Abstract
Sex-related differences in cardiovascular health and disease have been identified, with males having a higher incidence of cardiovascular events but females more likely to develop arrhythmias. Adverse fetal environments are now accepted as a cause for the development of cardiovascular diseases in adulthood, but sex-related differences in response to adverse fetal environments have not been extensively explored. The combination of both in utero and postnatal exposure to inflammation is highly relevant for the infant that is born preterm or has clinical complications at birth or in early postnatal life. We have previously observed cardiac contractile deficiencies and dysregulation of Ca2+-handling proteins in our model of maternal lipopolysaccharide (LPS) and neonatal hyperoxia exposures (LPS/O2). This investigation tested the hypothesis that there are sex-related differences in the adult pathologies after exposure to perinatal inflammation. Using pressure-volume assessments, males exposed to LPS/O2 had more pronounced contractile deficiencies than similarly exposed females, but females tended to have long PR intervals. While both sexes demonstrated decreases in α-myosin heavy chain and connexin 43 after LPS/O2 exposure compared with saline/room air controls, females indicated aberrant increases in microRNA 208a, microRNA 208b, and desmin expression. Our study supports our hypothesis that early life exposure to inflammation results in sex-dependent deficits in cardiovascular function.
NEW & NOTEWORTHY Sex-specific differences in cardiovascular disease are recognized, but the mechanisms and origins are not well understood. Adverse maternal environments can influence cardiac development and later cardiovascular disease. This study identifies sex-dependent differences in cardiac disease associated with perinatal inflammation.
Keywords: desmin, microribonucleic acid 208, myosin heavy chain
INTRODUCTION
Sex-related differences in cardiovascular disease have been recognized for many years (16, 25). Under physiological conditions, female hearts are smaller and have reduced stroke volumes and thus lower cardiac output but have higher heart rates and performance indexes and are less controlled by the autonomic nervous system than males (11). Furthermore, sex has a profound impact on the response to cardiac insults, and females have a lower incidence of cardiovascular events than males but are more likely to develop arrhythmias (3). In fact, long QT is observed at much higher rates in females than males, and this is associated with increased arrhythmogenic risk, including syncope, ventricular fibrillation, and sudden cardiac death (3, 13).
The contractile efficiency of the heart is largely dependent on myosin heavy chain (MHC) proteins and their respective function (20). Recent studies have identified microRNA (miR)-208 as an important regulator of cardiac function and more specifically MHC expression (4, 6). Most importantly, miR-208 regulates the development of myoblasts into cardiomyocytes during the late stages of cardiac development (6). Cardiac arrhythmias have been linked to both over- and underexpression of miR-208, resulting in a prolonged PR interval (4). Although the mechanisms responsible are not yet well defined, disruptions in miR-208 cause changes in the expression of connexins and several essential cardiac transcription factors such as GATA4 and HOP (4). Overexpression of miR-208a leads to hypertrophy, increases in β-MHC expression, abnormalities in cardiac rhythm, and fibrosis (26).
Cytoskeletal proteins such as desmin are associated with cardiac remodeling and contractile dysfunction (22). Both human and animal studies have identified changes in desmin content as a marker of contractile deficiency and severity of disease (17, 18, 23). Increases in desmin contents may be maladaptive and further contribute to pathology or may be compensatory in an attempt to rescue the dysfunction.
The incidence of idiopathic cardiomyopathies in both sexes remains significant, and patients with dilated left ventricles (LV) commonly present with systolic dysfunction, resulting in congestive heart failure, but the origins of the pathology are not obvious. Perinatal perturbations to the fetal environment such as nutritional deficits, hypoxia, subclinical maternal inflammation, or even overt infection or illness can significantly influence cardiac development and function (9). Systemic sources of maternal inflammation can include periodontal (31), urinary tract (7), or respiratory (8) infections. Maternal infections are often subclinical and even undetected but can be substantial contributors to inflammatory responses that affect specific organs at critical windows in development in the offspring (1, 29). Although the inflammation is maternal in origin, the fetus is exposed to inflammatory mediators via the circulation, including cytokines, chemokines, and/or lipid mediators. The consequences of adverse environmental cues during critical developmental windows in the heart are poorly understood.
