Significance
Autoimmune impairment of insulin-producing β cells in the pancreatic islet underlies pathogenesis of type 1 diabetes (T1D). The β-like cells differentiated from human pluripotent stem cells provide possible autologous cell replacement therapies for patients with T1D, and have been transplanted to various ectopic sites in rodents as therapeutic models. Here, we generated xenogeneic human pancreatic β-like cells in the mouse pancreas by orthotopic transplantation of stem cell-derived β-like cells into the pancreas of neonatal mice. This mouse model may provide an experimental system to study human pancreatic β cell biology and pathogenesis in vivo.
Keywords: humanized mice, human pluripotent stem cells, beta cells, diabetes
Abstract
Type 1 diabetes is characterized by autoimmune destruction of β cells located in pancreatic islets. However, tractable in vivo models of human pancreatic β cells have been limited. Here, we generated xenogeneic human pancreatic β-like cells in the mouse pancreas by orthotopic transplantation of stem cell-derived β (SC-β) cells into the pancreas of neonatal mice. The engrafted β-like cells expressed β cell transcription factors and markers associated with functional maturity. Engrafted human cells recruited mouse endothelial cells, suggesting functional integration. Human insulin was detected in the blood circulation of transplanted mice for months after transplantation and increased upon glucose stimulation. In addition to β-like cells, human cells expressing markers for other endocrine pancreas cell types, acinar cells, and pancreatic ductal cells were identified in the pancreata of transplanted mice, indicating that this approach allows studying other human pancreatic cell types in the mouse pancreas. Our results demonstrate that orthotopic transplantation of human SC-β cells into neonatal mice is an experimental platform that allows the generation of mice with human pancreatic β-like cells in the endogenous niche.
Type 1 diabetes (T1D) is a chronic disease that requires insulin therapies to control blood glucose levels in patients (1). T1D is considered to be an autoimmune disease resulting from destruction of insulin-producing pancreatic β cells by self-reacting immune cells and autoantibodies (1, 2). Extensive studies on nonobese diabetic (NOD) mice, a murine model of T1D, support the role of cytotoxic T cells in T1D progression (3). Furthermore, analysis of T1D susceptibility loci in human genetics studies localized T1D-associated SNPs in human leukocyte antigen (HLA) loci (4) and in enhancer regions active in immune cells (5). Moreover, antibodies against various autoantigens, including glutamic acid decarboxylase and insulin, have been identified in patients with T1D (6–8). Understanding interactions between immune cells and β cells in the native niche could be critical for characterizing disease mechanisms and for developing better treatment and preventative strategies (9).
Pluripotent stem cells, with their unlimited replicative capability and remarkable potential to differentiate into diverse cell types, provide a platform for the studying the pathogenesis of human diseases (10). Human pluripotent stem cells have been differentiated into β-like cells with some functional similarities to mature β cells through stepwise differentiation mimicking normal development (11–14). Combined with induced pluripotent stem cell (iPSC) technology (15), this opens the possibility of generating patient-specific β-like cells for autologous β cell replacement therapy. Although allograft rejection can be avoided with this approach, T1D patient-derived β-like cells, upon transplantation, will still be exposed to the preexisting disease-causing autoimmunity. In addition to developing immune protective encapsulation approaches (16), a major challenge is to understand the underlying mechanisms of immune destruction, which requires the generation of suitable in vivo experimental model systems.
Rodent models have been used to demonstrate that human pluripotent stem cell-derived β-like cells transplanted to ectopic sites, such as the kidney capsule (13), are able to control blood glucose levels. These studies are relevant for clinical application as most clinical pancreatic islet transplantations are at an ectopic site through portal vein infusion and subsequent liver embolization (17). Although the pancreas is not considered a clinical transplantation site for pancreatic islets, it could be used as a functionally more relevant site for the study of islet biology and pathogenesis. Compared with ectopic sites, the pancreas microenvironment may provide more physiological blood supply and oxygen levels needed for metabolism and long-term survival of transplanted β-like cells. In addition, the endogenous tissue microenvironment is critical for regulating the interaction of β cells with the immune system, and thus the pancreas may provide a more relevant physiological site for characterizing the immune response to β-like cells in T1D (9).
To mimic the native niche for engrafted human pancreatic β-like cells in mice, we generated mice with human pancreatic tissues in the mouse pancreas by orthotopic transplantation of β-like cells at the neonatal stage. Engrafted β-like cells expressed critical transcription factors and maturation markers for β cells and formed quiescent tissues without hyperplasia for months after transplantation. Endothelial cells in transplants were of host origin and close to the engrafted human cells. Glucose-stimulated release of human insulin was observed for months after transplantation. This approach may provide a platform to examine human β-like cell function and pathology in vivo, a critical step that allows dissecting mechanisms of T1D in mouse models. In addition, multiple other human pancreatic cell types were identified in pancreata of transplanted mice, suggesting that neonatal orthotopic transplantation could be used to study human pancreatic cell types other than β cells.
Results
Injection of Human Pancreatic β-Like Cells into Developing Mouse Embryos.
