Abstract
Syndecan-4 (Synd4) is a member of the membrane-spanning, glycocalyx-forming proteoglycan family. It has been suggested that Synd4 participates in renal fibrosis. We compared wild-type and fibrosis-prone endothelial sirtuin 1-deficient (Sirt1endo−/−) mice, the latter being a model of global endothelial dysfunction. We performed mass spectrometry analysis, which revealed that Synd4 was highly enriched in the secretome of renal microvascular endothelial cells obtained from Sirt1endo−/− mice upon stimulation with transforming growth factor-β1; notably, all detectable peptides were confined to the ectodomain of Synd4. Elevated Synd4 was due to enhanced NF-κB signaling in Sirt1endo−/− mice, while its shedding occurred as a result of oxidative stress in Sirt1 deficiency. Synd4 expression was significantly enhanced after unilateral ureteral obstruction compared with contralateral kidneys. Furthermore, hyperplasia of renal myofibroblasts accompanied by microvascular rarefaction and overexpression of Synd4 were detected in Sirt1endo−/− mice. The ectodomain of Synd4 acted as a chemoattractant for monocytes with higher levels of macrophages and higher expression levels of Synd4 in the extracellular matrix of Sirt1endo−/− mice. In vitro, ectodomain application resulted in generation of myofibroblasts from cultured renal fibroblasts, while in vivo, subcapsular injection of ectodomain increased interstitial fibrosis. Moreover, the endothelial glycocalyx was reduced in Sirt1endo−/− mice, highlighting the induction of Synd4 occurring in parallel with the depletion of its intact form and accumulation of its ectodomain in Sirt1endo−/− mice. On the basis of our experimental results, we propose that it is the Synd4 ectodomain per se that is partially responsible for fibrosis in unilateral ureteral obstruction, especially when it is combined with endothelial dysfunction.
NEW & NOTEWORTHY Our findings suggest that endothelial dysfunction induces the expression of syndecan-4 via activation of the NF-κB pathway. Furthermore, we show that syndecan-4 is shed to a greater amount because of increased oxidative stress in dysfunctional endothelial cells and that the release of the syndecan-4 ectodomain leads to tubulointerstitial fibrosis.
Keywords: endothelial glycocalyx, sirtuin 1, tubulointerstitial fibrosis, vascular endothelium
INTRODUCTION
Syndecans are a family of membrane-spanning proteoglycans with glycosaminoglycans covalently bound to their ectodomain and components of focal adhesion complexes recruited to their intracellular domain (10, 42). Syndecan-4 (Synd4) is a ubiquitous member of the family and is highly expressed in endothelial and epithelial cells in the kidney and other organs (19, 40). Its main functions include those of a coreceptor for fibroblast growth factor, binding and concentration on the cell surface of heparin-binding growth factors, such as VEGFs and PDGFs, and establishment of focal adhesions between the extracellular matrix and intracellular cytoskeleton. This explains the observations that cells deficient in Synd4 exhibit morphological abnormalities and migration defects (9), whereas mice with Synd4 knockout exhibited delayed wound repair and increased sensitivity to LPS and to myocardial ischemia (8, 14, 24). On this background, it has recently been reported that Synd4 knockout protects against tubulointerstitial fibrosis (33). Since the functions of Synd4 ectodomain, cleaved constitutively, but especially under inflammatory conditions, are probably distinct from those of the whole molecule membrane-spanning form, we sought to examine whether either of those could be incriminated in fibrogenesis.
On the basis of previous demonstration of the link between sirtuin 1 (Sirt1) and Synd4 through the regulation of NF-κB (15, 30, 37), we selected mice expressing catalytically defective Sirt1 in endothelial cells, which have been shown to represent a model of global endothelial dysfunction and exaggerated fibrogenesis (6, 39). Sirt1, a member of a larger family of deacetylases, is a NAD+-dependent enzyme with cytosolic and nuclear localization and a multitude of metabolic functions. Sirt1 activity and expression are reduced in aging and diverse diseases, from cardiovascular to neoplastic (12). Potente et al. (32) and our group (39) have previously established mice with the excised exon 4 of Sirt1, which disables its catalytic activity. When crossed with Tie-2/Cre mice, the abnormality of Sirt1-induced deacetylation is limited predominantly to endothelial cells. The Sirt1endo−/− mouse model has a broad applicability because endothelial Sirt1 deficiency is a constant companion of a broad range of chronic cardiovascular, metabolic, and renal diseases (6, 39). Sirt1-deficient endothelial cells exhibit all criteria of dysfunction, such as reduced endothelium-dependent vasorelaxation; microvascular rarefaction; impaired endothelial cell migration, sprouting, and matrilytic activity; premature senescence; and an exaggerated fibrotic response (6, 18, 32, 39). In view of the fact that Sirt1endo−/− mice have an exaggerated, endothelium-initiated fibrotic response, we examined the function of Synd4 under basal conditions and after a fibrogenic stimulus in these mice. Our findings are consistent with Synd4 ectodomain participation in renal fibrosis and highlight the existing mechanistic link between endothelial cells in their dysfunctional state, the loss of glycocalyx, and fibrogenesis via accumulation of Synd4 ectodomain in the renal extracellular matrix.
MATERIALS AND METHODS
Reagents and antibodies.
The following antibodies were used: CD31 (BD Pharmingen no. 553369, BD Biosciences), F4/80 (no. 14-4801-82, eBioscience), p65 (no. 8242S, Cell Signaling Technology), PDGF receptor-β (PDGFRβ; provided by Dr. W. Stallcup, Sanford Burnham Institute, La Jolla, CA), Synd4 (sc-12766, Santa Cruz Biotechnology), and α-smooth muscle actin (α-SMA; ab5694, Abcam).
The following reagents were used: collagenase type H (Roche), collagenase/dispase (Roche), DMEM (no. 30-2002, American Type Culture Collection), DMEM-F-12 (Gibco), Dynabeads anti-rat IgG (no. 11035, Invitrogen), Dynabeads anti-rabbit IgG (no. 11203D, Invitrogen), Iscove’s modified Dulbecco’s medium (IMDM; no. 12440-053, Invitrogen/Gibco), recombinant human insulin (no. 12585-014, Invitrogen/Gibco), human transferrin (no. 03-0124SA, Invitrogen/Gibco), recombinant mouse VEGF (PMG0115, Invitrogen/Gibco), basic fibroblast growth factor (PMG0035, Invitrogen/Gibco), epidermal growth factor (PMG8045, Invitrogen/Gibco), Matrigel (no. 356231, Corning), transforming growth factor (TGF)-β (no. 240-B-002, R&D Systems), PMA (P-8139, Sigma-Aldrich), and recombinant mouse Synd4 ectodomain (no. 6267-SD-050, R&D Systems).
