Abstract
The renal outer medullary potassium channel (ROMK; Kir1.1) plays an important role in Na+ and K+ homeostasis. ROMK knockout (KO) mice show a similar phenotype to Bartter’s syndrome of salt wasting and dehydration due to reduced Na-2Cl-K-cotransporter activity but not in ROMK1 KO mice. ROMK KO mice also show hydronephrosis; however, the mechanism of this phenotype has not been understood. We have previously demonstrated a gender-sex difference in hydronephrosis and PGE2 production in ROMK KO mice. In this study we compared the gender-sex difference in bladder hypertrophy and hydronephrosis in ROMK KO mice. The bladder weight, bladder capacity, and the thickness of urothelium in male ROMK KO showed average increased two to approximately fourfold greater than wild-type (WT) mice, but there was no difference in either female or ROMK1 KO mice. The thickness of the urothelium was 648.8 ± 33.2 µm vs. 302.7 ± 16.5 µm (P < 0.001) and the detrusor muscle 1,940.7 ± 98.9 µm vs. 1,308.2 ± 102.1 µm (P = 0.013), respectively, in 12-mo male ROMK KO mice compared with the same age WT mice. Western blotting detected ROMK expression at 45~48 kDa, and both ROMK1 and ROMK2 mRNA were detected by quantitative PCR in the bladder. Immunofluorescence staining showed ROMK stained in the bladder, ureter, and urethra in WT but not in KO. In addition, there was a correlation between the severity of hydronephrosis and the bladder weight in male but not in female ROMK KO mice. In conclusion, ROMK expressed in the urinary tract at both protein and mRNA levels; significant enlargement and hypertrophy of the bladder may contribute to hydronephrosis in male ROMK KO mice.
Keywords: detrusor muscle, gender-sex, KATP channel, kidney, knockout mice, urothelium
INTRODUCTION
The renal outer medullary potassium channel (ROMK) is an ATP-dependent potassium channel (Kir1.1) which forms apical K channels that play an important role in K+ recycling to support sodium and chloride absorption in the thick ascending limb (TAL) and in the regulation of K+ secretion in the collecting duct (10). It is highly expressed in the TAL of the loop of Henle, connecting segment (CNT), and cortical collecting duct. Mutations in the ROMK channel cause type II Bartter’s syndrome that presents with polyhydramnios and postnatal life-threatening volume depletion (10). ROMK knockout (KO) mice show a similar phenotype to Bartter’s syndrome of salt wasting and dehydration due to reduced Na-K-2Cl-cotransporter activity in the TAL (10). Three major ROMK isoforms were identified in the kidney: ROMK (1–3), with different lengths of the NH2 terminal (12, 26, 28). ROMK2 has the shortest NH2 terminal, whereas ROMK1 adds an additional 19 (rat) or 20 (mouse) residues. ROMK1 is expressed in the distal and collecting tubules and uniquely regulated by protein tyrosine kinase (PTK)/protein tyrosine phosphatase (PTP)-dependent endocytosis, which may be the mechanism for high-K-mediated enhanced ROMK channel activity in the collecting tubule (18, 19).
Previously we have demonstrated that total ROMK KO mice exhibited the same phenotypes as Bartter’s syndrome in humans (21), and that ROMK1 KO mice do not produce Bartter’s phenotype but exhibit impaired K excretion in the kidney (5). In addition to the Bartter’s phenotype, ROMK KO mice also show hydronephrosis (32), but the mechanism of this hydronephrosis is unknown. During the study of renal phenotypes, we have observed enlarged urinary bladder in ROMK KO mice. Since the amount of urine contained in bladders differed in each individual mouse at the time of the operation (Fig. 1), it would be difficult to know whether the enlarged size is due to more urine retained in the bladder. Therefore, we have measured and compared the bladder/body weight, bladder capacity, and the thickness of the bladder wall in WT and ROMK and ROMK1 KO mice. Results from these measurements are consistent with bladder hypertrophy in ROMK but not in ROMK1 KO mice.
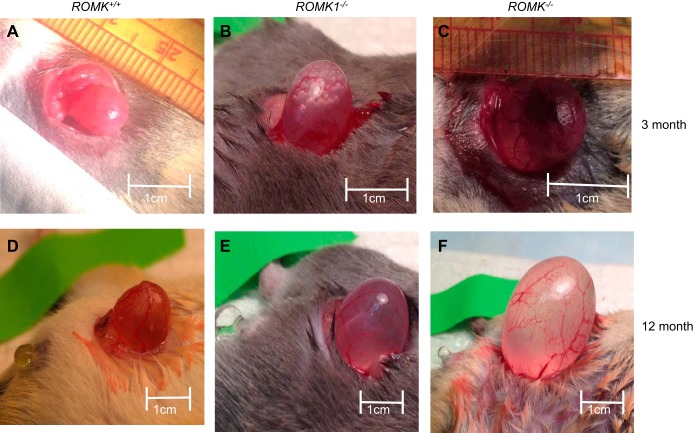
Fig. 1.
Comparison of urinary bladder size among ROMK+/+, ROMK1−/−, and ROMK−/− male mice at ages of 3 and 12 mo (n = 4, each group). The longest length from the bladder neck to dorm was measured after full filling of saline in the urinary bladder. The longest length from bladder neck to dorm is ~0.8–1 cm in ROMK+/+ mouse at both ages (A and D). The bladder length is more than 2 cm larger in 12-mo-old ROMK−/− mouse (C and F). The length of the urinary bladder was also significantly increased in ROMK1−/− mice over ROMK+/+ mice (B and E).
