Abstract
Long-term hypoxia (LTH) has a profound effect on pulmonary arterial vasoconstriction in the fetus and adult. Dysregulation in Ca2+ signaling is important during the development of LTH-induced pulmonary hypertension. In the present study, we tested the hypothesis that L-type Ca2+ channels (CaL), which are voltage dependent and found in smooth, skeletal, and cardiac muscle, are important in the adaptation of pulmonary arterial contractions in postnatal maturation and in response to LTH. Pulmonary arteries were isolated from fetal or adult sheep maintained at low or high altitude (3,801 m) for >100 days. The effects were measured using an L-type Ca2+ channel opener FPL 64176 (FPL) in the presence or absence of an inhibitor, Nifedipine (NIF) on arterial contractions, intracellular Ca2+ oscillations, and ryanodine receptor-driven Ca2+ sparks. FPL induced pulmonary arterial contractions in all groups were sensitive to NIF. However, when compared with 125 mM K+, FPL contractions were greater in fetuses than in adults. FPL reduced Ca2+ oscillations in myocytes of adult but not fetal arteries, independently of altitude. The FPL effects on Ca2+ oscillations were reversed by NIF in myocytes of hypoxic but not normoxic adults. FPL failed to enhance Ca2+ spark frequency and had little impact on spatiotemporal firing characteristics. These data suggest that CaL-dependent contractions are largely uncoupled from intracellular Ca2+ oscillations and the development of Ca2+ sparks. This raises questions regarding the coupling of pulmonary arterial contractility to membrane depolarization, attendant CaL facilitation, and the related associations with the activation of Ca2+ oscillations and Ca2+ sparks.
Keywords: arterial myocytes, Ca2+ oscillations, Ca2+ sparks, contraction, L-type Ca2+ channels
INTRODUCTION
Long-term hypoxemia, due to living at high altitude or other factors, increases the risk of developing pulmonary hypertension in adults as well as in infants who experience intrauterine hypoxemia. These diseases in adults and newborns are intractable, although there are a number of treatment options. Still, effective treatment of pulmonary hypertension in neonatal and adult patients is hampered by the lack of knowledge regarding the mechanisms that underlie the development of the disease.
A hallmark of pulmonary hypertension in newborns as well as adults is that the contractility of pulmonary arteries is dysregulated (4, 27, 44, 47). Arterial contraction is initiated by cytosolic Ca2+ increases within myocytes that line the vessel wall, and contractions are often affected by disease. L-type Ca2+ channels (CaL) are well regarded as being central to the rise in cytosolic Ca2+ and resultant arterial contractions. Channel activation facilitates Ca2+ influx subsequent to cell stimulation and membrane depolarization (28, 40). Dysregulation of CaL expression and function are important to the development of vascular hypertension and other vascular and nonvascular diseases (12).
Calcium signaling, required for myocyte contractions, is not simply a graded response depending on the strength of stimulation. Rather, calcium signals are highly dynamic and reliant on a combination of deterministic and stochastic processes. Our laboratory, and others', have previously reported that pulmonary arterial myocytes exhibit whole cell Ca2+ events that are spontaneous or can be activated by various forms of stimulation (34, 38, 43, 46). Although CaL activation and inhibition affect pulmonary arterial myocyte contractions by modifying intracellular Ca2+ events, the coupling between cell activation, Ca2+ responses, and the resulting myocyte contractions is not completely understood (46).
Membrane depolarization and CaL activity have also been shown to be coupled to Ca2+ sparks, which are subcellular localized cytosolic Ca2+ increases due to focal activation of ryanodine receptors (RyRs) on the sarcoplasmic reticulum (9, 10, 13). Indeed, depolarizing smooth muscle cells with elevated extracellular K+ is a routine method to activate RyRs and facilitate Ca2+ spark activity in various smooth muscle preparations including pulmonary arterial myocytes (16, 23, 49, 53). Myocyte depolarization with high extracellular K+ is also often used to assess the contribution of CaL to smooth muscle contraction. However, recent evidence illustrates that K+ depolarization induces pulmonary arterial contraction through the combined activation of CaL and Rho kinase (7, 28, 32). Although the synergistic interaction of CaL and Rho kinase by high K+ contributes to our understanding of excitation-contraction coupling, it complicates data interpretation with regard to unraveling the influence of CaL on Ca2+ oscillations, Ca2+ sparks, and pulmonary arterial contraction (20, 23, 28).
Pulmonary arterial vasoreactivity changes as animals mature into adulthood or when faced with stressors, such as hypoxia due to living at high altitude. Maturation and hypoxic stress influence the function of many different cell signaling systems including those involving calcium. This builds upon the general importance of Ca2+ signals to vessel reactivity and the role of CaL. With specific regard to high-K+-mediated depolarization; maturation increases pulmonary arterial contraction while long-term hypoxia increases contractions in newborns (4, 28). The presumption is that direct CaL activation as well as other Ca2+ signaling pathways influence Ca2+ oscillations and attendant contraction. Studying Ca2+ oscillations within the context of development and stress due to long-term, high-altitude exposure may provide insight into the mechanisms underlying the maturational and adaptive changes in pulmonary arterial vasoreactivity.
To address the combined influence of long-term hypoxia and maturation on Ca2+ signaling dynamics and vasoreactivity, we designed a series of experiments to enhance our understanding of the role that CaL has to cellular Ca2+ activity and vasoreactivity in the pulmonary vasculature. In these experiments, we manipulated CaL activity via direct pharmacological stimulation and inhibition as opposed to using high K+. We hypothesized that agonist-induced activation of CaL increases pulmonary arterial contractions with corresponding increases in Ca2+ oscillations and Ca2+ sparks and that postnatal maturation increases these Ca2+ signals and vasoreactivity. Additionally, we postulated that hypoxic stress modifies the involvement of CaL on the Ca2+ signals and vasoreactivity. These hypotheses were addressed by performing studies on vessels from fetal and adult sheep that were housed at either low or high altitude. Examinations were made regarding the relationships of long-term hypoxia and maturation on the activity of Ca2+ sparks, Ca2+ oscillations, and arterial contractions.
METHODS
Experimental animals.
Experimental procedures were performed on sheep arteries because the developmental progression of their lungs and the extent to which prenatal long-term hypoxia affects them are comparable to those in humans (27). These studies were in accordance with the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf), “The Guiding Principles in the Care and Use of Animals” approved by the Council of the American Physiological Society, and the Animal Care and Use Committee of Loma Linda University (IACUC-LLU). The tissue preparation, wire myography, and imaging experimental procedures and protocols were based on previously published methods (4, 15, 16). As indicated in our previous work, sheep were obtained from Nebeker Ranch (Lancaster, CA; raised at an elevation of 720 m above sea level), and normoxic animals were brought to LLU (353 m; arterial = 95 ± 5 Torr) for experimental study. Tissues were isolated from fetal normoxic (FN) and nonpregnant adult normoxic (AN) sheep. Animals in the long-term hypoxic experimental group were acclimatized to high altitude (3,801 m, = 60 ± 5 Torr) at the Barcroft Laboratory, White Mountain Research Station (Bishop, CA) for ~110 days (15, 16). The hypoxic ewes were then transported to LLU for experimental study. To maintain hypoxic conditions while at LLU in pregnant and nonpregnant sheep, a tracheal catheter was placed in the ewe shortly after arrival. The tracheal catheter allowed N2 to reach the animal at a rate adjusted to maintain at ~60 Torr, equivalent to the at the White Mountain Research Station (24). This was maintained until the time of the experimental study. Tissues were isolated from fetal hypoxic (FH) or nonpregnant adult hypoxic (AH) sheep (9).
For the fetal studies, within 1 to 5 days of arriving at LLU, pregnant sheep were eurthanized with an overdose of Euthasol (100 mg/kg pentobarbital sodium and 10 mg/kg phenytoin sodium; Virbac, Ft. Worth, TX), a proprietary euthanasia solution. Lungs were removed from the fetuses and used immediately for contractility and imaging experiments.
Tissue preparation.
As detailed previously, fourth to fifth branch order pulmonary arteries with internal diameters of ~500–700 μm were isolated from full-term fetal (138–141 days) or adult (~2 yr old) sheep from the different experimental groups (15, 16, 27, 28). The parenchyma was removed carefully from the pulmonary arteries for contractility studies, and the endothelium was disrupted by manually rotating the artery on a wire. The arteries were then cut into 5-mm long rings in ice-cold phosphate-free balanced salt solution (BSS) of the following composition (in mM): 126 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2 CaCl2, 10 glucose, pH 7.4 (adjusted with NaOH). Imaging studies were performed in BSS. All contraction studies were performed with a modified Krebs-Henseleit (KH) solution containing (in mM) 120 NaCl, 4.8 KCl, 1.2 K2HPO4, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, and 10 glucose. To depolarize the arteries, NaCl was omitted from the BSS or KH solutions and replaced with equimolar KCl.
Contraction studies.
The wire-mounted pulmonary arterial rings were suspended in organ baths (Radnoti Glass Instruments, Monrovia, CA) containing 5 or 10 ml of modified KH solution maintained at 37°C. Arteries in modified KH were aerated with 95% O2-5% CO2 (pH = 7.4). Each ring was suspended between two tungsten wires passed through the lumen. One wire was anchored to the glass hook at the bottom of the organ chamber, and the other was connected to a tissue hook attached to a low-compliance force transducer (Radnoti Glass Instruments) that measured isometric force (4, 15, 16). The transducers were connected to an analog-to-digital data interface (Powerlab 16/30; A/D Instruments, Colorado Springs, CO; or MP100, Biopac, Goleta, CA) attached to a computer, and changes in tension were recorded using Chart 7.0 (AD Instruments), or AcqKnowledge 3.9 (Biopac Systems). The acquired data were stored on laboratory microcomputers and backed up to magnetic media for later analysis. At the beginning of each experiment, vessels were equilibrated without tension for a minimum of 30 min. Tension was then applied to ~0.75 g and allowed to stabilize, as previously described (4, 15, 16).
