Abstract
The present study assessed the importance of immunity in angiotensin (ANG) II (5 ng·kg−1·min−1 iv)-mediated hypertension in Dahl salt-sensitive (SS) rats and SS rats deficient in T and B lymphocytes (SSRag1−/−) fed a 0.4% NaCl diet. Baseline mean arterial blood pressure (MAP) was not different between groups. ANG II infusion significantly increased MAP in both groups, although MAP increased more rapidly in SS rats, and the maximal MAP achieved was significantly greater in SS than SSRag1−/− rats (190 ± 3 vs. 177 ± 3 mmHg) after 12 days. Renal damage, as assessed by albumin excretion rate, was significantly increased after 12 days of ANG lI infusion in SS (from 32 ± 4 to 81 ± 9 mg/day) and SSRag1−/− (from 12 ± 2 to 51 ± 8 mg/day) rats; albumin excretion rate was significantly different between SS and SSRag1−/− rats at all points measured. After 9 days of recovery from ANG II, MAP was decreased to a greater extent in SSRag1−/− than SS rats (143 ± 5 vs. 157 ± 8 mmHg) compared with the peak MAP during ANG II infusion. At this same time point, albumin excretion rate was significantly lower in SSRag1−/− than SS rats (42 ± 8 vs. 66 ± 7 mg/day). Further studies demonstrated an increase in CD45+ total leukocytes, CD11b/c+ macrophages/monocytes, and CD3+ T cells in kidneys of ANG II- compared with vehicle-treated SS rats. The present data suggest that infiltrating T cells in the kidney exacerbate renal damage in ANG II-induced hypertension in SS rats maintained on a 0.4% NaCl diet, similar to results observed with a salt stimulus in SS rats.
Keywords: cytokines, hypertension, immune system, kidney, rats
INTRODUCTION
Hypertension is one of the most common treatable risk factors for many cardiovascular conditions, including stroke, atherosclerotic vascular disease, and congestive heart failure. In 2012, ~80 million American adults had hypertension; among these hypertensive individuals, only 54% have their blood pressure under control (25). This is likely due to the complex nature of the disease. One potential contributing factor is the immune system, which has been implicated in the pathogenesis of hypertension for multiple decades.
Numerous studies have implicated immunity in clinical and experimental hypertension (13, 15, 22, 23, 29). Lymphocytes (6, 7) and macrophages (26) have been observed in the kidneys of hypertensive animals and hypertensive humans (15), and pharmacological or genetic suppression of the immune system has been shown to attenuate the development of hypertension and kidney disease (2, 18, 20, 30). The role of immunity has been examined in experimental hypertension induced by a variety of maneuvers (13, 22, 23, 29). Perhaps the most definitive studies illustrating the importance of immune cells in hypertension were reported by Harrison and colleagues, who utilized adoptive transfer of immune cells in immunodeficient mutant mice to demonstrate the importance of T lymphocytes in the development of angiotensin (ANG) II-induced hypertension (12, 13).
Work in our laboratory has focused on the role of immune cells in the amplification of salt-sensitive hypertension in Dahl salt-sensitive (SS) rats (7, 8). Experiments have demonstrated that elevated NaCl intake in SS rats, through consumption of a high-salt (4.0% NaCl) diet, leads to increased immune cells in the renal interstitial space, a progressive elevation of arterial blood pressure, and renal damage (7, 8). These phenotypes are remarkably similar to those observed in hypertensive patients (10, 22). Subsequent experiments demonstrated that pharmacological or genetic approaches to inhibit the immune system result in attenuation of SS hypertension and renal damage.
The objective of the present study was to examine the role of adaptive immunity in the development and reversibility of ANG II-mediated hypertension and renal end-organ damage in SS rats maintained on a control/low-salt (0.4% NaCl) diet. Experiments were performed to assess the sensitivity of blood pressure and renal damage to an intravenous infusion of ANG II in SS rats and in SS rats with a deficit in T and B lymphocytes due to a mutation in the Rag1 gene (SS-Rag1em1Mcwi) (20). Accordingly, the present studies tested the hypothesis that a reduction in T and B cells would attenuate ANG II-induced hypertension and renal damage in the SS rat. As a corollary, we hypothesized that reversibility of the hypertension and renal damage would be impaired in SS rats with the full complement of T and B cells.
METHODS
Study Animals
Age-matched, male Dahl SS/JrHsdMcwi (SS) and SS-Rag1em1Mcwi (SSRag1−/−) rats were obtained from inbred colonies at the Medical College of Wisconsin. Breeding stock and study animals were fed purified AIN-76A rodent chow (Dyets, Bethlehem, PA) containing 0.4% NaCl from weaning and throughout the experimental protocol. All SS and SSRag1−/− rats were 9–12 wk of age during the study. In a single preliminary experiment, 9- to 12-wk-old male Sprague-Dawley (SD) rats (Harlan Sprague Dawley) were placed on the purified AIN-76A rodent diet containing 0.4% NaCl when they arrived at the Medical College of Wisconsin and maintained on that diet throughout the protocol. The Institutional Animal Care and Use Committee of the Medical College of Wisconsin approved all studies.
Surgical Preparation
Rats were surgically prepared as we previously described (18, 19, 21). The animals were deeply anesthetized with a mixture of ketamine (75 mg/kg ip), xylazine (10 mg/kg ip), and acepromazine (2.5 mg/kg ip); supplemental anesthesia was administered as needed. Aseptic technique was used to place polyvinyl catheters in the femoral artery for measurement of arterial pressure and in the femoral vein for infusions. The catheters were tunneled subcutaneously and exteriorized at the back of the neck in a lightweight tethering spring. The animals were kept warm on a warming table during and following surgery until they were fully awake. Buprenorphine (Buprenex, 0.1 mg/kg sc) and cefazolin (100 mg/kg im) were administered postoperatively to control pain and infection, respectively. After recovery from anesthesia, all rats were placed in individual stainless steel cages that permit daily measurement of arterial blood pressure and overnight urine collection; the animals were allowed to recover for 4–5 days before beginning an experimental protocol.
ANG II Infusion Rate
ANG II (Sigma; 5 ng·kg−1·min−1 iv) was continuously administered in 0.9% NaCl (0.5 ml/h iv) saline vehicle to experimental animals; control-treated rats were infused with saline alone (0.5 ml/h iv). Preliminary experiments in SS rats utilized a dose of ANG II (20 ng·kg−1·min−1 iv) that we previously used to induce hypertension in conscious, normotensive SD rats (3, 28). When compared with previous results in SD rats, 20 ng·kg−1·min−1 ANG II led to a rapid and exaggerated increase in blood pressure in Dahl SS and SSRag1−/− rats. Average mean arterial pressure (MAP) increased 30–40 mmHg, from 132 ± 3 to 171 ± 2 mmHg in SS rats (n = 14) and from 130 ± 3 to 160 ± 5 mmHg in SSRag1−/− rats (n = 11), after a single day of ANG II infusion and was only mildly altered from these levels over the following 10 days, with peak pressures of 192 ± 5 and 175 ± 9 mmHg after 12 days of infusion in the SS and SSRag1−/− rats, respectively. These preliminary results indicate enhanced sensitivity of blood pressure in SS rats to ANG II. The infusion rate of ANG II was then adjusted to 5 ng·kg−1·min−1 iv based on our previous observations that lower doses of ANG II had minimal effects on blood pressure in normotensive SD (11) or Brown Norway (34) rats but increased blood pressure in SS rats (34). To validate the minimal effects of this lower dose of ANG II in normotensive rats, a second preliminary study was performed in which SD rats were instrumented and infused with ANG II (5 ng·kg−1·min−1 iv, n = 6) or saline alone (12 ml/day iv, n = 6) for 12 days. Prior to ANG II infusion, no differences in baseline pressure were observed between the SD groups; moreover, MAP was unaltered from the baseline value of 121 ± 5 mmHg in SD rats after 12 days of ANG II infusion, although the final pressure was significantly greater in the ANG II-infused SD rats (133 ± 2 mmHg) than in the SD rats infused with saline alone (108 ± 7 mmHg).
