Abstract
Investigations of human conduit artery endothelial function via flow-mediated vasodilation (FMD) have largely been restricted to the reactive hyperemia (RH) technique, wherein a transient increase in shear stress after the release of limb occlusion stimulates upstream conduit artery vasodilation (RH-FMD). FMD can also be assessed in response to sustained increases in shear stress [sustained stimulus (SS)-FMD], most often created with limb heating or exercise. Exercise in particular creates a physiologically relevant stimulus because shear stress increases, and FMD occurs, during typical day-to-day activity. Several studies have identified that various conditions and acute interventions have a disparate impact on RH-FMD versus SS-FMD, sometimes with only the latter demonstrating impairment. Indeed, evidence suggests that transient (RH) and sustained (SS) shear stress stimuli may be transduced via different signaling pathways, and, as such, SS-FMD and RH-FMD appear to offer unique insights regarding endothelial function. The present review describes the techniques used to assess SS-FMD and summarizes the evidence regarding 1) SS-FMD as an index of endothelial function in humans, highlighting comparisons with RH-FMD, and 2) potential differences in shear stress transduction and vasodilator production stimulated by transient versus sustained shear stress stimuli. The evidence suggests that SS-FMD is a useful tool to assess endothelial function and that further research is required to characterize the mechanisms involved and its association with long-term cardiovascular outcomes.
NEW & NOTEWORTHY Sustained increases in peripheral conduit artery shear stress, created via distal skin heating or exercise, provide a physiologically relevant stimulus for flow-mediated dilation (FMD). Sustained stimulus FMD and FMD stimulated by transient, reactive hyperemia-induced increases in shear stress provide distinct assessments of conduit artery endothelial function.
Keywords: endothelium, exercise, flow-mediated dilation, nitric oxide, shear stress
INTRODUCTION
The endothelium is a critical determinant of vascular function and health and plays a key role in the regulation of vascular tone (18). Endothelial cells respond to blood flow-induced increases in vascular wall shear stress by increasing vasodilatory autocoid synthesis, leading to vascular smooth muscle relaxation [flow-mediated dilation (FMD)]. Shear stress is proportional to blood velocity and viscosity and inversely related to vessel diameter, whereas shear rate excludes the viscosity component and is frequently reported as a surrogate for shear stress (70). FMD after experimentally imposed increases in shear stress is often used as an index of endothelial function. The most popular technique used to evoke an increase in conduit artery shear stress for FMD assessment in humans involves a 5-min limb occlusion, achieved by inflating a pneumatic cuff to suprasystolic pressures (70, 86). The occlusion results in decreased resistance in the distal vasculature, such that upon cuff release there is a reactive hyperemia (RH) and transient surge of shear stress in the upstream artery, provoking FMD. RH-FMD has been shown to have some utility in predicting cardiovascular events (36, 42) and advanced the understanding that endothelial dysfunction precedes and contributes to the development of atherosclerosis (13). Although extensively studied, RH represents only one approach to augment shear stress for FMD assessment, and this transient shear stress profile is atypical in day-to-day life.
Sustained increases in shear stress represent a more common in vivo physiological occurrence; however, assessment of FMD in response to sustained increases in shear stress [sustained stimulus (SS)-FMD] has been used relatively infrequently to interrogate peripheral conduit artery endothelial function in humans in vivo. Sustained increases in conduit artery shear stress can be achieved by limb heating (46, 66), distal vasodilator infusion (60), and exercise (69, 100). Evidence suggests that transient and sustained shear stress stimuli may be differentially transduced by the endothelium (28) such that SS-FMD and RH-FMD may test different aspects of, and offer unique insights regarding, endothelial function. Indeed, several studies have suggested that RH-FMD and SS-FMD are impacted differently by various acute interventions [e.g., mental stress (84) or a high-fat meal (62)] and chronic conditions [e.g., smoking (25), obesity (81), or hypercholesterolemia (60)]. The nonuniform findings observed between transient and SS-FMD indicate that endothelial dysfunction undetected with RH-FMD may sometimes be detected with SS-FMD, and vice versa.
Techniques used to stimulate SS-FMD permit stimulus control. Thus, while an uncontrolled RH stimulus may differ between conditions or groups (55), hampering RH-FMD comparisons, it is usually possible to create a uniform sustained shear stress stimulus (or an overlapping range) (68, 92). Although limb heating and distal vasodilator infusion effectively increase upstream arterial shear stress, they have the disadvantage of being technically challenging, time consuming, and, in the case of infusion, invasive. Rhythmic handgrip exercise produces sustained increases in brachial artery shear stress by rapidly reducing downstream vascular resistance in the forearm. The resultant brachial artery vasodilation (SS-FMD) is stimulated by the mean increase in shear stress and does not seem to be influenced by the fluctuating shear stress pattern that occurs with contraction (48, 69). Furthermore, the vasodilation appears to be stimulated exclusively by shear stress, without a contribution from conducted vasodilation or attenuation due to any accompanying increases in sympathetic nervous activity or altered systemic hemodynamics (43, 69). Given that changes in shear stress in vivo are typically the result of exercise, rhythmic handgrip exercise represents a physiologically relevant stimulus profile. Although arteriolar dilation is typically more important for determining perfusion, conduit artery SS-FMD may become more relevant in arteries compromised by atherosclerosis (31, 32, 34, 37, 96).
In summary, present evidence suggests that SS-FMD and RH-FMD provide distinct information regarding endothelial function. The present review will describe the common experimental techniques used to assess SS-FMD and will synthesize the evidence regarding 1) SS-FMD as an index of peripheral conduit artery endothelial function in humans, highlighting comparisons with RH-FMD, and 2) potential differences in shear stress transduction and vasodilator production stimulated by transient versus sustained shear stress stimuli.
FMD: COMMON EXPERIMENTAL TECHNIQUES
Transient Shear Stress Stimulus (RH) FMD
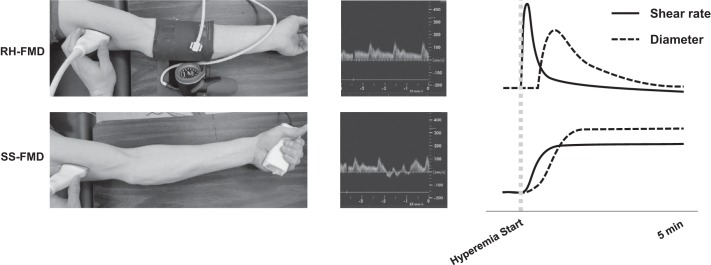
There are several published reviews (16, 38, 86) that have provided guidelines for RH-FMD assessment, and procedures will be reviewed only briefly here. RH is achieved via distal circulatory arrest induced by a prolonged suprasystolic occlusion. In a matter of minutes, downstream resistance is reduced, and, upon release of occlusion, this facilitates a high-flow environment in the feeding conduit artery, exposing the endothelium to an immediate, transient, uncontrolled increase in shear stress. The shear stress is laminar and almost exclusively antegrade. Peak hyperemia during RH generally occurs within the first 10 s of cuff release (71) before rapidly decaying, returning to near baseline levels by 2–3 min after release. The RH-FMD response is characterized as the peak change in diameter from baseline, which occurs well after the peak hyperemia (9). RH-FMD is most frequently assessed in the brachial artery (Fig. 1, top) and has been shown to be stable over multiple repeat trials (39, 68). With cuff placement on the wrist, calf, or thigh, RH-FMD can also be assessed in the radial, popliteal, and femoral arteries.
Fig. 1.
Schematic representation of reactive hyperemia flow-mediated vasodilation (RH-FMD; top) and handgrip exercise flow-mediated vasodilation [sustained stimulus (SS)-FMD; bottom] in a protocol designed to target a single shear rate. The solid lines represent the stimulus (shear stress); the dashed lines represent the response (vasodilation). The shear stress stimulus for RH-FMD is large and transient compared with a more gradual onset, sustained increase in shear stress with exercise-induced SS-FMD. The ultrasound velocity trace (middle) shows that the shear rate is entirely antegrade during RH, whereas it is mildly oscillatory during handgrip exercise because of contraction-induced impedance to flow. Increases in diameter correspond to the shear stress stimuli, with RH evoking a generally parabolic increase and decrease in diameter, whereas SS produces a slower, sustained increase in diameter.
