Abstract
Studies in our laboratory have shown that modest chronic increases in maternal cortisol concentrations over the last 0.20 of gestation impair maternal glucose metabolism and increase the incidence of perinatal stillbirth. Previous studies had found that an increase in maternal cortisol concentrations from 115 to 130 days of gestation in sheep increased both proliferation in fetal cardiomyocytes and apoptosis in the fetal cardiac Purkinje fibers. We hypothesized that the adverse effects of excess cortisol may result in defects in cardiac conduction during labor and delivery. In the present study, we infused cortisol (1 mg·kg−1·day−1) into late gestation pregnant ewes and continuously monitored fetal aortic pressure and ECG through labor and delivery. We found that, although the fetuses of cortisol infused ewes had normal late gestation patterns of arterial pressure and heart rate, there was a significant decrease in fetal aortic pressure and heart rate on the day of birth, specifically in the final hour before delivery. Significant changes in the fetal ECG were also apparent on the day of birth, including prolongation of the P wave and P-R interval. We speculate that chronic exposure to glucocorticoids alters cardiac metabolism or ion homeostasis, contributing to cardiac dysfunction, precipitated by active labor and delivery.
Keywords: cortisol, ECG, fetus, telemetry
INTRODUCTION
Glucocorticoids are essential for maturation of the fetal heart in late gestation (52). Nevertheless, experimental data from animal studies and clinical data from human studies indicate that the late gestation fetal heart may be especially susceptible to either early or excessive glucocorticoid signaling (9, 42). Overexposure to maternal glucocorticoids has been extensively studied in the context of antenatal glucocorticoid therapy for premature delivery; however, increased maternal secretion of endogenous corticosteroids can also contribute to the overexposure of fetal tissues (41, 44). Although Cushing’s syndrome is rare in pregnancy, the incidence of complications such as premature delivery, stillbirth, and intrauterine growth restriction are increased in these pregnancies, as well as those complicated by chronic maternal stress caused by adverse events such as violence or loss of a relative during pregnancy (34, 59, 61).
In previous studies using an ovine model of pregnancy, we have shown that modest, chronic increases in maternal cortisol concentration beginning at 115 days of gestation increase the thickness of the right ventricular and left ventricular free walls and increase fetal heart weight relative to fetal body weight at 130 days of gestation (term ~145 days) (45). These changes were not associated with an increase in fetal blood pressure or cardiac fibrosis and could be blocked by mineralocorticoid receptor (MR) antagonism. In the same fetuses, the cortisol overexposure significantly increased activated caspase-3 staining of Purkinje fibers in the intraventricular septum and subendocardial layers of the left ventricle; this effect of cortisol could be blocked by glucocorticoid receptor (GR) antagonism, suggesting that chronically increased fetal cortisol concentrations increased apoptosis of the fetal cardiac conduction system (19). Statistical modeling of gene expression in the 130-day fetal lamb septa indicated overexpression of pathways related to cellular calcium homeostasis and apoptosis (specifically muscle cell apoptosis) (50). Expanding on these studies, we found that cortisol treatment dramatically increased the incidence of stillbirth, particularly in the immediate perinatal period, when administered from 115 days of gestation to term (32). Transcriptomic analysis of septa collected during labor indicated changes in cardiac metabolism and mitochondrial function. Nearly one-fifth of the genes differentially regulated by cortisol in these hearts were associated with the mitochondria, and quantitative RT-PCR of mitochondrial DNA (mtDNA) from the left ventricle revealed significantly less mtDNA in cortisol compared with that in control fetuses (50). These results suggested that cardiac oxidative metabolism was adversely impacted by the chronic exposure to increased cortisol in these fetuses.
Together, our prior studies suggest that a modest yet chronic elevation in maternal cortisol concentration throughout late gestation might induce pathophysiological alterations in the late gestation fetal heart and cardiac conduction system. These deleterious changes appeared to become most apparent during the perinatal period, a time in which the fetal heart is normally subject to changes in metabolism, afterload, and ultimately cardiac work (25). We hypothesized that these effects of elevated maternal cortisol on fetal cardiac metabolism result in altered cardiac conduction that would be revealed by measurement of fetal ECG and would have deleterious effects on fetal heart rate (HR) and aortic pressure during labor and delivery.
METHODS
Rambouillet cross ewes and their lambs were studied. The University of Florida Institutional Animal Care and Use Committee approved all animal use for this study. All animals were housed individually in pens (24 ft2) within a temperature and light (lights on 0700–1900) controlled facility throughout the study period. Ewes were assigned to one of two groups of ewes at 115 days: a control group (n = 7 singleton pregnancies) with no infusion, and a group of ewes treated with cortisol (CORT; n = 5 singleton pregnancies; 1 mg·kg−1·day−1 of Solu-Cortef; hydrocortisone sodium succinate in sodium phosphate; Pfizer, New York, NY). An ambulatory infusion pump (3D Micro Infusion Pump; Strategic Applications, Lake Villa, IL) was used to deliver the infusion at a rate of 0.16 ml/h: before surgery the infusion was delivered through a jugular venous catheter, which was placed percutaneously (Angiocath, 18 g; Becton Dickinson), and after surgery a maternal femoral venous catheter was used for the infusion. This pattern of cortisol infusion was designed to mimic the chronic increases in maternal plasma cortisol concentrations occurring with chronic stress or Cushing’s syndrome during pregnancy. This same infusion rate of cortisol has been used in previous studies in this laboratory in which fetal cardiac enlargement was observed at 130 days of gestation, and increased incidence of stillbirth was evident at term (28, 32, 45).
At approximately day 118 (±1 SE) of gestation, surgery was performed under isoflurane anesthesia to place fetal and maternal catheters and a telemetry device for ECG and fetal aortic pressure measurement. The details of this procedure have been previously reported (4). A flow probe (6 mm 6PSS; Transonics, Ithaca, NY) was placed on the main uterine artery to allow observation of the uterine artery flow as an index of the progression of labor. The radiotelemetry device (DSI PA-D70 PCTP; Data Sciences International, Minneapolis, MN) was placed subcutaneously in the fetus to allow continuous measurement of fetal aortic pressure, amniotic pressure, fetal ECG, and temperature. The telemetry device included two pressure catheters and two ECG leads. The solid-tip ECG probe was placed into the right jugular vein of the fetus and advanced into the superior vena cava, with the tip just outside the right atrium, and the grounding lead for the ECG was attached to the skin of the thorax. One pressure catheter was inserted into the left carotid artery of the fetus and advanced into the aorta outside of the left ventricle. The other pressure catheter was used to measure amniotic fluid pressure; it was sutured to the skin with the tip exposed. Catheters for collection of fetal blood samples were also placed in the fetal tibial arteries and the maternal femoral arteries and veins; these were routed out of the ewes side and placed in a pocket.
Once recovered from anesthesia, the ewe was returned to her pen, and the acquisition component of the telemetry system was activated to allow continuous collection of data from the implanted device (acquisition rate 500 Hz per signal; using DataQuest A.R.T. software; Data Sciences). Ewes were allowed to move freely about the pen; the telemetry signal was collected by a repeater device on the ewe’s flank, and the signal transmitted to a receiver device on the front of the pen. Ewes were fed a diet of pelleted feed according to National Research Council standards adjusted for the ewe’s body weight and fetal gestational age. Ewes were treated at the end of surgery and for 2 days postoperatively with analgesic (flunixin meglamine, 1 mg/kg im, once daily; Merck Animal Health) and for 5 days postoperatively with antibiotic (Polyflex, 12–15 mg/kg, twice daily; Boehringer Ingelheim Vetmedica, St. Joseph, MO). Rectal temperature was measured twice a day for 5 days.
