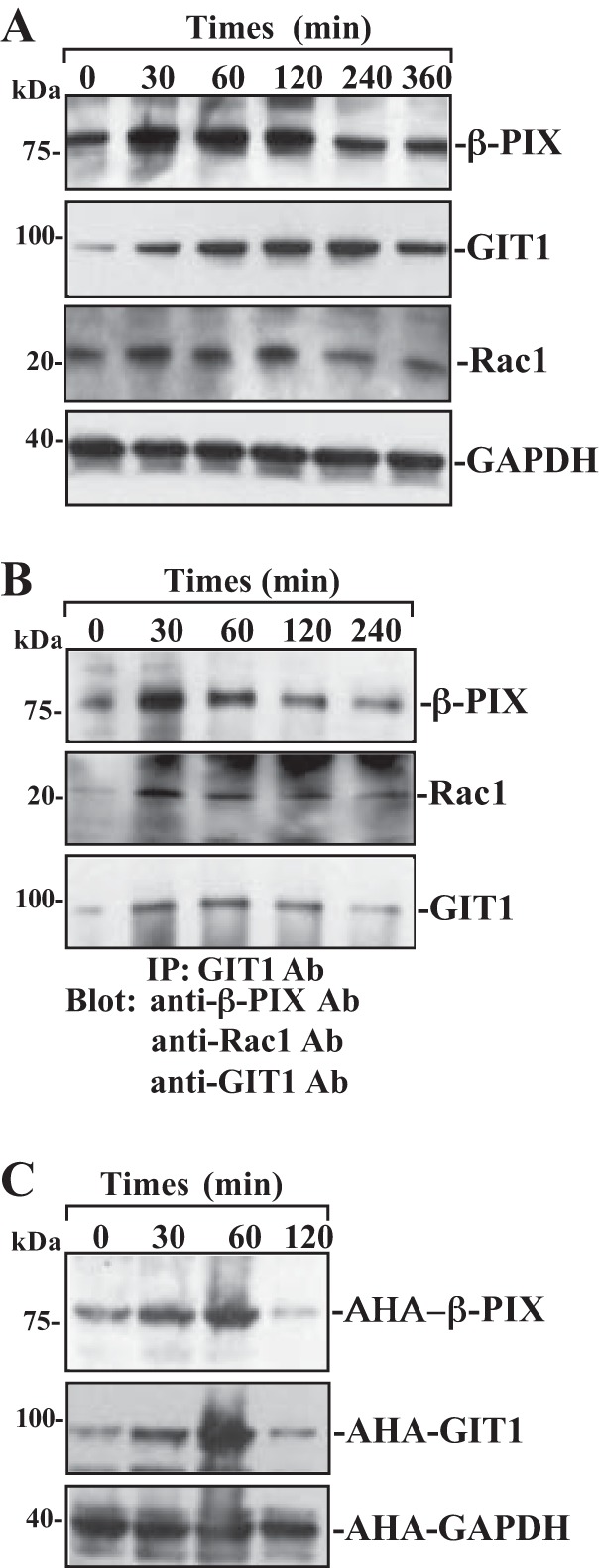
Changes in the levels of β-PIX, GIT1, Rac1, and β-PIX/GIT1/Rac1 association after wounding. After IEC-Cdx2L1 cells were grown to confluence, epithelial restitution was induced by removing part of the monolayer, as described in materials and methods. A: levels of β-PIX, GIT1, and Rac1 proteins in total cell lysates isolated at various times after wounding and detected by Western blot analysis. GAPDH immunoblotting was performed as an internal control for equal loading. B: levels of β-PIX, GIT1, and Rac1 proteins in IP materials by the anti-GIT1 Ab from the samples described in A. C: newly synthesized β-PIX and GIT1 proteins as measured by Click-IT protein analysis using l-azidohomoalanine (AHA) in samples described in A. Three experiments were performed that showed similar results.
β-PIX silencing reduces β-PIX/GIT1 complexes and represses epithelial restitution after wounding.
Specific siRNA targeting β-PIX mRNA (siβ-PIX) was used to cleave rat β-PIX mRNA and inhibit β-PIX expression in this study. Our initial study demonstrated that >95% of differentiated IEC-Cdx2L1 cells were positive when they were transfected with fluorescent FITC-conjugated siβ-PIX for 48 h. As shown in Fig. 3A, transfection with siβ-PIX for 48 h decreased β-PIX levels by >85%. To determine the specificity of siβ-PIX used in this study, we reprobed the membrane with anti-GIT1 antibody and showed that levels of GIT1 protein were not affected when cells were transfected with siβ-PIX. However, Rac1 levels were decreased following the inhibition of β-PIX protein. β-PIX silencing by transfection with siβ-PIX also decreased β-PIX/GIT1/Rac1 complexes (by ~60%) compared with those observed in control cells and cells transfected with control siRNA (C-siRNA) (Fig. 3B). Furthermore, decreased β-PIX/GIT1/Rac1 association by β-PIX silencing also suppressed cell migration (by ~45%) after wounding (Fig. 3C). Transfection with C-siRNA at the same concentrations showed no inhibitory effects on β-PIX expression and cell migration. In addition, neither siβ-PIX nor C-siRNA affected cell viability as measured by trypan blue staining (data not shown).
