Abstract
Brain function requires precise neural circuit assembly during development. Establishing a functional circuit involves multiple coordinated steps ranging from neural cell fate specification to proper matching between pre- and post-synaptic partners. How neuronal lineage and birth timing influence wiring specificity remains an open question. Recent findings suggest that the relationships between lineage, birth timing, and wiring specificity vary in different neuronal circuits. In this review, we summarize our current understanding of the cellular, molecular, and developmental mechanisms linking neuronal lineage and birth timing to wiring specificity in a few specific systems in Drosophila and mice, and review different methods employed to explore these mechanisms.
Introduction
Multiple developmental processes, including cellular specification, axon and dendrite targeting, and synaptic partner matching, must be tightly coordinated to ensure precise neural circuit assembly. Accordingly, many studies have focused on exploring the developmental mechanisms underlying wiring specificity, revealing, over the past several decades, numerous molecular and cellular mechanisms that regulate neural cell fate specification, axon guidance, and dendrite morphogenesis [1–3]. Synaptic partner matching, the final step in circuit assembly, remains relatively poorly understood, and underlying molecules and mechanisms are just being revealed [4–7].
In this review, we discuss how neuronal lineage and birth timing are linked to wiring specificity at the cellular and molecular levels. Progenitors undergo a series of cell proliferation and differentiation events in the process of generating postmitotic neurons. Cell lineage denotes this series of events for an individual cell or cell type. Here, we use the term lineage to refer to the last few rounds of cell divisions that generate postmitotic neurons from a proximal progenitor. Many molecular factors and cellular mechanisms synergize to ensure that each step, from progenitor proliferation to wiring of immature neurons, is tightly controlled. In some neuronal systems, different neuronal subtypes are sequentially generated from one progenitor or a pool of common progenitors, and birth order or birth timing can predict their cell fates and wiring patterns; we classify such lineage-related processes, which specify neuronal cell fate and wiring, as intrinsic mechanisms. In other neuronal systems, cell fate and consequent wiring patterns have been demonstrated to independent on lineage. As processes such as lateral inhibition, extracellular induction and stochastic regulation have been shown to play important roles in wiring these circuits, we classify these as extrinsic and stochastic mechanisms. In this review, we discuss how intrinsic, extrinsic, and stochastic mechanisms contribute to wiring specificity within lineages in both Drosophila and mouse nervous systems, using findings from six relatively well-studied systems and dividing these findings into intrinsic and extrinsic/stochastic sections based on our current understanding. We note that various combinations of intrinsic, extrinsic and stochastic mechanisms may be used in most or all developing neuronal systems; our categorizations of a specific system as using intrinsic or extrinsic/stochastic mechanisms below reflect either the biased use of one mechanism over the other or that our understanding of one mechanism is more complete than our understanding of the other in that system.
Intrinsic regulation of birth timing-dependent neural wiring
Some neuronal circuits appear to rely heavily on intrinsic mechanisms in the establishment of wiring specificity. Here we review how birth timing-related intrinsic factors guide development of wiring specificity in several model systems, including Drosophila olfactory projection neurons (PNs), mushroom body (MB) neurons and mouse cortical excitatory neurons. In reviewing findings from each system, we first describe the established relationships between cell lineage or birth timing and wiring specificity, and then summarize potential mechanisms at the molecular and cellular levels underlying such regulation.
Drosophila olfactory projection neurons
In the Drosophila olfactory system, 50 classes of olfactory receptor neurons (ORNs) form one-to-one connections with 50 classes of second-order projection neurons (PNs) in the antennal lobe in 50 discrete glomeruli [8–10]. Each PN class restricts its dendrites to one glomerulus and features a stereotyped axonal arborization pattern in the lateral horn, a higher brain center that processes olfactory information [11–15]. Drosophila PNs have provided an excellent system for investigating the relationship between cell lineage and wiring specificity. Studies of this system have demonstrated that dendrite targeting of different classes of PNs can be completely predicted from their birth order or timing within the PN lineage [12, 16, 17].
Using mosaic analysis with a repressible cell marker (MARCM; see Box), Jefferis et al. found that PNs are derived from three separate neuroblast lineages, named the anterodorsal, lateral and ventral lineages according to their cell bodies’ positions relative to the antennal lobe [12]. Anterodorsal and lateral PNs (adPNs and lPNs) are excitatory neurons that send their dendrites to single, distinct glomeruli, whereas ventral PNs (vPNs) are inhibitory GABAergic neurons that send their dendrites to one or more glomeruli [13, 18]. Within each lineage, one neuroblast repeatedly undergoes asymmetric division, giving rise to a new neuroblast and a ganglion mother cell, which divides again to generate two neurons (Fig. 1a). In the adPN and vPN lineages, only one of the two post-mitotic neurons survives and develops into a PN, while in the lPN lineage, both post-mitotic neurons survive, developing into one PN and one local interneuron [17, 19].
Fig. 1.
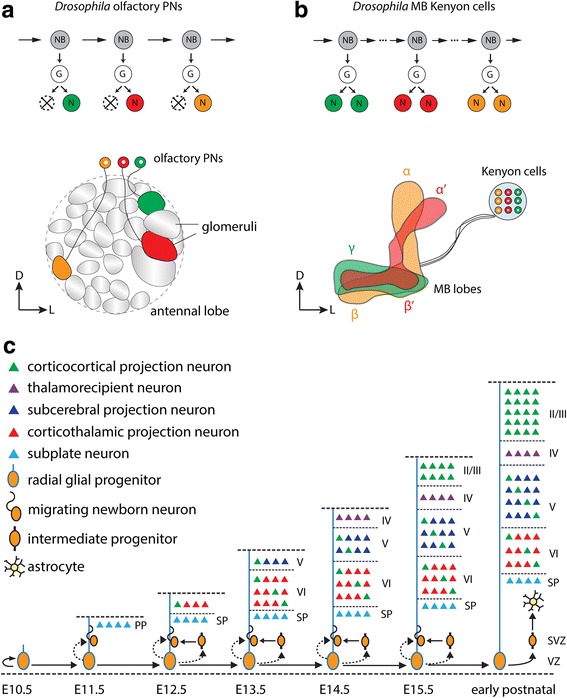
Intrinsic regulation of birth timing-dependent neural wiring. a and b In Drosophila, different types of olfactory projection neurons (PNs; a) and mushroom body (MB) Kenyon cells (KCs; b) are sequentially born from a common neuroblast (NB) in a stereotyped manner. In the anterodorsal PN (adPN) lineage, one of the postmitotic neurons undergoes apoptosis, so that only one PN is generated from one ganglion mother cell (GMC; labeled as G). Different PN classes send their dendrites to specific regions (glomeruli) in the antennal lobe. In the KC lineage, both postmitotic neurons resulting from GMC division survive and project their axons to the same MB lobe. D: dorsal; L: lateral. c In the developing mouse cortex, radial glia in the ventricular zone (VZ) divide asymmetrically to give rise to newborn projection neurons that populate progressively more superficial layers of the mature cortex and intermediate progenitors in the subventricular zone (SVZ), which themselves further divide to generate newborn projections neurons. Corticocortical projection neurons in layers II/III and scattered throughout layers V and VI project within the cortex; subcerebral projection neurons primarily occupying layer V project to subcortical structures such as the superior colliculus, pons and spinal cord; and corticothalamic projection neurons primarily occupying layer VI project to the thalamus. Radial glia produce astrocytes last, after filling in the cortex with projection neurons. Arrows represent postmitotic progeny; arrows with dotted lines represent possible postmitotic progeny. SP: subplate; PP: preplate; EX (e.g. E13.5): embryonic day X (days post conception, e.g. embryonic day 13.5) in mouse
Since MARCM allows temporal control over the induction of mCD8GFP-marked single cell clones [20], investigating the cell body position and target choice of single PNs induced at different times has allowed researchers to correlate PN classes with their lineage and birth order. Interestingly, within each lineage, different PN classes are born sequentially in a stereotyped order [12]. Two later studies using twin-spot MARCM, which allows labeling of sister clones from a common progenitor with two different fluorescent proteins [21], characterized the birth order of adPNs and lPNs more comprehensively. The authors captured every single neuron from one lineage based on birth order and identified several additional PN classes in both lineages not previously characterized [16, 17]. Meanwhile, twin-spot MARCM enabled the authors to deduce the number of cells in individual PN classes, revealing that each class comprises a stereotyped number of cells ranging from one to seven. Consistent with previous findings, both studies showed that lineage and birth order predict PN cell fate and dendrite targeting.
The stereotyped birth order of different PN classes suggests that there must be lineage-related intrinsic factors controlling cell fates of PNs and their dendritic targeting. What are these intrinsic factors? Transcription factors and cell-surface/secreted molecules are widely believed to be key factors regulating cell fate and wiring specificity, respectively. Accordingly, various transcription factors and cell-surface/secreted molecules have been shown to play crucial roles in regulating PN axon/dendrite targeting [5]. Recent findings suggest that transcription factors act within each lineage to specify different PN classes, and cell-surface/secreted molecules act downstream of transcription factors to directly execute the molecular processes underlying wiring specificity [22].
For example, Abnormal chemosensory jump 6 (Acj6) and Ventral veins lacking (Vvl, also called Drifter), two POU domain transcription factors, have been shown to be lineage-specific factors for adPNs and lPNs, respectively [23]. Acj6 and Vvl not only show lineage-specific expression patterns, but also are required for dendrite targeting of adPNs and lPNs, respectively. Loss of Acj6 in the adPNs or loss of Vvl in lPNs causes significant dendritic targeting defects. Misexpression of Acj6 in lPNs or Vvl in adPNs leads to aberrant targeting of PN dendrites to the glomeruli normally occupied by the other PN lineage. Acj6 also controls the axon terminal arborization of adPNs in the lateral horn, indicating that one transcription factor can affect wiring of both dendrites and axons in the same cell type. Additionally, Lim1, another POU domain transcription factor, is expressed in and required for dendrite targeting of vPNs but not for the other two lineages [24]. Since each lineage generates multiple PN classes, individual lineage factors are insufficient to specify different PN classes and corresponding axon/dendrite targeting. Indeed, additional lineage-specific transcription factors expressed in a subset of cells within a lineage, such as Islet and C15, have been identified [22, 24].
