Abstract
Cortical reorganization after stroke is thought to underlie functional improvement. Patterns of reorganization may differ depending on the amount of time since the stroke or the degree of improvement. We investigated these issues in a study of brain connectivity changes with aphasia therapy. Twelve individuals with chronic aphasia participated in a 6-week trial of imitation-based speech therapy. We assessed improvement on a repetition test and analyzed effective connectivity during functional magnetic resonance imaging of a speech observation task before and after therapy. Using structural equation modeling, patient networks were compared with a model derived from healthy controls performing the same task. Independent of the amount of time since the stroke, patients demonstrating behavioral improvement had networks that reorganized to be more similar to controls in two functional pathways in the left hemisphere. Independent of behavioral improvement, patients with remote infarcts (2–7 years poststroke; n = 5) also reorganized to more closely resemble controls in one of these pathways. Patients with far removed injury (>10 years poststroke; n = 3) did not show behavioral improvement and, despite similarities to the normative model and overall network heterogeneity, reorganized to be less similar to controls following therapy in a distinct right-lateralized pathway. Behavioral improvement following aphasia therapy was associated with connectivity more closely approximating that of healthy controls. Individuals who had a stroke more than a decade before testing also showed plasticity, with a few pathways becoming less like controls, possibly representing compensation. Better understanding of these mechanisms may help direct targeted brain stimulation.
Keywords: : aphasia, effective connectivity, neuroplasticity, speech therapy, stroke recovery
Introduction
One-third of all stroke patients present with some form of aphasia (Laska et al., 2001), with as many as one-fifth demonstrating persistent language impairment beyond 6 months. Severity is typically dependent on lesion extent, and outcome depends on myriad factors (Laska et al., 2001; Lazar et al., 2008), including therapy. Neurobiological studies of stroke recovery in humans have reported plasticity months after the infarction (Saur and Hartwigsen, 2012). Multiple compensatory mechanisms have been reported in individuals with aphasia, including functional recovery of the damaged region itself, activation of perilesional areas, and homologous right hemisphere activation (Saur and Hartwigsen, 2012). Correlation of multiple mechanisms with behavioral improvement implies a dynamic process for recovery in neural regions supporting language performance.
A systematic review of randomized controlled studies of aphasia intervention suggests that different behavioral interventions are generally comparable in efficacy (Brady et al., 2012). Most therapies are developed based on linguistic or psychological models, focusing on distinct deficits (e.g., syntax) or other therapeutic variables such as treatment intensity. From a biological standpoint, effective rehabilitative therapy relies on the ability to predict adaptive reorganization and to target specific neural pathways to promote recovery. This requires identification of affected neural networks on an individual basis or the targeting of circuits recognized as integral to language. Such therapies have been developed for aphasia (Baker et al., 2010; Lee et al., 2010; Marangolo and Caltagirone, 2014; Torres et al., 2013), and neuroimaging methods can be used to investigate treatment effects on the plasticity of language networks.
Functional neuroimaging permits noninvasive examination of global and regional plasticity in individuals with aphasia (Abel et al., 2015; Saur et al., 2010; Schlaug et al., 2009). A common observation is activation of regions that are not active for healthy controls performing the same language task: the interpretation of these regional activations is vexing as they have been viewed as either functionally adaptive (Perani et al., 2003) or obstructive (Postman-Caucheteux et al., 2010). Another metric of plasticity is altered connectivity (e.g., due to neural synchronization and directional influences) within neural networks. Only a few studies have investigated the dynamics of functional and effective connectivity during stroke recovery, with findings typically focusing on subcortical lesions and/or motor-related cortical activity (Bajaj et al., 2015; Rehme and Grefkes, 2013). To date, the use of functional neuroimaging methods to assess effective connectivity in aphasia therapy is limited (but see Abutalebi et al., 2009; Kiran et al., 2015).
In this study, we examine the effect of a behavioral intervention on reorganization of bihemispheric networks following left cortical stroke. Using structural equation modeling (SEM), network models for participants with aphasia were compared with a normative network model of language processing before, during, and after 6 weeks of intensive imitation-based aphasia therapy. We anticipated multiple patterns of neuroplasticity, depending on the degree of improvement and the time between the stroke and the testing. In particular, we expected participants with recent strokes and/or good therapeutic response to become more similar to healthy controls over the course of therapy, including stronger left lateralization of pathways used in repetition. On the other hand, we expected participants with more remote strokes and/or poor therapeutic response to demonstrate different reorganizational patterns, perhaps involving more right lateralization of these pathways.
