Significance
Methods for the chemoselective formation of peptide bonds in water generally leverage the exceptional reactivity of thiol nucleophiles. Here, we demonstrate that hydroxamic acids—incorporated into the side chain of asparagine or aspartic acid—can be leveraged analogously. N-terminal hydroxy-asparagine-peptides react with peptide-αthioesters to form “O-acyl hydroxamic acid isopeptides," which rearrange to peptide bonds via a facile O,N-acyl shift. The hydroxy-asparagine residue can be converted to either aspartic acid or asparagine, to furnish amide-linked ligation products comprising entirely canonical residues. Taken together, these observations form the basis of a strategy for peptide ligation that circumvents the requirement for Xaa-Cys ligation “sites.”
Keywords: chemical ligation, hydroxamic acid, O-acyl hydroxamic acid, acyl transfer, acyl shift
Abstract
The facile rearrangement of “S-acyl isopeptides” to native peptide bonds via S,N-acyl shift is central to the success of native chemical ligation, the widely used approach for protein total synthesis. Proximity-driven amide bond formation via acyl transfer reactions in other contexts has proven generally less effective. Here, we show that under neutral aqueous conditions, “O-acyl isopeptides” derived from hydroxy-asparagine [aspartic acid-β-hydroxamic acid; Asp(β-HA)] rearrange to form native peptide bonds via an O,N-acyl shift. This process constitutes a rare example of an O,N-acyl shift that proceeds rapidly across a medium-size ring (t1/2 ∼ 15 min), and takes place in water with minimal interference from hydrolysis. In contrast to serine/threonine or tyrosine, which form O-acyl isopeptides only by the use of highly activated acyl donors and appropriate protecting groups in organic solvent, Asp(β-HA) is sufficiently reactive to form O-acyl isopeptides by treatment with an unprotected peptide-αthioester, at low mM concentration, in water. These findings were applied to an acyl transfer-based chemical ligation strategy, in which an unprotected N-terminal Asp(β-HA)-peptide and peptide-αthioester react under aqueous conditions to give a ligation product ultimately linked by a native peptide bond.
Contemporary protein total synthesis invariably employs chemical ligation––the covalent coupling of two unprotected peptide segments by a chemoselective reaction, in aqueous solvent, to form a product linked by an amide bond or analog structure (1–3). Only a few reactions constitute the totality of the chemical ligation toolkit, highlighting the challenge of selective amide bond formation between unprotected peptides in water. Chemical ligation reactions that proceed in aqueous buffer include native chemical ligation (4) and oxime-forming ligation (5, 6); a variety of other chemistries, including Staudinger ligation (7, 8), α-ketoacid-hydroxylamine ligation (9, 10), pseudoproline-forming ligation (11), and Ser/Thr-forming ligation (12, 13), are conducted in organic or mixed aqueous/organic solvents.
In native chemical ligation, an N-terminal Cys-peptide undergoes thiol/thioester exchange with a peptide-αthioester to generate a thioester intermediate, which rearranges to a peptide bond via rapid S,N-acyl shift. Key to this approach is that aminolysis of the thioester—which would be essentially unreactive to amines at neutral pH—is facilitated by proximity-driven, entropic activation. The same principle was employed in two contemporary peptide-coupling strategies based on O,N-acyl transfer (14–16). However, both strategies were ultimately limited by slow acyl transfer rates.
Due to the facility of the original native chemical ligation chemistry, many methods have been developed to extend its applicability beyond Xaa-Cys ligation sites, by the use of various cysteine surrogates in place of an N-terminal cysteine (17–19). The abilities of certain nonthiol side-chain functionalities—including imidazoles (20) and carboxylates (21, 22)—to facilitate the aminolysis of a peptide-αthioester by N-terminal histidine or Asp/Glu-peptides have been documented. Otherwise, extensions of native chemical ligation have relied on the use of thiol nucleophiles to form S-acyl isopeptides, which undergo rapid S,N-acyl shifts through small rings. An exception is the use of selenocysteine (23–26) and peptide-αselenoesters (27, 28), which exhibit analogous but heightened reactivity.
We sought to expand the scope of nucleophiles that might be employed in acyl transfer-based chemical ligation, and to reinvestigate the possibility of O,N-acyl transfer across medium-size rings. Hydroxamic acids (29) were found to be sufficiently reactive to enable the formation of an O-acyl isopeptide from a peptide-αthioester and an N-terminal Asp(β-HA)-peptide at 5 mM concentrations in aqueous solvent, and without the need for protecting groups. Unexpectedly, the resulting O-acyl isopeptide products rearranged to native amide bonds via a facile O,N-acyl shift—apparently through a seven-member ring—with minimal interference from hydrolysis. These combined results were applied to the development of an amide-forming chemical ligation strategy involving reaction of an N-terminal Asp(β-HA)-peptide with a peptide-αthioester.