Our attempts to generate mouse-human chimeras by injection of naive human pluripotent cells into mouse blastocysts were unsuccessful (18). Similarly, injection of naive human pluripotent stem cells into pig blastocysts yielded only low contributions to the pig embryo (19). In contrast, in utero transplantation of human neural crest cells into embryonic day (E) 8.5 mouse embryos showed functional integration of the donor cells into the embryo and resulted in postnatal mice with pigment contribution from the human neural crest cells (20). To test whether introduction of pancreatic precursor cells into early embryos could be used to generate mice with human β cells in the pancreas, we generated HUES8 cells that have a constitutive CAGGS-GFP expression cassette knocked into the AAVS1 locus (HUES8-GFP) (21) (Fig. S1 A and B). HUES8-GFP cells expressed GFP uniformly and constitutively (Fig. S1C) and formed teratomas composed of tissues from three germ layers that retained GFP expression (Fig. S1D) upon injection into immunodeficient NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice (22), indicating a normal differentiation potential of the targeted cells. Furthermore, HUES8-GFP cells showed a normal karyotype (Fig. S1E).
We differentiated HUES8-GFP cells into definitive endodermal (DE) cells (Fig. S1F), which were transplanted into mouse embryos in utero at E8.5 following the protocol established previously for generating human-mouse neural crest chimeras (20) (Fig. S1G, Left). Consistent with the previous report (20), we observed transplanted GFP-positive cells frequently at the head or tail region of the embryos (Fig. S1G) but failed to detect human insulin in the plasma of transplanted mice (Fig. S1H). We conclude that injection of endodermal precursor cells into E8.5 embryos does not result in efficient engraftment of human β-like cells in transplanted mice.
To test whether transplantation at later developmental stages could generate mice carrying engrafted human pancreatic β-like cells in the mouse pancreas, we introduced cells at different stages of in vitro pancreatic β-cell differentiation into mouse embryos at different developmental stages from E12.5 to E16.5 (Fig. 1A). This approach failed to generate viable pups because recipient embryos were either absorbed or delivered as stillborn (Fig. 1B).
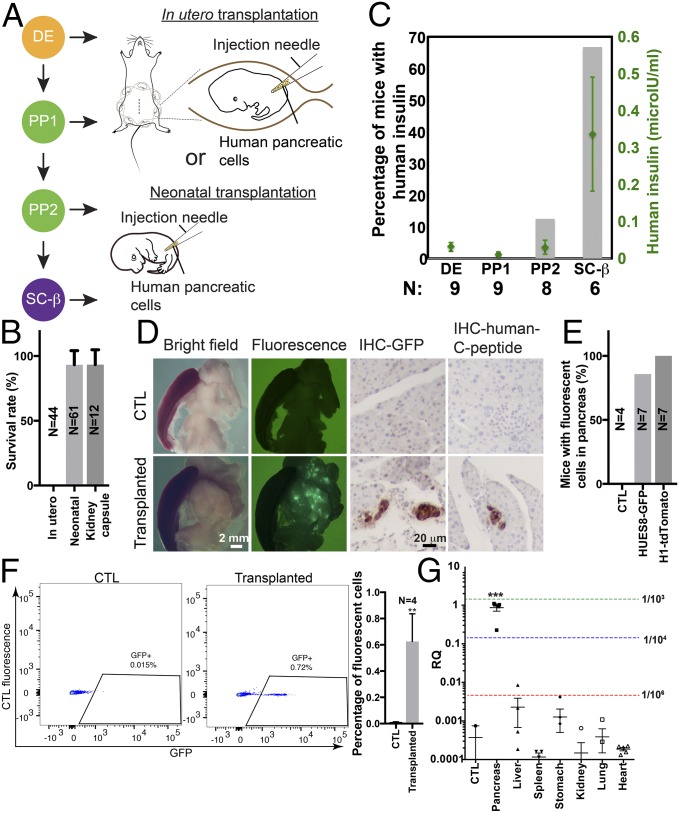
Fig. 1.
Transplantation of SC-β cells into neonatal mice leads to efficient functional engraftment of human pancreatic β-like cells. (A) Cells at different stages of pancreatic β-like cell differentiation were transplanted into the pancreatic regions of developing embryos in utero (Top) or in neonatal pups (Bottom). (B) Survival of mice transplanted in utero and postnatally. Neonatal denotes orthotopic transplantation into neonatal mice. Kidney capsule denotes transplantation under the kidney capsule of adult mice. (C) Human insulin levels from plasma samples of recipient mice were tested with human insulin-specific ELISA at 7 wk posttransplantation. The percentage of mice showing positive human insulin (>0.15 μIU/mL) was plotted as gray bars, and levels of human insulin were plotted as green dots. Error bars show SEM. N (below the bars) indicates the total number of mice analyzed. (D) Microscopic analysis (Left and Center Left) and immunohistochemistry with anti-GFP (Center Right, IHC-GFP) or anti-human C-peptide (Right, IHC-human C-peptide) antibodies of pancreata of control mice (CTL; Top) and NSG mice transplanted with SC-β cells differentiated from HUES8-GFP cells at the neonatal stage (Bottom). (White scale bar: 2 mm; black scale bar: 20 μm.) (E) Percentage of mice showing fluorescent human cells in mouse pancreata. All analyses in D and E were performed on 6-mo-old mice. (F) Flow cytometry analysis of GFP-positive cells from dissociated pancreata of CTL (Left) or 6-mo-old mice transplanted with SC-β cells differentiated from HUES8-GFP cells at the neonatal stage (Center). (Right) Percentage of GFP-positive cells was quantified. Error bars show SEM. **P < 0.01 (two-tailed t test). (G) Quantitative PCR analysis of human mitochondrial DNA in total DNA samples extracted from different organs of mice engrafted with human cells into the pancreas. Dashed green, blue, and red lines show relative quantification (RQ) signal from one human cell in 103, 104, and 105 mouse cells, respectively, from the standard curve. ***P < 0.001 (one-way ANOVA).