We have previously identified inflammatory and oxidant responses in the heart, liver, and lungs of newborn mice exposed to multiple perinatal stimuli (24, 27, 28). Newborn mice represent an immature physiology and are vulnerable to their perinatal environment. These studies investigated the effects of a subtle maternal systemic inflammation [intraperitoneal lipopolysaccharide (LPS)] such as found during maternal urinary or periodontal infections (1, 27) compounded by a relevant clinical intervention (85% O2) to identify the combined effects on sex-mediated differences in cardiac pathologies. In the present investigation, we analyzed sex-dependent differences in cardiomyopathy of the offspring at 10 mo of age. We hypothesized that the deficits in cardiac contraction previously observed in the LPS/O2-exposed mice are a result of abnormal development and/or defects in regulatory mechanisms related to the conduction and/or contractile machinery of the heart.
METHODS
Animal model.
All animals were handled in accordance with National Institutes of Health guidelines, and protocols were approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children’s Hospital (Columbus, OH). Male and female animals were paired, and the presence of a vaginal plug was designated as embryonic day (E) 1. On E16, dams were injected with LPS (80 µg/kg ip, serotype 0111:B4, no. 437627, Calbiochem) or an equal volume of saline. Newborn mice from saline- or LPS-injected dams were pooled and redistributed randomly to two dams (with similar E16 treatments) in separate cages within 12 h of birth. One litter of pups was exposed to 85% O2 for 2 wk and then returned to room air (RA), whereas the other litter of pups was maintained in RA. Body weights were recorded at the time of euthanasia (10 mo).
Pressure-volume loops.
A Millar conductance catheter was used to examine in vivo LV function. For this procedure, anesthetized mice (1.5% isoflurane) were ventilated, and a prewarmed conductance catheter probe was inserted in the right carotid artery and guided in the LV. Data collection was initiated after a 10/15-min time period for baseline stabilization. The Millar catheter uses conductance to determine relative volume units (RVU). After assessments, we converted RVU into ml using individual blood volumes. Measurements included end-systolic pressure (ESP), end-diastolic pressure (EDP), end-systolic volume (ESV), peak rate of pressure rise (dPmax), peak rate of pressure decline (dPmin), cardiac output (CO), ejection fraction (EF), and heart rate.
Quantitative real-time PCR.
Total RNAs were isolated from frozen LV tissue using an RNeasy Mini kit (Qiagen, Valencia, CA). cDNA was synthesized using a Maxima First Strand cDNA Synthesis Kit for quantitative RT-PCR (K1641, Thermo Scientific Fermentas, Glen Burnie, MD). Quantitative real-time PCR was performed using Maxima SYBR Green/ROX qPCR Master Mix (K0222, Thermo Scientific Fermentas) and the Mastercycler epgradient Realplex Real-Time PCR Detection System (Eppendorf, Hamburg, Germany). miR-208a and miR-208b were reverse transcribed using the Applied Biosystems High Capacity RNA-to-cDNA Kit. Quantitative PCR was performed using gene-specific Taqman Small RNA Assays and the Taqman Universal Master Mix III (Thermo Scientific Applied Biosystems, Foster City, CA) and normalized to β-actin.
Western immunoblot analysis.
LV tissues were homogenized, and proteins were separated on SDS-PAGE gels (75 μg) and transferred to PVDF membranes. Membranes were probed with antibodies to α-MHC (1:500, ab292, Abcam, Cambridge, MA), β-MHC [1:300, sc-71575 (6D592), Santa Cruz Biotechnology, Dallas, TX], connexin 43 (1:500, sc-9059, Santa Cruz Biotechnology), desmin (1:1,000, D1033, Sigma, St. Louis, MO), and species-specific secondary antibodies. Blots were developed using enhanced chemiluminescence (ECL Western Blotting Detection, GE Healthcare), and expression levels were quantified using Image Quant software (version 5.0, Molecular Dynamics, Sunnydale, CA). The density of the band for the protein of interest was normalized to the density of the total protein per lane using Ponceau stain.
Statistical analysis.
Data were tested for homogeneity of variances, log transformed when indicated, and analyzed by two-way ANOVA with Tukey post hoc analyses where appropriate using GraphPad PRISM 6 (GraphPad, La Jolla, CA). Data are expressed as means ± SE with significance noted at P < 0.05.
ARRIVE guidelines were followed for the experimental protocol, the interpretation of the data, and the writing of the manuscript.