Results and Discussion
Rearrangement of an O-Acyl Hydroxamic Acid Isopeptide to a Peptide Bond.
O-acyl hydroxamic acid isopeptide 1 rearranged to form native peptide 1′ upon standing in aqueous buffer at ambient temperature (Fig. 1A and SI Appendix, section 5). The reaction was pH-sensitive, exhibiting a maximum between pH 5 and 6 (Fig. 1B); it was substantially slower at pH 8, and did not proceed to any detectable extent at pH 2 (SI Appendix, Figs. S1 and S18). Potentially, the rate-pH maximum arises from a dependency on simultaneous deprotonation of the Nα group, and protonation of the O-acyl hydroxamic acid group of isopeptide 1.
Fig. 1.
An O-acyl hydroxamic acid isopeptide derived from Asp(β-HA) rearranges to a native peptide bond in aqueous buffer. (A) Reaction scheme. (B) First-order rate constants for O-acyl isopeptide rearrangement as a function of pH, obtained at each of two isopeptide concentrations. Rate constants were derived from fits of LC-MS peak areas to an integrated rate expression, as described in SI Appendix, section 5.
To confirm the intramolecular nature of the rearrangement, reaction time courses were carried out at two different isopeptide concentrations (Fig. 1B and SI Appendix, section 5). The observed reaction half-lives did not decrease when the isopeptide concentration was raised 10-fold, across a range of pH values. These data provide strong evidence that the reaction is first-order with respect to isopeptide, consistent with rearrangement by intramolecular acyl transfer (30).
At pH 6, the half-life (t1/2) for rearrangement of 1 was on the order of 15 min, compared with typical half-lives for O,N-acyl shifts of 2–4 h in dibenzofuran frameworks (14, 31), hours to days for ester-linked thiazolidines (16), 22 h for a perylene-based scaffold (32), and 3 h (with microwave heating) for medium to long-range shifts in serine isopeptides (33). Thus, the rate of O-acyl hydroxamic acid isopeptide rearrangement is notable. Rearrangements of serine, threonine, and related O-acyl isopeptides by O,N-acyl shift can also be rapid (half-lives of ∼30 s to ∼10 min at neutral or mildly alkaline pH) (34–38), but these proceed through five-member rings, which are expected to form ∼104-fold faster than seven-member rings (30). Facile rearrangement of Asp(β-HA) isopeptides is probably due in part to the heightened reactivity of a diacyl hydroxylamine compared with an alkyl ester. Acyl donor strength was demonstrated recently to increase the rate of transfer across a salicylaldehyde-derived template, which lends support to this hypothesis (39).
Several lines of evidence support the covalent structure of the isomeric reaction product 1′ as the indicated amide. First is the change in charge-state distribution observed upon conversion of O-acyl hydroxamic acid 1 to product: The most abundant charge state changes from [M+4H]4+ in 1 to [M+3H]3+ in the product 1′, consistent with the loss of a basic Nα group upon conversion. Second is the susceptibility of the Asp(β-HA) residue in 1′ to oxidative or reducing treatments to yield either Asp or Asn, respectively (see below). This behavior illustrates that a hydroxamic acid group has been revealed upon conversion of 1 to 1′. Third, and most compelling, is the observation that several amide products investigated were indistinguishable from authentic peptides, upon conversion of Asp(β-HA) to Asp (SI Appendix, Figs. S65 and S66).
Synthesis of an O-Acyl Hydroxamic Acid Isopeptide from a Peptide-αThioester and Hydroxy-Asparagine-Peptide.
We accessed the unique chemistry of O-acyl hydroxamic acid isopeptides by the reaction of Asp(β-HA) with thioesters (40). In a representative experiment, peptide-αthioester 2 (5 mM) was treated with 1.3 equivalents of N-terminal Asp(β-HA)-peptide 3 (6.5 mM) in 6 M guanidine hydrochloride, 200 mM phosphate, pH 9 buffer; after 15 min, 2 had been converted to O-acyl hydroxamic acid 1 (Fig. 2). The covalent structure of 1 was confirmed by reduction of the N-O bond in acetylated analog acetyl-1, which yielded products consistent with the indicated O-acyl connectivity (SI Appendix, Figs. S21 and S22).
Fig. 2.