Confocal microscopy studies.
Intracellular Ca2+ concentration was measured in pulmonary arterial myocytes in situ, with the Ca2+-sensitive dye Fluo-4 AM (Invitrogen, Carlsbad, CA) using a Zeiss 710 NLO laser scanning confocal imaging workstation (Thornwood, NY) with an inverted microscope (Zeiss Axio Observer), using procedures based on those previously described (4, 15, 16). Fluo-4 AM was dissolved in DMSO and added from a 1 mM stock solution to the arterial suspension at a final Fluo-4 concentration of 10 μM, along with 0.1% Pluronic F-127 for 1 h at room temperature in the dark in BSS. Arterial segments were then washed with BSS for 30 min to allow for dye esterification and cut into linear strips. The arterial segments were pinned to Sylgard (Ellsworth Adhesives, Germantown, WI) using fine insect dissecting pins and placed in an open-bath imaging chamber (Warner Instruments, Hamden, CT) mounted on the confocal imaging stage in an en face configuration. Cells were illuminated at 488 nm with a krypton-argon laser, and the emitted light was collected using a photomultiplier tube (PMT) with a band-limited spectral grating of range 493 to 622 nm and with full-frame images made every 700 ms. To ensure that the smooth muscle intracellular Ca2+ concentration was recorded, the pinhole was adjusted to provide an imaging depth of 5.4 μm. The sample was focused below the internal elastic lamina layer, which has significant autofluorescence when excited at 488 nm in this preparation. The imaging depth was roughly equivalent to that of an individual smooth muscle cell, based on morphological examination of fixed and live preparations. Thicker imaging sections accounted for sample ruffling, thus allowing for the examination of many more smooth muscle cells than otherwise would have been achieved at other tissue depths (16). Images were acquired using 12-bit sampling. Recordings were made using a water immersion ×63 Plan Apochromat, 1.4 NA objective. The imaging studies were performed in the following order to prevent experimental artifact due to irreversibility of drugs. Under basal conditions, a time series of 500 images (~210 s) was made to assess oscillatory activity in individual cells, which was followed by 30–50 line scans of 18.9 s that were made to measure Ca2+ spark activity, with one line being made in each cell. FPL 64176 (FPL) was then applied to the tissues and after ~5 min another set of time series and line scans was performed. Care was taken not to duplicate imaging in regions and cells due to the potential of photobleaching and laser-induced toxicity. Each set of time series and line scans required approximately 1 h of imaging time. On most occasions, a separate arterial segment from the same animal was selected and treated with the combination of FPL and nifedipine (NIF). This was done because of the potential for dye leakage or cell death, as the tissues were in the recording chamber for ~2 h by this point in the study. Time series and line scan recordings were then made on this segment. We then stimulated arteries with FPL or with the combination of FPL and NIF and examined dynamic changes in the Ca2+ signals. Fluo-4 was used in the present studies because we were primarily interested in the dynamics of the Ca2+ oscillations. However, technical limitations of using Fluo-4 to measure the intracellular Ca2+ concentration prevented us from quantifying basal or steady-state Ca2+ concentrations.
Oscillatory signal analysis.
Regions of interest with calcium oscillations were detected automatically post hoc, and the fractional fluorescence intensity was automatically calculated for presentation purposes using the LC Pro plugin for ImageJ (6, 11). False positives were excised from the final data set by visual analysis of the data.
Line scan analysis.
Ca2+ sparks were recorded in pulmonary arterial myocytes loaded with the Ca2+-sensitive dye Fluo-4 AM using a Zeiss LSM 710 NLO laser scanning confocal imaging workstation on an inverted microscope platform (Zeiss Axio Observer Z1). Arterial segments were loaded with Fluo-4 and handled as detailed above for the other confocal Ca2+ imaging studies. Arteries were illuminated at 488 nm with a krypton-argon laser, and emitted light was collected using a photomultiplier tube. Line scans were imaged at 529 lines per second with the emission signal recorded at 493–622 nm. The acquisition period for Ca2+ spark recordings was 18.9 s and the resultant pixel size ranged from 0.0148 to 0.0911 μm per pixel. To ensure that sparks within the cell were imaged, the pinhole was adjusted to provide an imaging depth of 2.5 μm; this is roughly equivalent to 50% of the width of the cell based on morphological examination of live preparations. Analysis was performed to characterize the percentage of scans with Ca2+ sparks, Ca2+ spark frequency, amplitude, and spatiotemporal characteristics using SparkLab 4.3.1. Threshold for spark detection was two standard deviations above the mean background noise. Before analysis, background fluorescence was subtracted from each image, assuming homogeneous background levels in each cell (17).
Triplicate sheep for each animal group were used for the spark experiments. The number of line scans that were performed ranged from 103 to 215 in each group, while the number of analyzed sparks ranged from 5 to 149. The exact number of lines, sparks, and animals are provided in the figure legends.
Chemical reagents and drugs.
Reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. The relevant CaL modulator drug doses were 1 µM BAY K8644 (BAYK), 1 µM FPL 64176 (FPL), and 10 µM NIF.
Correlations among Ca2+ events.
To quantify calcium transients that are correlated in time and space, we developed and used BASS, a custom set of physiology analysis tools written in Python (https://github.com/drcgw/BASS) program to cross-correlate all Ca2+ transients obtained in a given data record. To determine what correlation coefficient (Pearson’s r) was appropriate for correlation of each Ca2+ transient event, we performed a parameter space search for a family of correlated oscillations by using r coefficients from 0.1 to 1. After analyzing these oscillations, we chose r = 0.8 as the minimum threshold coefficient for transient events. To determine whether a transient occurred in a nearby smooth muscle cell, we chose a radius of 100 pixels (~23 μm), noting that the perimeter included most cells adjacent to a single cell. We used “friends and neighbors” as a more comprehensive form of analysis for calcium transients to correlate both temporal and spatial events. This analysis facilitates understanding of the local signaling network and provides greater characterization of calcium traffic between smooth muscle cells.
Statistical methods and sampling.
Statistical analyses and graphs of time-series recordings were made using GraphPad Prism 5.0 (La Jolla, CA). Summarized data presented as means ± SE (Fig. 1) or 95% confidence intervals (Figs. 2, 4, 6, 7, 9, and 10). Data were evaluated for normality before any comparative statistical analysis. The specific test used is denoted in the figure legends. P < 0.05 was generally considered statistically significant. The number of studies performed is provided as (n/N). Sample sizes are based on multiple individual measurements (n) that reflect analyses performed on individual arterial segments, the number of regions of interest that show calcium oscillations, or the number of line scans examined for Ca2+ sparks. The number of animals is given as N. The percentage of cells firing with Ca2+ sparks was computed as the number of individual line scans with events relative to the total number of line scans and is displayed in Fig. 9.
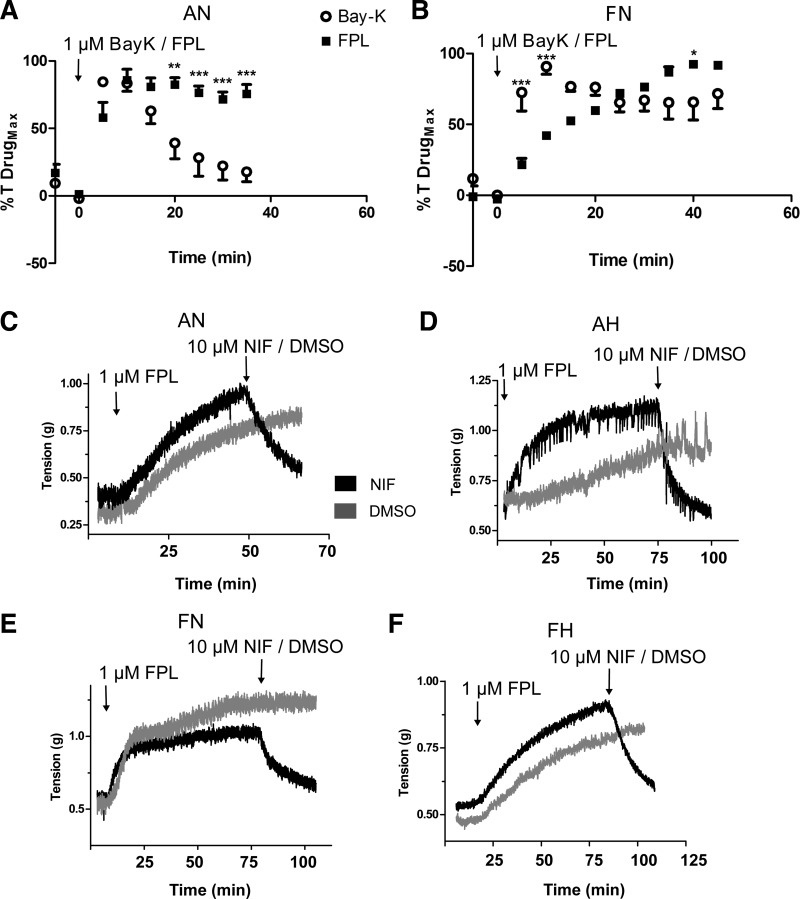
Fig. 1.
Pulmonary arterial contraction to BAY K8644 (BAYK) and FPL 64176 (FPL) are kinetically distinct. A and B: average isometric contractility response of pulmonary arterial rings exposed to 1 µM BAYK (open circles) and 1 µM FPL (closed squares) isolated from adult and fetal sheep under normoxic conditions. C–F: representative time series traces of the force developed for FPL in the presence of nifedipine (NIF; black) or vehicle (DMSO; gray) for pulmonary arteries from adult (C and D) and fetus (E and F). Values are means ± SE. Data were analyzed with a two-way ANOVA and Bonferroni posttest analysis. **P < 0.01, ***P < 0.001 between BAYK and FPL values at the same time point. FPL responses were obtained in 8/5 adult normoxic (AN) and 5/5 fetal normoxic (FN) arteries and animals, respectively. BAYK responses were from 12/4 AN and 5/5 FN arteries and animals, respectively.