RESULTS
Body and ventricular weights.
At 10 mo of age, animals were euthanized, and body weights and LV weights were obtained. There was an effect of sex on total body weights, with the saline/RA female animals being smaller than saline/RA male animals. There was also an effect of sex on LV/body weight, likely driven by the smaller body weights in female animals. Furthermore, there was an effect of LPS/O2 treatment on right ventricular weight to body weight and right ventricular-to-LV ratios (Table 1).
Table 1.
Body and ventricular weights
| Saline/Room Air |
Lipopolysaccharide/O2 |
||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | Two-Way ANOVA Effects | |
| Number of mice/group | 16 | 12 | 25 | 17 | |
| Body weight, g | 37 ± 1 | 34 ± 1 | 38 ± 1 | 35 ± 1 | P = 0.008 effect of sex |
| LV/body weight, mg/g | 2.91 ± 0.08 | 2.63 ± 0.15 | 2.90 ± 0.11 | 2.51 ± 0.13 | P = 0.009 effect of sex |
| RV/body weight, mg/g | 0.63 ± 0.03 | 0.61 ± 0.03 | 0.73 ± 0.03 | 0.67 ± 0.05 | P = 0.033 effect of treatment |
| RV-to-LV ratio | 0.22 ± 0.001 | 0.24 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.02 | P = 0.015 effect of treatment |
Values are means ± SE. LV, left ventricular; RA, right ventricular.
Pressure-volume loops.
Mice were anesthetized, and pressure-volume loop measurements were obtained as described above in methods. There were statistical differences due to sex in EDP, differences due to treatment in EDP, dPmax, dPmin, ESV, and EF, and interactions between treatment and sex in CO. Post hoc analyses indicated the male mice exposed to LPS/O2 were more severely affected by perinatal exposures than female mice (Table 2), and the deficits are in parameters of contractile function.
Table 2.
Pressure-volume loop measurements of cardiac function
| Saline/Room Air |
Lipopolysaccharide/O2 |
||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | Two-Way ANOVA Effects | |
| End-systolic pressure, mmHg | 94.30 ± 5.05 | 93.17 ± 4.10 | 84.77 ± 5.62 | 87.86 ± 3.78 | NS |
| End-diastolic pressure, mmHg | 2.86 ± 0.69 | 0.90 ± 0.15 | 1.16 ± 0.13* | 0.83 ± 0.05*# | P = 0.025 effect of treatment; P = 0.01 effect of sex; P = 0.04 interaction |
| Peak rate of pressure rise, mmHg/s | 10,283 ± 960.1 | 9,887 ± 710.1 | 6,226 ± 409.4* | 7,914 ± 638.7 | P = 0.001 effect of treatment |
| Peak rate of pressure decline, mmHg/s | −7,710 ± 424.6 | −7,788 ± 606.1 | −5,134 ± 544.8* | −6,661 ± 636.0 | P = 0.004 effect of treatment |
| End-systolic volume, µl | 37.79 ± 3.66 | 44.12 ± 2.92 | 60.14 ± 5.20* | 44.83 ± 6.46 | P = 0.024; P = 0.032 effect of treatment; interaction |
| Cardiac output, ml/min | 11.73 ± 1.38 | 8.84 ± 2.58 | 4.03 ± 1.51 | 10.34 ± 2.13 | P = 0.032 interaction |
| Ejection fraction, % | 37.53 ± 4.40 | 39.63 ± 3.95 | 26.70 ± 2.67 | 31.67 ± 1.97 | P = 0.014 effect of treatment |
| Heart rate, beats/min | 504 ± 20 | 481 ± 33 | 536 ± 32 | 497 ± 33 | NS |
Values are means ± SE; n = 5 mice/group. Pressure-volume loop measurements were conducted on 10-mo-old mice as described in methods. Data were analyzed by two-way ANOVA, and effects are indicated at the right. NS, not significant.
Difference in treatment, same sex;
difference in sex, same treatment (n = 4–6).
P to R wave distance from ECGs.
