Abstract
Traumatic Brain Injury (TBI) has been described as the “signature injury” of the Global War on Terror. Explosive blast TBI has become a leading cause of injury as a result of the widespread use of improvised explosive devices in Iraq and Afghanistan. We present a retrospective cross-sectional study of patients with blast-related mild TBI (mTBI, N = 303) seen at the Intrepid Spirit Concussion Recovery Center at Naval Medical Center Camp Lejeune. The objective was to predict outcomes of return to duty (RTD) vs. medical retirement via medical evaluation board (MEB), based on brain imaging, neuropsychological data, and history of mTBI. The motivation is to inform prognosis and target resources to improve outcomes for service members who are less likely to RTD through the standard treatment program. The RTD was defined operationally as individuals who completed treatment and were not recommended for medical retirement or separation for TBI or related sequelae. Higher scores on the Repeatable Battery for Neuropsychological Status (RBANS) test were associated positively with RTD (p = 0.001). A history of three or more lifetime concussions was associated negatively with RTD, when compared with one concussion (p = 0.04). Elevated apparent diffusion coefficient (ADC) in the anterior corona radiata was associated negatively with RTD (p = 0.04). A logistic regression model was used to classify individuals with RBANS and imaging data (n = 81) as RTD or MEB according to RBANS, ADC, and a history of multiple (≥3) concussions. The RBANS (p = 0.003) and multiple concussions (p = 0.03) were significant terms in the logistic model, but ADC was not (p = 0.27). The area under the receiver operating characteristic curve was 0.77 (95% confidence interval 0.66–0.86). These results suggest cognitive testing and TBI history might be used to identify service members who are more likely to be retired medically from active duty.
Keywords: : blast, mTBI, RBANS, return to duty, white matter
Introduction
Traumatic brain injury (TBI) has been described in the medical and lay literature for centuries, yet it remains difficult to predict the time course and extent of recovery. The clinical signs and symptoms of TBI are wide-ranging, but typically consist of headaches, vertigo, nausea and vomiting, disturbed sleep, cognitive deficits, and changes in memory and personality.1 While most persons with mild TBI (mTBI) recover fully, some individuals report ongoing symptoms including headache, insomnia, difficulty thinking, memory problems, attention deficits, mood swings, and frustration. An understanding of the long-term consequences of mTBI among active duty service members and veterans is essential to operational planning and the allocation of resources for treatment and long-term care and support.2
The diagnosis of complications caused by mTBI can be difficult at times because of the broad definition of mTBI and the nonspecific findings on history and physical examination, many of which overlap with post-traumatic stress disorder (PTSD), malingering, or mental health issues such as depression. In addition, there is no single indicator that is sensitive and specific for prognosis after mTBI.3–5
Different studies have found both cognitive performance and psychological symptoms are correlated with outcomes. In a 2000 study of 121 patients with mTBI who were all active-duty military personnel, a stepwise analysis revealed that age and three cognitive variables (verbal memory, verbal fluency, and a speed test of planning and strategy) were predictive of duty status, specifically whether service members were assigned to full or limited duties at the time of follow-up, 3–15 months after a documented mTBI. The selected measures correctly classified duty status 68.8% of the time.6 In contrast, a study of 50 civilian participants in a TBI rehabilitation program found that the Trail Making Test Part B (TMT-Part B) failed to predict three-month follow-up scores in the Mayo-Portland Adaptability Inventory-4 (MPAI-4).7 Beck depression scores, however, were predictive of three-month MPAI-4 scores, when controlling for baseline performance.
Psychological symptoms might be especially relevant to predicting outcomes in a military context, where mTBI is frequently the result of combat injuries that may also cause post-traumatic stress. There is a need to find relevant biomarkers and clinical sequelae within neurological, cognitive, and psychological domains, which can serve as prognostic indicators for service members who experience mTBI so that specific interventions can be developed to improve outcomes.
In this study, we analyzed retrospective data from the Naval Medical Center Camp Lejeune Intrepid Spirit Concussion Recovery Center (NMCCL ISCRC). The purpose of the study was to identify markers that predict outcomes from the ISCRC treatment program: either referral to a medical evaluation board (MEB) for retirement on medical grounds or a return to full duty (RTD). Most individuals who are RTD from the clinic return subsequently to their unit and resume previously assigned duties; others leave active duty because they have reached the end of their service commitments.
The study was approved by the Institutional Review Board (IRB) at the Naval Medical Center Portsmouth, VA, which serves as the IRB of record for Camp Lejeune. The data include neuroimaging measures, measures of symptom severity, and neuropsychological testing results. The outcome measure is the ability to RTD within the military at the conclusion of treatment. Specifically, we contrast those service members who RTD with those who are recommended for medical retirement based on unresolved TBI symptoms. The MEB makes the final decision on medical retirement. With rare exceptions, service members referred to the MEB from the ISCRC are ultimately medically retired from military service.
In the war in Iraq and Afghanistan, blasts are the leading cause of TBI with incidence reported to be as high as 20% in some units returning from the Iraqi or Afghan theater of operations.1,8 A recent review of mTBI literature found “there is minimal evidence that blast results in cognitive changes that are distinct from nonblast mechanisms of concussive injury.” There was a still small but “more sizeable” increase in self-reported psychological symptoms in mTBI caused by blast compared to nonblast injury, however.9 The frequent comorbidity and overlap in symptoms between TBI and PTSD presents a further challenge,10 particularly in military and veteran populations. Recent research using animal models of TBI suggest that blast may produce patterns of brain injury that are distinct from those caused by rapid deceleration.11,12
We did not compare directly blast and nonblast injury in this study; instead, we opted to study data from the population of service members seen at the ISCRC who had at least one overseas deployment and at least one mTBI caused by blast. This population is composed primarily of veterans of Operation Enduring Freedom (OEF) and/or Operation Iraqi Freedom (OIF). The purpose of this study was to determine what factors may predict long-term outcomes of RTD vs. medical retirement based on analysis of imaging, laboratory, neuropsychological data, and duty status.
Methods
Subjects
We obtained a sample of retrospective data from 313 previously deployed service members with blast injury, seen at the ISCRC from 2009 through 2014. Neuropsychological reports were available for 61 of the subjects referred to MEB, which included an assessment of the validity of the cognitive tests. Ten subjects were excluded because it was concluded that the cognitive test results were invalid. This reduced the total sample size to N = 303.
Patients treated at the ISCRC are referred typically by their medical officer or other healthcare provider. In some cases, patients self-refer for evaluation. The center currently receives about 40 new referrals per month. A nurse practitioner or physician assistant as well as a team of nurses evaluate the patient initially and review the history of brain injury. Subsequently, patients are evaluated by an interdisciplinary team: speech and language, physical therapy, occupational therapy, behavioral health, neurology, sports medicine, and pastoral counseling. Providers from each discipline determine whether the patient requires their specific services based on their symptoms and functional impairment.
After the initial evaluations, the patient and providers meet as a team to discuss the recommended treatment plan and next steps. Evaluation and care are provided in a collaborative environment that promotes physical, psychological, and spiritual health. Interdisciplinary care is facilitated by the colocation of these providers in the ISCRC. Follow-up treatment team meetings are scheduled every four weeks until discharge to reassess progress in the program, make necessary adjustments, and plan for eventual discharge. The typical patient moves through the program over a 12-week period. Patients are offered traditional medical treatment as well as complementary medical programs including yoga, iRest® meditation, cognitive behavioral therapy groups, acupuncture, mindfulness-based stress reduction, art therapy, therapeutic writing, equine facilitated communication, and therapeutic photography. According to internal ISCRC tracking data, approximately 80% of the service members who participate in the program return to full duty.
