Abstract
Preeclampsia is a pregnancy-specific disorder of maternal hypertension and reduced renal hemodynamics linked to reduced endothelial function. Placental ischemia is thought to be the culprit of this disease, as it causes the release of factors like tumor necrosis factor (TNF)-α that induce vascular endothelin-1 (ET-1) production. Interestingly, placental ischemia-induced hypertension in rats [reduced uterine perfusion pressure (RUPP) model] is abolished by ETA receptor blockade, suggesting a critical role for ET-1. Although it has been found that systemic induction of heme oxygenase (HO)-1 is associated with reduced ET-1 production and attenuated hypertension, it is unclear whether HO-1 directly modulates the increased ET-1 response to placental factors. We tested the hypothesis that HO-1 or its metabolites inhibit ET-1 production in human glomerular endothelial cells induced by serum of RUPP rats or TNF-α. Serum (5%) from RUPP hypertensive (mean arterial blood pressure 119 ± 9 mmHg) vs. normotensive pregnant (NP, 101 ± 6 mmHg, P < 0.001) rats increased ET-1 production (RUPP 168.8 ± 18.1 pg/ml, NP 80.3 ± 22.7 pg/ml, P < 0.001, n = 12/group). HO-1 induction [25 µM cobalt photoporphyrin (CoPP)] abolished RUPP serum-induced ET-1 production (1.6 ± 0.8 pg/ml, P < 0.001), whereas bilirubin (10 µM) significantly attenuated ET-1 release (125.3 ± 5.2 pg/ml, P = 0.005). Furthermore, TNF-α-induced ET-1 production (TNF-α 31.0 ± 8.4 vs. untreated 7.5 ± 0.4 pg/ml, P < 0.001) was reduced by CoPP (1.5 ± 0.8 pg/ml, P < 0.001) and bilirubin (10.5 ± 4.3 pg/ml, P < 0.001). These results suggest that circulating factors released during placental ischemia target the maternal glomerular endothelium to increase ET-1, and that pharmacological induction of HO-1 or bilirubin could be a treatment strategy to block this prohypertensive pathway in preeclampsia.
Keywords: bilirubin, inflammation, kidney, preeclampsia, women’s health
INTRODUCTION
Preeclampsia (PE) is a pregnancy-specific disorder characterized by new-onset maternal hypertension, endothelial dysfunction, and reductions in renal hemodynamics (37). It is the leading cause of mortality and morbidity (15, 20), but despite this, current treatment of the disease is limited to premature delivery of the baby and placenta. In addition to the immediate consequences of the complicated pregnancy, increased risk of cardiovascular disease to both mother and baby later in life has been shown in several studies (4, 9, 29, 34, 36). Therefore, it is imperative to develop a comprehensive understanding of this maternal disorder to assist in creating novel therapeutic strategies to treat the women with PE. Although the pathogenesis of this disease is not completely understood, placental ischemia is hypothesized to play a key role (2, 14, 38) by triggering the release of antiangiogenic factors such as soluble fms-like tyrosine kinase-1 (sFlt-1) and proinflammatory cytokines (tumor necrosis factor-α, TNF-α), into the maternal circulation (23, 36, 46). These soluble placental factors induce maternal hypertension by increasing endothelial production of endothelin-1 (ET-1), a potent vasoconstrictor (14, 18, 51). In addition, ET-1 also increases reactive oxygen species and inflammation and can cause fibrosis of vascular cells (35, 45). Since ET-1 has been proposed to be an important final common pathway linking placental ischemia and maternal cardiovascular dysfunction, modulation of ET-1 production may be a potential approach to treating preeclampsia.
