Abstract
Clinical studies have suggested that myocardial iron is a risk factor for left ventricular remodeling in patients after myocardial infarction. Ferroptosis has recently been reported as a mechanism of iron-dependent nonapoptotic cell death. However, ferroptosis in the heart is not well understood. Mechanistic target of rapamycin (mTOR) protects the heart against pathological stimuli such as ischemia. To define the role of cardiac mTOR on cell survival in iron-mediated cell death, we examined cardiomyocyte (CM) cell viability under excess iron and ferroptosis conditions. Adult mouse CMs were isolated from cardiac-specific mTOR transgenic mice, cardiac-specific mTOR knockout mice, or control mice. CMs were treated with ferric iron [Fe(III)]-citrate, erastin, a class 1 ferroptosis inducer, or Ras-selective lethal 3 (RSL3), a class 2 ferroptosis inducer. Live/dead cell viability assays revealed that Fe(III)-citrate, erastin, and RSL3 induced cell death. Cotreatment with ferrostatin-1, a ferroptosis inhibitor, inhibited cell death in all conditions. mTOR overexpression suppressed Fe(III)-citrate, erastin, and RSL3-induced cell death, whereas mTOR deletion exaggerated cell death in these conditions. 2′,7′-Dichlorodihydrofluorescein diacetate measurement of reactive oxygen species (ROS) production showed that erastin-induced ROS production was significantly lower in mTOR transgenic versus control CMs. These findings suggest that ferroptosis is a significant type of cell death in CMs and that mTOR plays an important role in protecting CMs against excess iron and ferroptosis, at least in part, by regulating ROS production. Understanding the effects of mTOR in preventing iron-mediated cell death will provide a new therapy for patients with myocardial infarction.
NEW & NOTEWORTHY Ferroptosis has recently been reported as a new form of iron-dependent nonapoptotic cell death. However, ferroptosis in the heart is not well characterized. Using cultured adult mouse cardiomyocytes, we demonstrated that the mechanistic target of rapamycin plays an important role in protecting cardiomyocytes against excess iron and ferroptosis.
Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/mtor-prevents-ferroptosis-in-cardiomyocytes/.
Keywords: cardiomyocyte, ferroptosis, iron, mechanistic target of rapamycin
INTRODUCTION
Adverse left ventricular (LV) remodeling is a major cause toward the pathogenesis of heart failure (HF) after acute myocardial infarction (MI). Clinical studies using cardiovascular magnetic resonance have suggested that myocardial iron around the infarct zone is a risk factor for adverse LV remodeling after acute MI in patients treated with a reperfusion therapy by percutaneous coronary intervention (9). While the cytotoxicity of excess iron is well known in the heart and many other cases, effective therapies that specifically target and manage excess iron in acute MI do not currently exist.
Iron is the most abundant transition metal in the human body and is important for numerous biological reactions (25). However, excessive iron is toxic to cells, mainly via iron-mediated formation of reactive oxygen species (ROS) by the Fenton reaction (45). Cardiac dysfunction is a prominent feature of iron overload diseases (36). Ferroptosis, which has recently been discovered as a novel type of regulated cell death, is dependent on intracellular iron and is distinct from apoptosis, necrosis, and autophagy (15). Class 1 ferroptosis inducers, including erastin and sulfasalazine, cause ferroptotic cell death by system Xc− inhibition that reduces intracellular glutathione (GSH) content and results in inactivating glutathione peroxidase-4 (GPX4), a lipid hydroperoxide detoxifying enzyme (10, 15, 50). Class 2 ferroptosis inducers, which directly inhibit GPX4 enzymatic activity without depleting intracellular GSH, include Ras-selective lethal 3 (RSL3) and DPI7 (also known as ML162) (10, 15, 50). Loss of GPX4 activity induces ROS production from lipid hydroperoxidation and eventually causes cell death (10, 50). Iron accelerates the lipid hydroperoxidation reaction and increases lipid ROS, which is suppressed by GPX4 in physiological setting (34, 49). Chelators such as 2,2-bipyridyl effectively inhibit erastin- and RSL3-induced ferroptosis (10, 15). A recent study (21) using ex vivo perfused hearts suggested that ferroptosis is a key toward the pathogenesis of ischemia-reperfusion (I/R) injury and that deferoxamine (DFO), a chelator, protects the heart against ex vivo I/R injury. However, the specific cellular mechanisms of ferroptosis in cardiomyocytes (CMs) are not well characterized.
Mechanistic target of rapamycin (mTOR) is a key effector in the insulin signaling pathway that regulates not only cell metabolism but cell survival (3, 44). Previously, we (1, 2) demonstrated that cardiac mTOR protects the heart against I/R injury. A previous report (5) showed that mTOR regulates iron homeostasis by modulating transferrin receptor 1 (TfR1) stability and altering cellular iron flux. Clinical studies have shown that chronic treatment with mTOR inhibitors such as sirolimus are associated with the development of microcytic anemia, suggesting that mTOR plays a key role in iron homeostasis (35, 39). However, the role of mTOR in cell survival against excess iron and ferroptosis in the heart remains undefined.
To explore the role of cardiac mTOR in iron-mediated cell death, we examined cell viability under excess iron and ferroptotic conditions using adult mouse CMs obtained from cardiac-specific mTOR transgenic (Tg) and knockout (KO) mice. We demonstrated that excess iron and ferroptosis inducers cause cell death and that a ferroptosis inhibitor, ferrostatin-1, prevents cell death caused by excess iron and ferroptosis inducers. We also show that mTOR overexpression inhibits cell death caused by excess iron and ferroptosis and that mTOR deletion exaggerates excess iron-induced and ferroptotic cell death. Our results indicate that mTOR is necessary and sufficient for preventing iron-mediated cell death.
MATERIALS AND METHODS
Animal models.
The animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the University of Hawaii (Honolulu, HI). Tg mice expressing hemagglutinin-tagged rat mTOR under the direction of the murine α-myosin heavy chain (α-MHC) promoter have been previously described in detail (43). mTOR Tg mice were back crossed to C57BL/6 mice for more than 18 generations. Male wild-type (WT) littermate or male age-matched WT mice were used as controls. For cardiac-specific KO (mTOR KO) mice, breeding pairs of floxed mTOR mice (mTORfl; B6.129S4-mTORtm1.2Koz/J) were obtained from Jackson Laboratories (Bar Harbor, ME) and inbred to generate mTORfl/fl mice. These mice were further inbred for more than 10 generations before use. These mice contain loxP sites that flank exons 1–5 of the mTOR gene (19). Since these exons contain the transcription start site, Cre-mediated deletion of these exons results in the loss of mTOR. CM-specific mTOR KO mice were then generated by crossing homozygous mTORfl/fl mice with homozygous transgenic mTORfl/fl mice expressing tamoxifen-inducible Cre recombinase fused to two mutated estrogen receptors under the transcriptional control of the α-MHC promoter (α-MHCmerCremer, Jackson Laboratory). Knockdown of mTOR was induced by administering tamoxifen chow (Harlan Laboratories, Indianapolis, IN) to male mice aged 6–8 wk. The chow consisted of 250 mg/kg tamoxifen, which provided 40 mg tamoxifen/kg body wt, assuming a normal 20- to 25-g body wt and 3- to 4-g intake. Mice were kept on this diet for 2 wk before a normal chow diet was resumed. Male age-matched Cre-negative (Cre−) mice were used as controls.
In vivo I/R model.
Male C57BL/6 mice aged 12–14 wk were subjected to I/R as previously described (2). Briefly, animals were anesthetized with ketamine + sevoflurane, intubated, and ventilated. A left thoracotomy was performed, and the left anterior descending coronary artery (LAD) was ligated with 8-0 silk sutures. After 30 min of ischemia, the LAD ligature was released, and reperfusion was visually confirmed. Operated mice were euthanized 1 wk after I/R for histological analysis for the fibrotic area, iron, and ferritin deposition (see below).
Histology.