Different expression levels of the same transcription factor can also help to specify PN classes. For example, a temporal gradient of Chinmo, a BTB-Zinc finger transcription factor, governs assignment of neuronal identity in both PN and mushroom body lineages (see below) [25]. Loss of Chinmo leads to a transformation of early-born neuronal fates to late-born neuronal fates, and misexpression of Chinmo causes the opposite effects. Interestingly, a recent study shows that in addition to lineage-specific transcription factors, two RNA-binding proteins, IGF-II mRNA-binding protein (Imp) and Syncrip (Syp), could also act as intrinsic factors to specify PN identity [26]. Imp and Syp show opposing temporal gradients across the progression of both PN and mushroom body lineages (see below), and they promote early and late neuronal fates, respectively. Imp and Syp appear to govern temporal neuronal fates at least partially through Chinmo. Another recent study reveals that the transcription factor Seven-up (Svp) is critical for establishing Imp/Syp temporal gradients [27]. In summary, PNs of specific classes, which target their dendrites to specific glomeruli, are born in an invariant order, and this process seems to be controlled by a combination of transcription factors and RNA-binding proteins.
Drosophila mushroom body Kenyon cells
Like Drosophila olfactory projection neurons (PNs), different types of Drosophila mushroom body (MB) intrinsic neurons, also known as Kenyon cells (KCs), are also born sequentially and in an invariant order (Fig. 1b), suggesting that lineage-related intrinsic factors also influence the progression of the MB lineage. The Drosophila MB is a higher order center for olfactory learning and memory and other brain functions such as sleep and courtship [28–32]. The MB contains four main parts: somata, calyx, peduncle and lobes. KC somata cluster in the dorsal posterior brain and send processes anteriorly, forming dendritic branches that comprise the calyx and then converge to form the peduncle. The axon bundle bifurcates at the anterior end of the peduncle to form dorsal (α and α’) and medial (β, β’ and γ) lobes (Fig. 1b). KCs are classified as γ, α’/β’ or α/β neurons, according to the lobes in which their axons terminate. All KCs originate from four neuroblasts in each hemisphere and each neuroblast generates an indistinguishable set of KCs. Clonal analysis using MARCM revealed that these three types of neurons are born sequentially from these common neuroblasts in a stereotyped order [33].
γ neurons are born first, before the midlarval stage; next, in the late larval stages, α’/β’ neurons are born; lastly, during the pupal stages, α/β neurons are born [33]. In the larval brain, both γ and α’/β’ neurons send axons to both dorsal and medial lobes. Whereas α’/β’ retain their bifurcated axon branches during metamorphosis, bifurcated axons of γ neurons degenerate in the early pupal stage and axon fragments are phagocytosed by the glial cells. γ neurons then extend axons only medially to form the adult γ lobe [33–36]. KC dendrites integrate inputs from projection neurons encoding olfactory, thermal, gustatory and visual stimuli [32, 37, 38], while MB output neurons elaborate segregated dendrites that form 15 distinct compartments in the MB lobes [32, 39]. In summary, three classes of KCs form connections with upstream and downstream partners, and current evidence suggests that lineage information fully predicts cell fate and wiring specificity.
Intrinsic factors such as Chinmo, Imp and Syp, which specify PN fates, also specify neuronal fates in the MB lineage [25, 26]. Interestingly, studies of the Drosophila embryonic ventral nerve cord suggest that sequential expression of another set of transcription factors (Hunchback/Hb, Kruppel/Kr, Pdm, and Castor/Cas) drives temporal cell fate specification [40]. These factors are transiently expressed in neuroblasts; inheritance by post-mitotic cells is what ultimately specifies cellular identities [40, 41]. Recent studies have also shown that optic lobe neuroblasts use a similar temporal patterning strategy featuring yet another set of molecules to control neural fate in the medulla [42, 43]. These findings suggest that different neuronal systems in the developing Drosophila central nervous system use analogous temporal patterning strategies that nevertheless employ different sets of molecules.
Several questions regarding the development of Drosophila PNs and KCs remain unaddressed. What other intrinsic factors and mechanisms control neuronal specification? How do multiple factors cooperate to specify different neuronal classes? How do intrinsic mechanisms ultimately control wiring specificity? One recent study that applied single-cell RNA-sequencing to Drosophila PNs shed light onto these questions, suggesting that combinations of transcription factors and cell-surface molecules may play a critical role in specifying different PN subtypes [22]. However, how these two sets of molecules interface remains unclear, and should be investigated in future studies.
Mammalian cortical excitatory neurons
Intrinsic mechanisms also regulate birth timing-dependent neural wiring in the developing mammalian brain. The role birth timing plays in organizing mammalian neuronal wiring is perhaps nowhere more apparent than in the developing cerebral cortex [44–48], which throughout embryonic and postnatal development forms a structure featuring six layers of excitatory neurons that largely project to different extra-cortical targets (Fig. 1c). Asymmetric divisions of individual radial glia (RG), the primary neural progenitor cells in the developing cortex [49], generate newborn excitatory neurons that migrate out from the ventricular zone along radial glial fibers, resulting in the formation of cortical columns [50]. RG also generate intermediate progenitor cells that also eventually differentiate into neurons [51–53]. Because the cortex develops in an inside-out manner, such that earlier-born neurons populate the deeper layers and progressively later-born neurons populate progressively more superficial layers, much work has investigated the relationship between birth timing and eventual cell position in various cortical layers. Astrocytes are born last, after all cortical neurons are born. Importantly, projection neurons that occupy different layers project to different targets: corticocortical projection neurons of layers II/III, V and VI project to the contralateral cortex; layer IV thalamorecipient neurons receive input from the thalamus and broadcast output to other layers (primarily layer II) of proximal cortex; layer V subcerebral projection neurons project to subcortical targets such as the superior colliculus, pons and spinal cord; and layer VI corticothalamic projection neurons project to the thalamus [54, 55]. Thus, these basic layer-specific projection patterns exemplify the effects of birth timing on both cell fate and neural wiring of cortex excitatory neurons.
The mechanisms underlying layer-specific neuronal specification appear to rely heavily on intrinsic properties of progenitor cells, and ongoing work investigates whether these properties apply uniformly to all RG. Two extreme models posit that a) timing is the sole determinant of the potential of a given RG cell, or b) pre-specified, potential-restricted RG subtypes preferentially generate neuronal subtypes with specific projection patterns. The most parsimonious model proposes that all progenitors have equal potential, and thus that birth timing is the only factor influencing progenitor competence. Support for this model comes from early transplantation studies in which early-stage progenitors transplanted into late-stage cortex could produce all neuronal subtypes, but late-stage progenitors transplanted into early-stage cortex could produce only superficial-layer subtypes [56–59]. These studies indicated that the competence of a given RG becomes progressively limited across cortical development, although later transplantation studies indicated that both intrinsic and environmental cues control RG competence [60, 61]. Retroviral labeling studies, in which early viral injections resulted in labeling of neurons of all layers and later viral injections resulted in labeling of superficial layer neurons, corroborated these results [62–65]. Finally, various in vitro approaches have recapitulated birth timing-dependent layering of cortical developmental processes [61, 66–68]. Taken together, these studies suggest that neuronal birthdate is an important determinant of neuronal positioning in the cortex, and thus of wiring patterns, but do not address the possibility of differences in the relative abundance of pre-specified, potential-restricted progenitor cells.
An alternative model, which still incorporates intrinsic, birth timing-dependent mechanisms, would posit that potential-restricted progenitors preferentially generate different neuronal subtypes, such that some progenitors give birth to neurons that predominantly populate lower layers while others give birth to neurons that predominantly populate more superficial layers. Sparse expression of subtype-specific transcription factors such as Fezf2, which defines adult subcortical projection neurons [69–71], and Cux1/Cux2, which define adult callosal projection neurons, suggests that different progenitor subsets may be at least partially committed to generating different neuronal subtypes [72, 73]. Further investigations of this hypothesis used Cre/CreER transgenic mouse lines (see Box) to trace Cux2+ and Fezf2+ lineages in order to investigate the eventual positions of neurons derived from Cux2+ and Fezf2+ progenitors. These studies yielded contradictory results, with an initial study reporting a population of cortical progenitors that preferentially generates neurons populating more superficial layers [74] and a subsequent study from another group using similar approaches, including experiments utilizing some of the same mice on different genetic backgrounds, reporting contrasting findings [75]. Taken together, these results highlight the necessity of careful performance and interpretation of fate-mapping experiments using mouse genetic tools [76, 77]. An additional study utilizing MADM-based clonal labeling provided evidence that RG divide in a stereotyped manner consistent with a more parsimonious, strictly timing-dependent model of cortical neurogenesis [78], but results from such MADM-based studies can potentially suffer from biases due to the genomic positioning of MADM cassettes; some loci may be more susceptible to recombination in certain cell types than others. Thus, while positioning of excitatory cortical neurons seems to be largely predicted by birthdate, the degree to which production of various projection neuron subtypes is limited to pre-specified progenitors remains an area of active investigation.
Recent studies of excitatory cortical neurogenesis have focused on the functional consequences of lineage-dependent cell positioning. Sister excitatory neurons in ontogenetic radial clones labeled by in utero intraventricular injection of eGFP-expressing retroviruses, for example, are preferentially connected and have stronger connections in the second and third postnatal weeks than unrelated neurons [79]. Furthermore, gap junctions mediate transient electrical coupling between sister excitatory neurons and are required for development of these preferential connections and subsequent similarity of functional response properties between sister neurons [80, 81], as predicted by prescient dye-tracing studies [82–85]. Such functional similarities may be most prominent in neurons born very closely in time, and thus most closely related by lineage [86, 87], although other factors, such as the distance between clones and thus the degree to which they share a developmental microenvironment may also predict functional connectivity patterns. Determining the relative contributions of lineage and local environmental factors will be difficult. Finally, as multiple reports have noted that neurons with similar response properties tend to be preferentially connected [88–91], it may be that lineage and birth timing predict preferential connectivity established by gap junctions together with shared response properties driven by thalamocortical specificity and plasticity-mediated maturation of functional corticocortical connections in the immature cortex [92]. However, the molecular mechanisms underlying these processes, thought to be executed at the length scale of spines [91], remain poorly understood. Taken together, these findings indicate that birth timing biases excitatory cortical neuron positioning and wiring, and that lineage relationships can predict functional connectivity and response properties.
Cortical interneurons, however, develop from distinct lineages originating in the medial ganglionic eminence, caudal ganglionic eminence and preoptic area [93, 94]. While several groups have been actively investigating the possible lineage-dependence of inhibitory interneuron positioning using a combination of viral fluorescent labeling and barcoding [95–101], differing results and divergent interpretations of common datasets highlight the need for careful application of lineage tracing tools (see Box) and analytic and statistical definitions and procedures. Thus, the possible lineage-dependence of cortical interneuron positioning and wiring has been the subject of intense investigation; additionally, any possible birth timing-dependence of cortical interneuron positioning and wiring is not fully understood and also warrants further study [102, 103]. Finally, studies of the developing vertebrate retina have also provided valuable insight into the intrinsic mechanisms underlying birth timing-dependent regulation of cell fate and wiring specificity, which has been extensively reviewed [104].