Fig. 3.
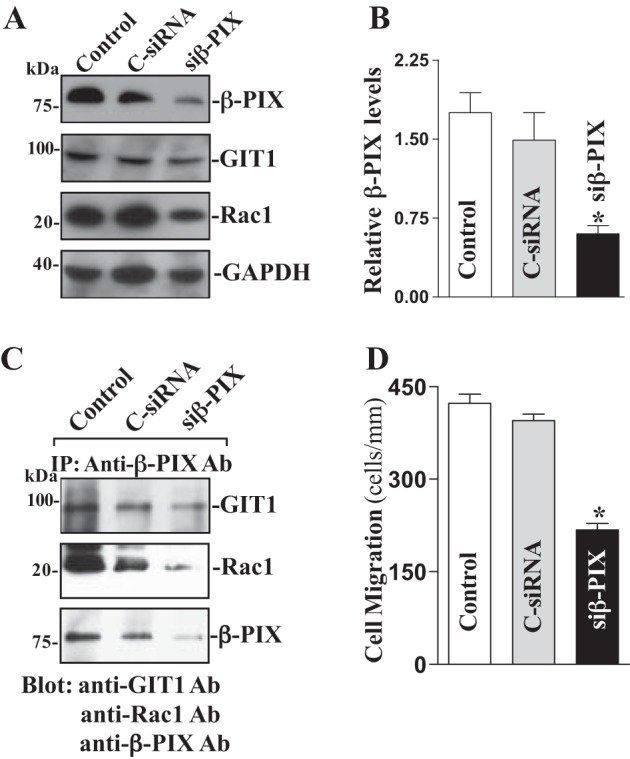
β-PIX silencing on β-PIX/GIT1/Rac1 interactions and cell migration. A: representative immunoblots of β-PIX, GIT1 and Rac1. IEC-Cdx2L1 cells were transfected with control siRNA (C-siRNA) or siβ-PIX by LipofectAMINE 2000, and whole cell lysates were harvested 48 h thereafter. Levels of β-PIX, GIT1, and Rac1 proteins were measured by Western immunoblot analysis, and GAPDH immunoblotting was performed as an internal control for equal loading. Three separate experiments were performed that showed similar results. B: quantitative analysis of β-PIX immunoblots by densitometry that were corrected for GAPDH loading from cells described above. Values are means ± SE. * P < 0.05 vs. C-siRNA-transfected cells. C: levels of GIT1, Rac1, and β-PIX proteins in IP materials by the anti-β-PIX Ab from the samples described in A. D: summarized data of cell migration 6 h after wounding in cells transfected with siβ-PIX for 48 h. Data are means ± SE from 6 dishes. *P < 0.05 vs. cells transfected with C-siRNA.
Ectopic overexpression of the β-PIX gene increases β-PIX/GIT1/Rac1 complexes and promotes cell migration.
To further define the role of β-PIX activity in the regulation of epithelial restitution, we examined the effect of ectopic overexpression of the β-PIX gene on levels of β-PIX/GIT1/Rac1 complex and cell migration in Caco-2 cells. This cell line was chosen for this study because it represents human epithelial cells and provides an excellent model for transient transfection. When Caco-2 cells were transfected with the expression vector containing the corresponding β-PIX cDNA under the control of the pCMV promoter, levels of β-PIX protein were increased approximately sevenfold at 48 and 72 h thereafter (Fig. 4A). The vector that lacked exogenous β-PIX cDNA (Null) was used as a negative control in this experiment and did not alter the β-PIX level. In addition, transfection with the β-PIX expression vector had no effect on levels of GIT1 protein. To determine whether increased levels of β-PIX enhanced β-PIX/GIT1/Rac1 association, whole cell lysates were immunoprecipitated with the specific anti-β-PIX antibody. As shown in Fig. 4B, levels of the β-PIX/GIT1/Rac1 complex were higher in the cells overexpressing β-PIX than those observed in cells transfected with control vector. Moreover, ectopically expressed β-PIX also enhanced cell migration after wounding (Fig. 4C). The number of cells migrating over the wounded edge in cells overexpressing β-PIX was increased by ~25% compared with control cells. These results indicate that increasing cellular β-PIX abundance promotes epithelial restitution after wounding by enhancing β-PIX/GIT1/Rac1 association.