Materials and Methods
Participants
Twelve participants with left hemisphere ischemic cortical strokes were recruited based on medical criteria and ability to participate in the therapy. Medical criteria included no prior history of neurological or psychiatric disease, including stroke. Selected demographics are listed in Table 1 for each participant. This study was carried out in accordance with recommendations of the Institutional Review Boards of The University of Chicago and University of California, Irvine, with written informed consent from all participants. All participants gave written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Participant Information
| Unconstrained model comparison pattern | ||||||||
|---|---|---|---|---|---|---|---|---|
| Participant number | Gender | Age at start of study (years) | Months poststroke (at start of study) | Lesion size (LH%) | Average AQ | Δ Repetition (%) | LH | RH |
| 4 | M | 63 | 7 | 7.52 | 20.8 | 0.4333 | Before | Both |
| 5 | M | 56 | 16 | 3.31 | 79.1 | 2.1723 | After | Both |
| 6 | M | 65 | 8 | 6.36 | 81.0 | 5.4872 | Neither | After |
| 9 | F | 46 | 28 | 17.36 | 76.3 | 12.0501 | Both | Before |
| 11 | M | 58 | 13 | 19.78 | 58.1 | 2.6294 | Both | Both |
| 13 | M | 36 | 78 | 12.35 | 77.8 | 8.5944 | Both | Neither |
| 14 | M | 37 | 51 | 10.06 | 68.3 | 8.1579 | Both | Before |
| 15 | M | 70 | 120 | 26.34 | 83.1 | −2.8251 | Both | Both |
| 16 | M | 58 | 29 | 3.25 | 93.8 | 1.8299 | Both | After |
| 17 | M | 57 | 130 | 13.52 | 77.8 | 2.5076 | Both | Both |
| 18 | M | 55 | 81 | 11.54 | 55.4 | 8.6429 | Before | Both |
| 19 | M | 42 | 123 | 5.21 | 84.4 | −0.8835 | After | Neither |
Select demographics, behavioral data, and results of unconstrained model comparisons (including pre-post therapy changes) for all participants. LH %, percent of left hemisphere lesioned. Average AQ, average over 4 testing sessions of Aphasia Quotient (AQ) composite score from Western Aphasia Battery-Revised (WAB; maximum = 100). Δ Repetition, change in score from repetition test between start and end of study. Both indicates participants generally resembled the normative model across all study time points; After indicates participants resembled the normative model during/after therapy but not before. Before indicates participants resembled the normative model before/during therapy but not after; Neither indicates no pattern to model comparison across time points.
RH, right hemisphere.
Image acquisition
Imaging was performed on a Siemens 3T Trio scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) at the Center for Advanced Magnetic Resonance Imaging (CAMRI) of Northwestern University in Chicago. Anatomical images used a T1-magnetization-prepared rapid gradient-echo sequence with the following parameters: TR = 2300 ms, TE = 3.36 ms, FA = 9°, and voxel size = 1 mm isotropic. Functional acquisition used an echo-planar imaging sequence with the following parameters: TR = 1500 ms, TE =20 ms, FA = 71°, FOV = 220 × 220 mm, 29 axial slices with 4-mm thickness (1-mm gap), and in-plane voxel size = 3.75 ×3.75 mm. Functional runs were 6:30 min (260 volumes). During the functional scan, participants performed an observation task, passively watching and listening to a video of a woman articulating four syllables: /pa/, /fa/, /ta/, and/θa/(tha). These syllables were chosen due to their varying articulatory profiles (i.e., visually distinct appearance). Functional runs contained 120 event-related randomized stimuli (30 of each syllable, jittered ISI = 0 to 12 sec, sequenced for deconvolution per optseq2; Greve, 2002).
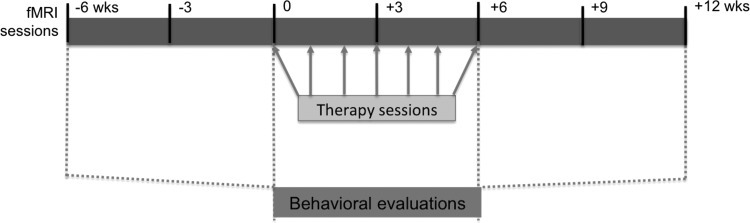
Participants underwent imaging sessions seven times over 18 weeks, beginning at week −6 and ending at week 12, with 3 weeks between imaging sessions. During weeks 0 to 6 of the study, participants completed three 30-min sessions of imitation-based aphasia therapy 6 days a week (Lee et al., 2010). Figure 1 depicts the study timeline.
FIG. 1.
Study timeline (total of 18 weeks), including imaging time points (top box), behavioral testing sessions (dashed lines), and the seven in-clinic therapy sessions (arrows). Imaging sessions occurred every 3 weeks. Behavioral evaluations occurred every 6 weeks. During the 6-week intensive therapy period, one session was conducted in the clinic weekly.
Behavioral assessment
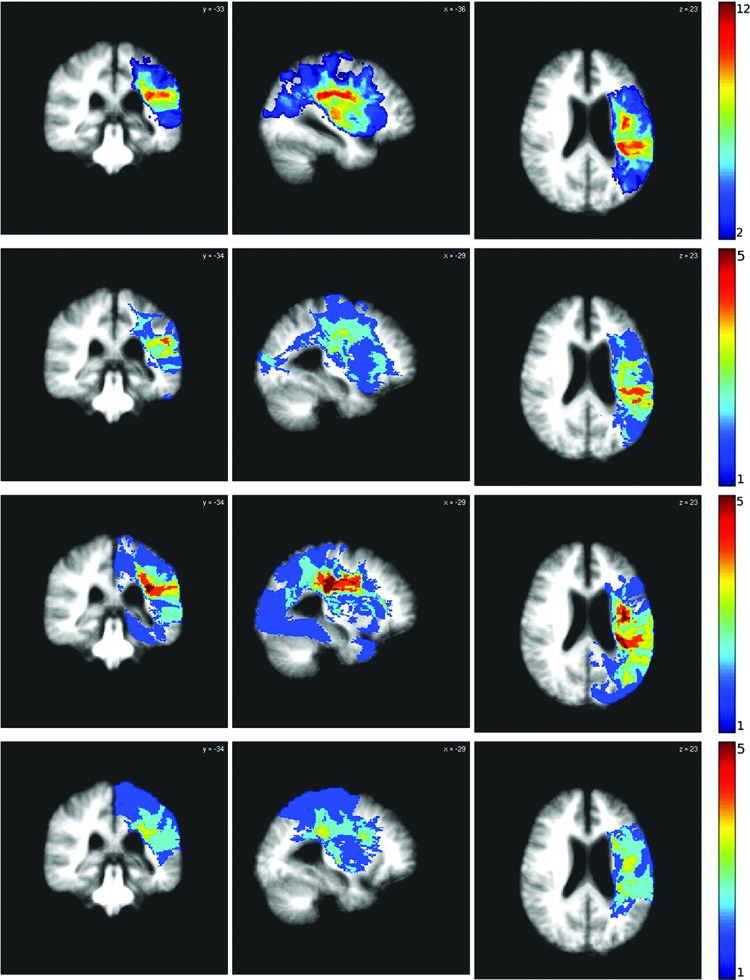
Additional speech and language testing was administered at four time points (6 weeks apart) over the 18-week study (weeks −6, 0, 6, and 12). This included administration of the Western Aphasia Battery (WAB)-Revised (Kertesz, 1982) and a repetition test mimicking the therapy task (for additional details, see Duncan et al., 2016). Figure 1 depicts the study timeline, including imaging, behavioral testing, and in-clinic therapy sessions. Additionally, three discrete participant subsets were derived based on time since stroke: recent (<16 months), moderately remote (2–7 years), and far removed (10+ years). These subgroups were derived holistically from the participant set, with the consideration that poststroke recovery is most dramatically evidenced in the short term (i.e., within 1 year of injury; Carmichael, 2003; Jenkins and Merzenich, 1987; Thulborn et al., 1999; Wolf et al., 2007), but some studies have demonstrated neurogenesis and significant neural plastic outcomes persisting for years after injury (Cramer, 2008; Gauthier et al., 2008; Kopp et al., 1999). Lesion overlap for the whole group and each of the three subgroups can be seen in Figure 2.