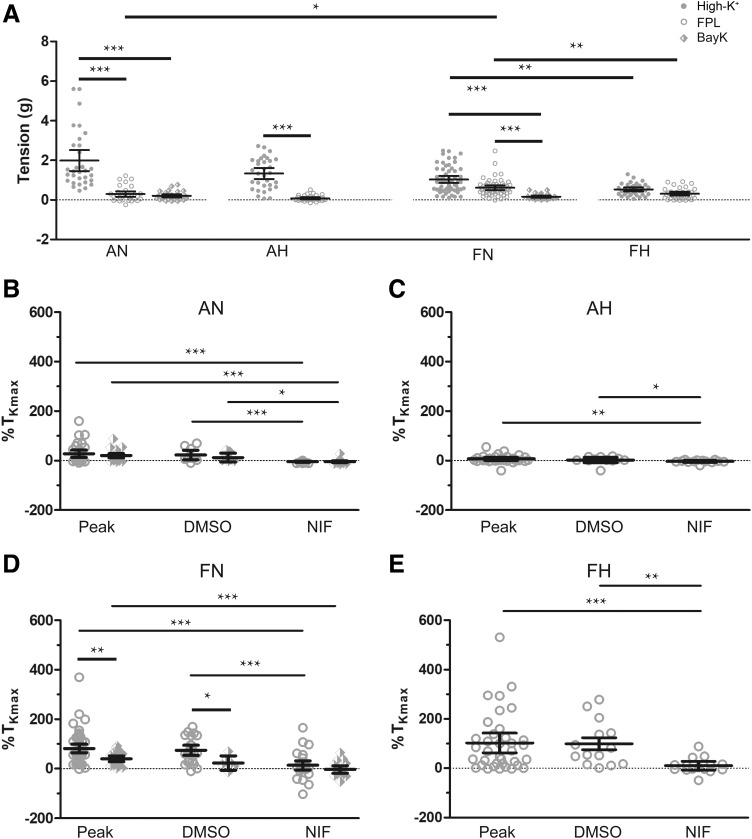
Fig. 2.
L-type Ca2+ channel (CaL) inhibition reduces BAY K8644 (BAYK) and FPL 64176 (FPL)-induced pulmonary arterial contraction. A: average tension due to high K+ (closed circles), 1 µM FPL (open circles), and 1 µM BAYK (diamond). B–E: average peak, DMSO-, or NIF nifedipine (NIF)-treated FPL (open circles) or BAYK (diamonds) induced contraction normalized to high K+. Values are means ± 95% confidence interval (CI). Data were analyzed by Kruskal-Wallis one-way ANOVA with Dunn's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. AN, adult normoxic, AH, adult hypoxic, FN, fetal normoxic, FH, fetal hypoxic. High-K+ responses were obtained from 31/7 AN, 32/7 AH, 56/9 FN, and 36/6 FH arteries and animals, respectively. FPL responses were obtained for AN 31/7 (Peak), 10/7 (DMSO), and 21/7 (NIF); FN 56/9 (Peak), 25/9 (DMSO), 31/9 (NIF); AH 32/7 and FH 35/6 arteries and animals, respectively. BAYK responses were obtained for AN 29/5 (peak), 7/5 (DMSO), 22/5 (NIF), and FN 23/5 (Peak), 6/5 (DMSO), and 17/5 (NIF) arteries and animals, respectively.
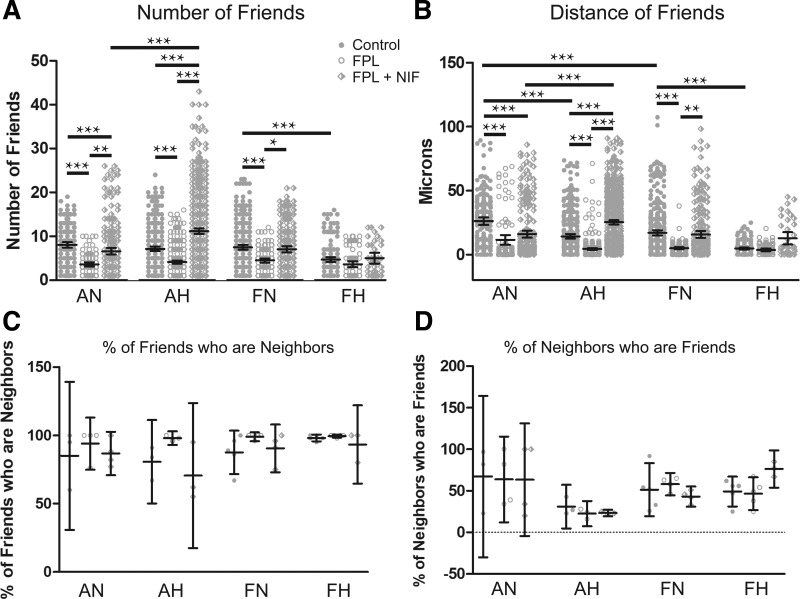
Fig. 4.
FPL 64176 (FPL) decreased the correlation among regions of interest (ROIs) with Ca2+ oscillatory events. A–D: arteries from each group were recorded en face and treated with DMSO (control, closed circles), 1 µM FPL (open circles), or 1 µM FPL with 10 µM nifedipine (NIF) (diamonds). They were analyzed for number of correlated ROIs, distance between correlated ROIs, percentage of nearby ROIs that were correlated, and percentage of correlated ROIs that were in nearby cells. Values are means ± 95% CI based on the number of ROIs (A and B) or number of animals (C and D). Data were analyzed by Kruskal-Wallis one-way ANOVA with Dunn's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. Measurements of correlated ROIs and distance between these ROIs were obtained from the following. Control responses were obtained from 189/3 AN, 349/3 AH, 372/5 FN, and 175/5 FH ROIs and animals, respectively. FPL responses were obtained from 97/3 AN, 370/3 AH, 131/5 FN, and 81/5 FH ROIs and animals, respectively. NIF responses were obtained from 252/3 AN, 633/3 AH, 229/5 FN, and 37/5 FH ROIs and animals, respectively. Percentage of neighboring correlated ROIs were obtained from the following. Control responses were obtained from 3/3 AN, 3/3 AH, 5/5 FN, and 5/5 FH experimental recordings and animals, respectively. FPL responses were obtained from 4/3 AN, 3/3 AH, 5/5 FN, and 5/5 FH experimental recordings and animals, respectively. FPL with NIF responses were obtained from 4/3 AN, 3/3 AH, 5/5 FN, and 5/5 FH experimental recordings and animals, respectively.
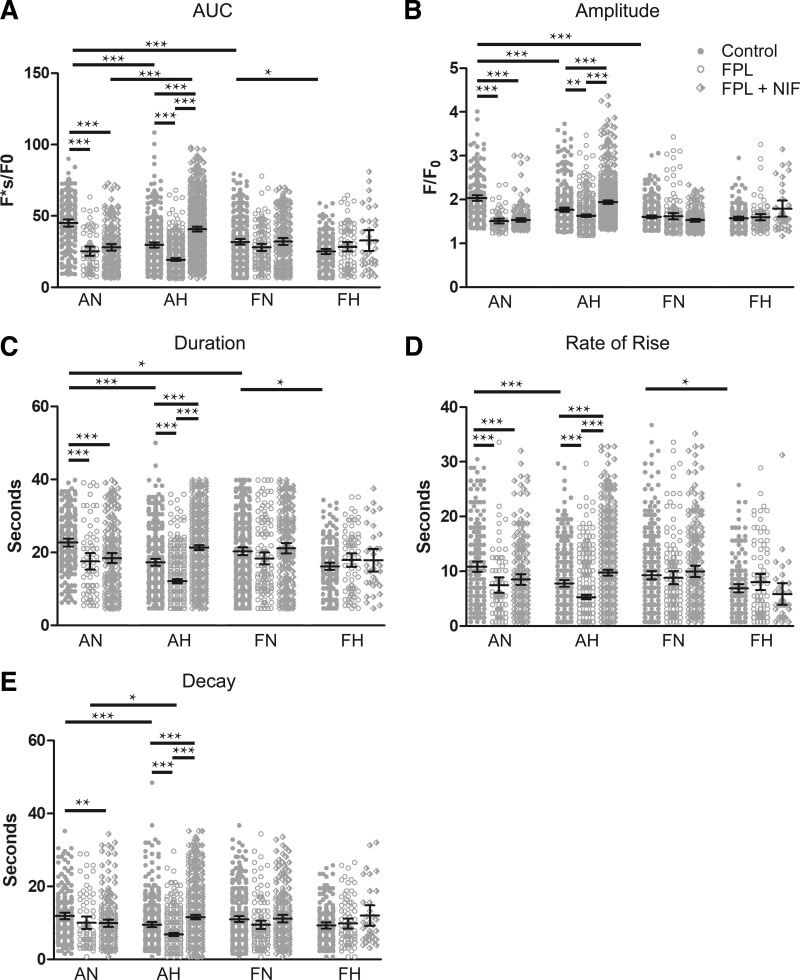
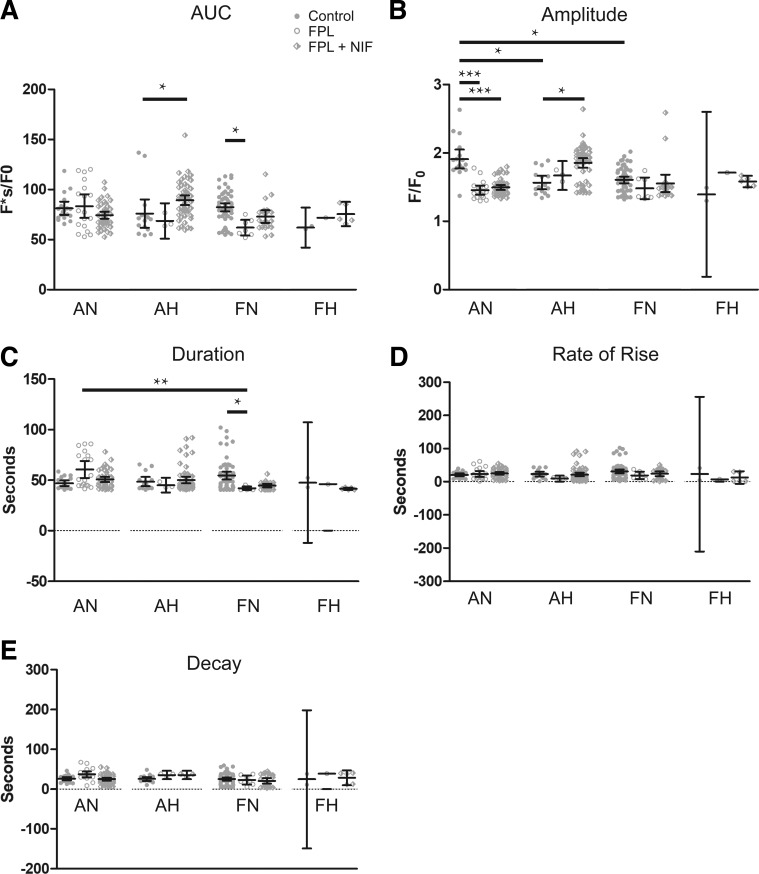
Fig. 6.