Urinalysis
Urine electrolytes were measured by flame photometry. Plasma creatinine values were measured with an assay based on the Jaffé reaction by autoanalyzer (ACE, Alfa Wassermann). Urine albumin was quantified with a fluorescence assay that utilized albumin blue 580 dye (Molecular Probes, Eugene, OR) and a fluorescence plate reader (model FL600, Bio-Tek, Winooski, VT).
Histological Analysis
At the conclusion of the experiments, blood was flushed from the kidneys, and a histological analysis of the renal tissue was performed as previously described (18, 19, 21). Tissue was fixed in 10% formaldehyde, embedded in paraffin, cut in 3-µm sections, mounted, and stained with Gomori one-step trichrome. Slides were photographed using a Nikon E-400 microscope fitted with a Spot Insight camera; digital micrographs were taken at different magnifications. Individual glomeruli (40 per rat) were evaluated using a semiquantitative index method and scored from 0 (best) to 4 (worst) on the basis of glomerulosclerosis and mesangial expansion, as we described previously (18, 19, 21). The percentage of outer medullary tissue containing blocked tubules filled with protein casts was quantified by determination of the proportion of red-stained structures in this region using Metamorph Image Analysis software (version 4.6, Universal Imaging Systems), as described elsewhere (18, 19, 21). Glomerular and medullary damage was scored in a blinded manner.
Immune Cell Isolation
Immune cells were isolated from the kidneys as previously described (14, 30, 31). Briefly, the kidneys were flushed with heparinized saline, and the tissue was minced and incubated in RPMI 1640 medium (Gibco) containing FBS (5%; Atlanta Biologicals), collagenase type IV (0.1%; Worthington), and DNase I (0.001%; Sigma-Aldrich). The cell suspension was filtered through 70- and 40-μm cell strainers, and mononuclear cells were separated by density gradient centrifugation over Histopaque 1083. Mononuclear cells were counted on a hemocytometer and incubated with phycoerythrin (PE)-Cy7-anti-CD45 (BioLegend) for leukocytes, peridinin chlorophyll protein complex (PerCP)-eFluor 710-anti-CD3 (eBioscience) for T lymphocytes, FITC-anti-CD8 (BioLegend) for cytotoxic T cells, allophycocyanin (APC)-Cy7-anti-CD4 (BioLegend) for T helper cells, and anti-CD11b/c, Alexa eFluor 660-anti-CD11b/c (eBioscience) for macrophages and monocytes. Cells were also incubated with PE-antitumor necrosis factor- α (TNF-α) (BD Bioscience), eFluor 660-anti-interferon-γ (IFN-γ) (eBioscience), and PE-Cy7-anti-IL-17 (eBioscience) to assess intracellular cytokines. Cell viability was assessed using 4′,6-diaminido-2-phenylindole or fixable viability dye eFluor 450 (eBioscience). The cells were analyzed by flow cytometry (model LSRII, BD Biosciences) using FACSDiva (BD Biosciences) and FlowJo (Tree Star) software.
Activated T Cell Analysis
To assess cytokine production, isolated kidney immune cells were resuspended at a concentration of 106/ml in RPMI 1640 medium supplemented with 25 mM HEPES (Gibco), 100 U/ml penicillin-streptomycin (Gibco), 2 mM l-glutamine (Gibco), 1 mM sodium pyruvate, MEM nonessential amino acid solution (Gibco), 0.00035% 2-mercaptoethanol (Sigma), and 15% heat-inactivated FBS (Atlanta Biologicals). The resuspended cells were incubated for 4 h at 37°C and 5% CO2 in the presence of cell stimulation cocktail (eBioscience) and brefeldin (BD GolgiPlug, BD Biosciences). After incubation, the cells were washed and incubated with the above-listed antibodies. To exclude dead cells from the analysis, cells were stained with fixable viability dye eFluor 450 (eBioscience) according to the manufacturer’s instructions. This approach, in which freshly isolated cells are stimulated before assessment of intracellular cytokines, is based on preliminary experiments performed in CD3+ cells from the blood or the kidney in which we found that the percentage of CD3+ cells staining positive for TNF-α, IFN-γ, and IL-17 in the absence of stimulation is <1% of total cells in freshly isolated peripheral T cells (from blood) or in cells isolated from the kidney of SS rats (n = 4–5/group) infused with saline or ANG II (5 ng·kg−1·min−1).
Protocols
Protocol 1: influence of ANG II infusion on MAP and renal damage.
Rats were surgically prepared as described above. The experimental period consisted of a 4-day control period of continuous infusion of isotonic saline (0.9% NaCl, 12 ml/day) followed by a 12-day treatment period consisting of infusion of saline vehicle or saline with ANG II (5 ng·kg−1·min−1 iv). This approach permits controlled daily administration of agents directly into the circulation as well as measurement of blood pressure with the use of instrumentation calibrated daily with an external reference. The rats were fed the control (0.4% NaCl) diet throughout the protocol. MAP was recorded daily from 12 PM to 4 PM. Overnight urine collections were performed on baseline saline infusion day 4 and ANG II infusion days 4, 8, and 12 for assessment of albumin excretion rate. After infusion day 12, the kidneys were flushed of blood and harvested for histological analysis and quantification of immune cell infiltration; the animals were subsequently euthanized.
Protocol 2: reversibility of ANG II-induced effects on blood pressure and renal damage.
Rats were surgically prepared as described above. The experimental period consisted of a 4-day control period of continuous infusion of saline (12 ml/day iv), a 12-day treatment period consisting of infusion of saline vehicle or saline with ANG II (5 ng·kg−1·min−1 iv), and a recovery period in which all rats were infused for an additional 9 days with saline alone (12 ml/day iv). The rats were fed the control (0.4% NaCl) diet throughout the protocol. MAP was recorded daily from 12 PM to 4 PM. Overnight urine collections were made on baseline saline infusion day 4, ANG II infusion days 4, 8, and 12, and recovery days 3, 6, and 9 for assessment of albumin excretion rates. At the end of the experimental period, the animals were euthanized, and the kidneys were flushed of blood and harvested for histological analysis and quantification of immune cell infiltration.
Protocol 3: influence of IL-17 on ANG II-induced hypertension and renal damage.
Rats were surgically prepared as described above. The experimental period consisted of a 4-day control period of continuous infusion of isotonic saline (0.9% NaCl, 12 ml/day iv) followed by a 12-day treatment period consisting of infusion of ANG II (5 ng·kg−1·min−1 iv) in saline vehicle (12 ml/day iv) or ANG II (5 ng·kg−1·min−1 iv) + recombinant mouse IL-17 receptor C (IL-17RC, 2,000 ng/day iv; R & D Systems). The dose of IL-17RC employed in these studies was determined following a pilot dose-response study based originally on the doses of IL-17RC demonstrated to effectively attenuate hypertension in a rat model of preeclampsia (4) and to attenuate renal fibrosis and neutrophil infiltration in a rat model of acute kidney injury (24). The dose used in the present study was observed to be the minimum dose that elicited a discernable functional response in the SS rat infused with ANG II. The rats were fed the control diet (0.4% NaCl) throughout the protocol. Overnight urine collections were performed on baseline saline infusion day 4 and ANG II infusion days 4, 8, and 12 for assessment of albumin excretion rate. After infusion day 12, the animals were euthanized, and the kidneys were flushed of blood and harvested for quantification of immune cell infiltration.
