Abstract
Declines in auditory nerve (AN) function contribute to suprathreshold auditory processing and communication deficits in individuals with normal hearing, hearing loss, hyperacusis, and tinnitus. Procedures to characterize AN loss or dysfunction in humans are limited. We report several novel complementary metrics using the compound action potential (CAP), a direct measure of summated AN activity. Together, these metrics may be used to characterize AN function noninvasively in humans. We examined how these metrics change with stimulus intensity and interpreted these changes within a framework of known physiological properties of the basilar membrane and AN. Our results reveal how neural synchrony and the recruitment of AN fibers with longer first-spike latencies likely contribute to the CAP, affect auditory processing, and differ with noise exposure history in younger adults with normal pure-tone thresholds. Moving forward, this new battery of metrics provides a crucial step toward new diagnostics of AN function in humans.
NEW & NOTEWORTHY Loss or inactivity of auditory nerve (AN) fibers is thought to contribute to suprathreshold auditory processing deficits, but evidence-based methods to assess these effects are not available. We describe several novel metrics that together may be used to quantify neural synchrony and characterize AN function in humans.
Keywords: auditory nerve, cochlear synaptopathy, compound action potential, phase locking value
INTRODUCTION
The healthy human cochlea contains ~30,000 auditory nerve (AN) fibers and represents the sole route from the inner ear to the central auditory system. All acoustic information must be encoded in the spike timing and rates of AN fibers. Studies in laboratory animals have shown that moderate noise exposure and aging can lead to a loss or inactivity of AN fibers (Furman et al. 2013; Kujawa and Liberman 2009, 2015; Schmiedt et al. 1996), especially those with low spontaneous rates (low-SR). Loss or inactivity of AN fibers can lead to compensatory changes throughout the auditory system and may disrupt suprathreshold auditory processes (Chambers et al. 2016). Despite a potential impact on suprathreshold processing, losses of up to 80% of AN synapses may not affect pure-tone detection thresholds (Lobarinas et al. 2013; Schuknecht and Woellner 1953). This suggests that standard clinical assessments of human hearing based on detection thresholds (“audiograms”) may appear normal despite significant AN loss. The loss of AN synapses, termed “cochlear synaptopathy,” has been hypothesized to underlie declines in suprathreshold processing in individuals with normal pure-tone thresholds and other auditory perceptual anomalies including hyperacusis and tinnitus (Hickox and Liberman 2014; Plack et al. 2014; Schaette and McAlpine 2011).
The compound action potential (CAP) is an extracellular potential reflecting the summed response of a population of AN fibers. Here we present several new metrics developed from the CAP response (Figs. 1 and 2), which may be used individually or in combination to noninvasively characterize AN function and fiber loss in humans. We introduce and validate five metrics derived from the averaged CAP response that exploit known differences in response patterns of high-SR and low-SR fibers. Compared with high-SR fibers, low-SR fibers have higher thresholds, larger dynamic ranges, better preservation of timing information, longer first-spike latencies, and slower conduction velocities (Bourien et al. 2014; Heil and Irvine 1997; Liberman 1978). Because of the effects of signal averaging, however, differences in spike timing of individual fibers result in an averaged response that is wider than either the single-trial responses or the individual action potentials. The width of this averaged response is dependent on the conduction velocity and fiber diameter of the contributing neurons, which vary depending on each fiber’s SR and location in the cochlea (Heil and Irvine 1997; Kiang 1975). Fibers with high SRs and higher characteristic frequencies are known to have shorter first-spike latencies than fibers with low SRs and lower characteristic frequencies (Heil and Irvine 1997). The onset of an averaged response reflects the single-trial responses with the shortest latency, or fibers with shorter first-spike latencies, whereas the peak latency and width of the response reflect the contribution of fibers with later first-spike latencies. These relationships form the basis of the classic finding that increasing stimulus intensity results in higher CAP amplitudes and shorter latencies (Thornton 1976). As intensity increases, peripheral auditory filter widths broaden, which has the effect of recruiting higher-frequency fibers located in the cochlear base. With increasing intensity, neural synchrony also increases and the population response of AN fibers has a shorter latency (Fig. 3A) (Lichtenhan and Chertoff 2008), resulting in higher CAP amplitudes and shorter latencies. We hypothesize that individual differences in the rate of change in amplitude and peak latency with increasing intensity may reflect differences in the ability to recruit low-SR fibers. That is, individuals who recruit relatively more fibers with increasing intensity will have steeper amplitude-intensity functions with broader responses that yield longer peak latencies (Fig. 3B). We propose that using this multimetric approach will result in improved ability to identify and characterize AN dysfunction in individuals.
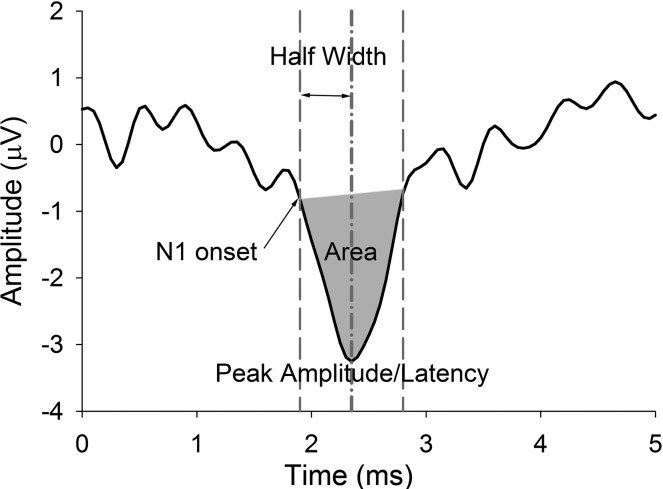
Fig. 1.
Representative CAP response from 1 participant and illustration of 5 metrics: peak amplitude, peak-to-baseline amplitude calculated in reference to the average baseline (−1 to 1 ms) (µV); peak latency, latency of the peak amplitude (ms); onset latency, 90% fractional peak latency at N1 onset (ms); half-width, time from onset latency to peak latency (ms); area, numerical integration from N1 onset to offset (µV/ms) (gray area).
Fig. 2.
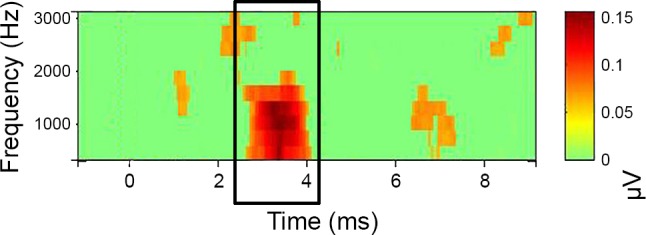
PLV time-frequency plot. Time-frequency analysis was used to estimate PLV of N1 (EEGlab) within linearly spaced frequencies from 625 to 3,120 Hz. x-Axis represents time (signal onset = 1 ms). y-Axis represents frequency. The strength of the PLV above baseline (green) is indicated by color (red). Window size was 1.6 ms, with a padratio of 2. PLVs were extracted from a 2-ms window surrounding the peak of N1.