Medium-duration Ca2+ oscillation activity is selectively reduced by FPL 64176 (FPL) in pulmonary arterial myocytes of adults. A–E: area under the curve, amplitude of the fractional fluorescence, duration, rate of rise, and decay of medium-duration Ca2+ oscillations exposed to DMSO (control, closed circles), 1 µM FPL (open circles), or 1 µM FPL with 10 µM nifedipine (NIF) (diamonds) for adult and fetal pulmonary arterial myocytes from sheep under normoxic and hypoxic conditions. Values are means ± 95% CI. Data were analyzed by Kruskal-Wallis one-way ANOVA with Dunn's multiple comparison test. **P < 0.01, ***P < 0.001. Control responses were obtained from 213/3 AN, 345/3 AH, 325/5 FN, and 174/5 FH ROIs and animals, respectively. FPL responses were obtained from 72/3 AN, 467/3 AH, 133/5 FN, and 84/5 FH ROIs and animals, respectively. NIF responses were obtained from 194/3 AN, 600/3 AH, 212/5 FN, and 34/5 FH ROIs and animals, respectively.
Fig. 7.
Long-duration Ca2+ oscillations are mildly affected by modifiers of L-type Ca2+ channel (CaL) activity. A–E: area under the curve, amplitude of the fractional fluorescence, duration, rate of rise, and decay of long-duration calcium oscillations exposed to DMSO (control, closed circles), 1 µM FPL 64176 (FPL) (open circles), or 1 µM FPL with 10 µM nifedipine (NIF) (diamonds) for pulmonary arterial myocytes of adult and fetal sheep recorded under normoxic and hypoxic conditions. Values are means ± 95% CI. Data were analyzed by one-way ANOVA with Dunn's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001. Control responses were obtained from 18/3 AN, 15/3 AH, 56/5 FN, and 2/5 FH ROIs and animals, respectively. FPL responses were obtained from 18/3 AN, 3/3 AH, 7/5 FN, and 1/5 FH ROIs and animals, respectively. NIF responses were obtained from 47/3 AN, 55/3 AH, 22/5 FN, and 5/5 FH ROIs and animals, respectively.
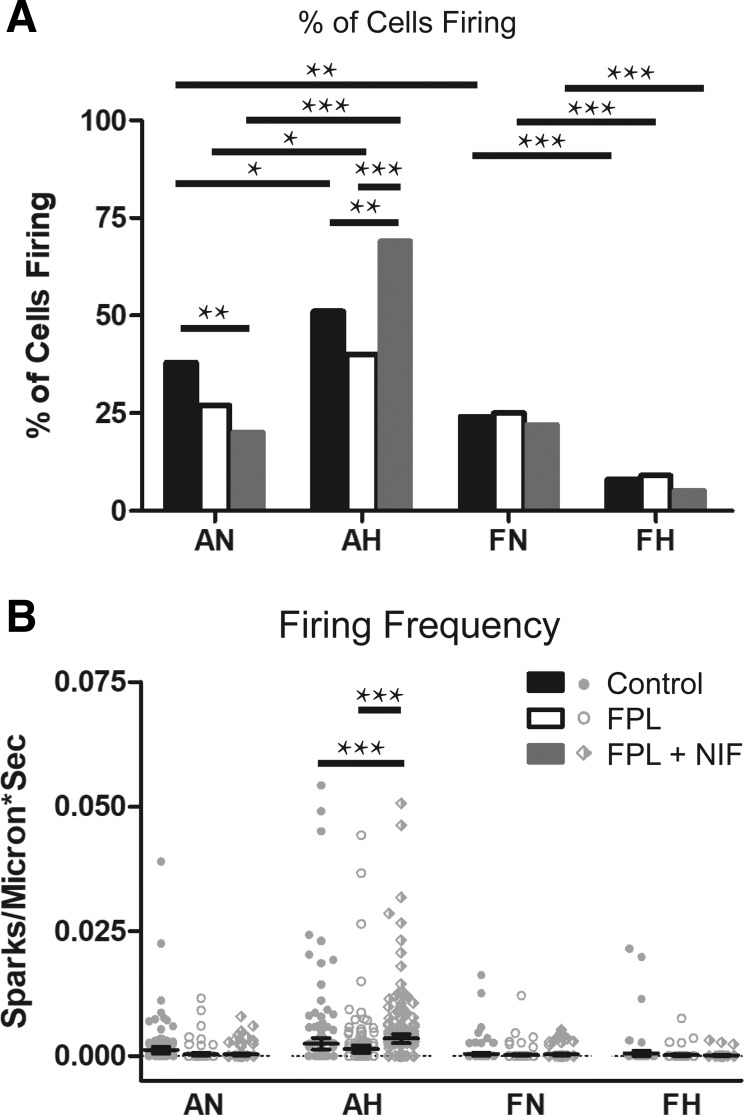
Fig. 9.
FPL 64176 (FPL) alone failed to substantially modify Ca2+ spark activity. A: percentage of cells with Ca2+ sparks. B: Ca2+ spark firing frequency when exposed to DMSO (control, closed bars and circles), 1 µM FPL (open bars and circles), or 1 µM FPL with 10 µM nifedipine (NIF) (gray bar, diamonds) for arterial myocytes from adult and fetal sheep under normoxic and hypoxic conditions. Values are means ± 95% CI. Data in A were analyzed by a χ2 test. **P < 0.01, ***P < 0.001, while data in B were analyzed by Kruskal-Wallis one-way ANOVA with Dunn's multiple comparison test for each group +P < 0.05, +++P < 0.001. Control responses were obtained from 55/141/3 AN, 142/167/3 AH, 19/153/3 FN, and 20/119/3 FH sparks, lines, and animals, respectively. FPL responses were obtained from 14/122/3 AN, 110/183/3 AH, 11/158/3 FN, and 5/103/3 FH sparks, lines, and animals, respectively. FPL with NIF responses were obtained from 14/133/3 AN, 280/216/3 AH, 16/152/3 FN, and 3/106/3 FH regions of sparks, lines, and animals, respectively.
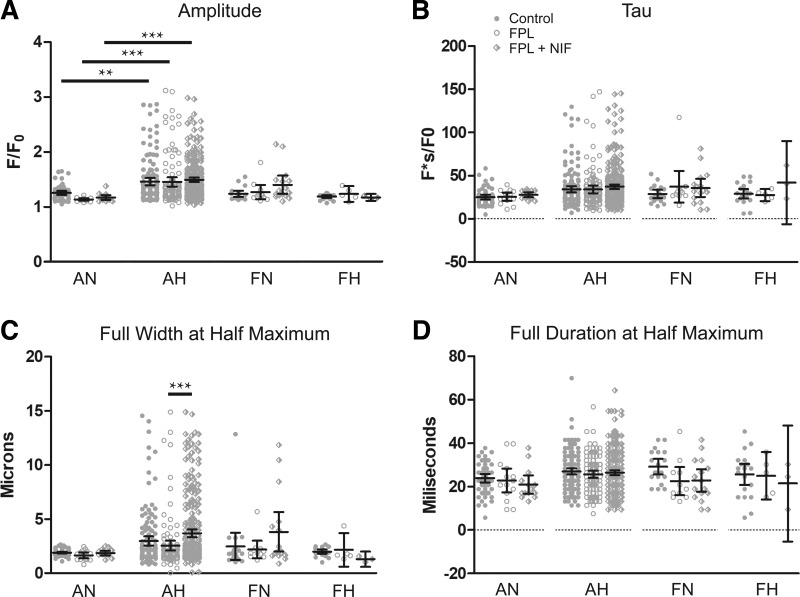
Fig. 10.
Magnitude and kinetics of Ca2+ sparks were modestly affected by FPL 64176 (FPL) or nifedipine (NIF) application. A–D: amplitude, tau, full width at half-maximum, and full duration at half-maximum exposed to DMSO (control, closed circles), 1 µM FPL (open circles), or 1 µM FPL with 10 µM NIF (diamonds) for Ca2+ spark events of arterial myocytes from adult and fetal sheep under normoxic and hypoxic conditions. Values are means ± 95% CI. Data were analyzed by a Kruskal-Wallis one-way way ANOVA with Dunn's multiple comparison test. *P < 0.05, ***P < 0.001. Control responses were obtained from 55/141/3 AN, 142/167/3 AH, 19/153/3 FN, and 20/119/3 FH sparks, lines, and animals, respectively. FPL responses were obtained from 14/122/3 AN, 110/183/3 AH, 11/158/3 FN, and 5/103/3 FH sparks, lines, and animals, respectively. NIF responses were obtained from 14/133/3 AN, 280/216/3 AH, 16/152/3 FN, and 3/106/3 FH regions of sparks, lines, and animals, respectively.