Statistical Analysis
Values are means ± SE. Data were assessed for significance using a t-test, a one-way repeated-measures analysis of variance (ANOVA), or a two-way repeated-measures ANOVA with an appropriate post hoc test as applicable. P < 0.05 was considered significant.
RESULTS
Influence of ANG II Infusion on MAP and Renal Damage
In SS and SSRag1−/− rats, no differences in MAP were detected before ANG II infusion when all rats were infused with saline vehicle (Fig. 1A). Moreover, blood pressure was not altered from that level in the SS or SSRag1−/− rats infused with vehicle during the experimental period while maintained on the 0.4% NaCl diet (n = 3–5/group). Continuous intravenous infusion of ANG II (5 ng·kg−1·min−1) led to a rapid and significant increase in MAP in SS (from 129 ± 1 to 200 ± 1 mmHg) and SSRag1−/− (from 120 ± 2 to 191 ± 6 mmHg) rats. MAP was significantly different between the ANG II-infused SS and SSRag1−/− rats, indicating greater sensitivity of blood pressure to ANG II in SS than SSRag1−/− rats. Urine albumin excretion rate, as an index of renal damage, was not different between the SSRag1−/− and SS rats during the baseline period and was not altered from the levels in the rats infused with vehicle throughout the experiment (Fig. 1B). Albumin excretion rate significantly increased in ANG II-infused SS and SSRag1−/− rats, although it was significantly lower in SSRag1−/− than SS rats (63 ± 5 vs. 108 ± 22 mg/day) on day 12 of the ANG II infusion. Steady-state sodium and potassium excretion rates were not different between the groups; sodium and potassium excretion averaged 2.7 ± 0.1 and 1.0 ± 0.1 meq/day, respectively, in the SS saline-treated group in the baseline period; the excretion rates were not different between the groups and did not change over the time course of the study. Similarly, no difference was noted in plasma creatinine concentration between the strains or treatment groups at the conclusion of the study; plasma creatinine averaged 0.34 ± 0.04 mg/dl in the SS rats treated with saline at the end of the experiment.
Fig. 1.
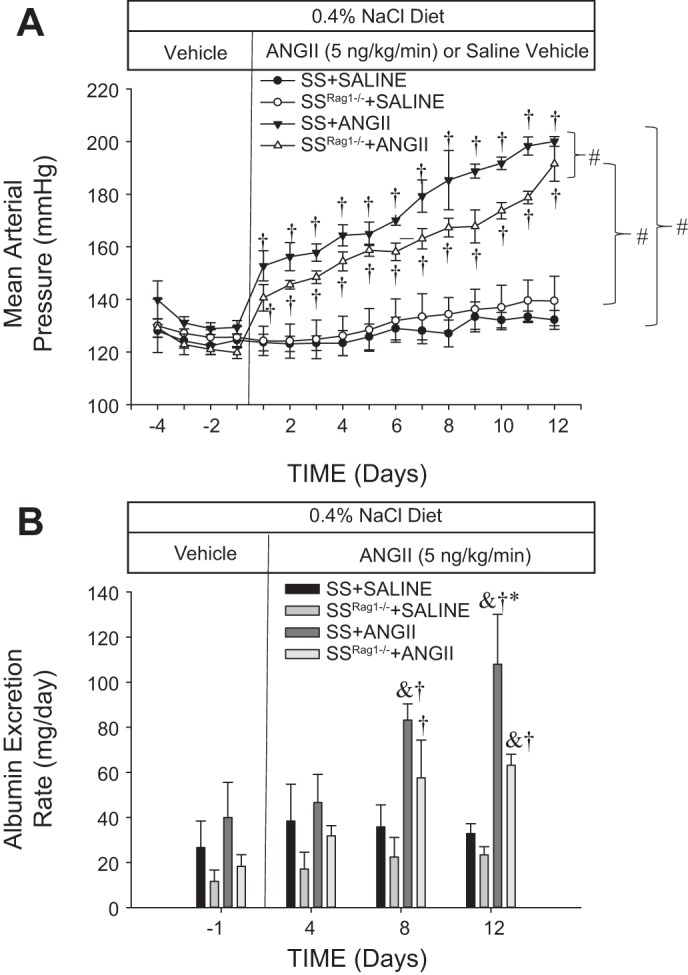
Mean arterial blood pressure (A) and albumin excretion rate (B) in Dahl salt-sensitive (SS) rats and SS rats deficient in T and B lymphocytes (SSRag1−/−) infused intravenously with 0.9% saline vehicle in a control period followed by an intravenous infusion of ANG II (5 ng·kg−1·min−1) or vehicle for 12 days. Rats were fed a 0.4% NaCl diet throughout the experiment. Values are means ± SE; n = 3–5/group. #P < 0.05 between groups (by ANOVA); †P < 0.05 vs. day −1, same group; *P < 0.05 vs. SSRag1−/−, same time; &P < 0.05 vs. saline, same strain and time.
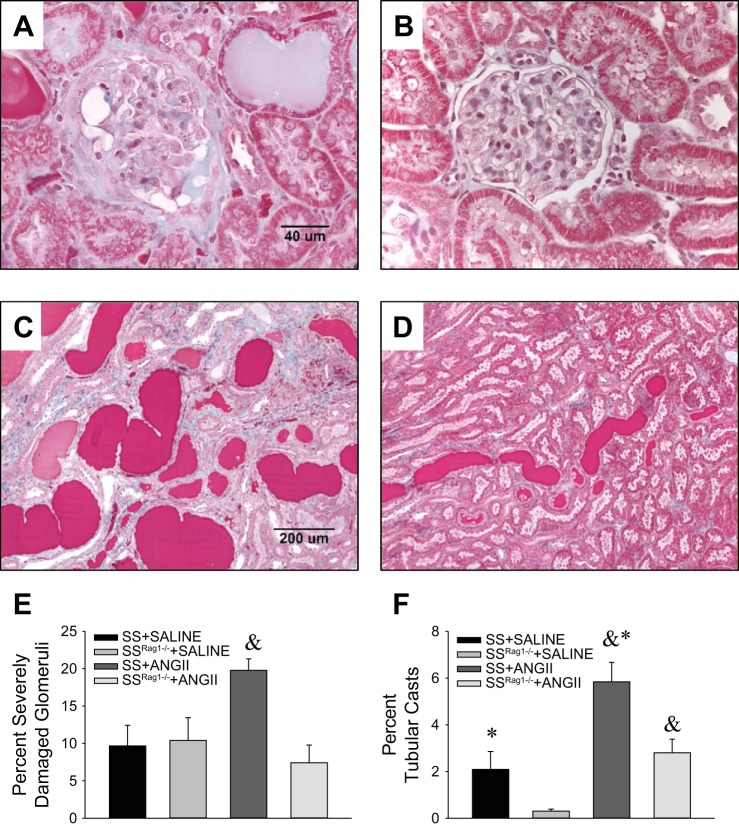
Representative histological sections of kidneys obtained from separate groups of SS or SSRag1−/− rats infused with ANG II while maintained on the low-salt diet are shown in Fig. 2 (n = 4–6/group). Consistent with previous reports of SS rats fed a high-salt diet, marked glomerular damage (blue fibrotic tissue and collapsed capillary structure) and blocked tubules in the outer medulla (red protein deposition casts) are readily apparent in the ANG II-infused SS rats. Compared with SSRag1−/− rats, the percentage of severely damaged glomeruli (glomerular damage score 4) and blocked and damaged tubules (percentage of protein casts in the outer medulla) were significantly increased in kidneys of SS rats infused with ANG II.
Fig. 2.
