Abstract
Knee injuries and chronic disorders, such as arthritis, affect millions of Americans, leading to missed workdays and reduced quality of life. Currently, after an initial diagnosis, there are few quantitative technologies available to provide sensitive subclinical feedback to patients regarding improvements or setbacks to their knee health status; instead, most assessments are qualitative, relying on patient-reported symptoms, performance during functional tests, and physical examinations. Recent advances have been made with wearable technologies for assessing the health status of the knee (and potentially other joints) with the goal of facilitating personalized rehabilitation of injuries and care for chronic conditions. This review describes our progress in developing wearable sensing technologies that enable quantitative physiological measurements and interpretation of knee health status. Our sensing system enables longitudinal quantitative measurements of knee sounds, swelling, and activity context during clinical and field situations. Importantly, we leverage machine-learning algorithms to fuse the low-level signal and feature data of the measured time series waveforms into higher level metrics of joint health. This paper summarizes the engineering validation, baseline physiological experiments, and human subject studies—both cross-sectional and longitudinal—that demonstrate the efficacy of using such systems for robust knee joint health assessment. We envision our sensor system complementing and advancing present-day practices to reduce joint reinjury risk, to optimize rehabilitation recovery time for a quicker return to activity, and to reduce health care costs.
Keywords: joint health, rehabilitation, wearable sensing
INTRODUCTION
Musculoskeletal injuries and chronic conditions are prevalent (25, 68), and the knee is one of the most frequently injured body parts (30). Persons participating in sports, occupational/military duty, or other high-performance activities are particularly susceptible to knee injuries because of the repetitive stress and high loads experienced by the joint. Nevertheless, the more sedentary populations also have a high risk of knee injury due in part to atrophied muscles leading to a destabilized joint (21). This large susceptible population along with the concentrated stresses put on the knee during physical activity leads to a high frequency of acute knee injury—a staggering 18 million patient visits occur per year in the United States (2). Beyond knee injuries, many Americans suffer from chronic degenerative joint diseases, such as osteoarthritis and rheumatoid arthritis, that affect their quality of life through knee pain or limitations. From 2013 to 2015, 53.3 million U.S. adults (22.7% of the population) were diagnosed with some form of arthritis. This number is predicted to rise to 78 million (26% of population) by 2040 (20). Thus the high number of patients with chronic knee (joint) conditions imposes a severe burden on healthcare systems, and a need exists for wearable technologies that can help manage the recovery and treatment of such joint injuries.
We are developing wearable sensing technologies that facilitate quantitative physiological measurements and interpretation of knee health status. Our sensing systems will enable longitudinal measurements of knee sounds, swelling, and activity context during clinical and field situations. Importantly, we are employing machine-learning algorithms to fuse these physiological measures into an individualized joint health score that will facilitate optimized progression of rehabilitation and/or personalization of care. Our system will be noninvasive, easy to use, and provide accurate and meaningful physiological information while performing standardized clinical evaluation or rehabilitation/physical training activities. Our vision is that this sensor system will complement and advance present-day practices to reduce reinjury risk, optimize recovery time for return to activity, and enable personalization of care for patients with joint injuries by using objective physiological data.
PRESENT-DAY STANDARDS FOR TREATMENT AND RECOVERY MANAGEMENT OF KNEE HEALTH
The gold standard for diagnosing and managing knee health (e.g., following an acute knee injury during rehabilitation) is a combination of physical exams and medical imaging. Imaging is not always feasible in austere environments and provides limited information for rehabilitation planning (e.g., duration of treatment interventions). Rehabilitation management is usually based on repeated physical exams focusing on subjective measures of pain, range of motion, edema, and crepitus while performing exam maneuvers (e.g., anterior drawer tests, pivot tests, etc.). These tests have positive predictive values of less than 20% (23). During this critical period of rehabilitation, the medical team, and patient, rely principally on subjective analysis, patient-reported symptoms, and functional activity levels of daily living to adjust the treatment regimen. These adjustments vary patient to patient and physician to physician. Scoring scales such as the International Knee Documentation Committee have been devised to transition this subjective procedure into a more objective analysis but have had mixed results in terms of effectiveness (67). Thus there is a compelling need for a more sensitive and objective system for monitoring joint injuries that could complement these existing approaches.
Multiple researchers have developed technologies for assessing knee health using wearable sensors, with the approaches primarily focusing on inertial sensing, gait assessment, or knee kinematics (4, 9, 14, 33, 46, 47, 57, 58, 65). The most common techniques leveraged inertial sensors placed on the thigh and calf to quantify knee angle as a function of time and to then assess gait impairment. The combination of body-worn inertial sensors yielded higher level information regarding movement of the joint that might potentially relate to underlying pathophysiology or rehabilitation status (14, 57). Although these approaches provide an advancement toward facilitating knee health monitoring outside of clinical settings, they generally do not provide sufficient depth of information regarding knee physiology and function required to properly assist in clinical decision making. Subtle changes in knee physiology or structure that can be used to quantify progress for knee rehabilitation, or to assess efficacy of treatment for an arthritic knee, are unfortunately not captured in a precise enough way to titrate care.
WEARABLE KNEE HEALTH SYSTEMS: SOUNDS, SWELLING, AND ACTIVITY CONTEXT
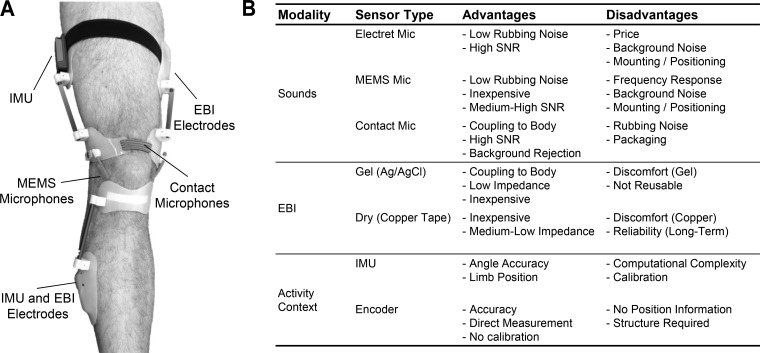
We have developed a multimodal sensing approach to wearable knee health assessment, using a combination of knee sounds, swelling, and activity context measurements. Our most recent sensing system prototype incorporates these three modalities and is shown in Fig. 1. Joint sounds are measured by a combination of miniature microelectromechanical systems (MEMS) microphones that sense sound pressure levels in air and piezoelectric contact microphones that measure the vibrations at the surface of the skin (54, 56, 59). Assessment of swelling is based on electrical bioimpedance (EBI) measurements, taken by using four electrodes incorporated into the device (19). Activity context is acquired based on inertial measurements, including assessment of the position of the limbs and angle of the joint (19, 49, 61). In addition, we are developing machine-learning algorithms that will provide a joint health score based on these measurements (18).
Fig. 1.
A: photo of our wearable knee health monitoring system prototype with joint sounds, swelling (using electrical bioimpedance, EBI), and activity context (using inertial measurement units, IMUs) measurement. B: table summarizing example sensor types available for each of the three measurement modalities used in our work: sounds, EBI, and activity context. For each sensor type, the advantages and disadvantages are provided.
Sounds.
Although physicians have historically examined for joint crepitus, little is known about these sounds quantitatively (32). The underlying biology, including the characterization of the source(s), the propagation of the sound from the bones, cartilage and ligaments to the surface of the skin, and the effects of structural disruptions (e.g., ligament tear) on the nature of the sounds have never been quantitatively studied. Nevertheless, we recognized these sounds as an important longitudinal measure of joint health that should be studied more carefully. Acoustical emissions can potentially provide in-depth information regarding the structural integrity of the joint and the health of the internal surfaces (bone and cartilage). Indeed, the use of acoustical emissions as a means of quantifying the health of structures is common in other domains such as civil and aerospace engineering (51); thus their use for characterizing joint health holds promise.