Information from clinical records, neuropsychological tests, and self-report measures were tested individually for association with MEB status. In addition, we derived quantitative measures of regional white matter integrity from the diffusion magnetic resonance imaging (MRI) images. Patient information was collected from a variety of sources. Some data were retrieved from the electronic Armed Forces Health Longitudinal Technology Application (AHLTA) medical record. The MRI data were retrieved from the picture archiving and communication system (PACS) at Camp Lejeune. Other information was retrieved manually from the patient's clinic notes, including reported symptoms and history of brain injury. All patient data were de-identified and coded with a study-specific identifier at Camp Lejeune before transfer to the University of Pennsylvania for analysis.
All of the variables in the data set have some degree of missing data. Patients seen more recently have more data because the use of standardized tests and questionnaires has expanded at ISCRC over the time span of the study. This means the statistical tests have different power because the sample size varies substantially. We identify the sample size for each test with a lower case n.
Population characteristics and medical histories
Limited demographic information and individual history were recorded where available. Patient demographics are summarized in Table 1. To protect patient privacy, demographic information was limited to sex, race, and marital status. Individual age was not recorded in the data set; based on internal clinic records, the average age of the population seen is 27.9 years with 48% of patients less than age 26. Before injury, all subjects were on full active duty and required to meet the physical and cognitive standards of active duty Marines.
Table 1.
Patient Characteristics and Traumatic Brain Injury History
| Variable name/MEB status | MEB | RTD | Other |
|---|---|---|---|
| Race, White | 27 | 65 | 27 |
| Race, Black | 1 | 5 | 2 |
| Race, Asian | 0 | 3 | 0 |
| Race, Other | 9 | 12 | 3 |
| Race, NA | 48 | 93 | 8 |
| Sex, Male | 83 | 176 | 40 |
| Sex, Female | 2 | 2 | 0 |
| Marital status, Married | 46 | 77 | 25 |
| Marital status, Single | 12 | 33 | 9 |
| Marital status, Divorced / separated | 9 | 18 | 3 |
| Marital status, NA | 18 | 50 | 3 |
| Number of deployments (median, interquartile range) | 2, 1–3 | 2, 1–3 | 2, 1–3 |
| 1 TBI blast | 15 | 52 | 11 |
| 2 TBI, blast | 12 | 18 | 6 |
| 2 TBI, mix blast and nonblast | 7 | 20 | 6 |
| 3+ TBI blast | 12 | 27 | 5 |
| 3+ TBI, mix blast and nonblast | 39 | 61 | 12 |
MEB, medical evaluation board; RTD, return to duty.
Information from clinical histories
The patient history contains, in narrative form, self-reported individual and family medical history including history of TBI, reports of symptoms, and other information related to the patient recorded by the examining physician. Blast TBI history was determined by patient report in the medical record. Following standard definitions, a TBI was classified as mild if the reported loss of consciousness and/or confusion and disorientation was shorter than 30 min. A data structure was defined to encode information as either numerical (e.g., number of reported blast injuries) or categorical (e.g., was there a blast injury caused by an improvised explosive device [IED], Yes/No). Records were reviewed manually, and the extracted data was recorded in a spreadsheet.
We recorded the number of head traumas from blast and lifetime total concussions, including injuries sustained before military service. In addition, we recorded details about blast exposures when they were present in the clinical notes, including devices of blast injury, the estimated proximity to the device, and the location of the individual at the time of injury.
Standardized tests and surveys
In addition to the narrative history, standardized tests and surveys were administered to some patients. Most of these have now become standard at the clinic. The tests included in the study are:
Alcohol Use Disorders Identification Test (AUDIT)
This is a 10-item questionnaire that screens for hazardous or harmful alcohol consumption, developed by the World Health Organization.13
Headache Impact Test (HIT-6)
This is a headache-related quality of life score.14 The HIT-6 is a six-item questionnaire that assesses an individual's perception of headache burden over the past month.15,16
Neurobehavioral Symptoms Inventory (NSI)
This is a questionnaire measuring self-reported symptoms of concussion.17 The variant of the NSI test used at Camp Lejeune is the “NSI-22,” containing 22 questions. Four of the symptoms (concentration, memory, difficulty making decisions, and slower thinking) are related to cognitive impairments. The NSI has been validated in a population of veterans from the conflicts in Afghanistan and Iraq.16 The NSI is currently being used by both the U.S. Department of Defense and the Veterans Administration as part of their Operation Iraqi Freedom/Operation Enduring Freedom TBI evaluation process of post-concussional symptoms and as a core outcome measure of concussion treatment.18–20
Patient Health Questionnaire-9 (PHQ9)
This is an assessment of depression where subjects are asked to rate the frequency in which they experienced eight depressive symptoms in the past two weeks; the ninth question addresses thoughts of suicide.21
Pittsburgh Sleep Quality Index (PSQI)
This is a self-rated questionnaire that assesses sleep quality and disturbances over a one-month time interval.22 Nineteen individual items generate seven component scores including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction.
PTSD Checklist, Military Version (PCLM)
This is a 17-item self-report checklist of PTSD symptoms for a military population based closely on the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria. Respondents rate each item from 1 (“not at all”) to 5 (“extremely”) to indicate the degree to which they have been bothered by that particular symptom over the past month. The PCLM has a recommended cutoff score of 50 for indicating a probable diagnosis of combat-related PTSD.23
Repeatable Battery for the Assessment of Neurological Status (RBANS)
This comprises 12 individual subtests (list learning, story memory, figure copy, line orientation, digit span, coding, picture naming, semantic fluency, list recall, list recognition, story recall, and figure recall) similar to those of independently developed neuropsychological measures. It provides a measure of functioning across a variety of cognitive domains, with an administration time of 30 min.24 Subtest raw scores are converted to index scores for six general areas: immediate memory, visuospatial/constructional ability, language, attention, delayed memory, and overall function.25 The RBANS is a widely used measure of cognitive functioning and has been validated to assess cognitive functioning among a variety of populations, including TBI,26 and has been used extensively in studies with the military population. In a study by Mckay and associates,27 the RBANS was demonstrated to be a clinically valid and reliable tool in the brief screening of individuals who experience a TBI.
MRI data
Neuroimaging data was acquired on a 1.5T Siemens Espree scanner. The standard neuroimaging protocol at the TBI clinic includes sagittal T1 and axial T2 and fluid attenuation inversion recovery (FLAIR) images and an axial diffusion-weighted imaging series. Diffusion was measured along three orthogonal planes, at b-values of (0, 500, and 1000) s mm2. The earliest FLAIR data (2009–2010) had a resolution of 0.94 × 0.94 × 6.5 mm3 (n = 62). The majority of images after 2010 had an in-plane resolution of 0.45 × 0.45 mm2 (n = 167), but some were acquired at intermediate resolutions of approximately 0.75 × 0.75 mm2 in plane and 6.5 or 5.2 mm-thick slices (n = 43). The diffusion images were almost all acquired at similar resolution, either 1.25 × 1.25 × 6.5 mm3 (n = 137) or 1.2 × 1.2 × 6.5 mm3 (n = 113), with a few later scans acquired at the highest resolution of 0.6 × 0.6 × 5.2 mm2 (n = 22). In all cases, apparent diffusion coefficients (ADC) were computed on the scanner, and these were used for analysis.
To analyze the ADC in white matter, we transferred regions of interest (ROIs) from a standard template brain to each subject brain by image registration, using the open-source Advanced Normalization Tools (ANTs) toolkit.28 The labels are defined in the International Consortium of Brain Mapping-diffusion tensor imaging-81 (ICBM-DTI-81) atlas and identify major white matter tracts in the MNI152 brain template space.29 Four ROIs were selected to test for association with MEB status: the genu of corpus callosum, the splenium of corpus callosum, the posterior limb of the internal capsule (bilateral), and the anterior corona radiata (ACR) (bilateral). These regions have been found to have anisotropy changes in multiple DTI studies of TBI.30 Other white matter ROIs including the cingulum and uncinate fasciculus were also abnormal in several studies in the meta-analysis, but the size and curvature of these structures made them too difficult to reliably segment in our data.