Generation of Mice with Human Pancreatic β-Like Cells Integrated into the Pancreas by Neonatal Orthotopic Transplantation.
As an alternative approach, we tested the feasibility of orthotopic transplantation by injecting fluorescent beads into the pancreas of neonatal pups with the spleen as a reference anatomical landmark (Fig. S2A). Pups transplanted with beads survived and displayed beads in the pancreas at 2 mo after transplantation (Fig. S2 B and C). When pancreatic cells derived from in vitro differentiation of human pluripotent stem cells were transplanted at the neonatal stage, we observed an improved survival rate of transplanted pups compared with in utero transplantation (Fig. 1B), suggesting that transplantation of human pancreatic cells into neonatal mice could be used to evaluate optimal differentiation stages of the donor cells.
To systematically assess the optimal in vitro differentiation stage of donor cells, we transplanted SOX17-expressing DE, PDX1-expressing pancreatic precursor (PP1), PDX1- and NKX6.1-expressing pancreatic precursor (PP2), and C-peptide– and NKX6.1-expressing stem cell-derived β (SC-β) cells (13) into neonatal pups. After 7 wk, human insulin levels in the plasma of mice were measured using a human insulin-specific ELISA (Fig. 1C). Mice transplanted with SC-β cells showed the highest level of human insulin (Fig. 1C, green dots). Also, the fraction of mice with human insulin in the plasma was highest in animals transplanted with SC-β cells (Fig. 1C, gray bars). Therefore, transplantation of SC-β cells into the pancreas of newborn mice was used in the following experiments unless otherwise stated.
We characterized the viability and function of human β-like cells integrated into the mouse pancreas. HUES8-GFP cells were differentiated into SC-β cells based on published protocols (13) and used as donor cells for transplantation. Expression of GFP was retained in cells at stage 6 of differentiation (Fig. S3), suggesting no obvious silencing of the CAGGS-GFP expression cassette. Analyses for NKX6.1/PDX1, NKX6.1/chromogranin A, and NKX6.1/C-peptide expression at stages 4, 5, and 6 of differentiation of HUES8-GFP showed the expected marker double-positive populations (Fig. S4, Bottom). The marker expression profiles indicate that the HUES8-GFP cells differentiated into SC-β similar to HUES8 cells.
We transplanted SC-β cells differentiated from HUES-GFP cells into newborn mice and detected green fluorescence signals in the pancreas of transplanted mice, but not in the pancreas of control mice, at 6 mo after transplantation (Fig. 1D, Left and Center Left and Fig. 1E). Consistently, immunostaining demonstrated the presence of GFP-expressing cells in the transplanted pancreas (Fig. 1D, Center Right). Furthermore, human C-peptide–expressing cells were detected in the transplanted pancreas but not in control samples (Fig. 1D, Right), demonstrating the presence of insulin-producing human β-like cells. To further confirm the presence of human cells in the transplanted mouse pancreas, we performed laser capture microdissection of the human C-peptide–positive cells and control cells showing negative human C-peptide antibody staining (Fig. S5A), extracted DNA samples from the microdissected regions, and performed quantitative PCR with human mitochondrial DNA-specific primers and an ultraconserved region-specific primer as a loading control (20). While no signal was detected in control samples, DNA extracted from microdissected human C-peptide–positive regions revealed the presence of human DNA at a level corresponding to about one human cell in 10–100 mouse cells (Fig. S5B). These results suggest that human β-like cells engraft into the mouse pancreas upon neonatal orthotopic transplantation.
We estimated the fraction of GFP-expressing human cells present in the mouse pancreas by dissociating pancreas tissue into single cells, followed by flow cytometry analysis. On average, about 0.6% of GFP-expressing cells was detected in transplanted pancreas samples, but not in control pancreas samples (Fig. 1F). To test whether another human pluripotent stem cell line could generate cells suitable for transplantation, we differentiated H1-OCT4-GFP cells (23) into pancreatic β-like cells (14), which were NKX6.1/PDX1 double-positive at stage 4 (Fig. S6A) and NKX6.1/C-peptide double-positive at stage 7 (Fig. S6B). A CAGGS-tdTomato cassette was knocked into the AAVS1 locus (Fig. S6 C–E) to produce cells that constitutively express tdTomato, and thus would allow detection of the human donor cells. We transplanted stage 7 cells differentiated from H1-OCT4-GFP/tdTomato–positive cells into the neonatal mouse pancreas. Fig. S7A shows the presence of human cells in mouse pancreata by anti-tdTomato and anti-human C-peptide–immunostaining. Furthermore, the presence of tdTomato-positive cells was confirmed by flow cytometry analysis (Fig. S7B). These results demonstrate that human β-like cells can be established in the mouse pancreas following neonatal orthotropic transplantation.