It has been reported that the ROMK potassium channel is present in rat urinary tract epithelia and muscle cells (30); however, whether ROMK is expressed in mouse urinary tract has not been studied. We characterized ROMK expressions at mRNA level by PCR at the protein level by Western blotting and also by immunostaining. Results from these studies clearly demonstrated that ROMK is expressed in the urinary bladder at both mRNA and protein levels. ROMK is also expressed in the luminal membrane of the ureter and urethra in WT but not in the KO mice. Previously we have demonstrated that female ROMK null mice manifest a more severe Bartter’s phenotype and hydronephrosis with higher PGE2 production in females than males (32). In that study the hydronephrosis was evaluated by the liquid retention rate (LRR) of the kidney, and urinary PGE2 concentration was measured in male and female WT and ROMK KO mice. It was found that PGE2 concentration increased 2.4-fold in female KO compared with WT, and the PGE2 excretion level is directly correlated to the level of LRR in females. In contrast, there was no significant increase of PGE2 production and no correlation between PGE2 excretion and hydronephrosis in male KO mice (32). In the present study, we found enlarged bladder and bladder hypertrophy only in males, although urine outputs were the same between male and female ROMK KO mice, further supporting the theory of different mechanisms involved in the development of hydronephrosis.
METHODS
Animals.
All experiments from animal work were conducted according to an Institutional Animal Care and Use Committee-approved protocol at Yale School of Medicine. ROMK KO mice were originally generated from Gary Shull’s laboratory at the University of Cincinnati (20), and ROMK1 KO mice were generated from our laboratory (21). All WT mice carried the same genetic background as the KO mice. We used mice aged 6 wk, 4 mo, and 12 mo to compare changes of urinary bladder according to age. All mice were maintained on standard mouse chow and tap water. Mice were anesthetized with an intraperitoneal injection of thiobutabarbital (0.25 mg/10 g body wt).
Measurement of bladder capacity and bladder weight.
After mice were anesthetized by the same method, the urinary bladder was exposed under a lower abdominal midline incision. A 30-gauge needle connected with a PE-30 tube was inserted into the bladder dorm after removal of all urine by compression of the urinary bladder. Normal saline was infused into the bladder at an infusion rate of 0.0096 ml/min by using a Harvard compact infusion pump, model 975. The infusion was stopped when a drop of saline was released from the urethra, and the infusion time was recorded. The bladder capacity was calculated according to the infusion time and infusion rate. To compensate for increased bladder capacity according to increased body weight, the ratio of bladder capacity (ml)/body wt (BW) (10 g) × 100 was calculated. The urinary bladders were retrieved by excision at bladder neck, and the weight was measured after removal of remaining urine and perivesical fat tissue.
Measurement of thickness of the urothelium and detrusor muscle layer.
The bladders were fixed in 2% paraformaldehyde for 24 h at 4°C, rinsed in PBS, and embedded in EPON solution. Embedded blocks were cut in 3-μm-thick sections and hematoxylin and eosin staining was applied. The stained slides were visualized and recorded with Nikon Eclipse E800 microscopy (5). The middle circular muscle layer in the detrusor muscle was selected, and the intensity was measured. The thickness was analyzed using ImageJ (6).
Immunolocalization of ROMK.
Immunofluorescence staining was used to study ROMK localization. Briefly, the sections were stained with antibodies of ROMK (ROMK anti-rabbit, a generous gift from Dr. Johannes Loffing at the University of Zurich, Switzerland). Antigen retrieval was applied during the staining process. After the staining was completed, anti-ROMK was detected with Alexa Fluor 594-conjugated goat anti-rabbit IgG (Sigma) (5).
RNA preparation and quantitative RT-PCR analysis.
Total RNA was isolated from mouse whole bladder by using TRIzol reagent (Invitrogen, Carlsbad, CA) and further purified by NucleoSpin RNA kit (Clontech). cDNA was synthesized using Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. Real-time PCR was performed on a STRATAGENE Mx3005p QPCR system (Agilent Technologies). The primers of mouse Romk1, Romk2 and Cyclophilin A for RT-PCR were synthesized by IDT and are listed as follows: Romk1, gctttgcccagcatggat (forward), atgtgagtgacaaaccatcttcga (reverse); Romk2, cacgtttaccccagcaatcc (forward), ccgagaacgcccaaatatgt (reverse); and Cyclophilin A, ttgcagacaaagttccaaagaca (forward), aagtcaccaccctggcacat (reverse) (7).
Western blot analysis.
Bladder tissues were homogenized with M-PER Mammalian Protein Extraction Reagent containing Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL). Membrane protein was isolated from mouse bladder by using a Mem-PER Plus kit (Thermo Scientific) according to the manufacturer’s protocol. Protein concentration was determined by Bradford assay. Equal amounts of protein samples were separated by SDS-PAGE by using 4–20% precast gels (Bio-Rad) and transferred to nitrocellulose membranes. Antibodies against ROMK (from Santa Cruz) and β-actin were used in Western blot detection at dilutions of 1:500 and 1:3,000, respectively. The immune complexes were detected with the enhanced chemiluminescence reagent kit.
Statistical analysis.
Data are expressed as means ± SD. Differences between experimental groups were assessed by the two-tail paired Student’s t-test. In each experiment, three replicate samples were analyzed for each group. The experiments of Western blotting and the quantitative PCR were repeated three or more times. The Fisher’s exact test was applied to compare positive or negative staining of the luminal membrane of umbrella cells. One-way ANOVA was used for comparison of multiple groups. Differences with P < 0.05 were considered statistically significant.
RESULTS
Enlarged urinary bladder in ROMK KO mice.