Animals.
The endothelial Sirt1-deleted mouse model was established by crossbreeding B6;129-Sirt1tm1Ygu/J mice with Tie2-Cre transgenic mice expressing Cre recombinase in vascular endothelial cells (both from Jackson Laboratory) (39). The resulting Sirt1endo+/− mice were mated with Sirt1Flox/Flox mice to gain endothelium-deleted Sirt1endo−/− mutant mice. All animal experiments were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and with approval by the New York Medical College Institutional Animal Care and Use Committee.
Isolation of renal microvascular endothelial cells or fibroblasts and preparation of cell-conditioned medium.
Primary renal microvascular endothelial cells (RMVECs) were isolated from adult mouse kidneys using magnetic beads. To prepare a single cell suspension, kidneys were decapsulated, minced, and incubated (37°C, 40 min) with collagenase type H (1 mg/ml), collagenase/dispase (1 mg/ml), 2% FBS, and penicillin-streptomycin (100 g/ml) in DMEM-F-12 medium. After digestion was stopped by 0.5 M EDTA (pH 7.5), the single cell suspension was filtered through a cell strainer (40 μm, Fisher Scientific) to remove glomeruli and arterioles, centrifuged, and resuspended in DMEM (American Type Culture Collection). Purified anti-CD31 antibodies were incubated with Dynabeads anti-rat IgG (4°C, overnight) before the cell isolation procedure. Cells were incubated with CD31 antibody-bead complex (4°C, 1 h), and a positive selection was performed by a Magnetic Particle Concentrator (Invitrogen).
Purified RMVECs were cultured on gelatin-coated plates in IMDM containing 15% FBS, 1% BSA, 0.01 mg/ml recombinant human insulin, 0.2 mg/ml human transferrin, 1× penicillin-streptomycin, and 100 ng/ml each of recombinant mouse VEGF, basic fibroblast growth factor, and epidermal growth factor at 37°C in a humidified 5% CO2 atmosphere. The purity of primary cultures was confirmed by staining for CD31. If its purity was <85%, a second positive selection was performed 5 days after the first cell isolation. Cells were used between passages 1 and 3.
Primary fibroblasts were isolated from kidneys of α-SMA-green fluorescent protein mice using the above-described methods. A kidney single cell suspension was incubated with the PDGFRβ antibody-bead complex (4°C, 1 h). PDGFRβ antibodies were preincubated with Dynabeads anti-rabbit IgG (4°C, overnight). Primary fibroblasts were cultured on Matrigel-coated plates in DMEM containing 10% FBS at 37°C in a humidified 5% CO2 atmosphere.
RMVECs from 1) control wild-type (WT) and 2) Sirt1endo−/− mice were expanded and exposed to TGF-β (5 ng/ml, 48-h incubation), and conditioned medium was collected. The latter was analyzed using tandem mass spectrometry (MS/MS). In brief, we aspirated the culture medium and added 10 ml of prewarmed serum-free IMDM per 10-cm dish. The serum-free medium was exchanged three times, each lasting for 30-min incubation in the CO2 incubator, and then replaced with IMDM containing 50 nM PMA for stimulation of protein secretion from cells, according to Tunica et al. (38). Cells were incubated for 1 h in the CO2 incubator; subsequently, conditioned medium was collected and centrifuged at 300 g for 5 min at 4°C, and supernatants were collected and stored at −80°C.
MS of the secretome of RMVECs.
Tryptic digests were analyzed using Thermo Scientific Q Exactive, and the tandem mass spectra were extracted. All MS/MS samples were analyzed using Mascot (version 2.4.1, Matrix Science, London, UK). Mascot was set up to search the Swiss-Prot 2015_07 database (selected for Mus musculus, 16,723 entries) with trypsin as the digestion enzyme. Mascot was searched with a fragment ion mass tolerance of 0.020 Da and a parent ion tolerance of 10.0 ppm. Carbamidomethyl of cysteine was specified in Mascot as a fixed modification. Oxidation of methionine was specified in Mascot as a variable modification.
Scaffold (version Scaffold_4.4.5, Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. The software normalizes the MS/MS data between samples. This allows us to compare abundances of a protein between samples. The normalization scheme used works for the common experimental situation where individual proteins may be upregulated or downregulated but the total amount of all proteins in each sample is about the same, as was the case in our experiments. There are two levels of “sample” in Scaffold. The MS level is the sample run through the mass spectrometer and the second level is the biological sample, and we performed replicates in both levels. Frequently, the biological sample is fractionated into multiple MS samples. Scaffold allows us to view the MS samples within a biological sample or to combine all MS samples into a single sample using the “MuDPIT” option. Normalization is done on the MS sample level. The normalization method that Scaffold uses is to sum the “unweighted spectrum counts” for each MS sample. These sums are then scaled so that they are all the same. The scaling factor for each sample is then applied to each protein group and adjusts its unweighted spectrum count to a normalized “quantitative value.”
Peptide identifications were accepted if they could be established at >80.0% probability to achieve a false discovery rate of <1.0% by the Scaffold Local false discovery rate algorithm. Protein identifications were accepted if they could be established at >28.0% probability to achieve a false discovery rate of <1.0% and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (27). Proteins that contained similar peptides and could not be differentiated on the basis of MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters. Proteins were annotated with Gene Ontology terms from the National Center for Biotechnology Information downloaded on July 21, 2015, and August 11, 2015 (2).
Unilateral ureteral obstruction and subcapsular injection of the Synd4 ectodomain.
Unilateral ureteral obstruction (UUO) was performed as previously described (17). Briefly, under general 2% isoflurane anesthesia, a left flank incision was made, and the left ureter was ligated with two 4-0 silk ties. Wounds were sutured, and the skin was closed. Mice were euthanized 10 days postoperatively.
For subcapsular injection of the Synd4 ectodomain, a left and right flank incision was made, the kidneys were visualized, and 10 µl of 1 µg/ml Synd4 ectodomain or 10 µl of vehicle solution (1× PBS) were injected at the lower pole of the left or right kidney, respectively. The wounds were sutured, and the skin was closed. Surgeries were performed under general 2% isoflurane anesthesia, and mice were euthanized 5 days after injection.
Fluorescence microscopy, immunocytochemistry, and immunohistochemistry.