Extrinsic and stochastic regulation of neural wiring
In other neural systems, birth timing and cell lineage do not appear to tightly constrain wiring patterns, suggesting that extrinsic and/or stochastic mechanisms play a more dominant role in regulating wiring specificity in these systems. Here, we discuss how such mechanisms influence the wiring specificity of Drosophila photoreceptor cells and olfactory receptor neurons (ORNs) and mouse cerebellar granule cells (GCs).
Drosophila photoreceptors
The Drosophila retina is a powerful model system for studying cell fate specification and wiring specificity. Current models suggest that cell fate specification of Drosophila photoreceptor cells involves a series of cell-cell interactions and some stochastic processes (Fig. 2a).
Fig. 2.
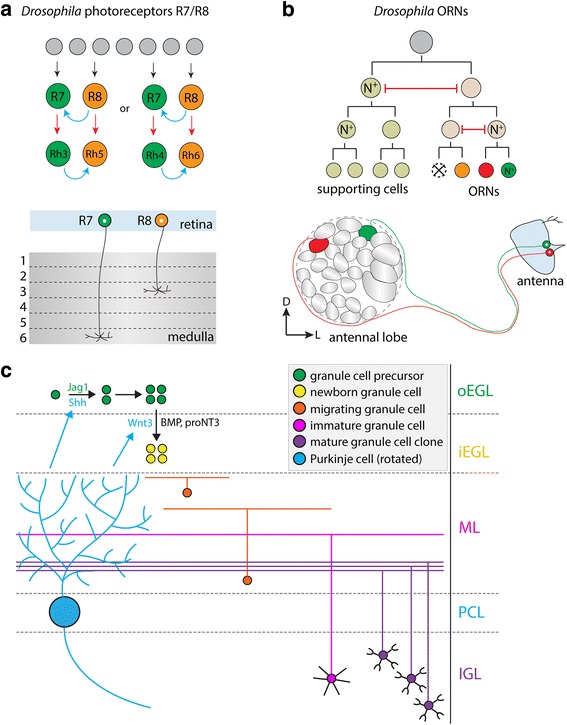
Extrinsic and stochastic regulation of neural wiring. a In the Drosophila retina, photoreceptors R7 and R8 (and R1-R6; not shown) are produced from a pool of progenitors. Cell-cell interactions (blue arrows) and stochastic mechanisms (red arrows) play critical roles in cell fate specification. Mature R7 and R8 cells project their axons to layers 6 and 3, respectively, of the medulla. Rhodopsin: Rh. b In the Drosophila olfactory receptor neuron (ORN) lineage, one progenitor cell within each sensillum undergoes several rounds of asymmetric division, giving rise to four non-neuronal supporting cells and between one and four ORNs depending on other events, such as cell death and glial fate adoption. Binary Notch signaling activation is iteratively employed, and lateral inhibition (red bars) is required for cell fate determination. Notch-ON (N+) and Notch-OFF ORNs send their axons to different glomeruli in the antennal lobe. D: dorsal; L: lateral. c In the developing mouse cerebellum, granule cell precursors (GCPs) in the outer external germinal layer (oEGL) undergo steady proliferation in a process promoted by Purkinje cell-derived Shh and GCP-derived Jag1. GCPs in the inner external germinal layer (iEGL) undergo a rapid burst of cell division before terminal differentiation, a process promoted by Wnt3 (expressed by Purkinje cells), BMP and proNT3. Migrating granule cells (GCs) then extend their parallel fiber axons into the molecular layer (ML), where they contact the dendritic arbors of developing Purkinje cells (rotated 90 degrees). Clones of mature GCs, which are born around the same time, project their parallel fiber axons to restricted depths of the ML. Parallel fibers of early-born GCs thus occupy the deepest depths of the ML while those of late-born GCs occupy the most superficial depths of the ML. PCL: Purkinje cell layer; IGL: internal granule layer
The Drosophila compound eye consists of around 800 identical units called ommatidia, and each ommatidium contains eight photoreceptors (R1-R8) arranged in a stereotyped pattern [105]. R1-R6 photoreceptors reside at the periphery of each ommatidium and project axons to the lamina, the first layer underneath the retina, where they form synaptic connections with lamina neurons. R7 and R8 photoreceptors reside in the center of the ommatidium and project their axons to the M6 and M3 layers of the medulla, the ganglion below the lamina, where they synapse with transmedullary neurons that send visual information to the lobula complex, a higher visual center. In developing ommatidia, the eight R neurons are generated in the following order: R8, R2/R5, R3/R4, R1/R6 and R7 [105, 106]. Interestingly, although eight classes of photoreceptors are produced in a fixed order, genetic mosaic analysis revealed that there is no lineage relationship between different classes [107]. These data suggest that inductive mechanisms, rather than cell lineage, specify Drosophila R cell fates. Below we review how cell-cell interactions and stochastic mechanisms specify R7 and R8 cell fates, as these cells have the best-characterized developmental mechanisms.
Two genes, sevenless and bride of sevenless (boss), are critical for R7 specification, as mutation of either leads to complete loss of R7 cells in all ommatidia [106, 108]. Mosaic analysis, allowing deletion of specific genes in one or several specific cells but not in neighboring cells, revealed more detailed mechanisms. Deletion of sevenless in non-R7 R cells does not affect the development of R7 cells, whereas deletion of sevenless in R7 cells always causes the transformation of R7 cells into non-neuronal cells, indicating that sevenless acts cell-autonomously. Conversely, boss acts cell-nonautonomously: its expression in R8 cells is indispensible for R7 development. Further molecular studies identified Boss as a 7-transmembrane ligand expressed in R8 cells, and Sevenless as a receptor tyrosine kinase expressed in R7 (and a few other cell types). Furthermore, the Ras/Raf/MAP kinase cascade acts downstream of the Sevenless receptor tyrosine kinase pathway that activates R7-specific genes [109, 110].
After R7 and R8 cells acquire their fates, cell type-specific rhodopsin (Rh) proteins are selectively expressed in those cells, allowing them to detect light of different wavelengths. Both R7 and R8 cells comprise two Rh-expressing subtypes: R7 cells can express Rh3 or Rh4 while R8 cells can express Rh5 or Rh6. These subtypes are paired precisely in ommatidia: 30% of ommatidia contain Rh3-expressing R7 paired with Rh5-expressing R8; 70% of ommatidia contain Rh4-expressing R7 paired with Rh6-expressing R8. Interestingly, the distribution of R7 subtypes seems to be regulated by the stochastic expression of transcription factor Spineless in R7 cells [111]. Spineless activates Rh4 and inhibits Rh3 expression in R7, and represses an unknown signal required to induce neighboring R8 cells to express Rh5. Conversely, Spineless-negative R7 cells express Rh3 and induce neighboring R8 cells to express Rh5. Consequently, Rh3-expressing R7 cells are always paired with Rh5-expressing R8 cells while Rh4-expressing R7 cells are always paired with Rh6-expressing R8 cells [111, 112].
As both inductive and stochastic mechanisms drive cell fate specification of Drosophila R7 and R8 cells, how, then, is cell fate specification linked to axon targeting? Several molecules have been shown to regulate R cell axon targeting, including trio, dock, Pak, insulin receptor (InR), Dscam, N-cadherin, Lar, Netrin/Frazzled, and Capricious [113–119]. While most of these factors have not been associated with cell specification mechanisms, Capricious provides an example of a molecule involved in both processes [120]. Capricious is a leucine-rich repeat transmembrane protein expressed in R8 cells but not in R7 cells. Gain- and loss-of function analyses suggest that Capricious regulates axon guidance in R8 cells. Strikingly, Capricious is activated by a transcription factor called Senseless, which is specifically expressed in R8 cells and acts as a key determinant for R8 cell fate by promoting R8-specifc rhodopsins and inhibiting R7-specific rhodopsins [120]. R7 cells express Prospero, another transcription factor, but downstream axon guidance molecules remain to be identified [120].
These findings suggest a model in which cell fate specification factors continually ensure that each cell type expresses a unique set of axon guidance molecules that drive wiring specificity. However, current studies largely focus on investigating either putative specification factors or ultimate wiring molecules. We expect that future studies integrating different techniques (see Box) will help to bridge investigation of both classes of molecules.
Drosophila olfactory receptor neurons
Olfactory receptor neurons (ORNs) are the primary sensory neurons of the Drosophila olfactory system. There are 50 classes of Drosophila ORNs (~ 1300 cells) whose cell bodies are located in the antenna or maxillary palp. Each ORN class is defined by expression of a single olfactory receptor (Or) or a unique combination of ionotropic receptors and by the glomerulus to which their axons target in the antennal lobe [121–125]. Two fundamental questions regarding the development and wiring of Drosophila ORNs remain to be addressed: How are Or genes regulated in different ORN classes? And how is Or regulation coordinated with stereotyped axon targeting? One simple solution is to use olfactory receptors to instruct the axon targeting; indeed, this strategy appears to drive the development of the mouse olfactory system [126–129]. However, it appears that Or genes do not drive axon targeting in Drosophila [130]. Below, we discuss these two events separately and then speculate about how they may be linked.
ORN specification appears to utilize a combination of intrinsic, extrinsic and stochastic mechanisms and consists of multiple sequential steps: pre-patterning of the antennal imaginal disc by larval and pupal patterning factors including Hedgehog, Wingless, and Decapentaplegic [131]; sensillar assignment by transcription factors Lozenge, Atonal and Amos [132–134]; and final specification by additional mechanisms such as lateral inhibition via Notch signaling, epigenetic processes and additional transcription factors [135–137]. Sensilla are hair structures that cover the antenna and maxillary palp and host ORNs and supporting cells. Since different sensilla and their subtypes are distributed in a stereotyped manner on the antenna and maxillary palp and are associated with specific ORN types, sensillar specification is likely controlled by intrinsic factors. However, the further specification of ORN types within individual sensilla involves extrinsic factors. Here we discuss the final step of ORN specification, which leads to Or expression.
Within each sensillum, one multipotent precursor cell undergoes several rounds of asymmetric division, giving rise to between one and four fully differentiated ORNs and four supporting cells (Fig. 2b). Binary segregation of Notch activity (ON or OFF) is iteratively used during each round of division to regulate temporal and final cell fates [138], echoing a mechanism reported to drive development of the Drosophila peripheral somatosensory system [139]. During the initial division, the Notch-ON daughter cell acquires the supporting cell precursor fate and the Notch-OFF daughter cell acquires the neuronal precursor fate. The last round of division in the neuronal precursor lineage produces two distinct ORNs, one Notch-ON and the other Notch-OFF, expressing two different olfactory receptors and sending axons to different glomeruli in the antennal lobe. Genetic activation or inhibition of Notch activity leads to generation of two Notch-ON ORNs or two Notch-OFF ORNs, respectively. For example, mutation of the Notch positive effector mastermind leads to the generation of two Notch-OFF ORNs that project to the same glomerulus. Conversely, mutation of the Notch antagonist numb results in two Notch-ON ORNs that also project to the same glomerulus. Thus, Notch signaling is required for ORN fate specification, likely through lateral inhibition [138]. The exact number of ORNs within one sensillum varies and seems to be regulated by other mechanisms, such as cell death and glial fate adoption [140]. In summary, as different ORN classes are not born sequentially, birth timing and lineage do not predict ORN fate, as with PNs and KCs; instead, fate specification of ORNs born within a single sensillum through asymmetric division of a common precursor involves Notch signaling-mediated lateral inhibition [138].