An enlarged bladder full of urine was often observed when opening the abdomen during the experiments in ROMK KO mice. Figure 1, A–F, shows images of bladders from WT, ROMK1, and ROMK knockout mice. Because bladder size can be different according to the amount of urine, we measured size of the urinary bladder and bladder capacity by filling with normal saline. The length of the bladder was measured from the bladder neck to the dome. It was 9.3 ± 0.8, 15.3 ± 1.4 mm (n = 4; P > 0.05), and 25.2 ± 2.6 mm (n = 4; P < 0.05) in WT, ROMK1 KO, and ROMK KO mice, respectively. In addition, the dome of the urinary bladder in ROMK KO mice reached to upper abdomen, and vertical length was longer than 2 cm in ROMK old mice (Fig. 1, C and F). Nevertheless, the bladder was enlarged two to three times in ROMK KO mice compared with WT at the same age, and there was no significant enlargement of bladder in ROMK1 KO mice compared with WT.
Increased bladder weight and capacity in ROMK KO mice.
Figure 2A shows the ratio of bladder and body weight in WT, ROMK1, and ROMK KO mice at the ages of 2, 4, and 12 mo. In WT mice, the ratio had slightly increased from 2 to 4 mo and remained the same from 4 to 12 mo. It was 0.96 ± 0.54, 1.36 ± 0.13% (n = 11; P > 0.05), and 1.39 ± 0.16% (n = 4; P > 0.05) in 2, 4, and 12 mo, respectively. The result is similar to the previous report that the bladder-body weight ratio was relatively constant from 4 to 12 mo in WT mice (15). In ROMK1 KO mice, the ratio was slightly higher compared with WT at 2 mo (1.45 ± 0.09 vs. 0.96 ± 0.54%; P < 0.05), but there were no significant differences between the KO and WT at age 4 and 12 mo (1.90 ± 0.47 vs. 1.36 ± 0.13%; P > 0.05; and 1.69 ± 0.33 vs. 1.39 ± 0.16%), respectively. In contrast, the bladder weight ratio was two to three times higher in ROMK KO than the WT (Fig. 2A). The increase was shown as early as 2 mo old (2.61 ± 0.43 vs. 0.95 ± 0.53%; n = 3; P = 0.024) and sustained to 12 mo old (4.05 ± 1.01 vs. 1.38 ± 0.15%; n = 6; P = 0.019) in ROMK KO and WT, respectively. In addition, unlike the WT and ROMK1 KO mice, the ratio was continuously increased at older ages in ROMK KO mice (from 2.61 ± 0.43% to 4.05 ± 1.01%; P < 0.05) at 2 and 12 mo, respectively. There was no significant difference in the bladder weight and body weight between 2 and 4 mo of ROMK KO mice (2.61 and 1.90%, respectively).
Fig. 2.
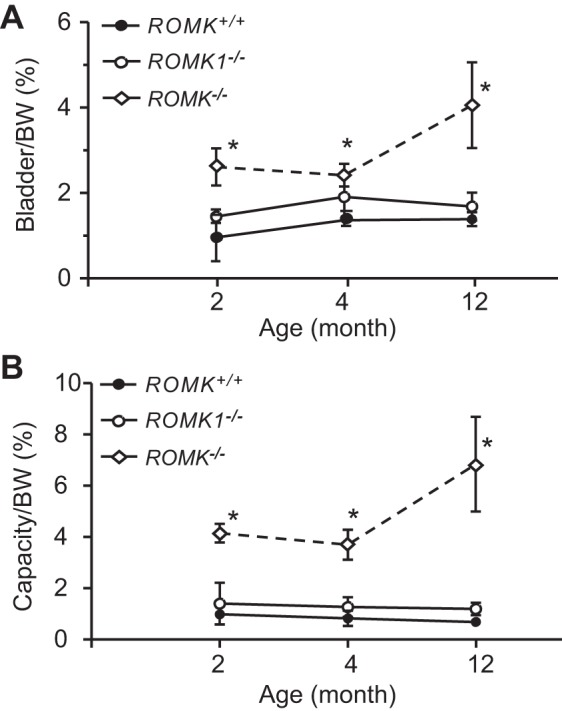
Comparison of urinary bladder weight and capacity between ROMK+/+, ROMK1−/−, and ROMK−/− male mice. A: bladder and body weight ratio (bladder weight/BW) was compared at the ages of 2, 4, and 12 mo in ROMK+/+, ROMK1−/−, and ROMK−/− male mice (n = 4 in each group). The bladder-to-body weight ratio was significantly higher in ROMK−/− mice than the WT control (ROMK+/+) at all ages. The bladder-to-body weight ratio was not significantly different between ROMK1−/− and ROMK+/+ mice. *P < 0.05 compared with the control. B: bladder capacity and body weight ratio (bladder capacity/BW ml/10 g BW × 100) was compared at the ages of 2, 4, and 12 mo in ROMK+/+, ROMK1−/−, and ROMK−/− mice (n = 4 in each group). Bladder capacity was measured by normal saline infusion with constant rate (0.0096 ml/min) into urinary bladder. The bladder capacity-to-body weight ratio was significantly higher in ROMK−/− mice than the WT control (ROMK+/+) at all ages. The bladder capacity-to-body weight ratio was not significantly different between ROMK1−/− and ROMK+/+ mice. *P < 0.05 compared with the control.