Transplanted Human Cells Preferentially Localize to the Pancreas.
To quantify the tissue distribution of the human donor cells, we extracted DNA from different visceral organs from transplanted mice and performed quantitative PCR analysis of human mitochondrial DNA. Consistent with the fluorescent microscopy analysis (Fig. 1D and Fig. S7A), we detected human mitochondrial DNA prominently in the pancreas (Fig. 1G) at a level corresponding to one human cell per 1,000–10,000 mouse cells, a range similar to the flow cytometry analysis shown in Fig. 1F. No human DNA was detected in other organs [detection limit of one human cell in 10,000 mouse cells (20)]. This significant enrichment of human mitochondrial DNA in the pancreas suggests that orthotopic transplantation generates mice with transplanted human cells enriched in the pancreas.
Expression of β Cell Markers in Engrafted Human β-Like Cells.
We analyzed the engrafted human β-like cells by immunofluorescence. Consistent with immunohistochemistry, GFP and human C-peptide signals were detected in the transplanted pancreas, but not in controls (Fig. 2A). Furthermore, the engrafted human β-like cells did not produce glucagon (Fig. 2B), suggesting formation of monohormonal β-like cells. Murine pancreatic islets were positive for insulin but negative for anti-human C-peptide antibody staining (Fig. 2C, Top) in contrast to cells from transplanted mice, where all human C-peptide–positive cells also expressed insulin (Fig. 2C, Bottom).
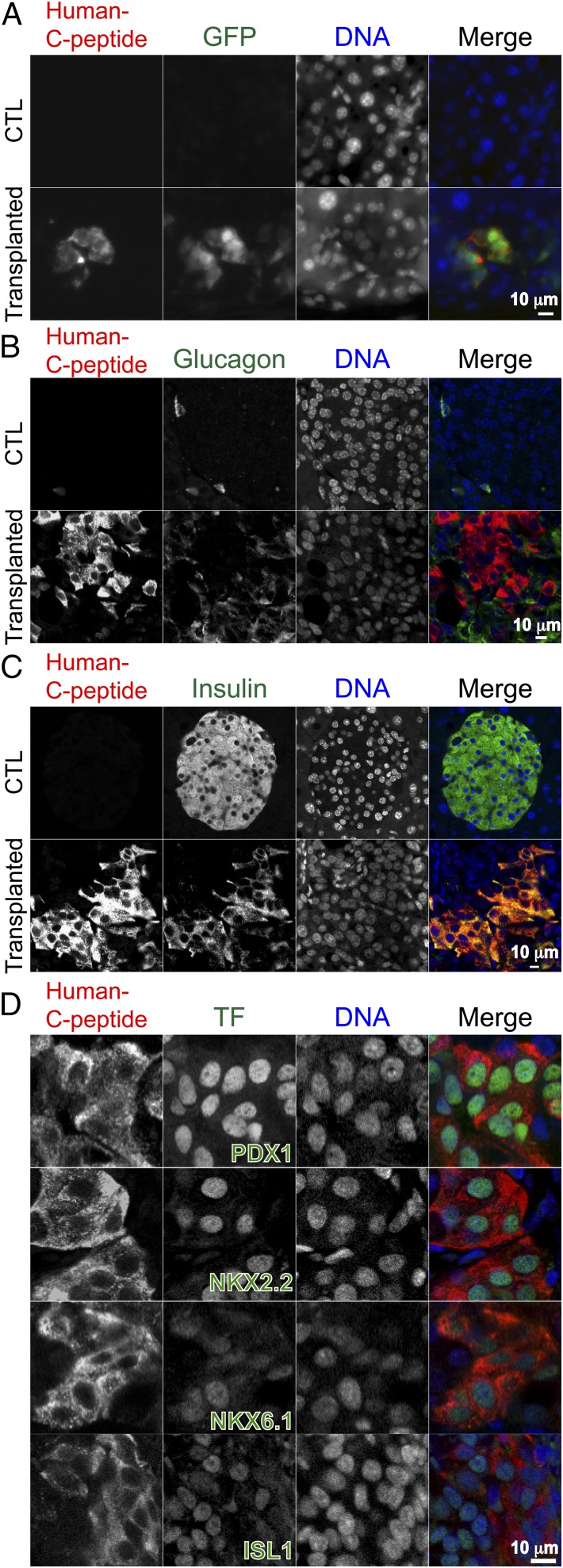
Fig. 2.
Characterization of engrafted human β-like cells in the mouse pancreas. (A) Representative fluorescent micrographs of anti-human C-peptide staining (red) and anti-GFP staining (green) in control (CTL) mouse pancreas (Top) and in the pancreas of 6-mo-old transplanted mice (Bottom). (B) Representative fluorescent micrographs of anti-human C-peptide (red) and antiglucagon antibody (green) staining in pancreatic tissue from CTL (Top) and a 6-mo-old mouse engrafted with human β-like cells into the pancreas (Bottom). (C) Fluorescent micrographs showing coexpression of C-peptide and insulin in human β-like cells engrafted in the mouse pancreas (Bottom), but not in β cells in the CTL mouse (Top). (D) Expression of β cell transcription factors PDX1, NKX2.2, NKX6.1, and ISL1 in β-like cells engrafted into the mouse pancreas. (Scale bars: 10 μm.)