FIG. 2.
Lesion overlap. The top row shows lesion overlap for the whole group. The remaining three rows show lesion distribution for the three subgroups: recent (<16 months), moderately remote (2–7 years), and far removed (10+ years). Color images available online at www.liebertpub.com/brain
Image preprocessing
Functional magnetic resonance imaging (fMRI) data processing was performed using AFNI (Cox, 1996). Preprocessing steps for the functional images included despiking, slice time correction, motion correction, and alignment to anatomical data. Multiple linear regression of functional data was performed with the observation task as the main regressor and nuisance variables comprising motion parameters and global, white matter, and ventricular signals. Time series were converted to percent signal change, which was deconvolved based on a contrast with the baseline, resulting in statistical activation maps rendered using a false discovery rate corresponding to p < 0.05 after multiple comparison correction.
We used virtual brain transplantation (VBT; Solodkin et al., 2010) to permit automated parcellation of the cortical surface despite lack of anatomical data in regions affected by stroke. For VBT, the nonlesioned right hemisphere was flipped and nonlinearly aligned to the lesioned left hemisphere. A hand-drawn lesion mask was then used to extract virtual tissue from the right hemisphere, which was then transplanted into the left hemisphere to facilitate use of FreeSurfer. VBT anatomical volumes were then processed in FreeSurfer (Fischl, 2012) by inflation of the cortical surface and registration to an average template.
Regions of interest (ROIs) were selected based on the anatomical model of speech observation and execution used by Mashal and associates (2012) and are listed in Table 2. For each participant, ROIs were identified by registering functional overlays from the observation task to the anatomical underlay. ROIs were defined by grouping of automated FreeSurfer parcellation of the cortical surface (Table 2). Parcellation maps were then projected back to volumetric space and aligned with functional data to extract time series from the peak voxel (maximum t-value) for each ROI (Walsh et al., 2008).
Table 2.
Anatomical Description and Boundaries for Regions of Interest
| ROI | Parcellated anatomical regions | Brodmann's areas | Delimiting landmarks |
|---|---|---|---|
| IP (inferior parietal) | Inferior parietal gyrus (angular) Inferior parietal gyrus (supramarginal) Intraparietal/parietal transverse sulcus |
39, 40 | A = Postcentral sulcus P = Sulcus intermedius secundus S = Superior parietal gyrus I = Horizontal posterior segment of the superior temporal sulcus |
| M1 (primary motor) | Central sulcus Postcentral gyrus |
1,2,3,4 | A = Precentral gyrus P = Postcentral sulcus S = Medial surface of the hemisphere I = Parietal operculum |
| postST (posterior superior temporal) | Superior temporal gyrus (planum temporale) Superior temporal gyrus (posterior) Superior temporal sulcus (posterior) |
41,42,22p | A = Vertical plane drawn from the anterior extent of the transverse temporal gyrus P = Angular gyrus S = Supramarginal gyrus I = Middle temporal gyrus |
| antST (anterior superior temporal) | Superior temporal gyrus (planum polare) Superior temporal sulcus (anterior) Precentral sulcus (ventral) |
22a | A = Inferior circular sulcus of insula P = Vertical plane drawn from the anterior extent of the transverse temporal gyrus S = Anterior horizontal ramus of the sylvian fissure I = Middle temporal gyrus |
| LPMCv (ventrolateral premotor) | Precentral gyrus (ventral) Inferior frontal gyrus (opercular) Inferior frontal sulcus (inferior) Precentral sulcus (dorsal) |
4,6v,44 | A = Anterior vertical ramus of the sylvian fissure P = Central sulcus S = Inferior frontal sulcus, extending a horizontal plane posteriorly across the precentral gyrus I = Anterior horizontal ramus of the sylvian fissure to the border with insular cortex |
| LPMCd (dorsolateral premotor) | Precentral gyrus (dorsal) Superior frontal gyrus (posterior) Superior frontal sulcus (posterior) |
6d | A = Vertical plane through the anterior commissure P = Central sulcus S = Medial surface of the hemisphere I = Inferior frontal sulcus, extending a horizontal plane posteriorly across the precentral gyrus |
ROI, region of interest.