One protein that has been shown to play an important role in pregnancy is heme oxygenase-1 (HO-1) (13, 25). Heme oxygenase exists as two primary isoforms, HO-1 and heme oxygenase-2 (HO-2), originating from nonallelic homologous genes. These enzymes catalyze the pro-oxidant heme into biliverdin, the rate-limiting step into its final conversion to bilirubin by biliverdin reductase. As a result of this conversion, three bioactive metabolites [elemental iron, carbon monoxide (CO), and bilirubin] are formed, which all have been shown to have distinct physiological functions (33, 39, 43, 44). Although HO-1 was discovered primarily as an enzyme involved in heme catabolism, increasing evidence shows that this enzyme has antioxidant, anti-inflammatory, and cytoprotective properties. In addition, the induction of HO-1 to increase the levels of its metabolites for the treatment of cardiovascular disease is an area of active research (3, 5, 28, 32, 54). CO is a potent vasodilator implicated in maintenance of vascular tone. Although ultimately conjugated and excreted as a waste product, bilirubin functions as a powerful antioxidant. Interestingly, in an animal model of PE {reduced uterine perfusion pressure (RUPP) model], activation of HO-1 attenuates placental ischemia-induced hypertension, accompanied by reductions in aortic ET-1 mRNA levels in rats (10); however, it is not known whether the HO-1 pathway has a direct effect to inhibit renal glomerular endothelial ET-1 production, which plays an important role in blood pressure regulation. Incidentally, it has been well documented that the placental factors released during PE induce ET-1 production from the glomerulus (6, 7). In addition, previous studies show that HO-1, CO, and bilirubin attenuate sFlt-1 production from placental villi explants under hypoxic conditions (12). Although systemic induction of HO-1 enzyme is associated with reduced ET-1, it is unclear whether HO-1 or its metabolites have a direct effect to modulate this ET-1 response to placental factors. Therefore, we tested the hypothesis that HO-1 or its metabolites inhibit ET-1 production in human glomerular endothelial cells induced by serum of RUPP rats or TNF-α.
To test this hypothesis, we examined the effects of serum from RUPP rats on ET-1 production from HGEnCs in the presence and absence of HO-1 and each of its metabolites: the HO-1 inducer, cobalt photoporphyrin (CoPP); bilirubin; a CO donor, CORM-A1; and biliverdin. Since TNF-α is a circulating factor released by the placenta during ischemia and is known to increase ET-1 production, we also determined whether HO-1 or its metabolites reduced TNF-α-induced ET-1.
METHODS
Animal model and blood collection.
All animal experiments were approved by the Institution Animal Care and Use Committee at the University of Mississippi Medical Center, before commencement. Sprague-Dawley time-pregnant rats (~12 wk old) were obtained on gestational day (GD) 10 and GD11 (Envigo, Indianapolis, IN), and were housed at the institutions laboratory animal facility. All animals were maintained on Teklad 8640 standard rat chow and water ad libitum for the duration of the study. On GD 14, rats were assigned to normotensive pregnant (NP, n = 12) or reduced uterine perfusion pressure (RUPP, n = 12) groups. The RUPP group underwent surgical procedure to place silver clips on the descending aorta (below kidneys) and uterine arteries to reduce uterine perfusion, as previously described (26). Indwelling carotid catheters were placed in all animals on GD 18, and mean arterial pressure (MAP) was measured on GD 19 via pressure transducers (MLT0699, ADInstruments, Colorado Springs, CO) using LabChart data-acquisition software (PowerLab, ADInstruments), as described previously (42). Blood was collected in Vacutainer tubes from each animal on GD 19, centrifuged, and serum stored at −20°C.
TNF-α ELISA assay.
TNF-α was measured in serum of all rats using a commercially available rat TNF-α ELISA kit (R&D Systems, Minneapolis, MN), which was performed as per the manufacturer’s instructions. Precision of intra- and interassays is ~5%, and the mean minimum detectable dose of TNF-α for this assay is 1.1 pg/ml.
Cell culture.
Conditionally immortalized human glomerular endothelial cells (HGEnCs) were cultured for these studies, as previously described (40). Briefly, cells were cultured in EGM-2MV media (Lonza, Walkersville, MD) supplemented with 5% FBS, VEGF, gEGF, gentamicin, IGF-1, ascorbic acid, hFGF, and hydrocortisone. Cells were cultured at 5% CO2 and 33°C until they became 70% confluent. In preparation for treatment, cells were seeded in 6-well plates at 0.2 × 106. Once confluent, cells were transferred to 37°C (5% CO2) for 5 days to allow for the temperature-sensitive simian virus 40 (SV40) expression to “switch-off” and for cells to adopt a mature phenotype.
Cell treatment.