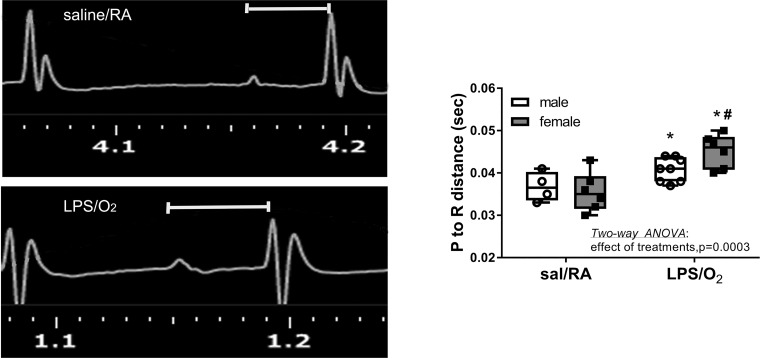
ECGs were assessed under sedation in spontaneously breathing mice and analyzed (Fig. 1). Whereas the P to R time ranged between 0.030 and 0.043 s in saline/RA control mice, this duration was extended to a range between 0.038 and 0.050 s in LPS/O2-treated mice with the longer times in female mice. Statistical differences were observed with LPS/O2 treatment, and post hoc analyses indicated individual differences between saline/RA and LPS/O2 treatment within the same sex.
Fig. 1.
ECG measurements. Distances from the P to R wave were measured in ECGs for male and female mice exposed to saline (sal)/room air (RA) and lipopolysaccharide (LPS)/O2. Data were analyzed by two-way ANOVA with Tukey’s post hoc analyses. Two-way ANOVA identified an effect of treatment, P = 0.0002, and individual differences were observed between saline/RA and LPS/O2 measurements, P < 0.05. n = 4–8 mice/group. *Different between treatments, same sex; #different between sexes, same treatment.
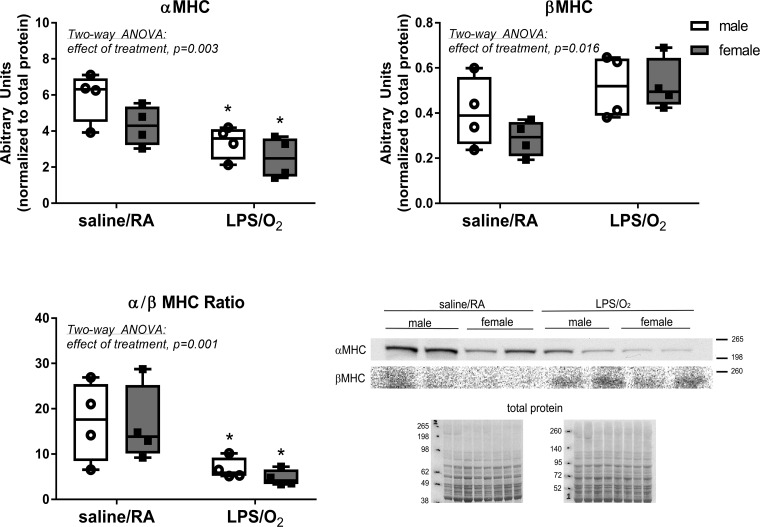
MHC protein.
LV MHC protein levels were measured by Western blot (Fig. 2). Analysis of α-MHC content indicated a decrease in LPS/O2-treated mice of both sexes with a statistical effect of treatment. Alternatively, β-MHC expression was modestly increased in LPS/O2-treated mice, also with a statistical effect of treatment.
Fig. 2.
Myosin heave chain (MHC) isoforms were determined by Western blot. Left ventricular homogenates were separated by SDS-PAGE, transferred to PVDF membranes, and probed with anti-α-MHC or anti-β-MHC antibodies. Membranes were quantified by densitometry. Data were analyzed by two-way ANOVA with Tukey’s post hoc analyses. Two-way ANOVA identified an effect of treatment, α-MHC, P = 0.003, and β-MHC, P = 0.016, and individual differences were observed between the saline/room air (RA) and lipopolysaccharide (LPS)/O2 measurements, P < 0.05. n = 4/group. *Different between treatments, same sex.