Kidneys were fixed in periodate-lysine-paraformaldehyde (PFA) and cryosectioned (8-μm-thick sections). Sections were blocked with 5% BSA in PBS for 1 h at room temperature followed by incubation with the primary antibody overnight at 4°C. The following primary antibodies were used: 1:100 dilution of CD31, 1:200 dilution of F4/80, 1:100 dilution of PDGFRβ, and 1:50 dilution of Synd4. Sections were incubated with the appropriate secondary antibody (Alexa Fluor 488, Alexa Fluor 594, and Alexa Fluor 647 at a 1:400 dilution) for 1 h at room temperature. Nuclei were counterstained with Hoechst or 4′,6-diamidino-2-phenylindole solution (1:1,000 dilution) for 10 min at room temperature.
Renal fibroblasts were fixed in 4% PFA for 15 min at room temperature after 48 h of treatment with 1 µg/ml of the Synd4 ectodomain or vehicle solution (1× PBS). Cells were permeabilized with 0.25% Triton X-100 for 15 min at room temperature and blocked with 5% BSA in PBS for 1 h. Afterward, cells were incubated with primary antibody (α-SMA, 1:200 dilution) for 1 h at 4°C followed by incubation with the appropriate secondary antibody (Alexa Fluor 488) for 1 h at room temperature. Nuclei were counterstained as described above.
RMVECs isolated from WT and Sirt1endo−/− mouse kidneys were fixed in 4% PFA for 15 min at room temperature after 48 h of treatment with 5 ng/ml TGF-β. Cells were blocked with 5% BSA in PBS for 1 h. Afterward, cells were incubated with primary antibody (p65, 1:200 dilution) diluted in PBS consisting of 3% FBS and 0.1% Triton X-100 for 30 min at room temperature followed by incubation with the appropriate secondary antibody (Alexa Fluor 594) for 45 min at room temperature according to Maguire et al. (22). Nuclei were counterstained as described above.
Images were acquired using a compound Nikon microscope (TE-2000U) equipped with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) or with a Nikon microscope (90i eclipse) equipped with a high-definition color camera (Nikon DS-Fi2). Images were analyzed using Adobe Photoshop and NIH ImageJ software.
Picrosirius red staining.
Kidneys were perfused with ice-cold PBS and removed, and midcoronal sections were fixed in 4% PFA overnight followed by incubation in 70% ethanol (EtOH) and embedment in paraffin. Paraffin sections (4 μm thick) were deparaffinized and rehydrated using the following series of washes: xylene 2 × 10 min, 100% EtOH 2 × 5 min, 95% EtOH 2 × 5 min, 70% EtOH 1 × 5 min, and distilled water 1 × 5 min. Sections were then stained with hematoxylin for 10 min followed by collagen staining with 5% picrosirius red (Sigma) diluted in 5% acetic acid at room temperature for 1 h. After washing the remnant picrosirius red with 0.5% acetic acid, tissue sections were dehydrated with the remaining picrosirius red with 0.5% acetic acid in 70% EtOH 1 × 30 s, 100% EtOH 1 × 30 s, and xylene 1 × 30 s and mounted in xylene. The fibrotic tubulointerstitial areas were imaged using polarizing microscopy and quantified using area fraction analysis by NIH ImageJ.
Dihydroethidium expression in RMVECs.
RMVECs were seeded on a 96-well plate precoated with 1% gelatin and pretreated with 50 μM Sirt1 inhibitor III (no. 566322, EMD Millipore) and 5 ng/ml TGF-β for 48 h. Cells were incubated with the superoxide-sensing fluoroprobe dihydroethidium (DHE; 1:1,000 dilution in DMEM) for 20 min at 37°C and washed twice with PBS, and the fluorescence intensity of DHE was measured.
Quantitative PCR analyses.
cDNA was isolated from whole kidney samples of uninjured contralateral kidneys or UUO kidneys. Total RNA was extracted using TRIzol (Invitrogen). Purity of RNA was determined by the ratio of absorbance at 260 nm to that at 280 nm. After cDNA was synthesized, quantitative PCR was performed by a Mx3000P qPCR system (Stratagene) and PerfeCta SYBR Green FastMix (Quanta Bioscience). Expression of each transcript was normalized to that of 18S rRNA (Rn18S). The following primers were used: Rn18S, forward primer 5′-AAGGAGACTCTGGCATGCTAAC-3′ and reverse primer 5′-CAGACATCTAAGGGCATCACAGAC-3′; and Synd4 (Mm00488527_m1, ThermoFisher Scientific). The fold change in gene expression was determined using the method (where Ct is threshold cycle).
Total volume of the glycocalyx.
We used the comparative analysis of volume of distribution of glycocalyx-permeating and glycocalyx-nonpermeating tracers, as developed by Nieuwdorp and colleagues (28, 29) and recently modified by us (35). A total of 100 µl of fluorescent-labeled dextran solution (3.75 mg/ml of 40-kDa dextran-Texas red and 3.75 mg/ml of 70-kDa dextran-FITC in 1% albumin-PBS solution) were injected into the left jugular vein of anesthetized male C57BL/6 mice. Blood samples (50 µl) were drawn from the right jugular vein at 3, 5, 10, and 15 min. The heparinized blood samples were then immediately centrifuged (10 min at 3,000 rpm), and 20 µl of plasma were collected and stored at −20°C until further analysis. Plasma samples were diluted to 1:10 in PBS in a 96-well plate. The fluorescent intensity was measured at 575/620 nm for 40-kDa dextran-Texas red and 485/540 nm for 70-kDa dextran-FITC. The concentrations of both dextrans in each sample were calculated from linear least-squares fitting determined by fluorescence measurement of serially diluted dextran solution in PBS. The concentration (C)-time (t) curve for each dextran was constructed and fitted with the following least-squares monoexponential function: C = C0e−λt. The initial volume of distribution for each dextran was calculated from the total amount of injected dextran (0.375 mg) divided by the concentration at time 0 (C0). The difference between the initial volume of distribution for each dextran represents the whole body glycocalyx volume (Vglycocalyx = 0.375/C1e−λ1t – 0.375/C2e−λ2t, t = 0). To illustrate with an example, ideal calculations approximate the 40-kDa monoexponential curve to be approximately C40 kDa = 0.48e−0.03t; ideal calculations approximate the 70-kDa curve to be C70 kDa = 0.52e−0.01t. The initial volumes of distribution (Vd) at t = 0 are 0.78 ml (0.375/0.48) and 0.72 ml (0.375/0.52) for 40- and 70-kDa dextran, respectively. Subtracting Vd,70 kDa from Vd,40 kDa revealed the glycocalyx volume to be 0.06 ml (60 µm3).