Midventricular short-axis heart sections (5 µm) from I/R model hearts were fixed in 4% paraformaldehyde and prepared for staining with Masson’s trichrome (Sigma-Aldrich, St. Louis, MO) to visualize fibrosis (43) or Prussian blue (free iron stain, Sigma-Aldrich) to examine iron deposition. Immunohistochemical stains were performed using primary antibodies directed against ferritin (Novus Biologicals, Littleton, CO). Signals were enhanced with an ABC kit (Vector Laboratories, Burlingame, CA).
CM isolation and culture.
Left ventricular CMs were isolated with the perfused heart method and cultured in special medium for adult mouse CMs, as previously described (32). Briefly, after mTOR Tg, mTOR KO, or control male mice (12–20 wk of age) had been deeply anesthetized, hearts were quickly excised, cannulated via the aorta, and perfused at constant flow. Hearts were first perfused for 2–3 min at 37°C with a Ca2+-free bicarbonate-based buffer containing (in mM) 120 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, 10 2,3-butanedione monoxime (Sigma-Aldrich), and 5 taurine (Sigma-Aldrich) followed by collagenase buffer containing 0.4 mg/ml collagenase type B (Roche, Indianapolis, IN), 0.3 mg/ml collagenase type D (Roche), and 0.03 mg/ml protease type XIV (Sigma-Aldrich). After collagenase and protease digestion, CMs were isolated by mechanically pulling them apart and filtered using a sterile 250-µm filter top. CMs were transferred to 0.06, 0.24, 0.6, and 1.2 mM Ca2+ transfer buffer step by step every 10 min, and an appropriate amount of rod-shaped CMs was then suspended in plating medium [medium 199 (Sigma-Aldrich), 5% FBS, 25 μM blebbistatin (Cayman Chemical, Ann Arbor, MI), 100 U/ml penicillin, and 100 μg/ml streptomycin] and plated onto laminin (Invitrogen, Carlsbad, CA)-coated 12-well plates. Two hours after incubation in the plating medium, CMs were cultured in culture medium [medium 199, 100 μg/ml BSA, insulin (5 mg/l)-transferrin (5 mg/l)-sodium selenite (5 μg/l) media supplement (ITS; Sigma-Aldrich), 25 μM blebbistatin, 100 U/ml penicillin, and 100 μg/ml streptomycin].
Ferric iron-citrate, erastin, RSL3, and ferrostatin-1 treatment.
Sixteen to twenty-four hours after the start of the CM cell culture, the culture medium was replaced with fresh culture medium with the reagents described below. CMs were exposed to reagents for 8–24 h. Ferric iron [Fe(III)-citrate (C6H5FeO7, Sigma-Aldrich)] was initially dissolved in 1× PBS and diluted to a final concentration of 0.1–2 mM. Erastin (Tocris Bioscience, Minneapolis, MN) was initially dissolved in DMSO (Sigma-Aldrich) and diluted to a final concentration of 20–50 μM. RSL3 (Selleck Chemicals, Houston, TX) was initially dissolved in DMSO and diluted to a final concentration of 1 μg/ml. Ferrostatin-1 (Sigma-Aldrich) was initially dissolved in DMSO and diluted to a final concentration of 10 μM.
Live/dead cell viability assay.
After experimental treatment conditions were finished, cells were stained with buffer containing 2 μM calcein AM (Life Technologies, Carlsbad, CA) and 4 μM ethidium homodimer-1 (Life Technologies) dissolved in maintenance medium for isolated CMs. Cells were stained for 30 min at room temperature. Live cells were visualized as green with excitation at 494 nm and emission at 517 nm, and dead cell nuclei were visualized as red with excitation at 517 nm and emission at 617 nm on an Olympus IX71 fluorescence microscope.
ROS production assay.
ROS production was measured as previously described (23). After treatment with Fe(III)-citrate or ferroptosis inducers, cells were treated with 25 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Life Technologies) in maintenance medium. After cells had been incubated at 37°C for 30 min, they were washed with PBS and replaced with medium without H2DCFDA. Intracellular ROS production was measured by detecting fluorescence of the fluorescent oxidized 2′,7′-dichlorofluorescein (DCF) byproduct with excitation at 495 nm and emission at 529 nm.
Western blot analysis.
Mouse hearts and other organs were harvested, snap frozen, and crushed in liquid nitrogen. The tissue was then homogenized in cold lysis buffer (Cell Signaling, Danvers, MA) as previously described (1, 2). Isolated CMs were snap frozen in liquid nitrogen. Cold lysis buffer was added, and the lysate was collected. Protein concentrations were measured by the Bradford method (Bio-Rad, Hercules, CA). SDS-PAGE was performed under reducing conditions on 4–20% gradient gels (Bio-Rad). Proteins were transferred to a PVDF membrane (Bio-Rad). After being blocked with 1% fish gelatin buffer (Sigma-Aldrich), membranes were incubated overnight at 4°C with primary antibodies. Blots were then incubated with donkey anti-rabbit IRDye-680RD or donkey anti-mouse IRDye-800CW secondary antibodies (LI-COR) for 1 h. Signals were visualized on an Odyssey scanner (LI-COR). Primary antibodies against cleaved caspase-3 (Cell Signaling), light chain 3B (LC3B; Cell Signaling), mTOR (Cell Signaling), Akt (Cell Signaling), phospho-Akt (Ser473, Cell Signaling), S6 ribosomal protein (Cell Signaling), phospho-S6 ribosomal protein (Ser235/236, Cell Signaling), hemagglutinin (Santa Cruz Biotechnology, Dallas, TX), TfR1 (ThermoFisher Scientific, Waltham, MA), ferroportin (Novus Biologicals), and GAPDH (Santa Cruz Biotechnology) were used for immunoblot analysis.
Statistical analysis.
Data are means ± SE. Group differences were analyzed using a two-tailed Student’s t-test or Welch’s t-test. For all analyses, P values of <0.05 were considered significant.
RESULTS
Iron induces CM cell death.
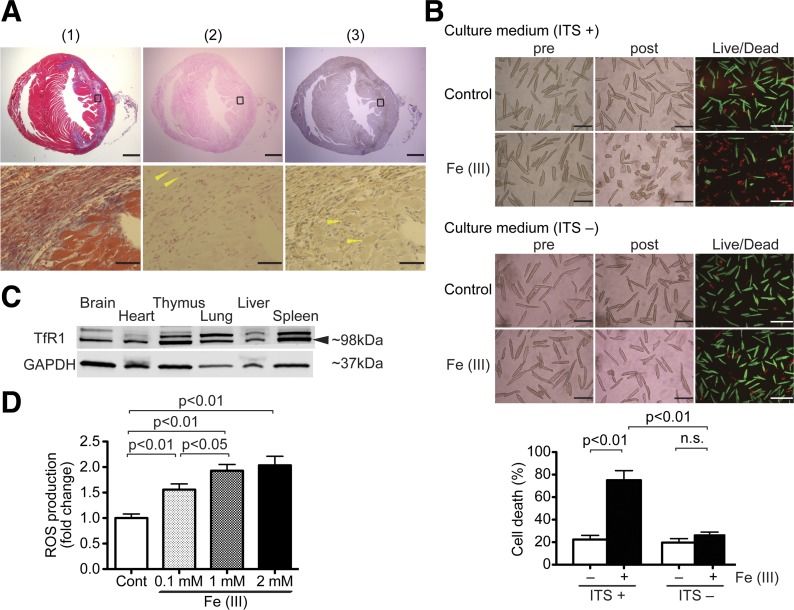
To assess iron accumulation in the heart after I/R injury, we examined the level of myocardial iron in mouse models of I/R injury generated by 30-min LAD ligation. As reported in our previous papers (1, 2, 27, 30), Masson’s trichrome staining showed that fibrotic scars extended from the initial infarct to the remote zone along myofibers in the midcardium (Fig. 1A-1). Prussian blue staining showed that iron was accumulated mainly in non-CMs, including endothelial cells, macrophages, and fibroblasts (Fig. 1A-2). To study cellular iron accumulation in CMs, we assessed cellular ferritin, a ubiquitous intracellular protein that stores iron (25). Immunohistochemistry with anti-ferritin staining demonstrated that ferritin accumulated along the scar area after I/R injury (Fig. 1A-3). Ferritin was stained in both CM and also non-CM cells (Fig. 1A-3). These findings suggest that iron accumulated in CMs around the myocardial scar in post-I/R hearts.