The uncontrolled stimulus that occurs with RH presents a challenge to FMD interpretation if different RH stimuli are experienced between groups or experimental conditions. A nonuniform stimulus is a real possibility given that several factors, including baseline conduit artery diameter and the impact of cardiovascular disease risk on microvascular function, can influence the magnitude of RH (46, 59, 66). If one group experiences a smaller RH-FMD in response to a smaller RH stimulus, it becomes unclear whether this group has inferior conduit artery endothelial function (55). Although ratio normalization, allometric scaling, and covariate analysis are used, there is currently no generally accepted practice to enable correction for group/condition differences in the RH stimulus when interpreting RH-FMD (86).
Sustained Shear Stress Stimulus FMD
Sustained increases in shear stress can be achieved via stimulating prolonged vasodilation of the vasculature downstream of the conduit artery of interest. For example, vasodilator infusion distal to the site of arterial diameter measurement has been used in the coronary and upper limb vasculatures (17, 60, 79). Continuous infusion of acetylcholine in the brachial artery at the antecubital fossa induces a dose-dependent, sustained rise in flow and shear stress in the proximal portion of the artery (60). However, the infusion of vasoactive drugs necessitates arterial cannulation and the presence of a physician, rendering the task expensive and invasive, thereby limiting its feasibility. Forearm or hand heating using a heating pad, hot water, or hot air offers an alternative to increase conduit artery shear stress in a “dose” (temperature)-dependent manner via downstream skin arteriolar vasodilation without altering systemic hemodynamics; however, attaining a steady-state increase in shear stress takes ~10–35 min (5, 46, 60, 66, 67, 69, 74). Skin heating has been used for the assessment of SS-FMD in different populations [e.g., men and women (46) or healthy vs. clinical conditions (3, 7, 94)] and/or by before versus after prolonged interventions [e.g., angiotensin converting enzyme (ACE) treatment in heart failure patients (45)], although the duration of the protocol and persistence of the skin dilation limits its utility for assessing SS-FMD before and after acute interventions (74).
Exercise produces an active hyperemia and, similar to both vasodilator infusion and skin heating, induces “dose” (intensity)-dependent increases in shear stress and consequently FMD (Figs. 1, bottom, and 2). Rhythmic handgrip exercise causes a rapid reduction in forearm vascular resistance, evoking an associated increase in brachial artery flow and thus shear stress. The increase in conduit artery shear stress is not instantaneous; rather, mean shear stress increases gradually before reaching a sustained, steady state in 30−60 s, returning to resting levels only after exercise completion.
Fig. 2.
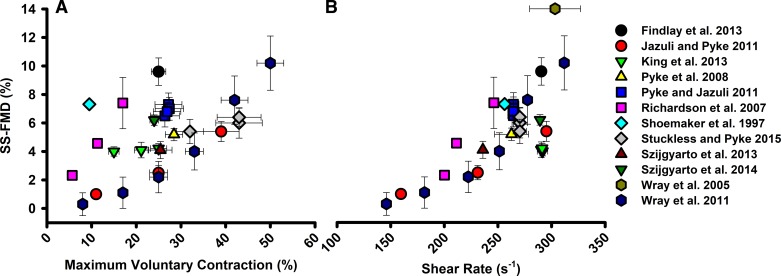
Synthesis of investigations implementing rhythmic handgrip exercise sustained shear stimulus flow-mediated vasodilation (SS-FMD). Studies included measured brachial artery diameter and maximal voluntary contraction (A) or mean shear rate (B) in young, healthy participants. Data are presented as means with SE bars and include 12 studies.
Primarily, two general rhythmic handgrip exercise protocols have been adopted to study SS-FMD. The first involves progressive increases in workload, at 3 min/workload separated by 1 min of rest (20, 72, 100). This technique elicits progressive increases in brachial artery vasodilation with increased workload and establishes within-subject shear rate-diameter (stimulus-response) relationships. The second technique, shown in Fig. 1, involves matching shear rate between and/or within subjects while subjects perform the handgrip exercise at an intensity required to produce the desired shear rate (1, 25, 43). The single-stimulus method typically involves sustaining a similar workload for 6−10 min, permitting the assessment of the time course of SS-FMD (67). When real-time Doppler velocity output to an external computer with data acquisition software is possible (40), velocity can be displayed as a moving average [e.g., a 1-s contraction to 5-s relaxation duty cycle could be monitored as a 6-s average moving forward in 1-s increments (49)]. Combined with baseline or real-time assessment of arterial diameter, the intensity of rhythmic handgrip contraction can be altered “on the fly” to either increase or decrease blood velocity to maintain a targeted shear rate (i.e., shear rate = 4 × mean velocity/vessel diameter; required velocity = target shear rate × vessel diameter/4). Several studies from our group have provided a description of this methodology (43, 49, 81).
Single versus progressive intensity handgrip protocols represent the two most commonly adopted techniques to evoke exercise-induced SS-FMD; however, a strength of the technique is its versatility, as it can be adapted to address the research question. Although exercise-induced SS-FMD is usually assessed in the upper limb, SS-FMD has been reported in the femoral arteries using cycling (30), single-leg kicking (33, 99), and plantar flexion exercise (48) to generate an increase in shear stress.
All methods of inducing SS-FMD afford some degree of stimulus control, enabling the generation of a similar stimulus magnitude between groups and conditions. With creation of the same stimulus over four trials, a corresponding stability of brachial artery SS-FMD during handgrip exercise has been demonstrated (68); however, superficial femoral artery SS-FMD during plantar flexion exercise increased systematically over four trials (48). Creation of a controlled stimulus facilitates isolation of macrovascular function and group/condition comparisons of SS-FMD. For example, researchers have used heating and exercise to create uniform stimuli to compare SS-FMD between groups with heterogeneous arterial diameters (46) and within individuals to compare reactivity in different-sized arteries (43).
Using Exercise to Elevate Shear Stress for FMD Assessment: Is the Conduit Artery Vasodilation Stimulated During Exercise Exclusively Flow Mediated? Putative Nonshear Stress Contributions to Conduit Artery Diameter Changes During Exercise
Exercise is the most commonly encountered stimulus for increases in muscle blood flow and the associated conduit artery shear stress. However, exercise is a complex stimulus that involves metabolite production and sympathetic nervous system activation. If these factors influence conduit artery diameter changes during exercise, changes in conduit artery diameter will not exclusively reflect SS-FMD/endothelial function. Indeed, conduit artery diameter changes during exercise of the downstream muscle mass have been described as distinct from FMD because of these concerns (72, 80).
Conducted vasodilation.
Exercise-induced vasodilation originating at terminal arterioles in activated skeletal muscle can conduct up the arterial tree (77, 97) via direct coupling between endothelial cells and/or smooth muscle cells through gap junctions (76). If vasodilation can conduct up to the level of the conduit artery, exercise-induced conduit artery vasodilation could be a combination of flow-mediated and conducted vasodilation. Pyke et al. (69) examined whether exercise-induced conducted vasodilation reached the brachial artery during rhythmic handgrip exercise. During exercise, simultaneous brachial arterial compression was applied to prevent any increase in brachial artery shear stress while maintaining muscle activation. If conducted signals contributed to exercise-induced brachial artery vasodilation, muscle activation, independent of changes in upstream shear stress, would be expected to produce increases in brachial artery diameter. However, eliminating the shear stress stimulus prevented any change in brachial artery diameter, suggesting that conducted vasodilation does not reach the brachial artery. Similarly, in the radial artery, using arterial compression to prevent increases in shear stress during hand muscle exercise prevented radial artery vasodilation (43). Prevention of increases in shear stress during forearm heating also prevents increases in brachial artery diameter (69). Therefore, the evidence to date suggests that the conduit artery dilation that occurs with distal heating and exercise is stimulated by shear stress without a contribution from conducted vasodilation, thus reflecting FMD. Studies of this phenomenon to date have been performed in the upper limb and included only young and healthy participants. Confirmation in the lower limb, and in additional populations, will help to strengthen the conclusion that conducted vasodilation does not reach the conduit artery.
Sympathetic nervous system activity.
Sympathetic activation can increase vascular tone and has the potential to restrict RH-FMD (22, 41). Exercise itself is sympathoexcitatory, and the sympathoexcitation could have an impact on SS-FMD stimulated by exercise. To examine whether rhythmic handgrip exercise-associated increases in sympathetic activity restrict SS-FMD, Pyke et al. (69) matched the mean shear rate in the brachial artery achieved with rhythmic handgrip exercise via forearm heating. Although the exercise trial increased both heart rate and mean arterial pressure, SS-FMD was similar to the passive forearm-heating condition, providing evidence that handgrip exercise SS-FMD is not affected by any concomitant sympathetic activation or associated alterations in systemic hemodynamics. To assess whether simultaneous superimposition of sympathoexcitation restricts SS-FMD, Stuckless and Pyke (83) performed SS-FMD stimulated by handgrip exercise-induced increases in shear stress during a cold pressor test. The cold pressor test increased mean arterial pressure from 98 ± 10 to 115 ± 10 mmHg, yet handgrip exercise SS-FMD remained unaltered, suggesting that SS-FMD is preserved during exaggerated sympathoexcitation. Collectively, this evidence suggests that sympathetic activation does not restrict SS-FMD. However, whether other sympathoexcitatory maneuvers restrict SS-FMD in healthy participants or if clinical populations experience blunted SS-FMD during sympathoexcitation merits future investigation.