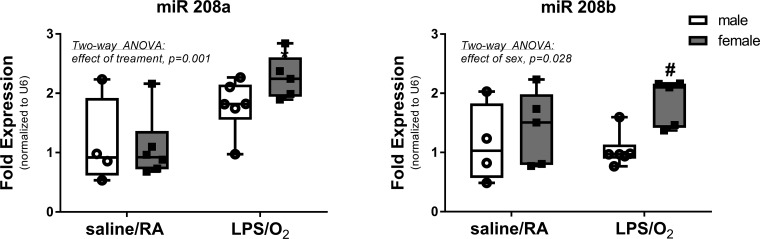
miR-208 expression.
miR-208a and miR-208b expressions were measured by RT-PCR in RNA isolations from the LV (Fig. 3). Statistical analyses indicated an effect of treatment in miR-208a, with greater expression in LPS/O2-treated female animals than LPS/O2-treated male and saline/RA-treated male or female animals. Analyses of miR-208b levels indicated an effect of sex, with female animals having higher levels than male animals in both groups.
Fig. 3.
Expression levels of micro-RNA (miR)-208 were measured by quantitative RT-PCR. RNA was isolated from left ventricles, and expression levels of both miR-208a and miR-208b were measured by PCR as described in methods. Data were analyzed by two-way ANOVA with Tukey’s post hoc analyses. Two-way ANOVA identified an effect of treatment, miR-208a, P = 0.0012, and an effect of sex miR-208b, P = 0.021, and individual differences were observed between the saline/room air (RA) and lipopolysaccharide (LPS)/O2 measurements, P < 0.05. n = 5–6/group. #Different between sexes, same treatment.
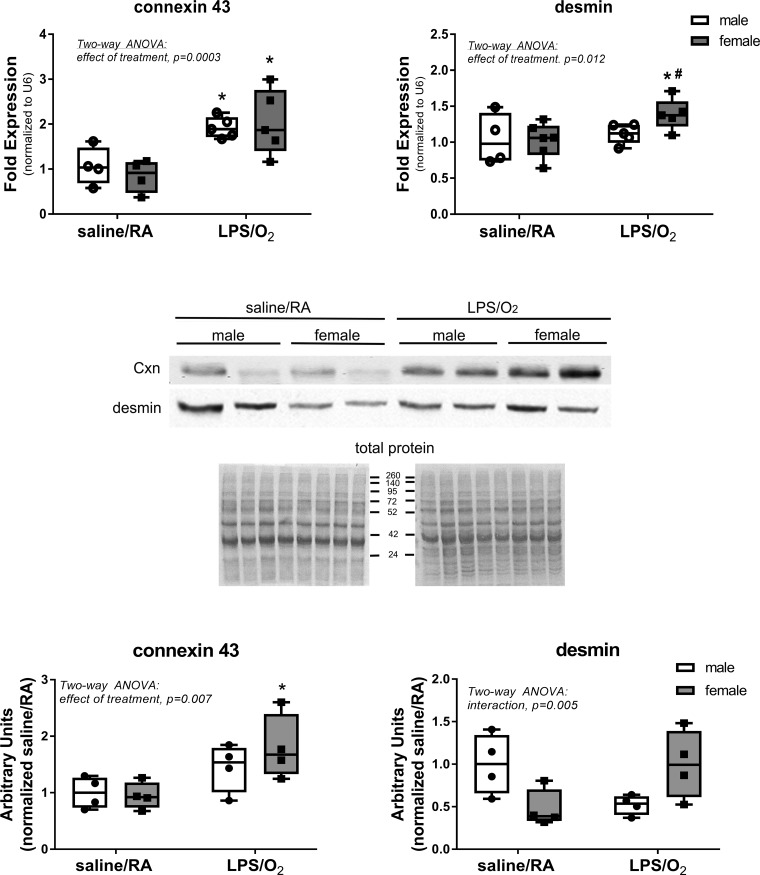
Conduction proteins.
Proteins that contribute to electrical conduction and structural integrity, connexin 43 and desmin, were assessed by RT-PCR and Western blot in the LV (Fig. 4). Connexin 43 message was elevated in both sexes, and protein levels were elevated in mice previously exposed to LPS/O2. Desmin mRNA expression was only increased in female mice previously exposed to LPS/O2, whereas LPS/O2-exposed male mice did not exhibit similar increases and desmin protein levels demonstrated lower levels in saline/RA-exposed female mice and higher levels in LPS/O2-exposed female mice than in similarly exposed male mice.
Fig. 4.