The bladder capacity was also measured in WT, ROMK1, and ROMK KO mice at ages 2, 4, and 12 mo. As shown in Fig. 2B, the ratio of bladder capacity (ml) per BW (10 g) × 100 was represented. In WT mice, similar to the ratio of bladder and body weight, the bladder capacity had no significant change among ages 2, 4, and 12 mo. It was 0.99 ± 0.03, 0.82 ± 0.30, and 0.69 ± 0.10 ml/10 g BW% at 2, 4, and 12 mo, respectively (P > 0.05). There was also no significant change of bladder capacity in ROMK1 KO at different ages and no statistical difference compared with the WT mice. The bladder capacity was 1.41 ± 0.82, 1.27 ± 0.38, and 1.19 ± 0.24 ml/10 g% in 2 mo, 4 mo, and 12 mo, respectively (P > 0.05). In contrast, the capacity was increased by 3.19-, 2.9-, and 8.9-fold in ROMK KO mice compared with the WT. It was 4.15 ± 0.36 and 0.99 ± 0.03 in 2 mo (P < 0.001); 3.71 ± 0.58 and 0.82 ± 0.30 in 4 mo (P = 0.005), and 6.84 ± 1.85 and 0.69 ± 0.10 ml/10 gm ×100 in 12-mo-old (P = 0.003) ROMK KO and WT mice respectively. In addition, the bladder capacity was increased by 84% from 4 to 12 mo in ROMK KO mice (P < 0.05).
Bladder hypertrophy in ROMK KO mice.
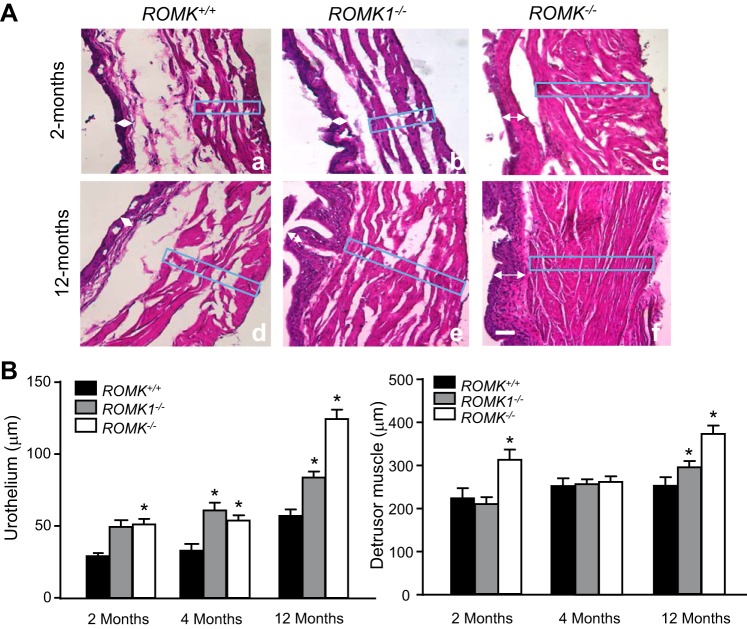
The thickness of the urothelium and the detrusor muscle layer were measured and compared between WT, ROMK1, and ROMK KO mice bladder at the ages of 2, 4, and 12 mo. Results are summarized in Fig. 3A which shows images from bladder sections with hematoxylin and eosin staining, and Fig. 3B shows the summary result of measured thickness of urothelium and detrusor muscle layer from WT, ROMK1, and ROMK KO mice at different ages. In these studies, at least 25 areas were randomly selected in each bladder, and the mean score was calculated under ×100 magnification. The thickness of the urothelium was demonstrated as the sum thickness of the urothelium and lamina propria. Because thickness of bladder is different among base, dorm, and both sides, the same numbers of each site were chosen and measured in WT control and the KO mice. The urothelium in ROMK KO mice was significantly thicker than ROMK WT mice in all ages. It was 30.61 ± 1.36 and 55.26 ± 4.08 (n = 24, P < 0.001) in 2-mo-old, 32.51 ± 4.99 and 54.28 ± 3.31 (n = 18, P < 0.001) in 4-mo-old, and 58.53 ± 3.19 and 125.42 ± 6.43 (n = 18, P < 0.001) in 12-mo-old WT and ROMK KO, respectively (Fig. 3B). The urothelium in ROMK1 KO mice was also significantly thicker than the WT in both the 4-mo-old and 12-mo-old mice, but there was no difference in 2-mo-old mice. Figure 3A also shows an increased thickness of detrusor muscle layer with higher muscle mass density in both young and old age of ROMK KO mice. Although the measurements show that the thickness of the detrusor muscle layer was increased by only 40 and 47% in ROMK KO compared with the WT (Fig. 3B), higher muscle mass density was clearly exhibited. This result also indicated muscle hypertrophy in the bladder of ROMK KO mice. A slight increase of the thickness of the detrusor muscle layer was also observed in the old age (12 mo) ROMK1 KO mice (Fig. 3B).
Fig. 3.
Comparison of the thickness of the urothelium and detrusor muscle layer between ROMK+/+, ROMK1−/−, and ROMK−/− male mice at the ages of 2, 4, and 12 mo. A: images of the bladder wall with hematoxylin and eosin staining from ROMK+/+ (a and d), ROMK1−/− (b and e), and ROMK−/− (c and f) mice at the different ages. The thickness was measured using ImageJ as indicated in the box area. Bladder wall hypertrophy is exhibited in ROMK−/− mice at both 2 (c) and 12 mo (f). B: statistical analysis and comparison of the thickness of the urothelium (left) and the detrusor muscle (right) in each group. The thickness of the urothelium was significantly increased in both ROMK−/− and ROMK1−/− mice at the ages of 4 and 12 mo and a significant increase in ROMK−/− over the ROMK1−/− mice (P < 0.05). Thickness of detrusor muscle layer (box) was significantly increased in ROMK−/− mice at ages of 2 and 12 mo. It was also increased in ROMK1−/− mice at age of 12 mo. *P < 0.05 compared with the control.
ROMK is expressed in the urinary track.
