Abstract
Metabolic syndrome (MetS) leads to cardiac vascular injury, which may reflect in increased retention of endothelial progenitor cells (EPCs). Coronary endothelial cell (EC) mitochondria partly regulate vascular function and structure. We hypothesized that chronic mitoprotection would preserve EC mitochondria and attenuate coronary vascular injury and dysfunction in swine MetS. Pigs were studied after 16 wk of diet-induced MetS, MetS treated for the last 4 wk with the mitochondria-targeted peptide elamipretide (ELAM; 0.1 mg/kg sc once daily), and lean controls (n = 6 each). Cardiac remodeling and function were assessed in vivo by multidetector-computed tomography (CT), and coronary artery and sinus blood samples were collected. EC mitochondrial density, apoptosis, oxidative stress, endothelial nitric oxide synthase immunoreactivity, myocardial microvascular density (three-dimensional microcomputed tomography), and coronary endothelial function (organ bath) were assessed ex vivo. The number and arteriovenous gradient of CD34+/KDR+ EPCs were calculated by FACS (a negative net gradient indicating EPC retention). MetS and MetS + ELAM pigs developed similar MetS (obesity, hyperlipidemia, insulin resistance, and hypertension). EC mitochondrial density decreased in MetS animals compared with lean animals but normalized in MetS + ELAM animals. ELAM also attenuated EC oxidative stress and apoptosis and improved subendocardial microvascular density. ELAM-induced vasculoprotection was reflected by decreased coronary retention of EPCs. ELAM also partly improved endothelial nitric oxide synthase immunoreactivity, coronary endothelial function, and vessel maturity, whereas myocardial perfusion was unaffected. Chronic mitoprotection improved coronary EC mitochondrial density and decreased vascular remodeling and dysfunction. However, additional mitochondria-independent mechanisms likely contribute to MetS-induced cardiac vascular injury.
NEW & NOTEWORTHY The present study shows that chronic mitoprotection preserved coronary endothelial cell mitochondria and decreased vascular injury, subendocardial microvascular loss, coronary retention of endothelial progenitor cells, and release of markers of vascular injury. However, myocardial perfusion remained blunted, suggesting that additional mitochondria-independent mechanisms likely contribute to metabolic syndrome-induced cardiac vascular injury.
Keywords: elamipretide, metabolic syndrome, microvessels, mitochondria, myocardium
INTRODUCTION
Metabolic syndrome (MetS) refers to a collection of risk factors for cardiovascular disease, including central obesity, high blood pressure, dyslipidemia, and impaired glucose metabolism (1). The prevalence of MetS has been escalating rapidly over the last three decades and has now reached epidemic proportions worldwide (33). Individuals with MetS have increased cardiovascular and all-cause mortality rates compared with those without as well as an increased incidence of cardiovascular disease, coronary heart disease, and stroke (20).
Studies in small and large animal models as well as in humans have shown that MetS imparts substantial functional and structural damage to the myocardial microvasculature. A high-fat diet promotes progressive impairment of coronary vascular function in rats, as reflected by reduced acetylcholine-induced relaxation of isolated coronary microvessels (24). Furthermore, we have shown that MetS blunted the density of subepicardial medium-size vessels in MetS-prone Ossabaws pigs (31) and impaired myocardial perfusion and oxygenation in domestic pigs (35). In line with this, the nonischemic myocardium from obese patients undergoing coronary artery (CA) bypass shows reduced coronary microvascular density compared with nonobese patients (7). However, the mechanisms underlying MetS-induced myocardial microvascular injury are incompletely understood.
Unlike high energy-demanding cardiomyocytes, endothelial cells (ECs) have a limited number of mitochondria, which represent only 5% of their cytoplasmic volume (3). Nevertheless, EC mitochondria modulate several important factors that regulate vascular function, possibly by modulating apoptosis, oxidative stress, Ca2+ signaling, and nitric oxide (NO) bioavailability (26). Therefore, strategies directed to preserve the morphology and function of EC mitochondria may alleviate MetS-induced myocardial vascular damage.
The novel mitochondria-targeted peptide elamipretide (ELAM), which targets and preserves the mitochondrial inner membrane phospholipid cardiolipin, has demonstrated important cardioprotective properties in experimental ischemic disease (25, 37). We have previously shown in swine that during renal revascularization that reversed renovascular hypertension, adjunct ELAM expedited myocardial microvascular and tissue repair (16). We have also shown that when renovascular hypertension was sustained, chronic treatment with ELAM successfully attenuated myocardial cellular and microvascular remodeling (10). The mechanisms of vascular injury evoked in MetS might differ from pathways activated in hypertension. However, the potential of ELAM for attenuating MetS-induced cardiac vascular injury is unknown. Therefore, this study tested the hypothesis that chronic treatment with ELAM would preserve EC mitochondria, attenuating cardiac vascular injury in porcine MetS.
MATERIALS AND METHODS
Study groups and experimental design.
All experiments were approved by the Institutional Animal Care and Use Committee and conformed with National Institutes of Health guidelines (Guide for the Care and Use of Laboratory Animals). Three-month-old premenstrual female domestic pigs (n = 18) were studied after 16 wk of observation. We opted to study female pigs, which are more resistant to the deleterious effects of MetS on the myocardial microvasculature relative to male pigs (5, 23). At baseline, six lean pigs were fed a standard diet and 12 MetS pigs were fed a high-fat/high-fructose diet (16.1% protein, 43.0% ether extract fat, and 40.8% carbohydrates, 5B4L, Purina Test Diet, Richmond, IN) (35) for a total of 16 wk.
Twelve weeks after commencement of the diet, six MetS pigs started a 4-wk treatment with chronic subcutaneous injections of ELAM (also known as Bendavia or MTP-131, Stealth BioTherapeutics, Newton Centre, MA) and 0.1 mg/kg in 1 ml of PBS once daily 5 days/wk (10, 11), whereas PBS vehicle was injected into the remaining six MetS and lean pigs.
After 4 wk of ELAM or vehicle, pigs were anaesthetized with 0.25 g im of tiletamine hydrochloride-zolazepam hydrochloride and 0.5 g of xylazine, and anesthesia was maintained with intravenous ketamine (0.2 mg·kg−1·min−1) and xylazine (0.03 mg·kg−1·min−1) (15). Fasting blood samples were obtained, and total cholesterol, HDL, LDL, triglycerides, glucose, and insulin measured by standard procedures. Insulin resistance was calculated with the homeostasis model assessment of insulin resistance (HOMA-IR) (35). Cardiac remodeling and function were assessed using multidetector computed tomography (MDCT), and arterial blood pressure was measured using an intra-arterial catheter during MDCT experiments.