Pancreatic β cells express key transcription factors critical for maintaining β cell identity, glucose sensing, and controlled release of insulin (24), with factors such as Pdx1 (25), Nkx2.2 (26), Nkx6.1 (27), and Isl1 (28) playing critical roles for β cell development and for maintaining β cell function. To examine the expression of these factors in engrafted human β-like cells, we performed immunofluorescence with an anti-human C-peptide antibody and antibodies against the different transcription factors (Fig. 2D). As expected, we observed expression of PDX1, NKX2.2, NKX6.1, and ISL1 in engrafted human β-like cells (Fig. 2D).
Ucn3 is a marker associated with β cell maturation (29). To test whether the engrafted β-like cells expressed UCN3, we performed immunofluorescence and found that it was expressed in a subset of engrafted β-like cells (Fig. S8A, Left). Furthermore, islet amyloid polypeptide (IAPP), another marker for β cell maturity (30), was detected in engrafted human β-like cells (Fig. S8A, Right). The engrafted human β-like cells were postmitotic, as indicated by the absence of the cell proliferation marker Ki-67 (31) in human C-peptide–positive cells (Fig. S9A). These data suggest that the transplanted mice harbor postmitotic human β-like cells with characteristics of functional maturity in the pancreas.
Engrafted Human Tissues Recruit Mouse Endothelial Cells.
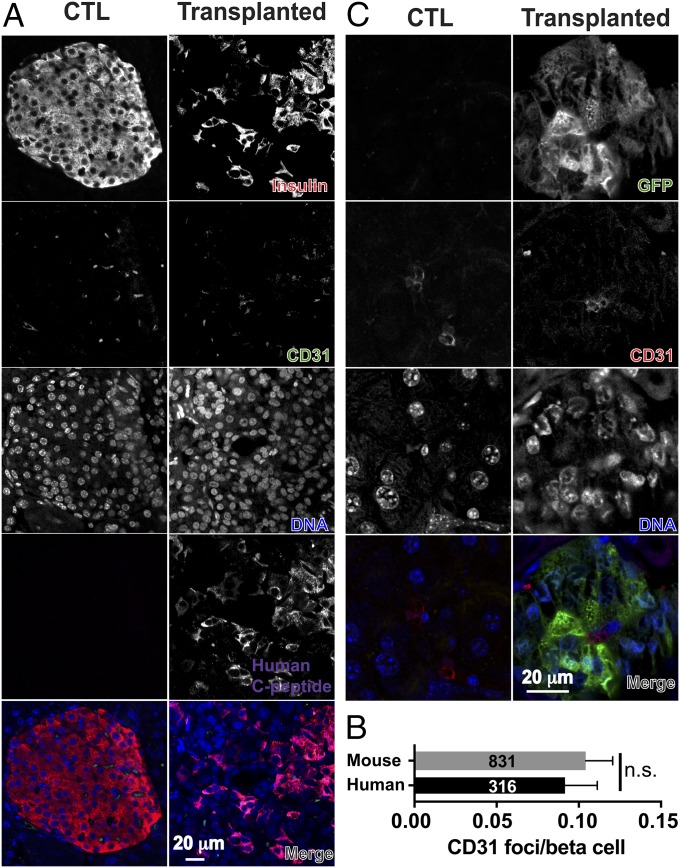
We examined vascularization of engrafted human β-like cells with anti-CD31 antibody staining (Fig. 3A). Quantification of CD31 foci numbers over insulin-producing cells revealed similar levels of vascularization around human pancreatic β-like cells as around mouse islets (Fig. 3B). Anti-GFP antibody staining localized GFP-negative, CD31-positive endothelial cells close to engrafted GFP-positive human cells (Fig. 3C), suggesting that the engrafted human cells recruited mouse endothelial cells.
Fig. 3.
Vascularization of engrafted human cells. (A) Representative fluorescent micrographs of anti-CD31 (green), antiinsulin (red), and anti-human C-peptide (magenta) staining in pancreatic islets of a control (CTL) mouse (Left) and of a transplanted mouse (Right). (B) Quantification of CD31 foci per mouse β cells (gray bar) or per human β-like cells (black bar) was plotted (n = 3 mice), and the total numbers of analyzed mouse β cells and human β-like cells are labeled. (C) Endothelial cells in the engrafted regions are of mouse origin, as indicated by the lack of expression of GFP in CD31-expressing cells. All analyses were performed on samples from 6-mo-old mice. n.s., not significant (t test).
Engraftment of Other Pancreatic Cell Types.
A subset of GFP-positive cells did not express C-peptide (Fig. 2A), suggesting that cell types other than β-like cells may have also been present in the mouse pancreas. To test this, we costained tissue slides with an anti-GFP antibody and antibodies for markers of other pancreatic cell types, and identified a significant number of GFP-expressing cells that were also glucagon-positive (Fig. S10A). Furthermore, somatostatin and pancreatic polypeptide expression was detected in GFP-positive cells (Fig. S10 B and C). Finally, exocrine amylase and the ductal cell marker CK19 were expressed in a subset of GFP-positive human cells (Fig. S10 D and E). In summary, these data suggest that in addition to β-like cells, other pancreatic cell types were present and survived after neonatal orthotopic transplantation.