Blood samples (8 ml) were collected from the ewe and fetus on ~125, 130, 135, 138, and 140 days of gestation and on the day of delivery, if possible. Fetal blood samples were analyzed for fetal blood gases arterial Po2, arterial Pco2, pH, (iSTAT Handheld; Abbott Point of Care, Princeton, NJ). Plasma was analyzed for maternal and fetal electrolytes (Roche Electrolyte Analyzer 9180), glucose, lactate (YSI model 2700 Glucose/Lactate Analyzer, Yellow Springs, OH), cortisol (EA65; Oxford Biomedical, Oxford, MI), and insulin (Alpco Ovine Insulin Kit, Alpco Diagnostics, Salem, NH), as previously described (19). After each sampling period, uterine blood flow was measured for at least 30 min using an ambulatory Bluetooth-based acquisition system (Transonics Physiogear; Transonics, Ithaca, NY). Uterine blood flow data were also continuously collected from ewes from 140 days until birth to monitor uterine contractions.
Analysis of the fetal aortic blood pressure and ECG was performed using DSI Dataquest Open A.R.T 4.31 and Ponemah 5.00 software. Aortic pressure was corrected by subtraction of the amniotic fluid pressure. Mean aortic pressure (MAP), systolic pressure (SP), diastolic pressure (DP), and HR were calculated from the aortic pressure wave form as 24-h means measured for 14 days before birth and 1-h means over the final 24 h of fetal life. The rate pressure product (RPP) was calculated from the SP and HR as an index of cardiac work. ECG parameters [P duration, PR, QR, QRS, corrected QT (QTc), and S-T intervals] were calculated for the 1-h interval between 0600 and 0700 for the 14 days before birth, and for each hourly interval over the final 24 h before birth. In all animals, the time of birth was confirmed using the telemetry record of fetal/neonatal temperature, which in all cases revealed a rapid decrease at the time of delivery. Details of the method and the data from the control animals have been previously reported (4).
The harmonics of fetal HR variability (HRV) was used as an index of the relative sympathetic and parasympathetic tone in control of HR. Power spectral density analysis was performed using Dataquest Open A.R.T 4.31 software using the interbeat interval derived from 10 min of fetal aortic pressure data occurring at the same time on each day (between 0600 and 0700) for 14 days before birth, or interbeat interval derived from 2 h of fetal ECG taken in the 2-h period before birth. The analysis used a nonparametric method (fast Fourier transform) to estimate the power of the spectrum for very low frequency (0.001–0.025 cycles/beat), low frequency (LF; 0.025–0.125 cycles/beat), medium frequency (0.125–0.2 cycles/beat), high frequency (HF; 0.2–0.5 cycles/beat), and very high frequency (0.5–1.0 cycles/beat) components; the standard deviation of the normal-to-normal R-R intervals (SDNN); and the root mean square of successive heartbeat interval differences (RMSSD) (33, 62, 70). The ratio of LF to HF and ratio of standard deviation of the normal-to-normal R-R intervals to root mean square of successive heartbeat interval differences were calculated as indexes of sympathetic tone relative to parasympathetic tone.
Two fetuses were excluded from the analysis. In the control group, we excluded one fetus that was hypoxic throughout the pregnancy. In the cortisol-infused group, we excluded one ewe that delivered a weak lamb at 135 days of gestation. In the control group, we included one ewe that was euthanized in labor due to dystocia, and one ewe that delivered a live lamb that died shortly after birth. In the CORT group, we included a ewe that delivered a live lamb that died within 20 min of birth. In this study, a total of three male and four female lambs were born to seven control ewes at 143 ± 1 days of gestation (range 139–148 days), and four male lambs and one female lamb were born to five cortisol-infused ewes at 144 ± 2 days of gestation (range 140–150 days).
Ewes and their lambs were euthanized as soon as possible after birth, between 10 and 480 min after birth (Euthasol, Fort Worth, TX), and measurements of the body and weights of the heart, liver, adrenal, lung, kidneys, perirenal fat, pancreas, and brain were performed.
Statistical analysis.
Analyses of fetal and maternal glucose, cortisol, lactate, electrolytes, fetal blood gases, MAP, SP, DP, HR, and ECG parameters were performed using two-way analysis of variance corrected for repeated measures across time. Between-treatment comparisons at individual time points were performed by post hoc t-test with Bonferroni adjustment for the number of comparisons of that variable or parameter. Because of the unequal variance across time points in fetal cortisol concentrations as a consequence of the steep increase in values near term, the values were log transformed and analyzed using two-way analysis of variance corrected for repeated measures over time. Two-way analysis of variance was used to analyze the fetal insulin concentration because of missing samples from some animals. Between-group comparison of fetal organ weights and morphometrics at necropsy were performed using Student’s t-test. All data are reported as means ± SE.
RESULTS
Maternal blood samples in late gestation.
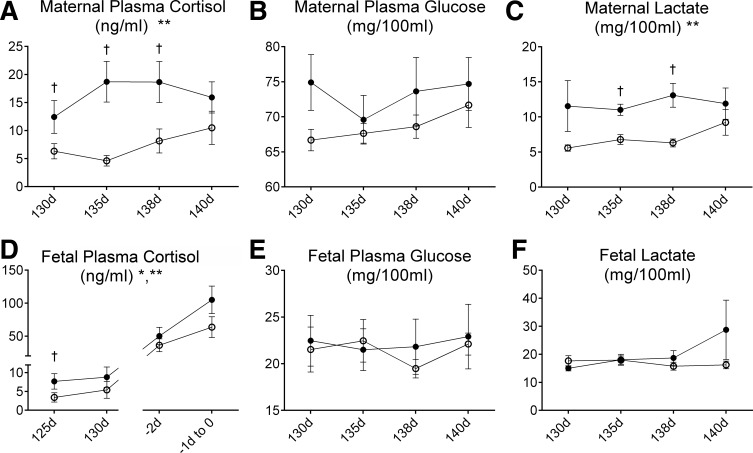
Infusion of 1 mg·kg−1·day−1 cortisol significantly increased maternal cortisol concentration (mean concentrations over the study: 16.4 ± 1.7 ng/ml in cortisol-infused ewes vs. 7.4 ± 1.4 ng/ml in control ewes (Fig. 1A). Maternal cortisol infusion did not significantly alter maternal plasma electrolytes, plasma proteins (PP), or insulin (Table 1); although plasma glucose concentrations tended to be higher in the CORT ewes, this increase was not significant (Fig. 1B). Cortisol infusion did significantly increase maternal lactate concentrations (Fig. 1C).
Fig. 1.
Plasma cortisol (A and D), glucose (B and E), and lactate (C and F) concentrations in control ewes (A–C) and their fetuses (○; D–F), or ewes that were infused with cortisol during pregnancy (1.0 mg·kg−1·day−1; A–C) and their fetuses (●; CORT group; D–F). *Significant overall effect of time. **Significant overall effect of maternal cortisol treatment. †Values significantly different between groups at the indicated time point. d, Days of gestation.
Table 1.
Maternal and fetal plasma electrolyte, protein, and insulin concentrations
| Maternal |
Fetal |
|||
|---|---|---|---|---|
| Control | Cortisol | Control | Cortisol | |
| Plasma Na+, meq/l | 147.6 ± 0.86 | 147.0 ± 0.9 | 140.5 ± 0.7 | 140.5 ± 0.6 |
| Plasma K+, meq/l | 4.13 ± 0.06 | 4.30 ± 0.07 | 4.08 ± 0.14 | 4.36 ± 0.16 |
| Plasma Ca2+, meq/l | 5.27 ± 0.09 | 5.31 ± 0.10 | 5.86 ± 0.17 | 6.07 ± 0.20 |
| Plasma protein, mg/ml | 6.29 ± 0.20 | 6.82 ± 0.24 | 4.00 ± 0.11 | 4.35 ± 0.13 |
| Plasma insulin, ng/ml | 1.47 ± 0.1 | 1.78 ± 0.091 | 0.464 ± 0.05 | 0.629 ± 0.04 |
Values are group means ± SE.