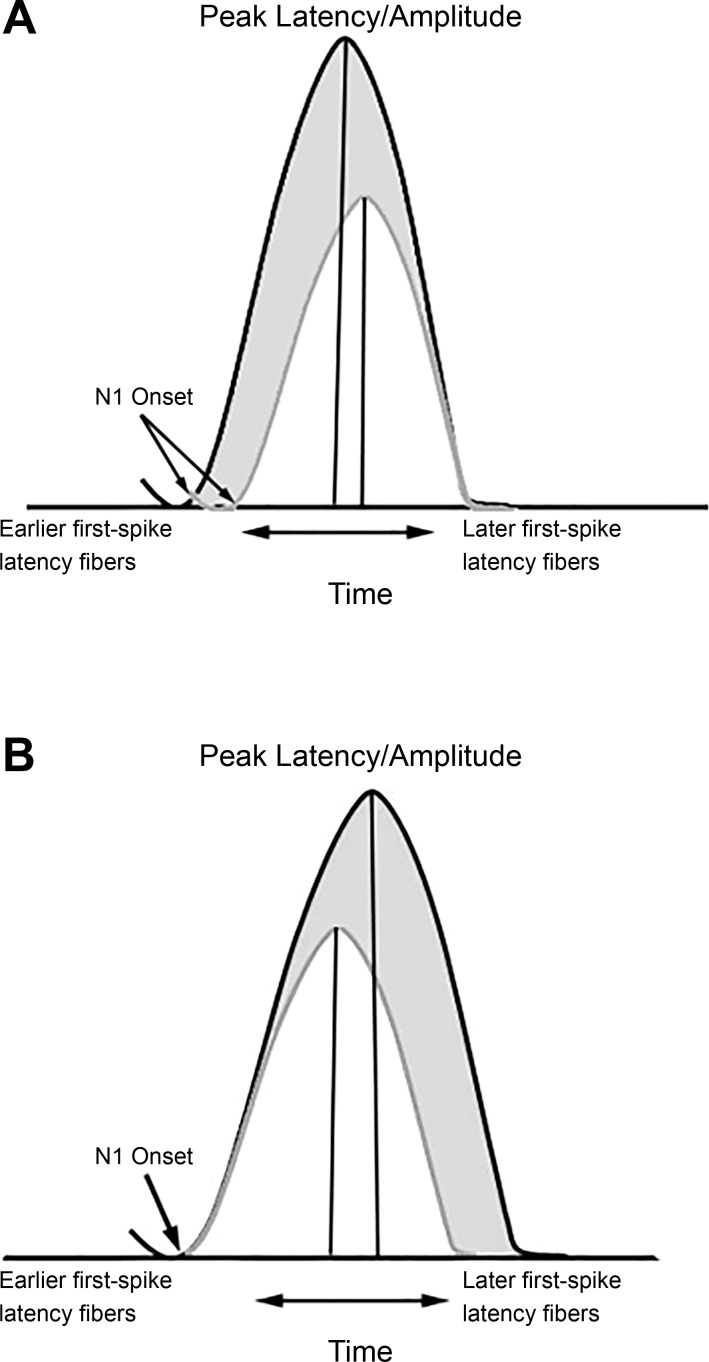
Fig. 3.
Association between amplitude and latency in the CAP response. A: schematic depicting the classic changes in the CAP response with increasing intensity within a participant (white to gray), showing shorter onset and peak latencies. As intensity increases, the probability of individual AN fibers firing increases; there is also increased neural synchrony and a basalward spread of excitation along the basilar membrane, resulting in the recruitment of higher-frequency AN fibers that have shorter first-spike latencies. Thus the shift in both the onset of the N1 response and the peak of the N1 response reflects a change across the neural population. The increase in amplitude with decreasing latency likely reflects improved synchrony and larger numbers of neurons activated with shorter first-spike latencies (higher frequency). B: schematic depicting the hypothesized contribution of later-onset low-SR fibers to the amplitude and latency of the CAP across participants in response to a high-level click (white and gray). Fibers with the shortest first-spike latency contribute to the onset of the response, whereas fibers with longer first-spike latencies contribute to the width and peak latency. Accordingly, larger N1 peak amplitudes, prolonged peak latencies, but similar onset times across participants are consistent with greater recruitment of AN fibers with varied and longer first-spike latencies in participants with the steepest growth of N1 amplitudes with increasing intensity (gray response).
In addition to the above-mentioned metrics drawn from the time-averaged CAP, we analyzed responses to individual stimuli in the time-frequency domain to quantify changes in neural synchrony across stimulus presentations. Similar to techniques previously used at the level of the brain stem and cortex (Clinard and Tremblay 2013; Harris et al. 2014; Zhu et al. 2013), we estimated the phase locking value (PLV) of the AN. PLV reflects the uniformity of the phase at a specific time and frequency across trials. Neural synchrony across trials is crucial in the generation of an averaged potential. Deficits in neural synchrony are hypothesized to contribute to several functional abnormalities, including age-related declines in auditory processing and auditory neuropathy.
Finally, we integrate these new measures to demonstrate how the recruitment of AN fibers with longer first-spike latencies and neural synchrony contribute to the CAP, affect auditory processing, and differ with noise exposure history. The development of objective, noninvasive measures has widespread clinical significance. A loss or inactivity of AN fibers may disrupt temporal processing and result in undersampling of the sound waveform, which may be particularly detrimental to understanding speech in competing noise (Lopez-Poveda 2014).
METHODS
Participants.
Twenty-six participants, aged 22–30 yr, were recruited from the Charleston community (mean age = 25.3 yr; 15 women, 11 men). Participants had pure-tone thresholds ≤ 20 dB HL at 250, 500, 1,000, 2,000, 3,000, 4,000, and 8,000 Hz; differences in thresholds between right and left ears did not exceed 15 dB HL at any frequency. Participants also had normal otoscopic findings and tympanometric measures. Participants provided written informed consent before participating in this Medical University of South Carolina Institutional Review Board-approved study.
CAP acquisition.
Measures were obtained with the participants seated in an acoustically and electrically shielded booth. The CAP was elicited by 100-µs clicks, rectangular pulses, alternating polarity, presented at 11.1/s in 10-dB steps ranging from 70 to 110 dB pSPL to the right ear through an insert earphone (ER-3c; Etymotic Research). According to their specifications, these earphones introduce a 1-ms delay; therefore stimulus presentation occurs at 1 ms rather than at 0 ms. CAPs were recorded in blocks of 1,100 trials (550 of each polarity). Each intensity was presented twice and was varied randomly across blocks from 70 to 110 dB SPL.
Electrical signals were recorded with a tympanic membrane electrode (Sanibel) as the active electrode, a surface electrode on the contralateral mastoid (inverting), and a low forehead electrode (ground). This montage optimizes activity from the AN, or N1. Participants slept and/or relaxed with their eyes closed during CAP recordings.
Neural activity was recorded continuously with a custom head stage (Tucker Davis Technologies; 30× gain) connected to the bipolar channels of a Neuroscan SynAmpsRT (AC mode, 2,010× gain) and digitized at a rate of 20,000 Hz. Neural activity was analyzed off-line in MATLAB with EEGlab and the ERPlab toolbox. Signals were band-pass filtered between 200 and 2,500 Hz. The filtered data were then epoched from −1 to 10 ms and baseline corrected to a −1 ms to 1 ms prestimulus baseline. Trials were identified and rejected on the basis of a moving window with peak-to-peak threshold detection of 50 µV. Epoched responses for the remaining trials were averaged. An N1 was considered present if it occurred within 2–4.5 ms after stimulus onset, was repeatable (identified in both runs at the same intensity), and was identified by two experienced reviewers who assessed the waveforms independently.
Onset latency and peak latency and amplitude.