Fig. 4.
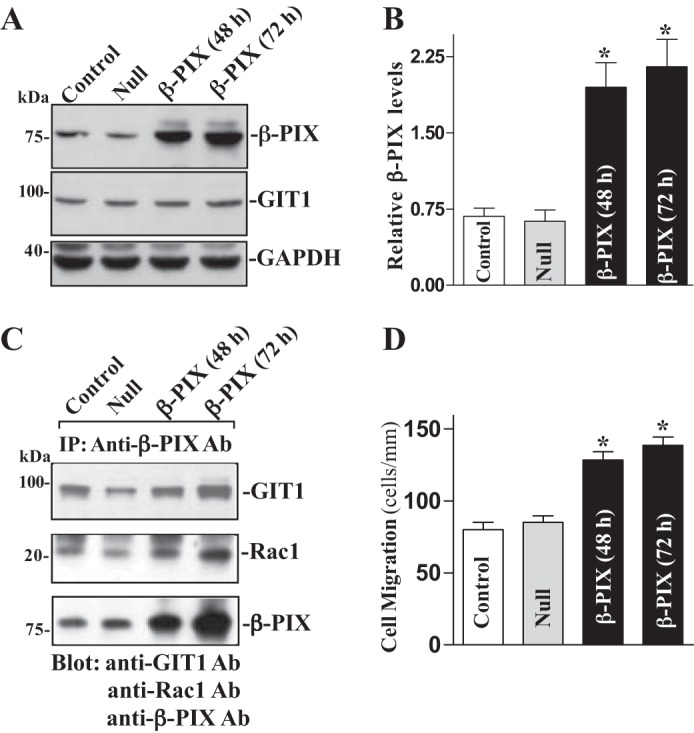
Ectopic overexpression of β-PIX on β-PIX/GIT1/Rac1 association and cell migration. A: representative immunoblots of β-PIX and GIT1. Caco-2 cells were transfected with β-PIX expression vector or empty vector (Null) by LipofectAMINE 2000 for 48 and 72 h, and levels of β-PIX and GIT1 proteins were examined by Western immunoblotting analysis. Three separate experiments were performed that showed similar results. B: quantitative analysis of β-PIX immunoblots by densitometry that were corrected for GAPDH loading from cells described above. Values are means ± SE. *P < 0.05 vs. Null cells. C: levels of GIT1, Rac1, and β-PIX proteins in IP materials by the anti-β-PIX Ab from the samples described in A. D: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE from 6 dishes. *P < 0.05 vs. cells transfected with Null.
Increased levels of cellular polyamines enhance β-PIX/GIT1 association and stimulates cell migration.
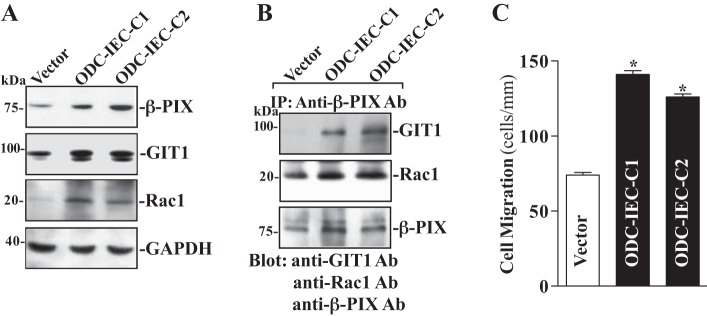
Polyamines, including spermidine, spermine, and their precursor putrescine, are organic cations found in all eukaryotic cells and act as biological regulators of gut mucosal repair after injury (48, 49, 51, 52). Our previous studies demonstrated that cellular polyamines promote intestinal epithelial restitution primarily by activating Rac1 signaling (36). To determine the effect of cellular polyamines on β-PIX and GIT1 expression and their interactions, two clonal populations of IEC cells stably overexpressing ODC (ODC-IEC) (38, 57) were used in this study. These stable ODC-IEC cells exhibited very high levels of ODC protein and a greater than 50-fold increase in ODC enzyme activity. Consistently, the levels of putrescine, spermidine, and spermine in ODC-IEC cells were increased ~12-fold, ~2-fold, and ~25%, respectively, compared with cells transfected with the control vector lacking ODC cDNA, as described in our previous studies (13, 57). As shown in Fig. 5, ODC-IEC cells displayed a substantial increase in the levels of β-PIX and GIT1 expression. The levels of β-PIX protein were increased more then threefold in stable ODC-IEC cells compared with those observed in cells transfected with the control vector. The level of GIT1 protein was increased by >50%. The increased expressions of β-PIX and GIT1 in ODC-transfected cells were not simply due to clonal variation, since two stable clones, ODC-IEC-C1 and ODC-IEC-C2, showed similar responses. Increasing the levels of cellular polyamines by ectopic ODC overexpression in stable ODC-IEC cells also increased β-PIX/GIT1 association (Fig. 5B) and stimulated the epithelial restitution, as indicated by an increase in cell migration after wounding (Fig. 5C). The number of cells migrating over the denuded area was increased by ~40% in stable ODC-IEC cells compared with those observed in control cells. These findings indicate that increased levels of cellular polyamines enhance β-PIX/GIT1/Rac1 association and stimulate epithelial restitution.