Effects of maternal cortisol on fetal blood samples in late gestation.
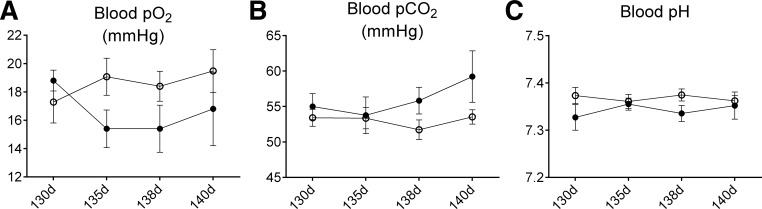
The fetal cortisol concentration was significantly increased by infusion of cortisol to the ewe. As expected, fetal cortisol concentrations significantly increased over time in both groups (Fig. 1D). Fetal plasma cortisol concentrations were significantly increased in the first sampling time at 125 days and were not significantly increased in fetuses at later gestational ages. Fetal plasma electrolytes, PP (Table 1), and packed cell volume were not changed by maternal cortisol infusion; packed cell volume did increase in both groups as gestation progressed (data not shown). Fetal plasma glucose, lactate concentrations (Fig. 1, E and F), arterial Po2, arterial Pco2, arterial pH (Fig. 2), and plasma insulin (Table 1) were also not significantly changed in the CORT fetuses compared with the control fetuses.
Fig. 2.
Blood Po2 (A), Pco2 (B), and pH (C) in fetuses of control ewes (○) or fetuses of ewes that were infused with cortisol during pregnancy (1.0 mg·kg−1·day−1; ●).
Effects of maternal cortisol on fetal organ weight and body morphometrics at necropsy.
Ponderal index was not increased in CORT fetuses compared with controls, despite significantly greater fetal birth weight, girth, and hindlimb measurements in CORT fetuses. Fetal weight relative to maternal weight was also not different between groups (Table 2). Heart weight was not significantly different between groups; however, left ventricular free wall thickness relative to tibial length and interventricular septal thickness relative to tibial length were both significantly greater in CORT newborns (Table 2). In the immediate postnatal period, CORT newborns had difficulty standing (3/5), had evidence of respiratory distress with incomplete inflation of lungs (3/5), and/or had a considerable amount of meconium staining (2/5) at birth. In contrast, only one of the control newborns was unable to stand after 30 min; all other control newborns were healthy, standing shortly after birth, and with little or no meconium staining evident.
Table 2.
Relative organ weights, cardiac wall thicknesses, and body morphometrics at necropsy in control and cortisol-exposed lambs
| Control | Cortisol | |
|---|---|---|
| Body weight, g | 3,892 ± 258 | 5,528 ± 504* |
| Crown to rump, cm | 54.2 ± 2.08 | 58.9 ± 2.25 |
| Girth, cm | 34.9 ± 1.1 | 39.2 ± 1.0* |
| Rear leg hock to hoof, cm | 19.2 ± 0.60 | 22.0 ± 0.71* |
| Ponderal index, g/cm3 | 2.53 ± 0.11 | 2.48 ± 0.11 |
| Relative weight, g/kg | ||
| Brain | 1.19 ± 0.09 | 1.06 ± 0.64 |
| Pituitary | 0.0464 ± 0.0082 | 0.0271 ± 0.0047 |
| Lung | 21.4 ± 2.2 | 1.73 ± 0.2.2 |
| Liver | 28.8 ± 5.1 | 24.3 ± 1.0 |
| Kidney | 2.93 ± 0.16 | 2.57 ± 0.09 |
| Adrenal | 0.0944 ± 0.0060 | 0.0872 ± 0.0011 |
| Perirenal adipose | 3.68 ± 0.44 | 3.39 ± 0.21 |
| Pancreas | 1.01 ± 0.10 | 0.76 ± 0.07 |
| Heart | 6.89 ± 0.48 | 6.35 ± 0.24 |
| Relative cardiac wall thickness, mm/cm tibial length | ||
| Left ventricular free wall | 0.383 ± 0.015 | 0.449 ± 0.016* |
| Septum wall thickness | 0.445 ± 0.016 | 0.520 ± 0.024* |
| Right ventricular free wall | 0.332 ± 0.011 | 0.315 ± 0.041 |
Values are group means ± SE.
Values significantly different between lambs of cortisol-infused ewes compared with control lambs (P < 0.05).
Effects of maternal cortisol on fetal blood pressure, HR, and ECG in late gestation.
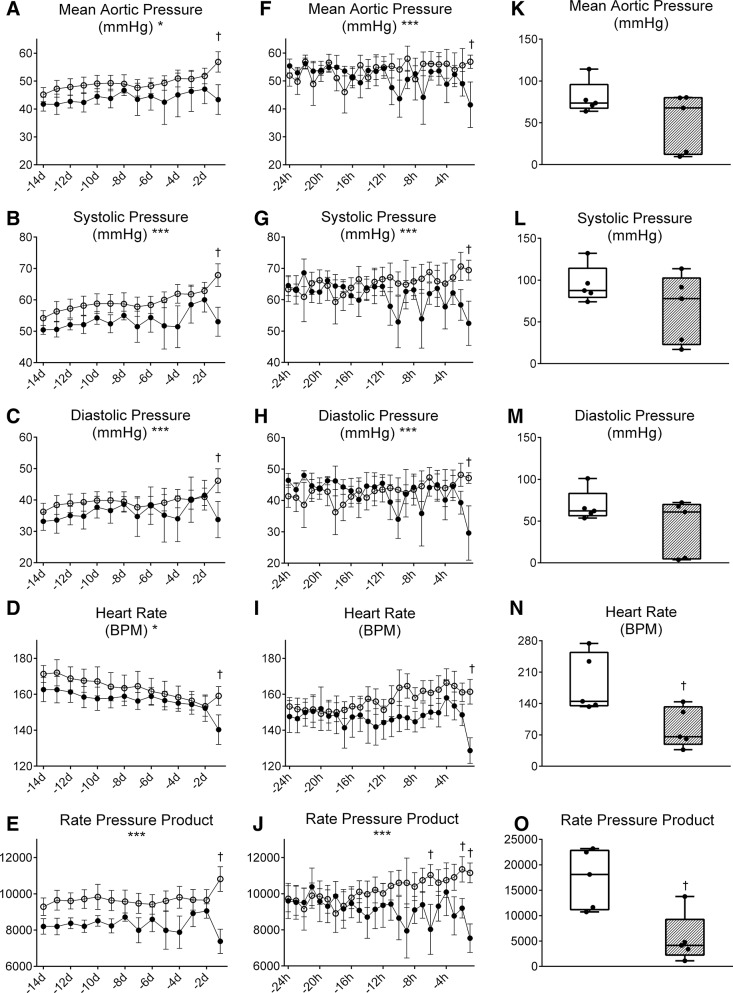
Maternal cortisol infusion did not alter the pattern of change of fetal MAP throughout the final 14 days of fetal life, but did significantly reduce MAP on the day of birth (43.4 ± 4.7 mmHg in CORT fetuses vs. 56.9 ± 4.0 mmHg in control fetuses; Fig. 3A). Maternal cortisol significantly altered the pattern of change in SP and DP over the 14 days. DP, SP, and PP all increase over time in both groups of fetuses, but the DP and SP did not increase further on the day of birth in the CORT fetuses as it did in the control fetuses (Fig. 3, B and C). Similarly, maternal cortisol infusion significantly altered the pattern of change in the RPP over the last 14 days (Fig. 3E). The RPP product was also significantly lower in the CORT fetuses on the day of birth.
Fig. 3.