Fig. 1.
Excess iron induces cardiomyocyte (CM) cell death. A: iron and ferritin accumulation in the mouse heart after ischemia-reperfusion (I/R) injury. This is a representative sample from 6 mice at 1 wk post-I/R injury. All samples displayed a similar staining pattern to the one shown here. Top, Masson’s trichrome staining (1), fibrotic areas stained with blue (2), and iron staining (3). Anti-ferritin-positive cells are stained with brown (anti-ferritin staining). Scale bars = 1 mm. Bottom, magnified images of regions indicated by squares in each heart section of the top images (1), Masson’s trichrome staining and iron staining (2), and free iron-positive cells stained with blue (3); arrowheads indicate iron-positive non-CMs. Scale bars = 50 µm. B: excess iron-induced CM cell death. Top, representative images of CM cell death induced by Fe(III)-citrate in the presence or absence of insulin-transferrin-sodium selenite (ITS) in culture medium. Adult mouse CMs isolated from wild-type (WT) mice were exposed to 1 mM Fe(III)-citrate or 1 mM sodium citrate for 24 h. Morphological changes in the CM cell culture were assessed before applying the agents (pre) and at the end of experiments (post). Live/dead assays, in which live cells stain with calcein AM (green) and nuclei of dead cells stain with ethidium homodimer-1 (red), were used to assess cell viability. This is a set of representative images among 4 independent experiments. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Total numbers of live cytosol (green) and dead nuclei (red) cells were counted in three low-power fields. More than 500 cells were counted in each condition. The percent cell death was calculated as dead cells/total cells; n = 4. C: representative immunoblots of transferrin receptor 1 (TfR1) expression in mouse organs. Arrow indicates immunoblots of TfR1. Blots are representative from 3 independent WT mice. D: reactive oxygen species (ROS) production after Fe(III)-citrate treatment in WT CMs. ROS production in WT CMs exposed to 0.1, 1, or 2 mM Fe(III)-citrate or control buffer (Cont) for 24 h was measured by 2′,7′-dichlorodihydrofluorescein diacetate; n = 6. n.s., Not significant.
Fig. 2.
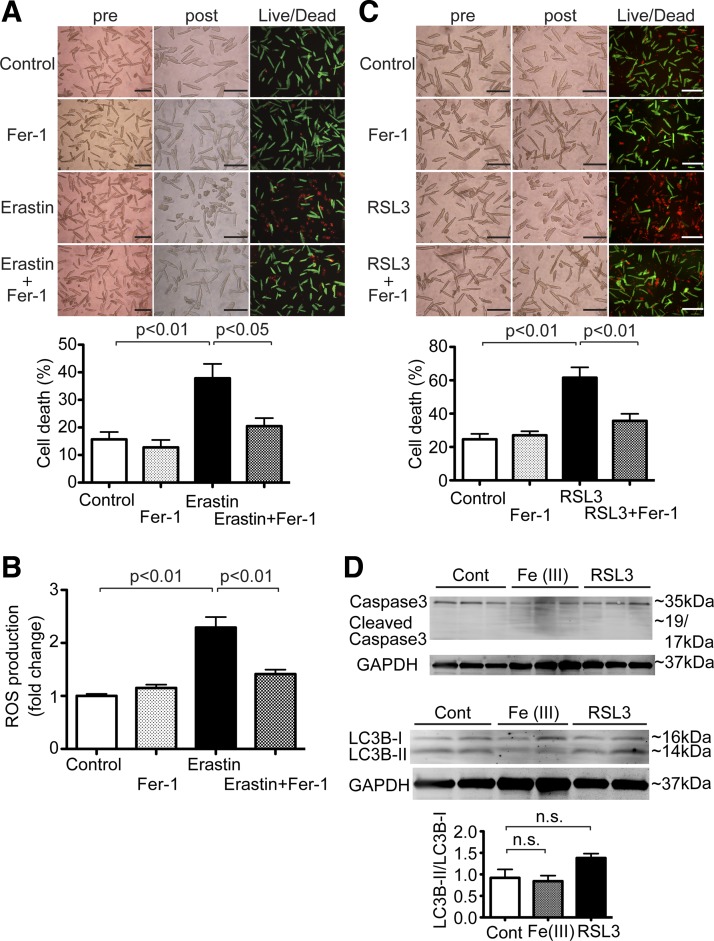
Ferroptosis in cardiomyocytes (CMs). A: erastin-induced CM cell death. Top, representative images of CM cell death induced by erastin. Adult mouse CMs isolated from wild-type (WT) mice were treated with 50 μM erastin or vehicle control in the presence or absence of 10 μM ferrostatin-1 (Fer-1) for 16 h. Morphological changes in CM cell culture were assessed before applying the agents (pre) and at the end of experiments (post). Cell death was assessed by a Live/Dead assay. Representative images are shown from 4 independent experiments. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1; n = 4. B: reactive oxygen species (ROS) production after erastin treatment in WT CMs. ROS production was assessed in WT CMs treated with 50 μM erastin or vehicle control in the presence or absence of 10 μM Fer-1 for 16 h. ROS production was measured by 2′,7′-dichlorodihydrofluorescein diacetate; n = 5. C: Ras-selective lethal 3 (RSL3)-induced CM cell death. Top, representative images of CM cell death induced by RSL3. Adult mouse CMs isolated from WT mice were treated with 1 μg/ml RSL3 or vehicle control in the presence or absence of 10 μM Fer-1 for 16 h. Representative images are shown from 6 independent experiments. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1; n = 6. D: expression levels of cleaved caspase-3 and light chain (LC)3B in WT CMs treated with vehicle control (Cont), 1 mM Fe(III)-citrate, or 1 μg/ml RSL3 for 16 h. Top, representative immunoblots of cleaved caspase-3 and LC3B in CMs. Bottom, densitometric quantitation of LC3B expression. Data are ratios of LC3B-II to LC3B-I. Control, n = 3; Fe(III), n = 3; RSL3, n = 3.
Fig. 3.
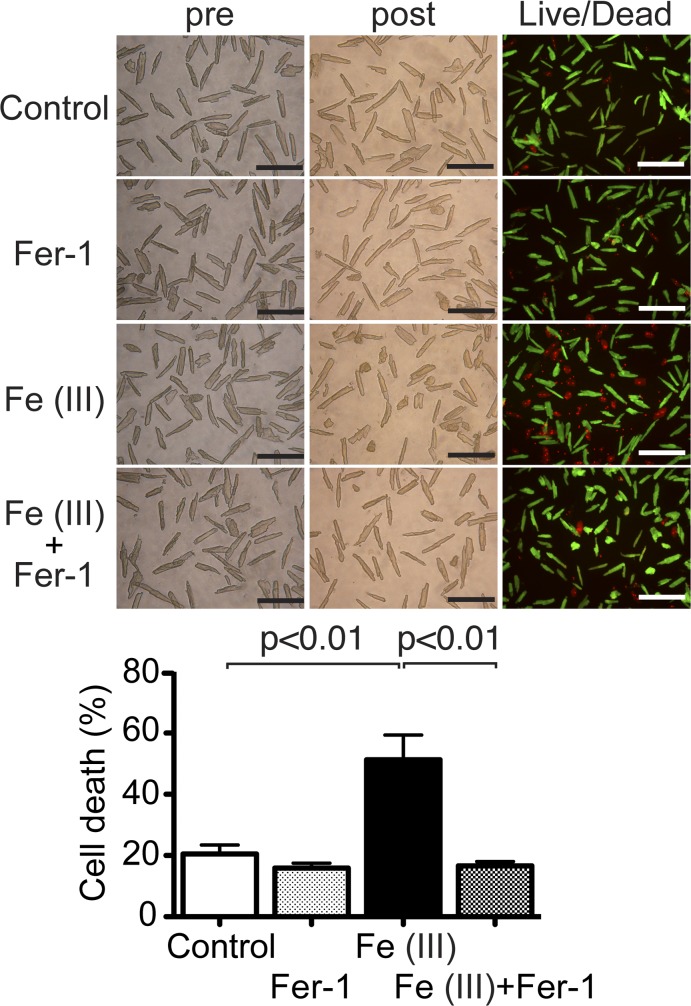
Ferrostatin-1 (Fer-1), a lipid reactive oxygen species (ROS) scavenger, inhibits excess iron-induced cell death in cardiomyocytes (CMs). Top, representative images of CM cell death induced by excess iron. Isolated CMs from wild-type (WT) adult mice were exposed to 1 mM Fe(III)-citrate or vehicle control for 24 h. To test the contribution of lipid ROS to iron-induced cell death, CMs in each group were cotreated with or without 10 μM Fer-1. Morphological changes in CM cell culture were assessed before applying agents (pre) and at the end of experiments (post). Cell death was assessed by Live/Dead assay. Representative images are shown from 5 independent experiments. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1; n = 5.