Notch signaling occurs in all sensilla, but only assigns ORNs to two classes: Notch-ON and Notch-OFF. Thus, there must be additional context-dependent factors that complement Notch signaling, providing each precursor with the potential to acquire a different fate. One possibility is that the initial or intermediate precursor cell retains an intrinsic cellular memory that Notch signaling acts on during each cell division. Indeed, two recent studies showed that a cellular memory could be imprinted upon precursors through epigenetic regulation. One study discovered that the chromatin modifier Hamlet modulates cellular responses to Notch signaling in a context-dependent manner and controls Or expression choice. Hamlet executes locus-specific modifications of histone methylation and histone density to control the accessibility of DNA binding protein at the Notch target promoter regions [141]. Another study showed that the transcriptional corepressor Atrophin regulates Or genes in Notch-ON ORNs by controlling histone 3 acetylation [142]. Thus, these data suggest that regulation of chromatin and epigenetic status provides more diverse contexts for Notch signaling to act on, allowing specification of more ORN classes. We anticipate that a more comprehensive investigation of the chromatin statuses of ORNs and their precursors, for example, at the single cell level, would greatly enhance our understanding of the epigenetic regulation of these processes.
Transcription factors also play critical roles in regulating Or expression in post-mitotic ORNs, demonstrating that intrinsic and stochastic Notch-mediated mechanisms together guide ORN specification. Acj6 was first identified via an olfactory behavioral screen in which the acj6 mutant displayed reduced jump responses to odor stimuli [143]. Acj6 is expressed in adult antenna and maxillary palp ORNs and is required for Or expression in a subset of ORN classes [144, 145]. Later work identified 13 alternative spliced isoforms of acj6, and overexpression of different isoforms in the acj6 mutant background revealed that different isoforms specify different ORNs [146]. Individual isoforms could positively or negatively regulate the expression of certain Or genes. Pdm3, another POU domain transcription factor, showed broad expression in ORNs, but is specifically required for the activation of one Or gene, Or42a [147]. Interestingly, Acj6 is also required for Or42a expression, and acj6 and pdm3 appear to genetically interact. These data suggest that a combinatorial code of different transcription factors may regulate expression of Or genes. Accordingly, another study identified six new transcription factors that, in combination with Acj6, regulate Or expression in different ORNs [148].
How do transcription factors regulate Or expression in post-mitotic ORNs? If transcription factors directly regulate expression of specific olfactory receptors, there should be binding motifs in Or promoter regions. Three lines of evidence support this idea. First, an artificial Or promoter fused to a reporter could recapitulate expression of the endogenous Or even if the promoter-fused reporter was not inserted into the endogenous locus [149], suggesting that cis-regulatory elements in the Or promoter regulate Or expression. Second, several Or promoters have been shown to share a common binding motif which could be bound by an activator or a repressor depending on the positioning of the motif within the promoter [149]. Third, a specific set of Or genes have been shown to have an acj6 binding motif [150].
Taken together, these studies suggest that ORN cell fate specification involves interplay between intrinsic, extrinsic and stochastic factors. While we have discussed how distinct mechanisms drive ORN specification, it remains unclear how these mechanisms relate to ORN axon targeting at earlier developmental stages. So far, a number of signaling pathways and molecules, including Sema-2b/PlexB and Hh signaling and N-Cadherin, Dscam, Robo, Ten-a/Ten-m and Toll-6/Toll-7, has been shown to regulate ORN axon targeting [5, 6, 151–156]. However, most of these factors have not been shown to regulate ORN fate. Interestingly, Acj6, in addition to regulating expression of certain Or genes, also regulates axon targeting of some ORN classes [157]. The exact mechanism underlying such regulation of axon guidance remains unclear and is presumably independent of regulation of Or expression. Another study reported that Notch signaling in Notch-ON ORNs suppresses the expression of Sema2b, a key regulator of ORN axon trajectory choice [152]. Since trajectory choice is a critical step in the process of ORN wiring specificity, this finding linked ORN fate determination and wiring specificity.
Many interesting questions remain: What other transcription factors independently regulate Or genes? What is the combinatorial code regulating Or expression? Are there common upstream factors that regulate both Or expression and wiring specificity molecules? We anticipate that systematic analysis of single ORN transcriptomes during development will help to address these questions.
Mammalian cerebellar granule cells
Inductive factors are well-documented to regulate differentiation, migration and wiring processes during development of the mammalian cerebellum. Like the cortex, the cerebellum is a layered structure with different cell types residing in different layers. Notably, cerebellar granule cells (GCs), small excitatory neurons packed into the internal granule cell layer, comprise over half of all neurons in mammalian brains. GCs send parallel fiber axons to the molecular layer, where they synapse onto dendritic spines studding the planar dendritic arbors of Purkinje cells, the inhibitory output projection neurons of the cerebellar cortex (Fig. 2c).
During prenatal cerebellar development, the rhombic lip generates granule cell progenitors (GCPs) that migrate to and undergo prolonged clonal expansion in the external germinal layer before exiting the cell cycle. GCPs then migrate through the developing molecular layer to form the internal granule layer, establish parallel fiber synapses with Purkinje cells and receive mossy fiber inputs via specialized dendritic claws (Fig. 2c; [158, 159]). As with cerebral cortical development, cerebellar cortical development proceeds in an “inside-out” fashion, as earlier-born GCs project their axons to deeper portions of the molecular layer and progressively later-born GCs project their axons to progressively more superficial depths [160–162]. GCP expansion seems to occur at a steady rate of around one or fewer divisions per day, followed by rapid expansion of clonally-related GCPs shortly before differentiation and migration [163].
Interestingly, single GCPs labeled at time points as early as E13.5 give rise to clones that project their axons to restricted depths of the molecular layer, indicating that these clones differentiate within a restricted time window (Fig. 2c; [164]). This finding suggests that clonally-related GCs may innervate nearby regions of a given Purkinje cell dendritic arbor [163], and while the functional significance of such lineage-related clonal axonal clustering remains unknown, one study reported spatially clustered patterns of parallel fiber activity during sensory processing that could facilitate generation of dendritic spikes, nonlinear postsynaptic calcium signaling and synaptic plasticity in Purkinje cells [165]. While the axons of GCs born around the same time project to restricted depths of the molecular layer, it remains unknown whether or not clonally- or birth timing-related GCs receive common mossy fiber inputs. To address this question, future studies should develop strategies to access early- and late-born granule cells and characterize their mossy fiber inputs.
Several secreted factors have been shown to regulate GCP differentiation, and thus to regulate the depth to which progeny GCs project their axons. One of the best-studied factors is Purkinje cell-derived sonic hedgehog (Shh), which serves to prolong GCP proliferation and inhibit GC differentiation [166–168]. Mutations in Shh and its downstream effectors have been observed in various forms of pediatric medulloblastoma, the most common pediatric brain tumor, which is caused by GCP over-proliferation. Shh signals via its canonical receptor Ptch1 and coreceptors Boc/Cdon and Gas1, which release Smo signaling in GCPs, leading to transcriptional activation via transcription factors Gli1 and Gli2 [169–172]. Additionally, in vitro studies revealed that GCP-derived Jag1 activates Notch2 signaling, which also supports proliferation [173].
Additionally, in vitro studies have identified secreted factors that promote GCP differentiation and migration. For example, BMP signaling inhibits GCP proliferation in vitro and induces differentiation by proteasome-mediated degradation of Math1, a transcription factor active in proliferating GCPs, and this signaling is disrupted in mouse models of medulloblastoma [174]. Wnt3, which is expressed in developing and adult Purkinje cells [175], also suppresses GCP proliferation and inhibits medulloblastoma growth, and does so by inhibiting transcriptional responsivity to both Shh and Math1 [176]. Interestingly, Wnt3 expression in Purkinje cells increases postnatally and is lost in mutants lacking GCs, implying that Wnt3 expression depends on interactions between GCs and Purkinje cells [175]. Finally, proNT3 promotes differentiation by inhibiting Shh-induced proliferation following activation of the pan-neurotrophin receptor p75 [177]. In vitro studies showed that proNT3 prevents Shh-induced proliferation of GCPs and upregulation of Shh pathways and genetic deletion of p75 in GCPs resulted in increased GCP proliferation [177]. However, the cellular source of the proNT3 required for this process remains unclear. Interestingly, GC-derived NT3 is also required for proper Purkinje cell dendritic morphogenesis [178], highlighting the multifunctionality of NT3 signaling in cerebellar development. Taken together, these studies reveal several secreted factors that promote GCP differentiation and migration yet primarily feature in vitro experiments, leaving the cellular sources of these factors indeterminate [179–182]. In the future, in vivo loss-of-function experiments utilizing cell-type specific Cre lines and floxed genes should be performed to recapitulate reported in vitro phenotypes.
Thus, various extracellular factors secreted by a variety of sources have been shown to regulate GC proliferation and differentiation, and thus also birth timing and axonal projection depth, as clonally-related GCs exit the cell cycle around the same time and thus also project their axons to restricted depths of the molecular layer. Specifically, these studies suggest that GCPs, unlike cortical progenitors, which divide asymmetrically, resulting in specification of postmitotic cell position and wiring based largely on birth timing (see transplantation studies described above), are highly sensitive to various local environmental cues secreted by Purkinje cells and GCPs themselves. Such cues either positively or negatively regulate GCP proliferation and differentiation, and future studies should focus on unambiguously identifying the cellular sources of these signals and the corresponding upstream mechanisms that in turn regulate activation of these signals.
Box: Methods for lineage tracing in developing neural circuits
To address the role neuronal lineage plays in establishing wiring specificity in a developing neural circuit, neurons belonging to a specific lineage must be unambiguously marked at specific developmental stages, enabling subsequent characterization of neuronal morphology and wiring. Moreover, gene disruption in a targeted neuronal population allows researchers to address the molecular mechanisms underlying circuit assembly. Here, we review several powerful approaches for lineage tracing in developing neural circuits and discuss how these may be combined with emerging methods for characterizing circuit organization.