In addition to the conventional measure of peak latency of the averaged CAP, we measured the latency at the onset of the CAP. The CAP peak-to-baseline amplitude and peak latency of the N1 were identified with visual overlay cursors on a computer monitor. Peak amplitude is measured in reference to the average baseline (2 ms before sound onset, −1 to 1 ms). Peak selection was performed by two independent reviewers and assessed for repeatability across multiple runs. The onset of the N1 of the CAP was calculated in ERPlab with the fractional peak latency function. The fractional peak latency is calculated from the peak, back in time, as the point at which the signal reaches 10% of the fraction of the peak. Rather than 0%, the 10% point was selected because of the influence of trial-by-trial latency variability, such that the onset time of an averaged evoked response will be substantially shorter than the average onset of the single trials, and the 10% peak amplitude point may be more representative of the average single-trial onset times (Luck 2004). Visually, this time point corresponded well with the “starting point” of the evoked response.
Half-width and area.
The half-width of the CAP response was calculated as the time (in ms) from the onset of the CAP to the peak latency. We used numerical integration to calculate the area of the CAP with units of microvolts per millisecond.
PLV.
PLV, also called intertrial coherence, is the relative number of responses at a particular phase and time relative to the stimulus onset (Delorme et al. 2007). An important advantage of PLV is that it is independent of differences in baseline levels of noise and signal power. In addition, given that it is a measure of neural synchrony, PLV may provide a human parallel to the phase-locking measures acquired in single-unit auditory neurophysiological measures in laboratory animals (Zhu et al. 2013) or simulated with predictions from physiologically inspired computational models of AN function (Zilany et al. 2009). PLVs were measured by time-frequency analysis performed with a continuous Morlet wavelet transform as implemented in EEGlab. This process is fully described in Delorme and Makeig (2004). Briefly, PLV measures take values ranging from 0, meaning absence of synchronization across trials, to 1, meaning perfect synchronization. Typically for n trials, if Fk(f,t) is the spectral estimate (i.e., the complex time-varying energy calculated in EEGlab via Morlet wavelet decomposition) of trial k at frequency f and time t:
PLV was computed with linearly spaced frequencies from 625 Hz to 2,500 Hz, with a padratio of 2 and a window size of 32 samples. PLVs were assessed relative to prestimulus baseline (−1 to 1 ms) (P < 0.001, bootstrap permutation). A single estimate of PLV was obtained for each subject as the median PLV across a 2-ms window surrounding the CAP peak response from 625 to 2,500 Hz. A color-coded representation of the PLV data is provided in Fig. 2, with PLV images across frequency, where significance levels are assessed using surrogate data by randomly shuffling the single-trial spectral estimates from different baseline latency windows.
Acoustic reflex measurements.
It has been suggested that CAP measures may be confounded by individual differences in thresholds of the acoustic reflex (Liberman et al. 2016; Valero et al. 2016). To test this hypothesis, acoustic reflex thresholds were measured in a subset of participants (N = 6) with the GSI TympStar (Viasys Health Care, Madison, WI). By using the external stimuli option, the same hardware used to measure CAPs was used to present a 1.5-s click train at levels ranging from 80 dB to 120 dB pSPL in 5-dB steps.
Interaural time difference digit segregation task.
We examined associations between CAP metrics, PLV, and a behavioral temporal processing task. The digit segregation task was previously found to relate to brain stem encoding and hypothesized to reflect differences in AN function (Bharadwaj et al. 2014). Recorded spoken digits of a female speaker were monotonized (184 Hz, close to the natural pitch of the voice). Each trial consisted of two simultaneous sequences of three spoken digits each (containing numbers between 1 and 4). Sequences were differentiated by their interaural time difference (ITD). The ITD was used to spatialize the digits to the right or left of midline. A visual cue (light on vote box) and an auditory cue (noise occurring at same ITD as the tokens) was presented 2 s before the onset of the digits, identifying the direction of the target stream (left or right based on ITD). Target direction was randomized on each trial. The ITD size in each trial was drawn uniformly from a set of ITDs of 100, 200, 300, 400, 800, and 1,200 µs. Each ITD was presented 210 times. After the digit sequence presentation, participants indicated the three digits of the target sequence with button presses. Feedback was provided as follows: a green light indicated that all three digits were identified correctly from the target stream; a yellow light indicated that two digits were identified correctly; and a red light indicated that fewer than two digits were identified correctly. The target digit stream was compared to the participant’s response, and each digit was recorded as correct or incorrect. The ITD digit segregation task was completed by 24 of 26 participants.
Noise exposure history.
To assess the relationship between self-reported noise exposure history and CAP metrics and PLV, participants completed a noise history questionnaire. This questionnaire asks participants to report whether they have a positive history for one or more noise exposure categories: 1) noisy work environments, 2) gun use, 3) loud music, 4) power tools, 5) farm machinery, and 6) sudden loud noises. Noise history questionnaires were completed by 25 of 26 participants. On the basis of their responses, participants were divided into two groups; those who answered “yes” to one or more of the noise history questions were included in the “exposed” group (N = 15; 8 women, 7 men), and the remaining participants were included in the “nonexposed” group (n = 10; 7 women, 3 men). Additional details on the noise history questionnaire are included in Dubno et al. (2013).
Data analyses.
Test-retest reliability of our CAP metrics was assessed with intraclass correlation coefficients (ICCs). Linear regression and repeated-measures analysis of variance (ANOVA) were used to assess the extent to which each metric changed with increasing intensity. We hypothesized that because of their higher thresholds and larger dynamic ranges, the contribution of low-SR AN fibers would be greater at higher than at lower intensities. To test this hypothesis, we examined associations (Pearson product correlations) between metrics at a higher intensity (110 dB pSPL) and a lower intensity (80 dB pSPL). Bootstrapping procedures with 10,000 estimates were used to produce 95% confidence intervals (CIs) for Pearson product correlations. We used linear regression and model testing to assess the independent contribution of our estimates of neural synchrony (PLV) and the contribution of AN fibers with longer onsets (peak latency at 110 dB pSPL) to the growth of N1 amplitude with increasing intensity. Associations between the N1 response, participants’ hearing thresholds, and temporal processing (ITD digit segregation task performance) were modeled with linear mixed-effects regression models, as further described in results. Differences in outcomes between the exposed and nonexposed noise history groups were assessed with independent-sample t-tests. To better visualize the variance across participants and the associations between variables, data are primarily displayed as scatterplots.
RESULTS
Test-retest reliability.
We first analyzed test-retest reliability of the six new metrics (PLV plus 5 from CAP). Click-evoked CAP recordings showed an N1 present in all 26 participants at levels of 80 dB pSPL and higher. A response was present at 70 dB pSPL in 24 of 26 participants. ICCs ranged from 0.68 to 0.98 for all six metrics across the two runs at the same intensity (statistically significant ICCs are ≥0.67). Test-retest reliability was also measured across sessions in two participants by rerecording the CAP 1 wk after the initial measures. The ICCs from the N1 metrics across sessions were >0.9 at intensities from 70 to 110 dB pSPL, suggesting high test-retest reliability across sessions for each CAP metric. The same trained audiologist placed the tympanic membrane electrode for all participants, likely increasing the repeatability across sessions.
CAP metrics: intensity functions.