Mean aortic pressure (A, F, and K), systolic pressure (B, G, and L), diastolic pressure (C, H, and M), heart rate (D, I, and N), and rate-pressure product (E, J, and O) during late gestation (A–E), the immediate perinatal period (F–J), and 10 min after birth (K–O) in fetuses of control ewes (○) or fetuses of ewes infused with cortisol during pregnancy (1.0 mg·kg−1·day−1; ●). *Significant effect of time. ***Significant interaction between the effects of maternal cortisol treatment and time. †Values significantly different between groups at the indicated time point.
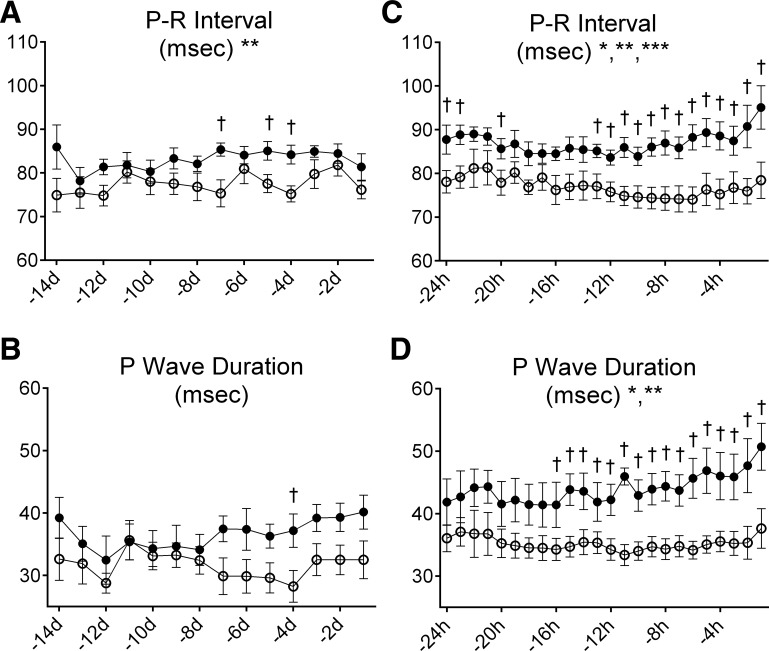
Fetal HR significantly decreased over the last 14 days in utero in both groups (Fig. 3D). Although maternal cortisol infusion did not significantly alter fetal HR over this period, the CORT fetuses had significantly lower HR on the day of birth (140 ± 7 beats/min in CORT fetuses versus 159 ± 6 beats/min in control fetuses). Overall, the P-R interval was significantly increased by maternal cortisol infusion (overall mean interval 83.0 ± 2.0 ms in CORT fetuses versus 77.5 ± 1.7 ms in control fetuses; Fig. 4A). There was no significant effect of cortisol in late gestation on the duration of the P wave: although the P-wave duration tended to be higher in the CORT fetuses, this was only significant 4 days before birth (Fig. 4B). The time course for the Q-R interval was also significantly altered by maternal cortisol infusion over late gestation; the overall mean interval in control fetuses was decreased from 13.1 ± 0.8 ms in control fetuses to 12.2 ± 1.1 ms, whereas in CORT fetuses the overall mean interval increased from 10.7 ± 0.91 to 12.9 ± 1.35 ms.
Fig. 4.
The duration of the P-R interval (A and C) and the P wave (B and D) during late gestation (A and B) and the immediate perinatal period (C and D) in fetuses of control ewes (○) or fetuses of ewes infused with cortisol during pregnancy (1.0 mg·kg−1·day−1; ●). *Significant effect of time. **Significant overall effect of maternal cortisol treatment. ***Significant interaction effect of maternal cortisol treatment and time. †Values significantly different between groups at the indicated time point.
Analysis of fetal HR harmonics indicated that there were no significant differences in very low frequency, LF, medium frequency, HF, very high frequency power, or the LF-to-HF ratio between the two groups when data for each morning of the final 14 days of fetal life were analyzed (Table 3), suggesting no major changes in relative contributions of sympathetic or parasympathetic tone to HR over this period in either group of fetuses.
Table 3.
Frequency and time measurements of the fetal heart rate variability in late gestation and in the immediate perinatal period
| Late Gestation |
Perinatal Period |
|||
|---|---|---|---|---|
| Control | Cortisol | Control | Cortisol | |
| VLF | 0.315 ± 0.010 | 0.297 ± 0.012 | NA | NA |
| LF | 0.135 ± 0.011 | 0.141 ± 0.013 | NA | NA |
| MF | 0.088 ± 0.010 | 0.084 ± 0.009 | NA | NA |
| HF | 0.047 ± 0.006 | 0.060 ± 0.007 | NA | NA |
| VHF | 0.028 ± 0.009 | 0.018 ± 0.007 | NA | NA |
| LF/HF | 3.48 ± 0.319 | 2.88 ± 0.378 | NA | NA |
| SDNN | NA | NA | 0.144 ± 0.020 | 0.198 ± 0.043 |
| RMSSD | NA | NA | 0.449 ± 0.037 | 0.725 ± 0.196 |
| SDNN/RMSSD | NA | NA | 0.314 ± 0.028 | 0.297 ± 0.045 |
Values are group means ± SE. VLF, very low frequency; LF, low frequency; MF, medium frequency; HF, high frequency; VHF, very high frequency; LF/HF, ratio of LF to HF; SDNN, standard deviation of N-N intervals; RMSSD, root mean square of successive differences; SDNN/RMSSD, ratio of SDNN to RMSSD; NA, not applicable.
Effects of maternal cortisol on fetal blood pressure, HR, and ECG in the 24 hours before birth.
Fetal MAP dropped significantly in the final hour before birth in the fetuses of cortisol-infused ewes (41.5 ± 5.6 mmHg in CORT fetuses vs. 56.9 ± 4.7 mmHg in control fetuses; Fig. 3F). The time courses for SP and DP were significantly altered by cortisol in the final 24 h before birth (Fig. 3, G and H); although overall the SP and DP were not different between the groups, DP and SP were significantly reduced in the CORT ewes compared with control ewes in the hour before birth (SP: 52.5 ± 5.3 mmHg in CORT fetuses vs. 69.4 ± 4.5 mmHg in control fetuses; DP: 29.6 ± 5.7 in CORT fetuses vs. 47.1 ± 4.8 mmHg in control fetuses). The time course for the RPP was also significantly altered by cortisol in the final 24 h before birth (Fig. 3J). The RPP was also significantly reduced in the CORT fetuses compared with control fetuses in the hour before birth (7,539 ± 709 in CORT fetuses vs. 11,153 ± 599 in control fetuses). HR was also significantly lower in the final hour before birth (129 ± 8 beats/min in CORT fetuses vs. 161 ± 7 beats/min in control fetuses; Fig. 3I). The reduction in HR and RPP that occurred before birth in CORT fetuses persisted in the 10 min immediately after birth (HR: 86 ± 20 beats/min in CORT newborns vs. 185 ± 29 beats/min in control newborns; RPP: 5,429 ± 2,171 in CORT newborns vs. 17,234 ± 2,622 in control newborns; Fig. 3, N and O). Although the mean aortic pressure, as well as SP and DP, were not significantly different between groups immediately after birth (Fig. 3, K–M), two of the weakest CORT newborns had extremely low aortic pressures (10 and 15 mmHg; Fig. 3K).