In summary, rhythmic handgrip exercise appears to be an effective means to elicit flow-mediated vasodilation, as putative confounders including conducted vasodilation and sympathetic activation do not appear to influence SS-FMD.
Using Exercise to Elevate Shear Stress for FMD Assessment: Does Exercise-Associated Retrograde Shear Stress Negatively Impact SS-FMD?
Unlike limb heating and distal vasodilator infusion, rhythmic exercise used to stimulate SS-FMD induces mildly oscillatory shear patterns, owing to contraction-induced impedances in blood flow followed by surges in antegrade flow during relaxation. Studies in humans have shown that brief exposure of a conduit artery to oscillatory shear stress (30 min) is sufficient to decrease RH-FMD (87, 90). Accordingly, whether the fluctuating shear pattern during rhythmic exercise mitigates SS-FMD was tested by matching mean shear rate in three conditions: 1) a handgrip exercise protocol (mildly oscillatory), 2) a forearm-heating protocol (steady shear stress, not oscillatory), and 3) forearm heating with rhythmic cuff inflation to mimic the shear rate pattern during handgrip exercise (mildly oscillatory without muscle activation) (69). If the mildly oscillatory pattern applied to the endothelium during rhythmic handgrip exercise or heating with rhythmic cuff inflation had a detrimental effect on SS-FMD, a greater vasodilation would be expected during forearm heating alone. However, both SS-FMD and the relationship between the increase in shear rate and SS-FMD was similar in each condition, suggesting that SS-FMD responds to the mean sustained shear rate rather than its pattern during exercise.
Further exploring the influence of shear rate pattern on SS-FMD, King et al. (49) compared the FMD response to varying handgrip duty cycles, again while sustaining a uniform mean shear rate. Duty cycles of 1-s contraction to 1-, 3-, and 5-s relaxation periods produced nearly identical FMD responses in the brachial artery, despite the 1:1 duty cycle creating a greater retrograde shear rate. Therefore, the mildly oscillatory shear pattern present during exercise does not appear to influence SS-FMD.
Comparisons Between FMD Stimulated by Sustained Versus Transient Increases in Shear Stress: Do RH-FMD and SS-FMD Provide Distinct Information About Endothelial Function?
A small number of studies have compared RH-FMD and SS-FMD in populations with increased cardiovascular risk or within individuals before versus after the intervention. These studies provide insight regarding whether FMD in response to transient versus sustained increases in shear stress should be viewed as interchangeable or distinct assessments of endothelial function (see summary in Table 1).
Table 1.
Summary of intervention and cross-sectional studies that have administered sustained shear stress flow-mediated dilation and, where applicable, reactive hyperemia flow-mediated dilation
| Artery | Technique | SS-FMD | RH-FMD | Reference | |
|---|---|---|---|---|---|
| Intervention | Arrows versus preintervention | ||||
| Prior antegrade shear stress | Brachial | Rhythmic handgrip | ↑ | 1 | |
| l-NMMA and EDHF receptor inhibition | Radial | Hand heating | ↓ | 5, 8 | |
| Angiotensin converting enzyme inhibitors in congestive heart failure | Radial | Hand heating | ↑ | 45 | |
| l-NMMA and aspirin | Radial | Hand heating | ↔ | 60 | |
| Endothelin type B receptor inhibition | Radial | Hand heating | ↓ | 6 | |
| Anemia correction in end-stage renal disease | Brachial | Forearm heating | ↑ | 94 | |
| Antioxidant | Brachial | Progressive handgrip | ↓ | 72 | |
| Antioxidant or exercise training in the elderly | Brachial | Progressive handgrip | ↑ | 20 | |
| Acute and chronic folic acid ingestion in the elderly | Brachial | Progressive handgrip | ↑ | 73 | |
| l-NMMA | Brachial | Progressive handgrip | ↓ | 100 | |
| l-NMMA in the elderly | Brachial | Progressive handgrip | ↓ | 93 | |
| Ascorbic acid and l-NMMA in the elderly | Brachial | Progressive handgrip | ↔ | 92 | |
| High-fat meal | Brachial | Rhythmic handgrip | ↔ | ↓ | 62 |
| Altered contraction duty cycles | Brachial | Rhythmic handgrip | ↔ | 49 | |
| Cold pressor test | Brachial | Rhythmic handgrip | ↔ | 83 | |
| Acute mental stress | Brachial | Rhythmic handgrip | ↔ | ↓ | 84 |
| Acute mental stress and cold pressor test | Brachial | Rhythmic handgrip | ↑ | 85 | |
| Cross-sectional | Arrows versus healthy controls | ||||
| Uncomplicated type I diabetes | Radial | Hand heating | ↓ | ↔ | 3 |
| Hypertension | Radial | Hand heating | ↓ | ↓ | 7 |
| Autosomal dominant polycystic kidney disease | Radial | Hand heating | ↓ | ↔ | 56 |
| End-stage renal disease | Brachial | Forearm heating | ↓ | 94 | |
| Hypercholesterolemia | Brachial | Distal acetylcholine infusion | ↔ | ↓ | 60 |
| Young smokers | Brachial | Rhythmic handgrip | ↓ | ↔ | 25 |
| Obese young adults | Brachial | Rhythmic handgrip | ↓ | ↔ | 81 |
| Systemic sclerosis | Brachial | Progressive handgrip | ↓ | 57 | |
SS-FMD, sustained shear stress flow-mediated dilation; RH-FMD, reactive hyperemia flow-mediated dilation; l-NMMA, NG-monomethyl-l-arginine.
RH-FMD Versus SS-FMD in Populations With Increased Cardiovascular Risk
Smokers.
Young male smokers with a short smoking history were found to have a significantly blunted handgrip exercise-induced SS-FMD compared with young male nonsmokers, whereas RH-FMD did not differ between groups (25). Neither the RH nor handgrip exercise shear rate stimulus differed between groups. Interestingly, the difference in SS-FMD between groups became more pronounced over time during the 10 min of steady-state shear rate. Therefore, endothelial responses to a transient increase in shear rate did not appear to be impaired; however, the response to sustained shear stress was blunted in young, otherwise healthy smokers. When assessing an older cohort (~40 yr old) with a longer smoking history, Gaenzer et al. (30) found that femoral artery FMD in response to cycle exercise (SS-FMD) and brachial artery RH-FMD were both impaired in smokers versus nonsmokers. It is possible that SS-FMD is initially impaired in individuals with a short smoking history and that with continued smoking responses to transient shear stress stimuli are also impaired (i.e., impaired RH-FMD). If so, SS-FMD may permit earlier identification of endothelial dysfunction in smokers, and this requires further research.
Type 1 diabetes.
Bellien et al. (3) found reduced radial artery RH-FMD and hand-heating SS-FMD in people with type 1 diabetes versus healthy control subjects. When solely assessing those with uncomplicated type 1 diabetes (without microangiopathic complications) however, FMD was impaired versus matched control subjects only in response to sustained shear stress (and when heating shear stress was similar between groups). This initial evidence from individuals with type 1 diabetes suggests that SS-FMD may be impaired earlier in the course of the pathology.
Hypertension.
In essential hypertension, endothelial/vascular dysfunction is often present in both the micro- and macrovasculature (54). Therefore, the hyperemic stimulus for RH-FMD may be blunted in essential hypertension compared with healthy control participants, complicating the interpretation of RH-FMD as an index of conduit artery endothelial function. For example, Bellien et al. (7) performed RH-FMD and hand-heating SS-FMD in the radial artery of patients with essential hypertension. RH-FMD was lower in patients with hypertension compared with matched control subjects; however, so was the duration of the postischemic hyperemia and hence the RH-FMD stimulus. The lower RH makes it more difficult to ascertain whether the smaller RH-FMD is truly due to impairment in the endothelium’s ability to transduce the transient stimulus (i.e., endothelial dysfunction) or simply due (or at least due in part) to the smaller stimulus. In this case, the impairment in RH-FMD was maintained with statistical adjustment for the stimulus, supporting the presence of endothelial dysfunction. Hand heating evoked equivalent increases in shear stress between groups, whereas SS-FMD was lower in patients with essential hypertension compared with matched control subjects, thus providing firm evidence of reduced conduit artery endothelial function. Taken together, this evidence suggests that endothelial responses to both transient and sustained increases in shear stress are impaired in essential hypertension.