A few days after the completion of in vivo experiments, pigs were euthanized with pentobarbital sodium (100 mg/kg iv Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI). Hearts were harvested, and a segment from the left ventricle (LV) was prepared for microcomputed tomography experiments, whereas the main branches of the CA were dissected and placed in Krebs solution for in vitro assessment of coronary endothelial function.
Cardiac function and remodeling.
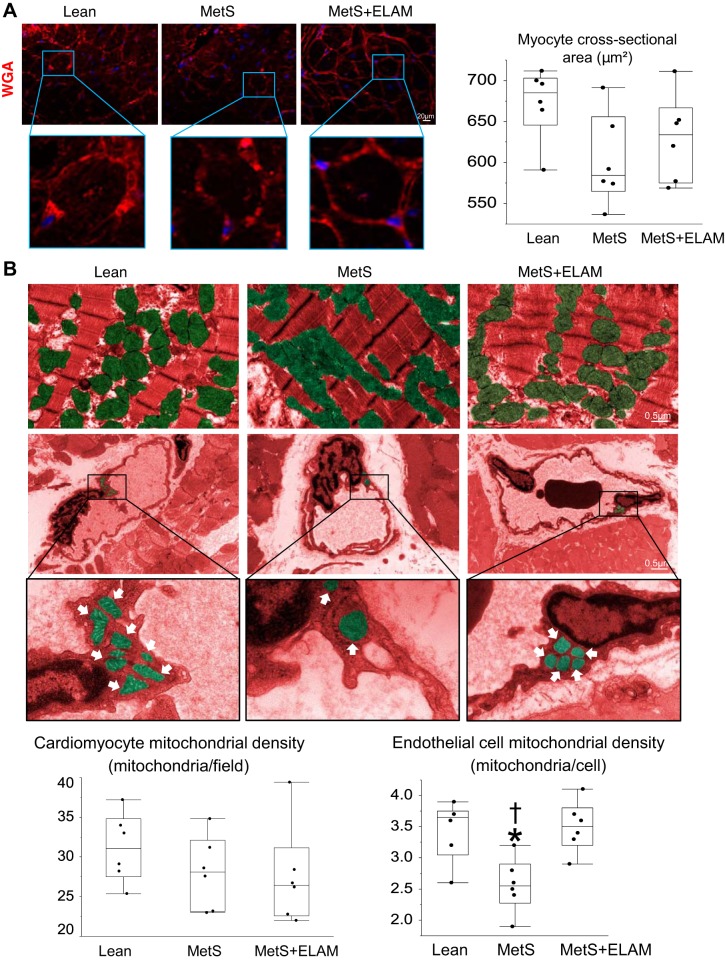
Cardiac output, stroke volume, ejection fraction, and LV muscle mass (LVMM) were assessed using MDCT (Somatom Sensation-128, Siemens Medical Solution, Forchheim, Germany). Myocardial perfusion was calculated from time-attenuation curves obtained from the anterior cardiac wall, as previously described (41), and coronary blood flow was calculated as perfusion × LVMM. Images were analyzed with the Analyze software package (Biomedical Imaging Resource, Mayo Clinic, MN) (18). Systemic vascular resistance (SVR) was calculated as mean arterial pressure × 80/(cardiac output). Myocyte cross-sectional area was assessed in mid-LV cross sections stained with wheat germ agglutinin.
Markers of vascular injury.
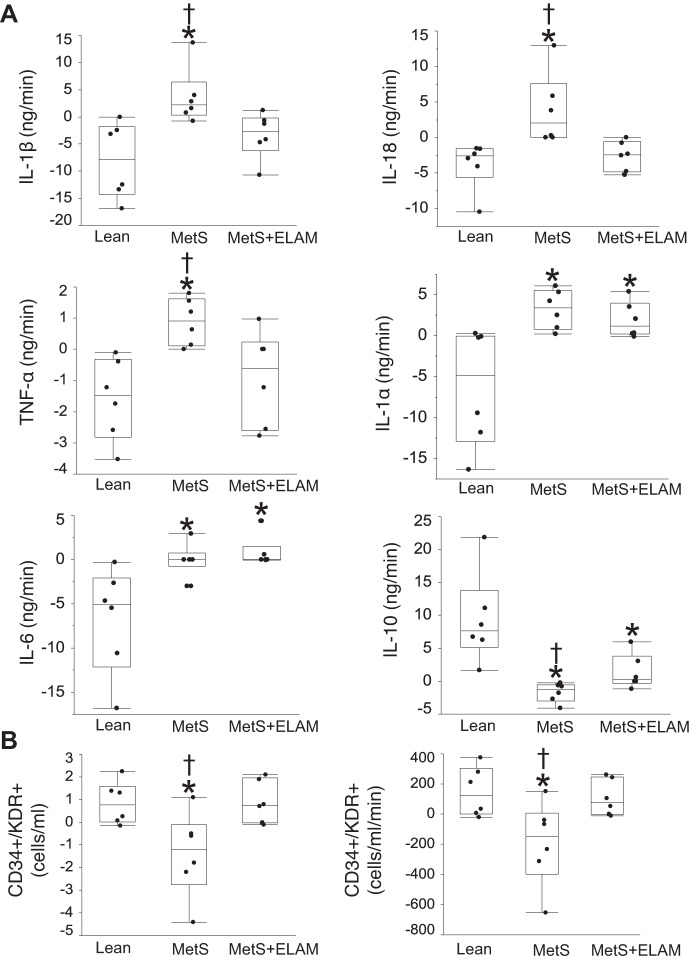
Before MDCT experiments, all pigs were anesthetized, and, under fluoroscopic guidance, catheters were advanced into the CA and coronary sinus (CS) to collect blood samples. Samples were centrifuged, and plasma aliquots were stored at −80°C until being assayed. CA and CS levels of IL-1β, IL-18, TNF-α, IL-1α, IL-6, and IL-10 were measured by luminex (Millipore, Billerica, MA) (12, 13). Their net release across the coronary circulation was then calculated by multiplying coronary blood flow by their transcardiac gradient (CA − CS), with negative gradients indicating retention (29).
Additionally, blood mononuclear cells were isolated from fresh blood by the density-gradient method and characterized for antigen expression of the endothelial progenitor cell (EPC) markers CD34 and KDR. CA and CS levels of CD34+/KDR+ EPCs were determined by FASCS per 100,000 events, as previously described (21). Transcardiac and net gradients of EPCs were then calculated.