After 5 days at 37°C, cells were serum-starved in EBM-2 media, supplemented only with gentamicin, for 48 h, followed by 12 h of treatment. An n = 1 was counted as one well in a 6-well plate. Each treatment was repeated to yield n = 12. To test the hypothesis that the HO-1 pathway has a direct effect to reduce ET-1 production from HGEnCs in response to placental factors, serum from 12 rats (NP or RUPP) was pooled and used for cell treatments. The subsequent groups were assigned: 5% NP serum, 5% RUPP serum, 5% NP serum + 25 µM cobalt photoporphyrin (CoPP, HO-1 inducer; Frontier Scientific, Logan, UT), 5% RUPP serum + 25 µM CoPP, 5% NP serum + 10 µM bilirubin (Frontier Scientific), 5% RUPP serum + 10 µM bilirubin, 5% NP serum + 100 µM carbon monoxide releasing molecule-A1 (CORM-A1), 5% RUPP serum + 100 µM CORM-A1, 5% NP serum + 10 µM biliverdin (Frontier Scientific), 5% RUPP serum + 10 µM biliverdin. To determine whether ET-1 production induced by the placental factor, TNF-α, can be blocked by HO-1 and its metabolites, the following groups were assigned: Untreated (No TNF-α), 10 ng TNF-α (R&D Systems), Untreated + 25 µM CoPP, 10 ng TNF-α + 25 µM CoPP, Untreated + 10 µM bilirubin, 10 ng TNF-α + 10 µM bilirubin, Untreated + 100 µM CORM-A1, 10 ng TNF-α + 100 µM CORM-A1, Untreated + 10 µM biliverdin, 10 ng TNF-α + 10 µM biliverdin. The concentrations used for CoPP (12, 53), bilirubin (16) CORMA-1 (31, 48), and biliverdin treatments are based on ranges previously published, by our group and others. Groups treated with CoPP were pretreated after only 24 h starving, followed by treatment 24 h later.
Endothelin-1 ELISA assay.
After the 12-h treatment period, media was collected and stored at −20°C. All samples were diluted (1:4) and ET-1 determination was carried out using a commercially available human ET-1 Quantikine ELISA kit (R&D Systems), as per the manufacturer’s instructions.
Statistical analysis.
All data are expressed as means + SD. Blood pressure, ET-1, and TNF-α content in NP and RUPP serum-treated groups were analyzed using Student’s t-test. The remaining data were analyzed using one-way ANOVA to determine if, and where, variance occurred between treatment groups, followed by Tukey’s post hoc comparisons. All data were analyzed and graphed using GraphPad Prism software (La Jolla, CA). A two-sided P value < 0.05 was considered statistically significant.
RESULTS
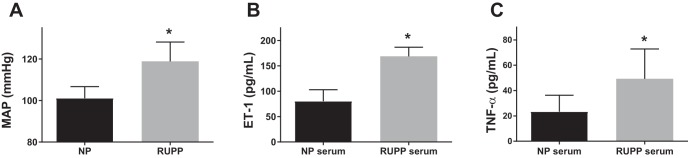
Mean arterial pressure was measured in NP (n = 12) and RUPP (n = 12) rats on GD 19. The RUPP rats had a significantly higher mean arterial pressure compared with the NP group (Fig. 1A; 119 ± 9 vs. 101 ± 6 mmHg, P < 0.001). In vitro, human glomerular endothelial cells (HGEnCs) treated with RUPP serum released significantly higher ET-1 than those treated with serum from NP rats (Fig. 1B; 168.8 ± 18.1 vs. 80.3 ± 22.7 pg/ml P < 0.001). The placental factor TNF-α was measured in rat serum and was significantly increased in RUPP rats compared with controls (Fig. 1C; 49.3 ± 23.6 vs. 23.4 ± 12.9 pg/ml P < 0.001).
Fig. 1.
Blood pressure and circulating factors in serum of normotensive pregnant (NP) and reduced uterine perfusion pressure (RUPP) rats. Data are shown for mean arterial pressure (MAP) (A), circulating endothelin-1 (ET-1) in serum (B), and circulating tumor necrosis factor-α (TNF-α) (C) of NP and RUPP rats at gestational day (GD) 19 (n = 12/group). All data are means + SD. Statistical analysis was performed using Student’s t-test. *P < 0.001.