Notably, most existing research in joint acoustical emissions measurement and characterization has focused on developing diagnostic techniques to differentiate “healthy” vs. “unhealthy” knee joints (8, 12), primarily as it concerns cartilage-based conditions such as osteoarthritis and chondromalacia. Shark et al. (50) measured acoustic emissions from the knee by using wide-band piezoelectric sensors, finding that osteoarthritic knees produce more frequent, louder, and longer duration acoustic emissions compared with healthy knees. Lee et al. (28) also evaluated osteoarthritic subjects and successfully classified three different conditions of the patellarfemoral joint. To achieve such outcomes, significant work has been devoted to developing empirical signal processing techniques for conditioning and classifying the joint sound signals. These algorithms have leveraged linear prediction and autoregressive modeling (28, 53), statistical parameter investigation (42), Fourier and time-frequency analysis (26, 28a), and wavelet decomposition (6, 63).
This prior work establishes a foundation for the use of acoustical emissions for characterizing knee health but is limited by its focus on “snapshot” diagnostics. The primary strength of acoustical emission-based analysis is its ability to provide longitudinal monitoring: monitoring a structure over time to detect changes in underlying health status. The main limitation precluding specific longitudinal knee acoustical emission measurements has been the lack of technologies for convenient, wearable measurement of joint sounds outside of clinical and laboratory settings. We demonstrated recently, for the first time, that miniature microphones could be used for wearable knee acoustical emission measurements with high reliability and repeatability (56). The results from these studies are described below (Acoustics) in detail.
Swelling.
Edema (swelling) is difficult to quantify in practice and is usually assessed using volume displacement (15), surface circumference measurements (52), medical imaging (13), and visual inspection. In the most severe cases, edema limits mobility of a joint and walking. Unfortunately, these techniques are not amenable to wearable or continuous longitudinal assessment of either edema following a musculoskeletal injury or titration of medication in patients with arthritis.
One technique that may enable such longitudinal assessments of knee edema is EBI, a measurement that has been studied for decades in various biomedical instrumentation applications such as assessing body composition and estimating total body water (11, 37), cardiac output (24, 27), and limb blood flow (38, 43). The principle behind EBI measurements is that the electrical impedance of different types of tissue, air, blood, and fluid are different, and thus modifying the composition of a volume of the body should affect the impedance of that volume in a predictable and quantifiable manner. A small, safe electrical current is inputted to a volume of tissue using electrodes on the surface of the skin, and the corresponding voltage differential across this volume of tissue is measured synchronously. To reduce the skin-electrode interface impedance (44), the measurement is typically conducted at excitation frequencies of 50 kHz or greater (50a, 39).
Nescolarde et al. (34, 35) showed that swelling of a joint can be quantified in laboratory/clinical settings by using bench-top EBI measurement instrumentation. Researchers have also evaluated EBI measured at multiple frequencies (i.e., spectroscopy) for assessing inflammation in patients with osteoarthritis with promising results (1, 36). Using EBI for assessing edema of the joint assumes that the swollen joint will contain a greater volume of fluid compared with normal, and thus the resistance would be lower than a healthy joint. Additionally, the damaged cell membranes associated with an acute injury can lead to reduced overall capacitance. The purpose of multifrequency spectroscopy-based approaches is to assess both intracellular and extracellular fluid levels via the same measurement.
Regardless of the frequency at which it is measured, the magnitude of the changes in EBI expected with edema/inflammation is quite small, on the order of several Ohms or less, and thus a high-resolution system is needed. It is also preferable to avoid gel electrodes and instead use dry electrodes (7), which can be implemented in textiles or other convenient and comfortable interfaces against the skin (31, 41). Unfortunately, such dry/textile electrodes have higher skin-electrode interface impedance compared with gel electrode counterparts. This combination of a small EBI difference associated with edema, a large skin-electrode interface impedance, and the requirement for a wearable system to have appreciable battery life has precluded wearable EBI systems for edema assessment from being available in research or commercial settings.
We have recently developed a wearable vector EBI measurement system with sufficient resolution to differentiate between a healthy joint and one with edema (following acute injury) that can operate with dry electrodes (17, 19). Importantly, by combining high-performance electronics with programmable and “smart” computing, our EBI system performs self-calibration, adjusts the current delivered to the body automatically to maximize signal quality, and records both the resistive and capacitive components of the tissue impedance. We have observed statistically significant differences in both the resistive and capacitive components for healthy compared with injured knee joints, as well as in athletes with acute knee injury immediately following the injury as compared with several months later after surgery and rehabilitation. These results are described below (Cross-sectional studies) in more detail.
Activity context via inertial measures.
The most important contextual parameters related to the joint health monitoring are determination of limb position, identification of movement type, and quantification of joint angle. Knowledge of the position of the limbs (e.g., straight vs. bent leg, seated vs. standing) was found to improve the accuracy and reliability of EBI measurements (19). Identifying the type of movement (e.g., walking vs. unloaded flexion/extension exercises) can provide a means of reducing stepping noise artifacts in joint sound measurements during walking (59). Joint angle measurements over time, taken simultaneously with acoustical emissions, can allow the patterns and characteristics of the emissions to be placed in the context of angle. For example, the angular location at which the loudest sounds occur has been found to be consistent among healthy subjects (56). Thus accurate contextual information related to these three aspects can improve the assessment of joint sounds and swelling via microphones and EBI.
In our work, we have employed inertial measurement units (IMUs) to measure these contextual parameters. IMUs typically include three-axis accelerometers, gyroscopes, and magnetometers to provide information regarding linear acceleration, angular velocity, and position of the point on the body at which the unit is placed. Position sensing has been demonstrated in a variety of scenarios, including with healthy subjects and patients during rehabilitation (16, 69). Our work recently demonstrated that activity classification is possible based on IMUs placed at the knee joint—one on the thigh and a second on the side of the calf (49, 60). Finally, several studies have shown that IMUs can provide accurate assessment of knee angles (9, 10, 48).
KNEE HEALTH SYSTEM: ENGINEERING AND RESULTS
We designed and implemented novel engineering approaches for the joint acoustical emissions and EBI sensing to ensure that high-quality and repeatable measurements could be obtained in a wearable form factor for facilitating longitudinal, quantitative knee health monitoring. We then employed the technologies developed by our laboratory in several clinically relevant applications to further evaluate the potential of our wearable knee health sensing systems.
Several engineering challenges which have precluded wearable joint health monitoring include 1) a lack of clarity as to which sensors and hardware will be most effective in robust measurement of biosignals; 2) ambiguity regarding the consistency and quality of these measurements; and 3) the excessive power consumption and data storage requirements for acquiring these physiological signals for continuous, at-home monitoring. The primary engineering efforts of our work addressed these issues to obtain high-quality and sensitive wearable measures of joint acoustical emissions and EBI.
Acoustics.