Mitochondrial and cardiolipin content.
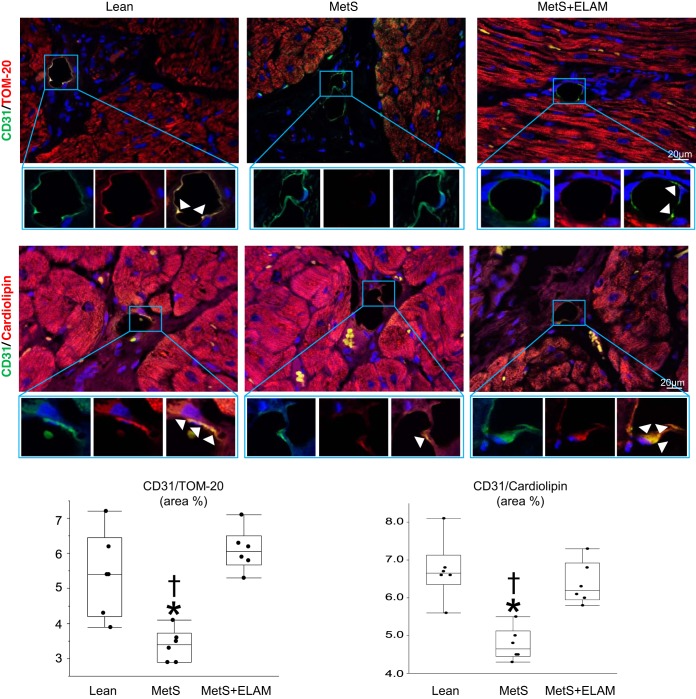
LV samples (2–3 mm3) were fixed with Trump’s fixative by transmission electron microscopy (Philips CM10 Transmission Electron Microscope) and processed at the Mayo Clinic’s electron microscopy core facility. The number of mitochondria distributed among myofibrils was counted in five randomly selected fields, and the number of mitochondria/cell was counted in randomly selected ECs (n = 20 in each sample). EC mitochondrial density was also assessed in LV sections by double immunofluorescence staining with CD31 and the mitochondrial marker translocase of outer membrane (TOM)-20. In each slide, double-positive areas were semiautomatically quantified in 15–20 fields (×40) and expressed as a fraction of the myocardial surface area, and the results from all fields were averaged. EC cardiolipin content was assessed by double immunofluorescence staining with CD31 and 10 N-nonyl acridine-orange (A1372; Invitrogen, Carlsbad, CA) and quantified in 15–20 random fields using a computer-aided image analysis program (ZEN 2012 blue edition, Carl Zeiss SMT, Oberkochen, Germany) (10).
EC apoptosis and oxidative stress.
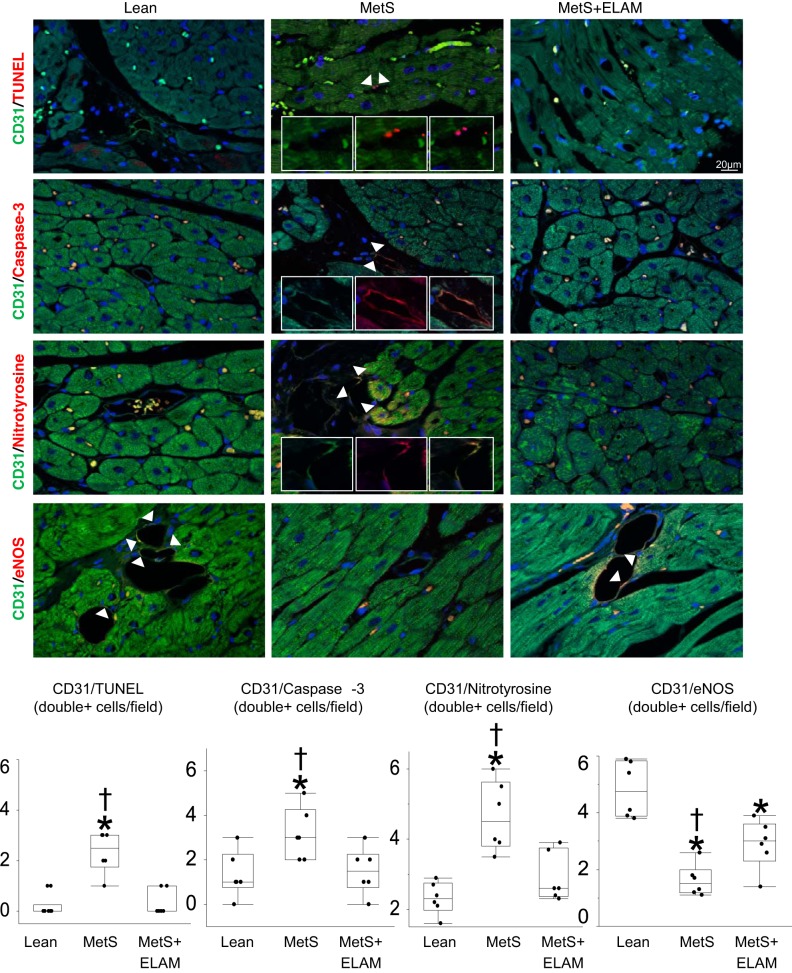
Myocardial EC apoptosis was assessed in LV sections double stained with CD31 and TUNEL as well as CD31 and caspase-3. Numbers of CD31/TUNEL+ and CD31/caspase-3+ cells were quantified and averaged in each group. EC oxidative stress was assessed by double immunofluorescence staining with CD31 and nitrotyrosine, and endothelial [endothelial NO synthase (eNOS)] immunoreactivity was assessed by CD31/eNOS staining; they were quantified using ZEN.
Microvascular structure.