Expression levels of connexin (Cxn) 43 and desmin were measured by quantitative RT-PCR. RNA was isolated from left ventricles, and expression levels of both connexin 43 and desmin were measured by PCR as described in methods. Data were analyzed by two-way ANOVA with Tukey’s post hoc analyses. Two-way ANOVA identified an effect of treatment, connexin 43, P = 0.0003, and an effect of treatment, desmin, P = 0.011, and individual differences were observed between the saline/room air (RA) and lipopolysaccharide (LPS)/O2 measurements for connexin 43 and individual differences between the female LPS/O2 measurements and all other points, P < 0.05. n = 5–6/group. *Different between treatments, same sex; #different between sexes, same treatment.
DISCUSSION
In addition to acknowledged risk factors related to lifestyle, adverse fetal environments are now accepted as a cause for the development of cardiovascular diseases in adulthood (2, 10, 12, 14, 30). The combination of both in utero and postnatal exposure to inflammation is highly clinically relevant for the infant that is born preterm or has complications at birth and early postnatal life. The goal of this study was to test the hypothesis that perinatal exposure to inflammation leads to adult cardiomyopathies and that there are sex-dependent differences in pathologies. Our earlier study (27) in this mouse model revealed smaller birth weights in mice that were born to LPS-injected dams, but sex-specific differences in body weight were only observable at 10 mo (Table 1). No differences in LV weights were identified, but cardiac function was substantially compromized in LPS/O2-exposed mice as indicated by increases in EDP and ESV and decreases in dPmax, dPmin, CO, and EF in mice of both sexes (Table 2). However, contractile deficits as exhibited by increased dPmax, dPmin, CO, and EF measurements were significantly more pronounced in male than in female animals, indicating a more severe contractile dysfunction in male animals. An interesting finding was that EF in the control group at 10 mo of age was marginally in the range of heart failure. We speculate that this observation is related to aging, since few studies have used C3H/HeN mice in cardiovascular studies, but age-related declines have been observed in other strains (15).
Further analysis of the pressure-volume measurements revealed increased LV diameter and decreased LV contractility in LPS/O2-treated mice, but both parameters were more severe in male mice. Similar to others that have reported female mice maintain contractility while male mice demonstrate progressive declines and pathological cardiac remodeling in models of myocardial infarction, our results suggest that males are more prone to the development of cardiac dysfunction (3, 5). Furthermore, cardiac hypertrophy is distinctly different in males than females, with male hearts exhibiting more severe contractile dysfunction and increased fibrosis (3).
Animal models and clinical studies have clearly highlighted that the female sex is less affected by the exposure to cardiovascular risk factors or experimental cardiovascular disease. In particular, the incidences of cardiomyopathies are lower in females (3). However, the mechanisms responsible for these differences are not clear. The present data suggest that some cardiomyopathies may be induced through adverse environmental events occurring during fetal development, and, like other cardiovascular pathologies, males are also more susceptible to insults occurring during perinatal development.
Sex differences in arrhythmias are well documented and occur at a much higher rate in females (13). In line with previous findings, we observed a prolonged P to R interval in mice exposed to perinatal LPS/O2, and this finding was more pronounced in female mice than in male mice (Fig. 1). Measurements of action potential in isolated cardiomyocytes have identified sex differences in ion channels that alter both conductivity and contraction in the heart (13). In addition to ion channel changes, alterations in Ca2+-handling properties of cardiomyocytes are linked to early depolarization, long QT syndrome, and mechanical dysfunction (13).
Expression of MHC subunits is developmentally regulated, and inappropriate expression is associated with cardiomyopathies (4). Decreases in α-MHC and increases in β-MHC are associated with cardiac hypertrophy and contractile dysfunction in mice. We observed pronounced decrease in α-MHC and a modest increase in β-MHC in both male and female mice that had been previously exposed to LPS/O2 (Fig. 2). These changes caused a substantial shift in the α-to-β-MHC ratio, indicating severe myocardial pathology (Fig. 2). The balance between α- and β-MHC protein expression is largely regulated by miR-208a and miR-208b. Overexpression of miR-208a leads to hypertrophy, increases in β-MHC expression, abnormalities in cardiac rhythm, and fibrosis (26). Mice deficient in miR-208a exhibit abnormal ECG profiles with missing P waves or prolonged P to R intervals and severe arrhythmias. In addition, miR-208 regulates genes involved with conduction, specifically connexins. Furthermore, miR-208a levels in the bloodstream have been identified as a reliable biomarker for myocardial infarction (6). These findings have led to manipulation of miR-208a as a potential therapeutic target (19). Inhibition of miR-208a, using antagomirs, has resulted in blocking cardiac remodeling, inhibition of fibrosis, and improved heart function in hypertension-induced heart failure models (4, 19, 21). We observed substantial increases in miR-208a and miR-208b expression in LPS/O2-exposed mice (Fig. 3), and this was associated with decreases in α-MHC and increases in β-MHC, respectively. We also observed increases in connexin 43 in male and female mice; however, desmin message levels were elevated only in female mice after LPS/O2 exposure, and desmin protein levels demonstrated an inverse interaction (Fig. 4). These data imply that the functional pathologies associated with altered MHC expression are likely contributing to the contractile deficits shown in Table 2 but may also be integral to the conduction deficits shown in Fig. 1.