RESULTS
Arterial contractions.
Direct activation of CaL with FPL or BAYK in arteries from normoxic animals elicited contractions, albeit with substantially different force development and kinetics, as shown in Fig. 1. Figure 1, A and B, show summary arterial tension time-series graphs for BAYK and FPL stimulation, respectively, in normoxic adult and fetal vessels. FPL caused a slow increase in contraction, whereas BAYK generally caused contraction to occur more rapidly. FPL-induced contraction tended to remain stable for extended periods, whereas that due to BAYK tended to desensitize, especially in arteries from adult sheep. Figure 1, C–F, shows representative arterial tension traces for FPL-induced contractions performed in tissues isolated from normoxic and hypoxic fetal and adult sheep. FPL contracted the arteries, whereas subsequent NIF treatment caused relaxation.
The maximal contraction to high K+, FPL, and BAYK varied depending on the animal’s age and exposure to long-term hypoxia, as summarized in Fig. 2. Figure 2A shows that, whereas arteries from adults developed robust tension in response to high K+, the tension due to FPL or BAYK was more limited. High K+ developed similar tension in fetal and adult normoxic vessels, but reactivity due to FPL was greater in fetal normoxic than in adult vessels. BAYK elicited contraction similarly to FPL in vessels from the adult normoxic group but generated less tension than FPL in the fetal normoxic group. Figure 2, B and D summarizes the high-K+ normalized contractile force elicited by FPL and BAYK in adult and fetal normoxic arteries and the relaxant response to NIF, a well described L-type Ca2+ channel antagonist that we have used previously to inhibit pulmonary vascular reactivity (27, 28). The data show the peak and the 15-min sustained, normalized force in the presence of DMSO (vehicle control) or NIF. The contraction due to FPL and BAYK relative to high K+ was generally greater in vessels from fetus compared with adult. Ensuing treatment with NIF reduced FPL-induced contraction to, and at times even below, baseline levels. This finding compares with the contraction due to high K+, in which NIF reduced contraction by ~50% (28). Figure 2, C and E, shows the influence of FPL on the contraction of arteries isolated from long-term hypoxic animals. The FPL-induced contraction in adult hypoxic animals was meager (under 7%) relative to high K+ (Fig. 2C). Figure 2E shows that the contraction due to FPL in fetal hypoxic vessels was nearly equivalent to that due to high K+ and was readily inhibited by NIF. Because the contraction elicited by FPL was more pronounced than that by BAYK and did not readily desensitize, FPL was used in subsequent experiments in which we examined Ca2+ oscillations and sparks.
CaL activation of whole cell Ca2+ responses.
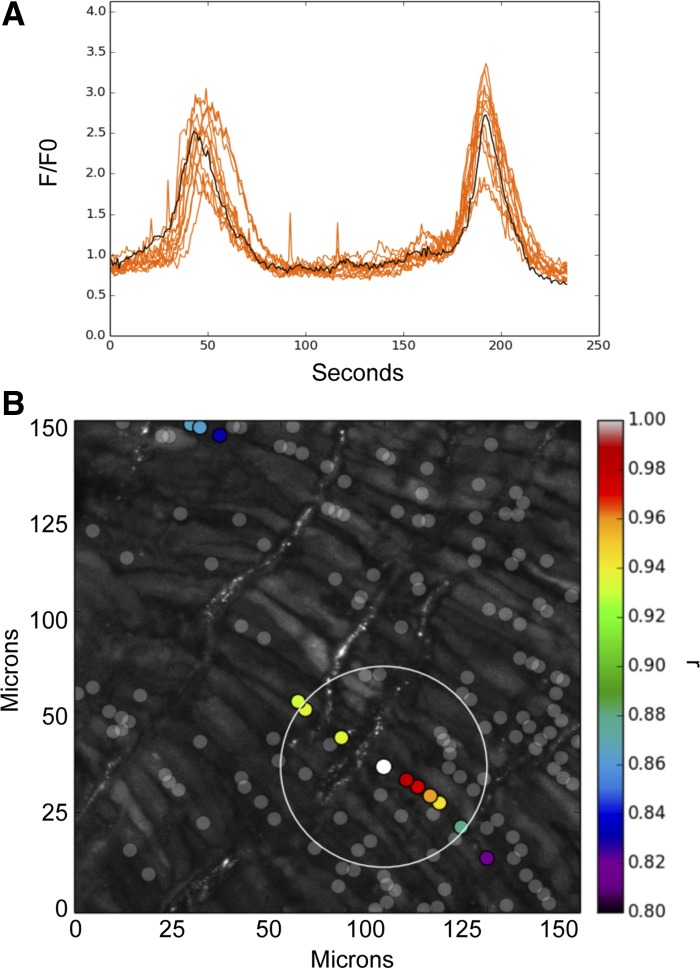
We then examined the influence of CaL activation on myocyte Ca2+ oscillation activity to address the hypothesis that CaL activation causes contraction through an increase in oscillatory activity. Figure 3 shows Ca2+ responses in arterial myocytes of an intact arterial segment acquired in situ using an en face preparation. This particular artery was isolated from an adult sheep and recorded under basal conditions. We observed spontaneous cytosolic Ca2+ increases in the pulmonary arterial myocytes that slowly decayed to baseline (Fig. 3A). Figure 3B shows the fluorescence of cells with increased Ca2+. Qualitatively, the increase in Ca2+ was similar for myocytes across all experimental groups. Calcium transients that were correlated temporally and spatially were plotted using correlation coefficients. Events with correlation coefficients greater than r = 0.8 are shown in Fig. 3B as red/orange regions of interest (ROIs) and plotted surrounding the cell of interest (white dot).
Fig. 3.
Representative Ca2+ responses in pulmonary arterial myocytes from an adult sheep recorded en face under basal conditions. A: fluorescence intensity tracing shows 2 spontaneous calcium oscillations. Regions of interest (ROIs) were plotted using correlation coefficients with highly correlated events colored red/orange and plotted surrounding the center ROI (black trace, white dot in B). B: maximum intensity projection for the fluorescence of cells in the area being recorded. Highly correlated events are colored red/orange and plotted surrounding the center ROI (white dot). Dark gray dots are ROIs that had spontaneous calcium oscillations that were not well correlated with the ROI shown by the white dot.
We then evaluated the interactions of the Ca2+ oscillations among smooth muscle cells by analyzing the temporal correlation of calcium transients, or “friendship,” between oscillations at different ROIs, higher correlation indicating a greater level of “friendship.” Figure 4 shows that hypoxia reduced friendship (Fig. 4A) and the distance between friends (Fig. 4B) in fetal arteries. FPL stimulation also decreased the number of ROIs with friends in both adult and fetal groups under normoxic conditions and just the adults under hypoxic conditions (Fig. 4A). Although FPL resulted in fewer friendships, those friends that remained were closer together (Fig. 4B). Subsequent NIF addition caused the number of friends to increase in all except for the fetal hypoxic group and increased the distance between friends in all groups. We subsequently examined spatial correlations between friends, a relationship characterized as “neighbors” (Fig. 4, C and D, respectively), with close spatial proximity meaning a higher neighbor score. Overall, the proportion of friends that were in close proximity did not change.
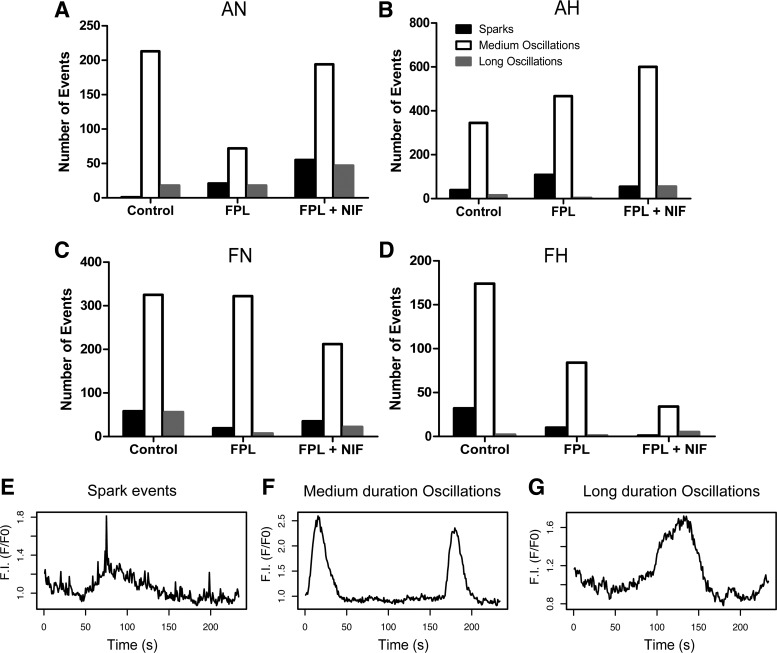
Upon visual observation of the Fluo-4 recordings, we saw events with three broadly defined duration characteristics. A minority of Ca2+ events were distinctly and visibly fast, often at our limit of detection, with durations less than 2 s, or below 2–3 acquisition points in the full-frame images. These rapid events were interpreted as Ca2+ sparks (Fig. 5E) (16, 23). For the sake of completeness and data transparency, these events were scored in the video recordings. Given the slow rate of acquisition of these recordings, Ca2+ sparks were subsequently examined by making high-speed line-scan recordings and performing post hoc analysis with SparkLab (17). A majority of Ca2+ events were between 4 and 40 s long (Fig. 5F), and we termed these medium duration events. Finally, some events lasted much longer than the medium duration Ca2+ oscillations (Fig. 5G). Thus, we divided the oscillations into three categories: Ca2+ sparks, which lasted less than 4 s, medium-duration oscillations of 4–40 s, and long-duration oscillations that lasted more than 40 s. The number of events in each category and their distribution are depicted in Fig. 5, A–D.