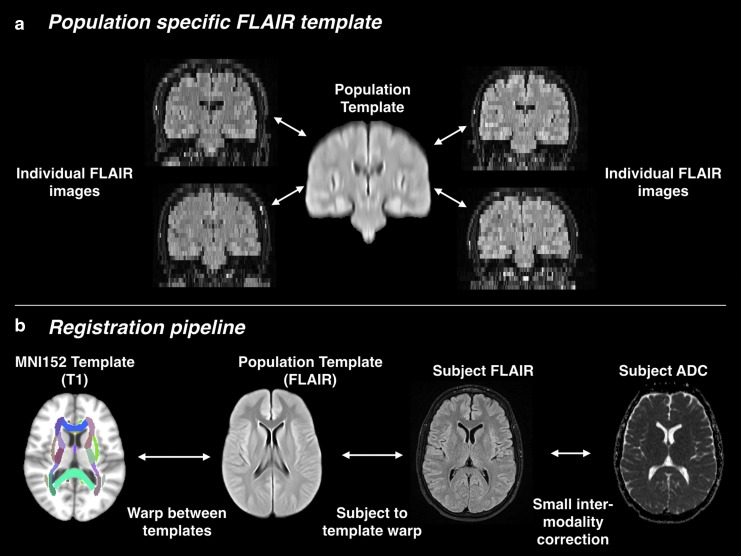
Transferring labels from the MNI152 template to individual subjects involves image registration of images with very different contrasts and spatial resolution. To meet this challenge, we broke the registration problem down into a series of simpler registrations, which were combined to map the white matter ROIs to each individual image. The first step was the construction of a population-specific template (Fig. 1). The population template was constructed by iteratively registering and averaging FLAIR images from 100 randomly chosen subjects.31 The population template has 1 mm3 spatial resolution, like the MNI152 template: by averaging information from a large number of subjects, we were able to produce a reasonable interpolation between the 6.5-mm–thick slices of the individual images.
FIG. 1.
The registration pipeline for apparent diffusion coefficient (ADC) analysis. (a) The population template is built from 100 randomly selected fluid attenuation inversion recovery (FLAIR) images. (b) The registration pipeline computes a series of transforms that are combined to warp ADC and white matter regions of interest to the subject space.
Each individual structural image was then registered to the population template. Because the population template is an average of images from the same population of subjects, using the same MRI imaging sequence and scanner, a population template helps accomplish unbiased and robust registration.32 The registration from the population template to MNI152 was computed once and applied consistently for all subject images.
The final registration shown in Figure 1 aligns the ADC image from each subject to the subject's FLAIR image. The two images are already closely aligned because they were acquired at the same time. A small correction accounts for patient motion and geometric distortions that differ between MRI sequences.
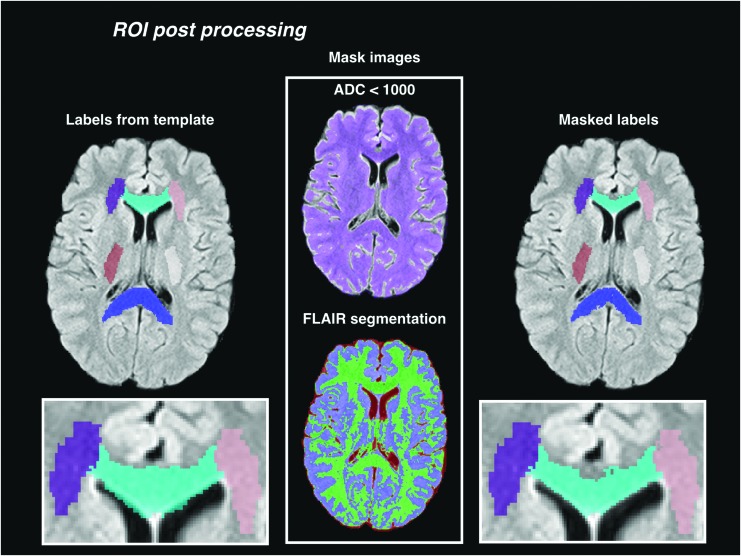
The subject FLAIR images were segmented into three tissue classes: cerebrospinal fluid (CSF), gray matter, and white matter, using the Atropos tool in ANTs.33 Each slice of the FLAIR image was segmented independently. A k-means classifier with three classes was used to initialize the segmentation. After each slice had been segmented, the mean intensities of gray matter, white matter, and CSF were computed over the whole brain, and the slice-by-slice segmentation was repeated with the k-means initialized consistently for each slice. On visual inspection, the segmentation appeared successful at segmenting CSF voxels from those containing gray or white matter; however, the separation of gray from white matter voxels was less reliable, especially in the internal capsule ROI (Fig. 2). Therefore, we did not exclude voxels within the white matter ROIs (defined by registration from the MNI template) if they were segmented as gray matter in the FLAIR image.
FIG. 2.
Region of interest (ROI) post-processing. The labels are first warped to the subject image from the template. The warped labels are refined by removing voxels where the apparent diffusion coefficient (ADC) is above a maximum threshold, or where the segmentation of the fluid attenuation inversion recovery (FLAIR) image classifies the voxel as cerebrospinal fluid.
After registration and segmentation of the native FLAIR images, we resampled the FLAIR images to a consistent resolution of 0.65 × 0.65 × 6.5 mm to minimize any bias in the resampling of the template ROIs into the subject FLAIR images. The registration warps were composed and applied once, transferring the white matter ROIs from MNI152 to the resampled subject FLAIR image with a single interpolation. The distortion-corrected ADC images were also resampled into the same FLAIR image space.
The ROIs were then masked with the FLAIR segmentations to remove voxels classified as CSF, and further masked with an ADC threshold to remove voxels with partial CSF volume. The ADC in normal white matter is relatively stable around 700 μm2 ms−1, and we removed voxels where the ADC was greater than 1000 μm2 ms−1 (Fig. 2). To verify that this threshold was well above normal white matter variance, we computed the group mean of each individual's median ADC in labeled voxels before masking (748 μm2 ms−1), and the mean interquartile range (IQR) (110 μm2 ms−1). The final masked labels were reviewed manually, and six of 278 ADC images were removed from the analysis because one or more of the ROI labels were of low quality because of registration or segmentation errors.
Statistical analysis
The main aim of this work was to construct a multivariate model that could predict MEB or RTD outcomes. Because currently there is no single prognostic indicator for mTBI, a variety of data is collected and assessed by the ISCRC medical team; however, these have not been combined previously into a multivariate statistical model. We hypothesized that a multivariate model could predict MEB or RTD outcomes. Models with too many variables, however, are liable to overfit to training data and generalize poorly. We therefore performed exploratory univariate tests and selected variables based on the univariate results as well as the availability and completeness of data and previous evidence in the TBI literature. The p values of the univariate tests are uncorrected for multiple comparisons.
Dichotomous categorical variables were tested for association with MEB status, using the “epiR” package (https://CRAN.R-project.org/package=epiR) in R (http://r-project.org). We report the incidence risk ratio (IRR, also called relative risk) of MEB referral between dichotomous groups, with a 95% confidence interval (CI) as well as a p value from a chi-square test for differences in outcome between groups with and without exposure to a hypothesized risk factor.
For example, consider a test of whether marital status is correlated with the incidence of MEB referral. Among married patients, 46 were referred to MEB and 77 RTD. The incidence risk of MEB is therefore 46 / (46+77) = 37.4%. The incidence risk differs from the odds (46/77 = 0.60), although the two become similar when the incidence risk is small. Among nonmarried patients, 21 were referred to MEB and 51 RTD, an incidence risk of 29.2%. This results in an IRR of 1.28, suggesting that married patients are more likely to be referred to MEB. The estimated 95% CI for the IRR is (0.84, 1.96), however, which spans 1.0, suggesting that the effect is not significant. The corresponding p value is 0.24, and we would not reject the null hypothesis that marital status is uncorrelated with MEB.