A–D: representative trichrome-stained sections of the renal cortex (×40 original magnification, A and B) and sections from the renal outer medulla (×10 original magnification, C and D) from SS (A and C) and SSRag1−/− (B and D) rats after 12 days of ANG II infusion (5 ng·kg−1·min−1 iv). E and F: percentage of severely damaged glomeruli and percentage of blocked tubules in the outer medulla from SS and SSRag1−/− rats infused with saline vehicle or ANG II for 12 days. Values are means ± SE; n = 4–6/group. *P < 0.05 vs. SSRag1−/−, same treatment; &P < 0.05 vs. saline, same strain.
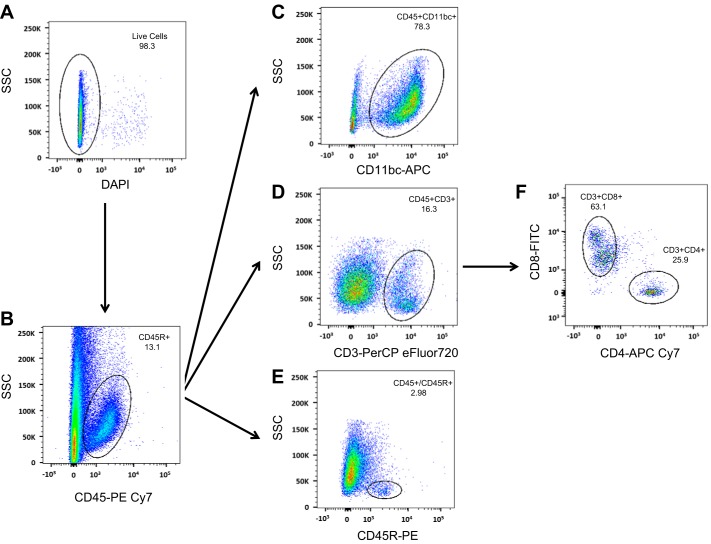
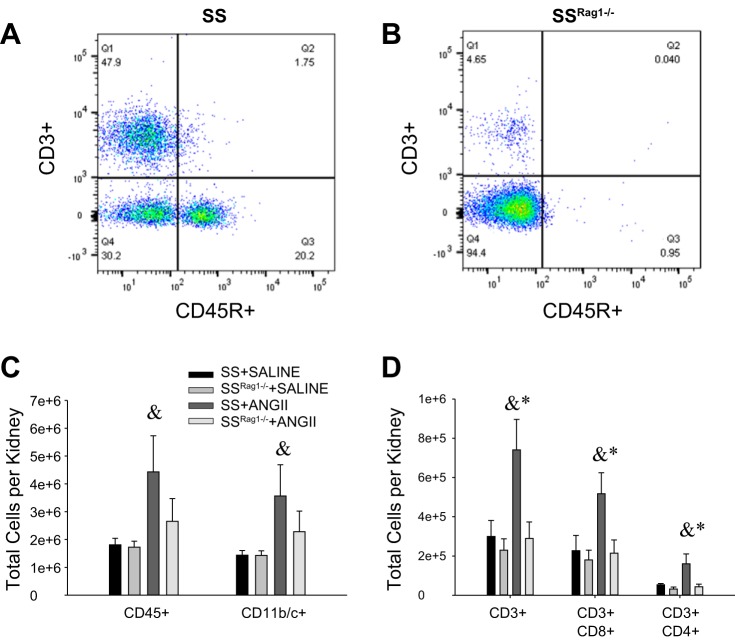
The gating strategy utilized to assess extracellular immune cell markers is illustrated in Fig. 3. To assess the immune cell phenotype of the rats, we used this strategy to perform a flow cytometric analysis on the blood of SS and SSRag1−/− rats (n = 8–10/group); representative plots identifying CD3+ T cells and CD45R+ B cells are provided in Fig. 4, A and B. CD11b/c+ cells in blood were not different between SSRag1−/− and SS rats (1.98 ± 0.19 and 2.16 ± 0.25 × 106 cells/ml, respectively), but there was an 85% reduction in CD45R+ B cells (0.98 ± 0.18 vs. 6.40 ± 0.92 × 105 cells/ml) and a 93% reduction in CD3+ T cells (0.11 ± 0.09 vs. 1.78 ± 0.24 × 106 cells/ml) in the SSRag1−/− rats compared with the SS rats (n = 8–10 rats/group, combined from different protocols in this study). These results are consistent with our previous report (20). Results of the examination of immune cells isolated from the kidneys of separate groups of rats infused with saline vehicle or saline with ANG II (5 ng·kg−1·min−1 iv) are provided in Fig. 4, C and D (n = 4–5/group). CD45+ total leukocytes, CD11b/c+ macrophages/monocytes, and total CD3+ T cells were increased in kidneys of ANG II-treated SS rats compared with vehicle-infused rats. Moreover, CD4+ and CD8+ T cells were increased in the ANG II-infused SS rats. Although CD45+ and CD11b/c+ cells tended to increase in ANG II-treated SSRag1−/− rats, the increase was not significant. Interestingly, the absolute number of CD3+ cells in the kidneys was not different between SS and SSRag1−/− rats infused with saline while maintained on the low-salt diet, despite a 95% reduction in circulating CD3+ cells in the SSRag1−/− rats (20). Additional experiments assessed the ability of CD3+ cells in the kidneys of the ANG II- and saline-infused SS rats to secrete cytokines (n = 10/group). Despite the increase in the absolute number of infiltrating CD3+CD4+ and CD3+CD8+ cells in the kidneys of ANG II-infused rats, no significant differences were observed between the groups when the percent distribution of CD3+CD4+ (Fig. 5A) or CD3+CD8+ (Fig. 5B) cells staining positive for the cytokines IL-17, IFN-γ, and TNF-α was compared. However, a significantly greater percentage of CD3+CD4+ IL-17+ than IFN-γ+ or TNF-α+ cells was observed. In CD3+CD8+ cells, IFN-γ was the predominant cytokine. The large percentage of CD3+ cells isolated from the kidneys that stained positive for cytokines contrasts with results observed in CD3+ T cells isolated from the blood of saline- or ANG II-infused animals (n = 4–5/group). The number of cells staining positive for these cytokines was significantly less in T cells isolated from the blood than in cells isolated from the kidney. The percentage of total CD3+ cells isolated from the blood of saline-infused rats with detectable levels of TNF-α, IFN-γ, and IL-17 following the identical stimulation protocol averaged 0.52 ± 0.26%, 0.26 ± 0.13%, and 0.43 ± 0.19%, respectively. Similarly, stimulated CD3+ cells isolated from the blood of ANG II-infused rats that express TNF-α, IFN-γ, and IL-17 averaged 0.59 ± 0.16%, 0.34 ± 0.07%, and 0.82 ± 0.14%, respectively.
Fig. 3.
Flow cytometry gating strategy to identify specific mononuclear cell types isolated from the kidney of SS rats. A: live cells were initially selected on the basis of 4′,6-diaminido-2-phenylindole-negative staining. B: from the cell population in A, a side scatter (SSC) vs. CD45 plot was used to identify leukocytes. C–E: of the CD45+ cells, subpopulations positive for CD11bc (macrophages and monocytes), CD3 (T lymphocytes), and CD45R (B lymphocytes) were identified. F: of the CD3+ cells, CD3+CD8+ (cytotoxic T cells) and CD3+CD4+ (T helper cells) were identified.
Fig. 4.