Renal disease.
Autosomal dominant polycystic kidney disease patients have cardiovascular complications that are thought to be related to the presence of abnormal polycystins in endothelial cells that may interfere with shear stress mechanotransduction; however, there are conflicting findings regarding whether patients with autosomal dominant polycystic kidney disease present conduit artery endothelial dysfunction as assessed by RH-FMD (15, 50). Lorthioir et al. (56) performed radial artery RH-FMD and hand-warming SS-FMD in patients with autosomal dominant polycystic kidney disease and observed an impaired SS-FMD but similar RH-FMD compared with control subjects with no difference in the shear stress stimuli (SS or RH) between groups. Thus patients with autosomal dominant polycystic kidney disease may have impairments in mechanotransduction of sustained, but not necessarily transient, shear stress.
Obesity.
Slattery et al. (81) examined a 10-min rhythmic handgrip exercise-induced SS-FMD and RH-FMD in lean and obese young men. A marked reduction in SS-FMD was observed in the obese group compared with the lean group (obese ~50% lower) without any difference in the standard 5-min occlusion RH-FMD. There were no group differences in the stimulus for SS-FMD, although there was a trend for a larger RH stimulus in the obese group. However, the lack of group difference in RH-FMD persisted after adjusted analysis to account for stimulus differences. Although other studies have reported impaired RH-FMD in obese populations (61), the results suggest that in this sample obesity was predominantly associated with an impairment in endothelium-dependent vasodilation in response to sustained shear stress.
Hypercholesterolemia.
Mullen et al. (60) compared RH-FMD and SS-FMD in hypercholesterolemic subjects. Brachial artery RH-FMD was lower in patients with hypercholesterolemia compared with healthy control subjects, despite a similar hyperemic stimulus, whereas brachial artery SS-FMD in response to stepwise increases in flow evoked by distal acetylcholine infusion was similar between groups. Exposure to high cholesterol, therefore, may have an adverse effect on the ability to transduce transient increases in shear stress but not sustained increases in shear stress.
Evidence of impairment in SS-FMD in other populations.
Several studies have examined SS-FMD in disease populations versus healthy controls without including an assessment of RH-FMD. Patients with end-stage renal failure present lower whole blood viscosity, chronically lower mean shear stress, and reduced hand-warming brachial artery SS-FMD compared with control subjects (lower FMD-shear stress slope in patients) (94). Chronic anemia correction, to increase blood viscosity and shear stress, enhanced heating-induced SS-FMD (improved the FMD-shear stress slope) (94). Systemic sclerosis is an autoimmune disease that results in fibrosis and is often associated with vascular dysfunction. Machin et al. (57) found that compared with healthy control subjects, patients with systemic sclerosis presented a lower SS-FMD in response to progressive handgrip exercise-induced increases in shear rate (57). Donato et al. (20) compared SS-FMD in young and elderly participants during a progressive handgrip exercise protocol. Elderly participants displayed a reduced SS-FMD over the whole range of shear rates and thus a reduced ability to transduce a sustained shear stress stimulus with aging, in agreement with numerous studies that have reported reduced RH-FMD with aging (14, 23, 75). Recently, both acute and chronic folic acid ingestion have been shown to improve progressive handgrip exercise SS-FMD in elderly adults (73). Joannides et al. (45) assessed radial artery SS-FMD achieved via stepwise hand warming in patients with congestive heart failure before and after treatment with ACE inhibitors. Treatment improved SS-FMD for a given increase in shear stress, thereby indicating a beneficial effect of ACE inhibition on this aspect of conduit artery endothelial function in heart failure patients.
In summary, several studies have noted distinct impacts of chronic conditions on RH-FMD versus SS-FMD. The potential for dissociation in FMD responses stimulated by transient and sustained shear stress in populations at increased cardiovascular risk may provide insight on the progression of endothelial dysfunction. Although evidence to date is limited, initial findings suggest that in some populations, SS-FMD can detect endothelial dysfunction that is not revealed by RH-FMD. In addition, these findings indicate that the impact of clinical conditions on FMD in response to a physiologically relevant pattern of shear stress during exercise cannot be reliably predicted from RH-FMD responses. The shear stress stimulus-specific heterogeneity of FMD deserves further exploration in larger numbers of distinct populations, and assessing responses to both transient and sustained increases in shear stress may provide a more comprehensive understanding of endothelial function. Whether SS-FMD impairment predicts cardiovascular disease risk/events is unknown, and this intriguing possibility requires investigation with prospective studies. Studies that include both RH and SS-FMD to enable comparison of their predictive value are also needed.
RH-FMD Versus SS-FMD Before and After Acute Stimuli
Differences between RH-FMD and SS-FMD have been observed not only among distinct populations but also within individuals in response to acute perturbations.
High-fat meal.
Padilla et al. (62) compared RH-FMD and SS-FMD 4 h after ingestion of low- and high-fat meals. When a low-intensity 5-min rhythmic handgrip exercise protocol (10% maximum voluntary contraction, 1-s contraction, 1-s relaxation) was adopted, no change in SS-FMD was observed after the high-fat meal, whereas RH-FMD was significantly reduced. Ingestion of a high-fat meal may therefore impair endothelial function in a manner that is specific to the transduction of transient increases in shear stress.
Mental stress.
Acute mental stress, elicited using the Trier social stress test, was shown to reduce RH-FMD, with no changes in handgrip exercise-induced SS-FMD 10 min after the test (84). Several other studies have also reported impaired RH-FMD after acute mental stress (64), whereas a followup SS-FMD investigation found a transient increase in handgrip exercise-induced SS-FMD 15 min after the Trier social stress test (85). The results of these studies suggest that acute mental stress has opposing effects on RH- and SS-FMD.
Antegrade shear stress.
Acute augmentation (30 min) of shear stress in the brachial artery, achieved via forearm heating, has previously been shown to potentiate RH-FMD (88). A similar forearm-heating intervention (40 min) enhanced handgrip exercise SS-FMD 15 min after the intervention compared with baseline (1). Thus, antegrade shear stress-mediated potentiation of endothelial function appears to improve FMD in response to both transient and sustained elevations in shear stress.
In summary, investigations that have measured FMD in response to both sustained and transient shear stress stimuli have often found unique responses to acute interventions. In combination with the disparate impact of various clinical conditions on RH-FMD and SS-FMD, it is tempting to speculate that distinct mechanisms or vasodilator pathways with a potential for heterogeneous vulnerability to vascular insults are involved in the FMD response to unique shear stress stimuli. However, the comparison of RH-FMD versus SS-FMD has often been performed in only one study for a given population or intervention. Therefore, replication of these findings in additional and larger studies is needed.
Mechanisms of Transient and Sustained Shear Stress FMD
The observation of distinct impacts of cardiovascular disease risk factors (e.g., smoking, diabetes, and obesity) and acute stimuli (e.g., mental stress and high-fat meals) on SS-FMD versus RH-FMD suggests that the endothelium distinguishes between these disparate shear stress stimuli (see summary schematic in Fig. 3). Shear stress is converted into biochemical signals by a variety of mechanosensitive structures at the luminal surface of the endothelium, including ion channels, plasma membrane receptors, G proteins, adhesion molecules, the cytoskeleton, caveolae, the glycocalyx, primary cilia, and the plasma membrane lipid bilayer (2). Signal transduction can lead to the production of numerous different vasodilators [e.g., nitric oxide (NO), prostaglandins, and EDHFs]. Specific vasodilator production and/or signaling pathways leading to the production of a given vasodilator appear to be dependent on the nature of the shear stress.
Fig. 3.