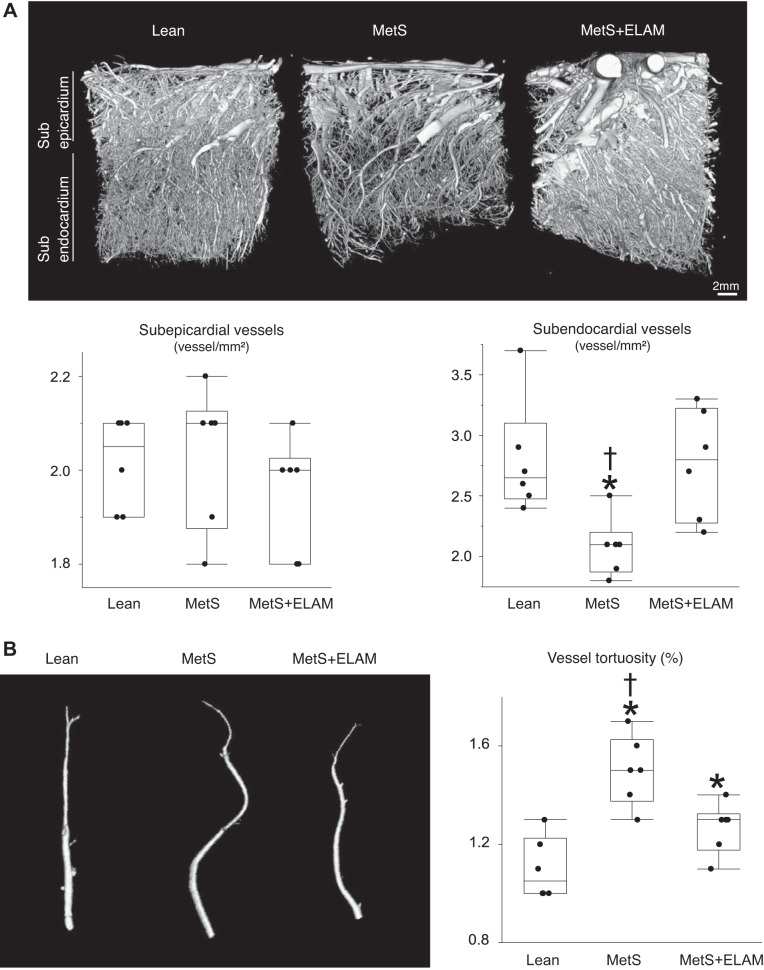
The LV wall was perfused under physiological pressure with an intravascular contrast agent (MV-122, Flow Tech, Carver, MA) through a branch of the left circumflex CA. A transmural portion (2 cm3) of the LV was scanned, and images were analyzed with Analyze. The spatial density of microvessels (20–500 μm) in the subepicardium and subendocardium as well as vessel tortuosity (an index of vessel immaturity) were calculated, as previously described (44).
Coronary endothelial function.
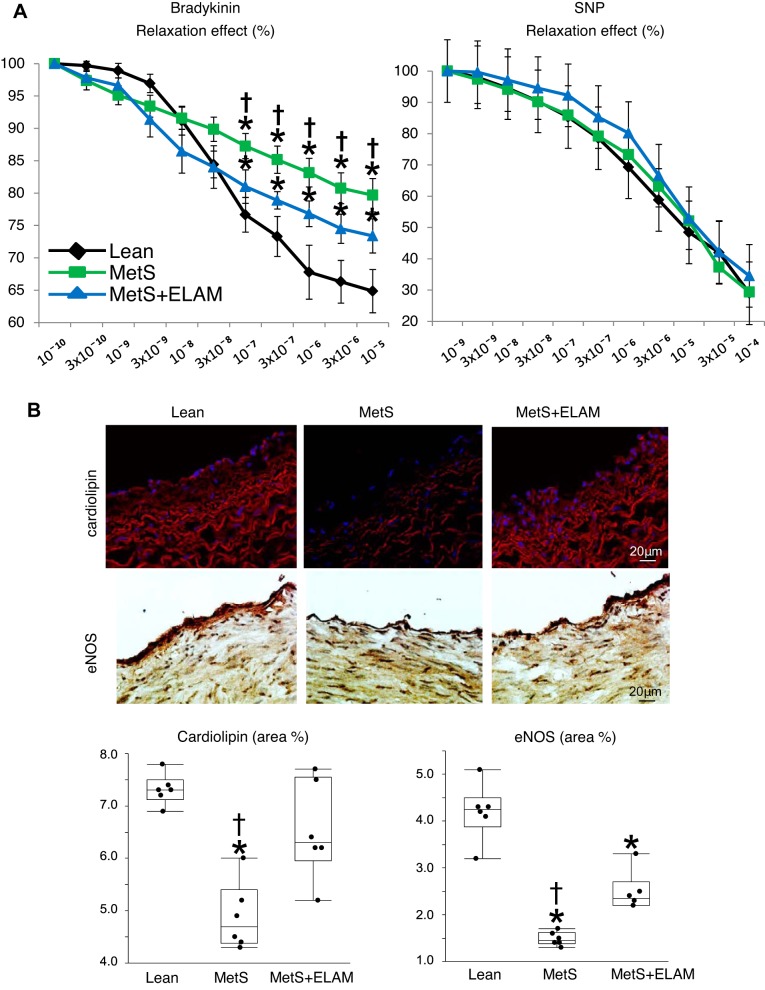
CAs were dissected immediately after euthanasia and placed in 25-ml organ chambers filled with Krebs solution (1 ring per animal per chamber). Rings were allowed to equilibrate for 30 min and then contracted with endothelin-1 (10ˉ7 M). The response to cumulative concentrations of endothelium-dependent bradykinin (10ˉ10−10ˉ5 M) or endothelium-independent sodium nitroprusside (SNP; 10ˉ10−10ˉ4 M) was studied, as previously described (22, 42, 43). In addition, cardiolipin content (10 N-nonyl-acridine-orange) and eNOS immunoreactivity (catalog no. ab5589, Abcam) were assessed in CA sections.
Statistical methods.
Statistical analysis was performed using JMP 10.0 software package (SAS Institute, Cary, NC). Data are presented as means ± SD or medians (interquartile ranges). Comparisons within and among groups were performed using a paired Student’s t-test and ANOVA, respectively. Data that did not follow a Gaussian distribution were compared with nonparametric tests (Wilcoxon and Kruskal Wallis). P values of <0.05 were considered statistically significant.
RESULTS
Systemic characteristics and cardiac function.
At the end of the study, body weight, mean blood pressure, and lipid fractions were higher in MetS pigs compared with lean pigs (Table 1). Fasting glucose did not differ among the groups, but fasting insulin and HOMA-IR levels were elevated in the MetS group, indicating prediabetic MetS. Heart rate was higher in the MetS group compared with the lean group, but cardiac output, stroke volume, and LV ejection fraction were similar among the groups, as were LVMM and SVR (Table 1). Myocardial perfusion similarly decreased in the MetS and MetS + ELAM groups. Myocyte cross-sectional area was similar among the groups (Fig. 1A).
Table 1.