Fig. 5.
Pulmonary arterial myocytes had 3 temporally distinct types of Ca2+ signaling events: Ca2+ sparks, medium-duration oscillations, and long-duration oscillations. A–D: absolute counts of each type of event exposed to DMSO (control), 1 µM FPL 64176 (FPL), or 1 µM FPL with 10 µM NIF for recordings from arteries isolated from each animal group. E–G: representative tracings from the AN control group of a Ca2+ spark, medium-duration oscillation, and a long-duration oscillation, respectively. In all groups, the majority of events were medium-duration oscillations.
Data were then separated into groups of medium- and long-duration oscillations for subsequent temporal analysis, as shown in Figs. 6 and 7, respectively. This analysis quantified the magnitude and kinetics of the fluorescent Ca2+ signals for the medium- and long-duration oscillations. Figure 6 provides summaries of the quantitative analysis for various aspects of the Ca2+ responses of medium-duration oscillations. Maturation decreased the area under the calcium response curve (AUC), amplitude, and duration of the Ca2+ response under control conditions. Hypoxia decreased each of the measured parameters in adult myocytes under control conditions. FPL application did not influence measured parameters in fetal myocytes; however, it decreased the AUC, amplitude, duration, rate of Ca2+ rise for the Ca2+ signals of ROIs in normoxic and hypoxic adult myocytes, and decay (rate of Ca2+ decay) in hypoxic adults (Fig. 6, A–E). Relative to control, NIF-treated myocytes had decreased AUC, amplitude, duration, and rate of rise, as well as decay in normoxic adults. However, in hypoxic adults NIF exposure increased each parameter of the Ca2+ oscillatory signals (Fig. 6, A–E). Interestingly, FPL treatment as well as exposure to NIF failed to modify Ca2+ signals in myocytes of fetuses.
We then quantified the magnitude and kinetics for the long-duration Ca2+ oscillations, events that lasted more than 40 s (Fig. 7, A–E). The amplitude of Ca2+ events under control conditions was greater in adult normoxic myocytes than in hypoxic adults and normoxic fetuses. The figure illustrates that FPL and NIF treatments had differential impacts on the long-duration oscillations compared with the medium-duration events. The AUC was increased modestly by the combination of FPL and NIF in adult hypoxic myocytes, as shown in Fig. 7A. However, FPL decreased the AUC in fetal normoxic myocytes. The peak amplitude of the Ca2+ response (Fig. 7B) was depressed in adult normoxic myocytes by either FPL or the combination of FPL and NIF. In comparison, the combination of FPL and NIF increased the peak amplitude in adult hypoxic myocytes. The duration of the Ca2+ response (Fig. 7C) was decreased in fetal normoxic myocytes by FPL treatment. The rate of rise (Fig. 7D) as well as decay (Fig. 7E) were unaffected by the experimental treatments in all groups.
CaL activation of Ca2+ sparks.
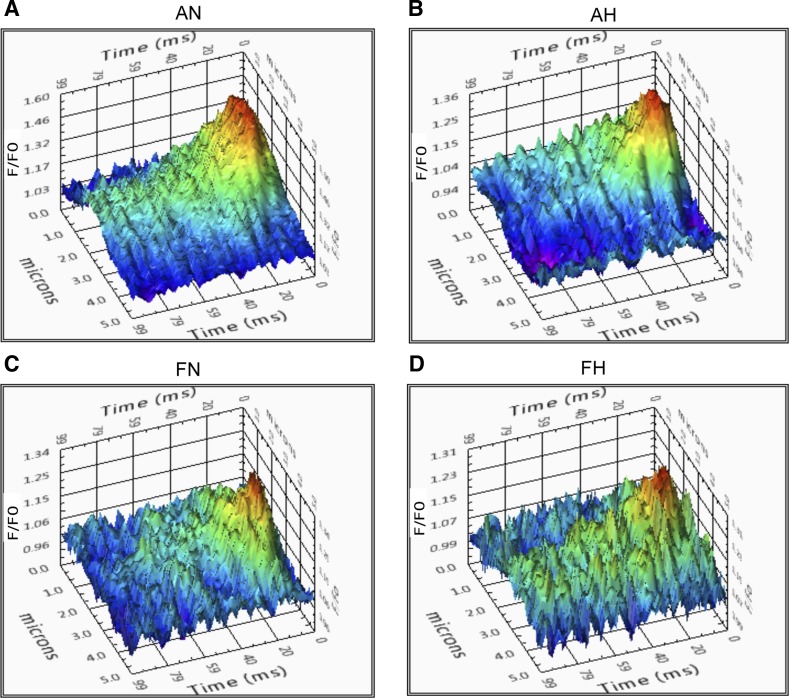
The last series of studies examined the influence of CaL activation on Ca2+ spark activity. Figure 8 shows representative Ca2+ spark tracings for control groups analyzed through SparkLab (Fig. 8, A–D). Many laboratories, including our own, have illustrated that membrane depolarization with moderately high concentrations of extracellular K+ can enhance Ca2+ spark activity (16, 21, 22, 50, 52). However, few studies have evaluated the influence of direct CaL activation on spark activation. Under control conditions a greater proportion of adult normoxic myocytes had Ca2+ sparks relative to fetuses. Under the control and both treatment conditions, hypoxia increased Ca2+ spark activity in adults but decreased activity in fetuses. This finding supports the influence of maturation and hypoxia on Ca2+ spark activity that was shown in our previous studies (16). Overall, direct CaL activation with FPL did not significantly modify the percentage of cells or the frequency of spark activation relative to baseline (Fig. 9, A and B). NIF treatment decreased the percentage of cells with sparks compared with baseline in adult normoxic cells but increased the prevalence of sparks in adult hypoxic cells. Spark frequency increased following the combination of FPL and NIF treatment in adult hypoxic myocytes (Fig. 9B). Interestingly, in adult hypoxic cells, the percentage of cells with sparks and their frequency was significantly lower following FPL treatment relative to combined FPL and NIF treatment (Fig. 9, A and B). Few sparks were recorded in fetal cells, and FPL or the combination of FPL and NIF failed to influence their activity.
Fig. 8.
Representative Ca2+ spark tracings for pulmonary arterial myocytes. A–D: fluo-4 fluorescence tracings of pulmonary arterial smooth muscle cells of adult and fetal sheep under normoxic and hypoxic conditions.
The magnitude and kinetics of Ca2+ sparks were quantified with the summary results shown in Fig. 10. Ca2+ spark amplitude was increased by hypoxia in adult myocytes for all three experimental conditions. The changes in spatial or temporal aspects to the Ca2+ sparks were modest following treatment with FPL or FPL with NIF. Ca2+ spark amplitude was slightly, but not significantly, reduced in adult normoxic myocytes following FPL or FPL with NIF. However, in adult hypoxic myocytes the Ca2+ spark amplitude for FPL and NIF was slightly elevated, but again not significantly, relative to the FPL group. Ca2+ spark full width at half-maximum (FWHM) was wider following FPL and NIF treatment relative to FPL for adult hypoxic myocytes. Ca2+ spark full duration at half-maximum (FDHM) and tau remained unchanged following treatment.
DISCUSSION
Because CaL conductance is a key requirement for arterial smooth muscle cell contraction, our experiments were designed to provide clarity about its importance in pulmonary arterial contractions and the coupling with Ca2+ responses. Our hypothesis was that contraction, L-type Ca2+ channel activity, and cellular Ca2+ responses are all correlated and interrelated. Stimulating L-type Ca2+ channels was predicted to elicit subcellular Ca2+ spark events and whole cell oscillatory Ca2+ responses directly proportional to pulmonary arterial contraction. Instead, subcellular and whole cell oscillatory Ca2+ responses were dissociated from contraction. These findings disprove the postulate and suggest that the relationship between calcium handling and contraction in pulmonary arteries is more complex than previously believed.
Arterial contractions.
The two chosen CaL agonists, FPL and BAYK, each caused arterial contractions that were sensitive to NIF. This was expected and illustrates that the agonists cause arterial contraction by increasing CaL-mediated Ca2+ influx. However, the kinetics of contraction elicited by each agonist was different, and this was developmentally dependent. FPL elicited a slow rise in the contraction, whereas that of BAYK was more rapid (Fig. 1). Secondarily, in arteries from adults, the contraction due to BAYK rapidly decayed, while the contraction in fetal vessels was well maintained (Fig. 1). The lack of decay in contractions due to BAYK in the fetal vessels suggests that development leads to distinct differences in the mechanism of action by which these two agonists enhance CaL activity. The differences in the two modulators likely reflect variation to CaL kinetics of activation and inactivation. Although not addressed in the present study, one possibility is that kinetic differences may be due to modifications in the CaL isoforms expressed in fetal versus adult pulmonary arterial myocytes. Indeed, there is evidence for developmental variation in CaL isoform expression in embryonic versus adult skeletal muscle (3). Embryonic skeletal muscles express a Cav1.1 splice variant that results in larger Ca2+ currents and modified excitation-contraction coupling relative to channels in adult skeletal muscle (3, 39). From a practical perspective, the rapid decay in the BAYK-induced contraction led us to believe that the induced Ca2+ signals may be short lived and preclude accurate assessment of CaL to Ca2+ spark coupling. Associated with this, the finding that FPL caused a more sustained contraction than BAYK suggests that FPL would be more conducive for Ca2+ imaging studies. Interestingly, the absolute as well as relative magnitudes of the contractions due to FPL versus high K+ were greater in the fetus relative to the adult (Fig. 2A), whereas the high K+ contractions were far greater in the adult relative to the fetus (28). Although not actively explored in the present studies, there are several possible explanations for this. As explained above, changes in the action of FPL could be due to genetic differences in the expression of CaL channel isoforms in the fetus relative to the adult (3, 39). Alternatively, the magnitude of FPL-induced activation of CaL is related to the resting membrane potential, where the CaL of more depolarized cells will respond more readily to FPL and contract to a greater extent than those that are relatively hyperpolarized (51). Vast differences in resting membrane potential seem unlikely in our preparations, as we previously showed that the dose-dependent contractile responses to varied K+ concentrations were similar between pulmonary arteries from fetuses and adults (28). Even so, facilitated CaL activity following hypoxia may have bearing on structural and functional malformations that lead to development of pulmonary hypertension, as there could be sustained increases in Ca2+ influx and resultant enhancement in contractility and activation of Ca2+-dependent transcription factors.