To investigate the effects of excess iron on CM cell death, adult CMs from WT mice were treated with 1 mM Fe(III)-citrate for 24 h. Live/dead viability assays, in which live cells stain with calcein AM (green) and nuclei of dead cells stain with ethidium homodimer-1 (red), demonstrated that Fe(III)-citrate significantly induced CM cell death compared with controls (Fig. 1B). This suggests that the iron accumulation observed in the I/R mouse model (Fig. 1A) induced CM cell death. Iron uptake into the cells is mostly regulated by TfR1, which is the form of transferrin-bound iron in the majority of cells. However, several tissues, including hepatocytes and cardiomyocytes, take up nontransferrin-bound iron (NTBI) without TfR1 regulation (8). For the adult mouse CM cultures in this study, we added the ITS media supplement, which contained 5 mg/l transferrin. Interestingly, there was no difference in cell death between Fe(III)-citrate treatment and vehicle control in the absence of ITS in the culture medium (Fig. 1B). Western blot analysis demonstrated that TfR1 is expressed in mice hearts (Fig. 1C). Those findings suggest that excess iron-induced cell death is caused by transferrin-bound iron via TfR1 and not by NTBI in adult mouse CMs. Excess iron is toxic to cells, mainly by iron-mediated formation of ROS via the Fenton reaction (45). Twenty-four hours after treatment with 0.1, 1, and 2 mM Fe(III)-citrate, we used H2DCFDA to measure ROS production in CMs from WT mice. We found that treatment with Fe(III)-citrate increased ROS generation in CMs in a dose-dependent manner (Fig. 1D).
Ferroptosis can be induced in CMs.
Erastin, a class 1 ferroptosis inducer, is a potent and selective inhibitor of system Xc− (cystine-glutamate antiporter) to induce ferroptosis (16). Erastin also induces cell death by directly targeting voltage-dependent anion channels 2 and 3 (VDAC2/3) (47). Ferroptosis is driven by the accumulation of toxic lipid ROS (10), and ferrostatin-1 is an inhibitor of ferroptosis and a lipid ROS scavenger (15). To test whether ferroptosis is induced in CMs, we treated adult mouse CMs with erastin for 16 h. Live/dead cell viability assays revealed that erastin significantly induced cell death compared with controls (P < 0.01; Fig. 2A). Cotreatment with ferrostatin-1 attenuated erastin-induced cell death (Fig. 2A). Sixteen hours after treatment with 40 µM erastin, we used H2DCFDA to measure ROS production in CMs from WT mice. We found that ROS generation was increased in erastin treatment compared with controls (P < 0.01; Fig. 2B). Cotreatment of erastin and ferrostatin-1 decreased ROS generation compared with erastin alone (Fig. 2B). RSL3, a class 2 ferroptosis inducer, induces ferroptosis by inactivating GPX4, an endogenous lipid ROS inhibitor, without upstream inhibition of system Xc− or VDAC2/3 (50). Treatment with RSL3 for 16 h induced cell death, and cotreatment with ferrostatin-1 inhibited ferroptotic cell death, much like ferrostatin-1’s effects on erastin treatment (Fig. 2C). To determine the type of cell death induced by excess iron or RSL3, we examined cleavage of caspase-3 for apoptosis (37) and expression levels of LC3B-II for autophagy (4). Neither excess iron nor RSL3 treatment increased cleavage of caspase3 (Fig. 2D), suggesting that iron-mediated cell death in adult CMs is nonapoptotic. Treatment with either excess iron or RSL3 did not change the ratio of LC3B-I and LC3B-II (Fig. 2D). Taken together, these findings showed that ferroptosis, a nonapoptotic form of regulated necrosis, occurred in adult CMs and that autophagy was not significantly activated in these settings.
Excess iron increases CM cell death and is inhibited by a lipid ROS scavenger.
A previous report (15) has demonstrated that ferrostatin-1 specifically inhibits erastin-induced ferroptosis by suppressing the function of the lipid repair enzyme GPX4. To test whether ferrostatin-1 protected CMs specifically against erastin-induced ferroptosis, but not excess iron-induced cell death, we treated CMs with both Fe(III)-citrate and ferrostatin-1. Interestingly, cotreatment with ferrostatin-1 inhibited Fe(III)-citrate-induced cell death (Fig. 3). This finding suggests that excess iron induces cell death, at least in part, by enhancing lipid ROS generation in adult CMs.
mTOR is necessary and sufficient in protecting against excess iron-induced CM cell death.
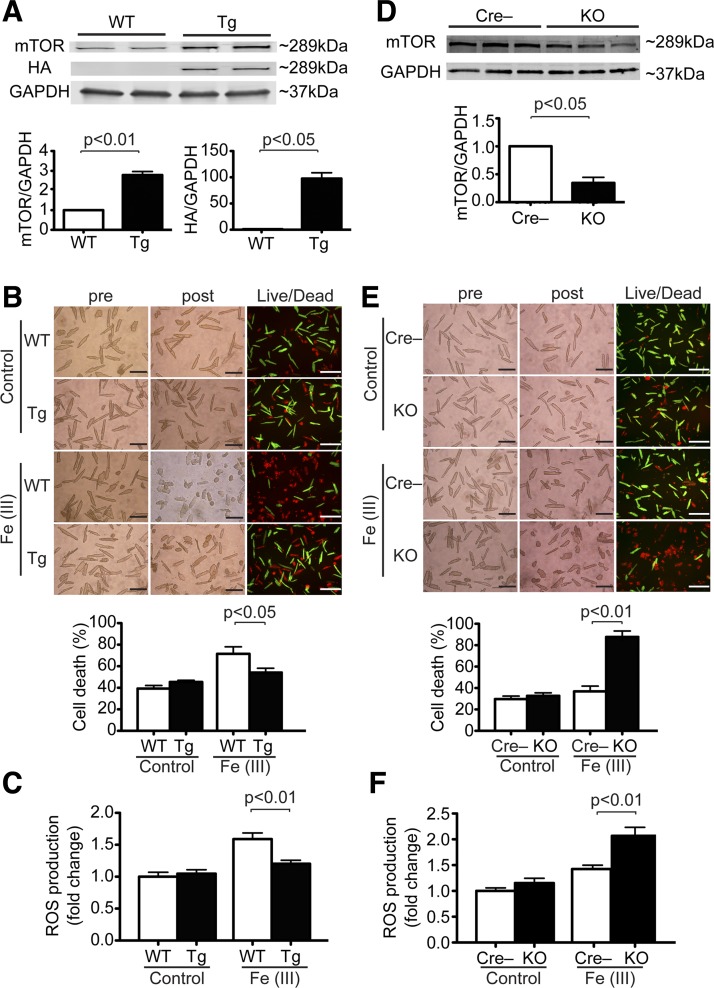
To test the role of mTOR on iron-mediated cell death in adult CMs, we used isolated CMs from cardiac-specific Tg mice overexpressing mTOR (mTOR Tg) or littermate control (WT) mice or cardiac-specific mTOR KO mice or Cre-negative control (Cre−) mice. As observed in our previous report (2), mTOR expression was about threefold higher in isolated CMs from mTOR Tg mice than from WT mice (Fig. 4A). Live/dead assays showed that treatment with Fe(III)-citrate for 24 h induced CM cell death and that mTOR overexpression attenuated Fe(III)-citrate-induced cell death (Fig. 4B). To assess the effects of mTOR on ROS production caused by excess iron, we used H2DCFDA to measure ROS production in CMs from WT or mTOR Tg mice after treatment with Fe(III)-citrate or control. We found that mTOR overexpression significantly inhibited iron-induced ROS production in CMs treated with Fe(III)-citrate (P < 0.01; Fig. 4C). These findings suggest that mTOR overexpression prevented iron-induced cell death, at least in part, by suppressing ROS production. To test the necessity of cardiac mTOR for cell survival against excess iron, CMs isolated from either Cre− or mTOR KO mice were exposed to Fe(III)-citrate for 24 h. mTOR expression in isolated CMs from mTOR KO mice was approximately one-third that in Cre− mice (Fig. 4D). Live/dead assays showed that significantly more CM cell death occurred in CMs from mTOR KO mice than from Cre− mice (Fig. 4E). Using DCFDA, we found that mTOR downregulation significantly increased iron-induced ROS production in CMs treated with Fe(III)-citrate (P < 0.01; Fig. 4F). These findings show that mTOR downregulation enhances Fe(III)-citrate-induced CM cell death, at least in part, by enhancing ROS production.