Statistical analysis.
Values are given as means ± SD unless otherwise mentioned. Data were analyzed using an independent t-test or ANOVA with post hoc analysis for multiple-group comparisons using the Bonferroni method. P values of <0.05 were considered statistically significant. All statistical analyses were performed with NCSS 10 (NCSS, Kaysville, UT).
RESULTS
Induction of Synd4 in the secretome of RMVECs of Sirt1endo−/− mice.
Considering the fact that Sirt1endo−/− mice have enhanced fibrogenesis, it is apparent that their dysfunctional endothelial cells transmit signals that activate fibroblasts (6, 18, 39). To determine the molecular identities of those signals, we performed unbiased proteomic analysis of cell secretomes. Specifically, we isolated RMVECs from WT and Sirt1endo−/− mice, collected conditioned media under resting conditions or after activation with TGF-β1 (5 ng/ml), and performed MS analysis, as we have previously reported (20). Analysis of peptides present in the trypsin-digested secretome of isolated endothelial cells revealed that Synd4 was highly enriched in the secretome of RMVECs obtained from Sirt1endo−/− mice upon stimulation with TGF-β1 (Fig. 1). This finding suggested that Synd4, and especially its soluble form, may represent one of the transmitted signals that activate fibroblasts and participate in the profibrogenic phenotype of these mice. The peptide sequences of Synd4 were not as highly detected as other proteins (Fig. 2); however, as a major proteoglycan component of the endothelial glycocalyx and an important binding partner, we decided to further investigate Synd4 in RMVECs and in UUO kidneys.
Fig. 1.
Syndecan-4 (Synd4) is elevated in the secretome of endothelial sirtuin 1-deficient (Sirt1endo−/−) mice. A: schematic representation of the secretome analysis. B: quantification of the spectral counts. MS/MS, tandem mass spectrometry; RMVECs, renal microvascular endothelial cells; TGF-β, transforming growth factor-β; WT, wild-type mice. ***P < 0.05.
Fig. 2.
Highest enriched proteins in the secretome of endothelial sirtuin 1-deficient (Sirt1endo−/−) mice. Compared with other proteins presented on the volcano plot, the number of peptides that were sequenced for syndecan-4 (Sdc4) was less. This impacts the position on the plot. In Fig. 1, where we compared the spectral counts of syndecan-4 in different groups, the difference was more pronounced. In other words, if we have a highly secreted protein, this will show a higher significance in the volcano plot than a protein that is secreted in lower amounts, even if the latter is secreted only in one treatment group, as was the case with syndecan-4. CT, control (wild type); ST, Sirt1−/− without transforming growth factor-β.
Synd4 expression in mouse kidneys under basal conditions and after UUO.
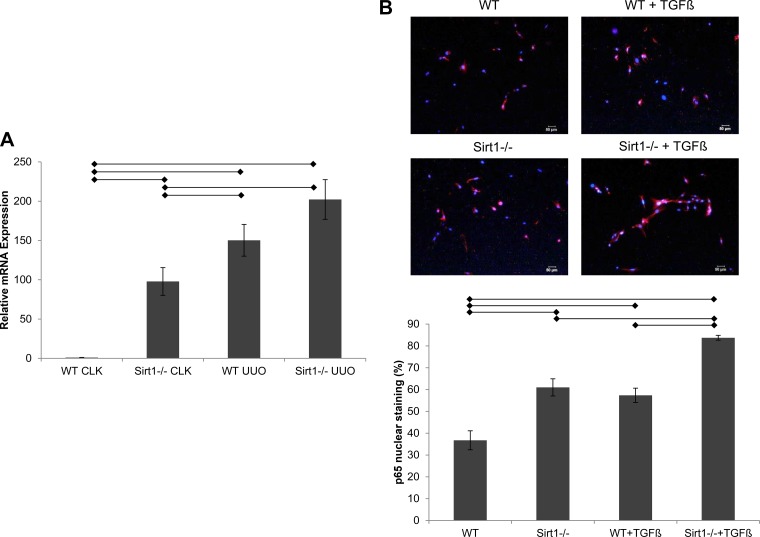
We examined the expression of Synd4 transcripts in mouse kidneys obtained from WT and Sirt1endo−/− mice, with or without UUO, and performed quantitative real-time PCR. We found that the Synd4 message level was almost 100-fold higher in Sirt1endo−/− mice than in WT mice under basal conditions. Moreover, in UUO kidneys, the relative mRNA expression was increased 150- and 200-fold in WT and Sirt1endo−/− mice, respectively (Fig. 3A). These results show not only an increase in the message level of Synd4 after UUO but also an increase in the uninjured Sirt1endo−/− kidney, supporting the hypothesis that endothelial Sirt1 deficiency induces the expression of Synd4.
Fig. 3.
Expression of syndecan-4 transcripts and p65 nuclear translocation. A: expression of syndecan-4 was examined by quantitative real-time PCR. cDNA was isolated from whole kidney samples of uninjured contralateral (CLK) or unilateral ureteral obstruction (UUO) kidneys of the respective groups (n = 5). Horizontal lines indicate statistical significance (P < 0.05). B: nuclear translocation of p65 in renal microvascular endothelial cells (RMVECs) of the respective groups. RMVECs with positive p65 nuclear stain were counted manually and divided by the total amount of RMVECs of the respective image (n = 4). Scale bar = 50 µm. Horizontal lines indicate statistical significance (P < 0.05). Sirt1−/−, sirtuin 1-deficient mice; TGF-β, transforming growth factor-β; WT, wild-type mice.
To account for the selective induction of Synd4 in Sirt1endo−/− mice, we performed experiments of NF-κB nuclear translocation. It has been established that the NF-κB response element is present in the promoter region of Synd4 (37). Moreover, Sirt1 was found to deacetylate p65 protein, disabling its transcriptional activity (4, 44) and thus inhibiting NF-κB-dependent inflammatory reactions (30, 44). Therefore, we inferred that in Sirt1-deficient cells, deacetylation of p65 is decreased, leading to its liberation and nuclear translocation. Hence, we examined p65 in RMVECs isolated from WT and Sirt1endo−/− mice. As shown in Fig. 3B, nuclear localization of p65 was significantly elevated in unstimulated RMVECs isolated from Sirt1endo−/− mice and further increased after stimulation with TGF-β1 (5 ng/ml). Notably, the difference in p65 nuclear localization between WT and Sirt1endo−/− RMVECs before and after stimulation with TGF-β1 remained constant, suggesting that it is the intrinsic Sirt1 deficiency that promotes p65 translocation. Collectively, these findings indicate the state of NF-κB-dependent Synd4 superinduction in Sirt1endo−/− mice and RMVECs and the appearance of a secretory form of Synd4 in conditioned media obtained from Sirt1-deficient RMVECs.