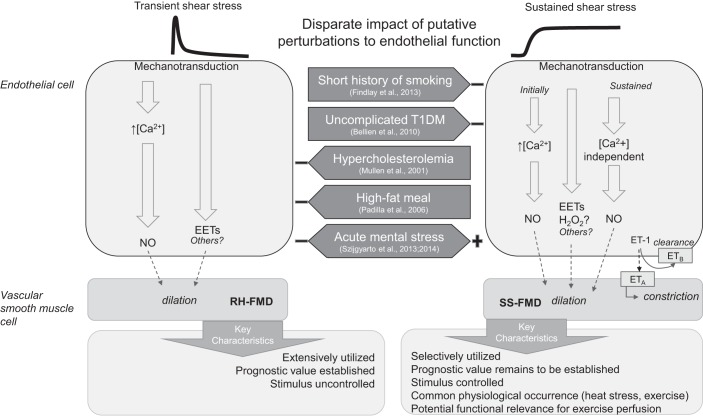
Summary schematic of sustained shear stimulus flow-mediated vasodilation (SS-FMD) compared with reactive hyperemia flow-mediated vasodilation (RH-FMD). The results from cell culture and isolated vessel studies suggest that transient and sustained shear stress are differentially transduced by the endothelium. Transient increases in shear stress are thought to activate endothelial nitric oxide (NO) synthase (eNOS) and produce NO via a Ca2+-dependent pathway, whereas sustained increases in shear stress activate eNOS in a distinct, Ca2+-independent pathway (28, 53). Hypothetically extrapolated to in vivo conduit artery studies, RH-FMD is depicted as reflecting primary recruitment of the Ca2+-dependent eNOS activation pathway. SS-FMD is depicted as initially eliciting Ca2+-dependent eNOS activation (if the initial increase in shear is abrupt); however, the prolonged stimulus is identified as relying to a greater extent on Ca2+-independent eNOS activation. There is evidence for involvement of endothelium-derived hyperpolarizing factors, namely epoxyeicosatrienoic acids (EETs), in both RH-FMD and SS-FMD (5, 26). Reactive oxygen species appear to contribute to SS-FMD (72), and H2O2 may be the responsible signaling molecule. There is emerging evidence that endothelin-1 (ET-1) binding to endothelial ET-1 type B (ETB) receptors contributes to SS-FMD by facilitating ET-1 uptake; when ET-1 binds to ET type A (ETA) receptors, vasoconstriction is promoted (4). Distinct RH-FMD versus SS-FMD pathways may be differentially affected by certain interventions and cardiovascular risk factors [smoking, type 1 diabetes mellitus (T1DM), hypercholesterolemia, high-fat meal, and acute mental stress], thereby providing a theoretical basis for the disparate findings of SS-FMD and RH-FMD. There is a need to incorporate both SS-FMD and RH-FMD in future studies to further understand the mechanistic and functional distinctions of SS-FMD and RH-FMD in the context of endothelial function.
Identification of the Primary Vasodilator(s) Responsible for Peripheral Conduit Artery FMD in Response to Transient and Sustained Shear Stress Stimuli
RH-FMD.
NO exerts several vasoprotective functions, and there has been considerable interest in evaluating the NO dependence of RH-FMD and thereby its utility as a functional assay of NO bioavailability (36, 70). Early investigation attempting to discern the contribution of NO to RH-FMD inhibited NO synthase with NG-monomethyl-l-arginine (l-NMMA), which resulted in complete abolition of radial artery RH-FMD upon distal cuff release (47). A similar effect was observed by Doshi et al. (21) in the brachial artery, where RH-FMD after release of distal cuff occlusion was completely abolished during l-NMMA infusion. Extending the distal cuff occlusion beyond 5 min increased the peak and total RH (44, 60, 65), and several studies, although not all (65), have indicated a diminished influence of l-NMMA on RH-FMD after distal cuff occlusion of >10 min in duration (4, 35, 51, 60). This suggests a dampened contribution of NO, or greater compensation or involvement from other vasodilators, in response to a larger, more prolonged RH stimulus.
However, the finding of predominant RH-FMD dependence on NO with even a “standard” 5-min distal cuff occlusion protocol has not been unanimous. For example, Pyke et al. (65) found no effect of l-NMMA infusion on radial artery RH-FMD, whereas Parker et al. (63) found only a modest mean reduction (~25%) with considerable interindividual variability in young men and women. In the brachial artery, Wray et al. (101) found that l-NMMA reduced RH-FMD by a third; however, after accounting for the reduction in shear rate stimulus during l-NMMA infusion, RH-FMD was similar with and without endothelial NO synthase (eNOS) inhibition. A meta-analysis of 20 investigations that have administered l-NMMA attributed ~70% of the RH-FMD response to NO, and this was consistent (~72%) when analysis was done on a subset of studies that used a standard technique (5-min distal occlusion in healthy volunteers) (35). However, the evidence of considerable interindividual variability indicates the potential for important involvement of other vasodilators and/or physiological redundancy. Although prostaglandins do not appear to make an important contribution (60, 63), the role of one EDHF pathway was identified in the radial artery.
Increases in shear stress increase endothelial intracellular Ca2+ concentration leading to the release of arachidonic acid from the cell membrane. Arachidonic acid can be subsequently metabolized by cytochrome P-450 (CYP) epoxygenases, generating epoxyeicosatrienoic acids (EETs), an EDHF, leading to smooth muscle cell hyperpolarization via several potential pathways including the activation of large-conductance Ca2+-activated K+ channels on vascular smooth muscle cells (12, 27). Fischer et al. (26) inhibited eNOS and an EET-producing enzyme (CYP2C9 inhibition via sulfaphenazole) separately and in combination in young healthy volunteers. Single inhibition of either enzyme reduced RH-FMD (~48 and ~36% for NO and EET, respectively), whereas coinfusion of inhibitors caused a further reduction without changing the hyperemic stimulus. Similar responses were observed in a separate group of patients with heart failure.
Taken together, these results suggest that the degree of NO dependence of RH-FMD varies and that EDHFs, specifically EETs, may make an important contribution. Further studies are required to elucidate the factors that influence interindividual variability in reliance on a particular vasodilator pathway.
SS-FMD.
Only one study has directly compared the mechanisms responsible for RH-FMD versus SS-FMD. Mullen et al. (60) assessed the involvement of NO in radial artery FMD in response to a variety of shear stress stimuli. Although l-NMMA nearly abolished radial artery RH-FMD in response to the hyperemia after release of a 5-min occlusion, FMD in protocols that involved a more sustained increase in shear stress (i.e., shear stress elevated by hand heating and hyperemia after release of 15 min of occlusion) were unaffected by NO synthase inhibition, suggesting that SS-FMD is mediated via non-NO mechanisms.
Studying the mechanisms of SS-FMD only, Bellien et al. (5) examined the contributions of both NO and an EDHF pathway. They observed a downward shift in the radial artery shear stress-SS-FMD relationship with either l-NMMA infusion or inhibition of EET production (via inhibition of CYP2C9 with fluconazole) or action (via inhibition of Ca2+-activated K+ channels with tetraethylammonium). Combined eNOS and EDHF pathway inhibition resulted in a further blunting of SS-FMD. The reduced SS-FMD during l-NMMA infusion conflicts with the findings of Mullen et al. (60); however, Bellien et al. (5) administered a higher dose of l-NMMA, which could have elicited a more complete eNOS inhibition. Using a similar protocol, Bellien et al. (7) reproduced these results in normotensive control participants but found that in patients with essential hypertension, inhibition of NO but not EET production significantly reduced their already smaller hand-heating SS-FMD. This suggests that there is a reduced contribution of EETs to SS-FMD in patients with hypertension.
SS-FMD may also reflect a balance between vasodilating and vasoconstricting factors. Endothelin-1 (ET-1) is a potent vasoconstrictor produced by endothelial cells that can be released in response to shear stress (52). The balance and distribution of ET-1 receptors determine its vasoactive effects. Vascular smooth muscle cell ET-1 type A and type B receptors elicit vasoconstriction (58, 78). In contrast, endothelial cell ET-1 type B receptor binding stimulates vasodilator formation and clears ET-1 from the circulation (i.e., they are involved in ET-1 uptake) (58, 89). Selective ET-1 type A receptor antagonism does not appear to influence RH-FMD in young, healthy individuals (82). However, Bellien et al. (6) found that in healthy men, ET-1 type B receptor blockade prevented a decrease in local ET-1 concentration during hand heating and reduced radial artery SS-FMD (the latter was prevented with coinhibition of ET-1 type A receptors). This suggests an important role for ET-1 type B receptors in ET-1 uptake and mitigating ET-1 constriction via ET-1 type A receptor binding during a sustained increase in shear stress. In patients with essential hypertension and other cardiovascular risk factors, the local ET-1 concentration was not found to decrease with heating, which may suggest an alteration in ET-1 release or uptake (6, 7). Whether a similar importance of ET-1 type B receptors in mitigating ET-1-mediated vasoconstriction is present in RH-FMD or in individuals at increased cardiovascular risk (i.e., hypertension) is unknown. There is, however, some evidence that ET-1 may contribute to decreases in RH-FMD with age (19). The role of vasoconstricting (ET-1) factors and receptor subtypes in SS-FMD and RH-FMD merits further investigation.