Systemic characteristics and cardiac function in experimental groups at 16 wk
| Parameter | Lean | MetS | MetS + ELAM |
|---|---|---|---|
| Body weight, kg | 70.0 ± 10.9 | 92.0 ± 3.8* | 91.7 ± 3.4* |
| Mean blood pressure, mmHg | 99.6 ± 9.7 | 122.8 ± 11.2* | 119.3 ± 2.2* |
| Total cholesterol, mg/dl | 82.5 (76.0–90.5) | 414.0 (331.0–506.8)* | 383.5 (306.8–410.3)* |
| HDL-cholesterol, mg/dl | 47.7 ± 5.9 | 134.1 ± 29.7* | 124.8 ± 37.7* |
| LDL-cholesterol, mg/dl | 33.8 (26.4–40.3) | 369.7 (213.4–489.6)* | 286.4 (262.6–468.1)* |
| Triglycerides, mg/dl | 7.8 ± 2.1 | 18.8 ± 3.4* | 21.2 ± 6.9* |
| Fasting glucose, mg/dl | 101.0 ± 2.7 | 103.7 ± 16.1 | 107.2 ± 12.5 |
| Fasting insulin, µU/ml | 0.4 (0.3–0.5) | 0.7 (0.7–0.8)* | 0.7 (0.7–0.8)* |
| HOMA-IR score | 0.6 (0.6–0.7) | 1.9 (1.5–1.9)* | 1.9 (1.8–2.0)* |
| Heart rate, beats/min | 74.6 ± 5.2 | 82.7 ± 4.4* | 81.0 ± 2.5* |
| Cardiac output, l/min | 5.4 ± 0.8 | 6.0 ± 1.0 | 5.7 ± 1.0 |
| Stroke volume, ml | 72.3 ± 5.4 | 71.1 ± 5.4 | 73.8 ± 5.7 |
| Ejection fraction, % | 58.8 ± 6.7 | 53.5 ± 5.4 | 54.4 ± 2.3 |
| Left ventricular muscle mass, g | 140.0 ± 23.9 | 146.5 ± 27.1 | 145.7 ± 24.6 |
| Systemic vascular resistance, mmHg⋅min⋅ml−1 | 1,443.7 ± 206.0 | 1,670.1 ± 343.5 | 1,708.5 ± 285.7 |
| Myocardial perfusion, ml·min−1·g−1 | 1.08 ± 0.15 | 0.85 ± 0.10* | 0.82 ± 0.20* |
Values are means or medians (interquartile ranges); n = 6 pigs/group. MetS, metabolic syndrome; ELAM, elamipretide; HOMA-IR, homeostasis model assessment of insulin resistance.
P < 0.05 vs. the lean group.
Fig. 1.
Metabolic syndrome (MetS) induces endothelial cell mitochondrial injury. A: left ventricular sections stained with wheat germ agglutinin (WGA) and quantification of myocyte cross-sectional area (n = 6/group). B: transmission electron microscopy and quantification of intermyofibrillar and endothelial cell mitochondrial density in study groups (n = 6/group). *P < 0.05 vs. the lean group; †P < 0.05 vs. the MetS + elamipretide (ELAM) group.
ELAM improves EC mitochondrial density.
The number of mitochondria interspersed among myofibrils was similar in all groups, yet EC mitochondrial density was considerably lower in the MetS group compared with the lean group but normalized in the ELAM-treated group (Fig. 1B). CD31/TOM-20 colocalization decreased in MetS pigs compared with lean pigs but was fully restored in MetS + ELAM pigs (Fig. 2). Similarly, EC cardiolipin content decreased in MetS pigs compared with lean pigs but was restored to normal levels in MetS + ELAM pigs (Fig. 2).
Fig. 2.
Elamipretide (ELAM) restores endothelial cell cardiolipin content. Representative double immunofluorescent staining (×40) for the endothelial marker CD31 (green) and the mitochondrial marker translocase of outer membrane (TOM)-20 (red) and the mitochondrial inner membrane phospholipid cardiolipin (red) show decreased endothelial mitochondria and cardiolipin expression (merge yellow) in metabolic syndrome (MetS), which was normalized in ELAM-treated pigs (n = 6/group). *P < 0.05 vs. the lean group; †P < 0.05 vs. the MetS + ELAM group.
ELAM attenuates EC oxidative stress and apoptosis.
Numbers of CD31/TUNEL-positive and CD31/caspase-3-positive cells, indicative of EC apoptosis, were elevated in MetS pigs but decreased to normal levels in ELAM-treated pigs (Fig. 3). The number of CD31/nitrotyrosine-positive cells also normalized in MetS + ELAM pigs, suggesting decreased EC oxidative stress. EC expression of eNOS was lower in MetS pigs compared with lean pigs but slightly improved in MetS + ELAM pigs (Fig. 3).
Fig. 3.
Elamipretide (ELAM) attenuates endothelial cell injury. Representative double immunofluorescent staining (×40) for CD31 (green) and TUNEL, caspase-3, nitrotyrosine, and endothelial nitric oxide synthase (eNOS; red). The number of endothelial cells (CD31, green) positive for TUNEL (red) and caspase-3 (red) was elevated in metabolic syndrome (MetS) animals but decreased in MetS + ELAM animals (n = 6/group). Treatment with ELAM restored the double immunoreactivity of nitrotyrosine (red) and CD31. Myocardial endothelial cell expression of eNOS decreased in MetS pigs but moderately improved in MetS + ELAM-treated pigs (n = 6/group). *P < 0.05 vs. lean pigs; †P < 0.05 vs. MetS + ELAM pigs.
Mitoprotection preserves the myocardial microvasculature.
The spatial density of subepicardial microvessels was comparable among the groups, whereas the density of subendocardial microvessels substantially decreased in MetS pigs but normalized in ELAM-treated pigs (Fig. 4A). Vessel tortuosity was higher in MetS pigs but slightly decreased in ELAM-treated pigs, suggesting partial improvement of vessel maturity (Fig. 4B).
Fig. 4.
Mitoprotection preserves the myocardial microvasculature. A: representative three-dimensional microcomputed tomography images of the left ventricle and quantification of spatial density of subepicardial and subendocardial microvessels (n = 6/group). B: vessel tortuosity was slightly higher in metabolic syndrome (MetS) pigs but decreased in MetS + elamipretide (ELAM) pigs (n = 6/group). *P < 0.05 vs. lean pigs; †P < 0.05 vs. MetS + ELAM pigs.
Mitoprotection partly attenuates coronary endothelial dysfunction.
The vasorelaxation response to bradykinin in CA vessel rings was impaired in the MetS group compared with the lean group but slightly improved in the MetS + ELAM group, whereas the response to SNP was unchanged (Fig. 5A). Cardiolipin content in CA sections was decreased in the MetS group but was restored in the MetS + ELAM group, associated with a partial improvement in eNOS immunoreactivity (Fig. 5B).