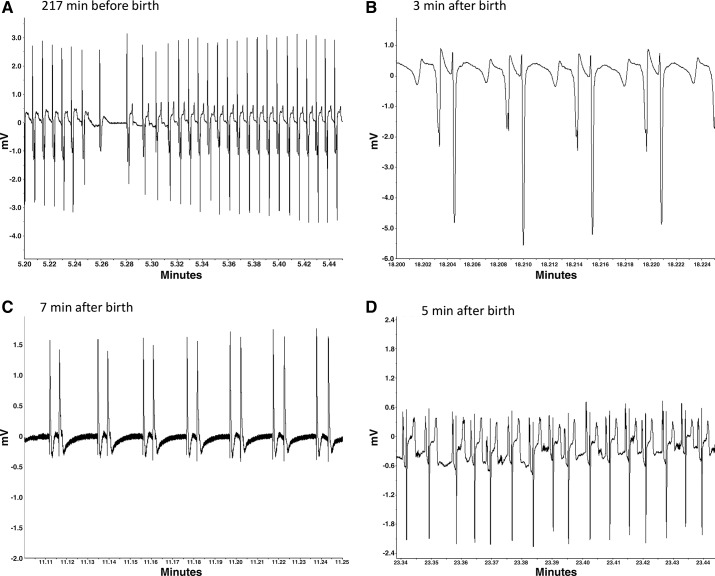
The duration of the fetal P wave and the P-R interval were significantly prolonged by maternal cortisol infusion over the last 24 h of fetal life (mean P-wave duration over 24 h: 44.0 ± 2.4 ms in CORT fetuses vs. 35.2 ± 2.1 ms in control fetuses; P-R interval: 87.0 ± 2.9 ms in CORT fetuses vs. 76.9 ± 2.4 ms in control fetuses; Fig. 4, C and D). Differences between groups for both of these variables were increased as the fetuses neared birth. Although the S-T interval was not overall significantly different in the last 24 h before birth, it was significantly greater in the final hour before birth in CORT fetuses (174 ± 6 ms in CORT fetuses vs. 157 ± 5 ms in control fetuses). Both groups of fetuses had similar patterns of decelerations occurring in the 2 h before birth, and measurements of the interbeat interval were not different (Table 3). Importantly, no fetus had a sinusoidal pattern or absent HRV occurring during this period. However, on the day of birth, and particularly in the immediate peripartal period, abnormalities in the ECG were evident in the CORT fetuses (Fig. 5), including atrioventricular (AV) block (Fig. 5A), notched P waves (Fig. 5B), atrial fibrillation (Fig. 5C), and abnormal ST segments (Fig. 5, A and D). In the hour before birth, AV block was evident in all of the CORT fetuses (5 of 5), with the most affected fetus displaying 44 instances, and with prominent atrial fibrillation after birth in that same lamb (as illustrated in Fig. 5C). In comparison only two of seven control fetuses had AV block. Notching or bifurcation of P waves was identified in four of five CORT fetuses compared with three of seven fetuses in the control group. Abnormal ST segments were identified in two of five CORT fetuses, with a “tombstone”-like T-wave shape, indicative of myocardial hypoxemia (65, 66) (Fig. 5D).
Fig. 5.
Examples of ECG abnormalities identified in four cortisol-treated fetuses or lambs at the time of birth. A: atrioventricular (AV) block (P wave with no associated QRS complex) and elevated ST segment in a fetus during active labor. B: notched (or split) P wave in a newborn lamb. C: atrial fibrillation in a newborn lamb who later succumbed. D: abnormal ST segment and notched P waves in a newborn lamb. Note that the shape of the ECG varies with the positioning of the lead on the chest wall relative to the lead secured within the jugular. Thus the shape can vary between fetuses and also within fetus, depending on the orientation of the fetus.
DISCUSSION
In the present study, we demonstrated that, as pregnancy progresses in late gestation, chronically elevated maternal cortisol concentrations detrimentally impact fetal cardiac function, particularly shortly before and after birth. There was no long-term effect of the maternal cortisol infusion on the normal maturation changes in HR or arterial blood pressure; that is, the normal decrease in fetal HR and increase in fetal MAP over late gestation progressed normally. However, the increases in HR and MAP during labor and delivery were blunted. Furthermore, there was a dramatic and precipitous decline in fetal MAP, HR, and cardiac work (the RPP) that became more pronounced as labor progressed and continued immediately after birth. The decline in fetal HR on the day of birth corresponded with slowing of atrial and AV conductions as both atrial (P-wave duration) and AV (P-R interval) conduction times were prolonged in the CORT fetuses. A parallel increase in the Q-R interval suggests that conduction through the Purkinje fibers of the intraventricular septum is also slowed. Importantly, the decrease in HR was not associated with pronounced alterations in sympathetic or parasympathetic cardiac tone, as measurements of HRV were not significantly different from those of the control fetuses. Our findings indicate that chronic exposure to excess cortisol in utero can detrimentally impact cardiac conduction, not only immediately before birth, but also shortly thereafter, and contribute to a reduction in cardiac output at the time of birth.
Thus our results confirm our hypothesis that excess maternal cortisol results in altered cardiac conduction that is revealed as alterations in the fetal ECG and adversely impacts fetal HR and aortic pressure during labor and delivery. Although testing hypotheses related to the cellular mechanism of the effect of excess maternal cortisol was not an objective of this study, we can speculate as to several possible mechanisms for this effect. It is notable that these effects of elevated maternal cortisol occur at a time when the fetal production of cortisol has been dramatically increased and is not significantly higher in the fetuses in the CORT group compared with the control fetuses. In this study, the fetal cortisol concentrations were only different in the 125-day sample; this is not surprising. Our laboratory has previously found that this treatment paradigm produces increased fetal cortisol in the first 10 days of treatment (45). These data are also consistent with the calculation that maternal cortisol accounts for nearly all of the fetal cortisol measured before 120 days, 37% of the fetal cortisol from 122 to 135 days, and only 12% after that time (24). Nevertheless, we found the effects on apoptosis and proliferation were mediated by GR or MR effects in the heart, although in our transcriptomic analysis we found that most of the genes altered were not ones with glucocorticoid response elements (50). This suggests that there may be both direct cortisol effects on the trajectory of maturation due to exposure before 130 days, and indirect effects through changes in substrate supply and/or metabolism in these fetuses.
We suspect that these effects on conduction may be the result of known GR effects on ion channel expression in the cardiac conduction pathway. Glucocorticoids are known to contribute to prolongation of the cardiac action potential (63, 64). Similar alterations have also been observed in the mouse when GR is conditionally overexpressed in cardiomyocytes; moreover, alterations in the ECG are evident, including elongated P-Q, QRS, and QTc intervals, and a substantially greater incidence of AV block (54). In contrast, when the GR was conditionally knocked out of cardiomyocytes in the fetal mouse, significant alterations in the macro- and microstructure of the compact myocardium were evident, along with changes in the expression for genes involved in calcium handling and cardiac metabolism (51, 52). Purkinje fibers of the adult human heart have been found to have high concentrations of corticosteroid-binding globulin (CBG; or transcortin) (56). Plasma CBG functions as a transporter of systemic cortisol. The function of CBG in Purkinje fibers is not well understood, but could potentially confer a protective effect or alternatively act as a delivery mechanism. Taken together, these findings highlight the critical importance of GR signaling in the normal development and maturation of key electrophysiological, metabolic, and structural pathways in the fetal heart. Therefore, the observed arrhythmias, including prolonged P and P-R intervals of the ECG are consistent with excessive GR signaling and suggest changes in maturation of cardiac calcium and/or potassium channels may play a role.