Reaction of a peptide-αthioester with an N-terminal Asp(β-HA)-peptide yields a peptide-αO-acyl hydroxamic acid isopeptide under mild aqueous conditions. Reaction scheme and LC-MS data showing conversion of thioester 2 to O-acyl hydroxamic acid isopeptide 1, by treatment with N-terminal Asp(β-HA)-peptide 3, at pH 9.
The rate at which hydroxamic acids react with thioesters would determine the practicality of chemical ligations based on these reagents, in the same way that thiol–thioester exchange between a Cys-peptide and a peptide-αthioester determines the rate of native chemical ligation (41). Accordingly, we directly compared the reactivity of peptide-αthioester 2 to a small panel of thiols and hydroxamic acids (SI Appendix, section 8). At pH 7, both acetyl hydroxamic acid and Asp(β-HA) were ∼10 or ∼30× less reactive to 2 relative to mercaptoethanesulfonate or cysteine, respectively. Thus, reaction of a peptide-αthioester with an N-terminal Asp(β-HA)-peptide was expected to be slower than reaction with an N-terminal Cys-peptide, but potentially fast enough to form the basis of a practical chemical ligation.
Chemical Ligation of Peptide-αThioesters and an Asp(β-HA)-Peptide.
Our kinetic data suggested two general strategies for chemical ligation involving capture of a peptide-αthioester by Asp(β-HA), and subsequent rearrangement of the resulting O-acyl isopeptide to give a native peptide bond. In the first strategy, a peptide-αthioester would be treated with an N-terminal Asp(β-HA)-peptide at a pH intermediate between the optima for each step. In the second strategy, the acyl capture step would be performed at mildly alkaline pH, where its rate is faster (pH 8–9); upon completion, the pH would be dropped to that optimal for the isopeptide rearrangement (pH 5–6). We first explored the one-step strategy.
Peptide-Ala-αthioester 2 (5 mM) was treated with 1.3 equivalents of N-terminal Asp(β-HA)-peptide 3 (6.5 mM) in 6 M guanidine hydrochloride, 200 mM phosphate, pH 7 buffer. After several hours, liquid chromatography-mass spectrometry (LC-MS) analysis of reaction mixture aliquots revealed a distribution of starting materials, intermediate O-acyl hydroxamic acid isopeptide 1, and ligation product 1′ (Fig. 3). The reaction was complete after 20 h, and was accompanied by formation of peptide-αcarboxylate derived from thioester 2 (compound 2b; ∼10%). A yield of 61% was obtained for ligation product 1′ after isolation by preparative HPLC, based on the limiting reagent 2.
Fig. 3.
Amide-forming chemical ligation of a peptide-αthioester and N-terminal Asp(β-HA)-peptide, at pH 7. The MS inset for the 2-h timepoint corresponds to isopeptide 1; the MS inset for the 20-h timepoint corresponds to amide product 1′. An isolated yield of 61% was obtained for 1′ after isolation by preparative HPLC.
To test the generality of the ligation, we studied the reactions of hydroxamic acid 3 with a range of peptide-Xaa-αthioesters (4a through 4g) at pH 7. Table 1 shows the yields of ligation products obtained after isolation by preparative HPLC (see SI Appendix, section 9 for LC-MS data). Reactions involving the Gly-αthioester, Ser-αthioester, and Tyr-αthioester were markedly faster than for the Ala-αthioester, and were complete within 15, 30, and 60 min, respectively. Reactions involving the Lys-αthioester and Trp-αthioester were intermediate in rate, being nearly complete within 2 h, and the Val-αthioester reacted sluggishly, as expected, requiring 48 h for complete conversion. In the case of the Lys-αthioester, lactam formation was observed to the extent of ∼10%. The tolerance of O-acyl hydroxamic acid isopeptide rearrangement to a variety of adjacent amino acid residues (Xaa-αO-acyl hydroxyamic acid) is in contrast to the rearrangements of S-acyl isopeptides derived from the Nα(ethanethiol) function (42), or of Staudinger ligation intermediates (3), which are facile only for Xaa-Gly and Gly-Xaa cases.
Table 1.