A and B: representative 2-dimensional plots of flow cytometric experiments identifying CD3+ T cells and CD45R+ B cells in SS and SSRag1−/− rats. C and D: total number of leukocytes (CD45+) and monocytes/macrophages (CD11b/c+) and total T lymphocytes (CD3+), CD8+ T lymphocytes, and CD4+ T lymphocytes in kidneys of SS and SSRag1−/− rats infused intravenously with 0.9% saline in a control period followed by intravenous infusion of ANG II (5 ng·kg−1·min−1) or 0.9% saline vehicle for 12 days. Values are means ± SE; n = 4–5/group. *P < 0.05 vs. SSRag1−/−, same treatment; &P < 0.05 vs. saline, same strain.
Fig. 5.
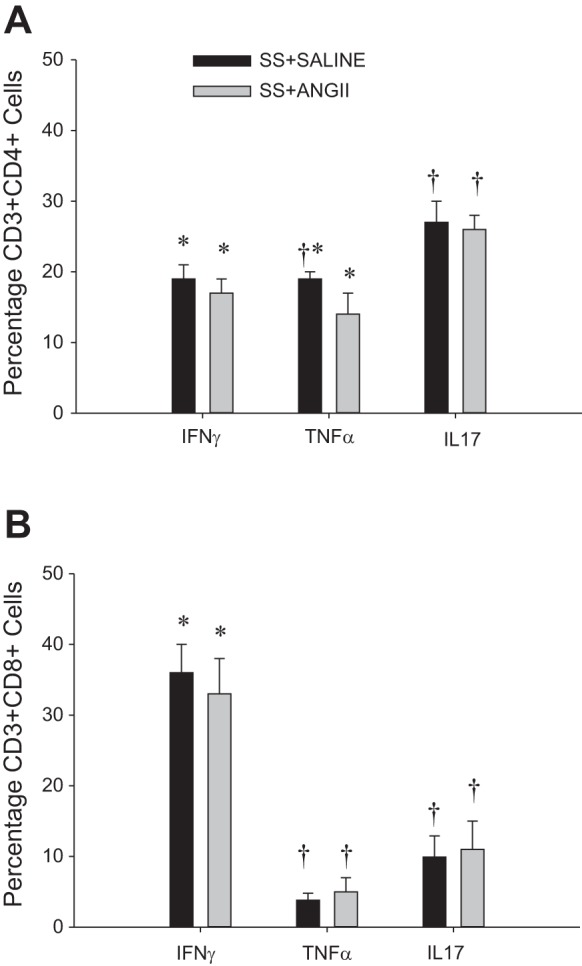
Percentage of CD4+ T lymphocytes (A) and CD8+ T lymphocytes (B) staining positive for interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin 17 (IL-7) in kidneys of SS rats infused intravenously with 0.9% saline in a control period followed by an intravenous infusion of ANG II (5 ng·kg−1·min−1) or 0.9% saline vehicle for 12 days. Values are means ± SE; n = 14–15/group. *P < 0.05 vs. IL17, same group; †P < 0.05 vs. IFN-γ, same group.
Protocol 2: Reversibility of ANG II-Induced Effects on Blood Pressure and Renal Damage
A set of experiments was performed to assess the reversibility of the ANG II-induced hypertension and renal damage in the SS and SSRag1−/− rats maintained on the 0.4% NaCl diet (n = 9–10/group). As demonstrated in protocol 1, there were no differences in MAP between the SS and SSRag1−/− rats in the control period, and ANG II infusion (5 ng·kg−1·min−1 iv) led to a significant increase in MAP in SS and SSRag1−/− rats, with a greater increase in pressure in the SS rats (Fig. 6A). When the ANG II infusion was switched back to saline alone, blood pressure in the SSRag1−/− rats significantly decreased to a level not different from control values. In contrast, although MAP decreased in the SS rats when ANG II infusion was stopped, blood pressure was maintained at a level greater than control values throughout the 9-day recovery period. The changes in albuminuria (Fig. 6B) largely reflected the changes in arterial pressure, although a difference in albumin excretion between the SS and SSRag1−/− rats was observed in the control period. Albumin excretion rate increased in both groups during ANG II infusion, with the highest absolute excretion rate observed in SS rats. Although albumin excretion rate tended to decrease in SS and SSRag1−/− rats following the return to saline infusion, the reversibility was only significant from day 12 of ANG II infusion in the SS rats at a single time point. Steady-state sodium and potassium excretion rates were not different between the SS and SSRag1−/− rats throughout the experiment (sodium and potassium excretion rates averaged 3.3 ± 0.3 and 1.1 ± 0.1 meq/day, respectively, in the SS rats during the baseline period). Plasma creatinine was not different between the SS and SSRag1−/− rats (0.49 ± 0.06 and 0.48 ± 0.02 mg/dl, respectively) at the conclusion of the experiment.
Fig. 6.
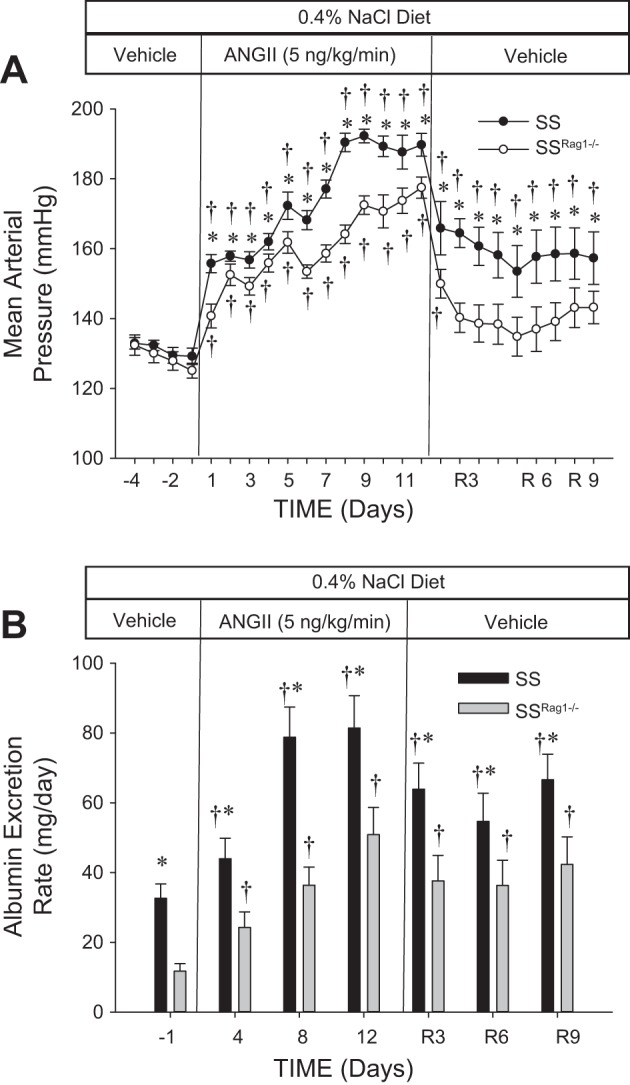
Mean arterial blood pressure (A) and albumin excretion rate (B) in SS and SSRag1−/− rats infused intravenously with 0.9% NaCl saline vehicle in a 4-day control period, followed by 12 days of ANG II infusion (5 ng·kg−1·min−1 iv) and a 9-day recovery period in which vehicle was infused. Rats were fed a 0.4% NaCl diet throughout the experiment. R3, R6, and R9, recovery days 3, 6, and 9. Values are means ± SE; n = 9–10/group. †P < 0.05 vs. day −1, same group; *P < 0.05 vs. SSRag1−/−, same time.