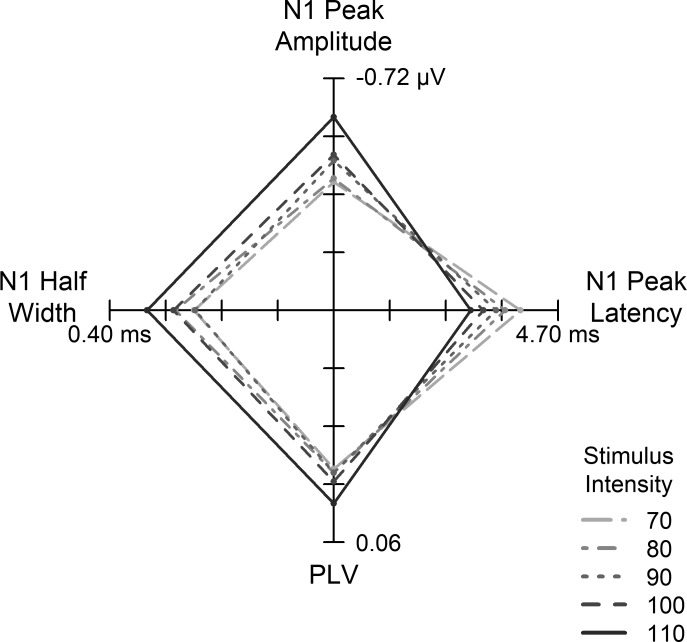
We used repeated-measures ANOVA to examine the extent to which each metric changed with increasing intensity. Mean CAP average waveforms are provided in Fig. 4. Individual subject data and mean regression lines are provided in Fig. 5. With increasing intensity, peak-to-baseline amplitude significantly increased (became more negative) [F(4,96) = 6.17, P < 0.001, = 0.77], peak latency [F(4,96) = 60.66, P < 0.001, = 0.98] and onset latency [F(4,96) = 42.75, P < 0.001, = 0.98] significantly decreased, and the width of the response (half-width) increased [F(4,96) = 2.73, P = 0.03, = 0.84]. The latency decrease and amplitude increase are consistent with previous reports (Dau 2003; Mehraei et al. 2016). PLV increased with increasing intensity [F(4,96) = 5.14, P = 0.001, = 0.96], consistent with reports in laboratory animals of improved neural synchrony with increasing intensity (Heil and Irvine 1997). Increased amplitudes with higher-intensity stimuli can be explained by increased PLV and the recruitment of higher threshold (low-SR) AN fibers. As described above, decreased onset and peak latencies are attributed to the broadening of peripheral auditory filter widths with increasing intensity. This broadening yields shorter first-spike latencies and an excitation pattern that peaks at higher frequencies along the basilar membrane (Harte et al. 2009). Our measure of CAP area was more variable. Although not significant, area showed the general trend of increasing with increasing intensity (P > 0.05). Figure 6 summarizes the multivariate pattern of intensity-dependent changes occurring among N1 peak amplitude, N1 peak latency, N1 half-width, and PLV.
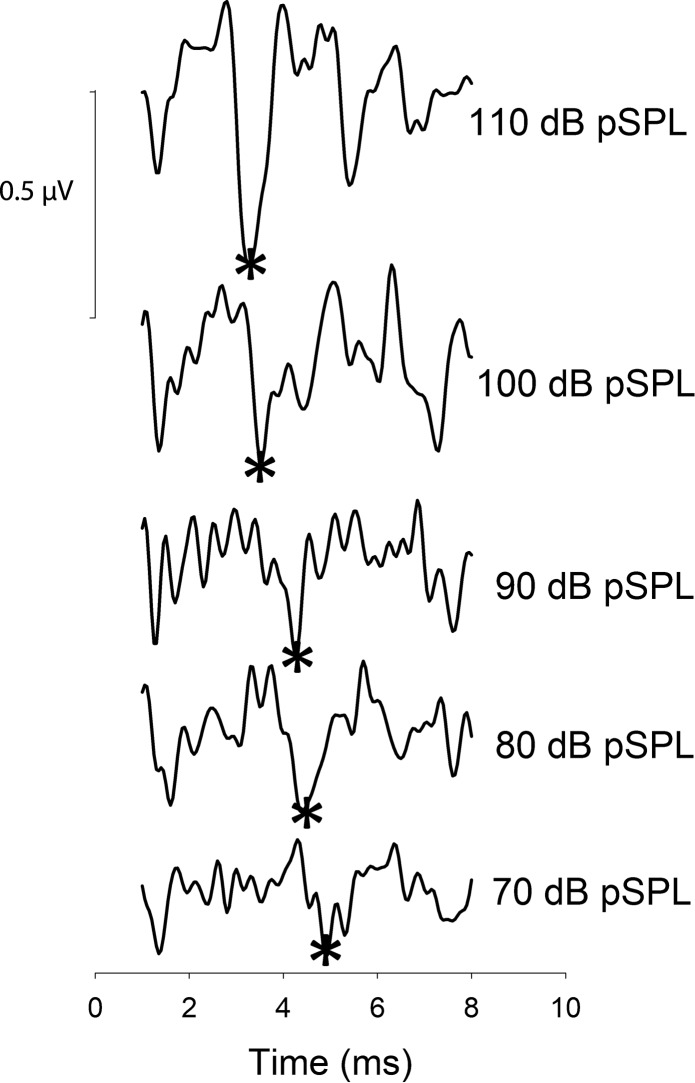
Fig. 4.
Group average CAP waveform for each intensity (70–110 dB pSPL). N1 peak is marked with an asterisk.
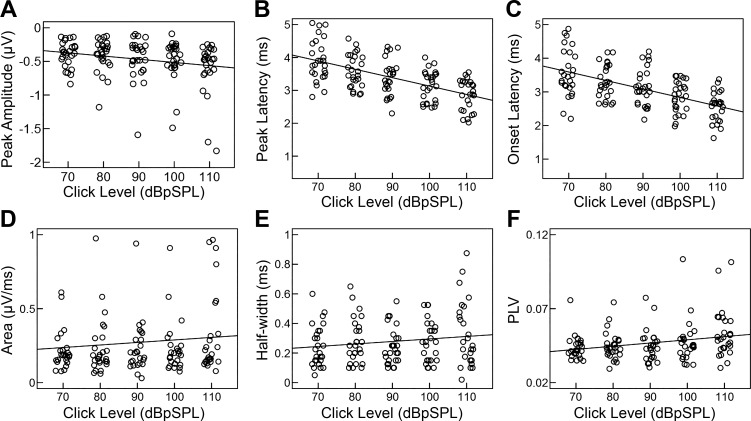
Fig. 5.
CAP metrics as a function of stimulus intensity. With increasing intensity, peak-to-baseline amplitude increased (became more negative) (A), peak and onset latencies decreased (B and C), and half-width increased (E), as expected. Although not significant, area increased with increasing intensity (D). PLVs increased with increasing intensity (F), indicating increased synchrony of N1. Individual data points were randomly jittered around each intensity for clarity in display. Each dot represents data from a single participant, and solid line represents the regression of the variables by intensity.
Fig. 6.
Association between CAP metrics across intensity: star plot with 4 axes illustrating the multivariate pattern of intensity-dependent changes across N1 peak amplitude, N1 peak latency, N1 half-width, and PLV. The coordinates of the origin are 0, 0. Higher intensities resulted in broader N1 half-widths, higher N1 peak amplitudes, shorter N1 peak latencies, and increased phase locking. Different line types indicate the intensity associated with each combination of N1 values.
CAP metrics: individual differences.