Fig. 4.
Mechanistic target of rapamycin (mTOR) protects against excess iron-induced cardiomyocyte (CM) cell death. A: expression levels of mTOR and hemagglutinin (HA) in CMs isolated from wild-type (WT) and mTOR transgenic (Tg) mice. Top, representative immunoblots of mTOR and HA in CMs. Blots are representative from 3 WT mice and 3 Tg mice. Bottom, densitometric quantitation of mTOR and HA expression. Data were normalized to GAPDH. WT, n = 3; Tg, n = 3. B: effects of excess iron on CMs from Tg mice. Adult CMs isolated from either WT or Tg mice were exposed to 2 mM Fe(III)-citrate or vehicle control for 24 h. Top, morphological changes in CM cell culture were assessed before applying the agents (pre) and at the end of experiments (post). Cell death was assessed by Live/Dead assay. Representative images are shown from 6 WT mice and 6 Tg mice. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1. WT, n = 6; Tg, n = 6. C: ROS production in WT and Tg CMs treated with 1 mM Fe(III)-citrate or vehicle control for 8 h. ROS production was measured by 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). WT, n = 4; Tg, n = 4. D: expression levels of cardiac mTOR in mTOR knockout (KO) mice. Top, immunoblots of mTOR in CMs isolated from Cre-negative control (Cre−) and mTOR KO mice. Blots are representative from 3 Cre− mice and 3 KO mice. Bottom, densitometric quantitation of mTOR expression in CMs in KO mice. Data were normalized to GAPDH. Cre−, n = 3; KO, n = 3. E: effects of excess iron in CMs from KO mice. Adult CMs isolated from either Cre− or KO mice were exposed to 1 mM Fe(III)-citrate or vehicle control for 24 h. Top, morphological changes and Live/Dead assay. Representative images are shown from 3 Cre− mice and 3 KO mice. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1. Cre−, n = 3; KO, n = 3. F: ROS production in Cre− and KO CMs treated with 1 mM Fe(III)-citrate or vehicle control for 8 h. ROS production was measured by H2DCFDA. Cre−, n = 3; KO, n = 3.
mTOR is necessary and sufficient against ferroptotic CM cell death.
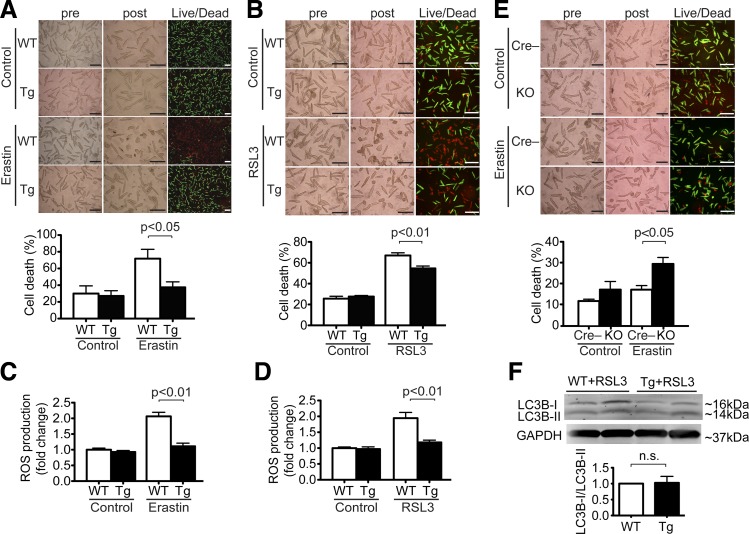
Although a recent study (51) showed that cellular cysteine and its derivative GSH cooperate to regulate mTOR and ferroptosis pathways, the relation between mTOR signaling and ferroptosis is not fully characterized. To test the sufficiency of cardiac mTOR in cell survival against ferroptosis, CMs isolated from either WT or mTOR Tg mice were exposed to erastin or RSL3 for 16 h. Live/dead assays showed that less CM cell death occurred in CMs from mTOR Tg mice than from WT mice (Fig. 5, A and B). These findings suggest that mTOR overexpression protects CMs against ferroptosis. We also found that mTOR overexpression significantly inhibited erastin- and RSL3-induced ROS production in adult CMs (Fig. 5, C and D). To test the necessity of cardiac mTOR in cell survival against ferroptosis, CMs isolated from either Cre− or mTOR KO mice were exposed to erastin for 16 h. Live/dead assays showed that significantly more CM cell death occurred in CMs from mTOR KO mice than from Cre− mice (Fig. 5E).
Fig. 5.
Mechanistic target of rapamycin (mTOR) protects cardiomyocytes (CMs) against ferroptosis. A: effects of erastin in CMs from mTOR transgenic (Tg) mice. Adult CMs isolated from wild-type (WT) or Tg mice were treated with 40 μM erastin or vehicle control for 16 h in CMs. Top, morphological changes in CM cell culture were assessed before applying agents (pre) and at the end of experiments (post). Cell death was assessed by Live/Dead assay. Representative images are shown from 5 WT mice and 4 Tg mice. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1. WT, n = 5; Tg, n = 4. B: effects of Ras-selective lethal 3 (RSL3) in CMs from Tg mice. Adult CMs isolated from WT or Tg mice were treated with 1 μg/ml RSL3 or vehicle control for 16 h in CMs. Top, morphological changes and Live/Dead assay. Representative images are shown from 6 WT mice and 6 Tg mice. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1. WT, n = 6; Tg, n = 6. C: reactive oxygen species (ROS) production in WT and Tg CMs treated with 40 μM erastin or vehicle control for 8 h. ROS production was measured by 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). WT, n = 6; Tg, n = 6. D: ROS production in WT and Tg CMs treated with 1 μg/ml RSL3 or vehicle control for 8 h. ROS production was measured by H2DCFDA. WT, n = 3; Tg, n = 3. E: effects of erastin in CMs from mTOR knockout (KO) mice. Adult CMs isolated Cre-negative control (Cre−) and KO mice were treated with 20 μM erastin or vehicle control for 16 h in CMs. Top, morphological changes and Live/Dead assay. Representative images are shown from 6 Cre− mice and 6 KO mice. Scale bars = 200 µm. Bottom, quantitative data of CM cell death. Percent cell death was calculated as described in Fig. 1. Cre−, n = 6; KO, n = 6. F: expression levels of light chain (LC)3B in CMs from WT and Tg mice treated with 1 μg/ml RSL3 for 16 h. Top, representative immunoblots of LC3B in CMs. Bottom, densitometric quantitation of LC3B expression. Data are ratios of LC3B-II to LC3B-I. WT, n = 3; Tg, n = 3.
mTOR inhibits autophagy mainly through phosphorylation of Unc-51-like kinase (ULK) (28). We assessed the possible contribution of autophagy to mTOR-mediated ferroptosis inhibition in CMs by measuring LC3 byproducts. There was no significant difference in the ratio of LC3B-II to LC3B-I between RSL3-treated CMs from WT mice and from Tg mice (Fig. 5F). Taken together, these findings suggest that mTOR is necessary and sufficient to protect CMs against ferroptotic cell death without affecting traditional autophagy pathways.