Clinical data revealed that increased desmin expression was observed in patients diagnosed with idiopathic dilated cardiomyopathy and compensated cardiac function, but desmin expression decreases with disease progression and ultimate cardiac failure (23). In our model, desmin message concentrations were increased in female mice but unchanged in male mice previously exposed to LPS/O2. Interestingly desmin protein levels in female mice tended to be lower than male mice in the control group and higher than male mice in the LPS/O2-exposed group. Whether increased expression of desmin is compensatory and protects females from contraction deficits or contributes to the changes in conduction, which is more severe in females, is unknown and beyond the scope of the present study. However, the possibility that desmin may be playing a significant role in cardiomyopathies originating from adverse perinatal environments is intriguing and a topic of further investigation.
In summary, the present study confirms the impact of perinatal perturbations on sex-specific cardiac dysfunction. Although in utero inflammation and postnatal oxygen exposure induce alterations in structural and contractile proteins, resulting in increased ECG durations and contractile dysfunction in both sexes, contractile dysfunction was more exaggerated in male mice. Molecular biological analyses revealed sex-related differences in expression levels of proteins involved in myocardial function and contraction, including increased desmin expression in female mice, potentially attenuating the development of cardiac dysfunction.
GRANTS
This work was supported by Grant VE 614/1–1 from the German Research Foundation (to M. Velten) and National Institutes of Health Grants R01-AT-006880 (to L. K. Rogers) and R01-ES-019923 (to L. E. Wold).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.V. and L.K.R. conceived and designed research; M.V., K.M.H., and L.K.R. performed experiments; M.V., K.M.H., and L.K.R. analyzed data; M.V., L.E.W., and L.K.R. interpreted results of experiments; M.V., K.M.H., L.E.W., and L.K.R. prepared figures; M.V. and L.K.R. drafted manuscript; M.V., L.E.W., and L.K.R. edited and revised manuscript; M.V., K.M.H., L.E.W., and L.K.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Maximilian Falinat.
REFERENCES
- 1.Bansal M, Khatri M, Kumar A, Bhatia G. Relationship between maternal periodontal status and preterm low birth weight. Rev Obstet Gynecol 6: 135–140, 2013. [PMC free article] [PubMed] [Google Scholar]
- 2.Bergvall N, Iliadou A, Johansson S, de Faire U, Kramer MS, Pawitan Y, Pedersen NL, Lichtenstein P, Cnattingius S. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation 115: 2931–2938, 2007. doi: 10.1161/CIRCULATIONAHA.106.674812. [DOI] [PubMed] [Google Scholar]
- 3.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res 118: 1294–1312, 2016. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772–2786, 2009. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci 75: 2181–2192, 2004. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J Mol Cell Cardiol 94: 107–121, 2016. doi: 10.1016/j.yjmcc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Freak-Poli R, Chan A, Tucker G, Street J. Previous abortion and risk of pre-term birth: a population study. J Matern Fetal Neonatal Med 22: 1–7, 2009. doi: 10.1080/14767050802531813. [DOI] [PubMed] [Google Scholar]
- 8.Getahun D, Ananth CV, Oyelese Y, Peltier MR, Smulian JC, Vintzileos AM. Acute and chronic respiratory diseases in pregnancy: associations with spontaneous premature rupture of membranes. J Matern Fetal Neonatal Med 20: 669–675, 2007. doi: 10.1080/14767050701516063. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev 81: 51–59, 2005. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Huxley R, Owen CG, Whincup PH, Cook DG, Colman S, Collins R. Birth weight and subsequent cholesterol levels: exploration of the “fetal origins” hypothesis. JAMA 292: 2755–2764, 2004. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- 11.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 31: 17–22, 2007. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koupilová I, Leon DA, McKeigue PM, Lithell HO. Is the effect of low birth weight on cardiovascular mortality mediated through high blood pressure? J Hypertens 17: 19–25, 1999. doi: 10.1097/00004872-199917010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lang CN, Menza M, Jochem S, Franke G, Perez Feliz S, Brunner M, Koren G, Zehender M, Bugger H, Jung BA, Foell D, Bode C, Odening KE. Electro-mechanical dysfunction in long QT syndrome: role for arrhythmogenic risk prediction and modulation by sex and sex hormones. Prog Biophys Mol Biol 120: 255–269, 2016. doi: 10.1016/j.pbiomolbio.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, Lithell UB, McKeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915−29. BMJ 317: 241–245, 1998. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S, Wang Y, Zhang X, Kong Q, Li C, Li Y, Ding Z, Liu L. HSP27 alleviates cardiac aging in mice via a mechanism involving antioxidation and mitophagy activation. Oxid Med Cell Longev 2016: 2586706, 2016. doi: 10.1155/2016/2586706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 17.Monreal G, Nicholson LM, Han B, Joshi MS, Phillips AB, Wold LE, Bauer JA, Gerhardt MA. Cytoskeletal remodeling of desmin is a more accurate measure of cardiac dysfunction than fibrosis or myocyte hypertrophy. Life Sci 83: 786–794, 2008. doi: 10.1016/j.lfs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Monreal G, Youtz DJ, Phillips AB, Eyman ME, Gorr MW, Velten C, Lucchesi PA, Wold LE, Gerhardt MA. Right ventricular remodeling in restrictive ventricular septal defect. J Mol Cell Cardiol 49: 699–706, 2010. doi: 10.1016/j.yjmcc.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124: 1537–1547, 2011. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadal-Ginard B, Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest 84: 1693–1700, 1989. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira-Carvalho V, Carvalho VO, Bocchi EA. The emerging role of miR-208a in the heart. DNA Cell Biol 32: 8–12, 2013. doi: 10.1089/dna.2012.1787. [DOI] [PubMed] [Google Scholar]
- 22.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301: 1–7, 2004. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Pawlak A, Gil RJ, Kulawik T, Pronicki M, Karkucińska-Więckowska A, Szymańska-Dębińska T, Gil K, Lagwinski N, Czarnowska E. Type of desmin expression in cardiomyocytes: a good marker of heart failure development in idiopathic dilated cardiomyopathy. J Intern Med 272: 287–297, 2012. doi: 10.1111/j.1365-2796.2012.02524.x. [DOI] [PubMed] [Google Scholar]
- 24.Rogers LK, Tipple TE, Nelin LD, Welty SE. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr Res 65: 33–38, 2009. doi: 10.1203/PDR.0b013e31818a1d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkranz-Weiss P, Tomek RJ, Mathew J, Eghbali M. Gender-specific differences in expression of mRNAs for functional and structural proteins in rat ventricular myocardium. J Mol Cell Cardiol 26: 261–270, 1994. doi: 10.1006/jmcc.1994.1029. [DOI] [PubMed] [Google Scholar]
- 26.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Nakamura M. Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J Card Fail 16: 404–410, 2010. doi: 10.1016/j.cardfail.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Velten M, Gorr MW, Youtz DJ, Velten C, Rogers LK, Wold LE. Adverse perinatal environment contributes to altered cardiac development and function. Am J Physiol Heart Circ Physiol 306: H1334–H1340, 2014. doi: 10.1152/ajpheart.00056.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velten M, Hutchinson KR, Gorr MW, Wold LE, Lucchesi PA, Rogers LK. Systemic maternal inflammation and neonatal hyperoxia induces remodeling and left ventricular dysfunction in mice. PLoS One 6: e24544, 2011. doi: 10.1371/journal.pone.0024544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidaeff AC, Ramin SM. From concept to practice: the recent history of preterm delivery prevention. Part II: Subclinical infection and hormonal effects. Am J Perinatol 23: 75–84, 2006. doi: 10.1055/s-2006-931803. [DOI] [PubMed] [Google Scholar]
- 30.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27: 358–368, 2009. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 113: 135–143, 2006. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]