Glucocorticoids are also important mediators of cellular metabolism and have known effects on skeletal muscle metabolism and function (16, 53, 60). Previously, our laboratory showed that CORT fetuses at term had significantly reduced mtDNA, and gene array data from hearts of similarly treated fetuses in early labor also indicated that many of the genes differentially regulated by maternal cortisol infusion were associated with changes in cardiac metabolism and mitochondrial function (50). In late gestation, the fetal cardiac mitochondria are in a dynamic state of maturational change as the number and volume of mitochondria increase as it prepares to make the switch from primarily utilizing carbohydrates in utero to fatty acids ex utero as the main energy sources for ATP production (6, 10). In cardiomyocytes, ATP is primarily consumed by ATPases that support the contractile machinery, Ca2+ reuptake, ion channels that maintain the membrane potential, and anabolic reactions (55). Discovery of glucocorticoid response elements in the mtDNA and that cytoplasmic GR are capable of translocating into the mitochondria have suggested steroid modulation of mitochondrial function (13, 17). Mitochondrial diseases in children and adults are associated with dilated and hypertrophic cardiomyopathies, heart failure, and ECG abnormalities, including prolonged QTc interval and AV block (2, 5, 35). In the present study, there were significant reductions in the expression of COX-4, the fourth subunit of cytochrome-c oxidase of the respiratory chain in the septum (3), suggesting that compromised mitochondrial function could contribute to the abnormalities in cardiac conduction at birth. Although the ultimate effect of maternal cortisol excess produced obvious deleterious effects on cardiac function compared with earlier studies, the incidence of fetal/newborn demise was somewhat reduced. In this study, only two of the five CORT newborns showing signs of imminent demise at birth. In a previous study in which noninstrumented fetuses were allowed to deliver, three of four ewes delivered dead fetuses before we stopped the study (32). In the subsequent study in which ewes were similarly treated with cortisol at 1 mg·kg−1·day−1 starting on day 115, when we performed necropsy in labor, 5 of 11 fetuses had succumbed (32). In that same study, which was conducted in Suffolk ewes, the maternal glucose concentrations were significantly elevated (by 17%); in the ewes whose fetuses died, maternal insulin was also significantly increased. This suggests that there was a more severe metabolic abnormality in that study, which could contribute to a more severe metabolic phenotype in the myocardium. In this study, there was no significant increase in the glucose in the cortisol-treated ewes; however, average glucoses were higher in both groups. We attribute this to a change in feeding initiated by the animal care staff, which included higher amounts of alfalfa hay than we had previously used in feeding.
It is not likely that the effects of cortisol are secondary to other well-characterized hypertensive effects of higher doses of cortisol or synthetic glucocorticoid administration in the fetus, or to effects on fetal growth per se. Both excess cortisol and high levels of stress reduce birth weight (15, 23) and increase fetal arterial pressure (21, 39, 58). In studies in which cortisol increases fetal arterial pressure, cardiac hypertrophy results; this is associated with an increased expression of angiotensinogen mRNA in the heart (39). In our laboratory’s studies, we have not observed any appreciable increase in fetal arterial pressure (29, 45). Our laboratory also has not found any increase in expression of angiotensinogen or the angiotensin type 2 receptor (46). Thus we think it is unlikely that these changes are mediated by changes in fetal insulin-like growth factor (IGF) or renin-angiotensin system activity. We also did not find an increase in the RPP, which is an index of cardiac work, suggesting that increased cardiac work does not contribute to the increased thickness of the left ventricular wall. However, as we initially found that MR are involved in the proliferation at day 130, and MR are known to mediate hypertrophy and fibrosis in adult hearts, we cannot exclude the possibility that a similar mechanism is involved in the fetal heart with chronic exposure to the cortisol concentrations achieved in our study.
Similarly, in our studies, we have not found a decrease in fetal weight, although this may be a type II error resulting from low numbers of animals. However, as our laboratory did not find any change in concentrations of IGFs, nor changes in IGF expression in the heart in previous studies (27, 47), the well-characterized effect of high-dose cortisol on growth appears not to be critical in the mechanism of cardiac dysfunction.
The maternal cortisol concentration used in this study is pathophysiologically relevant. The levels are similar to those our laboratory measured in previous studies in which we transported ewes to a laboratory from the animal facility before study (31, 68, 69), albeit sustained chronically. Although ovine plasma cortisol concentrations cannot be directly compared to human plasma cortisol concentrations because of differences in transcortin between the species (43), the receptors have similar affinities for cortisol (48). Free cortisol levels are comparable when measured at the human circadian nadir (nighttime), as the sheep has no circadian rhythm in cortisol (8). The estimated free cortisol levels in these ewes would be ~4 nM in the controls and 9–10 nM in the CORT. In normal pregnant women, nighttime salivary (free) cortisol levels average 4 nM, and the upper 95% confidence limit is 7 nM; in pregnant women with Cushing’s syndrome, the evening cortisol levels average 14 nM (38). Salivary free cortisol concentrations are higher in minority or low-income pregnant women (average evening ~5 nM in minorities) (11). In pregnant women, salivary cortisol at the time of diagnostic fetal MRI, an anxiety-producing event, is ~16.5 nM. Thus the concentrations we achieve at baseline are well within a range that would be expected with stress in pregnant women. We would predict that the concentrations produced by our cortisol infusion produce 60–70% occupancy of the ovine GR (48).
The changes in ECG, MAP, and HR occurring in the immediate perinatal period suggest that the fetal hearts chronically exposed to higher than normal cortisol may have a maladaptive or disruptive pattern of maturation, increasing the likelihood of failure when confronted with the considerable physiological challenges, and further increase in cortisol, occurring within labor and delivery. Nevertheless, the entirety of the cardiac effects observed in this study may not be exclusively due to the direct effect of hypercortisolemia on the fetal heart as there are likely other metabolic and endocrine changes secondary to the excess cortisol that are contributing factors. Further studies will be necessary to identify specific cellular changes that contribute to the phenotype.
Perspectives and Significance
We have shown adverse effects of chronically elevated maternal cortisol concentrations on the fetal cardiac conduction system. The results corroborate our laboratory’s previous findings (19, 32, 49, 50), which indicated an effect of maternal hypercortisolemia on the fetal cardiac conduction system. The present studies also suggest that these changes in cardiac conduction contribute to the increased incidence of perinatal death and are consistent with the patterns of ECGs observed in mitochondrial diseases and in ion channel defects, supporting a role of altered cardiac metabolism in the cardiac failure. Fetal adrenal production of cortisol begins at ~80% of gestation in both humans and sheep (7, 22, 40) as part of a tightly regulated process that is integral to tissue maturation and ex utero survival (36). During this last 20% of gestation, the ovine fetal heart undergoes a critical period of cardiac maturation as cardiomyocytes transition from primarily mononucleated proliferative cells to binucleated terminally differentiated tissue (30), suggesting that cardiac tissue may be susceptible to remodeling by either early, elevated, or prolonged glucocorticoid exposure during this time. Our laboratory (45), others (18), and the present study have shown that increases in maternal cortisol concentration increase the thickness of the fetal ventricular free walls and interventricular septum, indicating that these areas are particularly vulnerable to glucocorticoid overexposure during this period. Glucocorticoids have been shown to significantly increase fetal blood pressure, as well as increase the arterial pressure, bradycardia, and acidemia during a hypoxic episode (14, 20, 26, 57). In this present study, the period of increased cortisol appears to predispose the fetal heart to greater bradycardia in labor. Nevertheless, it is clear that glucocorticoid actions on cardiac function, while essential, are dose dependent, with adverse effects at high or prolonged elevated concentrations. In the adult hippocampus, corticosterone has been shown to have protective effects at low doses and adverse effects at high doses (1, 12). Our data indicate that this biphasic relationship also holds true for the effects of cortisol on the developing heart, with effects that could contribute to the increase in stillbirth rates associated with chronic maternal stress or Cushing’s syndrome in late pregnancy (18, 35, 37, 61, 67).
GRANTS
This work was funded by National Institutes of Health Grant HD057871 and the American Heart Association Grant 14GRNT20420048.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A., C.E.W., and M.K.-W. conceived and designed research; A.A., C.E.W., and M.K.-W. performed experiments; A.A. and M.K.-W. analyzed data; A.A., C.E.W., and M.K.-W. interpreted results of experiments; A.A. and M.K.-W. prepared figures; A.A., C.E.W., and M.K.-W. drafted manuscript; A.A., C.E.W., and M.K.-W. edited and revised manuscript; A.A., C.E.W., and M.K.-W. approved final version of manuscript.