Structural equation modeling
SEM was performed on the time series extracted from task-related ROIs using AMOS (SPSS, Inc., Chicago, IL). SEM, or path analysis, identifies connection strengths (coefficients) that best predict the observed variance to covariance relationship of the data with respect to a defined anatomical model. Coefficients derived from SEM analysis represent the change in functional activity of a target region per unit change in activity of a source region (McIntosh, 1998).
Participant models of activity during the observation task were compared with previously identified normative left and right hemisphere models for this same task (Mashal et al., 2012); nodes in this model were selected and validated based on anatomical landmarks and fMRI task-related activation maps derived from a healthy control population (n = 11). Patient models in the present study were constructed using this theoretical anatomical network model combined with the functional time series extracted from the peak voxel of each of the six ROIs in Table 2: primary sensorimotor cortices (M1), dorsolateral premotor cortex (LPMCd), ventrolateral premotor cortex (LPMCv), inferior parietal lobule (IP), posterior superior temporal gyrus/sulcus (postST), and anterior superior temporal gyrus/sulcus (antST).
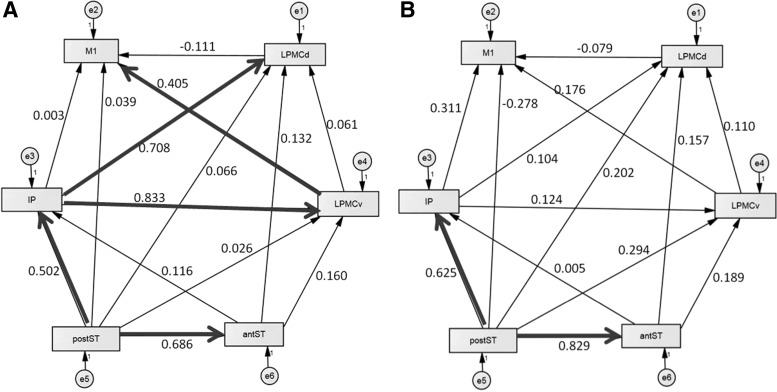
Covariance matrices were constructed for each participant using the peak voxel time series for each ROI. The network model (derived from the time series-based covariance matrix) was compared with the predicted model derived from the normative group result for healthy controls (Fig. 3A, B) for each participant at each imaging time point. Using the predicted model, structural equations relating weights and directional influence of network nodes were derived and iterative maximum likelihood solutions obtained. An acceptance (p ≥ 0.05) or rejection (p < 0.05) of the null hypothesis (i.e., no difference between predicted/observed models) was determined from the chi-squared (χ2) solution distribution (McIntosh and Gonzalez-Lima, 1994). Individual models that did not fit the normative model (i.e., failed to achieve the χ2 criteria for a good fit) at a particular time point were excluded from second-level analysis for that time point.
FIG. 3.
Normative model for speech observation task for the (A) left and (B) right hemispheres. Weights reflect model fit for an average control group. Highlighted pathways are those of significant (>0.4) weighting (reflecting directional influence/connectivity) for the observation task in the normal population. Region abbreviations are as follows: antST, anterior superior temporal gyrus/sulcus; IP, inferior parietal lobule; LPMCd, dorsolateral premotor cortex; LPMCv, ventrolateral premotor cortex; M1, primary sensorimotor cortices; postST, posterior superior temporal gyrus/sulcus.
Multiple group analysis was also performed on the individual network models in comparison with the normative model to identify specific pathways of significance. Individual models were compared with the normative group model using the stacked model method (McIntosh and Gonzalez-Lima, 1994), in which free models (i.e., all pathways are allowed to vary between groups) are compared systematically with constrained models (i.e., a single pathway is considered equal for each constrained model). Model comparison was assessed by comparing the goodness-of-fit (χ2) value for the free model with that of the constrained model, where significant difference is determined by a critical χ2 value (χ2diff) based on degrees of freedom in the statistical analysis to identify deviance from the normative model. If the model fit is better when a pathway is allowed to vary than when constrained, the models are considered significantly different and the pathway is considered to deviate from the normative model (Dick et al., 2010; McIntosh and Gonzalez-Lima, 1994). Variances of the pathway residuals (error) were included and limited to unity to reduce the total number of estimated parameters. As models must be identical for multiple group comparison, if functional data were missing due to lesion, a random time series was entered for that ROI and coefficients for all connecting paths were fixed to zero. As the analysis used the time series from the peak voxel within each ROI, exclusion due to lesion was neither common nor systematic among participants within subgroups (Fig. 2 shows overlap of lesion regions by subgroup).
Results
Select behavioral data from all participants are shown in Table 1. The majority of participants had average aphasia quotient (AQ) scores on the WAB in the 68–93-point range, with notable exceptions of participants 4, 11, and 18. Scores on the repetition test improved moderately (defined as positive change of at least 5%) between the start and end of the study (−6 to 12 weeks) for participants 6, 9, 13, 14, and 18. This subset of participants was considered to have behavioral improvement.
Unconstrained model comparisons revealed general conformity to the free normative model for most participants. A few individuals showed significantly higher or lower goodness-of-fit scores before or after therapy, and these are noted as such in Table 1. Fit to the normative model is a global measure of task-related network activation, with findings in Table 1 indicating (for the most part) that participants utilized the normative network during the task. In a few cases, there was no pattern of fit over the seven study time points (indicated as None in Table 1), which led to exemption of those time points that did not converge to the normative model skeleton (and were therefore not statistically valid for path-by-path comparisons).