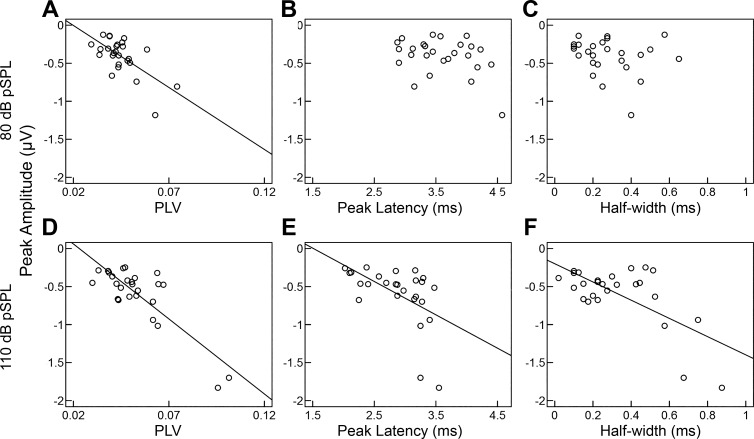
Studies of the CAP have suggested that the response amplitude of N1 is indicative of the quantity and integrity of AN fibers. We hypothesized that the relationship of CAP metrics at low and high intensities will vary across individuals and intensities based on individuals’ ability to recruit additional AN fibers as intensity increases. Accordingly, we determined how each metric related to N1 amplitude at a lower (80 dB pSPL) and a higher (110 pSPL) intensity. Stronger phase locking (PLV) was associated with larger N1 responses (Fig. 7) at lower (r = −0.68, P = 0.001, 95% CI = [−0.86, −0.30]) and higher (r = −0.85, P < 0.001, 95% CI = [−0.96, −0.27]) intensities. Given that phase locking is fundamental to the generation of an averaged event-related potential, PLV may explain some of the variance in event-related potential amplitudes throughout the auditory system. Consistent with this interpretation, we have previously reported associations between the amplitude of the auditory cortical evoked response and PLV (Harris et al. 2014). Our present results provide a novel metric to assess PLV at the level of the AN, which may be used to disambiguate differences attributed to synchrony from other contributing factors, such as the recruitment of additional AN fibers.
Fig. 7.
Scatterplot of N1 peak amplitude, CAP metrics, and PLV at 80 dB pSPL (A–C) and 110 dB pSPL (D–F). Peak amplitudes increased (became more negative) as PLV increased at low (A) and high (D) intensities, consistent with increased neural synchrony across stimulus trials generating larger N1 peak responses. Unexpectedly, larger peak amplitudes were also associated with longer N1 peak latencies and wider (half-width) responses, but only at the higher intensity (E and F). Peak amplitudes were not significantly correlated with onset latencies at either intensity (data not shown, P > 0.05), suggesting that larger peak amplitudes with prolonged peak latencies are not simply the result of delayed N1 responses. Solid line represents the regression line between the variables.
Not surprisingly, CAP area was significantly negatively associated with peak amplitude at low (r = −0.76, P < 0.001, 95% CI = [−0.93, −0.69]) and high (r = −0.68, P = 0.001, 95% CI = [−0.94, −0.27]) intensities (results not shown). However, CAP area was the only variable tested that did not show a significant change with intensity (Fig. 5D), likely because of increased variability in this measure. The area measurement is partially determined by the offset of the N1, which is dependent on the following P2 response and shape of the CAP response. The P2 response is more variable within and across participants than the N1 (data not shown) and may limit the diagnostic utility of this area measurement.
As described above, CAP amplitude increased (became more negative) and latency decreased with increasing intensity. However, these changes were not uniform across participants or CAP metrics, with some participants and metrics showing steeper rates of change than others. We hypothesized that these individual differences in the rate of change across variables may reflect differences in the integrity of the AN, specifically the recruitment of low-SR fibers. Latency (peak and onset) and amplitude metrics were independent of one another at lower intensities (P > 0.05). Larger peak amplitudes were associated with prolonged N1 peak latencies (r = −0.64, P = 0.003, 95% CI = [−0.82, −0.33]) and wider (half-width) responses, at higher intensities (r = −0.75, P < 0.001, 95% CI = [−0.90, −0.27]) (Fig. 7, E and F). Peak amplitudes were not significantly correlated with onset latencies at either intensity (not shown, P > 0.05), suggesting that larger peak amplitudes with prolonged peak latencies are not simply the result of delayed N1 responses. The amplitude and latency of the response to the onset of a stimulus are determined by the number and temporal pattern of responding neurons; their timing is calculated as the first-spike latency, which decreases with increasing SR and characteristic frequency (Heil and Irvine 1997). Therefore, prolonged peak latencies and larger amplitudes at higher but not lower intensities are consistent with the recruitment of low-SR fibers.
In addition to reductions in maximum amplitude, physiological findings with the CAP recorded from the AN in laboratory animals show shallow slopes of amplitude-intensity functions in those animals with a loss of AN fibers (Hellstrom and Schmiedt 1990, 1991; Schmiedt et al. 1996). Furthermore, while absolute differences in CAP amplitudes may stem in part from several factors unrelated to the AN, including placement of the electrode, tissue conductivity, and electrode resistance, differences in slope normalize these effects. We observed substantial differences in the slope of CAP amplitude-intensity functions across participants. We hypothesized that increased neural synchrony across trials (PLV) and/or the recruitment of low-SR fibers would contribute to individual differences in the increase in CAP amplitude at higher intensities. We used multiple linear regression and model testing to determine the extent to which PLV and N1 peak latency (110 dB pSPL) uniquely contributed to the growth of N1 amplitude with increasing intensity. Both PLV (P = 0.002) and peak latency (P = 0.008) remained strong predictors of N1 amplitude growth (multiple R2 = 0.59, P < 0.001). Model testing showed that including both PLV and peak latency significantly improved model fit compared with a model where only PLV [F(1,23) = 8.42, P = 0.008] or peak latency [F(1,23) = 12.07, P = 0.002] were included as predictors (Table 1). Therefore, the slope of N1 amplitude growth increased by 0.006 µV/dB for every 1-ms increase in N1 latency and by 0.023 µV/dB for each 10% increase in PLV. Moving forward, our multimetric approach may help characterize the underlying pathology present in patient populations or across age groups.
Table 1.
PLV and N1 latency associations with N1 amplitude growth
| β | Standardized β | t Score | P Value | |
|---|---|---|---|---|
| Model 1 | ||||
| PLV | −0.30 | −0.66 | −4.27 | <0.001 |
| Model 2 | ||||
| N1 peak latency | −0.009 | −0.61 | −3.72 | 0.001 |
| Model 3 | ||||
| PLV | −0.23 | −0.50 | −3.48 | 0.002 |
| N1 peak latency | −0.006 | −0.42 | −2.90 | 0.008 |
Model 1: multiple R2 = 0.43, P < 0.001. Model 2: multiple R2 = 0.38, P = 0.001. Model 3: multiple R2 = 0.59, P < 0.001.
Relationship to pure-tone thresholds and the acoustic reflex.
All participants had pure-tone thresholds ≤20 dB HL from 250 to 8,000 Hz (i.e., within clinically normal limits). Although some degree of variability was evident across participants, pure-tone thresholds at all frequencies were not significantly associated with neural metrics or results of the ITD digit segregation task (P > 0.05). With respect to the acoustic reflex, when stimuli reach a certain high intensity (which varies across individuals and stimuli), a reflex is initiated with an efferent (ipsilateral and contralateral) contraction of the stapedius muscle. This reflex restricts movement of the tympanic membrane, dampening the acoustic signal delivered to the cochlea. Therefore, individual differences in CAP metrics at low vs. high intensities could stem from differences in the level at which the acoustic reflex is elicited. However, none of the six participants tested exhibited an acoustic reflex within the range of levels used in the present experiment (70–110 dB pSPL); measured acoustic reflex thresholds ranged from 115 to >120 dB pSPL. Furthermore, complete lesion of cochlear efferent nerves in animal models fails to alter peak latencies of wave I of the auditory brain stem response (ABR) (equivalent to the CAP) (Maison et al. 2013), arguing against a contribution of the acoustic reflex to level-dependent changes in CAP metrics.
Relationship between suprathreshold temporal processing and CAP metrics.