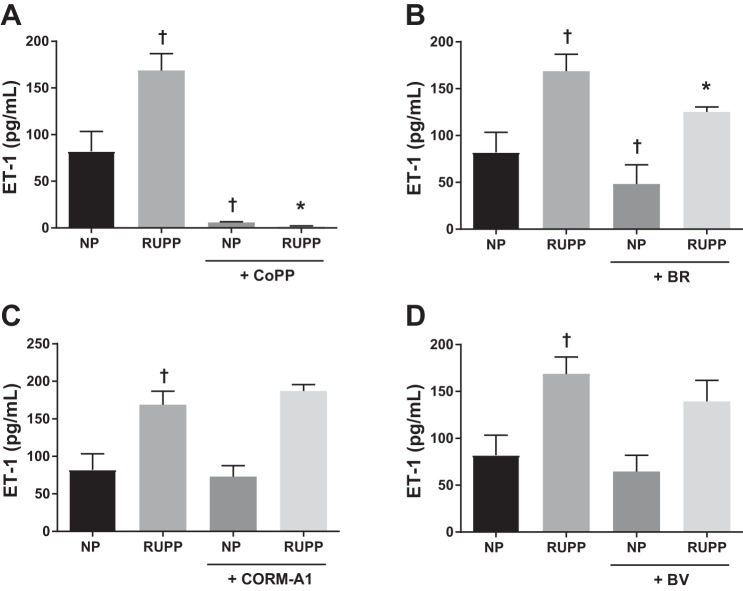
To determine whether HO-1 or its metabolites have a direct effect to reduce ET-1 production from RUPP-treated HGEnCs, cells were pretreated with CoPP, bilirubin, CORM-A1, and biliverdin. CoPP abolished ET-1 production by RUPP serum (Fig. 2A; RUPP, 168.8 ± 18.1 pg/ml, vs. RUPP + CoPP, 1.6 ± 0.8 pg/ml, P < 0.001). Bilirubin treatment attenuated RUPP serum-induced ET-1 production (Fig. 2B; BR, 125.3 ± 25.2 pg/ml, P = 0.005), whereas CORM-A1 and biliverdin did not have any effect on ET-1 production (Fig. 2, C, and D).
Fig. 2.
NP and RUPP-induced ET-1 production in human glomerular endothelial cells (HGEnCs). Data are shown for the effect of 25 µM CoPP (A), 10 µM bilirubin (BR) (B), 100 µM CORM-A1 (C), and 10 µM biliverdin (BV) treatment (D) on normotensive (NP) and RUPP serum (5%) induced ET-1 production in HGEnCs (n = 12/group). All data are means + SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. †P < 0.01 vs. NP group; *P < 0.001 vs. RUPP group.
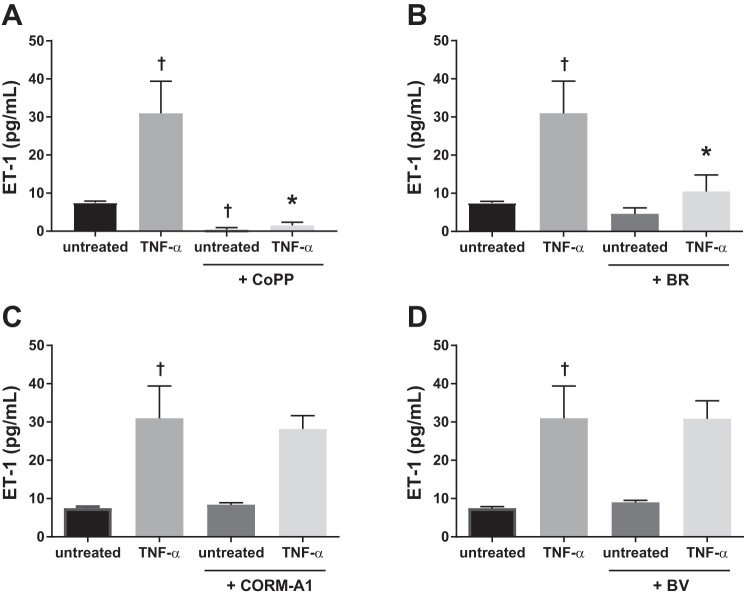
Since TNF-α is elevated in RUPP serum, we investigated the effect of direct stimulation by TNF-α on HGEnCs. TNF-α significantly increased ET-1 production compared with untreated cells (TNF-α 31.0 ± 8.4 pg/ml, Untreated 7.5 ± 0.4 pg/ml, P < 0.001). This effect was abolished in the presence of CoPP (1.5 ± 0.8 pg/ml, P < 0.001) (Fig. 3A) and significantly reduced in the presence of bilirubin (10.5 ± 4.3 pg/ml, P < 0.001) (Fig. 3B). CORM-A1 (Fig. 3C) and biliverdin (Fig. 3D) did not have any significant effect on TNF-α-induced ET-1 production.