The mTOR signaling pathway and cellular iron regulation.
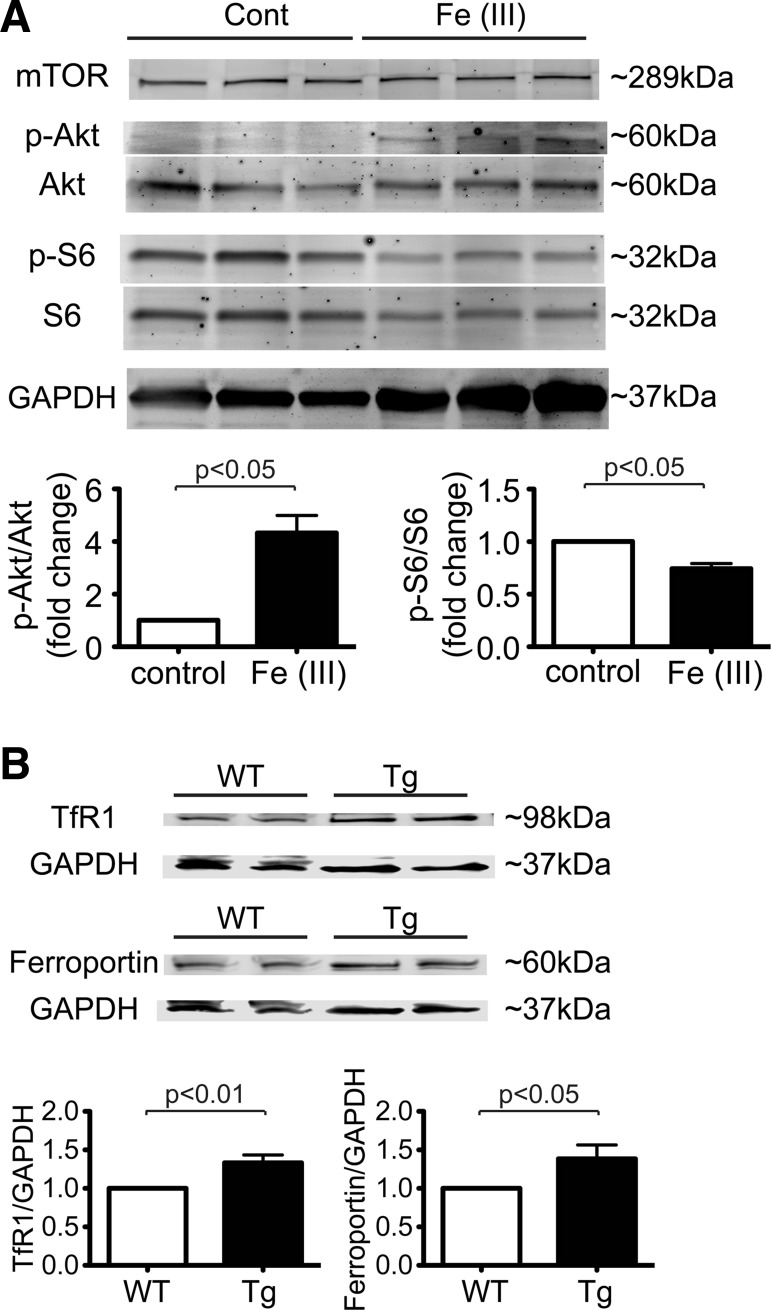
To determine mechanisms of mTOR in iron-mediated cell death, we examined activity in the mTOR signaling pathway. Interestingly, phosphorylation of Akt increased in CMs treated with excess iron (Fig. 6A). On the other hand, phosphorylation levels of S6 were decreased in excess iron compared with controls. Those findings suggest that in CMs treated with excess iron, mTOR complex 1 (mTORC1) was suppressed, whereas mTORC2 was activated.
Fig. 6.
Mechanistic target of rapamycin (mTOR) increases the expression levels of transferrin receptor 1 (TfR1) and ferroportin. A, top: representative immunoblots of mTOR signaling in cardiomyocytes (CMs). CMs isolated from wild-type (WT) mice were treated with 1 mM Fe(III)-citrate or vehicle control (Control) for 16 h. Immunoblot analysis was performed with the indicated antibodies. Bottom, densitometric quantitative analyses of phosphorylated (p-)Akt and p-S6 are shown with the ratio of p-Akt to total Akt (Akt) and the ratio of p-S6 to total S6 (S6). Data were normalized to those of control cells. Control, n = 3; Fe(III), n = 3. B, top: representative immunoblots of TfR1 and ferroportin in hearts harvested from WT and mTOR transgenic (Tg) mice without additional treatments. Blots are representative of 6 WT mice and 6 Tg mice for TfR1 and 9 WT mice and 9 Tg mice for ferroportin. Bottom, densitometric quantitation of TfR1 and ferroportin expression. Data were normalized to GAPDH. n = 6 WT mice and n = 6 mice for TfR1; n = 9 WT mice and n = 9 Tg mice for ferroportin.
A previous study (22) has demonstrated that mTOR plays an important role in systemic iron metabolism through mTOR targeting multiple proteins that regulate cellular iron transport. mTOR is especially important in regulating cellular iron homeostasis at the cellular level and does so primarily by regulating TfR1, an iron uptake receptor, and then by enhancing expression of ferroportin, an iron export protein (5). We assessed key proteins that mediate iron homeostasis in adult CMs from mTOR mice. Although there was no difference in ferritin expression between mTOR Tg and WT whole hearts under normal conditions, expression levels of TfR1 and ferroportin in the heart were much higher in mTOR Tg mice than in WT mice (Fig. 6B). These results imply that in CMs overexpressing mTOR, iron turnover is enhanced, with sustained homeostasis of cellular iron levels.
DISCUSSION
In the present study, we show that excess iron and two ferroptosis inducers, erastin and RSL3, cause cell death in isolated adult mouse CMs. Ferroptosis was recently introduced as an iron-dependent form of regulated cell death that is distinct from apoptosis, necrosis, and autophagy (15). We observed that the ferroptosis inhibitor ferrostatin-1 protects CMs against not only ferroptosis but also excess iron-induced cell death. We also demonstrated that cardiac mTOR is necessary and sufficient to protect CMs against those conditions.
A clinical study (9) using cardiac magnetic resonance showed that, in post-MI patients treated with reperfusion therapies, the presence of myocardial hemorrhage was associated with residual myocardial iron and LV remodeling. A previous report (38) has demonstrated iron deposition in peri-infarct and noninfarcted zones in in vivo mouse models of MI. Our study is consistent with those reports, as iron-positive staining was seen in non-CMs around the myocardial scar. We also found that expression of ferritin, an iron regulatory protein in the cell, increased in many CMs besides the infarct zone. Since the cellular level of iron regulates ferritin synthesis and degradation (13, 33), these findings suggest that iron overload occurs in CMs around the infarct zone in post-MI hearts. Interestingly, a small clinical study (11) with DFO, an iron chelator, in acute MI patients with percutaneous coronary intervention demonstrated no significant reduction in MI size or the 3-mo cumulative incidence of heart failure. On the other hand, a report (12) using in vivo animal models of I/R injury showed that 2,2-bipyridyl, a cell-permeable iron chelator, preserved cardiac function against I/R injury, whereas no significant effects were observed in the DFO-treated group. To define the role of chelators on cardiac function and cardiovascular events post-MI, understanding the mechanisms of iron-mediated cell viability is essential.