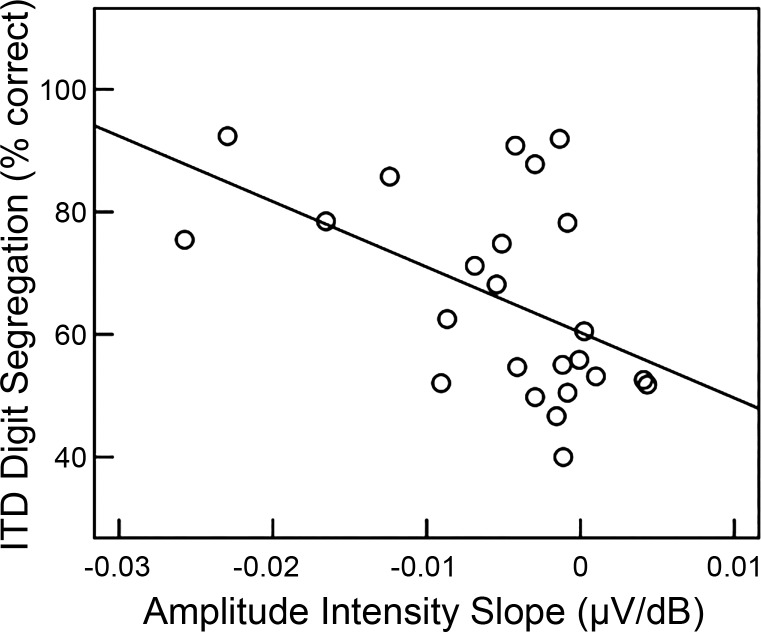
Overall, ITD digit segregation improved with increasing ITD [F(5,65) = 8.68, P < 0.001], but large individual differences in task performance were observed. Errors generally arose because participants incorrectly reported the digit from the masking stream that co-occurred with the target digit and not from omissions or lapses in intelligibility that would result in a misidentification. As results of previous studies have suggested, this pattern of errors is more likely to stem from individual differences in sensory encoding that make it difficult to spatially resolve the target from the masker rather than from lapses in attention or differences in working memory (Bharadwaj et al. 2015). To assess the relative contribution of AN function vs. traditional metrics of detection (pure-tone average), we entered the data into a generalized linear regression analysis. The data were modeled with a linear mixed-effects approach with a random effects term for participants. ITD, individual N1 amplitude-intensity slopes, and pure-tone average (500, 1,000, 2,000, and 4,000 Hz) were entered as explanatory variables and correct trials/ITD as the dependent variable. The coefficient for the pure-tone average term was not significant (P = 0.86). The ITD condition [t(108) = 5.09, P < 0.001] and N1 amplitude-intensity slope [t(22) = −2.26, P = 0.03] were significant predictors of performance. Interactions between N1 amplitude-intensity slope and ITD were not significant (P > 0.05) (Table 2). Post hoc Pearson product correlations showed that participants with steeper N1 amplitude-intensity slopes (more negative) had better performance on the ITD digit segregation task (r = −0.53, P = 0.02, 95% CI = [−0.79, −0.22]) (Fig. 8). ITD digit segregation is a difficult task with obvious demands on selective attention. However, our results suggest that individual differences in sensory processing, occurring as early as the AN, may also explain a significant portion of the variance in performance.
Table 2.
ITD level, N1 amplitude, and pure-tone average associations with ITD digit discrimination (model 1)
| Standardized β | t Score | P Value | |
|---|---|---|---|
| ITD level | 0.14 | 5.09 | <0.001 |
| N1 amplitude slope | −0.43 | −2.26 | 0.03 |
| Pure-tone average | −0.03 | 0.18 | 0.86 |
Marginal R2 = 0.19; conditional R2 = 0.91.
Fig. 8.
Scatterplot showing individual differences in average performance (% correct across ITD) on the ITD digit segregation task plotted against the slope of the CAP amplitude-intensity function. Twenty-four of 26 participants completed the ITD digit segregation task. Those participants with steeper slopes of N1 amplitude-intensity functions had better ITD digit segregation task performance.
Noise exposure history.
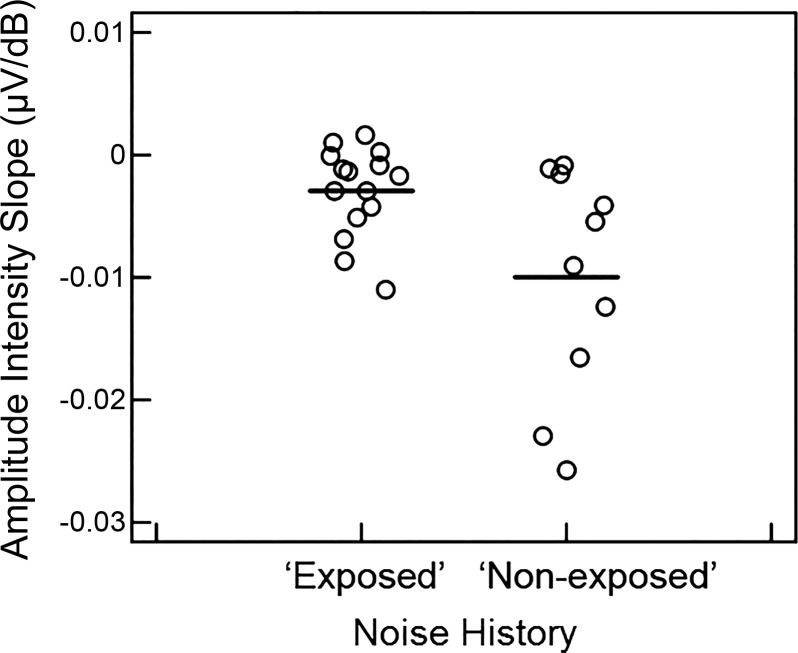
Noise history questionnaires indicated that 60% of participants had a positive history for one or more of the noise exposure categories and were included in the exposed group (see methods), with the most common positive response to loud music (N = 10) and gun use (N = 8). In contrast to previous studies, the likelihood of a positive noise history did not significantly differ with sex of the participant (P > 0.05). Although pure-tone thresholds ranged from −5 to 20 dB HL, pure-tone thresholds did not significantly differ between the two noise exposure groups at any frequency (P > 0.05). Despite substantial overlap between the groups, compared with the nonexposed group, the exposed group had significantly shallower N1 amplitude-intensity slopes [t(23) = 2.23, P = 0.03; Fig. 9] and narrower N1 responses [t(23) = −2.09, P = 0.048] with shorter peak N1 latencies [t(23) = −2.25, P = 0.03]. These effects were significant based on 95% CIs determined from bootstrap procedures with 10,000 permutations. There were no other significant differences between noise exposure groups, including PLV (P = 0.16).
Fig. 9.
Slopes of CAP amplitude-intensity functions for participants in exposed and nonexposed noise history groups. Participants in the exposed group exhibited significantly shallower slopes of N1 amplitude-intensity functions than the nonexposed group (P < 0.05). Horizontal lines indicate the mean slope of each group.
DISCUSSION
Growing evidence from animal models and human studies suggests that a loss or dysfunction of AN fibers, particularly those with low SRs, contributes to suprathreshold auditory processing deficits (Kujawa and Liberman 2009, 2015; Liberman and Liberman 2015; Schmiedt et al. 1996). The goal of the present experiment was to develop and validate new metrics from the CAP to better characterize AN function in humans. This was the first study to use a single-trial approach to assess the CAP response, calculating neural synchrony across trials. Our estimates of neural synchrony from the PLV of the CAP were assessed with good reliability in all participants and increased with signal intensity and peak amplitude, suggesting greater synchrony of neural discharges at higher intensities. These results are consistent with those from single-unit studies in laboratory animals that have shown a reduction in neural jitter with increasing intensity (Miller et al. 1999).