Immunohistochemical experiments, in agreement with the above data, showed that the expression of Synd4 in UUO was significantly increased (Fig. 4, A–D). In addition to this, UUO resulted in a significant expansion of the number of myofibroblasts, as judged by staining for PDGFRβ, and in microvascular rarefaction, as judged by CD31 staining, thus confirming our previous observations (18, 39). In Sirt1endo−/− mice, however, all these changes were dramatically amplified: accumulation of myofibroblasts, microvascular rarefaction, and overexpression of immunodetectable Synd4 were all significantly more profound in these mice, as was their fibrogenic response to UUO, as we have previously reported (18, 39). As shown in Fig. 4E, in normal kidneys, one-half of immunodetectable Synd4 was coexpressed with the endothelial marker CD31. In WT UUO kidneys, the proportion of Synd4 coexpressed with the endothelium was increased, in contrast to Sirt1endo−/− kidneys.
Fig. 4.
Syndecan-4 (Synd4) expression is increased in unilateral ureteral obstruction (UUO) kidneys (quadruple staining). A: representative images of PDGF receptor-β (PDGFRβ)-, CD31-, Synd4-, and Hoechst-stained contralateral (CLK) and UUO kidney sections from wild-type (WT) and endothelial sirtuin 1-deficient (Sirt1endo−/−) mice (n = 4). Scale bar = 10 µm. B: quantification of Synd4 fluorescence intensity per cell. A Synd4-stained image was merged with the respective Hoechst-stained image. Cells with Synd4-positive staining were counted manually. Finally, the measured total integrated density of Synd4 staining was divided by the respective total amount of Synd4-positive cells. Horizontal lines indicate statistical significance (P < 0.05). C: quantification of PDGFR intensity per cell. Horizontal lines indicate statistical significance (P < 0.05). D: quantification of CD31 intensity per cell. Horizontal lines indicate statistical significance (P < 0.05). E: costaining of Synd4 in CD31-positive cells. After the total amount of CD31-positive cells was counted, as previously described, the respective Synd4-stained image was merged with the already merged CD31/Hoechst image. Cells with CD31/Synd4-positive staining were counted manually plus cells with Synd4 staining only. With the total amount of CD31-positive and Synd4-positive cells, the percentage of the costained cells was calculated. *P < 0.05.
Following these immunohistochemical findings, we isolated RMVECs from WT and Sirt1endo−/− mouse kidneys, exposed them to 5 ng/ml TGF-β for 48 h, and stained RMVECs for Synd4. In both types of RMVEC, immunodetectable Synd4 increased after exposure to TGF-β, indicating increased synthesis by RMVECs (Fig. 5). Notably, the amount of Synd4 was significantly reduced in Sirt1-deficient RMVECs at the basal state compared with WT RMVECs. Considering the global elevation of Synd4 staining of UUO kidneys in these mice and the diverse levels of Synd4 in WT and Sirt1-deficient RMVECs, the question that arises is the identity of the compartment that is responsible for increased Synd4 staining.
Fig. 5.
Syndecan-4 (Synd4) expression increases in renal microvascular endothelial cells (RMVECs) after stimulation with transforming growth factor-β (TGF-β). A: representative images of CD31- and Synd4-stained RMVECs from wild-type (WT) and endothelial sirtuin 1-deficient (Sirt1endo−/−) mice with and without TGF-β stimulation (n = 4). Scale bar = 50 µm. B: quantification of the relative Synd4 fluorescence intensity. DAPI, 4′,6-diamidino-2-phenylindole. *P < 0.05.
Increased deposition of the Synd4 ectodomain in the extracellular matrix.
It has been previously demonstrated that the Synd4 ectodomain can be cleaved within the 15-amino acid juxtamembrane region by several sheddases, such as matrix metalloproteinase (MMP)-2, MMP-9, a disintegrin and metalloproteinase domain-containing protein-17 (ADAM17), a disintegrin and metalloproteinase with a thrombospondin type 1 motif-1 (ADAMTS1), ADAMTS4, thrombin, and plasmin, to be retained in the extracellular matrix (31). To verify whether elevated levels of immunodetectable Synd4 were attributable to the accumulation of ectodomain in the interstitial space, we next measured the immunofluorescence intensity of Synd4 staining in the interstitial compartment demarcated as nuclear stain-free areas between the tubules of UUO and contralateral control kidneys. Notably, the antibodies used were raised against the ectodomain, and kidney sections were nonpermeabilized. As shown in Fig. 6A, the intensity of Synd4 immunofluorescence localized to the interstitium of UUO kidneys was increased twofold and threefold in WT and Sirt1endo−/− mice, respectively. Indeed, these findings are in agreement with the MS data revealing elevated levels of Synd4 in the secretome of activated endothelial cells from Sirt1endo−/− mice.
Fig. 6.
Detection of the syndecan-4 (Synd4) ectodomain. A: quantification of the interstitial percentage of Synd4 and representative images of PDGF receptor-β (PDGFRβ)-, CD31-, Synd4-, and Hoechst-stained contralateral (CLK) and unilateral ureteral obstruction (UUO) kidney sections from wild-type (WT) and endothelial sirtuin 1-deficient (Sirt1endo−/−) mice (n = 4). Scale bar = 10 µm. Arrows indicate profound Synd4 staining in the interstitial space. A Synd4-stained image was merged with the respective Hoechst-stained image. The total area of the Synd4 signal was measured using ImageJ software. In the next step, the area of the Synd4 signal coexpressed with cells was quantified and subtracted from the total area to obtain the interstitial area. Finally, the percentage was calculated (n = 4). Horizontal lines indicate statistical significance (P < 0.05). B: protein sequences of Synd4 domains. Bold and underlined sequences were detected by mass spectrometry. Note that they cover 13% of all amino acids and are confined to the ectodomain sequence. C: representative images and quantification of dihydroethidium (DHE) fluorescence in renal microvascular endothelial cells treated with or without a Sirt1 inhibitor (n = 4). Scale bar = 50 µm. Horizontal lines indicate statistical significance (P < 0.05). TGF, transforming growth factor.
To further validate this assumption, we reanalyzed the proteomic findings and compared the detected peptide amino acid sequences to that of Synd4. We found that both detected sequences belong to the extracellular domain of Synd4 (Fig. 6B), suggesting that its ectodomain could be shed under UUO stress and predominantly in Sirt1endo−/− mice.