Fig. 3.
TNF-α-induced ET-1 production in HGEnCs. Data are shown for the effect of 25 µM CoPP (A), 10 µM bilirubin (BR) (B), 100 µM CORM-A1 (C), and 10 µM biliverdin (BV) treatment (D) on TNF-α (10 ng)-induced ET-1 production in HGEnCs (n = 12/group). All data are means + SD. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. †P < 0.001 vs. untreated group: *P < 0.001 vs. TNF-α group.
DISCUSSION
The HO-1 pathway plays an important role in healthy pregnancy, from vascular development of the uteroplacental unit (25) to growth of the fetus (13). In the current study, we found that HO-1 has a direct effect on glomerular endothelial cells to reduce ET-1 production. In a previous study, George et al. (10) reported significant decreases in hypertension, following CoPP treatment in RUPP rats, which could be explained in part, by the findings in this study. In addition, these data suggest HO-1 and bilirubin can directly attenuate ET-1 production induced by the placental factor, TNF-α.
Endothelial dysfunction, driven by soluble placental factors, is a hallmark of PE. Although we have previously reported that systemic induction of HO-1 enzyme is associated with reduced ET-1 production and attenuated placental ischemia induced hypertension, it is unclear whether HO-1 or its metabolites have a direct effect to modulate this ET-1 response to placental factors. In this study, we report that serum from hypertensive RUPP rats induces ET-1 production from glomerular endothelial cells. We also found that an HO-1 inducer acts directly on glomerular endothelial cells to reduce ET-1 production induced by serum from RUPP rats. Similarly, bilirubin treatment also attenuated ET-1 production. The exact mechanisms whereby HO-1 induction or bilirubin inhibits ET-1 production induced by RUPP serum is unknown but may be related to the ability of HO-1 induction and/or bilirubin to increase nitric oxide production (17, 28). It should also be noted that renal ET-1 is produced not only by glomerular endothelial cells, but several cell types. However, this study focused on endothelial cells since PE is a disease of vascular and endothelial dysfunction.
Although many cytokines, such as IL-6 and IL-17, are increased in PE and the RUPP model, one placental factor that drives the increase of ET-1 in the glomerular endothelial cells is the proinflammatory factor, TNF-α. Inflammatory cytokines are thought to be important in linking placental ischemia and cardiovascular dysfunction. Supporting a role for TNF-α in this disease are findings that plasma levels of TNF-α are elevated in women with PE (24, 46). Placental ischemia in pregnant animals is also associated with enhanced production of TNF-α, as we found here. In addition, chronic infusion of TNF-α into normal pregnant rats results in significant increases in arterial pressure and is also shown to activate the endothelin system in placental, renal, and vascular tissues (22). Furthermore, the administration of a selective ET type A receptor antagonist attenuates TNF-α and placental-ischemia-induced hypertension (21, 47). In this study, we show both HO-1 and bilirubin have a direct effect to attenuate TNF-α-induced ET-1 production in the glomerulus. In vivo, it is possible that damage to the glomerulus could also be reduced by HO-1. It has been shown in placental villous explants that TNF-α induces lactate dehydrogenase leakage, a marker of cell damage, which is attenuated in the presence of HO-1 (1). Although several studies have shown protection from the actions of TNF-α in the presence of both HO-1 and bilirubin, the mechanisms remain to be elucidated.