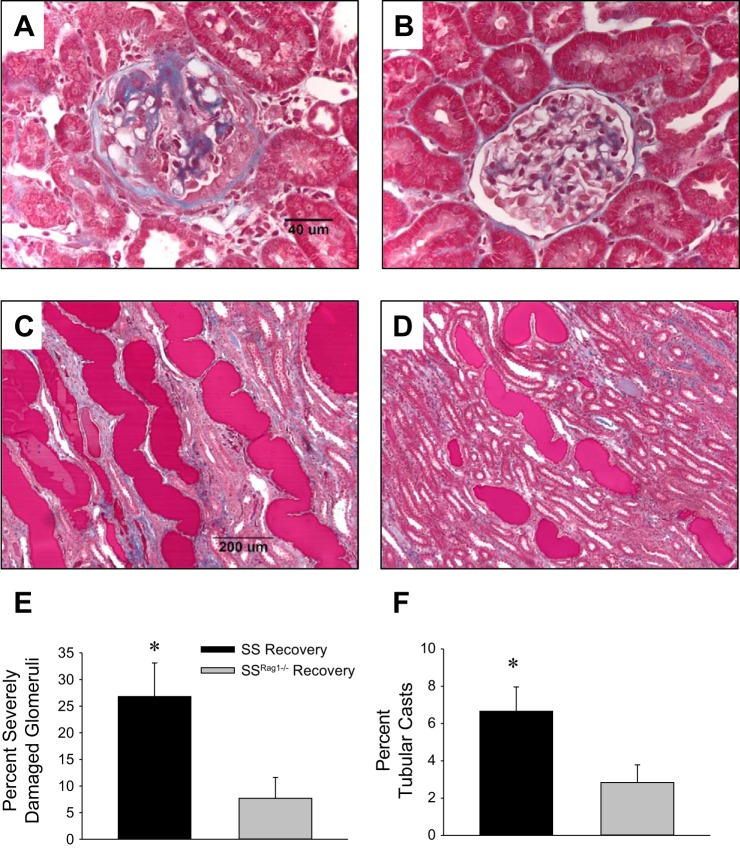
Representative histological sections of kidneys from SS and SS Rag1−/− rats following the 9-day saline recovery period are shown in Fig. 7. Scoring of glomerular and tubular damage indicated a greater percentage of severely damaged glomeruli (glomerular damage score 4) and blocked and damaged tubules (percentage of tubular protein casts) in the outer medulla of SS than SSRag1−/− rats (n = 5–6/group). An improvement of the glomerular and tubular damage scores was not observed when values in the rats following the 9-day recovery period were compared with those obtained from tissue harvested after 12 days of ANG II infusion in the SS and SSRag1−/− rats (Fig. 2). Notably, the glomerular injury score was not altered under any experimental condition in the SS Rag1−/− rats.
Fig. 7.
A–D: representative trichrome-stained sections of renal cortex (×40 original magnification, A and B) and renal outer medulla (×10 original magnification, C and D) from SS (A and C) and SSRag1−/− (B and D) rats after 9 days of recovery following a 12-day ANG II infusion (5 ng·kg−1·min−1 iv). E and F: percentage of severely damaged glomeruli and percentage of blocked tubules in the outer medulla (tubular casts) from SS and SSRag1−/− rats after 9 days of recovery. Values are means ± SE; n = 5–6/group. *P < 0.05 vs. SSRag1−/−.
Finally, a summary of the results of the examination of immune cells isolated from the kidneys of SS and SSRag1−/− rats after the 9-day recovery period is presented in Fig. 8 (n = 8–9/group). Although there tended to be fewer total leukocytes (CD45+) and total macrophages and monocytes (CD11b/c+) in the kidneys from SSRag1−/− than SS rats after the 9-day recovery period, the values were not significantly different (Fig. 8A). Total CD3+ cells and the number of CD4+ and CD8+ T cells in the kidney, however, were significantly reduced in the SSRag1−/− rats in the recovery period compared with the SS rats (Fig. 8B). A comparison between the values obtained after 12 days of ANG II infusion (Fig. 3) and the recovery period illustrated a significant reversal in the number of CD45+, CD11b/c+, and CD3+ cells (total, CD4+, and CD8+) in the kidneys from the SS rats following withdrawal of ANG II, indicating a reversal of the infiltration into the kidney. The number of CD45+ and CD11b/c+ cells in the kidneys from SSRag1−/− rats was also significantly decreased in the recovery period; although total CD3+, CD3+CD4+, and CD3+CD8+ cells tended to decrease in the kidneys from SSRag1−/− rats, the values were not significantly different from those observed following the ANG II infusion period.
Fig. 8.
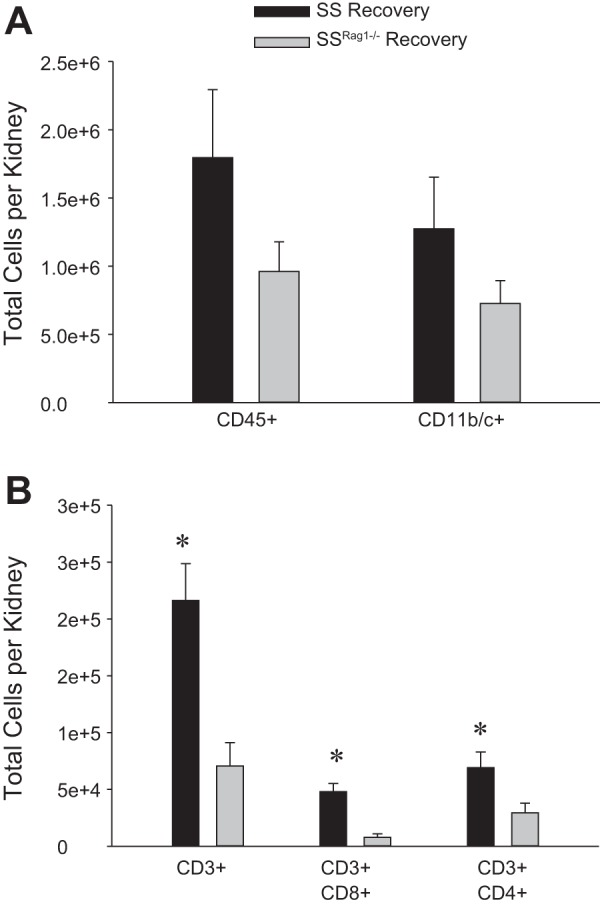
Total number of leukocytes (CD45+) and monocytes/macrophages (CD11b/c+) (A) and total T lymphocytes (CD3+), CD8+ T lymphocytes, and CD4+ T lymphocytes (B) in kidneys from SS and SSRag1−/− rats after 9 days of recovery following a 12-day ANG II infusion (5 ng·kg−1·min−1 iv). Values are means ± SE; n = 8–9/group. *P < 0.05 vs. SSRag1−/−.
Protocol 3: Influence of IL-17 on ANG II-Induced Hypertension and Renal Damage
A third set of experiments was performed to assess the influence of IL-17 on ANG II-induced hypertension and renal damage by coinfusion of mouse recombinant IL-17RC in combination with ANG II. No difference in baseline blood pressure was observed between the two groups of SS rats (Fig. 9A; n = 4–5/group). Moreover, compared with the final day of baseline measurements, MAP was significantly increased after day 1 of infusion of ANG II (5 ng·kg−1·min−1 iv) + saline vehicle (from 125 ± 3 to 148 ± 4 mmHg) or ANG II (5 ng·kg−1·min−1 iv) + IL-17RC (2,000 ng/day iv) (from 124 ± 2 to 142 ± 4 mmHg). MAP gradually continued to increase in both groups during the infusion period, but MAP was significantly greater in the group of SS rats infused with ANG II only than in the SS rats treated with ANG II + IL-17RC from day 6 through the conclusion of the experiment. Renal damage was assessed by albumin excretion rate in these experiments (Fig. 9B; n = 6–7/group). Baseline albumin excretion rate was not different between the different treatment groups, and ANG II infusion was accompanied by an increase in albumin excretion rate in both groups by day 12 of ANG II infusion. Notably, the increase in albumin excretion rate was attenuated in the ANG II-infused rats that also received IL-17RC. A subsequent analysis of cells infiltrating the kidney demonstrated no significant differences in total CD45+ leukocytes, CD11b/c+ macrophages and monocytes, or CD3+ T cells (data not shown).