Path-by-path network analysis within the normative model was examined using multiple group analysis. Unlike unconstrained model comparisons, these are pathway-level comparisons within a constrained model. Changes in effective connectivity over time within the known network can be indicative of plasticity on the participant level. Multiple group analysis results are shown in Tables 3 and 4; significant differences from the normative model are shown for each pathway individually. For the left hemisphere, postST→antST, IP→LPMCv, and IP→LPMCd pathway coefficients were significantly different from the normative model at all time points for all or nearly all participants (Table 4), while many other pathways showed patterns of change (Table 3; either becoming more or less like controls following therapy).
Table 3.
Changes in Effective Connectivity Associated with Therapy
| Participant number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathway | 4 | 5 | 6 | 9 | 11 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| Left hemisphere | ||||||||||||
| LPMCd→M1 | →C | →C | ||||||||||
| postST→IP | →C | →C | →C | →C | C→ | C→ | →C | C→ | ||||
| antST→LPMCv | →C | →C | ||||||||||
| LPMCv→M1 | C→ | C→ | →C | →C | →C | →C | →C | →C | →C | C→ | ||
| antST→IP | →C | C→ | ||||||||||
| Right hemisphere | ||||||||||||
| postST→IP | C→ | →C | C→ | C→ | C→ | |||||||
Results of multiple group analysis for both hemispheres, presented as path-by-path model comparisons for each patient, and indicating changes over the course of therapy. C→ indicates pathway was significantly different from normal model during/after therapy but not before; →C indicates pathway was significantly different from normal model before/during therapy but not after. Pathways that did not demonstrate significant change for at least two participants are omitted.
antST, anterior superior temporal gyrus/sulcus; IP, inferior parietal lobule; LPMCd, dorsolateral premotor cortex; LPMCv, ventrolateral premotor cortex; M1, primary sensorimotor cortices; postST, posterior superior temporal gyrus/sulcus.
Table 4.
Differences and Similarities in Effective Connectivity from the Normative Model
| Patient number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathway | 4 | 5 | 6 | 9 | 11 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| Left hemisphere | ||||||||||||
| IP→LPMCd | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | |
| IP→LPMCv | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ |
| postST→IP | Δ | Δ | Δ | |||||||||
| postST→antST | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ |
| LPMCv→M1 | Δ | Δ | ||||||||||
| LPMCd→M1 | C | Δ | C | Δ | C | C | C | C | C | C | ||
| LPMCd→LPMCv | C | C | C | C | C | C | C | C | C | C | C | |
| antST→LPMCv | C | C | C | C | C | C | C | C | C | C | ||
| postST→LPMCv | C | C | C | C | C | C | C | C | C | C | ||
| antST→IP | C | C | C | C | C | C | C | C | C | C | ||
| antST→LPMCd | C | C | C | C | C | C | C | C | C | C | C | C |
| postST→LPMCd | C | C | C | C | C | C | C | C | C | C | C | C |
| IP→M1 | C | C | C | C | C | C | C | C | C | C | C | |
| postST→M1 | C | C | C | C | C | C | C | C | C | C | C | C |
| Right hemisphere | ||||||||||||
| postST→IP | Δ | Δ | Δ | Δ | Δ | Δ | Δ | |||||
| postST→antST | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | |
| postST→M1 | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | Δ | |
| LPMCd→M1 | C | C | C | C | C | C | C | C | C | C | C | |
| LPMCv→LPMCd | C | C | C | C | C | C | C | C | C | C | C | |
| IP→LPMCd | C | C | C | C | C | C | C | C | C | C | C | C |
| IP→LPMCv | C | C | C | C | C | C | C | C | C | C | C | C |
| antST→LPMCv | C | C | C | C | C | C | C | C | C | C | C | C |
| LPMCv→M1 | C | C | C | C | C | C | C | C | C | C | C | C |
| postST→LPMCv | C | C | C | C | C | C | C | C | C | C | C | C |
| antST→IP | C | C | C | C | C | C | C | C | C | C | C | |
| antST→LPMCd | C | C | C | C | C | C | C | C | C | C | C | C |
| postST→LPMCd | C | C | C | C | C | C | C | C | C | C | C | C |
| IP→M1 | C | C | C | C | C | C | C | C | C | C | ||
Results of multiple group analysis for both hemispheres, presented as path-by-path model comparisons for each patient. Delta (Δ) indicates pathway was significantly different from the normative model at all study time points. C indicates pathway did not differ significantly from the control model at any study time point. Pathways that were consistently different or similar, respectively, for at least two participants are included.
As there were a total of seven imaging time points, patterns of change in each participant were defined based on comparison of the three time points preceding therapy sessions with the one during therapy and the three following therapy. Generally, one of the following three patterns was noted: (1) the first three time points were similar to controls, but subsequent time points were different from controls [C→] (Table 3); (2) the first three time points were different from controls, but subsequent time points were similar to controls [→C] (Table 3), and (3) no change was present between pre- and post-therapy time points [remaining consistently different (Δ) or similar (C) reported in Table 4].
Two effective connections, postST→IP and LPMCv→M1, showed patterns of change (denoted as C→ or →C in Table 3) in the majority of participants, with a few participants also showing changes in LPMCd→M1, antST→LPMCv, and antST→IP. Additionally, a few pathway coefficients (antST→LPMCd, postST→LPMCd, and postST→M1) appear to have no significant difference at any time point from the normative model in the left hemisphere (Table 4). In the right hemisphere, postST→M1 and postST→antST pathway coefficients were significantly different from the normative model at all time points for nearly all participants, with few other differences from the normative model for other pathways (Table 4).