Concerning SS-FMD stimulated by handgrip exercise, in agreement with the findings of Bellien et al. (5, 7), there is evidence that NO makes an important contribution (100). Wray et al. (100) infused l-NMMA in the brachial artery of young, healthy adults during 3 min of rhythmic handgrip exercise at six levels of increasing workload separated by 1 min of rest. SS-FMD was significantly lower during l-NMMA infusion at the three highest shear rates (workloads of ~30–50% maximum voluntary contraction) compared with control, highlighted by a ~70% lower SS-FMD at the highest shear rates. Importantly, shear rate was similar between control and l-NMMA infusion trials at each workload. Thus, in young, healthy adults, handgrip exercise SS-FMD in the brachial artery appears to be partially NO dependent. Using a similar progressive handgrip exercise technique with l-NMMA infusion in elderly (mean age: 69 yr) individuals, Trinity et al. (93) found a 30% lower SS-FMD at the highest workload (~50% maximum voluntary contraction) during l-NMMA infusion compared with control. Thus, the contribution of NO to SS-FMD appears to diminish with healthy aging.
There is also evidence of a vasodilatory role of reactive oxygen species (ROS) in SS-FMD that is influenced by aging. Progressive handgrip exercise SS-FMD was blunted after oral antioxidant ingestion without altering shear rate in young, healthy adults, suggesting that ROS play an important role in exercise-induced SS-FMD (72). This may involve H2O2, which is produced in response to shear stress exposure (10) and can cause vasodilation via NO-dependent and NO-independent (hyperpolarization) pathways (11). In contrast, oral antioxidant treatment and ascorbic acid infusion increased exercise-induced SS-FMD in sedentary elderly participants (20, 92). However, similar to the observations in younger adults, when elderly participants were exercise trained, the effect of antioxidant administration was reversed and resulted in attenuated SS-FMD.
The disparate influence of antioxidant administration on SS-FMD may reflect an age/training-related distinction in redox balance (20) wherein sedentary elderly are experiencing deleterious levels of oxidative stress and antioxidant administration improves the imbalance, permitting enhanced vasodilation, perhaps via an increase in NO bioavailability. In contrast, in young and trained elderly groups, in the absence of excess oxidative stress, antioxidant administration may interfere with ROS signaling involved in vasodilation (91). Whether ROS signaling functions in the same way for RH-FMD is unclear. Similar to the SS-FMD findings, antioxidant administration has been shown to improve RH-FMD in sedentary elderly participants (23). Furthermore, in elderly men, Wray et al. (98) found that RH-FMD was impaired by antioxidant administration after, but not before, 6 wk of exercise training. This is in contrast with the findings of Eskurza et al. (23), who reported that antioxidant administration did not alter RH-FMD in young or endurance-trained older men. The nature of the antioxidant administration (oral vs. infused, single vs. cocktail) may contribute to the conflicting results.
In summary, there is evidence indicating that both RH- and SS-FMD can have a NO- and EDHF (EET)-dependent component, although individual heterogeneity and cross talk between vasodilator pathways makes it difficult to clearly identify whether the proportionate contributions differ as shear stress duration increases. In addition, although NO appears to be involved in both RH-FMD and SS-FMD, the underlying pathways eliciting NO synthesis may be distinct (see The shear stress profile and Ca2+-dependent versus Ca2+-independent NO production below). Furthermore, there is evidence to suggest that handling of ET-1 by ET-1 type B receptors may be important for SS-FMD, as this remains unexplored for RH-FMD. Antioxidant administration studies have suggested that ROS signaling plays a vasodilatory role in SS-FMD in young and trained elderly groups. Although ROS signaling could also contribute to RH-FMD, sustained shear stress, perhaps in response to exercise in particular, may provide a more potent stimulus for endothelial ROS generation and involvement in conduit artery vasodilation (20, 72).
Additional studies directly comparing the mechanisms involved in RH-FMD versus SS-FMD are required in healthy and disease populations to define the importance of stimulus duration for the predominance of a given vasodilatory mechanism. Such studies examining SS-FMD must consider the potential for vasodilatory mechanisms to differ with different magnitudes of shear stress and/or to transition over time. For example, the predominant FMD mechanism may differ early and late in a sustained stimulus, and the timing or nature of the transition could be altered by disease. A better understanding of the mechanisms mediating FMD in response to RH versus sustained increases in shear stress is critical for interpretation of data as well for deciding which test may be most suitable based on the research question.
The shear stress profile and Ca2+-dependent versus Ca2+-independent NO production.
The profile of the shear stress stimulus appears to influence the Ca2+ dependence of resulting NO production. Human umbilical vein endothelial cells subjected to impulse, step, and ramped increases in flow demonstrate biphasic NO production. A step increase in shear stress causes an initial transient burst in NO production that is Ca2+, calmodulin, and G protein dependent (28, 53). Slower rates of onset and sustained shear stress appear to evoke a distinct stimulus for augmenting NO production that is Ca2+, calmodulin, and G protein independent (28, 53). One intriguing hypothesis is that Ca2+-dependent and Ca2+-independent eNOS regulation may localize in distinct pools of the enzyme. Plasma membrane eNOS appears to produce Ca2+-activated NO synthesis, whereas Golgi-associated eNOS is primarily activated by Ca2+-independent phosphorylation (29). Indeed, in isolated resistance vessels, plasma membrane eNOS, associated with Ca2+-dependent NO production in response to an abrupt increase in shear stress, appears to translocate to the cytosol and Golgi complex under conditions of prolonged shear stress, continuing to produce NO via a Ca2+-independent pathway (24). Functionally, plasma membrane-restricted eNOS is more susceptible to changes in membrane cholesterol and modified LDL compared with Golgi-associated eNOS (102). Whether distinct shear stress profiles preferentially target separate pools of eNOS, or other vasodilators, in humans is intriguing and would provide detailed biological insight on endothelial function in health and disease.
It is possible that RH-FMD and SS-FMD result in primarily Ca2+-dependent and Ca2+-independent NO production by plasma membrane versus Golgi or cytosol localized eNOS, respectively. The differential sources and pathways to increase NO raises the interesting possibility that distinct shear stimuli may provide information on NO bioavailability from unique intracellular sources, although work is needed to establish the link between in vitro and in vivo studies. Putative differences in mechanotransduction and vasodilator production pathways with distinct shear stress stimuli may underlie the apparent disparate sensitivity of RH-FMD and SS-FMD to certain conditions and acute interventions.
Summary and Conclusions
Although the majority of human FMD research has used transient increases in shear stress created with RH, limb heating, distal vasodilator infusion, and exercise provide effective means to create sustained increases in conduit artery shear stress for FMD assessment (SS-FMD). SS-FMD is physiologically relevant, reflecting how conduit arteries respond to heat and exercise stress. There is now an abundance of evidence suggesting that FMD in response to transient (RH-FMD) and sustained (SS-FMD) increases in shear stress can be impacted differently by various chronic conditions and acute interventions. In some cases, the evidence suggests that FMD impairment may be isolated to SS-FMD or that impairment in SS-FMD may develop before RH-FMD is impaired. Although more research is required to clarify the overlap/distinction in primary vasodilators, in vitro evidence indicates that the signaling pathways involved in transducing transient and sustained shear stress profiles differ, and this may underlie the distinct vulnerability of RH-FMD and SS-FMD to various vascular insults. Collectively, the evidence suggests that RH-FMD and SS-FMD reflect distinct aspects of endothelial function, and characterizing both responses may provide more comprehensive insight. Future research is required to clearly distinguish the signaling pathways involved and to explore the utility of SS-FMD, compared with RH-FMD, in predicting cardiovascular events.
GRANTS
J. Trembaly was supported by a Natural Sciences and Engineering Research Council of Canada CGS-D scholarship, and K. Pyke was supported by a Natural Sciences and Engineering Research Council of Canada Discovery grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.T. and K.E.P. conceived and designed research; J.C.T. prepared figures; J.C.T. drafted manuscript; J.C.T. and K.E.P. edited and revised manuscript; J.C.T. and K.E.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Trevor J. King for the assistance in preparing Fig. 2.