Comparison of Orthotopic and Heterotopic Transplantation.
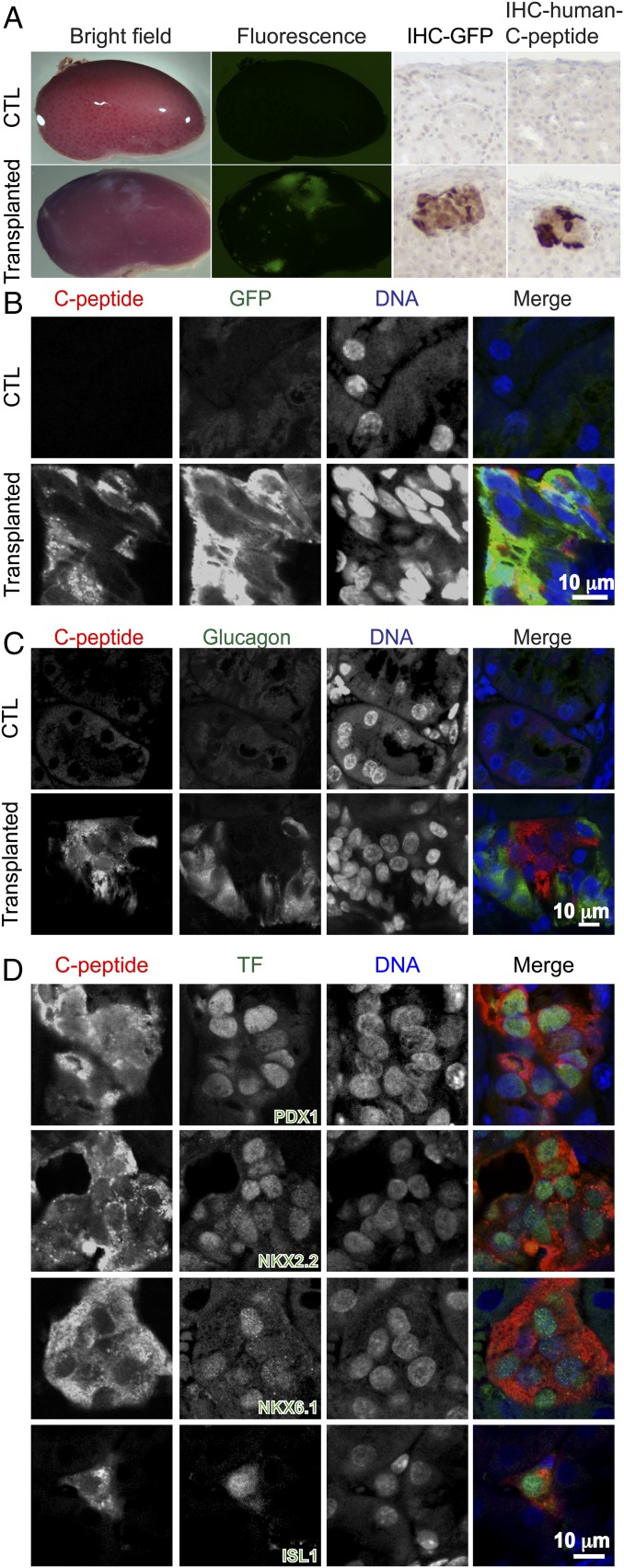
Transplantation of islet or β-like cells under the kidney capsule of mice is widely used to assess in vivo function of transplanted cells (17). We compared neonatal orthotopic with heterotopic transplantation of human SC-β cells under the kidney capsule using the same number of SC-β cells from the same batch of differentiated HUES8-GFP cells. Fig. 4A (Left and Center Left) shows the presence of GFP-expressing human cells in transplanted kidney, but not in control samples. Histological analysis with an anti-GFP antibody and anti-human C-peptide antibody staining revealed the presence of human β-like cells under the kidney capsule of transplanted mice (Fig. 4A, Center Right and Right). Similar to the engrafted cells in the pancreas, the β-like cells expressed GFP (Fig. 4B), but not glucagon (Fig. 4C), indicating monohormonal β-like cells. Furthermore, immunohistochemical analyses revealed the expression of transcription factors PDX1, NKX2.2, NKX6.1, and ISL1 in C-peptide–expressing cells (Fig. 4D) similar to the β-like cells transplanted into the pancreas. Immunofluorescence with anti-UCN3 and anti-IAPP detected the expression of maturation markers (Fig. S8B). Similar to cells engrafted in the pancreas, β-like cells under the kidney capsule did not express the proliferation marker Ki-67 (Fig. S9B). Moreover, glucagon, somatostatin, pancreatic polypeptide, amylase, and CK19-expressing cells were detected in the GFP-positive human cells (Fig. S11). Quantification revealed a similar ratio of C-peptide–expressing cells and glucagon-expressing cells in GFP-positive human cells engrafted in the pancreas and under the kidney capsule (Fig. S12 A and B). In addition, the ratio of NKX6.1-positive cells was similar in C-peptide–expressing cells engrafted in the pancreas or under the kidney capsule (Fig. S12C).