Another placental factor that contributes to ET-1 production in PE is sFlt-1; an antiangiogenic placental factor which also drives hypertension during PE. HO-1 has been shown to attenuate sFlt-1 induced hypertension and correct the angiogenic balance in vivo. Moreover, CORM-A1 and bilirubin treatment on cultured cells and placental villi significantly attenuate sFlt-1 production (8, 12), suggesting a role for the HO-1 pathway to block sFlt-1-induced ET-1. Future studies remain to be done to determine whether HO-1 has a direct effect to reduce sFlt-1 induced ET-1 in HGEnCs.
Acute treatment with the CO-donor CORM-A1 was not effective in reducing RUPP serum-induced ET-1 production in this study. Previous studies have demonstrated that chronic in vivo treatment of RUPP rats with CORMs for 5 days reduced blood pressure (11). In another study, low-dose CO in ambient air attenuated hypertension and proteinuria in an sFlt-1-infused preeclampsia-like mouse model (49). The effect of chronic CO induction to lower blood pressure in these models of PE may be mediated by the known vasodilatory properties of CO, which occur due to direct activation of soluble guanylyl cyclase (41). This mechanism is consistent with the results of our present study given that CORM treatment did not have a significant effect on RUPP- or TNFα-mediated ET-1 production. Exogenous CORMs have been considered as a therapeutic option for other cardiovascular disease due to their vasodilatory effects, effectively promoting angiogenesis (52), promoting cell survival in endothelial cells (27) and blocking ET-1 in umbilical vascular endothelial cells (30). Although the results of our present study demonstrate that acute CORMs are ineffective against blocking ET-1 production in glomerular endothelial cells exposed to placental factors, they reduce blood pressure in experimental animal models of PE.
The physiological and therapeutic effects of biliverdin and biliverdin reductase (BVR), which is responsible for converting biliverdin to bilirubin, have been examined in several models of ischemia-reperfusion and have been found to have antioxidant, anti-inflammatory and antiapoptotic properties (55). However, the role of biliverdin treatment in a model of placental ischemia has yet to be examined. Here, biliverdin treatment had no significant effect to blunt ET-1 release from either RUPP serum or TNF-α treatment. Several studies have demonstrated that, in the absence of BVR, administration of biliverdin is not able to decrease ROS production to an extent similar to bilirubin treatment (19, 56). The endothelin system was not examined in those studies. Overall, it is possible that bilirubin administration participates in attenuating RUPP serum and TNF-α-mediated ET-1 production via an antioxidant pathway, which is not altered by biliverdin in glomerular endothelial cells.
Perspectives and Significance
It is well established that the HO-1 pathway is involved in the development of a healthy pregnancy and fetus. PE has been correlated with low circulating levels of HO-1 (50). This likely contributes to impaired vascular relaxation and abnormal maternal blood pressure regulation. Indeed, the findings of this study suggest that HO-1 has a direct effect to inhibit ET-1 production induced by factors from preeclamptic rats as well as TNF-α in cultured glomerular endothelial cells. These data demonstrate that alterations in HO-1 levels during PE may mediate the increased levels in ET-1 production and contribute to glomerular endothelial cell injury. They also implicate that the HO-1 pathway could be an effective therapeutic target for PE.
GRANTS
This work was supported in part by the National Heart, Lung, and Blood Institute under the award numbers PO1-HL-051971 and 4R00-HL-130577–02, and an Institutional Development Award (IDeA) Grant P20-GM-104932 from the National Institute of General Medical Sciences (NIGMS), as well as the Research Core B of COBRE, a component of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.A.B., F.T.S., and J.P.G. conceived and designed research; B.A.B. performed experiments; B.A.B. analyzed data; B.A.B., F.T.S., D.E.S., and J.P.G. interpreted results of experiments; B.A.B. prepared figures; B.A.B. drafted manuscript; B.A.B., F.T.S., D.E.S., and J.P.G. edited and revised manuscript; B.A.B., F.T.S., S.C.S., D.E.S., J.M.R., R.S.V.G., and J.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Arany for technical expertise.