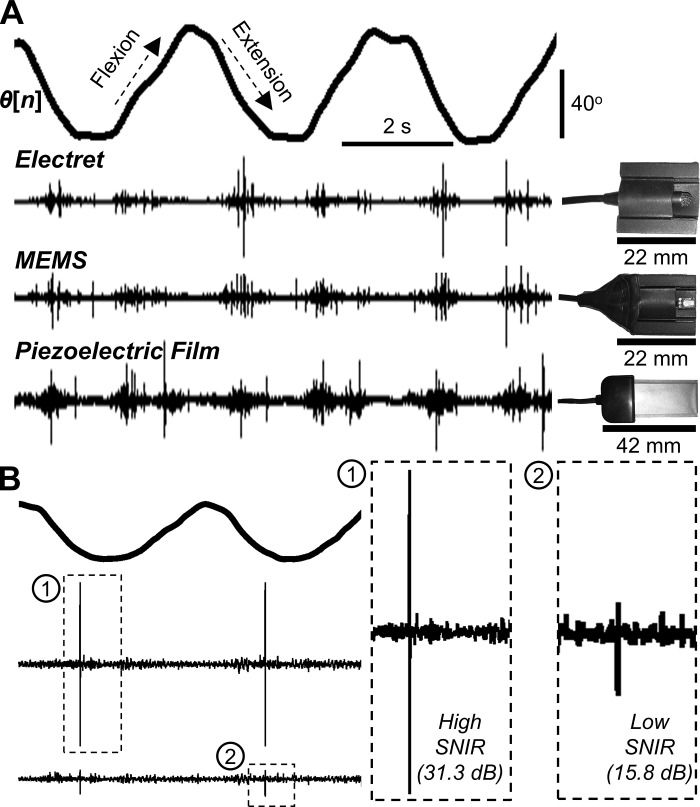
We investigated quantitatively which sensors, or combination of sensors, would provide high-quality and reliable recordings of acoustical emissions for a wearable system. The criteria for selecting sensors for a wearable device required that the microphone have 1) a small size, such that it is noninvasive; 2) broad bandwidth to acquire sounds that span low to high frequencies (i.e., 100 Hz to 20 kHz); 3) adequate coupling to the body in the case of contact microphones to ensure signal capture; 4) low power consumption to extend device battery life; and 5) low cost for widespread future patient use. We identified four types of microphones to examine their feasibility for detecting joint sounds: electret (COS11D, Sanken Microphone, Japan), MEMS (MP33AB01, STMicroelectronics, Geneva, Switzerland), piezoelectric film (SDT, Measurement Specialties, Hampton, VA), and piezoelectric accelerometer (BU-23173, Knowles, Itasca, IL) based microphones. The advantages and disadvantages of these microphones are summarized in Fig. 1B, and pictures and a comparison of these microphones are shown in Fig. 2A. We showed that the MEMS (low-cost) and electret (high-end) microphones showed high similarity in their ability to acquire joint sounds (56). Although theoretically the contact microphones should provide the best coupling to the skin and thus the highest quality pickup of acoustical emissions, we found that rubbing noise of the microphone against the skin could potentially degrade the recording quality in some instances. Thus an important finding of our work was that a hybrid combination of air (e.g., MEMS) and contact (e.g., BU-23173) microphones would be optimal for robust wearable acoustical emission sensing.
Fig. 2.
A comparison of different microphones investigated for wearable joint sound sensing. A: electret, MEMS, and piezoelectric film microphones simultaneously captured joint sounds during three repetitions of flexion/extension exercises as indicated by joint angle (θ[n]). The traces show that prominent acoustic emissions are captured by all three types of microphones. The electret and MEMS microphones show high degrees of similarities because both sensors capture airborne sounds. The piezoelectric film shows the highest similarity to the air microphones when capturing high-frequency sounds (clicks). The relative sizes of the microphones are shown on the right. B: an illustration of differences in signal-to-noise-and-interference ratio (SNIR) with two representative recordings. Two electret microphones were placed laterally and medially during two cycles of flexion/extension. The lateral microphone “1”—for this particular subject—exhibits high SNIR (31.3 dB) since the click is highly distinguishable from the baseline noise, whereas the medial microphone “2” shows a lower SNIR (15.8 dB) because the click’s amplitude is closer to that of the noise. [From Teague et al. (56).]
With this hardware and custom signal processing algorithms for detecting acoustic features, we demonstrated that high-quality and repeatable joint sound measures could be obtained. For physiological measurements, quality is often described using signal-to-noise-and-interference ratio (SNIR), which characterizes the prominence of the desired signal (e.g., joint sounds) in comparison with noise and interference that obscure this signal (e.g., rubbing or background noise). The SNIR was 11.7, 12.4, and 8.4 dB for the electret, MEMS, and piezoelectric microphones, respectively, indicating that all the sensors could still detect joint sounds at levels more than two times that of the underlying noise (56). Representation signals in Fig. 2B provide a visualization of SNIR values. Furthermore, we observed that significant acoustic emissions or “clicks”—characterized by their short duration (<1 ms), wide bandwidth (90% of spectral power between 1–10 kHz), and distinct shapes (59)—were measured simultaneously using electret, MEMS, and piezoelectric film-based microphones and could even be detected by air microphones (electret and MEMS) when placed 5 cm off the skin (54, 56). Importantly, we showed that our hardware could consistently detect these clicks at similar joint angles for repeated range of motion exercises, facilitating reliable longitudinal recordings of joint sounds for use in rehabilitation assessment algorithms (56).
We demonstrated that incorporating kinematic data could not only provide physiological context (e.g., joint angle) for emission of sounds, measured across articulating surfaces of the knee joint, but could also address the significant engineering challenges related to high sampling rate requirements, namely data storage limitations and power efficiency constraints. Compared with other biosignals, such as joint angle, audio data has high bandwidth and thus necessitates high sampling rates, which is computationally expensive for wearable system implementations. Consequently, continuous acquisition of joint sounds would quickly deplete data storage (microSD card memory) and the battery. To mitigate these issues, we gated the sampling of high-throughput, multichannel audio data with kinematic data. Custom activity classification algorithms analyzed low sample rate signals from low-power IMU sensors, recognized exercise motions of interest (e.g., seated knee flexion/extension and sit-to-stand test) in real time, and subsequently triggered the acquisition and saving of two channels of joint sounds and kinematic data (i.e., two three-channel accelerometer and gyroscope IMUs) to a microSD card (49, 55, 60).
We have also designed an algorithm to output a knee health score, using unsupervised machine learning (18). One of the most challenging aspects of knee acoustical emissions analysis is intersubject variability, likely due to structural and anatomical variability of the knee among individuals. Thus to generate a knee health score, we use the acoustical signals recorded from both the left and the right knees, expecting these two signals to be similar for healthy subjects but different for subjects with knee injuries.
In our algorithm, first the knee acoustical emissions are band-pass filtered (1–15 kHz), to remove rubbing and clothing noise (low frequency) and electronic noise beyond the useful information provided by the joint acoustical emissions (higher frequency). The signal is then standardized (zero mean and unity variance) and divided into 200-ms frames with 50% overlap. Sixty-four audio features (time and frequency domain) are extracted from each frame, and this information is stored in a matrix. The matrix (including information from both the left and the right knees) is then synthesized to produce a weighted k-Nearest-Neighbor graph. This k-Nearest-Neighbor graph has some regions with a high density of vertices that are referred to as communities within the graph. The Infomap community detection algorithm (45) is used to detect the communities within this graph. The number of communities detected, which is referred to as the graph community factor, is the knee health score.
Electrical bioimpedance for edema.