In the results section, we report the total numbers in the MEB and RTD group (nM and nR), the IRR, CI, and associated p value. For the example above, this would be nM = 67, nR = 128, IRR = 1.28, CI = (0.84, 1.96), p = 0.24. Full incidence risk data are listed in Table 2. Point estimates and CIs for both the IRR and odds ratio may be computed from this table using the “epi.2by2” function in epiR.
Table 2.
Categorical Variables Tested with “epiR”*
| Categorical variable | nM | nR | Total | Incidence risk (%) | Odds |
|---|---|---|---|---|---|
| Number of TBI 3+ | 51 | 88 | 139 | 36.7 | 0.58 |
| Number of TBI 1 | 15 | 52 | 67 | 22.4 | 0.29 |
| Number of TBI 1 or 2 | 34 | 90 | 124 | 27.4 | 0.38 |
| Distance to blast ≤3m | 21 | 37 | 58 | 36.2 | 0.57 |
| Distance to blast >3m | 18 | 33 | 51 | 35.3 | 0.55 |
| Location at blast: vehicle enclosed | 27 | 67 | 94 | 28.7 | 0.40 |
| Location at blast: dismounted | 23 | 46 | 69 | 33.3 | 0.50 |
| Location at blast: vehicle open | 8 | 20 | 28 | 28.6 | 0.40 |
TBI, traumatic brain injury.
Each test compares two rows of this table—for example, 3+ TBI vs. 1 TBI. The sample sizes for medical evaluation board (MEB) (nM) and return to duty (RTD) (nR) reported in the text are the sum of the two rows used in the test.
For continuous data, we performed two-sample, two-tailed t tests, without assuming equal variance between the MEB and RTD groups (Welch t test). The p values are reported directly by the “t test” function in R. The t statistics are positive where the sample mean of the MEB group μM is greater than that of the RTD group μR.
Finally, we combined variables of interest to predict MEB or RTD status using a binomial logistic regression, implemented in the “glm” method in R. In this model, the dependent variable is MEB or RTD status, encoded as 1 for MEB and 0 for RTD. The model estimates the probability of MEB referral given the variables of interest.
Results
Because of missing data, the sample size varies and is reported separately for each test. Data points are included in statistical tests only if all of the variables are not missing.
RTD and MEB referral outcomes
Of the 303 subjects, 85 were referred to MEB and 178 RTD. The remaining 40 did not have a definite status at the time of data collection, either because they were still in treatment or because they had left the military without being medically retired. Among the 85 subjects referred to MEB, the specific medical grounds for referral were recovered for 84 subjects, coded as TBI-neurological, psychiatric, orthopedic, or other reasons. The codes are summarized in Table 3. The TBI-neurological code was defined to include nonpsychiatric neurological diagnoses attributable to TBI. Psychiatric diagnoses, mainly PTSD, were coded separately. All 84 subjects had either a TBI or psychiatric diagnosis, and in 77 cases, one of these was the primary diagnosis.
Table 3.
Diagnostic Codes for Medical Evaluation Board Referral*
| Primary (cols) / Secondary MEB referral diagnosis | TBI-neurological | Psychiatric | Orthopedic | Other | Total secondary |
|---|---|---|---|---|---|
| TBI-neurological | 9 | 22 | 5 | 0 | 36 |
| Psychiatric | 28 | 5 | 1 | 0 | 34 |
| Orthopedic | 5 | 2 | 0 | 0 | 7 |
| Other | 1 | 1 | 0 | 1 | 3 |
| Total primary | 43 | 30 | 6 | 1 |
TBI, traumatic brain injury; MEB, medical evaluation board.
All subjects had either a TBI or psychiatric diagnosis and, in most cases, one of these was the primary diagnosis. Subjects with only one diagnosis are tabulated as having identical primary and secondary diagnoses.
Number of TBI
All service members included in this study have at least one blast-related TBI. The number of TBI from blast and nonblast mechanisms was gathered from patient self-reports as recorded in clinic notes and is listed in Table 1. Injury mechanisms other than blast were also relatively common—145 subjects reported at least one TBI not caused by a blast. The median total TBI was three, with IQR one to four.
Subjects with three or more total TBI, including blast and nonblast injuries (3+ TBI) were more likely to be referred to the MEB than those with a single TBI: nM = 66, nR = 140, IRR = 1.64, CI = (1.0, 2.69), p = 0.039. This particular test was based on an earlier study of active duty US Marines by Spira and colleagues34 who found that three or more lifetime concussions was associated with worse post-concussive symptom reporting compared with one or zero concussions. We also compared subjects with 3+ TBI with those with one or two TBI, which was not significant: nM = 85, nR = 178, IRR = 1.34, CI = (0.93, 1.92), p = 0.11.
Characteristics of blast injury
Many of the clinic notes included limited information about the circumstances of blast-related injuries, which we hypothesized may be related to outcomes. We recorded information on blast device, estimated distance from the device, and the location of the service member inside or outside a vehicle when the blast occurred (Table 2). Of 290 subjects with information about the blast device, the most common was an IED (254 subjects), followed by rocket propelled grenades (39 subjects). Because of the predominance of IED exposure in the data set, we did not perform statistical tests on blast device. Compared with the largest group, “vehicle enclosed,” dismounted or vehicle open location was not related to MEB (p = 0.53 and p = 0.99, respectively). There was no association with closest proximity to a blast device and MEB status (p = 0.92). The actual severity of the blast injury depends on many factors not accounted for in these tests, including the explosive power of the device. Most service members reported injuries from IEDs, a category that encompasses a wide range of explosive types and sizes.
Standardized questionnaires
Of the standardized test scores, only RBANS was significantly associated with MEB status. The results of the tests are summarized in Table 4. In addition to potential utility in predicting MEB referral, these tests offer some insight into the physical and psychological symptoms of clinic patients seeking treatment at the ISCRC. We briefly discuss each test in turn, with reference to diagnostic scales where available. The sample size and group averages below are for all patients in the data set, including those without a defined MEB or RTD status at the time of data collection. The tests in Table 4 include only those subjects with a defined MEB or RTD status.
Table 4.
Repeatable Battery for Neuropsychological Status Domain Scores*
| RBANS domain (nM, nR) | Mean score (MEB) | Mean score (RTD) | t | p |
|---|---|---|---|---|
| Composite (29,56) | 79.14 | 88.45 | −3.35 | 0.0013 |
| Attention (26,49) | 72.11 | 87.98 | −3.21 | 0.0023 |
| Delayed memory (25,48) | 79.16 | 87.94 | −2.08 | 0.043 |
| Immediate memory (24,49) | 80.29 | 87.61 | −1.96 | 0.055 |
| Language (25, 48) | 85.64 | 88.02 | −0.82 | 0.42 |
| Visuospatial / constructional (24,48) | 104.42 | 105.32 | −0.29 | 0.77 |
RBANS, Repeatable Battery for Neuropsychological Status; MEB, medical evaluation board; RTD, return to duty.
Mean scores for the RTD and MEB group, t statistics and p values of two-tailed tests for difference between the MEB and RTD sample means.
AUDIT, n = 90
the mean score was 7.04, and 32 subjects had scores of 8 or more, indicating “harmful” drinking behavior.
HIT6, n = 98
the mean score was 59.09, and 63 subjects reported “severe” impact of headaches (HIT6 ≥60).
NSI, n = 101
the mean score was 41.38, median 40. Diagnostic scales have not been established for the NSI, but a recent article by Soble and coworkers17 examined quantiles in several populations of Florida National Guard service members. Among service members with at least one deployment-related mTBI, the median NSI was 24. The median NSI in the ISCRC study population is 40, which may reflect the fact that patients referred to the ISCRC have worse symptoms than the average service member who has sustained an mTBI. In addition, the Florida mTBI cohort included only subjects who screened negative for both depression and PTSD, both of which are often comorbid with post-concussive symptoms.35
PHQ9, n = 125
The mean score was 13.69, consistent with moderate depression according to the test categories.