Although direct counts of low-SR and high-SR fibers are not possible in humans, we attempted to estimate the contribution of low-SR fibers by 1) using high signal intensities, where the contribution to the overall response of low-SR fibers is greater than at lower intensities, and 2) examining the growth of amplitude and the width of the CAP across these higher intensities. Even in our sample of younger adults with normal pure-tone thresholds, we found large individual differences in CAP response metrics and suprathreshold temporal processing. We found that neural synchrony at the level of the AN and longer peak latencies independently contributed to the growth of CAP amplitude with increasing intensity. By examining differential measures across intensity, we demonstrated that associations between amplitude and prolonged peak latency were stronger at higher levels, where CAP amplitude growth is dependent on the contribution of low-SR fibers, relative to lower levels. Although level-dependent increases in synchrony result in larger CAP responses, these changes do not account for level-dependent increases in N1 response latency and response width. Our examination of the onset of the response and the width of the CAP suggests that these differences in peak latency are not related to a delay or prolongation of the response but instead represent a wider response, consistent with the recruitment of slower-onset AN fibers. Together, these complementary metrics may provide a means to assess and differentiate the underlying neural pathologies of hearing loss and aging and a better understanding of the interrelated roles of neural dyssynchrony and AN degeneration and loss.
Of importance to the present study is the extent to which low-SR AN fibers contribute to the generation of the CAP response. A recent paper by Bourien et al. (2014) examined the contribution of AN fibers to the CAP; increasing doses of ouabain were delivered to the round window of Mongolian gerbils and guinea pigs. At 80 dB SPL, a selective loss of low-SR fibers appeared to have no effect on the CAP response. However, experimental manipulations, using both noise and furosemide, resulted in a loss or dysfunction of low-SR fibers and elicited robust changes in wave I of the ABR, including increased thresholds and decreased response amplitudes (Fernandez et al. 2015; Lang et al. 2010; Mehraei et al. 2016; Sergeyenko et al. 2013). The contribution of low-SR fibers to the CAP has also been demonstrated in the recovery of the CAP response from prior stimulation, both in animals where the distribution of low-SR fibers was estimated (Schmiedt et al. 1996) and in humans (Murnane et al. 1998). Importantly, the study by Bourien et al. (2014) assessed the contribution of low-SR fibers with stimuli presented at 80 dB SPL. At this intensity, our results are consistent with this finding, showing little to no association between response width or peak latency and amplitude. Associations among metrics reach significance at higher intensities, consistent with the higher thresholds of low-SR fibers, and intensities at which high-SR fibers are known to saturate (50–80 dB SPL) (Sachs and Abbas 1974).
In animals, cochlear synaptopathy and deficits in AN function can be identified via the suprathreshold amplitudes of the CAP response or wave I of the ABR (Fernandez et al. 2015; Sergeyenko et al. 2013). In humans, the use of CAP amplitudes as a marker of AN integrity is complicated by factors unrelated to AN integrity, including electrode location and resistance, head size, etc. To minimize these confounds, we adopted a multimetric and differential approach, examining the interaction of variables at multiple intensities. This approach is based on the assumption that the impact of low-SR fibers would be greater at higher than lower intensities. Recently, several additional methods have been proposed for assessing AN loss and function in humans. Liberman et al. (2016) suggested using the ratio of the summating potential (SP), generated by inner hair cells in the cochlea, to the CAP N1, based on the assumption that “hidden hearing loss” and cochlear synaptopathy would affect the AN (i.e., CAP response) while leaving the inner hair cells intact (i.e., the SP). While they did indeed find a significant difference in the SP-to-CAP ratio between their “noise-exposed” and “nonexposed” groups, these were driven by differences in the SP and not the CAP, complicating interpretation. The click stimulus, 200-Hz filter, and presentation rate in the present study were selected to optimize the CAP response; therefore we were unable to compare our metrics to the SP-to-CAP ratio because the SP could not be recorded reliably in several participants. In addition to measures from the AN, several studies have advocated for the use of brain stem measures, including the frequency following response and wave V of the ABR, to identify deficits in AN function (Bharadwaj et al. 2015; Mehraei et al. 2016). However, these responses are generated at the level of the brain stem, and individual differences could include AN function and central auditory system factors. This limitation is of particular significance when assessing different experimental or patient samples, as both age and experience are known to affect central neural function (Clinard et al. 2010; Clinard and Tremblay 2013; Krishnan et al. 2016; Wong et al. 2007). In contrast, our AN measures should reflect only AN function across experimental or patient samples, particularly after controlling for differences in peripheral auditory function using pure-tone thresholds or otoacoustic emissions.
Relationship to temporal processing.
Similar to previous studies employing the digit segregation task (Bharadwaj et al. 2015), we found large variations in performance, even across younger adults with normal pure-tone thresholds. This task was designed to limit nonsensory factors that affect selective attention demands during speech recognition in order to focus on the degree to which sensory processing differences impact performance. The ability to differentiate the two digit streams was dependent on the use of small ITD cues, thereby testing the fidelity of the temporal resolution in the auditory system. While individual differences in suprathreshold AN activity were predictive of performance, performance was unrelated to small differences in hearing thresholds. These results support the hypothesis set forth by Bharadwaj et al. (2015) that the fidelity of temporal feature encoding, even at early levels of the auditory system, may contribute to ITD stream segregation. The synchronous neural firing patterns of AN fibers are critical for encoding time-varying acoustic cues, particularly when speech is presented in the presence of a competing speech stream. A loss or dysfunction of the AN may result in a stochastic undersampling of speech streams (Lopez-Poveda and Barrios 2013), contributing to difficulties in segregating these streams.
Effects of noise on CAP responses.
Noise exposures are neuropathic and can lead to changes in CAP responses, including decreased peak latencies and decreased CAP amplitudes (Lichtenhan and Chertoff 2008; Maison et al. 2013). Even when the noise exposure is carefully calibrated, in previous studies of noise exposure in laboratory animals large individual differences are often observed. Recreational noise exposure is more difficult to quantify, but some estimates suggest that recreational exposures often exceed 100 dB SPL (Clark 1991). Noise history questionnaires used to assess noise exposure in humans are subjective in nature, particularly when assessing exposure to “loud” sounds, and are subject to recall bias. Despite these limitations, we observed significant differences between exposed and nonexposed groups, consistent with a loss or inactivity of AN fibers. These results are consistent with models developed by Lichtenhan and Chertoff (2008), suggesting that, after a noise exposure, even one that only results in a temporary elevation in hearing thresholds, peak latencies of the CAP decrease and the response is narrower because of fewer AN fibers contributing to the CAP response. Our results are also consistent with recent reports that have identified small but significant associations between self-reports of noise exposure and the CAP response (Bramhall et al. 2017; Liberman et al. 2016; Stamper and Johnson 2015). However, substantial overlap was observed between our exposed and nonexposed groups, and surprisingly there was no effect of the type of exposure, e.g., gun use vs. loud music, as has been reported previously (Bramhall et al. 2017). Similarly, a recent study employing a larger sample size (N = 126) failed to show an association between ABR wave I amplitudes and subjective reports of noise exposure (Prendergast et al. 2017).