Although wet (gel-based) electrodes provide lower noise electrical bioimpedance (EBI) measurements because of their lower skin-electrode impedance and adhesive-fixed (nonvariable) location, they are undesirable for wearable applications since they must continuously be replaced and may present discomfort for the wearer; we thus investigated the use of a dry electrode alternative. We designed a dry electrode consisting of an array of five rectangular copper-plated pieces (1.6 × 1.2 in.) (19). Regression curves comparing these dry electrodes with standard Ag/AgCl wet electrodes exhibited high correlation for resistive and reactive measures (r2 = 0.8 and 0.9, respectively), demonstrating their efficacy to reliably measure EBI for noninvasive applications (19). In the future we plan to conduct a similar experiment with different types of conductive textile electrodes as well to assess whether reliable measurements can also be acquired using textiles that would be both more comfortable and flexible.
We designed a high-resolution circuit for measuring static and dynamic bioimpedance such that both slowly (hours to days) and rapidly (~milliseconds) varying signals could be detected and used to obtain edema and blood flow information, thus providing insight to musculoskeletal and cardiovascular physiology. First, validation between expected and measured values showed a mean difference of 1.4% for magnitude and 1.8% for phase of the impedance values, providing accurate absolute (vs. relative) impedance value comparisons (17). Additionally, our EBI circuit has a dynamic range of 345 Ω, meaning that all anticipated knee impedances (40 to 80 Ω for resistive and −20 to −10 Ω for reactive)—influenced by joint size, amount of swelling, and vascular resistance—can be measured. Moreover, the circuit has a low noise floor of 0.018 and 0.055 mΩrms for resistive and reactive measures, respectively. This low noise floor combined with the high number of sampling bits allowed for high-resolution measurements of EBI.
To further improve the repeatability of EBI measurements, we accounted for several factors which could affect the accuracy of our circuit. We developed a self-calibration scheme to compensate for large errors due to board temperature- and current-related drift, phase delays, and other inaccuracies (17). Using this system, we assessed day-to-day repeatability of the absolute differences for left-to-right leg EBI (see Cross-sectional studies below for more information regarding the physiological significance of this metric) and found that variability (i.e., standard deviation) for healthy subjects (n = 5) was 2.5 Ω (resistive) and 1.2 Ω (reactive) (19).
We showed that gating EBI measurements with kinematic data improved the system’s ability to provide robust, longitudinal assessments of edema and can be used to increase its power efficiency, similarly to the approach we implemented for gating joint sound measurements. Because bioimpedance signals are dependent on the position and motion of the knee, measurements need to be recorded with the subject in similar positions to facilitate direct comparison of longitudinal data. As such, we developed a leg position identification algorithm to determine if the subject was in the optimal measurement posture (i.e., the subject seated with his leg extended and supported) using a binary decision rule to either accept or reject the EBI measurement during postprocessing (19). Moreover, relative to the kinematic sensors (i.e., IMUs), the EBI circuit consumes significantly more power, necessitating noncontinuous bioimpedance measurements. This optimal leg position detection algorithm can be implemented in real time to sustain battery life by only recording EBI when the subject is in the optimal position.
CLINICALLY RELEVANT HUMAN SUBJECTS STUDIES
We conducted three types of experiments and analyses with our wearable systems: 1) baseline studies on healthy subjects, 2) cross-sectional studies examining differences between knees of individuals with acute injury compared with healthy controls (no previous knee injury), and 3) longitudinal cohort studies evaluating the same individual following therapy (e.g., reconstructive surgery) and several months of rehabilitation. All studies were conducted with Institutional Review Board approvals. All participating subjects provided written, informed consent.
Baseline studies.
We performed basic physiologic studies with healthy subjects to improve the understanding of the parameters measured with the wearable knee health sensing systems. For the joint sound measurements, we characterized the angular location at which the most prominent acoustical events (clicks) were observed in healthy knees. Our hypothesis was that this position would be at a consistent phase of the range of motion during knee flexion/extension while seated, and that for healthy subjects this angular position would be consistent between the two knees. We confirmed this hypothesis, finding that the intraclass correlation coefficient (ICC) showed the reliability of the main acoustic event location for an individual cycle and mean of the cycles was highly similar across subjects [ICC (1, 1) = 0.94 with 95% confidence interval (CI) of 0.92–0.97 and ICC (1, k) = 0.99 with 95% CI of 0.98–0.99, respectively] and that the difference between left and right legs was not significant (P > 0.05) (56). Although the exact anatomical source and/or physiological mechanism eliciting the clicks at specific joint angles remains unknown, the fact that these sounds are repeatable and measurable has allowed for further testing in which specific correlates are explored. One such test, performing these cycles under a vertical load, has revealed a particularly interesting outcome.
We quantified the effects that vertical loading forces had on the acoustical emission patterns for a controlled exercise—vertical leg press with loads varying from 0 kg up to 100% of the subject’s body mass. Our hypothesis was that larger loads at the knee would increase the number and/or pressure of internal knee surfaces interacting with each other and thus affect the acoustical emissions accordingly. This also held true as the joint sounds changed proportionally as loading forces increased, reflected by a larger number of sound sources inside the knee (suggesting more surfaces interacting in a more complex manner). However, capturing this data in high-dimensional space required more mathematical complexity and complicated assumptions. Specifically, as described above for quantifying a knee health score, graph mining algorithms (k-Nearest-Neighbor graphs) were employed to embed the high-dimensional acoustical data feature space in lower dimensional space for visualization and reliable computation purposes (29, 40). Afterward, the Infomap algorithm (45) was applied to quantify the number of communities required to represent the extracted joint sound graph. The number of communities, which approximately estimates the heterogeneity of sound sources, increased for larger loads, confirming our hypothesis.
For validation of the EBI measurements, we characterized the changes in knee resistance associated with small modulation of local fluid volume in the knee based on heating and cooling of the skin. Our hypothesis was that the local resistance would increase with heating and decrease with cooling, as the local interstitial fluid volume, namely the precapillary to postcapillary volumes and concomitant resistance ratios, change with temperature. To investigate these changes, EBI was measured while local heating or cooling of the patella was conducted via hot and cold packs, respectively. We found that difference between the baseline resistance measurement and the measurements at 5, 10, and 15 min of heating and cooling was significant (P < 0.01) for both temperature modalities (19). Furthermore, this experiment showed that our circuit was sensitive to Ohm-level changes in tissue volume due to knee temperature, which may change because of environmental or activity-based factors.
Cross-sectional studies.
Because of the need for acute injury severity screening in austere environments or low-resource settings described above, we decided to investigate whether knee sounds and EBI measures could differentiate healthy vs. involved (injured or arthritic) knees. We performed two studies: 1) we measured joint sounds and EBI in two groups of athletes (those with acute knee injuries vs. healthy controls with no prior knee injury); and 2) we measured joint sounds from children with juvenile idiopathic arthritis (JIA) vs. healthy controls in a proof-of-concept study.
Our study on the group of college athletes (age ranging from 19 to 23 yr) in which knee joint sounds were acquired consisted of 33 healthy athletes (26 men) and nine athletes with acute unilateral knee injury (eight men). The injuries included torn anterior cruciate ligament (six subjects), torn lateral meniscus (one subject), and sprained medial collateral ligament (two subjects). We also collected EBI signals from 42 healthy subjects (27 men) and seven subjects with acute unilateral knee injuries (six men), all requiring subsequent corrective surgery. The difference between the subject demographics for the joint sound and EBI study is due to the unavailability of our acoustical sensing system in the earliest studies, whereas the EBI system was available for use in all subjects. Since our sample of athletes was derived from multiple sports (e.g., volleyball, football) and different positions (e.g., setter, offensive lineman), the mean (±SD) BMI for the healthy group was 27.0 ± 3.6 (range 21.1–36.3) and the injured group was 30.5 ± 4.2 (range 25.3–37.7), which were not significantly different (P > 0.05).