Pioneering techniques for neuronal lineage tracing include tissue transplantation and retroviral labeling [57, 183–187]. Prior to the development of genetic approaches, tissue transplantation allowed tracking of neural fates in developing nervous systems in situ. However, transplantation studies often require complicated, invasive embryonic surgical manipulations, limiting their resolution, flexibility, and applicability. Retroviral labeling strategies feature a retrovirus that infects a neuroblast and integrates its own genome into the host cell’s genome, resulting in inheritance of the viral payload by all progeny in the cell’s lineage. Recent approaches to retroviral labeling often utilize barcoded sequences as cell markers, expanding the throughput of viral lineage tracing and minimizing the likelihood of false clonal assignment. Consequently, retroviral labeling is still widely used for tracing neuronal lineage in developing mammalian neural systems.
Prototypical and subsequent genetic methods for clonal labeling have predominantly relied upon enzymatic DNA recombination by, most commonly, Flp and Cre recombinases. This recombination consists of removal of transcriptional terminator sequences flanked by unidirectional recognition target sequences (FRT and lox variants, respectively) or inversion of such sequences flanking an inverted reporter gene ORF, resulting in expression of reporter genes such as β-galactosidase (β-gal) or fluorescent proteins (Fig. 3a). DNA recombination is thus a simple and powerful genetic trick used widely in both invertebrate and vertebrate genetic model organisms for neuronal lineage tracing [188–196].
Fig. 3.
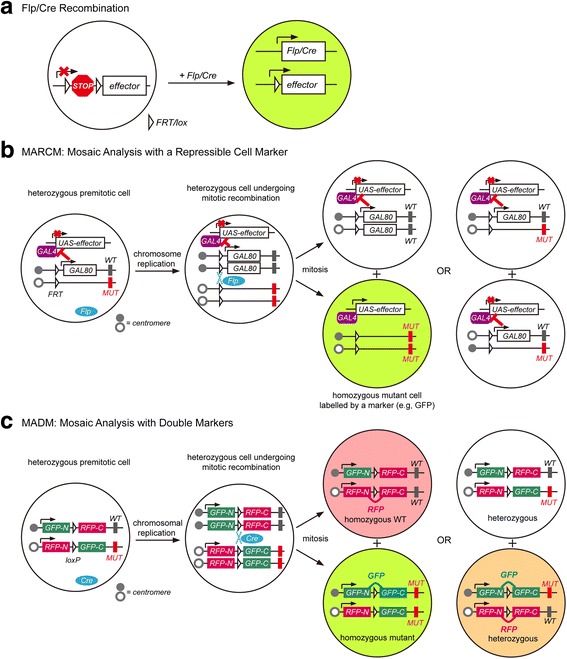
Genetic strategies for lineage analysis. a A transcriptional terminator (STOP) flanked by unidirectional FRT/lox sites blocks the expression of an effector/reporter gene such as GFP. In the cell population expressing Flp/Cre, the recombinase removes the terminator sequence to activate effector/reporter expression. b MARCM uses GAL80 to suppress marker expression driven by the GAL4-UAS binary expression system. The wild-type (WT), but not mutant (MUT), allele of the gene of interest is linked with GAL80. After Flp-mediated mitotic recombination, only the homozygous MUT progeny lose GAL80 and are labelled by marker gene expression. c In the original MADM configuration, N-terminal and C-terminal coding regions of GFP and RFP are segregated on homologous chromosomes. Cre-mediated mitotic recombination reconstitutes these coding regions to generate four distinct types of progeny (GFP+ only, RFP+ only, GFP+/RFP+ double-positive and unlabeled), in which fluorescent labeling corresponds to cellular genotype
Many improvements have been made to basic recombinase-based strategies. For example, while many early genetic strategies relied on β-gal expression, which allows for sensitive, robust histological labeling of clones, β-gal localizes mostly to neuronal somata and does not robustly label axons and dendrites. Fusing the coding sequence of tau, a microtubule binding protein, to β-gal results in improved axonal labeling [197, 198]. Furthermore, fluorescent proteins such as GFP and tdT diffuse more easily into neuronal processes, and their membrane-tethered derivatives, such as mCD8-GFP and mtdT, diffuse profusely into neuronal processes due to the high surface area-to-volume ratios of these compartments [20, 189], allowing single-process resolution mapping of neuronal morphology. Recombinase activity can also be targeted to specific cell populations and developmental timepoints. For example, Flp/Cre expression driven by specific enhancers, promoters and genetic loci allows genetic access to targeted cell populations. Additionally, Flp expression driven by a heat shock promoter (hs-Flp) in Drosophila allows control over the time window and scale of clonal induction by heat shock at different time points and with varying durations. Analogous temporal control over Cre recombinase activity can be achieved using the estrogen receptor-fused Cre (CreER) and specifying injection times and agonist dosages [199]. Moreover, recombinase-based intersectional methods allow greater genetic specificity, thus enhancing the resolution of neuronal fate mapping [200–203]. Finally, recombinase-based clonal labeling strategies that combine mosaic genetic analysis and lineage tracing, such as mosaic analysis with a repressible cell marker (MARCM) in Drosophila [20] and mosaic analysis with double markers (MADM) in mice [164], are widely used to study developing neural circuits.
MARCM takes advantage of the yeast binary expression system GAL4/UAS, in which expression of GAL4 protein results in expression of a genetic element downstream of the upstream activator sequence (UAS), and the corresponding suppressor protein GAL80, as well as Flp/FRT-mediated inter-chromosomal mitotic recombination, to generate genetically distinct daughter cells/clones: homozygous mutant cells lack GAL80 while heterozygous and homozygous wild-type (WT) cells express GAL80. Thus, expression of the marker protein driven by UAS can be limited to the mutant homozygous lineage (Fig. 3b), allowing mosaic analysis of neuronal morphology and wiring [12, 20, 21, 23, 204]. Several MARCM variants exist, including reverse MARCM, in which most cells have a given gene disruption and only a few, labeled cells remain wildtype [205]; twin-spot MARCM, in which clones of interest and sister clones are visualized with complementary fluorescent markers [21]; and Q-MARCM, which uses the Q repressible binary system orthogonal to the GAL4/UAS system [206, 207]. MARCM has been used extensively for sparse and single-cell labeling for clonal analysis, as well as dissection of cell-autonomous and non-cell-autonomous gene functions. Since various GAL4 and Flp driver lines can specify the cell-type and/or developmental stage MARCM targets, MARCM affords significant cell-type specificity and temporal resolution, and thus great flexibility for use in various Drosophila neural systems to study circuit assembly in WT conditions and to assess gene function in development, given the abundance of GAL4 and Flp driver lines available to the Drosophila community.
MADM utilizes mitotic inter-chromosomal recombination for reconstitution of the coding regions of two distinct effector genes that are inherited by separate sister cells. These genes are typically fluorescent proteins that allow generation of a color code representing the genetic status of subsequent daughter cells or clones; in the original MADM6 configuration, for example, homozygous mutant cells are green, homozygous WT cells are red and heterozygous cells are either yellow or unlabeled (Fig. 3c) [164, 208]. Thus, this technique allows cell-autonomous analysis of gene disruptions in sparsely labeled cells expressing one of two fluorescent reporters (e.g. GFP and tdTomato). Since MADM requires two different gene cassettes to be inserted into homologous chromosomal loci near centromeres, it is limited to genes distal to these cassettes on chromosomes into which these cassettes have been integrated, with corresponding MADM mice generated. MADM-mediated clonal analysis is often accomplished using CreER driver lines and providing pulses of tamoxifen or its chemical analogs at specific developmental stages. This adaptation increases temporal control over MADM-mediated clonal labeling and genetic manipulations. Moreover, use of different Cre lines extends cell-type specificity to MADM. Finally, MADM alleles may also express effector genes, such as the tetracycline transactivator protein, instead of fluorescent markers, allowing, for example, simultaneous generation of a lineage misexpressing a gene of interest and a homozygous mutant sibling lineage [208]. MADM has been applied to study a variety of developing neural structures including the developing cortex, hippocampus, thalamus, cerebellum and enteric nervous system [78, 98, 163, 178, 208–219], as well as adult neural stem cells [220]. Finally, mice are being generated to allow MADM access to all autosomes (S. Hippenmeyer, personal communications).
Following labeling and genetic manipulation of a given lineage, assessment of neuronal wiring can take various forms. Fluorescent imaging and physiological recording are common and complementary ways to characterize neuronal wiring patterns. Live imaging can also be applied to monitor real-time dynamics of a labelled lineage [104, 221–224]. Multicolor stochastic labeling methods, such as Brainbow, dBrainbow and MCFO, allow analysis of neuronal network architecture on a large scale [225–229]. Recent innovations in light-sheet microscopy, tissue clearing techniques and image processing and registration enable performance of automated, high-throughput reconstruction in intact mouse brains [230–244]. These new technologies may allow detection and characterization of clones following extremely sparse clonal labeling and thus may eclipse traditional, more laborious methods in large volume tissue samples. Using a barcode-sequencing strategy, two recent studies achieved large-scale lineage mapping in vivo [245, 246], which could be coupled with emerging in situ RNA sequencing methods [247, 248] to enable brain-wide profiling of neuronal lineages and connections. In addition to anatomical analysis, in vivo functional imaging with genetically encoded calcium and voltage sensors has been widely used to study neuronal physiology [80, 249–255], offering additional means to address the functional association of sibling neurons, in additional to more traditional physiological approaches [79, 81]. Moreover, single-cell RNA sequencing has been applied to developing brains to identify molecular signatures of different types of neurons and their transcriptomic dynamics [22, 256–258], allowing systematic investigation of how neuronal lineage defines the molecular consortium controlling wiring specificity. Combining advanced genetic strategies with scalable profiling techniques provides an unprecedented opportunity to discover new principles of lineage-dependent neural circuit assembly.
Summary and perspectives
Here we have discussed how neuronal lineage contributes to neural cell fate and wiring specificity in six different neuronal systems in Drosophila and mice. From birth to synaptic communication with appropriate upstream and downstream partners, a given neuron undergoes multiple steps to integrate into a functional neural circuit. Different neural systems have been observed to utilize different combinations of distinct intrinsic, extrinsic and stochastic mechanisms. Such a diversity of developmental mechanisms should be expected, given the diversity of information processing requirements these host neural systems attend to, and current investigations should both anticipate and appreciate discovery of new mechanisms that further enhance our understanding of these processes.
Understanding the mechanisms underlying neural cell fate specification and wiring specificity will be key to understanding how the brain develops and functions. While the diverse neural systems investigated have allowed discovery of a diversity of fate specification and wiring specificity mechanisms, undoubtedly many more remain undiscovered. Due to the complexity of the nervous system, most studies have focused on either how cell fate is specified within a lineage or how wiring patterns are established. Thus, future studies should aim to link these levels of analysis, and modern genetic tools combined with molecular profiling and anatomical characterization techniques should catalyze discovery of new mechanisms and principles underlying regulation of these developmental processes.