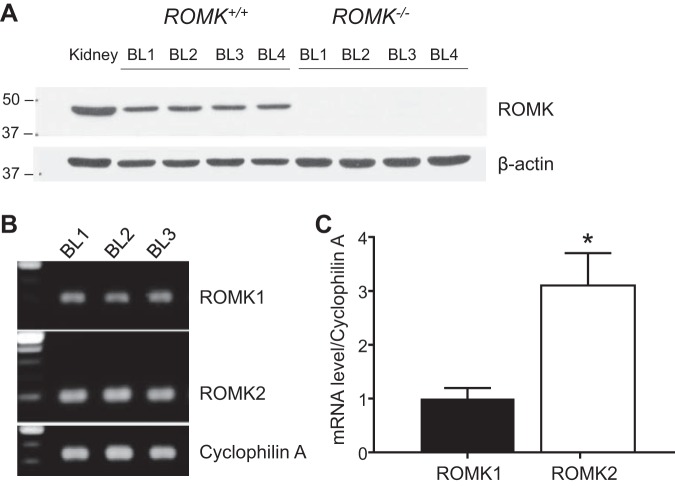
Western blot analysis, quantitative PCR, and immunofluorescence staining were performed to investigate whether ROMK is expressed in the bladder. Whole kidney and bladder tissues were used for the study. Kidney and bladder tissues were homogenized in M-PER reagent followed by protein extraction and were immune-blotted with a primary anti-ROMK antibody. Figure 4A illustrates a ROMK signal detected at 45~48 kDa in both kidney and urinary bladder tissues in ROMK WT but not in ROMK KO mice. To examine ROMK mRNA expression and compare the relative levels of ROMK1 and ROMK2 expression in the bladder of WT mice, quantitative PCR experiments were performed. As shown in Fig. 4B, both ROMK1 and ROMK2 were detected by the PCR in the bladder tissue, and ROMK2 expression was higher than the ROMK1. The quantitative RT-PCR result showed that the relative mRNA level of ROMK2 was two times higher than that of ROMK1 (n = 3, P < 0.05) in the bladder tissue (Fig. 4C).
Fig. 4.
ROMK expression in urinary bladder at the mRNA and protein levels. A: ROMK protein expression. Kidney tissue from ROMK+/+ was used as a positive control, and bladder tissue from the ROMK−/− mice was used as the negative control. β-Actin expression was served as a loading control (n = 4 in each group). BL, bladder. B and C: ROMK1 and ROMK2 mRNA expression measured by quantitative PCR using whole bladder preparations. Cyclophilin A expression was served as an internal control. *Significant difference between ROMK1 and ROMK2 (P < 0.05).
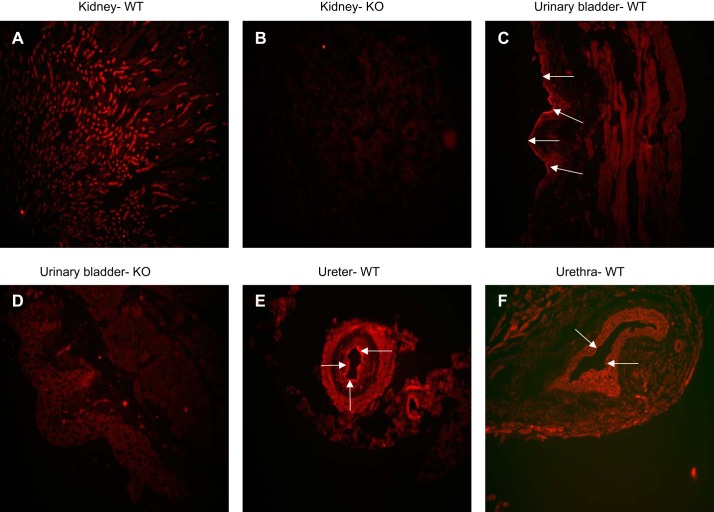
For immunolocalization of ROMK channel in the urinary tract, specimens from the kidney to urethra were embedded in EPON, cross-sectioned with 3 μm thickness, and stained with ROMK primary antibodies with 1:250 dilutions. ROMK expression in the kidney distal nephron in ROMK WT and KO mice was used as the positive and negative control for determining ROMK expression in the urinary tracts. Figure 5, A and B, displays that the renal cortex and outer medullary tubule was strongly stained by ROMK antibodies in ROMK WT, and it was not stained in ROMK KO mice kidney. Figure 5C shows that the luminal membrane of umbrella cells (arrows) were strongly stained and the detrusor muscle was moderately stained in the urinary bladder of ROMK WT mouse. Figure 5D shows some nonspecific ROMK staining in the bladder from ROMK KO mouse. In comparison with the nonspecific ROMK staining indicated in the ROMK KO tissue (Fig. 5), the specific ROMK positive staining was observed in the luminal membrane of the ureter (Fig. 5E) and also in the urethra (Fig. 5F).
Fig. 5.
Localizations of ROMK in urinary bladder, ureter, and urethra. ROMK expression was examined by immunofluorescence staining with rabbit-anti-ROMK antibody (1:250). Kidney stained with ROMK from ROMK+/+ was used as the positive control (A), and the kidney and bladder stained with ROMK from the ROMK−/− mice (B and D) were used as negative controls. ROMK is positively stained in the luminal membrane of the umbrella cells (arrows) and in the detrusor muscle of the bladder (C). Positive ROMK staining is also exhibited in the luminal membrane of the ureter (E) and the urethra (F). The background staining of ROMK is minimal (B and D).
Relationship between hydronephrosis and bladder hypertrophy.