Fig. 5.
Changes in levels of β-PIX, GIT1, and Rac1 expression, their association, and cell migration after increasing levels of cellular polyamines. A: changes in β-PIX, GIT1 and Rac1 protein levels in clonal (C) populations of stable line of IECs overexpressing ornithine decarboxylase (ODC) (ODC-IEC) cells and control cells (Vector). IEC-6 cells were infected with either the retroviral vector containing the sequence encoding mouse ODC cDNA or control retroviral vector lacking ODC cDNA. Clones resistant to the selection medium containing 0.6 mg/ml G418 were isolated and screened for ODC expression. Levels of β-PIX, GIT1, and Rac1 proteins were measured by Western blot analysis, and equal loading was monitored by GAPDH immunoblotting. Three separate experiments were performed that showed similar results. B: levels of GIT1, Rac1, and β-PIX proteins in IP materials by the anti-β-PIX Ab from samples described in A. C: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 vs. Vector cells.
Polyamine depletion decreases levels of β-PIX, Rac1, and GIT1 and repress cell migration.
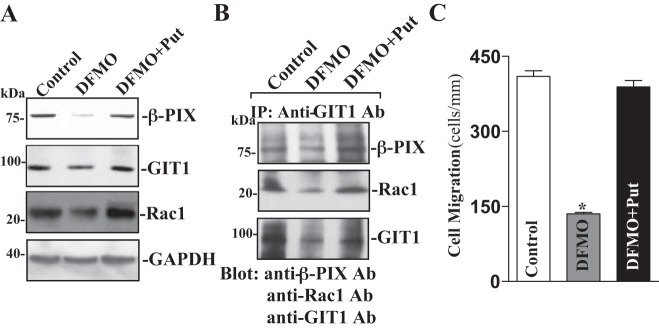
To further determine the role of endogenous polyamines in the regulation of the β-PIX/GIT1/Rac1 pathway, differentiated IEC-Cdx2L1 cells were exposed to 5 mM DFMO (a specific inhibitor of polyamine biosynthesis). As reported in our previous studies (12, 32), exposure to DFMO for 4 days completely depleted putrescine and spermidine and substantially decreased spermine content. As shown, polyamine depletion by DFMO inhibited expression of β-PIX, Rac1, and GIT1, and the levels of β-PIX, Rac1, and GIT1 proteins in polyamine-deficient cells were decreased by ~50% (Fig. 6A). Consistently, levels of β-PIX/GIT1/Rac1 complex were also decreased by polyamine depletion (Fig. 6B). In the presence of DFMO, addition of the exogenous polyamine putrescine (10 μM) to the cultures not only prevented the decreased levels of total β-PIX, Rac1, and GIT1 proteins but also restored the content of the β-PIX/GIT1/Rac1 complex to near normal level. Polyamine depletion-induced inhibition of β-PIX/GIT1/Rac1 association was also associated with decreases in cell migration after wounding (Fig. 6C). The number of cells migrating over the wounded edge was decreased by ~75% in DFMO-treated cells. Interestingly, restoration of levels of β-PIX/GIT1/Rac1 complex by exogenous putrescine given together with DFMO abolished the inhibition of cell migration in polyamine-deficient cells.
Fig. 6.
Changes in levels of β-PIX, GIT1, and Rac1 proteins, their association, and cell migration after polyamine depletion. A: representative immunoblots of β-PIX, GIT1, and Rac1 proteins. Cells were grown in DMEM containing l-α-difluoromethylornithine (DFMO; 5 mM) alone or DFMO plus putrescine (Put, 10 μM) for 4 days. Levels of β-PIX, GIT1, and Rac1 proteins were measured by Western blot analysis, and equal loading was monitored by GAPDH immunoblotting. Three separate experiments were performed that showed similar results. B: levels of β-PIX, GIT1, and Rac1 proteins in the complex IP by the anti-GIT1 Ab from samples described in A. C: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 vs. control cells or cells treated with DFMO plus Put.