REFERENCES
- 1.Ade CJ, Brown MG, Ederer AK, Hardy RN, Reiter LK, Didier KD. Influence of prior anterograde shear rate exposure on exercise-induced brachial artery dilation. Physiol Rep 3: e12414, 2015. doi: 10.14814/phy2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando J, Yamamoto K. Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. doi: 10.1093/cvr/cvt084. [DOI] [PubMed] [Google Scholar]
- 3.Bellien J, Costentin A, Dutheil-Maillochaud B, Iacob M, Kuhn J-M, Thuillez C, Joannides R. Early stage detection of conduit artery endothelial dysfunction in patients with type 1 diabetes. Diab Vasc Dis Res 7: 158–166, 2010. doi: 10.1177/1479164109360470. [DOI] [PubMed] [Google Scholar]
- 4.Bellien J, Iacob M, Eltchaninoff H, Bourkaib R, Thuillez C, Joannides R. AT1 receptor blockade prevents the decrease in conduit artery flow-mediated dilatation during NOS inhibition in humans. Clin Sci (Lond) 112: 393–401, 2007. doi: 10.1042/CS20060236. [DOI] [PubMed] [Google Scholar]
- 5.Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, Joannides R. Crucial role of NO and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension 48: 1088–1094, 2006. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- 6.Bellien J, Iacob M, Monteil C, Rémy-Jouet I, Roche C, Duflot T, Vendeville C, Gutierrez L, Thuillez C, Richard V, Joannidès R. Physiological role of endothelin-1 in flow-mediated vasodilatation in humans and impact of cardiovascular risk factors. J Hypertens 35: 1204–1212, 2017. doi: 10.1097/HJH.0000000000001307. [DOI] [PubMed] [Google Scholar]
- 7.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L, Vendeville C, Dreano Y, Mercier A, Thuillez C, Joannides R. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation 125: 1266–1275, 2012. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 8.Bellien J, Thuillez C, Joannides R. Role of endothelium-derived hyperpolarizing factor in the regulation of radial artery basal diameter and endothelium-dependent dilatation in vivo. Clin Exp Pharmacol Physiol 35: 494–497, 2008. doi: 10.1111/j.1440-1681.2008.04903.x. [DOI] [PubMed] [Google Scholar]
- 9.Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 10.Bretón-Romero R, González de Orduña C, Romero N, Sánchez-Gómez FJ, de Álvaro C, Porras A, Rodríguez-Pascual F, Laranjinha J, Radi R, Lamas S. Critical role of hydrogen peroxide signaling in the sequential activation of p38 MAPK and eNOS in laminar shear stress. Free Radic Biol Med 52: 1093–1100, 2012. doi: 10.1016/j.freeradbiomed.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 68: 26–36, 2005. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. doi: 10.1161/01.RES.78.3.415. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 15.Clausen P, Feldt-Rasmussen B, Iversen J, Lange M, Eidemak I, Strandgaard S. Flow-associated dilatory capacity of the brachial artery is intact in early autosomal dominant polycystic kidney disease. Am J Nephrol 26: 335–339, 2006. doi: 10.1159/000094402. [DOI] [PubMed] [Google Scholar]
- 16.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 17.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation 80: 458–465, 1989. doi: 10.1161/01.CIR.80.3.458. [DOI] [PubMed] [Google Scholar]
- 18.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 19.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. doi: 10.1042/cs1010629. [DOI] [PubMed] [Google Scholar]
- 22.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. doi: 10.1152/ajpheart.00771.2005. [DOI] [PubMed] [Google Scholar]
- 23.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa XF, González DR, Puebla M, Acevedo JP, Rojas-Libano D, Durán WN, Boric MP. Coordinated endothelial nitric oxide synthase activation by translocation and phosphorylation determines flow-induced nitric oxide production in resistance vessels. J Vasc Res 50: 498–511, 2013. doi: 10.1159/000355301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Findlay BB, Gupta P, Szijgyarto IC, Pyke KE. Impaired brachial artery flow-mediated vasodilation in response to handgrip exercise-induced increases in shear stress in young smokers. Vasc Med 18: 63–71, 2013. doi: 10.1177/1358863X13480259. [DOI] [PubMed] [Google Scholar]
- 26.Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Müller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. Eur J Heart Fail 9: 770–775, 2007. doi: 10.1016/j.ejheart.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res 49: 525–533, 2004. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Frangos JA, Huang TY, Clark CB. Steady shear and step changes in shear stimulate endothelium via independent mechanisms−superposition of transient and sustained nitric oxide production. Biochem Biophys Res Commun 224: 660–665, 1996. doi: 10.1006/bbrc.1996.1081. [DOI] [PubMed] [Google Scholar]
- 29.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem 279: 30349–30357, 2004. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 30.Gaenzer H, Neumayr G, Marschang P, Sturm W, Kirchmair R, Patsch JR. Flow-mediated vasodilation of the femoral and brachial artery induced by exercise in healthy nonsmoking and smoking men. J Am Coll Cardiol 38: 1313–1319, 2001. doi: 10.1016/S0735-1097(01)01575-3. [DOI] [PubMed] [Google Scholar]
- 31.Gielen S, Hambrecht R. Effects of exercise training on vascular function and myocardial perfusion. Cardiol Clin 19: 357–368, 2001. doi: 10.1016/S0733-8651(05)70222-8. [DOI] [PubMed] [Google Scholar]
- 32.Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation 103: e1–e6, 2001. doi: 10.1161/01.CIR.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 33.Gonzales JU, Miedlar JA, Parker BA, Proctor DN. Relation of femoral diameter, shear rate, and dilatory response to knee extensor exercise. Med Sci Sports Exerc 42: 1870–1875, 2010. doi: 10.1249/MSS.0b013e3181dd1c99. [DOI] [PubMed] [Google Scholar]
- 34.Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest 83: 1946–1952, 1989. doi: 10.1172/JCI114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ. Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension 63: 376–382, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 36.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 37.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123: 87–97, 2011. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris RA, Padilla J, Rink LD, Wallace JP. Variability of flow-mediated dilation measurements with repetitive reactive hyperemia. Vasc Med 11: 1–6, 2006. doi: 10.1191/1358863x06vm641oa. [DOI] [PubMed] [Google Scholar]
- 40.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002. doi: 10.1016/S0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 42.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 43.Jazuli F, Pyke KE. The impact of baseline artery diameter on flow-mediated vasodilation: a comparison of brachial and radial artery responses to matched levels of shear stress. Am J Physiol Heart Circ Physiol 301: H1667–H1677, 2011. doi: 10.1152/ajpheart.00487.2011. [DOI] [PubMed] [Google Scholar]
- 44.Joannides R, Bakkali el-H, Richard V, Benoist A, Moore N, Thuillez C. Evaluation of the determinants of flow-mediated radial artery vasodilatation in humans. Clin Exp Hypertens 19: 813–826, 1997. doi: 10.3109/10641969709083188. [DOI] [PubMed] [Google Scholar]
- 45.Joannides R, Bizet-Nafeh C, Costentin A, Iacob M, Derumeaux G, Cribier A, Thuillez C. Chronic ACE inhibition enhances the endothelial control of arterial mechanics and flow-dependent vasodilatation in heart failure. Hypertension 38: 1446–1450, 2001. doi: 10.1161/hy1201.096529. [DOI] [PubMed] [Google Scholar]
- 46.Joannides R, Costentin A, Iacob M, Compagnon P, Lahary A, Thuillez C. Influence of vascular dimension on gender difference in flow-dependent dilatation of peripheral conduit arteries. Am J Physiol Heart Circ Physiol 282: H1262–H1269, 2002. doi: 10.1152/ajpheart.00209.2001. [DOI] [PubMed] [Google Scholar]
- 47.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. doi: 10.1161/01.CIR.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 48.King TJ, Schmitter SM, Pyke KE. Assessment of flow-mediated dilatation in the superficial femoral artery using a sustained shear stress stimulus via calf plantar-flexion exercise. Exp Physiol 102: 725–737, 2017. doi: 10.1113/EP085980. [DOI] [PubMed] [Google Scholar]
- 49.King TJ, Slattery DJ, Pyke KE. The impact of handgrip exercise duty cycle on brachial artery flow-mediated dilation. Eur J Appl Physiol 113: 1849–1858, 2013. doi: 10.1007/s00421-013-2612-0. [DOI] [PubMed] [Google Scholar]