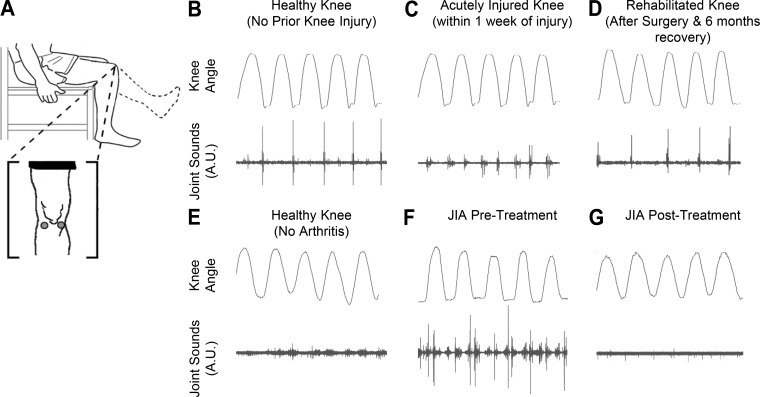
We found that the sounds emitted from the knees during movement were more heterogeneous for knees with recently incurred (within 2 wk) unilateral acute injury vs. healthy knees. The injury types comprised of torn anterior cruciate ligament, medial collateral ligament, and/or lateral meniscus. Whereas for healthy knees the prominent acoustical emissions occurred at similar knee angle locations for a given movement from cycle to cycle, injured knees produced more variable sounds. Specifically, we found that healthy knees exhibited more homogenous sounds than injured (acute) knees and that, during recovery, the injured knee sounds became more homogenous. In the context of our knee health score, we have found that the graphs constructed for healthy subjects are more homogenous in structure and therefore have fewer communities, whereas the graphs constructed from injured subjects were heterogeneous and had an increased number of communities. Therefore the knees of a healthy subject were found to emit similar and more consistent acoustical signatures, whereas the acoustical signatures emitted from the knees of an injured subject were more variable and different between the knees. This increased variability in knee sound patterns with injury can be observed in Fig. 3C compared with Fig. 3B.
Fig. 3.
Seated unloaded knee flexion/extension acoustical emission data in two cases: knee injury rehabilitation (B–D) and juvenile idiopathic arthritis (JIA; E–F). A: locations (lateral and medial sides of the patella) of the microphones, electret and piezoelectric for B–D and E–F, respectively. B: sex and age matched control’s (with no prior knee injuries) acoustic patterns. A repetitive click is seen at a similar joint angle throughout the range of motion. C: within a week of an operable ACL/meniscus tear we notice less consistent high frequency peaks. D: after surgery to repair the injury and 6 mo of rehabilitation, we see a return of an acoustic pattern like the healthy subject in B. E: sex and aged-matched control to the patient with JIA. F: this patient with JIA (13-yr-old girl) initially presented to the clinic with poorly controlled arthritis in her knee, and acoustic emissions from the knee at the time of presentation were recorded. G: patient with JIA after undergoing treatment with methotrexate and injected corticosteroids on a return visit. The acoustic profile appears to have reverted more closely to that of the healthy control in E. This improved acoustic pattern mirrors an improved clinical picture as described by the treating rheumatologist.
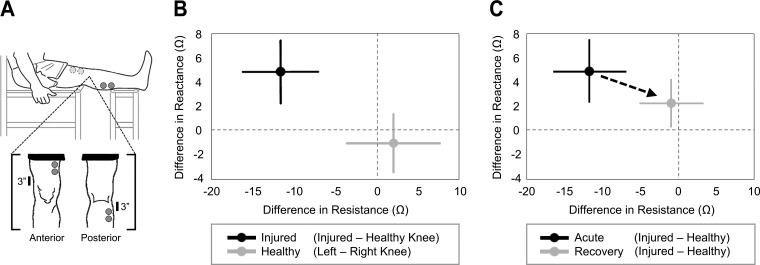
We demonstrated that the wearable EBI hardware could detect small differences in resistance and reactance between healthy and injured joints. The difference in resistance between the left and right knee for healthy subjects was 1.9 ± 5.6 Ω and in reactance was −1.1 ± 2.4 Ω. This is as expected since the knees for healthy subjects should, on average, yield approximately the same resistance and reactance values, and thus the differences should be close to zero. For injured subjects, the differences between knees were significantly greater (P < 0.05) for both resistance and reactance, with differences (injured knee minus healthy knee) of −11.8 ± 4.5 Ω and 4.9 ± 2.6 Ω, respectively. Thus the edema in the knee reduced the resistance (due to increased fluid in the knee, which is more conductive than other components of the tissue) and reduced the capacitance, thus causing the overall knee impedance to more closely resemble a purely resistive element. These results are summarized in Fig. 4B.
Fig. 4.
Electrical bioimpedance (EBI) studies comparing healthy, injured, and rehabilitated knees. A: a representation of the posture (leg fully extended and supported) and electrode placement for EBI measurements. B: cross-sectional comparison for the difference in resistance and reactance for healthy (gray) and injured (black) subjects. For healthy subjects (n = 42), the differences were calculated as that between the left and right knees, whereas for the injured subjects (n = 7) the differences were between the injured and contralateral knees. The differences for the healthy knees were approximately zero, indicating no differences in edema between the knees, and the differences between the knees for injured subjects were significantly greater (P < 0.05) for both resistance and reactance. The error bars denote one standard deviation. C: the differences in resistance and reactance for injured subjects (n = 7) over time as a function of rehabilitation. Longitudinal measurements were taken prospectively from the time of acute injury (within 1 mo but before surgery, shown in black) and at a second time measurement during rehabilitation (4 to 7 mo postsurgery, shown in gray). During the recovery process, the difference in edema between the two knees trends toward zero. The error bars denote one standard deviation. [From Hersek et al. (19).]
We performed a proof-of-concept clinical study to assess possible differences in joint sound measurements from patients with JIA compared with healthy controls (66). Representative results from one subject with systemic JIA (13-yr-old girl) and one age- and gender-matched healthy subject are shown in Fig. 3, E–G. Both subjects performed seated knee flexion/extension exercises (no load) with contact microphones for joint sound measurement placed on the lateral and medial sides of the patella. The sounds emitted from the knee of the subject with JIA were louder [higher root-mean-square (RMS) power] and included prominent peaks occurring periodically with each cycle. The results for the initial five subjects (three with JIA, two healthy controls) that have participated in these initial pilot studies support these representative results: the average RMS power of the group with JIA was 0.561 mG, whereas the healthy group had an average RMS power of 0.0196 mG. The average entropy of the diseased group was 0.763, whereas healthy was 0.363, indicating an increase in the disorder of the diseased joint. This indicates that there was an increased loudness and variability of joint sounds recorded in the population with JIA and that data from the healthy controls more tightly grouped together.
Longitudinal studies.
We performed joint sound and EBI measurements from the same cohort of injured athletes at a second time point: 4 to 6 mo following reconstructive surgery and rehabilitation, at which point the subjects could resume functional activities. A lower extremity functional scale questionnaire validated and utilized in clinical decision making (5) was completed by the subjects at each data collection. The self-reported survey assesses limitations in daily function and activities with 20 questions and has a maximum value of 80 when no limitations are present. The average (±SD) lower extremity functional scale for the first data collection (within 2 wk of the acute injury) was 36.1 ± 12.1 for the injured athletes, whereas for the second data collection (4 to 6 mo after surgery) the value improved significantly to 63.7 ± 11.0 (P < 0.01), indicating that the rehabilitation and surgery were successful in greatly improving the health of the knee.