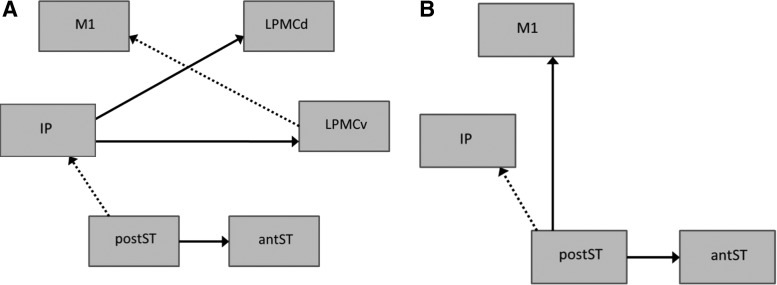
Figure 4 depicts comparative models for the left and right hemispheres, highlighting those pathways that had some pattern of change over the study. Aforementioned pathways that were significantly different from controls at all time points are shown in bold arrows in Figure 4. Despite variability in lesion location among participants and even within subgroups (Fig. 2), consistent patterns of reorganization within a subgroup are also represented. For participants with behavioral improvement (n = 5; participants 6, 9, 13, 14, and 18), left hemisphere LPMCv→M1 and postST→IP pathways consistently changed from being different from the normative model pretherapy to more similar post-therapy (with the exception of participant 9 postST→IP, which was not significantly different from the control model during pretherapy time points). A similar pattern was seen in the LPMCv→M1 pathway for those participants with moderately remote injury (n = 5; participants 9, 13, 14, 16, and 18). However, those with far removed injury (n = 3; participants 15, 17, and 19) showed the opposite pattern (i.e., more similar to controls before therapy than after) in the right hemisphere postST→IP pathway. Participants with recent injury (n = 4; participants 4, 5, 6, and 11) did not demonstrate any singular consistent pattern.
FIG. 4.
Comparative models for (A) left and (B) right hemispheres indicating paths significantly different from the normative group. Solid lines denote pathways that were consistently different from the normative model across participants and time points, while dashed lines denote pathways that showed significant changes within a subgroup of patients over the course of the study. (A) LPMCv → M1: patients 2–7 years poststroke (moderately remote injury) and those with behavioral improvement; postST → IP: patients with behavioral improvement; and (B) postST → IP: patients >10 years poststroke (far removed injury). Region abbreviations as in Figure 3.
Discussion
Several findings are reported in this study with regard to therapy-based aphasia recovery. First, we identified three functional pathways within a language network that were altered by injury and not recovered in response to therapy. Second, we observed distinct patterns of stroke recovery with respect to a normative model: reorganization of the network after therapy, such that some pathways demonstrated more similarity to those of controls after therapy than before, and others had the opposite result. Those participants with behavioral improvement, as well as those with moderately remote injury, recovered in a way that ultimately resembled the normative group more after therapy than before. However, participants with far removed injury (who did not show therapeutic benefit) also reorganized, but away from the normative model, that is, they resembled the normative group model more before therapy than after in one right-lateralized pathway. Third, some path coefficients did not differ significantly from the normative model at any time point (especially in the right hemisphere), implying that these pathways were not altered by stroke or recovery. Hemispheric differences in the results are also notable: the left hemisphere demonstrated much more plasticity in all participants. This might be expected from the inherent laterality of some important aspects of language processing (Heiss et al., 1999), although some aphasia recovery models would anticipate the majority of changes in the contralesional hemisphere (Anglade et al., 2014; Hartwigsen et al., 2013; Wan et al., 2014). Additionally, while some models suggest that right hemispheric contributions are most important for more severely affected participants (Heiss and Thiel, 2006; Turkeltaub et al., 2012), in this study, right hemispheric changes were found primarily in the participants with far removed injury, independent of severity, and were not associated with behavioral improvement.
These findings imply that individuals with poststroke aphasia have potentially different ways of reorganizing to remediate and/or compensate for regional loss of neuronal function. First, the fact that unconstrained model comparisons showed varying trends over the study indicates some amount of nonconformity, suggesting involvement of regions outside the normative skeleton derived from neurologically intact subjects. Time points that did not converge may represent a different kind of reorganization, with recruitment of regions external to the base model active during speech observation in healthy controls. Investigation of a more comprehensive model, including external regions, might benefit a fuller understanding of recovery.
The normative model used in this study reflected significant connections among regions engaged during language processing that are consistent with previous studies (Binder et al., 2000; Mashal et al., 2012; Wise et al., 2001). Of these, several left hemisphere pathways (postST→antST, IP→LPMCd, and IP→LPMCv) were significantly different from the normative group over all time points for all (or nearly all) participants, representing injured and nonrecovered connections. In the predicted model (Fig. 3), these pathways had significant directional influence (>0.4 or <−0.2), implying an important role in speech observation for healthy individuals. Thus, we can assume that disruption of these pathways by stroke leads to lower influence on neighboring regions, poorer audiovisual word observation, and other deficits in language processing. Alternatively, the fact that left postST→IP and LPMCv→M1 pathways (also of significant influence in the normative model) resembled controls after the study in the repetition-improved subgroup implies that plasticity in these pathways is associated with improved behavioral outcome. As repetition appears to be mediated largely by a left-lateralized posterodorsal stream (Saur et al., 2008), it is unsurprising that increasing normalcy of postST→IP connections would be implicated in improvement in this skill, and LPMCv→M1 may indicate a strengthening of motor planning with execution.