Conclusion
Not applicable.
Acknowledgements
We thank Jan Lui, Tongchao Li and Daniel Pederick for helpful comments on the manuscript. Hongjie Li is a Stanford Neuroscience Institute Interdisciplinary Scholar. S. Andrew Shuster is a Stanford Graduate Fellow and a National Science Foundation Graduate Research Fellow. Jiefu Li is a Vanessa Kong Kerzner Graduate Fellow and a Genentech Foundation Predoctoral Fellow. Liqun Luo is an HHMI investigator. Supported by NIH grants R01-DC005982 and R01-NS050580 to L.L.
Funding
This study is supported by funds from the Howard Hughes Medical Institute (Liqun Luo), Simons Foundation (Liqun Luo), National Science Foundation (Liqun Luo) and National Institutes of Health (Liqun Luo).
Availability of data and materials
Not applicable
Abbreviations
- adPN
Anterodoral projection neuron
- GC
Granule cell
- GCP
Granule cell progenitor
- KC
Kenyon cell
- lPN
Lateral projection neuron
- MADM
Mosaic analysis with double markers
- MARCM
Mosaic analysis with a repressible cell marker
- MB
Mushroom body
- Or
Olfactory receptor
- ORN
Olfactory receptor neuron
- PN
Projection neuron
- RG
Radial glia
- vPN
Ventral projection neuron
Authors’ contributions
HL, SAS, JL, and LL wrote the manuscript together. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongjie Li, Email: hongjie@stanford.edu.
S. Andrew Shuster, Email: sshuster@stanford.edu.
Jiefu Li, Email: jiefuli@stanford.edu.
Liqun Luo, Email: lluo@stanford.edu.
References
- 1.Jan Y-N, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed]
- 3.Kohwi M, Doe CQ. Temporal fate specification and neural progenitor competence during development. Nat Rev Neurosci. 2013;14:823–838. doi: 10.1038/nrn3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Hong W, Luo L. Genetic control of wiring specificity in the fly olfactory system. Genetics. 2014;196:17–29. doi: 10.1534/genetics.113.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong W, Mosca TJ, Luo L. Teneurins instruct synaptic partner matching in an olfactory map. Nature. 2012;484:201–207. doi: 10.1038/nature10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yogev S, Shen K. Cellular and molecular mechanisms of synaptic specificity. Annu Rev Cell Dev Biol. 2014;30:417–437. doi: 10.1146/annurev-cellbio-100913-012953. [DOI] [PubMed] [Google Scholar]
- 8.Su C-Y, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–241. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- 12.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 13.Marin EC, Jefferis GSXE, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 14.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 15.Jefferis GSXE, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H-H, Kao C-F, He Y, Ding P, Kao J-C, Lee T. A complete developmental sequence of a Drosophila neuronal lineage as revealed by twin-spot MARCM. PLoS Biol. 2010;8 10.1371/journal.pbio.1000461. [DOI] [PMC free article] [PubMed]
- 17.Lin S, Kao C-F, Yu H-H, Huang Y, Lee T. Lineage analysis of Drosophila lateral antennal lobe neurons reveals notch-dependent binary temporal fate decisions. PLoS Biol. 2012;10:e1001425. doi: 10.1371/journal.pbio.1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, et al. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79:917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S, Lai S-L, Yu H-H, Chihara T, Luo L, Lee T. Lineage-specific effects of notch/numb signaling in post-embryonic development of the Drosophila brain. Development. 2010;137:43–51. doi: 10.1242/dev.041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 21.Yu H-H, Chen C-H, Shi L, Huang Y, Lee T. Twin-spot MARCM to reveal the developmental origin and identity of neurons. Nat Neurosci. 2009;12:947–953. doi: 10.1038/nn.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Horns F, Wu B, Xie Q, Li J, Li T, et al. Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell. 2017;171:1206–1220.e22. doi: 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komiyama T, Johnson WA, Luo L, Jefferis GSXE. From lineage to wiring specificity. Cell. 2003;112:157–167. doi: 10.1016/s0092-8674(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 24.Komiyama T, Luo L. Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr Biol. 2007;17:278–285. doi: 10.1016/j.cub.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 25.Zhu S, Lin S, Kao C-F, Awasaki T, Chiang A-S, Lee T. Gradients of the Drosophila Chinmo BTB-zinc finger protein govern neuronal temporal identity. Cell. 2006;127:409–422. doi: 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Yang C-P, Sugino K, Fu C-C, Liu L-Y, Yao X, et al. Opposing intrinsic temporal gradients guide neural stem cell production of varied neuronal fates. Science. 2015;350:317–320. doi: 10.1126/science.aad1886. [DOI] [PubMed] [Google Scholar]
- 27.Ren Q, Yang C-P, Liu Z, Sugino K, Mok K, He Y, et al. Stem cell-intrinsic, seven-up-triggered temporal factor gradients diversify intermediate neural progenitors. Curr Biol. 2017;27:1303–1313. doi: 10.1016/j.cub.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 29.Séjourné J, Plaçais P-Y, Aso Y, Siwanowicz I, Trannoy S, Thoma V, et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci. 2011;14:903–910. doi: 10.1038/nn.2846. [DOI] [PubMed] [Google Scholar]
- 30.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 31.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, T-TB N, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 34.Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 38.Eichler K, Li F, Litwin-Kumar A, Park Y, Andrade I, Schneider-Mizell CM, et al. The complete connectome of a learning and memory centre in an insect brain. Nature. 2017;548:175–182. doi: 10.1038/nature23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waddell S. Reinforcement signalling in Drosophila; dopamine does it all after all. Curr Opin Neurobiol. 2013;23:324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- 41.Brody T, Odenwald WF. Cellular diversity in the developing nervous system: a temporal view from Drosophila. Development. 2002;129:3763–3770. doi: 10.1242/dev.129.16.3763. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, et al. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature. 2013;498:456–462. doi: 10.1038/nature12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki T, Kaido M, Takayama R, Sato M. A temporal mechanism that produces neuronal diversity in the Drosophila visual center. Dev Biol. 2013;380:12–24. doi: 10.1016/j.ydbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 45.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Custo Greig LF, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci. 2013;14:755–769. doi: 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao P, Sultan KT, Zhang X-J, Shi S-H. Lineage-dependent circuit assembly in the neocortex. Development. 2013;140:2645–2655. doi: 10.1242/dev.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 49.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 50.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 51.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 52.Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch Neurol. 2007;64:639–642. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- 53.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molyneaux BJ, Arlotta P, Fame RM, MacDonald JL, MacQuarrie KL, Macklis JD. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McConnell SK. Migration and differentiation of cerebral cortical neurons after transplantation into the brains of ferrets. Science. 1985;229:1268–1271. doi: 10.1126/science.4035355. [DOI] [PubMed] [Google Scholar]
- 57.McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 59.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 60.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 61.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 62.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 63.Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- 64.Price J, Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988;104:473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- 65.Reid CB, Liang I, Walsh C. Systematic widespread clonal organization in cerebral cortex. Neuron. 1995;15:299–310. doi: 10.1016/0896-6273(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 66.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 68.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 69.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen J-G, Rasin M-R, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 73.Zimmer C, Tiveron M-C, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 74.Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, et al. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JLR, Chen B. Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron. 2013;80:1167–1174. doi: 10.1016/j.neuron.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gil-Sanz C, Müller U. A new chapter in the life of cajal’s short-axon neurons: separation of interneuron siblings after birth. Neuron. 2015;87:909–911. doi: 10.1016/j.neuron.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 77.Eckler MJ, Nguyen TD, McKenna WL, Fastow BL, Guo C, Rubenstein JLR, et al. Cux2-positive radial glial cells generate diverse subtypes of neocortical projection neurons and macroglia. Neuron. 2015;86:1100–1108. doi: 10.1016/j.neuron.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, et al. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell. 2014;159:775–788. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu Y-C, Bultje RS, Wang X, Shi S-H. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Lu H, Cheng P, Ge S, Xu H, Shi S-H, et al. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature. 2012;486:118–121. doi: 10.1038/nature11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu Y-C, He S, Chen S, Fu Y, Brown KN, Yao X-H, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012;486:113–117. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peinado A, Yuste R, Katz LC. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- 83.Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 1993;3:488–498. doi: 10.1093/cercor/3.5.488. [DOI] [PubMed] [Google Scholar]
- 84.Yuste R, Nelson DA, Rubin WW, Katz LC. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron. 1995;14:7–17. doi: 10.1016/0896-6273(95)90236-8. [DOI] [PubMed] [Google Scholar]
- 85.Kandler K, Katz LC. Relationship between dye coupling and spontaneous activity in developing ferret visual cortex. Dev Neurosci. 1998;20:59–64. doi: 10.1159/000017299. [DOI] [PubMed] [Google Scholar]