REFERENCES
- 1.Almeida OF, Condé GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J 14: 779–790, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, Osame M, Tanaka H. Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation 91: 955–961, 1995. doi: 10.1161/01.CIR.91.4.955. [DOI] [PubMed] [Google Scholar]
- 3.Antolic A, Richards EM, Keller-Wood M.Modeling of gene expression in newborn heart following chronic maternal hypercortisolemia in late gestation (Abstract). In: 64th Annual Meeting of the Society for Reproductive Investigation, Orlando, FL, September 2017, vol. 24, suppl 1, p. 136A. [Google Scholar]
- 4.Antolic A, Wood CE, Keller-Wood M. Use of radiotelemetry to assess perinatal cardiac function in the ovine fetus and newborn. Am J Physiol Regul Integr Comp Physiol 313: R660–R668, 2017. doi: 10.1152/ajpregu.00078.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik R, Chae JH, Lee YM, Kang HC, Lee JS, Kim HD. Electrocardiography as an early cardiac screening test in children with mitochondrial disease. Korean J Pediatr 53: 644–647, 2010. doi: 10.3345/kjp.2010.53.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartelds B, Knoester H, Smid GB, Takens J, Visser GH, Penninga L, van der Leij FR, Beaufort-Krol GC, Zijlstra WG, Heymans HS, Kuipers JR. Perinatal changes in myocardial metabolism in lambs. Circulation 102: 926–931, 2000. doi: 10.1161/01.CIR.102.8.926. [DOI] [PubMed] [Google Scholar]
- 7.Bassett JM, Thorburn GD. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol 44: 285–286, 1969. doi: 10.1677/joe.0.0440285. [DOI] [PubMed] [Google Scholar]
- 8.Bell ME, Wood CE, Keller-Wood M, Kane C, Kluwe C, Manlove E, Taranovich C, Johnson J. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domest Anim Endocrinol 8: 245–254, 1991. doi: 10.1016/0739-7240(91)90060-W. [DOI] [PubMed] [Google Scholar]
- 9.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 341: 339–341, 1993. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 10.Brook WH, Connell S, Cannata J, Maloney JE, Walker AM. Ultrastructure of the myocardium during development from early fetal life to adult life in sheep. J Anat 137: 729–741, 1983. [PMC free article] [PubMed] [Google Scholar]
- 11.Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, Weber M, Pace T, Stafford B. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology 38: 1786–1796, 2013. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crochemore C, Lu J, Wu Y, Liposits Z, Sousa N, Holsboer F, Almeida OF. Direct targeting of hippocampal neurons for apoptosis by glucocorticoids is reversible by mineralocorticoid receptor activation. Mol Psychiatry 10: 790–798, 2005. doi: 10.1038/sj.mp.4001679. [DOI] [PubMed] [Google Scholar]
- 13.Demonacos C, Tsawdaroglou NC, Djordjevic-Markovic R, Papalopoulou M, Galanopoulos V, Papadogeorgaki S, Sekeris CE. Import of the glucocorticoid receptor into rat liver mitochondria in vivo and in vitro. J Steroid Biochem Mol Biol 46: 401–413, 1993. doi: 10.1016/0960-0760(93)90231-K. [DOI] [PubMed] [Google Scholar]
- 14.Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol 499: 217–226, 1997. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Garcia A. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosom Med 68: 747–753, 2006. doi: 10.1097/01.psy.0000238212.21598.7b. [DOI] [PubMed] [Google Scholar]
- 16.Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, Bevan S, Piva T, Wegener G, Newsholme EA. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J 321: 707–712, 1997. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 106: 3543–3548, 2009. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fayol L, Masson P, Millet V, Simeoni U. Cushing’s syndrome in pregnancy and neonatal hypertrophic obstructive cardiomyopathy. Acta Paediatr 93: 1400–1402, 2004. doi: 10.1111/j.1651-2227.2004.tb02943.x. [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Cardiovascular and endocrine responses to acute hypoxaemia during and following dexamethasone infusion in the ovine fetus. J Physiol 549: 271–287, 2003. doi: 10.1113/jphysiol.2002.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forhead AJ, Broughton Pipkin F, Fowden AL. Effect of cortisol on blood pressure and the renin-angiotensin system in fetal sheep during late gestation. J Physiol 526: 167–176, 2000. doi: 10.1111/j.1469-7793.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc 57: 113–122, 1998. doi: 10.1079/PNS19980017. [DOI] [PubMed] [Google Scholar]
- 23.Fowden AL, Szemere J, Hughes P, Gilmour RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. J Endocrinol 151: 97–105, 1996. doi: 10.1677/joe.0.1510097. [DOI] [PubMed] [Google Scholar]
- 24.Hennessy DP, Coghlan JP, Hardy KJ, Scoggins BA, Wintour EM. The origin of cortisol in the blood of fetal sheep. J Endocrinol 95: 71–79, 1982. doi: 10.1677/joe.0.0950071. [DOI] [PubMed] [Google Scholar]
- 25.Heymann MA, Iwamoto HS, Rudolph AM. Factors affecting changes in the neonatal systemic circulation. Annu Rev Physiol 43: 371–383, 1981. doi: 10.1146/annurev.ph.43.030181.002103. [DOI] [PubMed] [Google Scholar]
- 26.Jellyman JK, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep. J Physiol 567: 673–688, 2005. doi: 10.1113/jphysiol.2005.089805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen EC, Bennet L, Wood C, Vickers M, Breier B, Gunn AJ, Keller-Wood M. Loss of the pregnancy-induced rise in cortisol concentrations in the ewe impairs the fetal insulin-like growth factor axis. Reprod Fertil Dev 23: 665–672, 2011. doi: 10.1071/RD10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288: R54–R61, 2005. doi: 10.1152/ajpregu.00149.2004. [DOI] [PubMed] [Google Scholar]
- 29.Jensen E, Wood C, Keller-Wood M. The normal increase in adrenal secretion during pregnancy contributes to maternal volume expansion and fetal homeostasis. J Soc Gynecol Investig 9: 362–371, 2002. doi: 10.1177/107155760200900607. [DOI] [PubMed] [Google Scholar]
- 30.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol (1985) 102: 1130–1142, 2007. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 31.Keller-Wood M. Inhibition of stimulated and basal ACTH by cortisol during ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 271: R130–R136, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Keller-Wood M, Feng X, Wood CE, Richards E, Anthony RV, Dahl GE, Tao S. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 307: R405–R413, 2014. doi: 10.1152/ajpregu.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y, Okamura K, Watanabe T, Murotsuki J, Suzuki T, Yano M, Yajima A. Power spectral analysis for autonomic influences in heart rate and blood pressure variability in fetal lambs. Am J Physiol Heart Circ Physiol 271: H1333–H1339, 1996. doi: 10.1152/ajpheart.1996.271.4.H1333. [DOI] [PubMed] [Google Scholar]
- 34.László KD, Svensson T, Li J, Obel C, Vestergaard M, Olsen J, Cnattingius S. Maternal bereavement during pregnancy and the risk of stillbirth: a nationwide cohort study in Sweden. Am J Epidemiol 177: 219–227, 2013. doi: 10.1093/aje/kws383. [DOI] [PubMed] [Google Scholar]
- 35.Lev D, Nissenkorn A, Leshinsky-Silver E, Sadeh M, Zeharia A, Garty BZ, Blieden L, Barash V, Lerman-Sagie T. Clinical presentations of mitochondrial cardiomyopathies. Pediatr Cardiol 25: 443–450, 2004. doi: 10.1007/s00246-003-0490-7. [DOI] [PubMed] [Google Scholar]
- 36.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994. doi: 10.1071/RD9940141. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK. Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab 90: 3077–3083, 2005. doi: 10.1210/jc.2004-2361. [DOI] [PubMed] [Google Scholar]