To study whether there is a relative obstruction in enlarged bladder, which could be a mechanism for hydronephrosis of ROMK KO mice, we compared the severity of the hydronephrosis and the bladder hypertrophy measured by bladder weight. Kidney liquid retention rate (LRR) was measured as an index of the severity of hydronephrosis, which was established by our group previously (32). Our data showed that the LRR was <10% in both male and female kidneys from WT mice, and the LRR was significantly increased in ROMK KO mice kidney (32). Similar results were also obtained from the new measurements. As shown in Fig. 6, the LRR was ~5% and the bladder weight was ~0.05 g in both male and female WT mice. The LRR was in the range of 20 to 40% in both male and female ROMK KO, which was significantly higher compared with the WT mice. In addition to the increased LRR, the bladder weight was also higher in male KO mice. There is a positive relationship between the hydronephrosis and the bladder hypertrophy in male KO mice (R2 = 0.8614) but not in female KO mice (R2 = 0.01307). Although LRR was even higher in female than the male KO mice, the bladder weight still remained in the normal range, resulting in no relationship between hydronephrosis and bladder hypertrophy in female KO mice (Fig. 6).
Fig. 6.
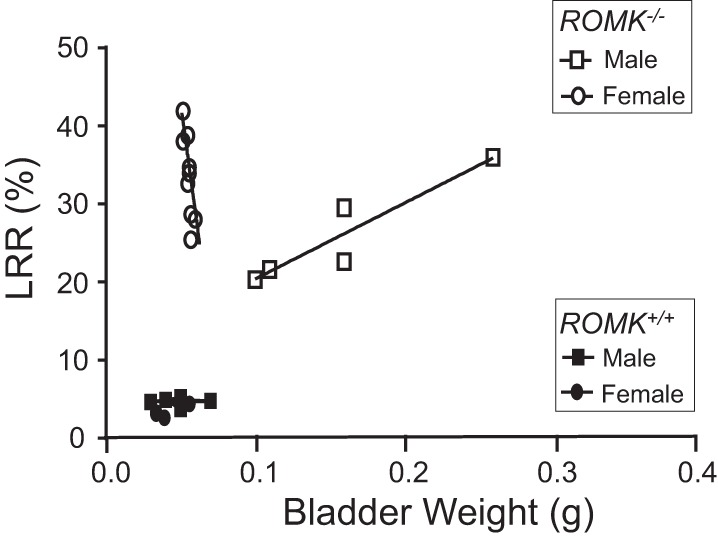
Relationship of hydronephrosis and the bladder weight in male and female ROMK−/− mice. Hydronephrosis was evaluated by the liquid retention rate (LRR) as reported previously (32). Kidney and bladder were collected from ROMK+/+ and ROMK−/− mice at the ages of 4 and 12 mo. There was no significant difference in LRR or bladder weight between male and female WT mice. In contrast, the LRR levels are significantly higher in both male and female ROMK−/− mice. The bladder weight was not different in female KO compared with the WT, even with 12-mo-old (n = 4) female KO mice. There was a direct correlation of the LRR and the bladder weight in male KO (R2 = 0.8614) but not in female KO (R2 = 0.01307) mice.
DISCUSSION
Mechanisms of Bartter’s phenotype produced in ROMK KO mice have been well established (10, 29, 31); however, how the hydronephrosis is developed in these mice is not clear. Previously, we have demonstrated that female ROMK KO mice had severe Bartter’s phenotype and severe hydronephrosis with higher PGE2 production (32). In this study, we examined whether ROMK is expressed in the urinary tract and whether there is a relationship between the enlarged bladder and the severity of hydronephrosis in ROMK KO mice. Experimental results showed that ROMK is expressed in the bladder, ureter, and urethra at both mRNA and the protein levels, and there is a direct relationship between hydronephrosis and bladder weight in male but not in female ROMK KO mice. Bladder hypertrophy, indicated by increased bladder size, weight, and the thickness of both urothelium and detrusor muscle layer, exists in male but not in female ROMK KO mice. There is less significant phenotype on bladder size, weight, and the thickness of the bladder wall in ROMK1 KO, which does not produce Bartter’s phenotype, than in ROMK KO mice. The mechanism of the gender difference in the bladder phenotype of ROMK KO mice needs to be further investigated.
The ROMK KO mouse has impaired functions of NKCC2-mediated NaCl absorption in the TAL, like the effect of loop diuretics furosemide, and this is the mechanism of the polyuria in ROMK KO mice (20). Unlike the total ROMK KO, the ROMK1 KO mice do not produce Bartter’s phenotype, since the ROMK1 is not expressed in the TAL (3); therefore, the NKCC2 function is not reduced in these mice (5). It is not surprising that we found no significant difference in the bladder weight and bladder capacity between WT and the ROMK1 KO mice (Fig. 2), and also no hydronephrosis in the kidney (5). Comparison of ROMK1 and ROMK2 isoforms expression by quantitative PCR showed three times more ROMK2 than ROMK1 expressed in the bladder (Fig. 3), suggesting ROMK2 can functionally compensate for the absence of ROMK1 in the bladder.
Major physiological functions of the bladder facilitate the storage and voiding of urine, which is controlled by the contraction and relaxation of the detrusor smooth muscle (DSM) in the bladder wall (1). Many families of K+ channels, including voltage-gated K+ (KV) channels, Ca2+-activated K+ (KCa) channels, and inward-rectifying ATP-sensitive K+ (Kir, Kcnj1, KATP) channels, are expressed and functional in DSM (23). ROMK (KATP, Kir1.1) belongs to the family of inward-rectifying ATP-sensitive K+ channels (11). We have demonstrated in this study that ROMK is expressed in both mRNA and protein levels (Fig. 3). Immunofluorescence staining showed that ROMK was strongly expressed in the luminal membrane of urothelium and was also moderately expressed in the detrusor muscle in WT mice. Expression patterns of ROMK in the urinary tract were similar to those previously reported from rat (30); however, the functional role of ROMK in these locations needs to be determined.