PSQI, n = 61
almost every subject with a PSQI score, 57 of 61, had scores greater than 5, indicating poor sleep quality.
PCLM, n = 146
the mean score was 48.62, and 58 subjects had scores above 50, consistent with a diagnosis of PTSD.
RBANS n = 117
the mean score was 84.6. The RBANS scoring algorithm includes adjustment for subject age and is calibrated such that the mean result is 100 in a US adult civilian population.
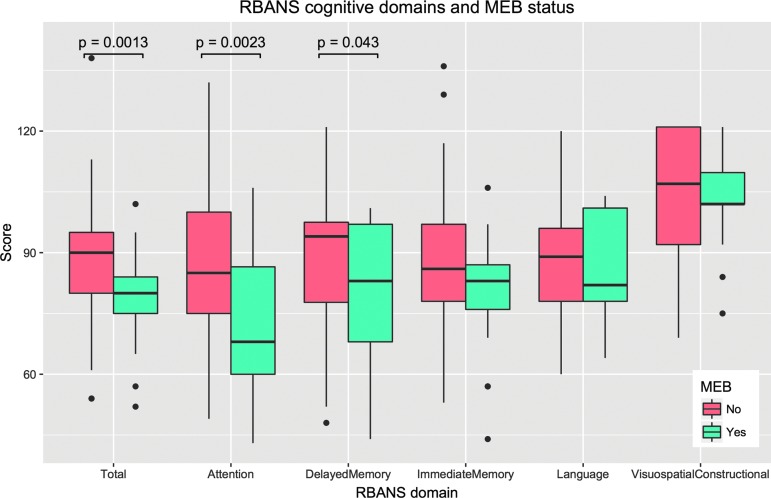
The scores were significantly lower in the MEB compared with the RTD group: (nM = 29, nR = 56, μM = 79.14, μR = 88.45, t = −3.35, p = 0.0013). One subject in the RTD group had an unusually high score (RBANS = 138, z = 3.71); however, the effect was robust to the removal of this point (p = 0.002). We hypothesized that the difference between the groups might be concentrated in particular cognitive domains within the RBANS. To test this, the individual cognitive domain scores were recovered where available, n = 75 subjects. We ran t tests on each domain score; the results are listed in Table 4 and plotted in Figure 3. Attention and delayed memory domain scores were significantly different between the MEB and RTD groups.
FIG. 3.
Repeatable Battery for Neuropsychological Status (RBANS) overall performance and domain scores. RBANS total scores were significantly lower for the medical evaluation board (MEB) group. Within individual domains, attention and immediate memory were significantly lower in the MEB group. Boxes show interquartile range (IQR) with a black line indicating the median. Whiskers extend to 1.5 * IQR; points outside this range are shown as black circles.
Neuroimaging Results
The white matter ROIs were transferred from the template to the individual image space as described in the Methods section. Quality of registration and segmentation was assessed by reviewing manually the deformed labels in four ROIs commonly associated with reduced white matter fractional anisotropy (FA) after TBI: the splenium and genu of corpus callosum, the ACR (bilateral), and the posterior limb of the internal capsule (bilateral). A total of 272 subjects had FLAIR and ADC images suitable for inclusion in the analysis. Of these, 240 had known MEB status (nM = 77, nR = 163).
We first tested overall mean ADC, averaged over all voxels in the four ROIs. Group mean ADC was 749.22 μm2 s−1. We computed z-scores on the composite ADC, and identified one outlier measurement, ADC = 674.01 μm2 s−1, z = −3.70. Without this point, the remaining z-scores are in the range of −2.47 to 2.76. This data point was excluded from the analysis, resulting in a sample size of n = 239. We note that the effect of including this outlier data point would be to increase the contrast between the RTD and MEB groups, because mean ADC is higher in the MEB group (Table 5).
Table 5.
Differences in Mean Apparent Diffusion Coefficient between Medical Evauation Board and Return to Duty Groups in Four Regions of Interest*
| Composite | 738.64 | 14.32 | 2.54e-08 | 23.98 | 9.6e-06 | 5.50 | 0.034 |
| Bilateral posterior limb of internal capsule | 697.75 | 9.39 | 1.73e-04 | 24.55 | 4.94e-06 | 2.56 | 0.070 |
| Bilateral anterior corona radiata | 727.07 | 12.44 | 9.30e-05 | 29.93 | 1.14e-05 | 6.80 | 0.037 |
| Genu of corpus callosum | 746.98 | 10.35 | 7.89e-04 | 26.19 | 7.55e-05 | 3.74 | 0.24 |
| Splenium of corpus callosum | 776.74 | 22.96 | 8.28e-11 | 15.88 | 0.028 | 4.35 | 0.22 |
From linear regression including apparent diffusion coefficient (ADC) resolution as a categorical covariate with three levels (LOW, INT, HIGH). The medical evaluation bord (MEB) term is significant in the anterior corona radiata, but the trend indicates higher ADC in the MEB group for all of the regions of interest.
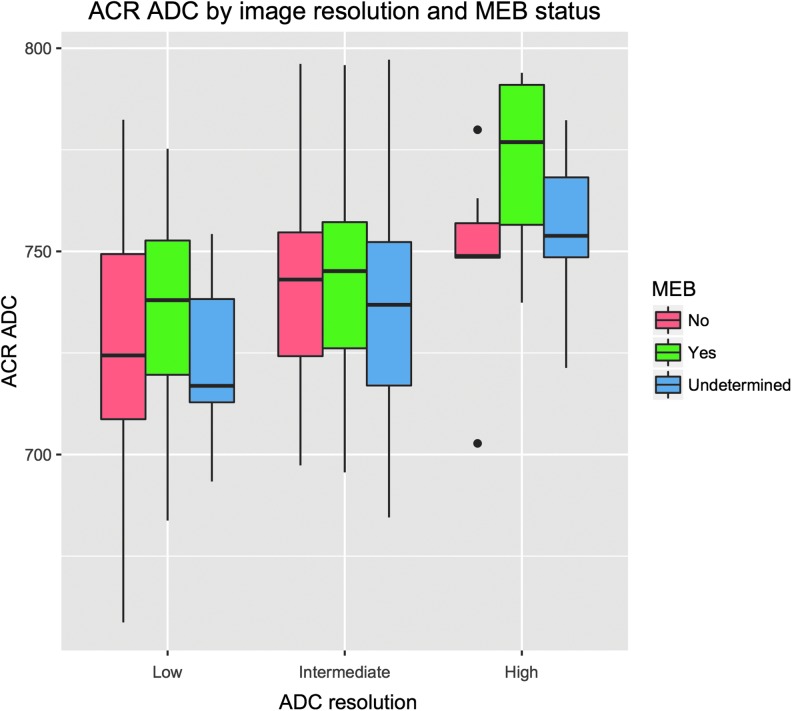
Next, we investigated whether the spatial resolution of the ADC scan was correlated with the composite ADC. The scan resolution was encoded as a factor with three levels: low (1.25 1.25 × 1.25 × 6.5 mm3, n = 113, nM = 34, nR = 69), intermediate (1.20 × 1.20 × 6.5 mm3, n = 136, nM = 36, nR = 86), or high (0.6 × 0.6 × 5.2 mm, n = 22, nM = 7, nR = 7). A boxplot of composite ADC against ADC resolution is shown in Figure 4. Linear regression shows a significant effect for both intermediate and high resolution: both levels were significant at p < 1E-7, and the model R2 = 0.16. We therefore included this factor as a covariate in the regional tests of ADC against MEB status. The covariates were significant in all the regional analyses.
FIG. 4.