A second set of findings was related to pathways that changed to become either more similar to or more different from the normative model after therapy, when compared to before. Patterns were noted in subgroups of participants only; there was no reorganization common to all participants. As stated above, the subgroup that showed behavioral improvement had left hemisphere LPMCv→M1 and postST→IP pathways conforming more greatly to the normative model after therapy; this implies functional recovery in these participants due to restoration of the level of effective influence in healthy controls. A subset of participants with moderately remote injury (2–7 years) also appeared to resemble the normative model more after therapy in the LPMCv→M1 pathway; however, those with very remote injury (10+ years) resembled the control group less after therapy than before in the right hemisphere postST→IP pathway. All participants in the moderately remote injured group reorganized to resemble controls more, regardless of behavioral improvement, possibly indicating specific recovery in a pathway receptive to targeting for improved speech. Notably, participants with far removed injury exhibited plasticity in response to therapy even a decade poststroke, although behavioral recovery in stroke is typically thought to plateau within the first 18 months. Furthermore, reorganization at this time point appears to occur through com-pensatory mechanisms, evidenced by deviation from the control group and reorganization within the contralesional hemisphere. Such compensation may be necessary in this subgroup uniquely as the injury from stroke may have permanently affected otherwise dynamic pathways (such as those identified with the moderately remote injury and behaviorally improved groups). However, it is possible that this reorganization may reflect a form of long-term maladaptation, such as learned nonuse (Taub et al., 2006).
The role of structural pathways in aphasia recovery is relevant to the development of transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) as potential adjunctives to aphasia therapy. TMS and tDCS have been applied in a number of studies of aphasia, typically either inhibiting or stimulating regions involved in language to observe subsequent behavioral outcomes (Naeser et al., 2011; Schlaug et al., 2011; Turkeltaub et al., 2012). Studies have achieved moderate improvements between pre- and poststimulation aphasia (Galletta et al., 2011; Martin et al., 2009; Naeser et al., 2010), but primarily identify targets on the basis of anatomical hypotheses. If effective connectivity can identify specific brain regions as functionally necessary for aphasia recovery at various stages, the timing or combination with behavioral therapy could be refined for augmentation with stimulation.
Sharma and associates (2009) examined the distal effects of subcortical infarct on cortical networks by relating motor recovery in subcortical stroke participants to cortical motor network connectivity. Limited connectivity was found in extended motor networks, even with behavioral improvement and normal regional activity. Neither reorganization of networks, as measured by effective connectivity between motor-related regions, nor activation extent alone directly reflected functional recovery. The implication of multiple modes of recovery reinforces similar findings in the current study.
Recently, Kiran and associates (2015) examined therapy-induced changes in effective connectivity between regions active during language tasks in seven individuals with chronic aphasia following stroke using dynamic causal modeling. Interestingly, she and her colleagues found the most consistent changes in connectivity for the left inferior frontal gyrus (IFG), partially included in our LPMCv, and which also demonstrated connectivity changes in our study for individuals with behavioral improvement or moderately remote injury. Deactivation of the left IFG has also been found following therapy (Abel et al., 2015). With consideration of that study, our findings of greater effective connectivity of LPMCv→M1 with behavioral improvement may indicate that these changes are qualitative (i.e., reflecting relative influence within the network) rather than quantitative (i.e., absolute level of activation).
Limitations of this study include constraint to left hemisphere ischemic cortical stroke and the heterogeneity of participants within our small subgroups. The variability in performance and reorganized networks could be due to contributions of a number of factors, including age, time since stroke, previous therapy treatment, and extent of lesion. If such demographic or medical factors were constrained, or if the study size was expanded, group effects could possibly emerge. Additionally, as the fMRI task was purely observational and did not require behavioral response, it is uncertain whether intrascanner factors, such as variable attention, may have affected our analysis.
Conclusions
As a study of effective connectivity, the findings reported here reveal network relationships of interest that activation or functional connectivity alone cannot identify. Furthermore, since results are based on a known language network subserving the same task for healthy control subjects, continuity is implied for our participants; that is, changes in effective connectivity between regions can be categorically identified as plasticity within this network. The effective connections identified in this study could be used in targeted therapy for individuals with aphasia and, if behaviorally successful and corroborated by additional imaging findings, would provide support for the idea that recovery results from greater cortical connectivity. This study also demonstrates plasticity in response to therapy in participants who are more than ten years poststroke, through a possibly unique compensatory mechanism. Finally, variability in recovery and compensation for neuronal loss in these individuals supports the need for personalized evaluation of stroke patients for both clinical and research considerations.
Acknowledgments
This research was supported by the National Institute of Deafness and other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) under Grants R01-DC007488 and R33-DC008638, the James S. McDonnell Foundation under a grant to the Brain Network Recovery Group (A.R. McIntosh, PI), and Mr. William Rosing, Esq. All speech and language evaluations were coordinated by Dr. Leora Cherney at the Rehabilitation Institute of Chicago (RIC) and performed by her staff at RIC. The research staff at The University of Chicago included Blythe Buchholz and Robert Fowler, who helped coordinate the project, and Dan Rodney, who authored the IMITATE software. Dr. Ana Solodkin supervised the drawing of lesion masks. The support of these individuals is gratefully acknowledged, as are the participants and families who generously participated in this research.
Author Disclosure Statement
No competing financial interests exist.