The heterogeneity in joint sound recordings substantially decreased following surgery and rehabilitation, with the joint sounds beginning to again resemble healthy knee recordings of the contralateral limb (Fig. 3, B–D). The prominent features of the acoustical signals occurred periodically and synchronously with joint angle for uniform exercises [unloaded knee flexion/extension (Fig. 3A)]. During the acute injury period (<1 wk postinjury), the joint would experience high levels of inflammation. This inflammatory cascade and associated cytokines return back to normal by day 7 (22). In addition, the mechanics of the joint would be altered both by the structural damage of the ACL/meniscus tear and by the subjects’ resistance of movement due to pain. This inflammation and restricted movement may be responsible for the reduction in sounds seen in Fig. 3C. After rehabilitation/surgery, the knee’s acoustic profile more closely resembles the uninjured, age-matched control with the aforementioned periodic spikes in signal.
We found that the differences in impedance between the knees were significantly reduced following surgery and rehabilitation (P < 0.05). The difference in resistance between the knees (injured side minus healthy side) decreased from −11.8 ± 4.5 to −1.0 ± 4.0 Ω, and the corresponding difference in reactance decreased from 4.9 ± 2.6 to 2.3 ± 2.0 Ω. Thus the EBI measurements were closer to the corresponding values for the healthy controls, with a reduction in the differences between the two knees (injured and contralateral side). These results are summarized in Fig. 4C.
For one subject with JIA, we conducted the measurements at a second time point: 3 mo following the initiation of therapy with methotrexate and injected corticosteroid therapy. The joint sounds measured from this subject are shown in Fig. 3, E–G. Compared with the prior recording, the amplitude of the sounds decreased substantially, and the measurement more closely resembled the recordings from the healthy controls.
FUTURE VISION AND NEXT STEPS
Our completed research in wearable knee health sensing sets a foundation for several future efforts. These include scientific studies aimed at advancing the understanding of the fundamental aspects of joint sounds. Although EBI has been measured for several decades in various applications, and is well understood, localized measurements with EBI coupled with joint sounds have not been sufficiently explored. Machine-learning algorithms are being designed to combine measured physiological data, including sounds, swelling, and activity context, to generate a total knee health score for the user. This knee health score could then be employed to determine, for example, when specific activities could be resumed or intensities of activities could be increased safely by the user while rehabilitating a knee injury. The score could also provide another objective decision aid to physicians for titrating care; for example, a pediatric rheumatologist treating a patient with JIA could determine quantitatively whether a therapy is, or is not, effective for that patient and change the clinical course accordingly. Finally, larger clinical studies expanding on the work presented here should be conducted to further assess the hypotheses related to joint rehabilitation following acute injury or treatments for patients with arthritis. Our study of joint sounds and EBI were conducted on a group of college athletes, and the question of impact of age on these acoustical emissions remains a topic of interest for future studies. All three areas—basic scientific experiments, machine-learning algorithm design, and clinical studies—could be undertaken in parallel such that synergistic progress can be made.
The research thus far has focused on the knee joint for several reasons. Knee injuries are prevalent, and pain, symptoms, and limitations are commonly encountered in the knee for patients with arthritis. The knee is a large, uniaxial hinge joint, thus multiple sensors can readily be placed on the surface of the skin to acquire data. Compared with the hip or ankle, the knee has fewer degrees of freedom, allowing for a more controlled setting within which the measurements could be studied. Nevertheless, in future efforts we plan to study other joints as well, such as the elbow and the shoulder.
A major advantage for wearable joint health sensing systems is the fact that inexpensive and frequent measurements can be obtained outside of clinical settings, including in austere environments and in a noninvasive manner. Field-based screening of injury severity could provide great benefit both for athletics (e.g., on the sidelines of a football game) or for occupational/military medicine applications as a tool for triage. Bone and cartilage loss is a concern for astronauts, for example, even with physical training used as a countermeasure (64). Monitoring joint health during long-term space missions can provide an indication of the state of such processes on an individualized basis. Although diagnoses should still be made based on a combination of physician examination and medical imaging, such screening and personalized monitoring approaches can complement these diagnoses to improve patient management.
We envision the combination of joint sounds, swelling, and activity context measures providing a physiological biomarker of joint health—an index that could potentially be used for titrating care, personalizing interventions, and determining an index of overuse from physical training and injury risk avoidance. Although such physiological biomarkers are commonly used in other domains, such as for cardiovascular medicine, few technologies exist for providing quantitative wearable measurements of joint health, thus creating an exciting future opportunity.
GRANTS
This material is based on work supported in part by the Defense Advanced Research Projects Agency, Arlington, VA, under Contract No. W911NF-14-C-0058; in part by National Institute of Biomedical Imaging and Bioengineering Grant 1R01 EB-023808, as part of the NSF/NIH Smart and Connected Health Program; and in part by the Georgia Tech/Children’s Healthcare of Atlanta Center for Pediatric Innovation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
O.T.I., D.C.W., C.N.T., S.H., M.B.P., M.M.-S., G.F.K., and M.N.S. conceived and designed research; O.T.I., D.C.W., C.N.T., S.H., and M.B.P. analyzed data; O.T.I., D.C.W., C.N.T., S.H., M.B.P., M.M.-S., G.F.K., and M.N.S. interpreted results of experiments; O.T.I., D.C.W., C.N.T., S.H., and M.N.S. prepared figures; O.T.I., D.C.W., C.N.T., S.H., and M.N.S. drafted manuscript; O.T.I., D.C.W., C.N.T., S.H., M.B.P., M.M.-S., G.F.K., and M.N.S. edited and revised manuscript; O.T.I., D.C.W., C.N.T., S.H., M.B.P., M.M.-S., G.F.K., and M.N.S. approved final version of manuscript; D.C.W., C.N.T., S.H., and M.B.P. performed experiments.
REFERENCES
- 1.Alvarenga R, Souza M.. Assessment of knee osteoarthritis by bioelectrical impedance. 2003 Proc 25th Annu Int Conf IEEE, 2003, p. 3118–3121. [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons Information About Musculoskeletal Conditions (Online). http://www.aaos.org/research/stats/patientstats.asp [3 Nov. 2015].