Apparent diffusion coefficient (ADC) in the anterior corona radiata (ACR) for scans at different spatial resolutions. Subjects with undetermined medical evaluation board (MEB) status were not included in the regression analyses, but are plotted here as further data points showing that imaging resolution is correlated with the measured ADC. Boxes show interquartile range (IQR) with a black line indicating the median. Whiskers extend to 1.5 * IQR; points outside this range are shown as black circles.
Other factors that may affect the ADC include the FLAIR resolution and changes in the MRI hardware, software, or operating procedures over the five-year period included in the study. These effects are difficult to study independently of ADC resolution because of correlations between the FLAIR resolution, ADC resolution, and the timing of acquisition. We attempted to minimize any FLAIR resolution effect by resampling the FLAIR images to a consistent resolution before warping the ROIs and measuring the ADC.
Linear regression was run for all four regions, with ADC as the dependent variable, and ADC resolution and MEB status as independent variables. In Table 5, the mean ADC is listed for the RTD group with low resolution ADC scans, along with the regression coefficient, t value, and p value for the image resolution covariate and the MEB status. The mean ADC varies across the four regions and is consistently highest in the CC-genu and CC-splenium, regardless of scan resolution. This may reflect inclusion of more CSF in those ROIs, because of their large surface area in proximity to the lateral ventricles. The MEB term has a positive coefficient in all four regions, indicating higher ADC in the MEB group, but the difference is only significant in the ACR (p = 0.037).
Multivariate model of MEB or RTD outcome
The predictor variables for the logistic regression were chosen based on the univariate tests: RBANS, ADC in the ACR, and a history of three or more TBI. To control for ADC image resolution, we regressed ADC against image resolution using all available ADC data and used the residuals as input to the logistic regression. The sample size for the logistic regression was n = 81. Although three or more TBI did not correlate significantly with MEB in the univariate analysis (except when subjects with two TBI were excluded), we included it in the logistic model because it trended in the expected direction (more head injuries associated with an increased likelihood of MEB), and previous studies have implicated multiple traumas as a risk factor for poor recovery from mTBI.
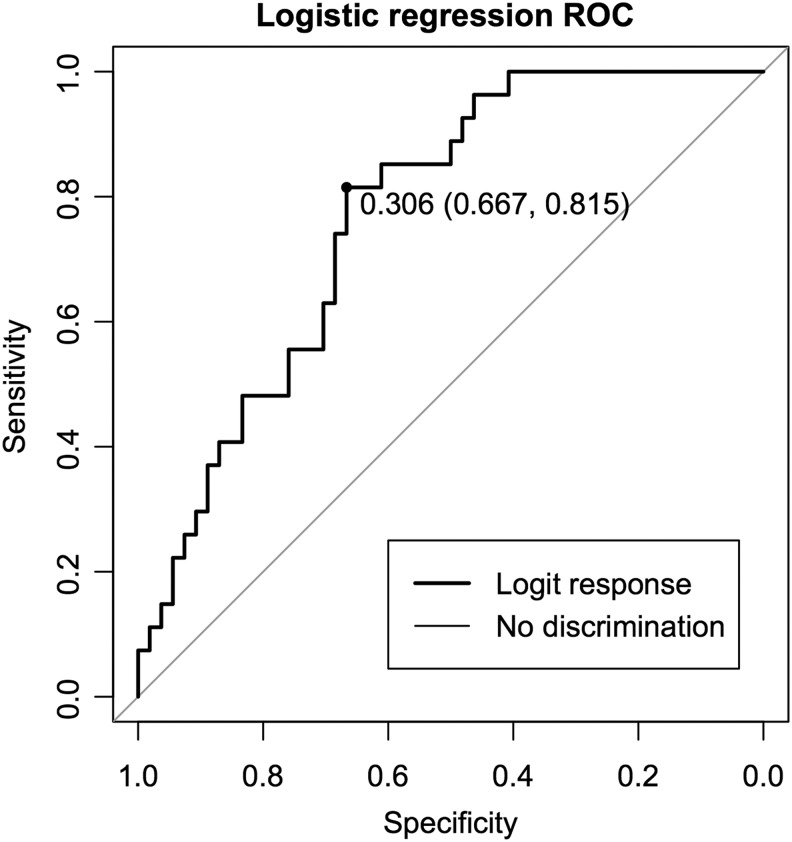
As in the univariate analysis, RBANS performance is the most significant predictor (β = −0.073, z = −2.96, p = 0.0031). Three or more head trauma was also a significant predictor (β = 1.20, z = 2.13, p = 0.033), but the ACR ADC was not significant (β = 0.013, z = 1.11, p = 0.27). To evaluate the logistic model as a classifier, we computed a receiver operating characteristic (ROC) curve with the “pROC” package36 in R. The ROC curve plots sensitivity against (1 – specificity) for different classifier thresholds of the logistic regression output, as shown in Figure 5.
FIG. 5.
Logistic regression output used as a classifier. The curve shows the specificity and sensitivity of different classification thresholds. The highlighted point has the maximum sum of sensitivity and specificity along the curve. ROC, receiver operating characteristic.
The highlighted point on the curve is placed at the point that maximizes the sum of sensitivity and specificity. At this threshold, the sensitivity is 0.815, meaning 81.5% of the subjects who went to MEB are classified as MEB. The specificity is 0.667, meaning 66.7% of subjects who RTD are correctly classified as RTD. The area under the curve (AUC) is 0.77. A perfect classifier has an AUC of 1.0, while a classifier performing no better than chance has an AUC of 0.5. A CI for the AUC was computed using bootstrap resampling of the classifier data; the 95% CI was (0.66, 0.86) after 2000 stratified bootstrap iterations.
We further tested the AUC by permutation testing. For each of 2000 trials, the MEB data were permuted, and the same logistic model was fit to the permuted data and used as a classifier. We define the p value for this test as the fraction of permuted data sets where the ROC AUC exceeded the value in the actual data. The permuted AUC exceeded the actual AUC in 4/2000 iterations, p = 0.002. To illustrate the relative importance of each variable, we computed AUC for models with one term left out (Table 6). The AUC is highest when the ADC is left out (AUC = 0.76) and lowest when RBANS is left out (AUC = 0.67).
Table 6.
Classification of Medical Evaluation Board or Return to Duty Status from Logistic Regression Models with One of the Predictors Dropped from the Full Model*
| Model predictors | Sensitivity | Specificity | AUC (95% CI) |
|---|---|---|---|
| RBANS, ADC, 3+ TBI | 0.82 | 0.67 | 0.77 (0.66, 0.86) |
| RBANS, 3+TBI | 0.78 | 0.70 | 0.76 (0.66, 0.98) |
| RBANS, 3+TBI | 0.78 | 0.70 | 0.76 (0.66, 0.98) |
| RBANS, ADC | 0.80 | 0.63 | 0.74 (0.62, 0.85) |
| ADC, 3+ TBI | 0.70 | 0.59 | 0.67 (0.54, 0.79) |
RBANS, Repeatable Battery for Neuropsychological Status; ADC, apparent diffusion coefficient; TBI, traumatic brain injury.
The classification threshold is chosen to maximize the sum of sensitivity and specificity. The area under the receiver operating characteristic curve (area under the curve [AUC]) is higher for a better classifier. The AUC is lowest when RBANS is left out of the model.
Discussion
As noted by Radomski and associates,37 fitness for duty in the deployed environment after an injury is a difficult clinical decision. The service member is evaluated in the context of the particular mission at hand and the demands of the service member's occupational specialty. Clinical factors taken into consideration include report of symptom resolution, neurological and physical examination findings, results of exertional and balance testing, a functional assessment, and neurocognitive assessment. In most cases, mTBI symptoms resolve spontaneously over the days and weeks after injury, and the service member can resume normal duties and activities. Some service members, however, experience persistent symptoms that degrade their physical performance and cognition, judgment, and emotional regulation.