In addition to ROMK, the large-conductance calcium-activated potassium channel named “big K+” or “Maxi-K” (BK or Maxi-K) expresses and regulates K transport in the kidney collecting tubules (8, 22). The BK channel is also highly expressed in DSM and is the most important physiologically relevant K+ channel that regulates DSM function (13). Genetic deletion of the BKβ1 subunit significantly decreases single BK channel open probability, causing detrusor overactivity and related urinary incontinence (24). In the kidney we have previously demonstrated that iberiotoxin-sensitive Maxi-K channel activity is increased in the collecting tubule of ROMK KO mice (2). However, whether the Maxi-K channel activity is also altered and reduces detrusor reactivity to urine storage in the bladder in ROMK KO mice is unknown.
The mechanism of the hydronephrosis in ROMK KO mice is not clear, even though higher urine volume can be one of the causes of hydronephrosis, e.g., bilateral nonobstructive urinary dilatation in nephrogenic diabetes insipidus (DI) (17). As reported, AQP2 KO mice suffer from severe hydronephrosis, presumably as a consequence of an inability to cope with the extreme polyuria (21, 27). Although the urine volume was five times higher in ROMK KO than WT mice in both sexes (21), this high level of daily urine output (5.8 ml/24 h) was markedly smaller than that in nephrogenic DI (27) and in aquaporin-2 KO mice (15 ml/12 h.) (21, 27). Our present result shows that the urinary bladder size, weight, and capacity were increased two to four times in ROMK KO compared with ROMK WT at the same age, and were constantly sustained through the age of 2 to 12 mo. The thickness of urothelium and detrusor muscle layer was also increased significantly in ROMK KO mice. This result demonstrated the bladder dilatation and hypertrophy in male ROMK KO mice and suggests that the relative urinary tract obstruction of urine output can be an additional mechanism of the hydronephrosis in male ROMK KO mice. Since ROMK is also expressed in the urethra and the urinary tract, whether or not the longer urinary tract could cause obstruction of urine output with polyuria and impaired ROMK function in male ROMK KO mice is not clear.
We have found direct correlations between bladder weight and hydronephrosis in male but not in female ROMK KO mice (Fig. 6), although females produced the same Bartter’s phenotypes as males and had even more severe hydronephrosis than male KO mice (4). As shown in Fig. 6, females have higher LRR but with normal bladder weight, and there was no relationship between LRR and the bladder weight in female ROMK KO mice. As we demonstrated before, females exhibited more severe hydronephrosis evaluated by the LRR compared with the male ROMK KO mouse (32). The urinary PGE2 excretion increased 2.4-fold in female KO compared with the WT, and the PGE2 excretion level is directly correlated to the level of LRR in females (32). In contrast, there was no significant increase of PGE2 production and no correlation between PGE2 excretion and the hydronephrosis in male KO mice (32). Different mechanisms of hydronephrosis may exist in male and female KO mice. We suggest that higher PGE2 levels in female and impaired urinary tract function in male contribute to the developmental of hydronephrosis in ROMK KO mice. In the kidney, a higher level of PGE2 may contribute to severe hydronephrosis (33). In the bladder, high levels of PGs decrease functional bladder capacity and micturition volume with detrusor muscle overactivity (25). PGE2 increases DSM constriction in strips isolated from rabbit urinary bladder dome and base in a dose-dependent manner (9). PGE2 is also known to inhibit the proliferation of smooth muscle cells (14, 16). Taken together, whether or not increased PGE2 function in the bladder can prevent bladder hypertrophy and polyuria-induced relative bladder obstruction in female ROMK KO mice needs to be studied.
New observations from the study include the following: 1) ROMK is expressed in the luminal membrane of ureter and urethra, and the mRNA expression of ROMK2 is higher than ROMK1 isoform in the bladder; 2) enlarged urinary bladder with urothelial and detrusor muscle hypertrophy exists in male ROMK KO mice; 3) there is a direct correlation of bladder weight and the severity of hydronephrosis in male but not in female ROMK KO mice; and 4) there is no bladder hypertrophy either in ROMK1 or in female ROMK KO mice. These studies indicated that relatively lower efficiency of urination might be occurring in male ROMK KO mice. Higher urinary PGE2 excretion in female ROMK KO mice, as we reported previously, may be a mechanism for increased efficiency of urination and preventing bladder hypertrophy. Whether or not ROMK contributes to the smooth muscle constriction in the urinary tract system needs to be studied.
Perspectives and Significance
Mechanisms of ROMK channel deletion, which produced Bartter’s phenotypes in total ROMK but not in ROMK1 KO mice, have been well established. However, the mechanism of hydronephrosis produced in ROMK Bartter’s mice has not been understood. We have previously demonstrated a gender-sex difference in hydronephrosis and PGE2 production in ROMK KO mouse. The more severe hydronephrosis, which is correlated to the higher level of urinary PGE2 excretion, existed in female but not in male ROMK KO mice. In this study we demonstrated the gender-sex difference in bladder hypertrophy and hydronephrosis in ROMK KO mice. Our data show that urinary bladder hypertrophy is susceptible in male but protected in female, and there was a correlation between the severity of hydronephrosis and the bladder weight in male but not in female ROMK KO mice. This novel observation indicated the importance of a gender-sex difference in health and disease, since it may also be involved in different mechanisms between male and females in other diseases in addition to ROMK Bartter’s syndrome.