While ferroptosis is defined as an iron-dependent nonapoptotic form of cell death in multiple cell lines (14), ferroptosis in CMs has not been characterized well. Erastin, a class 1 ferroptosis inducer, induces cell death by inhibiting system Xc− (16) and binding with VDAC2/3 (47). As previously reported, there is no expression of system Xc− in the heart (29); nevertheless, we found that erastin still induced cell death in CMs. This suggests that the effects of erastin on ferroptosis are probably due to inhibition of VDAC rather than system Xc− inhibition. RSL3, a class 2 ferroptosis inducer, inhibits GPX4, an endogenous lipid ROS inhibitor, which results in the induction of ferroptosis (49). Our present study using adult mouse CMs demonstrated that erastin and RSL3 induced CM cell death and that ferrostatin-1 prevented cell death against both classes of ferroptosis inducers. Our findings suggest that ferroptosis occurs in adult CMs in a manner similar to that seen in other cell types. To assess cell death in this study, we used Live/death cell viability assays, in which ethidium homodimer-1 is taken up through permeabilized membranes in necrotic cells (48). As seen in other cell lines (24), ferroptosis inducer did not cleave caspase-3, suggesting that ferroptosis in adult CMs is independent of apoptosis. On the other hand, a previous report (24) by another group showed that an autophagy inhibitor did not suppress erastin-induced ferroptotic cell death. Our experiments are consistent with that study, as we did not see autophagic activity during ferroptotic cell death in adult mouse CMs. A recent study (20) showed that in other cells ferroptosis is an autophagic cell death process. Our current results suggest that may not be the case with adult CMs, but further studies will better define the role of autophagy, if any, on ferroptosis in CMs.
Ferroptosis is considered a type of nonapoptotic cell death without iron overload (15). A previous study (46) showed that, in the liver, iron overload increases the expression of Slc7a11, a major component of system Xc−, and that iron overload-induced liver damage was rescued by ferrostatin-1. Ferrostatin-1 inhibits lipid peroxidation but not mitochondrial ROS formation or lysosomal membrane permeability (42). Therefore, ferrostatin-1 is considered a ferroptosis inhibitor. Our data showed that both excess iron and ferroptosis inducers caused CM cell death, and those effects are inhibited by ferrostatin-1. In the present study, we demonstrated an increase in total ROS generation. Although we tried to assess lipid ROS byproducts such as 4-hydroxynonenal and malondialdehyde (12), we could not see a sufficient amount of them due to the limited amount of cells yielded from the adult CM culture. However, the significant effect of ferrostatin-1 on inhibition of ROS (Fig. 2B) suggests that lipid ROS plays an important role in pathophysiological features caused by excess iron and ferroptosis inducers. A previous report (21) has demonstrated that transferrin and TfR1 are required for ferroptosis. We observed that TfR1 was expressed in the heart at comparable levels with the liver. Since Fe(III) bound to citrate is the major form of NTBI in plasma (17), we used Fe(III)-citrate to study the effects of excess iron. While NTBI is readily taken up by certain cell types, including hepatocytes and CMs (18), our data suggested that transferrin-bound Fe(III) seems to be a major trigger for excess iron-induced cell death and for ferroptosis. Even though the role of iron in ferroptosis remains unclear, those findings suggest that excess iron-induced cell death shares common cell death mechanisms with ferroptotic cell death in CMs, including acceleration of lipid ROS generation and iron incorporation via TfR1. Since ferroptosis is classified as a form of regulated cell death, our findings could provide a new therapeutic strategy for preventing CM cell death resulting from triggers such as hemochromatosis and MI.
We observed that cardiac mTOR overexpression attenuated both excess iron-induced cell death and ferroptosis and was accompanied by a decrease in ROS production. Previously, we have reported that adverse left ventricular remodeling in in vivo I/R injury was significantly suppressed in the mTOR Tg mice that were used in this study (2), suggesting that the cardioprotective role of cardiac mTOR in left ventricular remodeling was caused, at least in part, by suppressing excess iron-induced cell death and ferroptosis. In addition, we showed that mTOR downregulation enhanced excess iron-induced cell death and ferroptosis in CMs. These results indicate that mTOR is necessary to protect CMs against ferroptotic cell death. mTOR forms two complexes, mTORC1 and mTORC2, both of which have distinct mechanisms and effects (44). mTORC1 is rapamycin sensitive and phosphorylates p70S6K and 4E-binding protein-1. In contrast, mTORC2 is the kinase responsible for the phosphorylation and activation of Akt. Our study showed that in CMs, excess iron suppressed mTORC1 signaling, whereas mTORC2 activation was increased. As described above, a report (20) has shown that autophagic signaling mediates ferroptosis in some settings. Since the mTORC1 signaling pathway suppresses autophagy, inhibition of mTORC1 by excess iron may be a key pathophysiological change in cell death via activation of autophagy. However, we did not observe significant change in LC3B-II in this study. Further studies are required to define the role of autophagy in ferroptosis.
A previous report (5) has shown that rapamycin, a mTORC1 inhibitor, primarily reduces the expression of TfR1, followed by a reduction in cellular iron levels, activation of the iron regulatory protein-1/2 (IRP1/2) system, and compensatory downregulation of ferroportin to restore iron balance. In our study, the expression of TfR1 and ferroportin was higher in mTOR Tg mice than in WT mice. Ferroportin is the only known mammalian iron-exporting protein and is important for cellular iron homeostasis in the heart (31). This suggests that in mTOR Tg mice, iron turnover is accelerated and leads to cellular iron reduction due to increased iron export. In other words, mTOR regulates iron homeostasis in the heart and has cardioprotective effects against ferroptosis. On the other hand, mTOR KO mice and control mice did not have a significant difference in expression of TfR1 and ferroportin in this study (data not shown). Since the hearts and their cellular iron-regulatory proteins in this study were under physiological conditions without iron overload, expression levels of TfR1 and ferroportin might not have been changed in mTOR KO mice. Further studies for understanding the role of mTOR in cellular iron regulation are required.
Mitochondrial permeability transition pore (mPTP)-dependent necrosis, a type of regulated necrosis, is defined as the formation of the mPTP and subsequent permeabilization of the inner mitochondrial membrane (26). While Ca2+ overload in mitochondria is a major trigger of mPTP opening (7), overproduction of ROS also induces prolonged mPTP opening (41). Many reports have shown that accelerated iron uptake leads to excess ROS levels due to the Fenton reaction (45). Although the role of mTOR in regulated mPTP-dependent necrosis in CMs is not well characterized, previous reports have demonstrated that mTOR is located in the mitochondrial outer membrane and is directly or indirectly bound to key proteins in cell survival and metabolism, such as VDAC1 and Bcl-xL (6, 40). Those findings suggest that mTOR might protect the heart by reduction of ROS not only at cellular lipid membranes but also at mitochondria. Further studies to assess the role of mTOR in mitochondrial ROS production are necessary.
In conclusion, ferroptosis and excess iron are significant causes of cell death in CMs, and mTOR plays an important role in preventing these types of ROS-mediated cell death, at least in part by regulating iron metabolism. Understanding the effects of mTOR and other signaling pathways in preventing iron-mediated cell death will provide a new approach for patients with acute MI.
GRANTS
This work was supported, in part, by a research grant from Kochi Organization for Medical Reformation and Renewal, Japan (to Y. Baba), a research grant from the Mitsukoshi Health and Welfare Foundation, Japan (to T. Suhara), National Institutes of Health (NIH) Training Grant T32-HL-115505 (to B. K. Shimada) and Grants P30-GM-103341 and P20-GM-113134 (to T. Matsui) and G12-MD-007601 to the Histopathology Core, University of Hawaii at Manoa.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.B. and T.M. conceived and designed research; Y.B., J.K.H., B.K.S., K.M.H., J.D.W., H.A., and K.S.M. performed experiments; Y.B., J.K.H., K.M.H., and T.M. analyzed data; Y.B., J.K.H., and T.M. interpreted results of experiments; Y.B., J.K.H., B.K.S., K.M.H., and T.M. prepared figures; Y.B., J.K.H., and T.M. drafted manuscript; Y.B., J.K.H., B.K.S., T.S., M.K., H.K., and T.M. edited and revised manuscript; Y.B., J.K.H., B.K.S., K.M.H., T.S., M.K., J.D.W., H.A., K.S.M., H.K., and T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Toshinori Aoyagi for technical assistance.