It has been previously demonstrated that activity of ADAM17, one of the major Synd4 sheddases, is induced by oxidative stress (41). Therefore, we inquired whether increased oxidative stress in Sirt1-deficient endothelial cells contributed to the shedding of Synd4. To accomplish this, we treated WT RMVECs with 50 μM Sirt1 inhibitor III for 48 h and measured superoxide anion production using DHE. RMVECs treated with the Sirt1 inhibitor showed an eightfold higher DHE fluorescence intensity compared with RMVECs without Sirt1 inhibition (Fig. 6C). The addition of TGF-β further potentiated the intensity of DHE fluorescence in Sirt1-inhibited RMVECs. These findings support our hypothesis of greater oxidative stress contributing to greater shedding of Synd4 in Sirt1-deficient endothelial cells.
In addition, it has been previously demonstrated that cleaved ectodomain of Synd4 represents a powerful chemoattractant for monocytes/macrophages (36). Hence, we examined F4/80-positive infiltrating cells in the kidneys (Fig. 7). F4/80 fluorescence was marginal in all uninjured kidneys. In UUO kidneys of WT mice, F4/80 staining was elevated 2.5-fold. A dramatic 10-fold increase in infiltrating F4/80-positive cells was detected in UUO kidneys of Sirt1endo−/− mice. Moreover, ~25% Synd4-positive cells were detected in areas enriched in F4/80-positive cells in WT UUO kidneys, whereas almost 50% of Synd4-positive cells localized in the areas enriched in F4/80-positive cells in Sirt1endo−/− UUO kidneys. These data are consistent with the chemotactic potential of the Synd4 ectodomain and its excessive accumulation in the interstitium of obstructed Sirt1endo−/− mouse kidneys.
Fig. 7.
Triple staining of unilateral ureteral obstruction (UUO) kidneys. F4/80 expression was enhanced in UUO kidneys of endothelial sirtuin 1-deficient (Sirt1endo−/−) mice. A: representative images of F4/80- and syndecan-4 (Synd4)-stained contralateral (CLK) and UUO kidney sections from wild-type (WT) and Sirt1endo−/− mice (n = 4). Scale bar = 10 µm. No permeabilization was used. B: quantification of F4/80 intensity per cell. A F4/80-stained image was merged with the respective Hoechst-stained image. Cells with F4/80-positive staining were counted manually. Finally, the measured total integrated density of F4/80 staining was divided by the respective total amount of F4/80-positive cells. Horizontal lines indicate statistical significance (P < 0.05). C: costaining of Synd4 in F4/80-positive cells. After the total amount of F4/80-positive cells was counted, as previously described, the respective Synd4-stained image was merged with the already merged F4/80/Hoechst image. Cells with F4/80/Synd4-positive staining were counted manually plus cells with Synd4 staining only. With the total amount of F4/80-positive and Synd4-positive cells, the percentage of the costained cells was calculated.
Sirt1 deficiency and induced Synd4 shedding are associated with an impaired endothelial glycocalyx.
Considering the role of proteoglycans and specifically Synd4 in the maintenance of the integrity of the glycocalyx, where it serves as a scaffold, we next argued that if indeed the cell surface expression of Synd4 is reduced in Sirt1endo−/− mice, this could lead to a reduction in the glycocalyx. Thus, we examined the total volume of this structural layer in the whole animal using a fluorophore dilution technique, as detailed above in materials and methods. The premise behind this experiment was that if the above conclusions regarding an imbalance between the reduced quantity of intact Synd4 and increased extracellular pool of its ectodomain in Sirt1endo−/− mice is correct, that should affect the structure critically enriched in it, the endothelial glycocalyx. Toward this end, glycocalyx-permeating 40-kDa dextran-Texas red and glycocalyx-nonpermeating 70-kDa dextran-FITC were coinjected, and the volume of distribution for each was determined during the fast equilibration period of 3–5 min. As shown in Fig. 8A, the volume of the endothelial glycocalyx was significantly reduced already at the basal state in Sirt1endo−/− mice. This finding is consistent with the presented body of evidence highlighting the induction of Synd4 occurring in parallel with the depletion of its intact form and accumulation of its extracellular fragment, the ectodomain, in Sirt1endo−/− mice and explains the lower levels of Synd4 in Sirt1-deficient RMVECs under basal conditions. In fact, the observed impairment of the endothelial glycocalyx in these mice may, in part, explain their vasculopathic phenotype (6, 18, 32, 39).
Fig. 8.
Sirtuin deficiency affects the endothelial glycocalyx, and the syndecan-4 (Synd4) ectodomain induces fibrosis. A: glycocalyx volume was diminished in endothelial sirtuin 1-deficient (Sirt1endo−/−) mice. Quantification of the glycocalyx volume (n = 5). *P < 0.05. Data are expressed as medians, interquartile ranges, and ranges. B: α-smooth muscle actin (α-SMA) was increased in renal fibroblasts after exposure to 1 µg/ml of the Synd4 ectodomain (n = 5). Scale bar = 50 µm. *P < 0.05. C: increased interstitial fibrosis after subcapsular injection of 1 µg/ml of the Synd4 ectodomain (n = 4). Scale bar = 10 µm. *P < 0.05. GFP, green fluorescent protein; WT, wild-type mice.
Effects of the Synd4 ectodomain on renal fibroblasts.
To elucidate the possible profibrogenic effects of the ectodomain on renal fibroblasts, we isolated renal fibroblasts and exposed them to 1 µg/ml of the Synd4 ectodomain in accordance with Schellings et al. (34). Forty-eight hours after exposure, we fixed the cells and stained for α-SMA. We observed a ninefold increase of the α-SMA signal after Synd4 ectodomain exposure, indicating a higher transition of fibroblasts to myofibroblasts (Fig. 8B). Furthermore, we performed a subcapsular injection of 1 µg/ml of the Synd4 ectodomain and stained for picrosirius red 5 days after injection. As shown in Fig. 8C, the amount of interstitial fibrosis was greater in Synd4 ectodomain-treated kidneys compared with control kidneys. These results emphasize the pathogenic potential of the Synd4 ectodomain, even in the absence of other noxious stimuli, to induce a profibrotic phenotype.