References
- Abel S, Weiller C, Huber W, Willmes K, Specht K. 2015. Therapy-induced brain reorganization patterns in aphasia. Brain 138(Pt 4):1097–1112 [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Rosa PA, Tettamanti M, Green DW, Cappa SF. 2009. Bilingual aphasia and language control: a follow-up fMRI and intrinsic connectivity study. Brain Lang 109:141–156 [DOI] [PubMed] [Google Scholar]
- Anglade C, Thiel A, Ansaldo AI. 2014. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj 28:138–145 [DOI] [PubMed] [Google Scholar]
- Bajaj S, Butler AJ, Drake D, Dhamala M. 2015. Brain effective connectivity during motor-imagery and execution following stroke and rehabilitation. Neuroimage Clin 8:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. 2010. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke 41:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, et al. 2000. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10:512–528 [DOI] [PubMed] [Google Scholar]
- Brady MC, Kelly H, Godwin J, Enderby P. 2012. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev 5:CD000425. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. 2003. Plasticity of cortical projections after stroke. Neuroscientist 9:64–75 [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173 [DOI] [PubMed] [Google Scholar]
- Cramer SC. 2008. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63:272–287 [DOI] [PubMed] [Google Scholar]
- Dick AS, Solodkin A, Small SL. 2010. Neural development of networks for audiovisual speech comprehension. Brain Lang 114:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ES, Schmah T, Small SL. 2016. Performance variability as a predictor of response to aphasia treatment. Neurorehabil Neural Repair 30:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. 2012. FreeSurfer. Neuroimage 62:774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta EE, Rao PR, Barrett AM. 2011. Transcranial magnetic stimulation (TMS): potential progress for language improvement in aphasia. Top Stroke Rehabil 18:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. 2008. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke 39:1520–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN. Optseq2 [software]. 2002. Available from: https://surfer.nmr.mgh.harvard.edu/optseq/ Last accessed March15, 2018
- Hartwigsen G, Saur D, Price CJ, Ulmer S, Baumgaertner A, Siebner HR. 2013. Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proc Natl Acad Sci U S A 110:16402–16407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. 2006. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang 98:118–123 [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. 1999. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 45:430–438 [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. 1987. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res 71:249–266 [DOI] [PubMed] [Google Scholar]
- Kertesz A. 1982. The Western Aphasia Battery. New York: Grune and Stratton [Google Scholar]
- Kiran S, Meier EL, Kapse KJ, Glynn PA. 2015. Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Front Hum Neurosci 9:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp B, Kunkel A, Muhlnickel W, Villringer K, Taub E, Flor H. 1999. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport 10:807–810 [DOI] [PubMed] [Google Scholar]
- Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. 2001. Aphasia in acute stroke and relation to outcome. J Intern Med 249:413–422 [DOI] [PubMed] [Google Scholar]
- Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. 2008. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry 79:530–534 [DOI] [PubMed] [Google Scholar]
- Lee J, Fowler R, Rodney D, Cherney L, Small SL. 2010. IMITATE: an intensive computer-based treatment for aphasia based on action observation and imitation. Aphasiology 24:449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, et al. 2009. Overt naming fMRI pre- and post-TMS: two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang 111:20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Caltagirone C. 2014. Options to enhance recovery from aphasia by means of non-invasive brain stimulation and action observation therapy. Expert Rev Neurother 14:75–91 [DOI] [PubMed] [Google Scholar]
- Mashal N, Solodkin A, Chen EE, Dick AS, Small SL. 2012. A network model of observation and imitation of speech. Front Psychol 3:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. 1998. Understanding neural interactions in learning and memory using functional neuroimaging. Ann N Y Acad Sci 855:556–571 [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. 1994. Structural equation modeling and its application to network analysis in functional brain imaging. Human Brain Mapping 2:2–22 [Google Scholar]
- Naeser MA, Martin PI, Lundgren K, Klein R, Kaplan J, Treglia E, et al. 2010. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cogn Behav Neurol 23:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Theoret H, Kobayashi M, Fregni F, Nicholas M, et al. 2011;. MS suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain Lang 119:206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, et al. 2003. A fMRI study of word retrieval in aphasia. Brain Lang 85:357–368 [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. 2010. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci 22:1299–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Grefkes C. 2013. Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol 591(Pt 1):17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Hartwigsen G. 2012. Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Arch Phys Med Rehabil 93:S15–S25 [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. 2008. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A 105:18035–18040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Ronneberger O, Kummerer D, Mader I, Weiller C, Kloppel S. 2010. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain 133(Pt 4):1252–1264 [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. 2009. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci 1169:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Wan CY. 2011. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol Rev 21:288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Baron JC, Rowe JB. 2009. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol 66:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A, Hasson U, Siugzdaite R, Schiel M, Chen EE, Kotter R, et al. 2010. Virtual brain transplantation (VBT): a method for accurate image registration and parcellation in large cortical stroke. Arch Ital Biol 148:219–241 [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. 2006. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys 42:241–256 [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. 1999. Plasticity of language-related brain function during recovery from stroke. Stroke 30:749–754 [DOI] [PubMed] [Google Scholar]
- Torres J, Drebing D, Hamilton R. 2013. TMS and tDCS in post-stroke aphasia: integrating novel treatment approaches with mechanisms of plasticity. Restor Neurol Neurosci 31:501–515 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, et al. 2012. The right hemisphere is not unitary in its role in aphasia recovery. Cortex 48:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RR, Small SL, Chen EE, Solodkin A. 2008. Network activation during bimanual movements in humans. Neuroimage 43:540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Zheng X, Marchina S, Norton A, Schlaug G. 2014. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca's aphasia. Brain Lang 136:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. 2001. Separate neural subsystems within ‘Wernicke's area’. Brain 124(Pt 1):83–95 [DOI] [PubMed] [Google Scholar]
- Wolf SL, Newton H, Maddy D, Blanton S, Zhang Q, Winstein CJ, et al. 2007. The Excite Trial: relationship of intensity of constraint induced movement therapy to improvement in the wolf motor function test. Restor Neurol Neurosci 25:549–562 [PubMed] [Google Scholar]