Fig. 4.
Human SC-β cells transplanted under the mouse kidney capsule. (A, Left and Center Left) Microscopy analysis of green fluorescence signal in control (CTL) kidney (Top) or kidney transplanted with SC-β differentiated from HUES8-GFP cells from 7.5-mo-old mice (6 mo after transplantation to 1.5-mo-old mice) (Bottom). (A, Center Right and Right) Immunohistochemistry analysis with an anti-GFP antibody (IHC-GFP) or an anti-human C-peptide antibody (IHC-human C-peptide) of samples from the CTL mouse (Top) and the transplanted mouse (Bottom). (B) Representative fluorescent micrographs showing the presence of GFP- and C-peptide–expressing human cells in transplanted kidney (Bottom), but not in CTL (Top). (C) Representative fluorescent micrographs showing C-peptide–expressing cells engrafted under the kidney capsule did not express glucagon. (D) Expression of transcription factors PDX1, NKX2.2, NKX6.1, and ISL1 in engrafted C-peptide–expressing β-like cells under the kidney capsule. (Scale bars: 10 μm.)
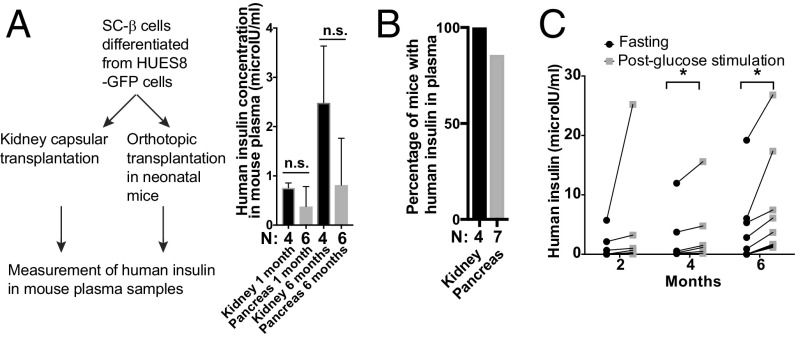
We compared human insulin levels in NSG mice heterotopically transplanted with human SC-β cells under the kidney capsule or orthotopically transplanted into the pancreas (Fig. 5A). Production of human insulin was detected in the serum of mice for months after transplantation, although the fraction of insulin-positive mice was higher in heterotopic than in orthotopic transplantation, suggesting a higher variability in mice transplanted with SC-β cells into the pancreas (Fig. 5B).
Fig. 5.
Functional characterization of human β-like cells engrafted into the mouse pancreas. (A, Left) Experimental design to compare the same number of pancreatic β-like cells transplanted under the kidney capsule or into the neonatal pancreas. (A, Right) Comparison of human insulin in mouse blood from the two transplantation methods. N (below the bars) indicates the total number of mice analyzed. (B) Ratio of mice showing detectable human insulin in the blood circulation 1 mo after kidney capsular transplantation or neonatal orthotopic transplantation. N (below the bars) indicates the total number of mice analyzed. (C) GSIS measurements at different time posttransplantation in mice orthotopically transplanted with human pancreatic β-like cells at the neonatal stage. After a 16-h fasting period, mouse plasma samples were collected (fasting insulin levels shown by black dots), glucose solution was injected i.p. (2 g per 1 kg of body weight), and plasma was collected 30 min postglucose injection (postglucose stimulation, shown by gray squares). n.s., not significant (t test). *P < 0.05 (paired t test).
Long-Term Function of Engrafted Human β-Like Cells.
To assess long-term survival and function of the human β-like cells, we performed an in vivo glucose-stimulated insulin secretion (GSIS) assay using ELISA-based measurements of human insulin levels in plasma samples collected from another cohort of mice orthotopically transplanted with SC-β cells as neonates. At 2 mo posttransplantation, we observed a modest increase of human insulin secretion upon glucose stimulation. The difference between fasting and postglucose stimulation levels was, however, significant at 4 mo posttransplantation (Fig. 5C). These data are consistent with the expression of key β cell transcription factors and maturation markers. In summary, these data suggest that the human β cells engrafted into the mouse pancreas remain functional over multiple months after transplantation.
Discussion
In this study, we used orthotopic transplantation of SC-β cells into the pancreas of neonatal mice to generate mice harboring human pancreatic β-like cells in the pancreas. Engrafted human cells recruited mouse endothelial cells and comprised β-like cells (expressing β cell transcription and maturation factors) and multiple other human pancreatic cell types (based on marker expression). Orthotopically transplanted mice showed glucose-regulated release of human insulin for months after transplantation.
Transplantation of aggregates of human pluripotent stem cell-derived pancreatic precursor cells embedded in type I collagen into the splenic lobe of adult NSG mice was used previously to evaluate maturation of pancreatic precursor cells (32). Similar to that study, we obtained monohormonal β-like cells by orthotopic transplantation of single-cell suspensions of SC-β cells into the neonatal pancreas (Fig. 2B). Importantly, our present study provides evidence that transplantation of in vitro-differentiated SC-β cells into the neonatal pancreas resulted in establishment of postmitotic human β-like cells that showed glucose-responsive release of human insulin into mouse blood (Fig. 5C).