- 38.Lopes LM, Francisco RP, Galletta MA, Bronstein MD. Determination of nighttime salivary cortisol during pregnancy: comparison with values in non-pregnancy and Cushing’s disease. Pituitary 19: 30–38, 2016. doi: 10.1007/s11102-015-0680-3. [DOI] [PubMed] [Google Scholar]
- 39.Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288: R567–R574, 2005. doi: 10.1152/ajpregu.00556.2004. [DOI] [PubMed] [Google Scholar]
- 40.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci 997: 136–149, 2003. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 41.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol 10: 391–402, 2014. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 42.Mulder EJ, de Heus R, Visser GH. Antenatal corticosteroid therapy: short-term effects on fetal behaviour and haemodynamics. Semin Fetal Neonatal Med 14: 151–156, 2009. doi: 10.1016/j.siny.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Murphy BE. Human fetal serum cortisol levels related to gestational age: evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. Am J Obstet Gynecol 144: 276–282, 1982. doi: 10.1016/0002-9378(82)90579-8. [DOI] [PubMed] [Google Scholar]
- 44.Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol 6: 285–292, 2001. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- 45.Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419–427, 2008. doi: 10.1677/JOE-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late-gestation ewes decreases fetal cardiac expression of 11β-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am J Physiol Regul Integr Comp Physiol 291: R1708–R1716, 2006. doi: 10.1152/ajpregu.00294.2006. [DOI] [PubMed] [Google Scholar]
- 47.Reini SA, Wood CE, Keller-Wood M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patterns 9: 122–128, 2009. doi: 10.1016/j.gep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards EM, Hua Y, Keller-Wood M. Pharmacology and physiology of ovine corticosteroid receptors. Neuroendocrinology 77: 2–14, 2003. doi: 10.1159/000068335. [DOI] [PubMed] [Google Scholar]
- 49.Richards EM, Rabaglino MB, Antolic A, Wood CE, Keller-Wood M. Patterns of gene expression in the sheep heart during the perinatal period revealed by transcriptomic modeling. Physiol Genomics 47: 407–419, 2015. doi: 10.1152/physiolgenomics.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards EM, Wood CE, Rabaglino MB, Antolic A, Keller-Wood M. Mechanisms for the adverse effects of late gestational increases in maternal cortisol on the heart revealed by transcriptomic analyses of the fetal septum. Physiol Genomics 46: 547–559, 2014. doi: 10.1152/physiolgenomics.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, Smith GL, Chapman KE. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1α. Cell Death Differ 22: 1106–1116, 2015. doi: 10.1038/cdd.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran CM, Szumska D, Michailidou Z, Richardson J, Owen E, Watt A, Morrison H, Forrester LM, Bhattacharya S, Holmes MC, Chapman KE. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet 22: 3269–3282, 2013. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 53.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 48: 2119–2130, 2005. doi: 10.1007/s00125-005-1886-0. [DOI] [PubMed] [Google Scholar]
- 54.Sainte-Marie Y, Nguyen Dinh Cat A, Perrier R, Mangin L, Soukaseum C, Peuchmaur M, Tronche F, Farman N, Escoubet B, Benitah JP, Jaisser F. Conditional glucocorticoid receptor expression in the heart induces atrio-ventricular block. FASEB J 21: 3133–3141, 2007. doi: 10.1096/fj.07-8357com. [DOI] [PubMed] [Google Scholar]
- 55.Saks VA, Ventura-Clapier R, Leverve X, Rossi A, Rigoulet M. What do we not know of cellular bioenergetics?—a general view on the state of the art. Mol Cell Biochem 184: 3–9, 1998. doi: 10.1023/A:1006881301354. [DOI] [PubMed] [Google Scholar]
- 56.Schäfer HH, Gebhart VM, Hertel K, Jirikowski GF. Expression of corticosteroid-binding globulin CBG in the human heart. Horm Metab Res 47: 596–599, 2015. doi: 10.1055/s-0034-1389957. [DOI] [PubMed] [Google Scholar]
- 57.Schwab M, Coksaygan T, Nathanielsz PW. Betamethasone effects on ovine uterine and umbilical placental perfusion at the dose used to enhance fetal lung maturation. Am J Obstet Gynecol 194: 572–579, 2006. doi: 10.1016/j.ajog.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 58.Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp Physiol 77: 709–717, 1992. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- 59.Valladares E, Peña R, Ellsberg M, Persson LA, Högberg U. Neuroendocrine response to violence during pregnancy—impact on duration of pregnancy and fetal growth. Acta Obstet Gynecol Scand 88: 818–823, 2009. doi: 10.1080/00016340903015321. [DOI] [PubMed] [Google Scholar]
- 60.Venkatesan N, Lim J, Bouch C, Marciano D, Davidson MB. Dexamethasone-induced impairment in skeletal muscle glucose transport is not reversed by inhibition of free fatty acid oxidation. Metabolism 45: 92–100, 1996. doi: 10.1016/S0026-0495(96)90205-X. [DOI] [PubMed] [Google Scholar]
- 61.Vilar L, Freitas MC, Lima LH, Lyra R, Kater CE. Cushing’s syndrome in pregnancy: an overview. Arq Bras Endocrinol Metabol 51: 1293–1302, 2007. doi: 10.1590/S0004-27302007000800015. [DOI] [PubMed] [Google Scholar]
- 62.Wang H-M, Huang S-C. SDNN/RMSSD as a surrogate for LF/HF: a revised investigation. Model Sim Eng 2012: 8, 2012. doi: 10.1155/2012/931943. [DOI] [Google Scholar]
- 63.Wang L, Feng ZP, Duff HJ. Glucocorticoid regulation of cardiac K+ currents and L-type Ca2+ current in neonatal mice. Circ Res 85: 168–173, 1999. doi: 10.1161/01.RES.85.2.168. [DOI] [PubMed] [Google Scholar]
- 64.Whitehurst RM Jr, Zhang M, Bhattacharjee A, Li M. Dexamethasone-induced hypertrophy in rat neonatal cardiac myocytes involves an elevated L-type Ca(2+)current. J Mol Cell Cardiol 31: 1551–1558, 1999. doi: 10.1006/jmcc.1999.0990. [DOI] [PubMed] [Google Scholar]
- 65.Wibbens B, Bennet L, Westgate JA, De Haan HH, Wassink G, Gunn AJ. Preexisting hypoxia is associated with a delayed but more sustained rise in T/QRS ratio during prolonged umbilical cord occlusion in near-term fetal sheep. Am J Physiol Regul Integr Comp Physiol 293: R1287–R1293, 2007. doi: 10.1152/ajpregu.00373.2007. [DOI] [PubMed] [Google Scholar]
- 66.Wibbens B, Westgate JA, Bennet L, Roelfsema V, De Haan HH, Hunter CJ, Gunn AJ. Profound hypotension and associated electrocardiographic changes during prolonged cord occlusion in the near term fetal sheep. Am J Obstet Gynecol 193: 803–810, 2005. doi: 10.1016/j.ajog.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 67.Wisborg K, Barklin A, Hedegaard M, Henriksen TB. Psychological stress during pregnancy and stillbirth: prospective study. BJOG 115: 882–885, 2008. doi: 10.1111/j.1471-0528.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 68.Wood CE. Are fetal adrenocorticotropic hormone and renin secretion suppressed by maternal cortisol secretion? Am J Physiol Regul Integr Comp Physiol 255: R412–R417, 1988. doi: 10.1152/ajpregu.1988.255.3.R412. [DOI] [PubMed] [Google Scholar]
- 69.Wood CE. Negative-feedback inhibition of fetal ACTH secretion by maternal cortisol. Am J Physiol Regul Integr Comp Physiol 252: R743–R748, 1987. doi: 10.1152/ajpregu.1987.252.4.R743. [DOI] [PubMed] [Google Scholar]
- 70.Wood CE, Powers Fraites M, Keller-Wood M. Blockade of PGHS-2 inhibits the hypothalamus-pituitary-adrenal axis response to cerebral hypoperfusion in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 296: R1813–R1819, 2009. doi: 10.1152/ajpregu.90917.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]