REFERENCES
- 1.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, Bussolati B, St John J. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Mol Med 6: 391–409, 2000. [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 3.Bakrania B, Du Toit EF, Wagner K-H, Headrick JP, Bulmer AC. Pre- or post-ischemic bilirubin ditaurate treatment reduces oxidative tissue damage and improves cardiac function. Int J Cardiol 202: 27–33, 2016. doi: 10.1016/j.ijcard.2015.08.192. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisht K, Wegiel B, Tampe J, Neubauer O, Wagner K-H, Otterbein LE, Bulmer AC. Biliverdin modulates the expression of C5aR in response to endotoxin in part via mTOR signaling. Biochem Biophys Res Commun 449: 94–99, 2014. doi: 10.1016/j.bbrc.2014.04.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185–F1194, 2008. doi: 10.1152/ajprenal.00442.2007. [DOI] [PubMed] [Google Scholar]
- 7.Craici IM, Wagner SJ, Weissgerber TL, Grande JP, Garovic VD. Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int 86: 275–285, 2014. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115: 1789–1797, 2007. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 44: 708–714, 2004. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 10.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George EM, Cockrell K, Arany M, Stec DE, Rimoldi JM, Gadepalli RSV, Granger JP. Carbon monoxide releasing molecules blunt placental ischemia-induced hypertension. Am J Hypertens 30: 931–937, 2017. doi: 10.1093/ajh/hpx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George EM, Colson D, Dixon J, Palei AC, Granger JP. Heme oxygenase-1 attenuates hypoxia-induced sFlt-1 and oxidative stress in placental villi through its metabolic products CO and bilirubin. Int J Hypertens 2012: 486053, 2012. doi: 10.1155/2012/486053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George EM, Warrington JP, Spradley FT, Palei AC, Granger JP. The heme oxygenases: important regulators of pregnancy and preeclampsia. Am J Physiol Regul Integr Comp Physiol 307: R769–R777, 2014. doi: 10.1152/ajpregu.00132.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 15.Gruslin A, Lemyre B. Pre-eclampsia: fetal assessment and neonatal outcomes. Best Pract Res Clin Obstet Gynaecol 25: 491–507, 2011. doi: 10.1016/j.bpobgyn.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Guo P, Qi X, Yuan R, Li H, Gao X, Wang J, Zhang B. Tet1-mediated DNA demethylation involves in neuron damage induced by bilirubin in vitro. Toxicol Mech Methods 25: 1–7, 2007. doi: 10.1080/15376516.2017.1357775. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Hamano H, Satoh A, Horinouchi Y, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Aihara K, Tsuchiya K, Tamaki T. Bilirubin exerts pro-angiogenic property through Akt-eNOS-dependent pathway. Hypertens Res 38: 733–740, 2015. doi: 10.1038/hr.2015.74. [DOI] [PubMed] [Google Scholar]
- 18.Jain A. Endothelin-1: a key pathological factor in pre-eclampsia? Reprod Biomed Online 25: 443–449, 2012. doi: 10.1016/j.rbmo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Münzel T, Daiber A. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol 49: 186–195, 2010. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 367: 1066–1074, 2006. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 21.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9: 480–485, 2007. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 22.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension 52: 1161–1167, 2008. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 24.Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 70: 412–427, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Levytska K, Kingdom J, Baczyk D, Drewlo S. Heme oxygenase-1 in placental development and pathology. Placenta 34: 291–298, 2013. doi: 10.1016/j.placenta.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 303: H1–H8, 2012. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XM, Peyton KJ, Durante W. Ammonia promotes endothelial cell survival via the heme oxygenase-1-mediated release of carbon monoxide. Free Radic Biol Med 102: 37–46, 2017. doi: 10.1016/j.freeradbiomed.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzone GL, Rigato I, Tiribelli C. Unconjugated bilirubin modulates nitric oxide production via iNOS regulation. Biosci Trends 4: 244–248, 2010. [PubMed] [Google Scholar]