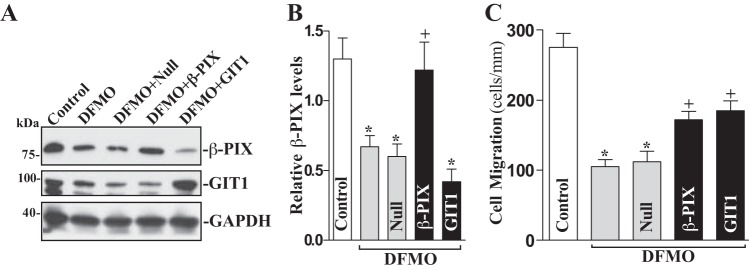
Moreover, ectopic overexpression of β-PIX and GIT1 also prevented inhibition of cell migration induced by polyamine depletion (Fig. 7). In this study, cells were exposed to DFMO for 2 days and then transfected with either the β-PIX or GIT1 expression vector for 48 h in the presence of DFMO. Ectopic overexpression of β-PIX and GIT1 significantly rescued cell migration after wounding in polyamine-deficient cells (Fig. 7B). The number of cells migrating over the denuded area was increased by ~25% in polyamine-deficient cells transfected with either the β-PIX or GIT1 expression vector compared with DFMO-treated cells transfected with empty vector (Null). Taken together, these results indicate that polyamines enhance intestinal epithelial restitution after injury, at least partially, by activating β-PIX/GIT1/Rac1 signaling pathway.
Fig. 7.
Effect of β-PIX and GIT1 overexpression on cell migration in polyamine-deficient cells. A: representative immunoblots of β-PIX and GIT1. Cells were exposed to 5 mM DFMO for 2 days and then transfected with either β-PIX or GIT1 expression vector or empty vector (Null) for 48 h in the presence of DFMO. Levels of β-PIX and GIT1 protein levels were measured by Western blot analysis, and equal loading was monitored by GAPDH immunoblotting. Three experiments were performed that showed similar results. B: quantitative analysis of β-PIX immunoblots by densitometry that were corrected for GAPDH loading from cells described above. Values are means ± SE. *P < 0.05 vs. control cells; +P < 0.05 vs. DFMO alone. C: summarized data showing cell migration 6 h after wounding in cells described in A. Values are means ± SE of data from 6 dishes. *P < 0.05 vs. control cells; +P < 0.05 vs. DFMO alone.
DISCUSSION
Early epithelial restitution in the GI mucosa occurs by sloughing of the damaged epithelial cells and migration of remaining viable cells from areas adjacent to or just beneath the injured surface to cover the wounded area (7, 26, 47). Although precise regulation of epithelial restitution to reseal superficial wounds is crucial for the maintenance of mucosal integrity under physiological and pathological conditions, the exact mechanism underlying this primary repair modality remains poorly understood. Our previous studies have shown that small GTPases such as RhoA and Rac1 regulate cell migration after injury by controlling cytoskeletal reorganization, and this process is tightly regulated by intracellular polyamines (6, 33, 36). Here, we provide new evidence showing that the β-PIX/GIT1 signaling pathway is also involved in regulating IEC migration after wounding by interacting with Rac1, thereby advancing our understanding of the mechanism of early mucosal restitution. Among the salient findings in this study is the discovery that β-PIX directly interacts with GIT1 in IECs, and the induced β-PIX/GIT1 complex is necessary for stimulation of cell migration after wounding. In addition, our findings also reveal that polyamines positively modulate expression of β-PIX and GIT1 in IECs and stimulate epithelial restitution, at least partially, by enhancing β-PIX/GIT1-mediated Rac1 signaling.
The results reported herein clearly indicate that β-PIX physically interacted with GIT1 and formed the β-PIX/GIT1/Rac1 complex. This induced association after wounding promotes epithelial restitution, since silencing β-PIX by transfection with siβ-PIX inhibited formation of β-PIX/GIT1/Rac1 complexes and suppressed cell migration. In contrast, ectopic overexpression of β-PIX increased β-PIX/GIT1/Rac1 association and stimulated cell migration over the wounded area. Consistent with our current observations, Rac1 activation is shown to sufficiently stimulate cell migration after wounding in IECs (36). Kuo et al. (20) reported that β-PIX is required for the Rac1 activity and rapid nascent adhesion turnover, leading to the negative regulation of focal adhesion maturation, lamellipodial protrusion, and enhancement of cell migration in human foreskin fibroblast cells. Park et al. (27) found that β-PIX is a predominant Rac-GEF, whereas Rac1 activation is abolished by silencing β-PIX. Restoration of β-PIX expression by genetic manipulation restores the ability of migration in mesenchymal stromal cells isolated from patients of amyotrophic lateral sclerosis (18). Disrupted function of β-PIX is also involved in the inhibition of cell migration following the use of anti-inflammatory drugs such as rosiglitazone and AS601245 in humans (4).