- 4.Atallah L, Jones GG, Ali R, Leong JJH, Lo B, Guang-Zhong Y. Observing recovery from knee-replacement surgery by using wearable sensors. Body Sensor Networks, 2011 Int Conf, 2011, p. 29–34. [Google Scholar]
- 5.Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther 79: 371–383, 1999. [PubMed] [Google Scholar]
- 6.Cai S, Yang S, Zheng F, Lu M, Wu Y, Krishnan S. Knee joint vibration signal analysis with matching pursuit decomposition and dynamic weighted classifier fusion. Comput Math Methods Med 2013:904267, 2013. doi: 10.1155/2013/904267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi YM, Jung TP, Cauwenberghs G. Dry-contact and noncontact biopotential electrodes: methodological review. IEEE Rev Biomed Eng 3: 106–119, 2010. doi: 10.1109/RBME.2010.2084078. [DOI] [PubMed] [Google Scholar]
- 8.Chu ML, Gradisar IA, Railey MR, Bowling GF. Detection of knee joint diseases using acoustical pattern recognition technique. J Biomech 9: 111–114, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Cooper G, Sheret I, McMillian L, Siliverdis K, Sha N, Hodgins D, Kenney L, Howard D. Inertial sensor-based knee flexion/extension angle estimation. J Biomech 42: 2678–2685, 2009. doi: 10.1016/j.jbiomech.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Favre J, Aissaoui R, Jolles BM, de Guise JA, Aminian K. Functional calibration procedure for 3D knee joint angle description using inertial sensors. J Biomech 42: 2330–2335, 2009. doi: 10.1016/j.jbiomech.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Forbes GB, Simon W, Amatruda JM. Is bioimpedance a good predictor of body-composition change? Am J Clin Nutr 56: 4–6, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Frank CB, Rangayyan RM, Bell GD. Analysis of knee joint sound signals for non-invasive diagnosis of cartilage pathology. IEEE Eng Med Biol Mag 9: 65–68, 1990. doi: 10.1109/51.62910. [DOI] [PubMed] [Google Scholar]
- 13.Frobell RB, Le Graverand MP, Buck R, Roos EM, Roos HP, Tamez-Pena J, Totterman S, Lohmander LS. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage 17: 161–167, 2009. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Glaros C, Fotiadis DI, Likas A, Stafylopatis A. A wearable intelligent system for monitoring health condition and rehabilitation of running athletes. Inf Technol Appl Biomed 2003 4th Int IEEE EMBS Spec Top Conf, 2003, p. 276–279. [Google Scholar]
- 15.Guex JJ, Perrin M. Edema and leg volume: methods of assessment. Angiology 51: 9–12, 2000. doi: 10.1177/000331970005100103. [DOI] [PubMed] [Google Scholar]
- 16.Hamdi MM, Awad MI, Abdelhameed MM, Tolbah FA. Lower limb gait activity recognition using Inertial Measurement Units for rehabilitation robotics. 2015 Int Conf Adv Robotics (ICAR), 2015, p. 316–322. [Google Scholar]
- 17.Hersek S, Töreyin H, Inan OT. A robust system for longitudinal knee joint edema and blood flow assessment based on vector bioimpedance measurements. IEEE Trans Biomed Circuits Syst 10: 545–555, 2016. doi: 10.1109/TBCAS.2015.2487300. [DOI] [PubMed] [Google Scholar]
- 18.Hersek S, Pouyan MB, Teague CN, Sawka MN, Millard-Stafford ML, Kogler GF, Wolkoff P, Inan OT. Acoustical emission analysis by unsupervised graph mining: a novel biomarker of knee health status. IEEE Trans Biomed Eng . In press. doi: 10.1109/TBME.2017.2743562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersek S, Toreyin H, Teague CN, Millard-Stafford ML, Jeong HK, Bavare MM, Wolkoff P, Sawka MN, Inan OT. Wearable vector electrical bioimpedance system to assess knee joint health. IEEE Trans Biomed Eng 64: 2353–2360, 2017. doi: 10.1109/TBME.2016.2641958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self‐reported doctor‐diagnosed arthritis and arthritis‐attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol 68: 1582–1587, 2016. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med Sci Sports Exerc 34: 838–844, 2002. doi: 10.1097/00005768-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10: 93–96, 2003. doi: 10.1016/S0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 23.Jackson JL, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med 139: 575–588, 2003. doi: 10.7326/0003-4819-139-7-200310070-00010. [DOI] [PubMed] [Google Scholar]
- 24.Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circ Physiol 293: H583–H589, 2007. doi: 10.1152/ajpheart.00195.2007. [DOI] [PubMed] [Google Scholar]
- 25.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. BMJ 322: 1079–1080, 2001. doi: 10.1136/bmj.322.7294.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan S, Rangayyan RM, Bell GD, Frank CB. Adaptive time-frequency analysis of knee joint vibroarthrographic signals for noninvasive screening of articular cartilage pathology. IEEE Trans Biomed Eng 47: 773–783, 2000. doi: 10.1109/10.844228. [DOI] [PubMed] [Google Scholar]
- 27.Kubicek WG, Patterson RP, Witsoe DA. Impedance cardiography as a noninvasive method of monitoring cardiac function and other parameters of the cardiovascular system. Ann N Y Acad Sci 170: 724–732, 1970. doi: 10.1111/j.1749-6632.1970.tb17735.x. [DOI] [Google Scholar]
- 28.Lee J-H, Jiang C-C, Yuan T-T. Vibration arthrometry in patients with knee joint disorders. IEEE Trans Biomed Eng 47: 1131–1133, 2000. doi: 10.1109/10.855942. [DOI] [PubMed] [Google Scholar]
- 28a.Lee TF, Lin WC, Wu LF, Wang HY.. Analysis of vibroarthrographic signals for knee osteoarthritis diagnosis. Genetic Evol Comput 2012 Sixth Int Conf, Kitakushu, Japan: IEEE, 2012, p. 223–228. doi: 10.1109/ICGEC.2012.60. [DOI] [Google Scholar]
- 29.Levine JH, Simonds EF, Bendall SC, Davis KL, Amir AD, Tadmor MD, Litvin O, Fienberg HG, Jager A, Zunder ER, Finck R, Gedman AL, Radtke I, Downing JR, Pe’er D, Nolan GP. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell 162: 184–197, 2015. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee 13: 184–188, 2006. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Medrano G, Ubl A, Zimmermann N, Gries T, Leonhardt S.. Skin electrode impedance of textile electrodes for bioimpedance spectroscopy. In: 13th International Conference on Electrical Bioimpedance and the 8th Conference on Electrical Impedance Tomography, edited by Scharfetter H, Merwa R. Berlin: Springer, 2007, p. 260–263. [Google Scholar]
- 32.Mollan RA, McCullagh GC, Wilson RI. A critical appraisal of auscultation of human joints. Clin Orthop Relat Res 170: 231–237, 1982. [PubMed] [Google Scholar]
- 33.Munro BJ, Campbell TE, Wallace GG, Steele JR. The intelligent knee sleeve: a wearable biofeedback device. Sens Actuators B Chem 131: 541–547, 2008. doi: 10.1016/j.snb.2007.12.041. [DOI] [Google Scholar]
- 34.Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Effects of muscle injury severity on localized bioimpedance measurements. Physiol Meas 36: 27–42, 2015. doi: 10.1088/0967-3334/36/1/27. [DOI] [PubMed] [Google Scholar]
- 35.Nescolarde L, Yanguas J, Lukaski H, Alomar X, Rosell-Ferrer J, Rodas G. Localized bioimpedance to assess muscle injury. Physiol Meas 34: 237–245, 2013. doi: 10.1088/0967-3334/34/2/237. [DOI] [PubMed] [Google Scholar]
- 36.Neves EB, Pino AV, de Almeida RM, de Souza MN. Knee bioelectric impedance assessment in healthy/with osteoarthritis subjects. Physiol Meas 31: 207–219, 2010. doi: 10.1088/0967-3334/31/2/007. [DOI] [PubMed] [Google Scholar]