In summary, we provide new metrics that describe CAP responses to characterize AN function in humans in vivo. Future studies will be designed to confirm underlying mechanisms and apply these CAP metrics and behavioral measures to characterize age- and hearing loss-related changes in AN function. Together, these complementary metrics may provide a means to assess and differentiate the underlying neural pathologies of human presbyacusis and better understand the interrelated roles of neural dyssynchrony and AN degeneration and loss. Although our results are broadly consistent with a role of low-SR fibers and changes in low-SR fiber populations that can occur with noise exposure, these differences observed in AN function among younger adults may simply represent typical developmental variability/experiential or genetic/epigenetic factors not assessed in the present study rather than direct effects of cochlear synaptopathy. More research is needed to rule out these contributing factors and confirm the associations between CAP metrics and AN function. One approach to the validation of these measures is the use of computational models of AN function in humans or laboratory animals. For example, in animal models of cochlear synaptopathy substantial lesions in the AN resulted in near-normal thresholds. However, differences in N1 amplitude of the CAP at suprathreshold intensities and model estimates of the number of AN fibers significantly correlated with measures of surviving AN area (Earl and Chertoff 2010).
GRANTS
This work was supported (in part) by National Institutes of Health (NIH) Grants R01 DC-014467 and P50 DC-00422. The project also received support from the South Carolina Clinical and Translational Research Institute with an academic home at the Medical University of South Carolina, NIH Grant UL1 RR-029882. This investigation was conducted in a facility constructed with support from NIH Research Facilities Improvement Program Grant C06 RR-14516.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.C.H. conceived and designed research; K.C.H., C.M.M., and J.W.D. performed experiments; K.C.H. and C.M.M. analyzed data; K.C.H. and J.R.D. interpreted results of experiments; K.C.H. and K.I.V. prepared figures; K.C.H. drafted manuscript; K.C.H., K.I.V., C.M.M., J.W.D., and J.R.D. edited and revised manuscript; K.C.H., K.I.V., C.M.M., J.W.D., and J.R.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Richard Schmiedt for his early review and help in developing these projects.
REFERENCES
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci 35: 2161–2172, 2015. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci 8: 26, 2014. doi: 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol 112: 1025–1039, 2014. doi: 10.1152/jn.00738.2013. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP, Griest SE. Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear Hear 38: e1–e12, 2017. doi: 10.1097/AUD.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WW. Noise exposure from leisure activities: a review. J Acoust Soc Am 90: 175–181, 1991. doi: 10.1121/1.401285. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL. Aging degrades the neural encoding of simple and complex sounds in the human brainstem. J Am Acad Audiol 24: 590–599, 2013. doi: 10.3766/jaaa.24.7.7. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res 264: 48–55, 2010. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dau T. The importance of cochlear processing for the formation of auditory brainstem and frequency following responses. J Acoust Soc Am 113: 936–950, 2003. doi: 10.1121/1.1534833. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 34: 1443–1449, 2007. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 14: 687–701, 2013. doi: 10.1007/s10162-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl BR, Chertoff ME. Predicting auditory nerve survival using the compound action potential. Ear Hear 31: 7–21, 2010. doi: 10.1097/AUD.0b013e3181ba748c. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci 35: 7509–7520, 2015. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110: 577–586, 2013. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Vaden KI Jr, Dubno JR. Auditory-evoked cortical activity: contribution of brain noise, phase locking, and spectral power. J Basic Clin Physiol Pharmacol 25: 277–284, 2014. doi: 10.1515/jbcpp-2014-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte JM, Pigasse G, Dau T. Comparison of cochlear delay estimates using otoacoustic emissions and auditory brainstem responses. J Acoust Soc Am 126: 1291–1301, 2009. doi: 10.1121/1.3168508. [DOI] [PubMed] [Google Scholar]
- Heil P, Irvine DR. First-spike timing of auditory-nerve fibers and comparison with auditory cortex. J Neurophysiol 78: 2438–2454, 1997. doi: 10.1152/jn.1997.78.5.2438. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Compound action potential input/output functions in young and quiet-aged gerbils. Hear Res 50: 163–174, 1990. doi: 10.1016/0378-5955(90)90042-N. [DOI] [PubMed] [Google Scholar]
- Hellstrom LI, Schmiedt RA. Rate/level functions of auditory-nerve fibers in young and quiet-aged gerbils. Hear Res 53: 217–222, 1991. doi: 10.1016/0378-5955(91)90055-E. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 111: 552–564, 2014. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang NY. Stimulus representation in the discharge patterns of auditory neurons. In: The Nervous System: Human Communication and Its Disorders, edited by Tower DB. New York: Raven, 1975, p. 81–96. [Google Scholar]
- Krishnan A, Gandour JT, Suresh CH. Language-experience plasticity in neural representation of changes in pitch salience. Brain Res 1637: 102–117, 2016. (Erratum. Brain Res 1644: 308, 2016). doi: 10.1016/j.brainres.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330: 191–199, 2015. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Jyothi V, Smythe NM, Dubno JR, Schulte BA, Schmiedt RA. Chronic reduction of endocochlear potential reduces auditory nerve activity: further confirmation of an animal model of metabolic presbyacusis. J Assoc Res Otolaryngol 11: 419–434, 2010. doi: 10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol 16: 205–219, 2015. (Erratum. J Assoc Res Otolaryngol 16: 221, 2015). doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63: 442–455, 1978. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11: e0162726, 2016. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenhan JT, Chertoff ME. Temporary hearing loss influences post-stimulus time histogram and single neuron action potential estimates from human compound action potentials. J Acoust Soc Am 123: 2200–2212, 2008. doi: 10.1121/1.2885748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res 302: 113–120, 2013. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA. Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Front Neurosci 8: 348, 2014. doi: 10.3389/fnins.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios P. Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Front Neurosci 7: 124, 2013. doi: 10.3389/fnins.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. Ten simple rules for designing ERP experiments. In: Event-Related Potentials: a Methods Handbook, edited by Handy TC. Cambridge, MA: MIT Press, 2004. [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci 33: 5542–5552, 2013. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci 36: 3755–3764, 2016. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Rubinstein JT. An empirically based model of the electrically evoked compound action potential. Hear Res 135: 1–18, 1999. doi: 10.1016/S0378-5955(99)00081-7. [DOI] [PubMed] [Google Scholar]
- Murnane OD, Prieve BA, Relkin EM. Recovery of the human compound action potential following prior stimulation. Hear Res 124: 182–189, 1998. doi: 10.1016/S0378-5955(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends Hear 18: 2331216514550621, 2014. doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ. Effects of noise exposure on young adults with normal audiograms I: electrophysiology. Hear Res 344: 68–81, 2017. doi: 10.1016/j.heares.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J Acoust Soc Am 56: 1835–1847, 1974. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31: 13452–13457, 2011. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 76: 2799–2803, 1996. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC. Hearing losses following partial section of the cochlear nerve. Laryngoscope 63: 441–465, 1953. doi: 10.1288/00005537-195306000-00001. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33: 13686–13694, 2013. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear 36: 172–184, 2015. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AR. Statistical properties of electrocochleographic responses and their use in clinical diagnosis. In: Electrocochleography, edited by Ruben RJ, Elberling C, Salomon G. Baltimore, MD: University Park, 1976, p. 257–276. [Google Scholar]
- Valero MD, Hancock KE, Liberman MC. The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res 332: 29–38, 2016. doi: 10.1016/j.heares.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci 10: 420–422, 2007. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bharadwaj H, Xia J, Shinn-Cunningham B. A comparison of spectral magnitude and phase-locking value analyses of the frequency-following response to complex tones. J Acoust Soc Am 134: 384–395, 2013. doi: 10.1121/1.4807498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MS, Bruce IC, Nelson PC, Carney LH. A phenomenological model of the synapse between the inner hair cell and auditory nerve: long-term adaptation with power-law dynamics. J Acoust Soc Am 126: 2390–2412, 2009. doi: 10.1121/1.3238250. [DOI] [PMC free article] [PubMed] [Google Scholar]