Fig. 9.
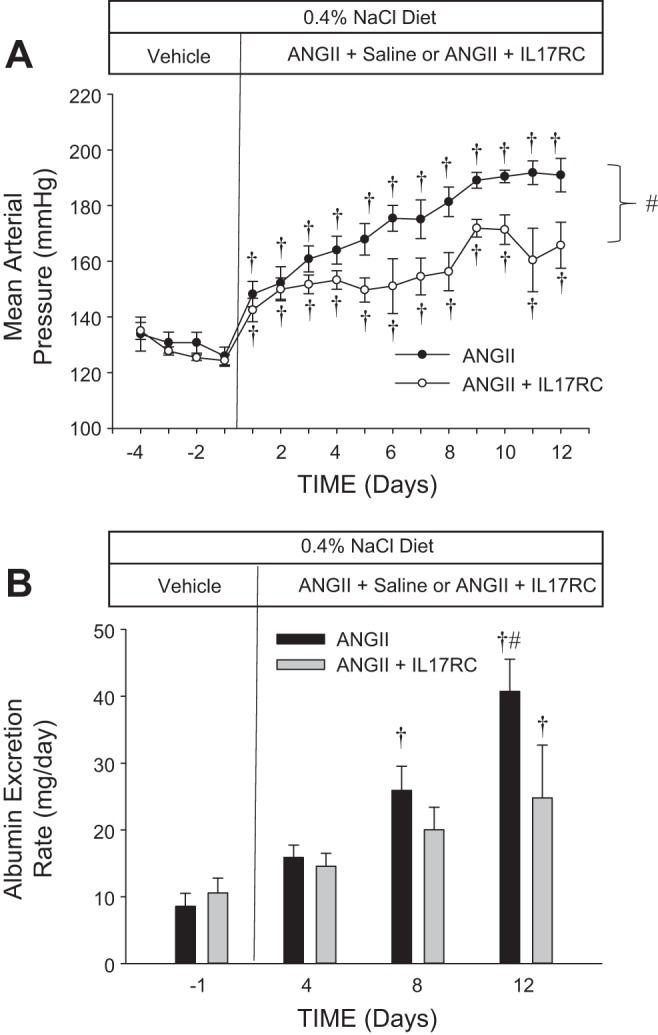
Mean arterial blood pressure (A) and albumin excretion rate (B) in SS rats infused intravenously with 0.9% NaCl saline vehicle in a 4-day control period, followed by a 12-day ANG II (5 ng·kg−1·min−1 iv) infusion with saline vehicle or recombinant mouse IL-17 receptor C (IL-17RC, 2,000 ng/day iv). Rats were fed a 0.4% NaCl diet throughout the experiment. Values are means ± SE; n = 4–5/group. †P < 0.05 vs. day −1, same group; #P < 0.05 between groups (by ANOVA).
DISCUSSION
The primary aim of this study was to address the development and reversibility of ANG II-induced hypertension and renal end-organ damage in SS rats with and without intact T and B lymphocytes. The ANG II-infused SS rats demonstrated an increase in the number of total leukocytes, including T cells and macrophages, in the kidney, which accompanied the development of elevated blood pressure, albuminuria, and renal glomerular and tubular damage. In SS rats lacking circulating T and B cells, the SSRag1−/− rats, the hypertensive response to ANG II was enhanced compared with SD rats, but the absolute increase in blood pressure after 12 days of ANG II infusion was attenuated in the SSRag1−/− rats compared with the control SS rats. These studies indicate that the presence of the full complement of T and/or B cells is required for the full hypertensive effect of ANG II, which is in general agreement with the reports of Guzik et al. in the Rag1−/− mouse (12). This study extends those observations and demonstrates a greater degree of renal damage in the SS rat with an intact immune system as assessed by albuminuria and histological damage, indicating the importance of immune mechanisms in the development of end-organ damage in ANG II-induced hypertension.
These experiments also examined the reversibility of ANG II-induced hypertension and renal end-organ damage. At the end of a 9-day recovery period following the 12-day ANG II infusion, we observed an absolute decrease in the number of infiltrating T cells and macrophages in the kidneys of the SS rats that was accompanied a significant decrease in MAP and a tendency for albumin excretion rate to decrease in the SS rat. Although these parameters were decreased or tended to decrease in the SS rats during the recovery period, there was still an elevation of MAP and albuminuria in the SS rats above that observed in the control period, indicating that the disease phenotype was not fully reversible in the time period of this experiment. In contrast, the SSRag1−/− rats, with a deficit in T and B cells, showed a reversibility of MAP to levels not different from those observed in the rat during the control period at the end of the recovery period when ANG II infusion had been discontinued. In contrast to the reversibility of MAP, the albumin excretion rate tended to decrease but was not significantly changed from the levels observed at the end of the ANG II infusion in the SSRag1−/− rats. Moreover, the glomerular injury in the SS rats and the tubular damage, as indicated by tubular casts, were unchanged in the SS and SSRag1−/− rats after 9 days of recovery following the ANG II infusion, demonstrating that the renal damage is not as quickly reversible as blood pressure. The glomerular damage index in the SSRag1−/− rats was unaltered by ANG II and, thus, was unaffected in the recovery period. The reversal data indicate that adaptive immune mechanisms participate in the development of hypertension and related end-organ damage but also serve to maintain elevated blood pressure and renal damage following removal of a hypertensive stimulus.
An additional observation of this study is that SS rats, when maintained on a low-NaCl diet, develop a significant elevation of arterial blood pressure with accompanying renal damage when infused with a relatively low dose of ANG II. This dose of ANG II (5 ng·kg−1·min−1) had a minimal effect on blood pressure and albuminuria in SD rats and is much lower than the dose previously used to induce hypertension in normotensive rats (3, 28), indicating that the SS rat is much more sensitive to systemic infusion of ANG II than normotensive animals, as previously reported (34). Interestingly, the SSRag1−/− rats, which have less sensitivity to hypertension and renal damage than SS rats when fed an elevated NaCl diet, also demonstrated an enhanced sensitivity to ANG II compared with SD rats, especially during the initial 2 days of the ANG II infusion. These results indicate that the SS genetic background confers an enhanced sensitivity to ANG II. The sensitivity to other vasoconstrictors was not assessed in this study, although previous reports have demonstrated greater sensitivity of the renal vasculature to ANG II in SS rats than the normotensive Brown Norway (5).
Results of the present study demonstrate that T cells in the kidney produce TNF-α, IFN-γ, and IL-17, cytokines that have been implicated in hypertension and associated renal damage. Previous experiments in mice that exhibited an impaired ability to produce TNF-α and IFN-γ and an inability to elicit a Th1 response (35) showed an ANG II-induced elevation in blood pressure similar to that observed in wild-type controls, but associated renal damage was reduced in these animals. Further studies demonstrated an attenuated ANG II-mediated increase in blood pressure and albuminuria in TNF-α-knockout mice, indicating that TNF-α may function as a major cytokine contributing to hypertension and renal end-organ damage (35). Separate studies in IL-17−/− mice demonstrated a marked attenuation of ANG II-induced hypertension, vascular dysfunction, and superoxide production compared with wild-type control mice (17). In the kidneys of SS rats fed a high-salt diet, we previously demonstrated that the infiltrating T cells can also serve as a rich source of cytokines (30) and NADPH oxidase (8). The current study indicates that significantly more CD4+ T cells in the kidney stain positive for IL-17, although a significant number are also positive for IFN-γ and TNF-α; in contrast, the CD8+ T cells isolated from the kidney of SS rats predominantly stained for IFN-γ among the cytokines assessed. Results of the present experiment with IL-17RC, which are consistent with observations in mice (17), indicate that IL-17 participates in development of ANG II-induced hypertension and renal damage. Since other experiments in this study demonstrated that T cells in the kidney stain positive for IL-17 while the genetic reduction of T and B cells attenuated ANG II-induced hypertension, it is possible that the source of IL-17 is the infiltrating immune cells. We did not, however, determine other potential sources of IL-17; further studies are required to demonstrate the role of IL-17 produced specifically by T cells in this model.