GRANTS
This research is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-099284 (to T. Wang), P01 DK-17433 Core E (to T. Wang), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01058722).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.-M.K. and T.W. conceived and designed research; J.-M.K., S.X., X.G., H.H., and T.W. performed experiments; J.-M.K., S.X., X.G., H.H., K.D., and T.W. analyzed data; J.-M.K., S.X., and T.W. interpreted results of experiments; J.-M.K., S.X., and T.W. prepared figures; J.-M.K. and T.W. drafted manuscript; J.-M.K., S.X., X.G., K.D., and T.W. edited and revised manuscript; J.-M.K., S.X., X.G., H.H., K.D., and T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. DeRen Shao for helping with the IF staining and Leah Sanders for editing this manuscript.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 3.Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol Renal Physiol 268: F1132–F1140, 1995. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 4.Cantone A, Yang X, Yan Q, Giebisch G, Hebert SC, Wang T. Mouse model of type II Bartter’s syndrome. I. Upregulation of thiazide-sensitive Na-Cl cotransport activity. Am J Physiol Renal Physiol 294: F1366–F1372, 2008. doi: 10.1152/ajprenal.00608.2007. [DOI] [PubMed] [Google Scholar]
- 5.Dong K, Yan Q, Lu M, Wan L, Hu H, Guo J, Boulpaep E, Wang W, Giebisch G, Hebert SC, Wang T. Romk1 knockout mice do not produce Bartter phenotype but exhibit impaired K excretion. J Biol Chem 291: 5259–5269, 2016. doi: 10.1074/jbc.M115.707877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010. doi: 10.1073/pnas.1015751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol 517: 1–14, 2009. doi: 10.1002/cne.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hanawa A. [Roles of prostaglandin on rabbit vesicourethral smooth muscle contraction]. Nihon Hinyokika Gakkai Zasshi 82: 1256–1264, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens 12: 527–532, 2003. doi: 10.1097/00041552-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 13.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008. doi: 10.1152/ajpcell.00001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PR, Armour CL, Carey D, Black JL. Heparin and PGE2 inhibit DNA synthesis in human airway smooth muscle cells in culture. Am J Physiol Lung Physiol 269: L514–L519, 1995. doi: 10.1152/ajplung.1995.269.4.L514. [DOI] [PubMed] [Google Scholar]
- 15.Jost SP. Postnatal growth of the mouse bladder. J Anat 143: 39–43, 1985. [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MA, Thompson CS, Angelini GD, Morgan RJ, Mikhailidis DP, Jeremy JY. Prostaglandins and cyclic nucleotides in the urinary bladder of a rabbit model of partial bladder outlet obstruction. Prostaglandins Leukot Essent Fatty Acids 61: 307–314, 1999. doi: 10.1054/plef.1999.0105. [DOI] [PubMed] [Google Scholar]
- 17.Korzets A, Sachs D, Gremitsky A, Gershkovitz R, Farrage G, Chlibowsky A, Erlich N. Unexplained polyuria and non-obstructive hydronephrosis in a urological department. Nephrol Dial Transplant 19: 2410–2412, 2004. doi: 10.1093/ndt/gfh229. [DOI] [PubMed] [Google Scholar]
- 18.Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology (Bethesda) 20: 140–146, 2005. doi: 10.1152/physiol.00044.2004. [DOI] [PubMed] [Google Scholar]
- 19.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter’s syndrome. J Biol Chem 277: 37871–37880, 2002. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper MA, Wang W, Giebisch G, Shull GE, Hebert SC. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter’s) knockout mice. J Biol Chem 277: 37881–37887, 2002. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pácha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Physiol 261: F696–F705, 1991. doi: 10.1152/ajprenal.1991.261.4.F696. [DOI] [PubMed] [Google Scholar]
- 23.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2011. doi: 10.1038/nrurol.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-Subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahnama’i MS, van Kerrebroeck PE, de Wachter SG, van Koeveringe GA. The role of prostanoids in urinary bladder physiology. Nat Rev Urol 9: 283–290, 2012. doi: 10.1038/nrurol.2012.33. [DOI] [PubMed] [Google Scholar]
- 26.Seldin DW, Giebisch GH. The Kidney: Physiology and Pathophysiology. Philadelphia, PA: Williams & Wilkins, 2000. [Google Scholar]
- 27.Shi PP, Cao XR, Qu J, Volk KA, Kirby P, Williamson RA, Stokes JB, Yang B. Nephrogenic diabetes insipidus in mice caused by deleting COOH-terminal tail of aquaporin-2. Am J Physiol Renal Physiol 292: F1334–F1344, 2007. doi: 10.1152/ajprenal.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuck ME, Bock JH, Benjamin CW, Tsai TD, Lee KS, Slightom JL, Bienkowski MJ. Cloning and characterization of multiple forms of the human kidney ROM-K potassium channel. J Biol Chem 269: 24261–24270, 1994. [PubMed] [Google Scholar]
- 29.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 30.Spector DA, Yang Q, Klopouh L, Deng J, Weinman EJ, Steplock DA, Biswas R, Brazie MF, Liu J, Wade JB. The ROMK potassium channel is present in mammalian urinary tract epithelia and muscle. Am J Physiol Renal Physiol 295: F1658–F1665, 2008. doi: 10.1152/ajprenal.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T. Renal outer medullary potassium channel knockout models reveal thick ascending limb function and dysfunction. Clin Exp Nephrol 16: 49–54, 2012. doi: 10.1007/s10157-011-0495-0. [DOI] [PubMed] [Google Scholar]
- 32.Yan Q, Yang X, Cantone A, Giebisch G, Hebert S, Wang T. Female ROMK null mice manifest more severe Bartter II phenotype on renal function and higher PGE2 production. Am J Physiol Regul Integr Comp Physiol 295: R997–R1004, 2008. doi: 10.1152/ajpregu.00051.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshioka W, Aida-Yasuoka K, Fujisawa N, Kawaguchi T, Ohsako S, Hara S, Uematsu S, Akira S, Tohyama C. Critical role of microsomal prostaglandin E synthase-1 in the hydronephrosis caused by lactational exposure to dioxin in mice. Toxicol Sci 127: 547–554, 2012. doi: 10.1093/toxsci/kfs115. [DOI] [PubMed] [Google Scholar]