DISCUSSION
Surprisingly little information exists on heparan sulfate proteoglycans in kidney disease. The role of proteoglycans and one of their major members, Synd4, in a variety of pathological processes has been a subject of a score of investigations. Synd4 transcript and protein expression are known to be induced by proinflammatory cytokines, in the processes of tissue remodeling and wound healing (1, 3, 7, 11, 23). Overexpression of Synd4 preserves heart function in a model of myocardial infarction (43). Synd4 knockout mice show delayed wound repair, increased LPS-induced mortality, and susceptibility to cardiac rupture after myocardial infarction (8, 14, 24). It has been argued that these deleterious effects of Synd4 deletion could be due, at least in part, to the absence of its extracellular ectodomain (37), rather than the absence of the whole Synd4 molecule.
The data presented herein show that endothelial Sirt1 deficiency, which aggravates renal fibrosis in UUO (39), serves as a superinducer of this proteoglycan. We observed a dramatic >6-fold increase in Synd4 in the secretome of TGF-β-activated microvascular endothelial cells isolated from kidneys of Sirt1endo−/− mice, and we confirmed Synd4 induction in UUO kidneys. Despite the upregulation of Synd4, a major proteoglycan component of the endothelial glycocalyx and a binding partner of glycosaminoglycans comprising this structure, the expression of the glycocalyx was dramatically reduced in Sirt1endo−/− mice. The above finding suggests that the integrity of this membrane-spanning glycocalyx-scaffolding molecule may be impaired, which is in accordance with our findings of decreased levels of Synd4 in Sirt1-deficient RMVECs. Pari passu, the experiments revealed a novel consequence of Sirt1 deficiency, loss of the endothelial glycocalyx, thus adding to a long list of endothelial abnormalities in this condition. One of the likely scenarios that explain the paradox of increased synthesis of Synd4 and its reduced cell-associated expression with enhanced appearance in the secretome of dysfunctional RMVECs is suggested by our finding of an 8- and 14-fold increase in superoxide generation under basal conditions and after cell stimulation, respectively. This is consistent with the known role of Sirt1 in deacetylating forkhead box O1 (FoxO1), a DNA-binding protein necessary for posttranslational modification of this transcription factor, which is required for its active modification and enhanced cellular defense against oxidative stress (5, 26). Deficient Sirt1 expression or activity in diverse vasculopathies (25) results in the sustained oxidative stress. The latter, in turn, leads to activation of a redox-sensitive sheddase, ADAM17 (41). Activation of this enzyme is ultimately responsible for the shedding of Synd4 (16).
Remarkably, promoter regions of both Sirt1 and Synd4 harbor NF-κB response element (37). In fact, Sirt1 deacetylates p65 protein and disables its transcriptional activity (4, 44). Consistent with this, activation of Sirt1 results in the inhibition of NF-κB-dependent inflammatory reactions (30, 44). The opposite is also true: inhibition of Sirt1 enhances NF-κB signaling, which induces activation of heparanase, one of the multiple targets of this transcription factor (15), and further degradation of the endothelial glycocalyx. These complex relations between Sirt1 and NF-κB may explain why Synd4 is upregulated in Sirt1endo−/− mice, as shown here (Fig. 9). UUO, by providing an additional independent stimulus for the activation of NF-κB signaling, further enhances Synd4 expression but at the same time activates even more mechanisms of its shedding and digestion of heparan sulfate. These mechanisms lead to potential pharmacological targets. Treatment with Sirt-activating compounds would inhibit NF-κB signaling and therefore lower Synd4 expression. The administration of antioxidants would reduce the activity of sheddases and therefore decrease the amount of shed Synd4 ectodomain.
Fig. 9.
Schematic representation of the proposed relations between sirtuin 1 (Sirt1), NF-κB, and syndecan-4 (Synd4). A: normally, Sirt1 deacetylates p65 and therefore prevents its nuclear translocation. HA, hyaluronic acid; HS, heparan sulfate. B: in Sirt1 deficiency, p65 translocates to the nucleus and increases the transcription of Synd4. Moreover, increased oxidative stress in Sirt1 deficiency induces the activity of a disintegrin and metalloproteinase domain-containing protein-17 (ADAM17) sheddase, leading to a greater amount of shedding and consequently to accumulation of interstitial form of cleaved Synd4, increased chemoattraction, and fibrosis.
It is intriguing to compare our findings with previously published data demonstrating that Synd4 knockout protects against tubulointerstitial fibrosis and results in reduced tissue transglutaminase cross-linking activity (33). Superficially, it contrasts with our findings implicating Synd4 in microvascular rarefaction and fibrosis. Actually, however, both sets of data emphasize the role played by the extracellularly deposited ectodomain of Synd4 in orchestrating fibrogenesis. It should be noted that Synd4 knockout mice, used in those and other studies, were generated through deletion of exons II and III with a portion of exon IV, all encoding for the ectodomain (13). Hence, both the loss of function (in Synd4 knockout) and gain of function, albeit accompanied by increased shedding (in Sirt1 deficiency), studies argue in favor of profibrotic actions of the Synd4 ectodomain.
The physiology of the Synd4 ectodomain is noteworthy because it is highly distinct from functions of the whole molecule. The ectodomain of Synd4 promotes collagen cross-linking and activates proinflammatory signaling cascades, stimulating immune cell infiltration and therefore exhibiting a central role in chemotaxis (21, 36). With the reduction of the endothelial glycocalyx and therefore reduced luminal barrier, immune cell infiltration is exhibited even more. On the basis of our experimental results and the existing body of published work, we speculate that it is the Synd4 ectodomain rather than Synd4 per se that is partially responsible for fibrosis in UUO and especially so when it is combined with endothelial dysfunction. It is this phenomenon that highlights the existing mechanistic link between dysfunctional endothelial cells and fibrogenesis via the loss of the glycocalyx and accumulation of the Synd4 ectodomain in the renal extracellular matrix.
GRANTS
This work was supported in part by the Dr. Werner Jackstädt Foundation (to M. Lipphardt); “ILJIN” Faculty Research Assistance Program of Yonsei University College of Medicine for 2013 Grant 6-2013-0068 and Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Science, ICT and Future Planning Grant NRF-2013R1A1A1010863 (to J. W. Song); American Heart Association Grant 12SDG9080006, American Society of Nephrology Grant 010973-101, and the Westchester Community Foundation-Renal Clinical Fund (New York Community Trust) (to B. B. Ratliff); and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54602, DK-052783, and DK-45462 (to M. S. Goligorsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L., H.D., G.A.M., and M.S.G. conceived and designed research; M.L., J.W.S., H.D., and M.S.G. performed experiments; M.L., J.W.S., H.D., and M.S.G. analyzed data; M.L., J.W.S., B.B.R., H.D., G.A.M., and M.S.G. interpreted results of experiments; M.L., J.W.S., H.D., and M.S.G. prepared figures; M.L. and M.S.G. drafted manuscript; M.L., J.W.S., B.B.R., H.D., G.A.M., and M.S.G. edited and revised manuscript; M.L., J.W.S., B.B.R., H.D., G.A.M., and M.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Yujiro Kida for the preliminary exploration of UUO mice.