It has been reported that β-PIX/GIT1 association and their complex scaffolding regulate a wide variety of cellular processes through interaction with many molecules, pathways, and signaling partners (10, 56). The β-PIX/GIT1 complex serves as a dynamic link between cell adhesion and/or migration and cytoskeletal reorganization (21, 22). On the other hand, β-PIX/GIT1 complex also acts as an upstream signaling effector that is initiated by GPCRs and integrins (10, 50). Although β-PIX and GIT1 are tightly associated, there is evidence showing that β-PIX and GIT1 proteins also function independently (10, 56). Induction of GIT1 increases Cdc42/Rac1-dependent membrane protrusion and spreading during migration in fibroblasts (17). Our findings suggest that β-PIX/GIT1 complex is necessary for Rac1 activation after wounding. GIT1 is widely studied protein that exists almost entirely full-length form and is mostly restricted to epithelial cells and vasculature, whereas GIT2 is extensively alternatively spliced (short and long forms) in a tissue-specific manner (10, 16). Because of limited information available about basal levels, sizes, and function of GIT2 in IECs, its exact role in intestinal epithelial restitution after wounding should be fully investigated in a separate study in the future.
The present study also indicates that polyamines are required for expression of β-PIX and GIT1 in IECs. An increasing body of evidence indicates that polyamines are intimately implicated in a wide variety of distinct biological functions (24, 38, 48, 49, 57) and that they enhance early mucosal restitution in the GI mucosa (51, 52). Our previous studies show that cellular polyamines stimulate epithelial cell migration after wounding primarily by activating small GTPases (6, 33, 36), but the exact process by which polyamines regulate small GTPases remains unclear. The present study provides new evidence indicating that polyamines activate Rac1 by increasing its interaction with β-PIX/GIT1 complex, since induced β-PIX/GIT1 association by increasing cellular polyamines in ODC-IEC cells increased the levels of β-PIX/GIT1/Rac1 complex (Fig. 5B). Polyamines also regulate the activity of small GTPases by altering stromal interaction molecule 1 (STIM1)/transient receptor potential canonical 1 (TRPC1)-mediated Ca2+ signaling (34, 38, 49) and c-Jun/PLC-γ1 pathways (35, 53).
In summary, our results indicate that β-PIX physically interacts with GIT1 and Rac1 and forms the β-PIX/GIT1/Rac1 complex in IECs. The increase in the levels of this complex after injury is essential for stimulation of cell migration during intestinal epithelial restitution. Specific inhibition of β-PIX prevents β-PIX/GIT1/Rac1 association and represses cell migration after wounding, whereas ectopically expressed β-PIX increases formation of the β-PIX/GIT1/Rac1 complexes and stimulates epithelial restitution. Our results further show that polyamines regulate expression of β-PIX and GIT1 in IECs and that depletion of cellular polyamines decreases cellular abundances of β-PIX and GIT1 proteins and reduces their association with Rac1. This study also provides new evidence showing that polyamines stimulate intestinal epithelial restitution, at least partially, by altering the β-PIX/GIT1/Rac1 signaling pathway. Because cellular levels of β-PIX and GIT1 increase dramatically after injury and their association with Rac1 is crucial for stimulation of cell migration, these findings suggest that the β-PIX/GIT1/Rac1 pathway plays an important role in maintaining GI epithelial integrity under physiological and pathological conditions.
GRANTS
This work was supported by Merit Review Awards from the Department of Veterans Affairs (J. N. Rao, D. J. Turner, and J.-Y. Wang) and by National Institutes of Health Grants DK-57819, DK-61972, and DK-68491 (J,-Y. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R., H.K.C., S.R.W., and M.Q. performed experiments; N.R., H.K.C., S.R.W., D.J.T., J.-Y.W., and J.N.R. analyzed data; N.R., H.K.C., D.J.T., J.-Y.W., and J.N.R. interpreted results of experiments; N.R. and J.N.R. prepared figures; N.R. and J.N.R. drafted manuscript; D.J.T., J.-Y.W., and J.N.R. approved final version of manuscript; J.-Y.W. and J.N.R. conceived and designed research; J.-Y.W. edited and revised manuscript.