The present study addressed the contribution of T and/or B cells to ANG II-induced hypertension but did not address the mechanisms independent of adaptive immunity that led to the increase in blood pressure and development of renal damage in the SSRag1−/− rats. It is possible that ANG II-induced increases in reactive oxygen species (32) or other factors mediate the increase in pressure in the SSRag1−/− rats. Alternatively, the effects of ANG II to mediate sodium retention and vasoconstriction [reviewed elsewhere (33)] may serve as the mediator(s) of the increase in arterial pressure in the SS and SSRag1−/− rats. The direct physiological effects of ANG II to constrict the vasculature, leading to a decrease in glomerular filtration rate and renal blood flow, the direct effects of ANG II to stimulate proximal tubular sodium reabsorption, and the indirect effects of ANG II (via aldosterone) to stimulate distal nephron sodium reabsorption are likely mediators of the elevation of blood pressure during ANG II infusion. We further speculate that the above-mentioned effects of ANG II lead to an initial elevation of arterial pressure accompanied by barotrauma in the kidney; this renal damage then results in an adaptive immune response that exacerbates the disease. This hypothesis is consistent with our recent observations that servo control of renal perfusion pressure (i.e., maintaining arterial pressure at low-salt levels with the use of an inflatable cuff on the aorta) when SS rats were fed a high-salt diet attenuated infiltration of immune cells into the kidney compared with a kidney exposed to elevated renal perfusion pressure (9). This concept is supported by observations in mice in which administration of a vasodilator and a diuretic to prevent ANG II-induced hypertension prevented infiltration of immune cells into the kidney and other organs (16). Moreover, the renal damage that accompanies ANG II-induced hypertension in normotensive SD rats was attenuated if an increase in renal perfusion pressure was prevented (27).
The decrease in the number of T cells and macrophages in the kidneys of the SS and SSRag1−/− rats following the 9-day recovery period after ANG II infusion compared with the number of T cells and macrophages in the kidneys of SS rats after 12 days of ANG II infusion demonstrates that the deleterious effects of immune cell infiltration are potentially reversible. In the present example, the withdrawal of ANG II and the significant reduction in MAP accompanied this reversal. Previous studies from our group have demonstrated that an elevation of renal perfusion pressure is a contributing factor in the infiltration of immune cells into the kidney of the SS rat (9). The reduction in perfusion pressure in the recovery period could help explain the reduction in immune cells in the kidney, although the mechanisms mediating the reduction in cells in the recovery period in the SS and SSRag1−/− rats are unclear, and additional work remains to determine the mechanisms at play in this model. A related, surprising observation is the presence of a significant number of CD3+ cells in the kidneys of the SSRag1−/− rats, despite the significant number of circulating CD3+ cells in these rats. This observation, which is consistent with our previous reports in this model following a high-salt stimulus (20), is not explained but may indicate preferential trafficking of CD3+ cells to the kidney. Regardless, and consistent with results of our previous study with a high-salt stimulus (20), the SSRag1−/− rats exhibit a significant attenuation of hypertension and end-organ damage, which is associated with a significant reduction of CD3+ cells in the kidney following the hypertensive stimulus.
One additional concern is the potential effect of the NaCl in the infusate to alter blood pressure in the present study. Since this amount of sodium intake is fairly small (~3 meq/day) compared with the amount of sodium in the high-salt (4.0% NaCl) diet (>10 meq/day), it should not have played a major role in the hypertensive response. As a control for the sodium intake from the infusion, saline-infused SS and SSRag1−/− rats demonstrated no change in blood pressure or albumin excretion throughout the course of the study, indicating a minimal effect of NaCl or other components of the infusate on development of the ANG II-dependent disease phenotype in these experiments.
Perspectives and Significance
The present studies demonstrate that immune cells, presumably infiltrating the kidney, play a role in amplification of ANG II-induced hypertension and end-organ damage in SS rats, similar to the results observed with a salt stimulus in the SS rats. Moreover, the hypertension is reversible following withdrawal of the ANG II infusion, but the renal damage was not abated in the initial week following the hypertensive stimulus. Interestingly, the number of infiltrating immune cells in the kidney was reversed following withdrawal of the ANG II infusion, indicating that the elevation of blood pressure and/or the direct effects of ANG II are mediating the infiltration of immune cells into the kidney.
GRANTS
This work was partially supported by National Institutes of Health Grants DK-96859 and HL-116264 and American Heart Association Grant 15SFRN2391002 to D. Mattson. B. Wade was supported by National Heart, Lung, and Blood Institute Training Grant HL-007852.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W. performed experiments; B.W., G.P., and D.L.M. analyzed data; B.W., G.P., and D.L.M. interpreted results of experiments; B.W. and D.L.M. prepared figures; B.W., G.P., and D.L.M. edited and revised manuscript; B.W., G.P., and D.L.M. approved final version of manuscript; G.P. and D.L.M. conceived and designed research; D.L.M. drafted manuscript.
REFERENCES
- 1.Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 283: F1132–F1141, 2002. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 2.Bataillard A, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of an immunosuppressive agent, cyclophosphamide, in genetically hypertensive rats of the Lyon strain. Int J Immunopharmacol 11: 377–384, 1989. doi: 10.1016/0192-0561(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 3.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol Scand 183: 309–320, 2005. doi: 10.1111/j.1365-201X.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 4.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. doi: 10.1161/01.HYP.37.2.456. [DOI] [PubMed] [Google Scholar]
- 6.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008. [Erratum. Am J Physiol Renal Physiol 298: F1286, 2010.] doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased blood pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Twin studies in Barbados. Hypertension 15: 803–809, 1990. doi: 10.1161/01.HYP.15.6.803. [DOI] [PubMed] [Google Scholar]
- 11.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW Jr. Effects of daily sodium intake and angiotensin II upon cortical and medullary blood flow in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1317–R1323, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 125: 130–138, 2014. [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 20.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741, 2005. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 22.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehrotra P, Collett JA, McKinney SD, Stevens J, Ivancic CM, Basile DP. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: compensatory role of natural killer cells in athymic rats. Am J Physiol Renal Physiol 312: F385–F397, 2017. doi: 10.1152/ajprenal.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members: American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polichnowski AJ, Cowley AW Jr. Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009. doi: 10.1161/HYPERTENSIONAHA.109.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajapakse NW, De Miguel C, Das S, Mattson DL, Mattson DL. Exogenous l-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension 52: 1084–1090, 2008. doi: 10.1161/HYPERTENSIONAHA.108.114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Iturbe B. Autoimmunity in the pathogenesis of hypertension. Hypertension 67: 477–483, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06418. [DOI] [PubMed] [Google Scholar]
- 30.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudemiller NP, Lund H, Priestley JRC, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65: 111–1117, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 33.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol 4: 1201–1228, 2014. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szentiványi M Jr, Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW Jr. Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R266–R272, 2002. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]