Fig. 5.
Mitoprotection partly attenuates coronary endothelial dysfunction. A: endothelium-dependent (left) and -independent (right) relaxation of coronary segments (1 ring per animal per chamber) from lean, metabolic syndrome (MetS), and MetS + elamipretide (ELAM) pigs (n = 6/group). B: representative images and quantification of cardiolipin expression (red) and endothelial nitric oxide synthase (eNOS) immunoreactivity in coronary artery sections (n = 6/group). *P < 0.05 vs. lean pigs; †P < 0.05 vs. MetS + ELAM pigs.
Markers of vascular injury and repair.
The net gradient of proinflammatory IL-1β, IL-18, and TNF-α increased in MetS pigs compared with lean pigs but decreased in MetS + ELAM pigs, suggesting decreased cardiac release of inflammatory cytokines (Fig. 6A). The release of IL-1α and IL-6 was similarly elevated in MetS and MetS + ELAM pigs, whereas release of the anti-inflammatory cytokine IL-10 decreased in MetS pigs and slightly improved in MetS + ELAM pigs.
Fig. 6.
Markers of vascular injury and progenitor cell retention. A: net gradient of the inflammatory cytokines IL-1β, IL-18, TNF-α, IL-1α, IL-6, and IL-10 in the study groups (n = 6/group). B: transcardiac and net gradients of CD34+/KDR+ showing retention of endothelial progenitor cells in metabolic syndrome (MetS) pigs but not in MetS + elamipretide (ELAM) pigs (n = 6/group). *P < 0.05 vs. lean pigs; †P < 0.05 vs. MetS + ELAM pigs.
Transcardiac and net gradients of CD34+/KDR+ EPCs were negative in the MetS group, suggesting retention of EPCs, but did not differ from the lean group with MetS + ELAM treatment (Fig. 6B).
DISCUSSION
The present study shows that chronic mitoprotection improved coronary EC mitochondrial density and cardiolipin content in experimental MetS, which were associated with protection of the coronary circulation. Treatment with ELAM attenuated EC oxidative stress and apoptosis, improved coronary endothelial function, and restored subendothelial microvascular density. ELAM-induced vasculoprotection was reflected by decreased coronary release of markers of vascular injury and retention of EPCs. However, eNOS immunoreactivity, coronary endothelial function, and vessel maturity were only partly improved, and myocardial perfusion was unaffected by the mitoprotection. These observations underscore the role of mitochondria in regulating the cardiac circulation in experimental MetS and suggest that nonmitochondrial mechanisms are likely implicated in MetS-induced cardiac vascular dysfunction.
Mounting experimental studies have shown that mitochondrial dysfunction might contribute to several forms of cardiac injury and vascular disease (14). Cardiolipin is an anionic phospholipid constituent of the inner mitochondrial membrane that plays a key role in cristae formation and mitochondrial function (40). Peroxidation and loss of cardiolipin favor its dissociation from cytochrome c on the inner membrane and the formation of the mitochondrial permeability transition pore. Consequent release of cytochrome c into the cytosol, in turn, activates the caspase pathway and initiates apoptosis (6, 36). Furthermore, cardiolipin loss triggers the production and release of mitochondrial reactive oxygen species that further activate NADPH oxidases, promoting forward-feeding oxidative stress (27).
ELAM is a synthetic tetrapeptide that selectively concentrates in the inner mitochondrial membrane, binding and preventing peroxidation of cardiolipin. In recent years, several studies have demonstrated the efficacy of ELAM in experimental heart disease (25, 37). Chronic treatment with ELAM improved systolic function, normalized plasma biomarkers, and reversed myocardial mitochondrial abnormalities in dogs with advanced heart failure (37). In addition, we have shown in pigs with renovascular hypertension that treatment with ELAM with or without adjunct renal revascularization attenuates myocardial remodeling and diastolic dysfunction (10, 16), underscoring its cardioprotective properties in hypertension.
This study shows that ELAM confers vasculoprotective effects in a large animal model that recapitulates many features of human MetS, a condition associated with significant cardiac vascular injury (20). Although ECs contain a small number of mitochondria (3), these organelles modulate several aspects of EC function (26). Here, we found that long-term monotherapy with ELAM in MetS pigs successfully improved EC mitochondrial number and restored cardiolipin content, which, in turn, decreased EC apoptosis and oxidative stress, reflected by the decreased numbers of CD31/caspase-3-positive and CD31/nitrotyrosine-positive cells.
MetS-induced EC apoptosis and oxidative stress may have impacted on myocardial microvascular architecture. We found that despite preserved numbers of subepicardial vessels, subendocardial spatial density decreased in the MetS group compared with the lean group. In patients with microvascular disease, subendocardial hypoperfusion is associated with intense myocardial ischemia (34), suggesting that selective loss of subendocardial vessels may have important functional implications. Notably, treatment with ELAM restored subendocardial microvascular density, possibly by blunting vascular cell apoptosis and oxidative stress. Furthermore, increased vessel tortuosity in MetS reflected microvascular immaturity. Despite normalization of subendocardial microvasculature density, vessel tortuosity decreased only partly in MetS + ELAM pigs, suggesting incomplete restoration of myocardial microvascular structure.
Several observations support the notion of coronary vascular dysfunction in our MetS pigs, including impaired vasorelaxation response to bradykinin and decreased eNOS immunoreactivity in CA vessel rings. In patients with CA disease, the injured myocardium releases inflammatory signals and activates mechanisms to repair the damaged endothelium (29), including homing and retention of bone marrow-derived circulating EPCs, contributing to EC regeneration and neoangiogenesis (8). The present study indicates that net release of several proinflammatory cytokines across the coronary circulation increased in MetS pigs compared with lean pigs, whereas release of anti-inflammatory IL-10 was blunted. MetS-induced cardiac vascular injury was also associated with retention of EPCs within the coronary circulation, in line with observations in patients with chronic ischemic heart failure (32). Increased circulating levels of IL-6 and IL-1β have been shown to impair EPC function after acute myocardial infraction (38). Therefore, MetS-induced increased net release of these proinflammatory cytokines might have limited EPC reparative effects.