NIF inhibited CaL-induced contractions predictably. Interestingly, NIF reduced FPL-mediated contraction far more substantially than it did in our previously published studies where we evaluated high K+-induced contractions (28). Although not conclusive, this finding supports the premise that there are fundamental differences between contractions due to direct CaL activation versus those due to high K+. Direct CaL-induced contractions with FPL and BAYK appear to depend predominantly on NIF-sensitive pathways, as one would expect. This finding contrasts the K+-induced contraction that we and others have previously shown is also dependent on Rho kinase activation (7, 28). Taken together, these data suggest that high K+ activates the Rho kinase pathway distinctly from the activation of CaL, although the present series of studies cannot discount the potential that there is cross-talk between these two pathways (32, 41). The finding that FPL-elicited reactivity is equal to that of high K+ in arteries from fetuses but substantially subdued in arteries from adults further suggests that Ca2+-sensitization pathways or other pathways important to high K+ contraction are upregulated following birth (28). Our current studies also provide additional evidence supporting our earlier work showing that NIF-sensitive CaL-dependent contractions were preserved following antenatal long-term hypoxia (Fig. 2) (28).
Whole cell models of Ca2+ signals indicate that the interplay between cytosolic Ca2+ influx and removal is dynamic, an effect that can have complex influences on Ca2+ oscillations and on the average cytosolic Ca2+ concentration (14, 25, 35). In particular, Ca2+ oscillations are dependent on the balance of extracellular influx and extrusion as well as intracellular release and uptake pathways that regulate steady-state and dynamic changes in the cytosolic Ca2+ concentration. These studies illustrate that CaL activation with FPL increases Ca2+ influx into the cytosol, as there is an increase in the NIF-sensitive contraction. FPL-induced activation of CaL therefore skews the balance, favoring Ca2+ influx over efflux pathways. Whole cell mathematical models of Ca2+ signaling dynamics further illustrate that Ca2+ oscillations are dependent on the stability of the system. These models show that perturbations in Ca2+ fluxes can lead to highly complex Ca2+ signaling behavior, far beyond simple changes in amplitude or period of the oscillatory Ca2+ signal (35). Instead of a straightforward mechanism in which decreasing or increasing CaL activity causes corresponding changes to the oscillatory Ca2+ signaling behavior, the myocytes that we examined have reasonably stable oscillatory signals during FPL stimulation. This finding suggests that there may be important differences between acute and long-term modulations of cellular steady-state and dynamic Ca2+ levels that serve distinct cellular functions.
The present series of studies examined Ca2+ signaling between individual myocytes in the arterial wall and examined potential differences due to hypoxia or postnatal maturation. The general premise is that communication between cells can coordinate and synchronize cellular activity. This type of coordination occurs readily in the gut, where the myenteric plexus allows for sequential firing of circular and longitudinal smooth muscle, which elicits peristaltic waves (36). Coordination among cells within the vessel walls, in comparison, is largely unknown, although it is well established that pulmonary vessels have tonic as opposed to phasic contraction (7, 46). One possibility is that coordination of Ca2+ signals between cells may provide for tonic as well as phasic contractile responses. Our data, however, illustrate that the role of CaL to contraction is unlikely to be related to enhancing coordination of firings between cells in the pulmonary arterial wall. What is more, the finding that FPL decreased coordinated ROI activity in all groups suggests that CaL activation may restrict ROI coordination. Of note, because the average distance between these coordinated ROIs was smaller than the average width of a pulmonary artery smooth muscle cell, most of the coordinated ROIs found after FPL application arose within individual cells and were not likely due to synchronized firings among separate cells (Fig. 4B). Rather, FPL activation resulted in few if any coordinated ROIs, possibly impairing the communication between cells identified by our ROIs. Overall, these findings are somewhat counter to the prevailing theory that Ca2+ oscillation activity is proportional and directly associated with contractions. This paradox hints at still-elusive cellular mechanisms important to the function and coordination of Ca2+ signals within active tissues.
The whole cell Ca2+ events with unique temporal characteristics (Fig. 5) reveal the presence of varied and presumably functionally distinct Ca2+ oscillations. The typical medium-duration whole cell Ca2+ oscillations that we and others routinely examine are not sufficient to explain the complexity of the role Ca2+ has on contraction as well as other cellular functions (2, 18, 19, 33, 42). Our data suggest that direct CaL activation with FPL does not enhance contractions of pulmonary arteries by influencing Ca2+ oscillations in individual myocytes. Whole cell Ca2+ oscillations in myocytes of adults appear to be inhibited by FPL and hypoxic conditions. Collectively, these results suggest a lack of coupling between CaL-dependent contractions and Ca2+ oscillations. Interestingly, the number of ROIs with Ca2+ oscillations was not reduced by FPL in fetal vessels, suggesting that the mechanism responsible for the suppression of oscillations may not be in place before birth.
The lack of impact by FPL and NIF on the long-duration oscillations (Fig. 7) emphasizes that these are distinct Ca2+ events, which are regulated by different Ca2+ flux pathways as opposed to the medium-duration oscillations, although the present study was not designed to resolve the origin of these long-duration Ca2+ oscillations. Because the long-duration events were infrequent, examining the physiological relevance of these events will be challenging. The finding that long-duration Ca2+ oscillation properties were similar across all groups suggests that these events are produced by an underlying mechanism that is not impacted by either maturation or hypoxia. FPL tends to decrease the amplitude of the oscillations in adult normoxic and decrease the AUC and duration of the Ca2+ oscillations in fetal normoxic myocytes, suggesting that the longer Ca2+ events are only partially dependent on CaL. These data imply that there is an indirect effect of CaL activity on long-duration Ca2+ oscillations (Fig. 7, A–C). Physiologically, longer-lasting Ca2+ oscillations have been studied for their roles in synaptic transmission in neurons and fertilization of eggs (31, 45). However, long-duration Ca2+ oscillations have not been examined for their importance in pulmonary arterial myocytes; therefore, understanding the role of these longer Ca2+ oscillations requires further investigation.
CaL activation of Ca2+ sparks.
The finding that FPL failed to enhance the Ca2+ spark activity is somewhat surprising and is counter to the widely held belief that Ca2+ sparks are activated by CaL. Previously, we showed that modest membrane depolarization with 30 mM K+ enhanced Ca2+ spark activity. Similarly, BAYK and 20 mM K+ both enhanced Ca2+ sparks in rat pulmonary arterial myocytes (30). One possibility is that FPL, BAYK, and K+ depolarization each activates CaL via different biophysical mechanisms. In the case of elevated K+, the membrane depolarizes and the channels undergo transitions from open to inactive, as well as to intermediate closed states. In comparison, FPL increases the number of CaL open channels by decreasing channel closure rates (37). In addition, different mechanisms have been proposed to explain low and high coupling fidelity that is observed in various scenarios. This includes the importance of full activation of voltage-gated calcium channels, the effect of membrane voltage, and the time constant of RyR activation (1, 29, 48).
There are noted differences between our work with FPL and that performed with BAYK in pulmonary arterial myocytes of rats (30). These distinctions may be due to subtle differences in how these compounds influence the CaL activity or potentially the loading state of Ca2+ in the sarcoplasmic reticulum. In ferret cardiac tissues, CaL activation with BAYK prolongs the time constant of RyR activation, consistent with the observed decrease in the efficacy of CaL currents to induce further Ca2+ release from the sarcoplasmic reticulum upon application of BAYK (26). Because BAYK does not directly influence RyR pharmacokinetics, it has been suggested that this phenomenon is the result of reduced sarcoplasmic reticulum Ca2+ content, which is a plausible explanation for our findings with FPL (29). Accordingly, an increase in the Ca2+ concentration in the sarcoplasmic reticulum, and concomitant effects on RyR activation, may also explain the paradoxical effect of NIF, which enhances the percentage of cells exhibiting Ca2+ sparks.
We began these experiments with the expectation that direct CaL activation would enhance both Ca2+ oscillations and spark activity, correlating with contraction. Instead, what we found is an extraordinary divergence from that expectation. Our experiments suggest there is an uncoupling between Ca2+ oscillations and cellular contractile events. The findings imply that Ca2+ oscillations may not underlie the role of CaL in mediating contractions of sheep pulmonary arterial myocytes. This challenges our understanding of the role of Ca2+ oscillations in smooth muscle contraction and provokes a myriad of new research trajectories. Stimulating CaL increased contractions as we presumed, but it decreased whole cell oscillatory Ca2+ signaling events, which was unexpected.
Perspectives and Significance
Taken together, the present series of studies provide novel insight into the interplay of Ca2+ entry and Ca2+ oscillatory signaling in myocytes of the intact arterial wall as well as the impact of maturation and LTH. The presented data provide details that while CaL is important to contractility it is not likely mediated through Ca2+ oscillations. Thus, the oscillatory events likely encode for other types of cell function. Potentially the Ca2+ oscillations may be important for the activation of transcription factors, which would modulate arterial reactivity through changes in the expression of various gene products. Beyond raising critical questions regarding the physiological importance of these Ca2+ oscillations, the data also lead to questions regarding the interplay between extracellular Ca2+ entry and intracellular Ca2+ release and coordination of signals among myocytes in the arterial wall. The present study illustrates that the contraction due to direct CaL stimulation is well developed before birth, which is unlike the contraction due to K+-induced membrane depolarization. The fetus appears to be somewhat protected from the impact of LTH with regard to effects of CaL activation on contractions and Ca2+ signals.