In this study, characteristics associated with a reduced likelihood of RTD included low performance on the RBANS, increased ADC in the ACR, and multiple concussions. Within the RBANS test, performance in the attention and delayed memory domains were significantly associated with MEB outcome. These univariate associations are uncorrected for multiple comparisons, and with the exception of RBANS, are marginally significant (0.01 < p < 0.05). The purpose of the univariate testing was to assist variable selection for the multivariate model, in which RBANS and multiple concussions were significant predictors of MEB status, but the ADC was not significant.
Neuroimaging results
Previous studies have shown differences in white matter between mTBI and control populations, including mTBI caused by blast.38,39 In a study of military veterans with blast-related mTBI, Miller and colleagues40 found a significant correlation between distributed white matter abnormalities and post-concussive symptoms. In this study, we found significant differences related to RTD or MEB status only in the ACR, and the effect size was small (Table 5). The ADC images are quite limited in their ability to quantify the diffusion in white matter, compared with DTI.41 Because of the low spatial resolution of the ADC images, we limited our analysis to four regions of interest. The choice of regions was guided by the TBI literature; however, these previous studies were mostly on civilian populations, and it is possible that the spatial distribution of white matter injuries is different in the military population exposed to blast. Diffusion anisotropy in the ACR has been linked to attentional control.42 In the present study, the RBANS results showed significant correlation between MEB and attention (p = 0.0023). It is possible that the ACR ADC correlation with MEB is also related to attention; however, ACR ADC was not directly correlated with RBANS attention scores (p = 0.27).
Defining the relationships between white matter abnormalities, domain specific cognitive impairments, and a complex measure of functional recovery like readiness for military duty remains a challenging problem. Further exploration of these trends is planned for future studies, using improved diffusion imaging to better characterize and localize white matter pathology.
Multiple concussions
Spira and coworkers34 found that Marines with three or more lifetime concussions reported lower performance or worse symptoms on a range of cognitive and post-concussive tests, when compared with service members with one or zero concussions. The population in the study by Spira and coworkers34 was also active duty Marines but included Marines with no history of TBI, and excluded Marines not on full duty. We performed a similar analysis by comparing service members with one TBI with those with three or more, and we found that multiple mTBI is associated with a reduced likelihood of RTD. One limitation of the use of number of mTBI in prognosis is that we must rely usually on patient recall of the number of injuries and their severity. More complete reporting and the adoption of reliable wearable technology to measure mTBI exposure may improve prediction.
RBANS performance
In the univariate tests, RBANS score was the strongest predictor of MEB versus RTD outcome. The sample size of subjects with RBANS data was smaller (n = 85) than for tests of neuroimaging (n = 239) or 3+ vs. 1 TBI (n = 206). It remained the strongest result in the logistic model, however, including the (n = 81) subjects who had data for RBANS, 3+ TBI, and neuroimaging.
Post-concussive symptoms, depression, and PTSD
There is growing recognition in the literature that post-concussive and psychological symptoms frequently co-occur, presenting distinct challenges to both diagnosis and treatment.34,37,43,44 In this study, we had data for the HIT-6, NSI, the PCL-M, PHQ-9, and PSQI on a subset of the patients. Scores on these tests did not correlate with RTD status, but they show that many service members in both the RTD and MEB groups are affected by symptoms of headache, depression, sleep disorder, and post-traumatic stress.
Multivariate model
A logistic regression model was used to classify individuals as RTD or MEB according to RBANS, ADC, and a history of multiple (≥3) concussions with approximately 82% sensitivity and 67% specificity. The accuracy of this model was evaluated only via bootstrap and permutation testing in the same data set, and the sample for this model was relatively small, n = 81. An independent sample is required to test generalizability of the model. Variable selection based on univariate tests may lead to the inclusion of noninformative variables because of type I error, or the exclusion of variables that would be predictive in the context of a multivariate model. Missing data also complicated the selection of variables and precluded much exploration of different multivariate models. With a larger sample of complete multivariate data, machine learning techniques could be applied to train and test a more powerful predictive model.
Conclusions
Improving the prediction of prognosis after mTBI is important to better direct treatment resources and improve long-term outcomes. The decision to return someone to full duty has potentially life changing implications. That individual will participate in demanding, potentially dangerous training, and may be placed in harm's way by commanders while on full duty. The decision to recommend medical retirement also cannot be taken lightly. As well as ending the individual's military career, his or her unit is deprived of a skilled and usually experienced team member.
Whether or not a service member ultimately returns to full duty, accurate prognosis is highly valuable to the patient and the military as a whole. The sooner the individual and the unit know with a high degree of certainty that the person is likely to return to duty, the better the person will be able prepare to get back into training with the unit and accomplish any mission assigned. If it can be ascertained quickly that an individual is not likely to be returned to full duty, he or she can be removed rapidly from the unit and placed on a limited duty status. This allows the unit to procure a replacement quickly who can begin training with the unit, optimizing combat effectiveness, and allows the individual to devote time and energy to treatment and rehabilitation, maximizing the potential for recovery and successful reintegration into the civilian community.
While we are far from the long-term goal of a model that can inform reliably command decisions, we believe these results demonstrate preliminary evidence that cognitive, patient history, and imaging data might be combined to predict RTD in a military population. As a retrospective study, we were limited in the available data and did not account for many variables potentially of interest including age, education, length of military service, and covariance between imaging, cognitive performance, and symptoms of PTSD. RBANS was the strongest predictor of MEB status. Computer-based neuropsychological screening tests that correlate well with more comprehensive neuropsychological assessments, such as the RBANS,27 have potential to identify subtle cognitive deficits without initially requiring the skills of a neuropsychologist. The suitability of RBANS for prediction of outcomes in other mTBI populations must be evaluated independently.
Acknowledgments
This work was supported by the Defense Advanced Research Projects Agency (DARPA) and the US Army Research Office under grant number W911NF1010093, and the National Institutes of Health under grant P30-NS045839.