Despite significant endothelial cell injury, overall cardiomyocyte morphology was preserved in MetS pigs. Previous studies have shown that microvessels may undergo vascular remodeling at an early stage of the disease progression and may present an early subclinical culprit in the pathogenesis of coronary microvascular disease in diabetes and MetS (19, 28). In line with this, we found that despite preserved cardiac function, MetS induced subendocardial microvascular rarefaction and coronary endothelial dysfunction. Contrarily, LVMM and myocyte cross-sectional area (wheat germ agglutinin staining) were similar among the groups, arguing against myocardial remodeling. Taken together, these finding suggest that MetS-induced EC injury precedes myocardial remodeling.
Notably, treatment with ELAM normalized both transcardiac and net EPC gradients, implying that mitoprotection attenuated cardiac vascular damage and consequently decreased EPC recruitment and retention. On the other hand, treatment with ELAM attenuated the release of IL-1β, IL-18, and TNF-α, yet the release of IL-10 was partially improved and release of IL-1α and IL-6 remained unchanged. Likewise, ELAM moderately improved, but did not normalize, in vitro coronary arterial endothelial function, denoted by responses to bradykinin, likely due to increased eNOS immunoreactivity and NO availability. Furthermore, myocardial perfusion remained blunted in ELAM-treated pigs, suggesting that additional mitochondria-independent mechanisms likely contribute to MetS-induced cardiac vascular dysfunction. Finally, vessel maturity, eNOS immunoreactivity, and coronary endothelial function were only partially improved in the MetS + ELAM group. Therefore, alterations in these pathways may partly explain the residual mild myocardial ischemia observed in ELAM-treated pigs.
Our model exhibits some notable similarities and differences with human MetS. In our model, MetS developed in young animals over a 16-wk period, which is shorter than the usual disease duration in humans. MetS pigs developed obesity and hypertriglyceridemia, accompanied by a spontaneous increase in blood pressure and emergence of insulin resistance (HOMA-IR), indicating successful development of MetS. Contrarily, glucose levels remained unaltered, indicating nondiabetic MetS. Although lipoprotein profiles in pigs have similarities to those in humans, HDL levels are elevated in MetS pigs, possibly due to decreased cholesteryl ester transfer protein activity (2, 9), which facilitates transport of cholesteryl esters and triglycerides between the lipoproteins. Cardiac function remained unaltered, possibly due to the short duration and young age of the animals. Nevertheless, a high-cholesterol/high-carbohydrate diet for 16 wk sufficed to develop MetS and promoted subendocardial microvascular rarefaction and endothelial dysfunction. Therefore, our MetS model is useful to study the molecular mechanisms and underlying pathways implicated in the initial development of MetS-induced myocardial vascular damage.
It is well known that hypertension develops more rapidly and becomes more severe in male animals than in female animals in several models of the disease. Previous studies in rats have shown that female animals are more protected against the prooxidant effect of a high-sucrose diet (5). Likewise, acetylcholine-induced endothelium-dependent relaxation is more pronounced in female compared with male spontaneously hypertensive rat aortas (23), suggesting that female animals are more protected from the effects of MetS compared with male animals and less prone to develop endothelial dysfunction. Therefore, in the present study, we opted to use female pigs to test whether the deleterious effects of MetS on the myocardial microvasculature outweigh this sex-based protection. Indeed, we found that 16 wk of high-fat/high-fructose diet decreased subendocardial microvascular density and impaired coronary endothelial function in female pigs, underscoring considerable myocardial microvasculature damage in early MetS.
Importantly, to avoid variability due to estrous cycle (4), we studied premenstrual female domestic pigs.
This study has some caveats. Our study design did not allow us to define the effects of individual components of MetS on the myocardial microvasculature. Given that systemic treatment with ELAM attenuated cardiac EC injury and conferred significant vasculoprotection, it is likely to have exerted protective effects on other organs, such as the skeletal muscle (30, 39), although its effects on the skeletal muscle microvasculature remain unknown. We have recently shown in swine MetS that mitoprotection with ELAM preserves renal structure and function (17) as well as the renal microvasculature. Therefore, despite preserved SVR, MetS induces EC injury and microvascular remodeling in the heart and kidneys of MetS pigs, which can be improved by chronic cardiolipin restoration. Further studies with longer observation periods may be needed assess the myocardial microvasculature and effectiveness of ELAM in MetS.
In summary, the present study shows that chronic treatment with ELAM preserved coronary EC mitochondria and decreased vascular injury. ELAM-induced vasculoprotection was reflected in improved subendocardial microvascular density, decreased coronary retention of EPCs, and decreased release of markers of vascular injury. However, ELAM partly improved eNOS expression, coronary endothelial function, and vessel maturity, and myocardial perfusion remained blunted. Hence, the mitochondrion may exert a more prominent influence on coronary microvascular structural integrity than on its function. These observations underscore the role of mitochondria in regulating the coronary circulation in experimental MetS and the benefits of mitoprotection to preserve EC integrity and myocardial microvascular density. However, additional mitochondria-independent mechanisms likely contribute to MetS-induced cardiac vascular injury.
GRANTS
This work was supported by a grant from Stealth BioTherapeutics and National Institutes of Health Grants DK-106427, DK-104273, HL-123160, and DK-102325.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.O.L. and A.E. conceived and designed research; F.Y., A.F.H., C.M.F., and A.E. performed experiments; F.Y., A.F.H., C.M.F., and A.E. analyzed data; F.Y., A.L., L.O.L., and A.E. interpreted results of experiments; F.Y. and A.E. prepared figures; F.Y. and A.E. drafted manuscript; A.F.H., C.M.F., A.L., L.O.L., and A.E. edited and revised manuscript; F.Y., A.F.H., C.M.F., A.L., L.O.L., and A.E. approved final version of manuscript.