REFERENCES
- 1.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol 39: 505–528, 2007. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777, 1999. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem 284: 2657–2671, 2009. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol 180: 973–983, 2012. doi: 10.1016/j.ajpath.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26: 89–114, 2010. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 8.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest 107: R9–R14, 2001. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elfenbein A, Rhodes JM, Meller J, Schwartz MA, Matsuda M, Simons M. Suppression of RhoG activity is mediated by a syndecan 4-synectin-RhoGDI1 complex and is reversed by PKCα in a Rac1 activation pathway. J Cell Biol 186: 75–83, 2009. doi: 10.1083/jcb.200810179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elfenbein A, Simons M. Syndecan-4 signaling at a glance. J Cell Sci 126: 3799–3804, 2013. doi: 10.1242/jcs.124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Götte M. Syndecans in inflammation. FASEB J 17: 575–591, 2003. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 12.Haigis MC, Guarente LP. Mammalian sirtuins: emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Tsuzuki S, Nakamura E, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency impairs focal adhesion formation only under restricted conditions. J Biol Chem 275: 5249–5252, 2000. doi: 10.1074/jbc.275.8.5249. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem 276: 47483–47488, 2001. doi: 10.1074/jbc.M106268200. [DOI] [PubMed] [Google Scholar]
- 15.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25: 1939–1948, 2013. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Kawahara R, Lima RN, Domingues RR, Pauletti BA, Meirelles GV, Assis M, Figueira AC, Paes Leme AF. Deciphering the role of the ADAM17-dependent secretome in cell signaling. J Proteome Res 13: 2080–2093, 2014. doi: 10.1021/pr401224u. [DOI] [PubMed] [Google Scholar]
- 17.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24: 559–572, 2013. doi: 10.1681/ASN.2012080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kida Y, Zullo JA, Goligorsky MS. Endothelial sirtuin 1 inactivation enhances capillary rarefaction and fibrosis following kidney injury through Notch activation. Biochem Biophys Res Commun 478: 1074–1079, 2016. doi: 10.1016/j.bbrc.2016.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell 5: 797–805, 1994. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipphardt M, Song JW, Matsumoto K, Dadafarin S, Dihazi H, Müller G, Goligorsky MS. The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int 92: 558–568, 2017. doi: 10.1016/j.kint.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunde IG, Herum KM, Carlson CC, Christensen G. Syndecans in heart fibrosis. Cell Tissue Res 365: 539–552, 2016. doi: 10.1007/s00441-016-2454-2. [DOI] [PubMed] [Google Scholar]
- 22.Maguire O, Collins C, O’Loughlin K, Miecznikowski J, Minderman H. Quantifying nuclear p65 as a parameter for NF-κB activation: correlation between ImageStream cytometry, microscopy, and Western blot. Cytometry A 79: 461–469, 2011. doi: 10.1002/cyto.a.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J 277: 3876–3889, 2010. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, Kojima T, Tsutsui H, Uede T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ Res 108: 1328–1339, 2011. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 25.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13, 2007. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563, 2004. doi: 10.1016/S0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 27.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55: 1127–1132, 2006. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55: 480–486, 2006. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Yu H, Huang S, Zhu P. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One 11: e0147034, 2016. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piperigkou Z, Mohr B, Karamanos N, Götte M. Shed proteoglycans in tumor stroma. Cell Tissue Res 365: 643–655, 2016. doi: 10.1007/s00441-016-2452-4. [DOI] [PubMed] [Google Scholar]
- 32.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarpellini A, Huang L, Burhan I, Schroeder N, Funck M, Johnson TS, Verderio EA. Syndecan-4 knockout leads to reduced extracellular transglutaminase-2 and protects against tubulointerstitial fibrosis. J Am Soc Nephrol 25: 1013–1027, 2014. doi: 10.1681/ASN.2013050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schellings MW, Vanhoutte D, van Almen GC, Swinnen M, Leenders JJ, Kubben N, van Leeuwen RE, Hofstra L, Heymans S, Pinto YM. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension 55: 249–256, 2010. doi: 10.1161/HYPERTENSIONAHA.109.137885. [DOI] [PubMed] [Google Scholar]
- 35.Song JW, Zullo JA, Liveris D, Dragovich M, Zhang XF, Goligorsky MS. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther 361: 115–121, 2017. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strand ME, Aronsen JM, Braathen B, Sjaastad I, Kvaløy H, Tønnessen T, Christensen G, Lunde IG. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J Mol Cell Cardiol 88: 133–144, 2015. doi: 10.1016/j.yjmcc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Strand ME, Herum KM, Rana ZA, Skrbic B, Askevold ET, Dahl CP, Vistnes M, Hasic A, Kvaløy H, Sjaastad I, Carlson CR, Tønnessen T, Gullestad L, Christensen G, Lunde IG. Innate immune signaling induces expression and shedding of the heparan sulfate proteoglycan syndecan-4 in cardiac fibroblasts and myocytes, affecting inflammation in the pressure-overloaded heart. FEBS J 280: 2228–2247, 2013. doi: 10.1111/febs.12161. [DOI] [PubMed] [Google Scholar]
- 38.Tunica DG, Yin X, Sidibe A, Stegemann C, Nissum M, Zeng L, Brunet M, Mayr M. Proteomic analysis of the secretome of human umbilical vein endothelial cells using a combination of free-flow electrophoresis and nanoflow LC-MS/MS. Proteomics 9: 4991–4996, 2009. doi: 10.1002/pmic.200900065. [DOI] [PubMed] [Google Scholar]
- 39.Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol 25: 276–291, 2014. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuong TT, Reine TM, Sudworth A, Jenssen TG, Kolset SO. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J Histochem Cytochem 63: 280–292, 2015. doi: 10.1369/0022155415568995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol 182: 2449–2457, 2009. doi: 10.4049/jimmunol.0802770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol 13: 578–583, 2001. doi: 10.1016/S0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 43.Xie J, Wang J, Li R, Dai Q, Yong Y, Zong B, Xu Y, Li E, Ferro A, Xu B. Syndecan-4 over-expression preserves cardiac function in a rat model of myocardial infarction. J Mol Cell Cardiol 53: 250–258, 2012. doi: 10.1016/j.yjmcc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]