The authors thank Dr. Charles Egan for assistance with the MRI data collection and Ms Helen Braswell for her invaluable work in organizing, collecting and recording the data.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Terrio H., Brenner L.A., Ivins B.J., Cho J.M., Helmick K., Schwab K., Scally K., Bretthauer R., and Warden D. (2009). Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23 [DOI] [PubMed] [Google Scholar]
- 2.Iverson G.L., Langlois J.A., McCrea M.A., and Kelly J.P. (2009). Challenges associated with post-deployment screening for mild traumatic brain injury in military personnel. Clin. Neuropsychol. 23, 1299–1314 [DOI] [PubMed] [Google Scholar]
- 3.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 4.Bazarian J.J., Blyth B., and Cimpello L. (2006). Bench to bedside: evidence for brain injury after concussion—looking beyond the computed tomography scan. Acad. Emerg. Med. 13, 199–214 [DOI] [PubMed] [Google Scholar]
- 5.Defense and Veterans Brain Injury Center Position Paper. Identification and Treatment of Combat Related Traumatic Brain Injury (TBI). (2006). Defense and Veterans Brain Injury Center: Silver Spring, Md [Google Scholar]
- 6.Drake A.I., Gray N., Yoder S., Pramuka M., and Llewellyn M. (2000). Factors predicting return to work following mild traumatic brain injury: a discriminant analysis. J. Head Trauma Rehabil. 15, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 7.Scott K.L., Strong C.A., Gorter B., and Donders J. (2016). Predictors of post-concussion rehabilitation outcomes at three-month follow-up. Clin. Neuropsychol. 30, 66–81 [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld J.V., McFarlane A.C., Bragge P., Armonda R.A., Grimes J.B., and Ling G.S. (2013). Blast-related traumatic brain injury. Lancet Neurol. 12, 882–893 [DOI] [PubMed] [Google Scholar]
- 9.Nelson N.W., Davenport N.D., Sponheim S.R., and Anderson C.R. (2015). Blast-Related mild traumatic brain injury: Neurosychological Evaluations and Findings, in: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Kobeissy F.H. (ed). CRC Press/Taylor & Francis: Boca Raton, FL: [PubMed] [Google Scholar]
- 10.Tanev K.S., Pentel K.Z., Kredlow M.A., and Charney M.E. (2014). PTSD and TBI co-morbidity: scope, clinical presentation and treatment options. Brain Inj. 28, 261–270 [DOI] [PubMed] [Google Scholar]
- 11.Stemper B.D., Shah A.S., Budde M.D., Olsen C.M., Glavaski-Joksimovic A., Kurpad S.N., McCrea M., and Pintar F.A. (2016). Behavioral outcomes differ between rotational acceleration and blast mechanisms of mild traumatic brain injury. Front. Neurol. 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuckerman A., Ram O., Ifergane G., Matar M.A., Sagi R., Ostfeld I., Hoffman J.R., Kaplan Z., Sadot O., and Cohen H. (2017). Controlled low-pressure blast-wave exposure causes distinct behavioral and morphological responses modelling mild traumatic brain injury, post-traumatic stress disorder, and comorbid mild traumatic brain injury-post-traumatic stress disorder. J. Neurotrauma 34, 145–164 [DOI] [PubMed] [Google Scholar]
- 13.Conigrave K.M., Hall W.D., and Saunders J.B. (1995). The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction 90, 1349–1356 [DOI] [PubMed] [Google Scholar]
- 14.Kosinski M., Bayliss M.S., Bjorner J.B., Ware J.E., Jr, Garber W.H., Batenhorst A., Cady R., Dahlof C.G., Dowson A., and Tepper S. (2003). A six-item short-form survey for measuring headache impact: the HIT-6. Qual. Life Res. 12, 963–974 [DOI] [PubMed] [Google Scholar]
- 15.Coeytaux R.R., Kaufman J.S., Chao R., Mann J.D., and Devellis R.F. (2006). Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J. Clin. Epidemiol. 59, 374–380 [DOI] [PubMed] [Google Scholar]
- 16.King P.R., Donnelly K.T., Donnelly J.P., Dunnam M., Warner G., Kittleson C.J., Bradshaw C.B., Alt M., and Meier S.T. (2012). Psychometric study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev. 49, 879–888 [DOI] [PubMed] [Google Scholar]
- 17.Soble J.R., Silva M.A., Vanderploeg R.D., Curtiss G., Belanger H.G., Donnell A.J., and Scott S.G. (2014). Normative Data for the Neurobehavioral Symptom Inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin. Neuropsychol. 28, 614–632 [DOI] [PubMed] [Google Scholar]
- 18.Defense and Veterans Brain Injury Center. (2014) Information Notice. Neurobehavioral Symptom Inventory (NSI): Recommendations for Scoring and Serial Administration for Concussion Outcomes Health Care Outcomes Standardization. Department of the Army: Silver Spring, Md [Google Scholar]
- 19.Wilde E.A., Whiteneck G.G., Bogner J., Bushnik T., Cifu D.X., Dikmen S., French L., Giacino J.T., Hart T., Malec J.F., Millis S.R., Novack T.A., Sherer M., Tulsky D.S., Vanderploeg R.D., and von Steinbuechel N. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 91, 1650–1660e17 [DOI] [PubMed] [Google Scholar]
- 20.Meterko M., Baker E., Stolzmann K.L., Hendricks A.M., Cicerone K.D., and Lew H.L. (2012). Psychometric assessment of the Neurobehavioral Symptom Inventory-22: the structure of persistent postconcussive symptoms following deployment-related mild traumatic brain injury among veterans. J. Head Trauma Rehabil. 27, 55–62 [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K., Spitzer R.L., and Williams J.B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., Berman S.R., and Kupfer D.J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 [DOI] [PubMed] [Google Scholar]
- 23.Ruggiero K.J., Del Ben K., Scotti J.R., and Rabalais A.E. (2003). Psychometric properties of the PTSD Checklist-Civilian Version. J. Trauma. Stress 16, 495–502 [DOI] [PubMed] [Google Scholar]
- 24.Duff K., Hobson V.L., Beglinger L.J. and O'Bryant S.E. (2010). Diagnostic accuracy of the RBANS in mild cognitive impairment: limitations on assessing milder impairments. Arch. Clin. Neuropsychol. 25, 429–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randolph C., Tierney M.C., Mohr E., and Chase T.N. (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 20, 310–319 [DOI] [PubMed] [Google Scholar]
- 26.McKay C., Wertheimer J.C., Fichtenberg N.L., and Casey J.E. (2008). The repeatable battery for the assessment of neuropsychological status (RBANS): clinical utility in a traumatic brain injury sample. Clin. Neuropsychol. 22, 228–241 [DOI] [PubMed] [Google Scholar]
- 27.McKay C., Casey J.E., Wertheimer J. and Fichtenberg N.L. (2007). Reliability and validity of the RBANS in a traumatic brain injured sample. Arch. Clin. Neuropsychol. 22, 91–98 [DOI] [PubMed] [Google Scholar]
- 28.Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., and Gee J.C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54, 2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Hua K., Faria A.V., Mahmood A., Woods R., Toga A.W., Pike G.B., Neto P.R., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P., and Mazziotta J. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40, 570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tustison N.J., Cook P.A., Klein A., Song G., Das S.R., Duda J.T., Kandel B.M., van Strien N., Stone J.R., Gee J.C., and Avants B.B. (2014). Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage 99, 166–179 [DOI] [PubMed] [Google Scholar]
- 32.Avants B.B., Yushkevich P., Pluta J., Minkoff D., Korczykowski M., Detre J., and Gee J.C. (2010). The optimal template effect in hippocampus studies of diseased populations. NeuroImage 49, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avants B.B., Tustison N.J., Wu J., Cook P.A., and Gee J.C. (2011). An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9, 381–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spira J.L., Lathan C.E., Bleiberg J., and Tsao J.W. (2014). The impact of multiple concussions on emotional distress, post-concussive symptoms, and neurocognitive functioning in active duty United States marines independent of combat exposure or emotional distress. J. Neurotrauma 31, 1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borg J., Holm L., Cassidy J.D., Peloso P.M., Carroll L.J., von Holst H., and Ericson K. (2004). Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43 Suppl, 61–75 [DOI] [PubMed] [Google Scholar]
- 36.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C., and Muller M. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radomski M.V., Weightman M.M., Davidson L.F., Finkelstein M., Goldman S., McCulloch K., Roy T.C., Scherer M., and Stern E.B. (2013). Development of a measure to inform return-to-duty decision making after mild traumatic brain injury. Mil. Med. 178, 246–253 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., and Brody D.L. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 364, 2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riedy G., Senseney J.S., Liu W., Ollinger J., Sham E., Krapiva P., Patel J.B., Smith A., Yeh P.H., Graner J., Nathan D., Caban J., French L.M., Harper J., Eskay V., Morissette J., and Oakes T.R. (2016). Findings from structural MR imaging in military traumatic brain injury. Radiology 279, 207–215 [DOI] [PubMed] [Google Scholar]
- 40.Miller D.R., Hayes J.P., Lafleche G., Salat D.H., and Verfaellie M. (2016). White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Map. 37, 220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierpaoli C., Jezzard P., Basser P.J., Barnett A., and Di Chiro G. (1996). Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648 [DOI] [PubMed] [Google Scholar]
- 42.Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., Suh M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131, 3209–3221 [DOI] [PubMed] [Google Scholar]
- 43.Vasterling J.J., Bryant R.A., and Keane T.M. (2012). PTSD and Mild Traumatic Brain Injury. Guilford Press: New York [Google Scholar]
- 44.Kennedy C.H., Porter Evans J., Chee S., Moore J.L., Barth J.T., and Stuessi K.A. (2012). Return to combat duty after concussive blast injury. Arch. Clin. Neuropsychol. 27, 817–827 [DOI] [PubMed] [Google Scholar]