- 86.Ohtsuki G, Nishiyama M, Yoshida T, Murakami T, Histed M, Lois C, et al. Similarity of visual selectivity among clonally related neurons in visual cortex. Neuron. 2012;75:65–72. doi: 10.1016/j.neuron.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 87.Smith GB, Fitzpatrick D. Specifying cortical circuits: a role for cell lineage. Neuron. 2012;75:4–5. doi: 10.1016/j.neuron.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko H, Hofer SB, Pichler B, Buchanan KA, Sjöström PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cossell L, Iacaruso MF, Muir DR, Houlton R, Sader EN, Ko H, et al. Functional organization of excitatory synaptic strength in primary visual cortex. Nature. 2015;518:399–403. doi: 10.1038/nature14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harris KD, Mrsic-Flogel TD. Cortical connectivity and sensory coding. Nature. 2013;503:51–58. doi: 10.1038/nature12654. [DOI] [PubMed] [Google Scholar]
- 91.Lee W-CA, Bonin V, Reed M, Graham BJ, Hood G, Glattfelder K, et al. Anatomy and function of an excitatory network in the visual cortex. Nature. 2016;532:370–374. doi: 10.1038/nature17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, et al. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 94.Bandler RC, Mayer C, Fishell G. Cortical interneuron specification: the juncture of genes, time and geometry. Curr Opin Neurobiol. 2017;42:17–24. doi: 10.1016/j.conb.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown KN, Chen S, Han Z, Lu C-H, Tan X, Zhang X-J, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marín O. Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci. 2013;16:1199–1210. doi: 10.1038/nn.3485. [DOI] [PubMed] [Google Scholar]
- 97.Harwell CC, Fuentealba LC, Gonzalez-Cerrillo A, Parker PRL, Gertz CC, Mazzola E, et al. Wide dispersion and diversity of clonally related inhibitory interneurons. Neuron. 2015;87:999–1007. doi: 10.1016/j.neuron.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayer C, Jaglin XH, Cobbs LV, Bandler RC, Streicher C, Cepko CL, et al. Clonally related forebrain interneurons disperse broadly across both functional areas and structural boundaries. Neuron. 2015;87:989–998. doi: 10.1016/j.neuron.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sultan KT, Han Z, Zhang X-J, Xianyu A, Li Z, Huang K, et al. Clonally related gabaergic interneurons do not randomly disperse but frequently form local clusters in the forebrain. Neuron. 2016;92:31–44. doi: 10.1016/j.neuron.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turrero García M, Mazzola E, Harwell CC. Lineage relationships do not drive MGE/PoA-derived interneuron clustering in the brain. Neuron. 2016;92:52–58. doi: 10.1016/j.neuron.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mayer C, Bandler RC, Fishell G. Lineage is a poor predictor of interneuron positioning within the forebrain. Neuron. 2016;92:45–51. doi: 10.1016/j.neuron.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Butt SJB, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 103.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJB, Battiste J, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neurosci. 2014;15:615–627. doi: 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- 105.Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- 107.Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 108.Reinke R, Zipursky SL. Cell-cell interaction in the Drosophila retina: the bride of sevenless gene is required in photoreceptor cell R8 for R7 cell development. Cell. 1988;55:321–330. doi: 10.1016/0092-8674(88)90055-4. [DOI] [PubMed] [Google Scholar]
- 109.Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991;67:701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- 110.Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–397. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
- 111.Wernet MF, Mazzoni EO, Çelik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnston RJ, Desplan C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu Rev Cell Dev Biol. 2010;26:689–719. doi: 10.1146/annurev-cellbio-100109-104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 114.Garrity PA, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky SL. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85:639–650. doi: 10.1016/s0092-8674(00)81231-3. [DOI] [PubMed] [Google Scholar]
- 115.Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 116.Song J, Wu L, Chen Z, Kohanski RA, Pick L. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- 117.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 118.Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 119.Gong Q, Rangarajan R, Seeger M, Gaul U. The netrin receptor frazzled is required in the target for establishment of retinal projections in the Drosophila visual system. Development. 1999;126:1451–1456. doi: 10.1242/dev.126.7.1451. [DOI] [PubMed] [Google Scholar]
- 120.Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 122.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 123.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 124.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GSXE, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feinstein P, Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–831. doi: 10.1016/j.cell.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 127.Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314:657–661. doi: 10.1126/science.1131794. [DOI] [PubMed] [Google Scholar]
- 128.Nakashima A, Takeuchi H, Imai T, Saito H, Kiyonari H, Abe T, et al. Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell. 2013;154:1314–1325. doi: 10.1016/j.cell.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- 130.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 131.Royet J, Finkelstein R. Establishing primordia in the Drosophila eye-antennal imaginal disc: the roles of decapentaplegic, wingless and hedgehog. Development. 1997;124:4793–4800. doi: 10.1242/dev.124.23.4793. [DOI] [PubMed] [Google Scholar]
- 132.Gupta BP, Flores GV, Banerjee U, Rodrigues V. Patterning an epidermal field: Drosophila lozenge, a member of the AML-1/runt family of transcription factors, specifies olfactory sense organ type in a dose-dependent manner. Dev Biol. 1998;203:400–411. doi: 10.1006/dbio.1998.9064. [DOI] [PubMed] [Google Scholar]
- 133.Gupta BP, Rodrigues V. Atonal is a proneural gene for a subset of olfactory sense organs in Drosophila. Genes Cells. 1997;2:225–233. doi: 10.1046/j.1365-2443.1997.d01-312.x. [DOI] [PubMed] [Google Scholar]
- 134.Goulding SE, Zur Lage P, Jarman AP. Amos, a proneural gene for Drosophila olfactory sense organs that is regulated by lozenge. Neuron. 2000;25:69–78. doi: 10.1016/s0896-6273(00)80872-7. [DOI] [PubMed] [Google Scholar]
- 135.Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet. 2004;20:453–459. doi: 10.1016/j.tig.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 136.Rodrigues V, Hummel T. Development of the Drosophila olfactory system. Adv Exp Med Biol. 2008;628:82–101. doi: 10.1007/978-0-387-78261-4_6. [DOI] [PubMed] [Google Scholar]
- 137.Barish S, Volkan PC. Mechanisms of olfactory receptor neuron specification in Drosophila. Wiley Interdiscip Rev Dev Biol. 2015;4:609–621. doi: 10.1002/wdev.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Endo K, Aoki T, Yoda Y, Kimura K, Hama C. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat Neurosci. 2007;10:153–160. doi: 10.1038/nn1832. [DOI] [PubMed] [Google Scholar]
- 139.Jan YN, Jan LY. Genetic control of cell fate specification in Drosophila peripheral nervous system. Annu Rev Genet. 1994;28:373–393. doi: 10.1146/annurev.ge.28.120194.002105. [DOI] [PubMed] [Google Scholar]
- 140.Sen A, Kuruvilla D, Pinto L, Sarin A, Rodrigues V. Programmed cell death and context dependent activation of the EGF pathway regulate gliogenesis in the Drosophila olfactory system. Mech Dev. 2004;121:65–78. doi: 10.1016/j.mod.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Endo K, Karim MR, Taniguchi H, Krejci A, Kinameri E, Siebert M, et al. Chromatin modification of notch targets in olfactory receptor neuron diversification. Nat Neurosci. 2011;15:224–233. doi: 10.1038/nn.2998. [DOI] [PubMed] [Google Scholar]
- 142.Alkhori L, Öst A, Alenius M. The corepressor Atrophin specifies odorant receptor expression in Drosophila. FASEB J. 2014;28:1355–1364. doi: 10.1096/fj.13-240325. [DOI] [PubMed] [Google Scholar]
- 143.Ayer RK, Carlson J. Acj6: a gene affecting olfactory physiology and behavior in Drosophila. Proc Natl Acad Sci U S A. 1991;88:5467–5471. doi: 10.1073/pnas.88.12.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ayer RK, Carlson J. Olfactory physiology in the Drosophila antenna and maxillary palp: acj6 distinguishes two classes of odorant pathways. J Neurobiol. 1992;23:965–982. doi: 10.1002/neu.480230804. [DOI] [PubMed] [Google Scholar]
- 145.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 146.Bai L, Carlson JR. Distinct functions of acj6 splice forms in odor receptor gene choice. J Neurosci. 2010;30:5028–5036. doi: 10.1523/JNEUROSCI.6292-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tichy AL, Ray A, Carlson JR. A new Drosophila POU gene, pdm3, acts in odor receptor expression and axon targeting of olfactory neurons. J Neurosci. 2008;28:7121–7129. doi: 10.1523/JNEUROSCI.2063-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jafari S, Alkhori L, Schleiffer A, Brochtrup A, Hummel T, Alenius M. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLoS Biol. 2012;10:e1001280. doi: 10.1371/journal.pbio.1001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miller CJ, Carlson JR. Regulation of odor receptor genes in trichoid sensilla of the Drosophila antenna. Genetics. 2010;186:79–95. doi: 10.1534/genetics.110.117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee M-H, Salvaterra PM. Abnormal chemosensory jump 6 is a positive transcriptional regulator of the cholinergic gene locus in Drosophila olfactory neurons. J Neurosci. 2002;22:5291–5299. doi: 10.1523/JNEUROSCI.22-13-05291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ward A, Hong W, Favaloro V, Luo L. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron. 2015;85:1013–1028. doi: 10.1016/j.neuron.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Joo WJ, Sweeney LB, Liang L, Luo L. Linking cell fate, trajectory choice, and target selection: genetic analysis of Sema-2b in olfactory axon targeting. Neuron. 2013;78:673–686. doi: 10.1016/j.neuron.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chou Y-H, Zheng X, Beachy PA, Luo L. Patterning axon targeting of olfactory receptor neurons by coupled hedgehog signaling at two distinct steps. Cell. 2010;142:954–966. doi: 10.1016/j.cell.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006;9:349–355. doi: 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- 155.Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
- 156.Jhaveri D, Saharan S, Sen A, Rodrigues V. Positioning sensory terminals in the olfactory lobe of Drosophila by Robo signaling. Development. 2004;131:1903–1912. doi: 10.1242/dev.01083. [DOI] [PubMed] [Google Scholar]
- 157.Komiyama T, Carlson JR, Luo L. Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nat Neurosci. 2004;7:819–825. doi: 10.1038/nn1284. [DOI] [PubMed] [Google Scholar]
- 158.Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. Boca Raton: CRC. 1996.