GRANTS
This material is based on work supported by National Science Foundation Grant MRI 0923559 and National Institutes of Health Grants HD-069746 (to S. M. Wilson), HL-085887 (to M. S. Taylor), P01 HD-031226, and R01 HD-003807 (to L. D. Longo), P01 HD-083132 (to L. Zhang). Additional funding for research reported in this publication was provided by Grants for Research and School Partnerships. Additional support for the Advanced Imaging and Microscopy Core was provided by the Loma Linda University School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S., M.R., A.B., C.W., A.D., J.P., C.G.W., and S.M.W. analyzed data; C.S., M.R., A.B., C.W., A.D., J.P., L.D.L., L.Z., C.G.W., and S.M.W. interpreted results of experiments; C.S., M.R., A.B., C.W., J.P., C.G.W., and S.M.W. prepared figures; C.S., C.G.W., and S.M.W. drafted manuscript; C.S., M.R., A.B., C.W., A.D., M.F., M.S.T., J.P., L.Z., C.G.W., and S.M.W. edited and revised manuscript; M.R., A.B., C.W., C.G.W., and S.M.W. performed experiments; L.D.L., C.G.W., and S.M.W. conceived and designed research; C.S., M.R., A.B., C.W., A.D., M.F., M.S.T., J.P., L.Z., C.G.W., and S.M.W. approved final version of manuscript.
REFERENCES
- 1.Altamirano J, Bers DM. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ Res 101: 590–597, 2007. doi: 10.1161/CIRCRESAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- 2.Bading H, Hardingham GE, Johnson CM, Chawla S. Gene regulation by nuclear and cytoplasmic calcium signals. Biochem Biophys Res Commun 236: 541–543, 1997. doi: 10.1006/bbrc.1997.7037. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti B, Tuluc P, Mastrolia V, Dlaska C, Flucher BE. Physiological and pharmacological modulation of the embryonic skeletal muscle calcium channel splice variant CaV1.1e. Biophys J 108: 1072–1080, 2015. doi: 10.1016/j.bpj.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blood AB, Terry MH, Merritt TA, Papamatheakis DG, Blood Q, Ross JM, Power GG, Longo LD, Wilson SM. Effect of chronic perinatal hypoxia on the role of Rho-kinase in pulmonary artery contraction in newborn lambs. Am J Physiol Regul Integr Comp Physiol 304: R136–R146, 2013. doi: 10.1152/ajpregu.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum-Johnston C, Thorpe RB, Wee C, Romero M, Brunelle A, Blood Q, Wilson R, Blood AB, Francis M, Taylor MS, Longo LD, Pearce WJ, Wilson SM. Developmental acceleration of bradykinin-dependent relaxation by prenatal chronic hypoxia impedes normal development after birth. Am J Physiol Lung Cell Mol Physiol 310: L271–L286, 2016. doi: 10.1152/ajplung.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broughton BRS, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 10.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol 115: 653–662, 2000. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis M, Qian X, Charbel C, Ledoux J, Parker JC, Taylor MS. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am J Physiol Cell Physiol 303: C236–C243, 2012. doi: 10.1152/ajpcell.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh D, Syed AU, Prada MP, Nystoriak MA, Santana LF, Nieves-Cintrón M, Navedo MF. Calcium channels in vascular smooth muscle In: Advances in Pharmacology, by Khalil RA. New York: Academic, 2017, chapt. 2, p. 49–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res 83: 1104–1114, 1998. doi: 10.1161/01.RES.83.11.1104. [DOI] [PubMed] [Google Scholar]
- 14.Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L905–L914, 2008. doi: 10.1152/ajplung.00053.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson AS, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18: 948–962, 2011. doi: 10.1177/1933719111401660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadley SR, Blood Q, Rubalcava M, Waskel E, Lumbard B, Le P, Longo LD, Buchholz JN, Wilson SM. Maternal high-altitude hypoxia and suppression of ryanodine receptor-mediated Ca2+ sparks in fetal sheep pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 303: L799–L813, 2012. doi: 10.1152/ajplung.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol 35: 1843–1851, 2015. doi: 10.1161/ATVBAHA.115.305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 19.Holowka D, Wilkes M, Stefan C, Baird B. Roles for Ca2+ mobilization and its regulation in mast cell functions: recent progress. Biochem Soc Trans 44: 505–509, 2016. doi: 10.1042/BST20150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabr RI, Toland H, Gelband CH, Xia Wang X, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 122: 21–30, 1997. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 22.Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol Cell Physiol 274: C1755–C1761, 1998. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- 23.Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 280: C22–C33, 2001. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- 24.Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD. Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169: 701–707, 1993. doi: 10.1016/0002-9378(93)90646-Z. [DOI] [PubMed] [Google Scholar]
- 25.Keizer J, De Young G. Effect of voltage-gated plasma membrane Ca2+ fluxes on IP3-linked Ca2+ oscillations. Cell Calcium 14: 397–410, 1993. doi: 10.1016/0143-4160(93)90044-7. [DOI] [PubMed] [Google Scholar]
- 26.McCall E, Bers DM. BAY K 8644 depresses excitation-contraction coupling in cardiac muscle. Am J Physiol Cell Physiol 270: C878–C884, 1996. doi: 10.1152/ajpcell.1996.270.3.C878. [DOI] [PubMed] [Google Scholar]
- 27.Papamatheakis DG, Chundu M, Blood AB, Wilson SM. Prenatal programming of pulmonary hypertension induced by chronic hypoxia or ductal ligation in sheep. Pulm Circ 3: 757–780, 2013. doi: 10.1086/674767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papamatheakis DG, Patel JJ, Blood Q, Merritt TT, Longo LD, Wilson SM. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ 2: 41–53, 2012. doi: 10.4103/2045-8932.94832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poláková E, Zahradníková A Jr, Pavelková J, Zahradník I, Zahradníková A. Local calcium release activation by DHPR calcium channel openings in rat cardiac myocytes. J Physiol 586: 3839–3854, 2008. doi: 10.1113/jphysiol.2007.149989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remillard CV, Zhang W-M, Shimoda LA, Sham JSK. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L433–L444, 2002. doi: 10.1152/ajplung.00468.2001. [DOI] [PubMed] [Google Scholar]
- 31.Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci 13: 157–168, 2012. doi: 10.1038/nrn3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93: 548–556, 2003. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 33.Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26: 300–312, 2016. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimoda LA, Undem C. Interactions between calcium and reactive oxygen species in pulmonary arterial smooth muscle responses to hypoxia. Respir Physiol Neurobiol 174: 221–229, 2010. doi: 10.1016/j.resp.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith GD, Lee RJ, Oliver JM, Keizer J. Effect of Ca2+ influx on intracellular free Ca2+ responses in antigen-stimulated RBL-2H3 cells. Am J Physiol Cell Physiol 270: C939–C952, 1996. doi: 10.1152/ajpcell.1996.270.3.C939. [DOI] [PubMed] [Google Scholar]
- 36.Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol 312: G1–G14, 2017. doi: 10.1152/ajpgi.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanislav B, Philipp K, Eugen T, Steffen H. Direct estimation of CaV1.2 gating parameters: quantification of voltage sensor-pore transductions and their modulation by FLP 64176. Curr Mol Pharmacol 8: 87–94, 2015. doi: 10.2174/1874467208666150507100256. [DOI] [PubMed] [Google Scholar]
- 38.Subedi KP, Paudel O, Sham JSK. Detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 306: C659–C669, 2014. doi: 10.1152/ajpcell.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J 96: 35–44, 2009. doi: 10.1016/j.bpj.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol Cell Physiol 285: C1377–C1385, 2003. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- 41.del Valle-Rodríguez A, López-Barneo J, Ureña J. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J 22: 4337–4345, 2003. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuong B, Hogan-Cann ADJ, Alano CC, Stevenson M, Chan WY, Anderson CM, Swanson RA, Kauppinen TM. NF-κB transcriptional activation by TNFα requires phospholipase C, extracellular signal-regulated kinase 2 and poly(ADP-ribose) polymerase-1. J Neuroinflammation 12: 229, 2015. doi: 10.1186/s12974-015-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 25: 628–635, 2001. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- 45.Whitaker M. Calcium at fertilization and in early development. Physiol Rev 86: 25–88, 2006. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol 144: 252–264, 2005. doi: 10.1038/sj.bjp.0706077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol (1985) 104: 1786–1792, 2008. doi: 10.1152/japplphysiol.01314.2007. [DOI] [PubMed] [Google Scholar]
- 48.Zahradníková A, Kubalová Z, Pavelková J, Györke S, Zahradník I. Activation of calcium release assessed by calcium release-induced inactivation of calcium current in rat cardiac myocytes. Am J Physiol Cell Physiol 286: C330–C341, 2004. doi: 10.1152/ajpcell.00272.2003. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W-M, Lin M-J, Sham JSK. Endothelin-1 and IP3 induced Ca2+ sparks in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol 44, Suppl 1: S121–S124, 2004. doi: 10.1097/01.fjc.0000166226.03712.4f. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W-M, Yip K-P, Lin M-J, Shimoda LA, Li W-H, Sham JSK. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 51.Zheng W, Rampe D, Triggle DJ. Pharmacological, radioligand binding, and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol Pharmacol 40: 734–741, 1991. [PubMed] [Google Scholar]
- 52.Zheng Y-M, Wang Q-S, Liu Q-H, Rathore R, Yadav V, Wang Y-X. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res 45: 469–479, 2008. doi: 10.1159/000127438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y-M, Wang Q-S, Rathore R, Zhang W-H, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang Y-X. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]