- 29.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 58: 709–715, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 30.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest 96: 2676–2682, 1995. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J 19: 284–286, 2005. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 32.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graça-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation 112: 1030–1039, 2005. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 33.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003. doi: 10.1016/S1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.Paauw ND, Joles JA, Spradley FT, Bakrania B, Zsengeller ZK, Franx A, Verhaar MC, Granger JP, Lely AT. Exposure to placental ischemia impairs postpartum maternal renal and cardiac function in rats. Am J Physiol Regul Integr Comp Physiol 312: R664–R670, 2017. doi: 10.1152/ajpregu.00510.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock JS, Pollock DM. Endothelin, nitric oxide, and reactive oxygen species in diabetic kidney disease. Contrib Nephrol 172: 149–159, 2011. doi: 10.1159/000329054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856–2869, 2011. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM, Druzin M, Gaiser RR, Granger JP, Jeyabalan A, Johnson DD, Karumanchi S, Lindheimer M, Owens MY, Saade GR, Sibai BM, Spong CY, Tsigas E, Joseph GF, O’Reilly N, Politzer A, Son S, Ngaiza K. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 38.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens 2: 72–83, 2012. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 40.Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O’Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006. doi: 10.1038/sj.ki.5000277. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt HHHW. NO, CO, and OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett 307: 102–107, 1992. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- 42.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 1: e00081, 2013. doi: 10.1002/phy2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987. [DOI] [PubMed] [Google Scholar]
- 44.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 6: 841–849, 2004. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 45.Sung LC, Chao HH, Chen CH, Tsai JC, Liu JC, Hong HJ, Cheng TH, Chen JJ. Lycopene inhibits cyclic strain-induced endothelin-1 expression through the suppression of reactive oxygen species generation and induction of heme oxygenase-1 in human umbilical vein endothelial cells. Clin Exp Pharmacol Physiol 42: 632–639, 2015. doi: 10.1111/1440-1681.12412. [DOI] [PubMed] [Google Scholar]
- 46.Szarka A, Rigó J Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11: 59, 2010. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330.e1–330.e4, 2011. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urquhart P, Rosignoli G, Cooper D, Motterlini R, Perretti M. Carbon monoxide-releasing molecules modulate leukocyte-endothelial interactions under flow. J Pharmacol Exp Ther 321: 656–662, 2007. doi: 10.1124/jpet.106.117218. [DOI] [PubMed] [Google Scholar]
- 49.Venditti CC, Casselman R, Young I, Karumanchi SA, Smith GN. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One 9: e106502, 2014. doi: 10.1371/journal.pone.0106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venditti CC, Smith GN. Involvement of the heme oxygenase system in the development of preeclampsia and as a possible therapeutic target. Womens Health (Lond) 10: 623–643, 2014. doi: 10.2217/WHE.14.54. [DOI] [PubMed] [Google Scholar]
- 51.Verdonk K, Saleh L, Lankhorst S, Smilde JEI, van Ingen MM, Garrelds IM, Friesema ECH, Russcher H, van den Meiracker AH, Visser W, Danser AHJ. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 65: 1316–1323, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 52.Volti GL, Sacerdoti D, Sangras B, Vanella A, Mezentsev A, Scapagnini G, Falck JR, Abraham NG. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal 7: 704–710, 2005. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 53.Warrington JP, Coleman K, Skaggs C, Hosick PA, George EM, Stec DE, Ryan MJ, Granger JP, Drummond HA. Heme oxygenase-1 promotes migration and β-epithelial Na+ channel expression in cytotrophoblasts and ischemic placentas. Am J Physiol Regul Integr Comp Physiol 306: R641–R646, 2014. doi: 10.1152/ajpregu.00566.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wegiel B, Gallo D, Csizmadia E, Roger T, Kaczmarek E, Harris C, Zuckerbraun BS, Otterbein LE. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proc Natl Acad Sci USA 108: 18849–18854, 2011. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wegiel B, Otterbein LE. Go green: the anti-inflammatory effects of biliverdin reductase. Front Pharmacol 3: 47, 2012. doi: 10.3389/fphar.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young SC, Storm MV, Speed JS, Kelsen S, Tiller CV, Vera T, Drummond HA, Stec DE. Inhibition of biliverdin reductase increases ANG II-dependent superoxide levels in cultured renal tubular epithelial cells. Am J Physiol Regul Integr Comp Physiol 297: R1546–R1553, 2009. doi: 10.1152/ajpregu.90933.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]