REFERENCES
- 1.Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem 273: 23633–23636, 1998. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 2.Baird D, Feng Q, Cerione RA. The Cool-2/α-Pix protein mediates a Cdc42-Rac signaling cascade. Curr Biol 15: 1–10, 2005. doi: 10.1016/j.cub.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 3.Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell 16: 4316–4328, 2005. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerbone A, Toaldo C, Minelli R, Ciamporcero E, Pizzimenti S, Pettazzoni P, Roma G, Dianzani MU, Ullio C, Ferretti C, Dianzani C, Barrera G. Rosiglitazone and AS601245 decrease cell adhesion and migration through modulation of specific gene expression in human colon cancer cells. PLoS One 7: e40149, 2012. doi: 10.1371/journal.pone.0040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell 18: 253–264, 2006. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung HK, Rathor N, Wang SR, Wang JY, Rao JN. RhoA enhances store-operated Ca2+ entry and intestinal epithelial restitution by interacting with TRPC1 after wounding. Am J Physiol Gastrointest Liver Physiol 309: G759–G767, 2015. doi: 10.1152/ajpgi.00185.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dignass AU, Tsunekawa S, Podolsky DK. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology 106: 1254–1262, 1994. doi: 10.1016/0016-5085(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 8.Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem 277: 5644–5650, 2002. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- 9.Feng Q, Baird D, Cerione RA. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/α-Pix. EMBO J 23: 3492–3504, 2004. doi: 10.1038/sj.emboj.7600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol 19: 234–244, 2008. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giehl K, Keller C, Muehlich S, Goppelt-Struebe M. Actin-mediated gene expression depends on RhoA and Rac1 signaling in proximal tubular epithelial cells. PLoS One 10: e0121589, 2015. doi: 10.1371/journal.pone.0121589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, Wang J-Y. Polyamines regulate β-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am J Physiol Cell Physiol 283: C722–C734, 2002. doi: 10.1152/ajpcell.00054.2002. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: C1174–C1187, 2003. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Harter JL. Critical values for Duncan’s new multiple range test. Biometrics 16: 671–685, 1960. doi: 10.2307/2527770. [DOI] [Google Scholar]
- 15.Hiroyasu S, Stimac GP, Hopkinson SB, Jones JCR. Loss of β-PIX inhibits focal adhesion disassembly and promotes keratinocyte motility via myosin light chain activation. J Cell Sci 130: 2329–2343, 2017. doi: 10.1242/jcs.196147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci 119: 1469–1475, 2006. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 17.Jones NP, Katan M. Role of phospholipase Cgamma1 in cell spreading requires association with a β-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol 27: 5790–5805, 2007. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh SH, Huh YM, Noh MY, Kim HY, Kim KS, Lee ES, Ko HJ, Cho GW, Yoo AR, Song HT, Hwang S, Lee K, Haam S, Frank JA, Suh JS, Kim SH. β-PIX is critical for transplanted mesenchymal stromal cell migration. Stem Cells Dev 21: 1989–1999, 2012. doi: 10.1089/scd.2011.0430. [DOI] [PubMed] [Google Scholar]
- 19.Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar WH. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci 110: 2417–2427, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Kuo JC, Han X, Hsiao CT, Yates JR III, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13: 383–393, 2011. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loo TH, Ng YW, Lim L, Manser E. GIT1 activates p21-activated kinase through a mechanism independent of p21 binding. Mol Cell Biol 24: 3849–3859, 2004. doi: 10.1128/MCB.24.9.3849-3859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manabe R, Kovalenko M, Webb DJ, Horwitz AR. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci 115: 1497–1510, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192, 1998. doi: 10.1016/S1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 24.McCormack SA, Viar MJ, Johnson LR. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am J Physiol 264: G367–G374, 1993. doi: 10.1152/ajpgi.1993.264.2.G367. [DOI] [PubMed] [Google Scholar]
- 25.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 173: 587–589, 2006. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, β Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol 24: 4384–4394, 2004. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pignatelli J, LaLonde SE, LaLonde DP, Clarke D, Turner CE. Actopaxin (α-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J Biol Chem 287: 37309–37320, 2012. doi: 10.1074/jbc.M112.385229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci USA 95: 14082–14087, 1998. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Premont RT, Perry SJ, Schmalzigaug R, Roseman JT, Xing Y, Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal 16: 1001–1011, 2004. doi: 10.1016/S0898-6568(04)00023-3. [DOI] [PubMed] [Google Scholar]