REFERENCES
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645, 2009. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Barter PJ, Ha YC, Calvert GD. Studies of esterified cholesterol in sub-fractions of plasma high density lipoproteins. Atherosclerosis 38: 165–175, 1981. doi: 10.1016/0021-9150(81)90113-1. [DOI] [PubMed] [Google Scholar]
- 3.Barth E, Stämmler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol 24: 669–681, 1992. doi: 10.1016/0022-2828(92)93381-S. [DOI] [PubMed] [Google Scholar]
- 4.Breedt E, Lacerda L, Essop MF. Trimetazidine therapy for diabetic mouse hearts subjected to ex vivo acute heart failure. PLoS One 12: e0179509, 2017. doi: 10.1371/journal.pone.0179509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busserolles J, Mazur A, Gueux E, Rock E, Rayssiguier Y. Metabolic syndrome in the rat: females are protected against the pro-oxidant effect of a high sucrose diet. Exp Biol Med (Maywood) 227: 837–842, 2002. doi: 10.1177/153537020222700918. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1366: 139–149, 1998. doi: 10.1016/S0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Somaratne JB, Prior DL, Yii M, Kenny JF, Newcomb AE, Kelly DJ, Black MJ. Obesity is associated with lower coronary microvascular density. PLoS One 8: e81798, 2013. doi: 10.1371/journal.pone.0081798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogle CR, Wainman DA, Jorgensen ML, Guthrie SM, Mames RN, Scott EW. Adult human hematopoietic cells provide functional hemangioblast activity. Blood 103: 133–135, 2004. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Tall AR, Daemen MJAP, Falk E, Fisher EA, García-Cardeña G, Lusis AJ, Owens AP III, Rosenfeld ME, Virmani R; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Basic Cardiovascular Sciences . Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 37: e131–e157, 2017. doi: 10.1161/ATV.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 10.Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS, Textor SC, Lerman A, Lerman LO. Restoration of mitochondrial cardiolipin attenuates cardiac damage in swine renovascular hypertension. J Am Heart Assoc 5: e003118, 2016. doi: 10.1161/JAHA.115.003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014. doi: 10.1093/cvr/cvu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Gloviczki ML, Tang H, Gössl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 34: 540–548a, 2013. doi: 10.1093/eurheartj/ehs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 27: 4153–4161, 2012. doi: 10.1093/ndt/gfs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eirin A, Lerman A, Lerman LO. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J 35: 3258–3266, 2014. doi: 10.1093/eurheartj/ehu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC, Lerman LO. A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60: 1242–1249, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199919. [DOI] [PubMed] [Google Scholar]
- 16.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO. Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens 32: 154–165, 2014. doi: 10.1097/HJH.0b013e3283658a53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eirin A, Woollard JR, Ferguson CM, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO. The metabolic syndrome induces early changes in the swine renal medullary mitochondria. Transl Res 184: 45–56, 2017. doi: 10.1016/j.trsl.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eirin A, Zhu XY, Ferguson CM, Riester SM, van Wijnen AJ, Lerman A, Lerman LO. Intra-renal delivery of mesenchymal stem cells attenuates myocardial injury after reversal of hypertension in porcine renovascular disease. Stem Cell Res Ther 6: 7, 2015. doi: 10.1186/scrt541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fornoni A, Raij L. Metabolic syndrome and endothelial dysfunction. Curr Hypertens Rep 7: 88–95, 2005. doi: 10.1007/s11906-005-0080-6. [DOI] [PubMed] [Google Scholar]
- 20.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 119: 812–819, 2006. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Gössl M, Mödder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, Lerman LO, Khosla S, Lerman A. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J 31: 2909–2914, 2010. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res 51: 762–766, 2001. doi: 10.1016/S0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 23.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension 25: 517–523, 1995. doi: 10.1161/01.HYP.25.4.517. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt TL, Oltman CL. Progression and reversal of coronary and mesenteric vascular dysfunction associated with obesity. Obesity (Silver Spring) 22: 2193–2200, 2014. doi: 10.1002/oby.20837. [DOI] [PubMed] [Google Scholar]
- 25.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH III, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J Am Heart Assoc 1: e001644, 2012. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res 112: 1171–1188, 2013. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Münzel T, Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20: 247–266, 2014. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labazi H, Trask AJ. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol Res 123: 114–121, 2017. doi: 10.1016/j.phrs.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension 51: 127–133, 2008. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Kaneki M, Andreas J, Tompkins RG, Martyn JA. Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 36: 580–585, 2011. doi: 10.1097/SHK.0b013e3182366872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ZL, Ebrahimi B, Zhang X, Eirin A, Woollard JR, Tang H, Lerman A, Wang SM, Lerman LO. Obesity-metabolic derangement exacerbates cardiomyocyte loss distal to moderate coronary artery stenosis in pigs without affecting global cardiac function. Am J Physiol Heart Circ Physiol 306: H1087–H1101, 2014. doi: 10.1152/ajpheart.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luu B, Leistner DM, Herrmann E, Seeger FH, Honold J, Fichtlscherer S, Zeiher AM, Assmus B. Minute myocardial injury as measured by high-sensitive troponin t serum levels predicts the response to intracoronary infusion of bone marrow-derived mononuclear cells in patients with stable chronic post-infarction heart failure: insights from the TOPCARE-CHD registry. Circ Res 120: 1938–1946, 2017. doi: 10.1161/CIRCRESAHA.116.309938. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 16: 1–12, 2015. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 34.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 346: 1948–1953, 2002. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 35.Pawar AS, Zhu XY, Eirin A, Tang H, Jordan KL, Woollard JR, Lerman A, Lerman LO. Adipose tissue remodeling in a novel domestic porcine model of diet-induced obesity. Obesity (Silver Spring) 23: 399–407, 2015. doi: 10.1002/oby.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 17: 2202–2208, 2003. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 37.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail 9: e002206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahrivari M, Wise E, Resende M, Shuster JJ, Zhang J, Bolli R, Cooke JP, Hare JM, Henry TD, Khan A, Taylor DA, Traverse JH, Yang PC, Pepine CJ, Cogle CR; Cardiovascular Cell Therapy Research Network (CCTRN) . Peripheral blood cytokine levels after acute myocardial infarction: IL-1β- and IL-6-related impairment of bone marrow function. Circ Res 120: 1947–1957, 2017. doi: 10.1161/CIRCRESAHA.116.309947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 12: 763–771, 2013. doi: 10.1111/acel.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050, 2014. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urbieta Caceres VH, Lin J, Zhu XY, Favreau FD, Gibson ME, Crane JA, Lerman A, Lerman LO. Early experimental hypertension preserves the myocardial microvasculature but aggravates cardiac injury distal to chronic coronary artery obstruction. Am J Physiol Heart Circ Physiol 300: H693–H701, 2011. doi: 10.1152/ajpheart.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson SH, Best PJ, Lerman LO, Holmes DR Jr, Richardson DM, Lerman A. Enhanced coronary vasoconstriction to oxidative stress product, 8-epi-prostaglandinF2 alpha, in experimental hypercholesterolemia. Cardiovasc Res 44: 601–607, 1999. doi: 10.1016/S0008-6363(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 43.Wilson SH, Simari RD, Best PJ, Peterson TE, Lerman LO, Aviram M, Nath KA, Holmes DR Jr, Lerman A. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol 21: 122–128, 2001. doi: 10.1161/01.ATV.21.1.122. [DOI] [PubMed] [Google Scholar]
- 44.Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol 18: 1209–1217, 2007. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]