- 37.Nuñez C, Gallagher D, Visser M, Pi-Sunyer FX, Wang Z, Heymsfield SB. Bioimpedance analysis: evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med Sci Sports Exerc 29: 524–531, 1997. doi: 10.1097/00005768-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Nyboer J, Kreider MM, Hannapel L. Electrical impedance plethysmography: a physical and physiologic approach to peripheral vascular study. Circulation 2: 811–821, 1950. doi: 10.1161/01.CIR.2.6.811. [DOI] [PubMed] [Google Scholar]
- 39.Patterson RP. Fundamentals of impedance cardiography. IEEE Eng Med Biol Mag 8: 35–38, 1989. doi: 10.1109/51.32403. [DOI] [PubMed] [Google Scholar]
- 40.Pouyan M, Nourani M. Clustering single-cell expression data using random forest graphs. IEEE J Biomed Health Inform 21: 1172–1181, 2017. doi: 10.1109/JBHI.2016.2565561. [DOI] [PubMed] [Google Scholar]
- 41.Rai P, Kumar PS, Oh S, Kwon H, Mathur GN, Varadan VK, Agarwal MP. Smart healthcare textile sensor system for unhindered-pervasive health monitoring. Nanosensors, Biosensors Info-Tech Sensors Syst 8344: 83440E–83440E, 2012. doi: 10.1117/12.921253. [DOI] [Google Scholar]
- 42.Rangayyan RM, Wu YF. Screening of knee-joint vibroarthrographic signals using statistical parameters and radial basis functions. Med Biol Eng Comput 46: 223–232, 2008. doi: 10.1007/s11517-007-0278-7. [DOI] [PubMed] [Google Scholar]
- 43.Risacher F, Jossinet J, McAdams ET, McLaughlin J, Mann Y, Schmitt M, Matias A, Jarry R. Impedance plethysmography for the evaluation of pulse-wave velocity in limbs. Med Biol Eng Comput 31: 318–322, 1993. doi: 10.1007/BF02458053. [DOI] [PubMed] [Google Scholar]
- 44.Rosell J, Colominas J, Riu P, Pallas-Areny R, Webster JG. Skin impedance from 1 Hz to 1 MHz. Biomed Eng IEEE Trans 35: 649–651, 1988. [DOI] [PubMed] [Google Scholar]
- 45.Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA 105: 1118–1123, 2008. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito H, Watanabe T, Arifin A.. Ankle and knee joint angle measurements during gait with wearable sensor system for rehabilitation. In: World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany, edited by Dössel O, Schlegel W. Berlin: Springer, 2009, p. 506–509. [Google Scholar]
- 47.Schulze M, Liu T-H, Xie J, Zhang W, Wolf K-H, Calliess T, Windhagen H, Marschollek M. Unobtrusive ambulatory estimation of knee joint angles during walking using gyroscope and accelerometer data-a preliminary evaluation study. Biomed Health Informatics 2012 IEEE-EMBS Int Conf, p. 559–562. [Google Scholar]
- 48.Seel T, Raisch J, Schauer T. IMU-based joint angle measurement for gait analysis. Sensors (Basel) 14: 6891–6909, 2014. doi: 10.3390/s140406891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah S, Toreyin H, Inan OT, Hasler J. Reconfigurable analog classifier for knee-joint rehabilitation. 2016 IEEE 38th Annual International Conference, 2016, p. 4784–4787. [DOI] [PubMed] [Google Scholar]
- 50.Shark L-K, Chen H, Goodacre J. Discovering differences in acoustic emission between healthy and osteoarthritic knees using a four-phase model of sit-stand-sit movements. Open Med Inform J 4: 116–125, 2010. doi: 10.2174/1874431101004010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Methodological guidelines for impedance cardiography. Psychophysiology 27: 1–23, 1990. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 51.Staszewski WJ, Boller C, Tomlinson GR. Health Monitoring of Aerospace Structures: Smart Sensor Technologies and Signal Processing. West Sussex: John Wiley, 2004. [Google Scholar]
- 52.Stranden E. A comparison between surface measurements and water displacement volumetry for the quantification of leg edema. J Oslo City Hosp 31: 153–155, 1981. [PubMed] [Google Scholar]
- 53.Tavathia S, Rangayyan RM, Frank CB, Bell GD, Ladly KO, Zhang YT. Analysis of knee vibration signals using linear prediction. IEEE Trans Biomed Eng 39: 959–970, 1992. doi: 10.1109/10.256430. [DOI] [PubMed] [Google Scholar]
- 54.Teague C, Hersek S, Toreyin H, Millard-Stafford ML, Jones ML, Kogler GF, Sawka MN, Inan OT. Novel approaches to measure acoustic emissions as biomarkers for joint health assessment. In: IEEE Body Sensor Networks. Cambridge, MA: IEEE, 2015. [Google Scholar]
- 55.Teague CN, Hersek S, Conant JL, Gilliland SM, Inan OT. Wearable knee health rehabilitation assessment using acoustical emissions. In: AIP Conference Proceedings. Melville, NY: AIP Publishing, 2017, p. 070008. [Google Scholar]
- 56.Teague CN, Hersek S, Toreyin H, Millard-Stafford ML, Jones ML, Kogler GF, Sawka MN, Inan OT. Novel methods for sensing acoustical emissions from the knee for wearable joint health assessment. IEEE Trans Biomed Eng 63: 1581–1590, 2016. doi: 10.1109/TBME.2016.2543226. [DOI] [PubMed] [Google Scholar]
- 57.Toffola LD, Patel S, Ozsecen MY, Ramachandran R, Bonato P. A wearable system for long-term monitoring of knee kinematics. Biomed Health Informatics 2012 IEEE-EMBS Int Conf, 2012, p. 188–191. [Google Scholar]
- 58.Tognetti A, Lorussi F, Bartalesi R, Quaglini S, Tesconi M, Zupone G, De Rossi D. Wearable kinesthetic system for capturing and classifying upper limb gesture in post-stroke rehabilitation. J Neuroeng Rehabil 2: 8, 2005. doi: 10.1186/1743-0003-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Töreyin H, Jeong HK, Hersek S, Teague CN, Inan OT. Quantifying the consistency of wearable knee acoustical emission measurements during complex motions. IEEE J Biomed Health Inform 20: 1265–1272, 2016. doi: 10.1109/JBHI.2016.2579610. [DOI] [PubMed] [Google Scholar]
- 60.Töreyin H, Jeong HK, Hersek S, Teague CN, Inan OT. Real-time activity classification in a wearable system prototype for knee health assessment via joint sounds. 2016 IEEE 38th Annu Int Conf, 2016, p. 3113–3116. [DOI] [PubMed] [Google Scholar]
- 61.Toreyin H, Shah S, Hersek S, Inan OT, Hasler J. Proof-of-concept energy-efficient and real-time hemodynamic feature extraction from bioimpedance signals using a mixed-signal field programmable analog array. In: IEEE Biomedical and Health Informatics Conference. Orlando, FL: IEEE, 2017. [Google Scholar]
- 63.Umapathy K, Krishnan S. Modified local discriminant bases algorithm and its application in analysis of human knee joint vibration signals. IEEE Trans Biomed Eng 53: 517–523, 2006. doi: 10.1109/TBME.2005.869787. [DOI] [PubMed] [Google Scholar]
- 64.Vico L, Collet P, Guignandon A, Lafage-Proust M-H, Thomas T, Rehailia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355: 1607–1611, 2000. doi: 10.1016/S0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe T, Saito H.. Tests of wireless wearable sensor system in joint angle measurement of lower limbs. Eng Med Biol Soc EMBC 2011 Annu Int Conf IEEE, 2011, p. 5469–5472. [DOI] [PubMed] [Google Scholar]
- 66.Whittingslow DC, Semiz B, Ponders L, Wiens A, Inan OT, Prahalad S. Analysis and implications of non-invasive knee acoustical emissions in juvenile idiopathic arthritis: a case study. In: Pediatric Rheumatology Symposium. Houston, TX: Am College of Rheumatol, 2017. [Google Scholar]
- 67.Winterstein AP, McGuine TA, Carr KE, Hetzel SJ. Comparison of IKDC and SANE outcome measures following knee injury in active female patients. Sports Health 5: 523–529, 2013. doi: 10.1177/1941738113499300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 81: 646–656, 2003. [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng H, Black ND, Harris ND. Position-sensing technologies for movement analysis in stroke rehabilitation. Med Biol Eng Comput 43: 413–420, 2005. doi: 10.1007/BF02344720. [DOI] [PubMed] [Google Scholar]