REFERENCES
- 1.Aoyagi T, Higa JK, Aoyagi H, Yorichika N, Shimada BK, Matsui T. Cardiac mTOR rescues the detrimental effects of diet-induced obesity in the heart after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 308: H1530–H1539, 2015. doi: 10.1152/ajpheart.00008.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303: H75–H85, 2012. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyagi T, Matsui T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Curr Pharm Des 17: 1818–1824, 2011. doi: 10.2174/138161211796390976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol 221: 117–124, 2010. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayeva M, Khechaduri A, Puig S, Chang HC, Patial S, Blackshear PJ, Ardehali H. mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab 16: 645–657, 2012. doi: 10.1016/j.cmet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN. mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci USA 110: 12526–12534, 2013. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner C, Moulin M. Physiological roles of the permeability transition pore. Circ Res 111: 1237–1247, 2012. doi: 10.1161/CIRCRESAHA.112.265942. [DOI] [PubMed] [Google Scholar]
- 8.Brissot P, Ropert M, Le Lan C, Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820: 403–410, 2012. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA, Fontana M, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Kellman P, Moon JC, Hausenloy DJ. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging 9: e00940, 2016. doi: 10.1161/CIRCIMAGING.116.004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci 73: 2195–2209, 2016. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan W, Taylor AJ, Ellims AH, Lefkovits L, Wong C, Kingwell BA, Natoli A, Croft KD, Mori T, Kaye DM, Dart AM, Duffy SJ. Effect of iron chelation on myocardial infarct size and oxidative stress in ST-elevation-myocardial infarction. Circ Cardiovasc Interv 5: 270–278, 2012. doi: 10.1161/CIRCINTERVENTIONS.111.966226. [DOI] [PubMed] [Google Scholar]
- 12.Chang HC, Wu R, Shang M, Sato T, Chen C, Shapiro JS, Liu T, Thakur A, Sawicki KT, Prasad SV, Ardehali H. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol Med 8: 247–267, 2016. doi: 10.15252/emmm.201505748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Domenico I, Vaughn MB, Li L, Bagley D, Musci G, Ward DM, Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J 25: 5396–5404, 2006. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon SJ. Ferroptosis: bug or feature? Immunol Rev 277: 150–157, 2017. doi: 10.1111/imr.12533. [DOI] [PubMed] [Google Scholar]
- 15.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B III, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3: e02523, 2014. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans RW, Rafique R, Zarea A, Rapisarda C, Cammack R, Evans PJ, Porter JB, Hider RC. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem 13: 57–74, 2007. doi: 10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 18.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med 366: 348–359, 2012. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 19.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 24: 9508–9516, 2004. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res 26: 1021–1032, 2016. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59: 298–308, 2015. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan P, Wang N. Mammalian target of rapamycin coordinates iron metabolism with iron-sulfur cluster assembly enzyme and tristetraprolin. Nutrition 30: 968–974, 2014. doi: 10.1016/j.nut.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB. Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol 282: H656–H664, 2002. doi: 10.1152/ajpheart.00726.2001. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520: 57–62, 2015. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 283: 65–87, 2011. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Karch J, Molkentin JD. Regulated necrotic cell death: the passive aggressive side of Bax and Bak. Circ Res 116: 1800–1809, 2015. doi: 10.1161/CIRCRESAHA.116.305421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz MY, Kusakari Y, Aoyagi H, Higa JK, Xiao CY, Abdelkarim AZ, Marh K, Aoyagi T, Rosenzweig A, Lozanoff S, Matsui T. Three-dimensional myocardial scarring along myofibers after coronary ischemia-reperfusion revealed by computerized images of histological assays. Physiol Rep 2: e12072, 2014. doi: 10.14814/phy2.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 1512: 335–344, 2001. doi: 10.1016/S0005-2736(01)00338-8. [DOI] [PubMed] [Google Scholar]
- 30.Kusakari Y, Xiao CY, Himes N, Kinsella SD, Takahashi M, Rosenzweig A, Matsui T. Myocyte injury along myofibers in left ventricular remodeling after myocardial infarction. Interact Cardiovasc Thorac Surg 9: 951–955, 2009. doi: 10.1510/icvts.2009.206524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakhal-Littleton S, Wolna M, Carr CA, Miller JJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D, Stillion R, Holdship P, Larner F, Tyler DJ, Clarke K, Davies B, Robbins PA. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proc Natl Acad Sci USA 112: 3164–3169, 2015. doi: 10.1073/pnas.1422373112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao R, Jain M. Isolation, culture, and functional analysis of adult mouse cardiomyocytes. Methods Mol Med 139: 251–262, 2007. doi: 10.1007/978-1-59745-571-8_16. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Theil EC. Ferritins: dynamic management of biological iron and oxygen chemistry. Acc Chem Res 38: 167–175, 2005. doi: 10.1021/ar0302336. [DOI] [PubMed] [Google Scholar]
- 34.Magtanong L, Ko PJ, Dixon SJ. Emerging roles for lipids in non-apoptotic cell death. Cell Death Differ 23: 1099–1109, 2016. doi: 10.1038/cdd.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, Grandaliano G. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 82: 908–912, 2006. doi: 10.1097/01.tp.0000235545.49391.1b. [DOI] [PubMed] [Google Scholar]
- 36.Marsella M, Borgna-Pignatti C, Meloni A, Caldarelli V, Dell’Amico MC, Spasiano A, Pitrolo L, Cracolici E, Valeri G, Positano V, Lombardi M, Pepe A. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: a T2* magnetic resonance imaging study. Haematologica 96: 515–520, 2011. doi: 10.3324/haematol.2010.025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 6: 1028–1042, 1999. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 38.Omiya S, Hikoso S, Imanishi Y, Saito A, Yamaguchi O, Takeda T, Mizote I, Oka T, Taneike M, Nakano Y, Matsumura Y, Nishida K, Sawa Y, Hori M, Otsu K. Downregulation of ferritin heavy chain increases labile iron pool, oxidative stress and cell death in cardiomyocytes. J Mol Cell Cardiol 46: 59–66, 2009. doi: 10.1016/j.yjmcc.2008.09.714. [DOI] [PubMed] [Google Scholar]
- 39.Przybylowski P, Malyszko JS, Macdougall IC, Malyszko J. Iron metabolism, hepcidin, and anemia in orthotopic heart transplantation recipients treated with mammalian target of rapamycin. Transplant Proc 45: 387–390, 2013. doi: 10.1016/j.transproceed.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 40.Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci USA 106: 22229–22232, 2009. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schriewer JM, Peek CB, Bass J, Schumacker PT. ROS-mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J Am Heart Assoc 2: e000159, 2013. doi: 10.1161/JAHA.113.000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136: 4551–4556, 2014. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol 299: C1256–C1266, 2010. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suhara T, Baba Y, Shimada BK, Higa JK, Matsui T. The mTOR signaling pathway in myocardial dysfunction in type 2 diabetes mellitus. Curr Diab Rep 17: 38, 2017. doi: 10.1007/s11892-017-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90: 1–37, 2016. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, Li G, Yang L, Liu W, Min J, Wang F. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66: 449–465, 2017. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447: 865–868, 2007. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ 13: 1815–1826, 2006. doi: 10.1038/sj.cdd.4401813. [DOI] [PubMed] [Google Scholar]
- 49.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331, 2014. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol 26: 165–176, 2016. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu X, Long YC. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci Rep 6: 30033, 2016. doi: 10.1038/srep30033. [DOI] [PMC free article] [PubMed] [Google Scholar]