- 31.Quaroni A, Wands J, Trelstat RL, Isselbacher KJ. Epithelial cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao JN, Li J, Li L, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol 277: G1149–G1158, 1999. doi: 10.1152/ajpgi.1999.277.6.G1149. [DOI] [PubMed] [Google Scholar]
- 33.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JXJ, Wang JY. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 280: C993–C1007, 2001. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 34.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JXJ, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol 290: G782–G792, 2006. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- 35.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007. doi: 10.1152/ajpgi.00282.2006. [DOI] [PubMed] [Google Scholar]
- 36.Rao JN, Liu SV, Zou T, Liu L, Xiao L, Zhang X, Bellavance E, Yuan JX, Wang JY. Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-(γ)1 after wounding. Am J Physiol Cell Physiol 295: C1499–C1509, 2008. doi: 10.1152/ajpcell.00232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao JN, Rathor N, Zou T, Liu L, Xiao L, Yu TX, Cui YH, Wang JY. STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-mediated Ca2+ signaling after wounding. Am J Physiol Cell Physiol 299: C579–C588, 2010. doi: 10.1152/ajpcell.00066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao JN, Rathor N, Zhuang R, Zou T, Liu L, Xiao L, Turner DJ, Wang JY. Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am J Physiol Cell Physiol 303: C308–C317, 2012. doi: 10.1152/ajpcell.00120.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao JN, Wang JY. Regulation of Gastrointestinal Mucosal Growth (2nd ed.). (Granger ND, Granger J, editors). San Rafael, CA: Morgan and Claypool, 2016, p. 1–135. [PubMed] [Google Scholar]
- 40.Rathor N, Chung HK, Wang SR, Wang JY, Turner DJ, Rao JN. Caveolin-1 enhances rapid mucosal restitution by activating TRPC1-mediated Ca2+ signaling. Physiol Rep 2: e12193, 2014. doi: 10.14814/phy2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathor N, Zhuang R, Wang JY, Donahue JM, Turner DJ, Rao JN. Src-mediated caveolin-1 phosphorylation regulates intestinal epithelial restitution by altering Ca2+ influx after wounding. Am J Physiol Gastrointest Liver Physiol 306: G650–G658, 2014. doi: 10.1152/ajpgi.00003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren XD, Wang R, Li Q, Kahek LA, Kaibuchi K, Clark RA. Disruption of Rho signal transduction upon cell detachment. J Cell Sci 117: 3511–3518, 2004. doi: 10.1242/jcs.01205. [DOI] [PubMed] [Google Scholar]
- 43.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 44.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6: 167–180, 2005. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 45.Rutten MJ, Ito S. Morphology and electrophysiology of guinea pig gastric mucosal repair in vitro. Am J Physiol 244: G171–G182, 1983. doi: 10.1152/ajpgi.1983.244.2.G171. [DOI] [PubMed] [Google Scholar]
- 46.Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, Reichardt LF, Fuchs E. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol 176: 667–680, 2007. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- 48.Seiler N, Raul F. Polyamines and the intestinal tract. Crit Rev Clin Lab Sci 44: 365–411, 2007. doi: 10.1080/10408360701250016. [DOI] [PubMed] [Google Scholar]
- 49.Timmons J, Chang ET, Wang JY, Rao JN. Polyamines and gut mucosal homeostasis. J Gastrointest Dig Syst 2, Suppl 7: 001, 2012. [PMC free article] [PubMed] [Google Scholar]
- 50.Valdes JL, Tang J, McDermott MI, Kuo JC, Zimmerman SP, Wincovitch SM, Waterman CM, Milgram SL, Playford MP. Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction. J Biol Chem 286: 39403–39416, 2011. doi: 10.1074/jbc.M111.260802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol 259: G584–G592, 1990. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- 52.Wang JY, Johnson LR. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100: 333–343, 1991. doi: 10.1016/0016-5085(91)90200-5. [DOI] [PubMed] [Google Scholar]
- 53.Wang PY, Wang SR, Xiao L, Chen J, Wang JY, Rao JN. c-Jun enhances intestinal epithelial restitution after wounding by increasing phospholipase C-γ1 transcription. Am J Physiol Cell Physiol 312: C367–C375, 2017. doi: 10.1152/ajpcell.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci 25: 3379–3388, 2005. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Zhang Y, Xiao L, Yu TX, Li JZ, Rao JN, Turner DJ, Gorospe M, Wang JY. Cooperative repression of insulin-like growth factor type 2 receptor translation by microRNA 195 and RNA-binding protein CUGBP1. Mol Cell Biol 37: e00225-17, 2017. doi: 10.1128/MCB.00225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou W, Li X, Premont RT. Expanding functions of GIT Arf GTPase-activating proteins, PIX Rho guanine nucleotide exchange factors and GIT-PIX complexes. J Cell Sci 129: 1963–1974, 2016. doi: 10.1242/jcs.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou T, Rao JN, Liu L, Xiao L, Cui YH, Jiang Z, Ouyang M, Donahue JM, Wang JY. Polyamines inhibit the assembly of stress granules in normal intestinal epithelial cells regulating apoptosis. Am J Physiol Cell Physiol 303: C102–C111, 2012. doi: 10.1152/ajpcell.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]