- 50.Kocaman O, Oflaz H, Yekeler E, Dursun M, Erdogan D, Demirel S, Alisir S, Turgut F, Mercanoglu F, Ecder T. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 43: 854–860, 2004. doi: 10.1053/j.ajkd.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Kooijman M, Thijssen DHJ, de Groot PCE, Bleeker MWP, van Kuppevelt HJM, Green DJ, Rongen GA, Smits P, Hopman MTE. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells Am J Physiol Heart Circ Physiol 264: H150–H156, 1993. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 53.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol Cell Physiol 266: C628–C636, 1994. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 54.Lauer T, Heiss C, Preik M, Balzer J, Hafner D, Strauer BE, Kelm M. Reduction of peripheral flow reserve impairs endothelial function in conduit arteries of patients with essential hypertension. J Hypertens 23: 563–569, 2005. doi: 10.1097/01.hjh.0000160213.40855.b7. [DOI] [PubMed] [Google Scholar]
- 55.Lee JF, Barrett-O’Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 102: 278–284, 2016. doi: 10.1136/heartjnl-2015-308403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorthioir A, Joannidès R, Rémy-Jouet I, Fréguin-Bouilland C, Iacob M, Roche C, Monteil C, Lucas D, Renet S, Audrézet M-P, Godin M, Richard V, Thuillez C, Guerrot D, Bellien J. Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int 87: 465–472, 2015. doi: 10.1038/ki.2014.241. [DOI] [PubMed] [Google Scholar]
- 57.Machin DR, Clifton HL, Garten RS, Gifford JR, Richardson RS, Wray DW, Frech TM, Donato AJ. Exercise-induced brachial artery blood flow and vascular function is impaired in systemic sclerosis. Am J Physiol Heart Circ Physiol 311: H1375–H1381, 2016. doi: 10.1152/ajpheart.00547.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol 84: 147–162, 2012. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 60.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. doi: 10.1161/01.RES.88.2.145. [DOI] [PubMed] [Google Scholar]
- 61.Ne JYA, Cai TY, Celermajer DS, Caterson ID, Gill T, Lee CMY, Skilton MR. Obesity, arterial function and arterial structure - a systematic review and meta-analysis. Obes Sci Pract 3: 171–184, 2017. doi: 10.1002/osp4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. A comparison between active- and reactive-hyperaemia-induced brachial artery vasodilation. Clin Sci (Lond) 110: 387–392, 2006. doi: 10.1042/CS20050328. [DOI] [PubMed] [Google Scholar]
- 63.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 301: H1118–H1126, 2011. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: evidence, mechanisms and importance. Int J Psychophysiol 88: 124–135, 2013. doi: 10.1016/j.ijpsycho.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O’Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010. doi: 10.1152/ajpheart.00571.2009. [DOI] [PubMed] [Google Scholar]
- 66.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 67.Pyke KE, Hartnett JA, Tschakovsky ME. Are the dynamic response characteristics of brachial artery flow-mediated dilation sensitive to the magnitude of increase in shear stimulus? J Appl Physiol 105: 282–292, 2008. doi: 10.1152/japplphysiol.01190.2007. [DOI] [PubMed] [Google Scholar]
- 68.Pyke KE, Jazuli F. Impact of repeated increases in shear stress via reactive hyperemia and handgrip exercise: no evidence of systematic changes in brachial artery FMD. Am J Physiol Heart Circ Physiol 300: H1078–H1089, 2011. doi: 10.1152/ajpheart.00736.2010. [DOI] [PubMed] [Google Scholar]
- 69.Pyke KE, Poitras V, Tschakovsky ME. Brachial artery flow-mediated dilation during handgrip exercise: evidence for endothelial transduction of the mean shear stimulus. Am J Physiol Heart Circ Physiol 294: H2669–H2679, 2008. doi: 10.1152/ajpheart.01372.2007. [DOI] [PubMed] [Google Scholar]
- 70.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 72.Richardson RS, Donato AJ, Uberoi A, Wray DW, Lawrenson L, Nishiyama S, Bailey DM. Exercise-induced brachial artery vasodilation: role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 73.Romero SA, Gagnon D, Adams AN, Moralez G, Kouda K, Jaffery MF, Cramer MN, Crandall CG. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am J Physiol Heart Circ Physiol 313: H658–H666, 2017. doi: 10.1152/ajpheart.00234.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Savage MV, Brengelmann GL. Reproducibility of the vascular response to heating in human skin. J Appl Physiol 76: 1759–1763, 1994. doi: 10.1152/jappl.1994.76.4.1759. [DOI] [PubMed] [Google Scholar]
- 75.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 77.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol 536: 937–946, 2001. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 89: 1203–1208, 1994. doi: 10.1161/01.CIR.89.3.1203. [DOI] [PubMed] [Google Scholar]
- 79.Shiode N, Morishima N, Nakayama K, Yamagata T, Matsuura H, Kajiyama G. Flow-mediated vasodilation of human epicardial coronary arteries: effect of inhibition of nitric oxide synthesis. J Am Coll Cardiol 27: 304–310, 1996. doi: 10.1016/0735-1097(95)00465-3. [DOI] [PubMed] [Google Scholar]
- 80.Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 35: 125–131, 1997. doi: 10.1016/S0008-6363(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 81.Slattery DJ, Stuckless TJR, King TJ, Pyke KE. Impaired handgrip exercise-induced brachial artery flow-mediated dilation in young obese males. Appl Physiol Nutr Metab 41: 528–537, 2016. doi: 10.1139/apnm-2015-0459. [DOI] [PubMed] [Google Scholar]
- 82.Spieker LE, Hürlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Lüscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 105: 2817–2820, 2002. doi: 10.1161/01.CIR.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 83.Stuckless TJ, Pyke KE. The impact of a cold pressor test on brachial artery handgrip exercise-induced flow-mediated dilation. Vasc Med 20: 409–416, 2015. doi: 10.1177/1358863X15586473. [DOI] [PubMed] [Google Scholar]
- 84.Szijgyarto IC, King TJ, Ku J, Poitras VJ, Gurd BJ, Pyke KE. The impact of acute mental stress on brachial artery flow-mediated dilation differs when shear stress is elevated by reactive hyperemia versus handgrip exercise. Appl Physiol Nutr Metab 38: 498–506, 2013. doi: 10.1139/apnm-2012-0328. [DOI] [PubMed] [Google Scholar]
- 85.Szijgyarto IC, Poitras VJ, Gurd BJ, Pyke KE. Acute psychological and physical stress transiently enhances brachial artery flow-mediated dilation stimulated by exercise-induced increases in shear stress. Appl Physiol Nutr Metab 39: 927–936, 2014. doi: 10.1139/apnm-2013-0384. [DOI] [PubMed] [Google Scholar]
- 86.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 88.Tinken TM, Thijssen DHJ, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tirapelli CR, Casolari DA, Yogi A, Montezano AC, Tostes RC, Legros E, D’Orléans-Juste P, de Oliveira AM. Functional characterization and expression of endothelin receptors in rat carotid artery: involvement of nitric oxide, a vasodilator prostanoid and the opening of K+ channels in ETB-induced relaxation. Br J Pharmacol 146: 903–912, 2005. doi: 10.1038/sj.bjp.0706388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Totosy de Zepetnek JO, Ditor DS, Au JS, MacDonald MJ. Impact of shear rate pattern on upper and lower limb conduit artery endothelial function in both spinal cord-injured and able-bodied men. Exp Physiol 100: 1107–1117, 2015. doi: 10.1113/EP085056. [DOI] [PubMed] [Google Scholar]
- 91.Trinity JD, Broxterman RM, Richardson RS. Regulation of exercise blood flow: role of free radicals. Free Radic Biol Med 98: 90–102, 2016. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Zhao J, Richardson RS. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am J Physiol Heart Circ Physiol 310: H765–H774, 2016. doi: 10.1152/ajpheart.00817.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trinity JD, Wray DW, Witman MAH, Layec G, Barrett-O’Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305: R893–R899, 2013. doi: 10.1152/ajpregu.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verbeke FH, Agharazii M, Boutouyrie P, Pannier B, Guérin AP, London GM. Local shear stress and brachial artery functions in end-stage renal disease. J Am Soc Nephrol 18: 621–628, 2007. doi: 10.1681/ASN.2006040400. [DOI] [PubMed] [Google Scholar]
- 96.Vita JA, Hamburg NM. Does endothelial dysfunction contribute to the clinical status of patients with peripheral arterial disease? Can J Cardiol 26, Suppl A: 45A–50A, 2010. doi: 10.1016/S0828-282X(10)71062-X. [DOI] [PubMed] [Google Scholar]
- 97.Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol 273: H156–H163, 1997. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]
- 98.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 99.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol 99: 81–86, 2005. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 100.Wray DW, Witman MAH, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. doi: 10.1152/ajpheart.01115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wray DW, Witman MAH, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol 26: 1015–1021, 2006. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]