- 159.Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, et al. Consensus paper: cerebellar development. Cerebellum. 2016;15:789–828. doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–397. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 161.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 162.Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–513. [DOI] [PubMed]
- 163.Espinosa JS, Luo L. Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J Neurosci. 2008;28:2301–2312. doi: 10.1523/JNEUROSCI.5157-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 165.Wilms CD, Häusser M. Reading out a spatiotemporal population code by imaging neighbouring parallel fibre axons in vivo. Nat Commun. 2015;6:6464. doi: 10.1038/ncomms7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 167.Dahmane N, Ruiz i, Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 168.Wallace VA. Purkinje-cell-derived sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 169.Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- 170.Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nat Rev Genet. 2006;7:841–850. doi: 10.1038/nrg1969. [DOI] [PubMed] [Google Scholar]
- 171.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- 172.Izzi L, Lévesque M, Morin S, Laniel D, Wilkes BC, Mille F, et al. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 174.Zhao H, Ayrault O, Zindy F, Kim J-H, Roussel MF. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 2008;22:722–727. doi: 10.1101/gad.1636408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Salinas PC, Fletcher C, Copeland NG, Jenkins NA, Nusse R. Maintenance of Wnt-3 expression in Purkinje cells of the mouse cerebellum depends on interactions with granule cells. Development. 1994;120:1277–1286. doi: 10.1242/dev.120.5.1277. [DOI] [PubMed] [Google Scholar]
- 176.Anne SL, Govek E-E, Ayrault O, Kim JH, Zhu X, Murphy DA, et al. WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation. PLoS One. 2013;8:e81769. doi: 10.1371/journal.pone.0081769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zanin JP, Abercrombie E, Friedman WJ. Proneurotrophin-3 promotes cell cycle withdrawal of developing cerebellar granule cell progenitors via the p75 neurotrophin receptor. elife. 2016;5 10.7554/eLife.16654. [DOI] [PMC free article] [PubMed]
- 178.Joo W, Hippenmeyer S, Luo L. Dendrite morphogenesis depends on relative levels of NT-3/TrkC signaling. Science. 2014;346:626–9. [DOI] [PMC free article] [PubMed]
- 179.Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, et al. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature. 2017;545:350–354. doi: 10.1038/nature22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, et al. Netrin1 produced by neural progenitors, not floor plate cells, is required for axon guidance in the spinal cord. Neuron. 2017;94:790–799.e3. doi: 10.1016/j.neuron.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamauchi K, Yamazaki M, Abe M, Sakimura K, Lickert H, Kawasaki T, et al. Netrin-1 derived from the ventricular zone, but not the floor plate, directs hindbrain commissural axons to the ventral midline. Sci Rep. 2017;7:11992. doi: 10.1038/s41598-017-12269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hand RA, Kolodkin AL. Netrin-mediated axon guidance to the CNS midline revisited. Neuron. 2017;94:691–693. doi: 10.1016/j.neuron.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 183.Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- 184.Hallonet MER, Douarin NM. Tracing Neuroepithelial cells of the mesencephalic and Metencephalic alar plates during cerebellar ontogeny in quail - chick chimaeras. Eur J Neurosci. 1993;5:1145–1155. doi: 10.1111/j.1460-9568.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 185.Cepko C, Ryder EF, Austin CP, Walsh C, Fekete DM. Lineage analysis using retrovirus vectors. Meth Enzymol. 1995;254:387–419. doi: 10.1016/0076-6879(95)54027-x. [DOI] [PubMed] [Google Scholar]
- 186.Sanes JR. Analysing cell lineage with a recombinant retrovirus. Trends Neurosci. 1989;12:21–28. doi: 10.1016/0166-2236(89)90152-5. [DOI] [PubMed] [Google Scholar]
- 187.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O’Gorman S, Fox DT, Wahl GM. Recombinase-mediated gene activation and site-specific integration in mammalian cells. Science. 1991;251:1351–1355. doi: 10.1126/science.1900642. [DOI] [PubMed] [Google Scholar]
- 189.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 190.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hashimoto M, Mikoshiba K. Neuronal birthdate-specific gene transfer with adenoviral vectors. J Neurosci. 2004;24:286–296. doi: 10.1523/JNEUROSCI.2529-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci U S A. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rodriguez CI, Dymecki SM. Origin of the precerebellar system. Neuron. 2000;27:475–486. doi: 10.1016/s0896-6273(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 195.Zinyk DL, Mercer EH, Harris E, Anderson DJ, Joyner AL. Fate mapping of the mouse midbrain-hindbrain constriction using a site-specific recombination system. Curr Biol. 1998;8:665–668. doi: 10.1016/s0960-9822(98)70255-6. [DOI] [PubMed] [Google Scholar]
- 196.García-Moreno F, Vasistha NA, Begbie J, Molnár Z. CLoNe is a new method to target single progenitors and study their progeny in mouse and chick. Development. 2014;141:1589–1598. doi: 10.1242/dev.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Callahan CA, Thomas JB. Tau-beta-galactosidase, an axon-targeted fusion protein. Proc Natl Acad Sci U S A. 1994;91:5972–5976. doi: 10.1073/pnas.91.13.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 199.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dymecki SM, Kim JC. Molecular neuroanatomy’s “Three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 202.Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. In: Guide to techniques in mouse development, part B: mouse molecular genetics. 2nd ed: Elsevier; 2010. p. 183–213. 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed]
- 203.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 205.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 206.Potter CJ, Luo L. Using the Q system in Drosophila melanogaster. Nat Protoc. 2011;6:1105–1120. doi: 10.1038/nprot.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tasic B, Miyamichi K, Hippenmeyer S, Dani VS, Zeng H, Joo W, et al. Extensions of MADM (mosaic analysis with double markers) in mice. PLoS One. 2012;7:e33332. doi: 10.1371/journal.pone.0033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hippenmeyer S, Youn YH, Moon HM, Miyamichi K, Zong H, Wynshaw-Boris A, et al. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron. 2010;68:695–709. doi: 10.1016/j.neuron.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Beattie R, Postiglione MP, Burnett LE, Laukoter S, Streicher C, Pauler FM, et al. Mosaic analysis with double markers reveals distinct sequential functions of Lgl1 in neural stem cells. Neuron. 2017;94:517–533.e3. doi: 10.1016/j.neuron.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 211.Xu H-T, Han Z, Gao P, He S, Li Z, Shi W, et al. Distinct lineage-dependent structural and functional organization of the hippocampus. Cell. 2014;157:1552–1564. doi: 10.1016/j.cell.2014.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shi W, Xianyu A, Han Z, Tang X, Li Z, Zhong H, et al. Ontogenetic establishment of order-specific nuclear organization in the mammalian thalamus. Nat Neurosci. 2017;20:516–528. doi: 10.1038/nn.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Henner A, Ventura PB, Jiang Y, Zong H. MADM-ML, a mouse genetic mosaic system with increased clonal efficiency. PLoS One. 2013;8:e77672. doi: 10.1371/journal.pone.0077672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lasrado R, Boesmans W, Kleinjung J, Pin C, Bell D, Bhaw L, et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science. 2017;356:722–726. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- 216.Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang S-B, Mclemore KD, Tasic B, Luo L, Jan YN, Jan LY. Kv1.1-dependent control of hippocampal neuron number as revealed by mosaic analysis with double markers. J Physiol. 2012;590:2645–2658. doi: 10.1113/jphysiol.2012.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hippenmeyer S, Johnson RL, Luo L. Mosaic analysis with double markers reveals cell-type-specific paternal growth dominance. Cell Rep. 2013;3:960–967. doi: 10.1016/j.celrep.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Muzumdar MD, Luo L, Zong H. Modeling sporadic loss of heterozygosity in mice by using mosaic analysis with double markers (MADM) Proc Natl Acad Sci U S A. 2007;104:4495–4500. doi: 10.1073/pnas.0606491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming G, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Özel MN, Langen M, Hassan BA, Hiesinger PR. Filopodial dynamics and growth cone stabilization in Drosophila visual circuit development. elife. 2015;4 10.7554/eLife.10721. [DOI] [PMC free article] [PubMed]
- 222.He J, Zhang G, Almeida AD, Cayouette M, Simons BD, Harris WA. How variable clones build an invariant retina. Neuron. 2012;75:786–798. doi: 10.1016/j.neuron.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suzuki SC, Bleckert A, Williams PR, Takechi M, Kawamura S, Wong ROL. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc Natl Acad Sci U S A. 2013;110:15109–15114. doi: 10.1073/pnas.1303551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Godinho L, Williams PR, Claassen Y, Provost E, Leach SD, Kamermans M, et al. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron. 2007;56:597–603. doi: 10.1016/j.neuron.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 225.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 226.Cai D, Cohen KB, Luo T, Lichtman JW, Sanes JR. Improved tools for the Brainbow toolbox. Nat Methods. 2013;10:540–547. [PubMed] [Google Scholar]
- 227.Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–422. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hampel S, Chung P, McKellar CE, Hall D, Looger LL, Simpson JH. Drosophila Brainbow: a recombinase-based fluorescence labeling technique to subdivide neural expression patterns. Nat Methods. 2011;8:253–259. doi: 10.1038/nmeth.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci U S A. 2015;112:E2967–E2976. doi: 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chung K, Wallace J, Kim S-Y, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 232.Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, et al. Mapping of brain activity by automated volume analysis of immediate early genes. Cell. 2016;165:1789–1802. doi: 10.1016/j.cell.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Richardson DS, Lichtman JW. Clarifying tissue clearing. Cell. 2015;162:246–257. doi: 10.1016/j.cell.2015.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157:726–739. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 235.Sung K, Ding Y, Ma J, Chen H, Huang V, Cheng M, et al. Simplified three-dimensional tissue clearing and incorporation of colorimetric phenotyping. Sci Rep. 2016;6:30736. doi: 10.1038/srep30736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, et al. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods. 2016;13:859–867. doi: 10.1038/nmeth.3964. [DOI] [PubMed] [Google Scholar]
- 237.Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim S-Y, et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell. 2015;163:1500–1514. doi: 10.1016/j.cell.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu AKL, Lai HM, Chang RCC, Gentleman SM. Free of acrylamide sodium dodecyl sulphate (SDS)-based tissue clearing (FASTClear): a novel protocol of tissue clearing for three-dimensional visualization of human brain tissues. Neuropathol Appl Neurobiol. 2017;43:346–351. doi: 10.1111/nan.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li W, Germain RN, Gerner MY. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D) Proc Natl Acad Sci U S A. 2017;114:E7321–E7330. doi: 10.1073/pnas.1708981114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ku T, Swaney J, Park J-Y, Albanese A, Murray E, Cho JH, et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat Biotechnol. 2016;34:973–981. doi: 10.1038/nbt.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim S-Y, Cho JH, Murray E, Bakh N, Choi H, Ohn K, et al. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules. Proc Natl Acad Sci U S A. 2015;112:E6274–E6283. doi: 10.1073/pnas.1510133112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ke M-T, Nakai Y, Fujimoto S, Takayama R, Yoshida S, Kitajima TS, et al. Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent. Cell Rep. 2016;14:2718–2732. doi: 10.1016/j.celrep.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 243.Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 2015;18:1518–1529. doi: 10.1038/nn.4107. [DOI] [PubMed] [Google Scholar]
- 244.Lee E, Choi J, Jo Y, Kim JY, Jang YJ, Lee HM, et al. ACT-PRESTO: rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci Rep. 2016;6:18631. doi: 10.1038/srep18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science. 2016;353:aaf7907. doi: 10.1126/science.aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pei W, Feyerabend TB, Rössler J, Wang X, Postrach D, Busch K, et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature. 2017;548:456–460. doi: 10.1038/nature23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ke R, Mignardi M, Pacureanu A, Svedlund J, Botling J, Wählby C, et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods. 2013;10:857–860. doi: 10.1038/nmeth.2563. [DOI] [PubMed] [Google Scholar]
- 248.Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Deisseroth K, Schnitzer MJ. Engineering approaches to illuminating brain structure and dynamics. Neuron. 2013;80:568–577. doi: 10.1016/j.neuron.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yang HH, St-Pierre F, Sun X, Ding X, Lin MZ, Clandinin TR. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell. 2016;166:245–257. doi: 10.1016/j.cell.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544:96–100. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016;19:1142–1153. doi: 10.1038/nn.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 254.Peron S, Chen T-W, Svoboda K. Comprehensive imaging of cortical networks. Curr Opin Neurobiol. 2015;32:115–123. doi: 10.1016/j.conb.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 255.Yang W, Yuste R. In vivo imaging of neural activity. Nat Methods. 2017;14:349–359. doi: 10.1038/nmeth.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pollen AA, Nowakowski TJ, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas CR, et al. Molecular identity of human outer radial glia during cortical development. Cell. 2015;163:55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, et al. Single-cell RNA-Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Crocker A, Guan X-J, Murphy CT, Murthy M. Cell-type-specific transcriptome analysis in the Drosophila mushroom body reveals memory-related changes in gene expression. Cell Rep. 2016;15:1580–1596. doi: 10.1016/j.celrep.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