We found that the same number of dissociated SC-β cells injected under the kidney capsule yielded higher levels of human insulin in the serum compared with neonatal orthotopic transplantation. This is similar to previous results, where injection of more mouse islets was needed after intrapancreatic transplantation as compared with transplantation under the kidney capsule to restore blood sugar levels in diabetic NRG-Akita mice (33). Enriching β-like cells before transplantation may increase engraftment efficiency.
Our attempts to establish human pancreatic cells in chimeric mice by in utero injection of DE cells into gastrulation-stage embryos at E8.5 failed to produce functional engraftment of the human donor cells. Our results suggest that human β-like cells may be the most appropriate donor cells in orthotopic transplantation to establish human β-like cells in mouse models.
Mechanistic understanding of human β-cells under normal physiological conditions and in disease is critical for more effective therapy and preventative strategies of conditions such as T1D. Recent progress in generating patient-specific iPSCs (34) and in differentiation of pluripotent stem cells to functional pancreatic β-like cells (13, 35) not only establishes autologous β cell replacement as a potential therapy but also provides opportunities to study human β cell biology. Generation of human-mouse chimeras is of interest for the investigation of human diseases as it provides a platform for studying both normal physiology and disease pathology under in vivo conditions. Although cell transplantation into the pancreas is considered to be clinically impractical due to possible complications, such as pancreatitis, the mouse model described here represents a tractable in vivo system of human pancreatic cells in the endogenous niche and will be useful to address critical questions of human β cell biology. This may contribute to elucidating the mechanisms of how T1D-associated genetic variants (4, 5) contribute to T1D pathology and may facilitate the development and assessment of novel therapies and preventative approaches.
Materials and Methods
A detailed description of experimental procedures and analyses is provided in SI Materials and Methods. Briefly, we transplanted human pluripotent stem cell-derived β-like cells into immunodeficient NSG mice (22). Functions of engrafted cells were investigated using an in vivo GSIS assay (13) and immunohistochemistry analyses (36).
Animals were used in accordance with the protocols approved by the Animal Research Regulation Committee at the Whitehead Institute and guidelines from the Department of Comparative Medicine at Massachusetts Institute of Technology.
Supplementary Material
Acknowledgments
We thank D. Fu, R. Flannery, E. Engquist, and Y. Yu for excellent technical assistance; members of the R.J. laboratory for advice and discussion; J. Kenty, K. Narayan, and other members of the D.A.M. laboratory for discussion; and W. Salmon (W. M. Keck Biological Imaging Facility) and E. Vasile (Koch Institute for Integrative Cancer Research) for assistance with imaging and laser capture microdissection microscopy. This study was supported by a generous gift from Liliana and Hillel Bachrach and, in part, by NIH Grants R01-CA084198, 5R01-MH104610-16, R01-GM114864, RF1-AG048029, and U19-AI3115135; NIH/National Institute of Child Health and Human Development Grant R37-HD045022; and NIH/National Institute of Neurological Disorders and Stroke Grant 1R01-1NS088538-01 (all to R.J.). The work in the D.A.M. laboratory was supported by the Harvard Stem Cell Institute and the JBP Foundation. D.A.M. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: R.J. is a cofounder and advisor of Fate Therapeutics, Fulcrum Therapeutics, and Omega Therapeutics. D.A.M. is the founder of Semma Therapeutics.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702059115/-/DCSupplemental.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serreze DV, Leiter EH. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr Opin Immunol. 1994;6:900–906. doi: 10.1016/0952-7915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet. 2015;47:898–905. doi: 10.1038/ng.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onengut-Gumuscu S, et al. Type 1 Diabetes Genetics Consortium Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–386. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baekkeskov S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156, and erratum (1990) 347:782. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 7.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322:1555–1560. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- 8.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2:a007781. doi: 10.1101/cshperspect.a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman RS, et al. An evolving autoimmune microenvironment regulates the quality of effector T cell restimulation and function. Proc Natl Acad Sci USA. 2014;111:9223–9228. doi: 10.1073/pnas.1322193111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 12.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 13.Pagliuca FW, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Vegas AJ, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95:1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 18.Theunissen TW, et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell. 2016;19:502–515. doi: 10.1016/j.stem.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017;168:473–486.e15. doi: 10.1016/j.cell.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MA, et al. Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proc Natl Acad Sci USA. 2016;113:1570–1575. doi: 10.1073/pnas.1525518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hockemeyer D, et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 23.Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- 24.Pan FC, Wright C. Pancreas organogenesis: From bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 26.Sussel L, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 27.Sander M, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 28.Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 29.Blum B, et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30:261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezania A, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 32.McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM. The basic helix-loop-helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes. 2015;64:2497–2505. doi: 10.2337/db14-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brehm MA, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgammanull Ins2Akita mice. Diabetes. 2010;59:2265–2270. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochedlinger K, Jaenisch R. Induced pluripotency and epigenetic reprogramming. Cold Spring Harb Perspect Biol. 2015;7:a019448. doi: 10.1101/cshperspect.a019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, et al. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest. 2014;124:2172–2187. doi: 10.1172/JCI71103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.