Isolated yields of HPLC-purified model ligation products YNFRXaa-D(β-HA)FYKLS-CONH2, obtained by ligation at pH 7
| Entry | Xaa | Ligation products | Isolated yield, % |
| 1 | Ala | 1′ | 61 (7.8 mg) |
| 3 | Leu | 5a | 41 (13 mg) |
| 2 | Gly | 5b | 73 (22 mg) |
| 4 | Val | 5c | 29 (5.7 mg) |
| 5 | Ser | 5d | 50 (13 mg) |
| 6 | Tyr | 5e | 41 (6.9 mg) |
| 7 | Lys | 5f | 39 (8.8 mg) |
| 8 | Trp | 5g | 64 (10 mg) |
A two-step ligation strategy was also explored, with the goal of decreasing the reaction time. Peptide-αthioester 2 (5 mM) was again treated with 1.3 equivalents of N-terminal Asp(β-HA)-peptide 3 (6.5 mM) in 6 M guanidine hydrochloride, 200 mM phosphate buffer, but this time at pH 8. After 15 min, peptide-αthioester 2 had been converted quantitatively to intermediate O-acyl hydroxamic acid isopeptide 1, and the reaction mixture was acidified to pH 6, to facilitate rearrangement of isopeptide 1 to peptide product 1′. The rearrangement was complete within 2 h (SI Appendix, Fig. S47), and ligation product 1′ was isolated in 75% yield after preparative HPLC (compared with 61% for the one-step approach).
A two-step ligation was also carried out using peptide-Leu-αthioester 4a, on an analytical scale. Similar to the case of Ala thioester 2, two-step ligation with 4a was complete after a total of 2 h 45 min (45 min at pH 8, and 2 h at pH 6; SI Appendix, Fig. S48). Similar quantities of peptide-αcarboxylate were formed by both the one-step and two-step procedures (∼5–10%), suggesting that this minor side product may be a general feature of the ligation (SI Appendix, Figs. S40 and S48).
Conversion of Asp(β-HA) to Asp or Asn.
The utility of Asp(β-HA)-mediated chemical ligation would be extended if an Asp(β-HA) residue could be converted into either Asp or Asn, postligation. We identified conditions for these transformations, and tested them on portions of the model ligation products 5a through 5g (Fig. 4 and SI Appendix, sections 11 and 12). Overall yields for ligation and subsequent conversion of Asp(β-HA) to either Asp or Asn—which include two preparative HPLC isolations—ranged from 15 to 54%, with an average of 32% (Table 2).
Fig. 4.
Conversion of an Asp(β-HA) residue to either Asn or Asp. (A) Reaction schemes. (B) LC-MS analysis of starting material and crude reaction mixtures, for a representative substrate.
Table 2.
Isolated yields for the transformation of model ligation products YNFRXaa-D(β-HA)FYKLS-CONH2 to either YNFRXaa-DFYKLS-CONH2 or YNFRXaa-NFYKLS-CONH2
| Entry | Xaa | Asn peptides | Isolated yield, % | Overall yield, % | Asp peptides | Isolated yield, % | Overall yield, % |
| 1 | Ala | 1a | 71 (3.5 mg) | 43 | 1b | 79 (4.4 mg) | 48 |
| 3 | Leu | 6a | 69 (3.3 mg) | 28 | 7a | 62 (3.5 mg) | 25 |
| 2 | Gly | 6b | 56 (2.8 mg) | 41 | 7b | 46 (5.2 mg) | 34 |
| 4 | Val | 6c | 74 (3.2 mg) | 21 | 7c | 91 (2.3 mg) | 26 |
| 5 | Ser | 6d | 65 (3.3 mg) | 33 | 7d | 48 (3.0 mg) | 24 |
| 6 | Tyr | 6e | 82 (3.8 mg) | 34 | 7e | 80 (2.5 mg) | 33 |
| 7 | Lys | 6f | 40 (2.2 mg) | 16 | 7f | 38 (1.7 mg) | 15 |
| 8 | Trp | 6g | 84 (3.0 mg) | 54 | 7g | 45 (1.9 mg) | 29 |
Overall yields for ligation and subsequent transformation of Asp(β-HA)—involving two HPLC purifications—are also shown.
Reduction of Asp(β-HA) to Asn was optimally achieved by treatment with Zn, at 37 °C, in 1 M ascorbic acid (Fig. 4 and SI Appendix, section 11). A variety of conditions, including Zn in acetonitrile/water (0.1% trifluoroacetic acid), Zn in acetic acid, and Zn in 1 M HCl were attempted with generally poorer reproducibility (43, 44). The reduction required ∼20 h for completion, and seemed to work better on smaller scale. We anticipate that given sufficient impetus, milder and homogeneous reaction conditions for the reduction of a hydroxamic acid to a carboxamide could be found.
Conversion of Asp(β-HA) to Asp proved straightforward. Nagasawa and coworkers (45) reported the liberation of benzoic acid and nitrous oxide from benzohydroxamic acid, under nitrosation conditions. Because nitrosation of unprotected peptide-αhydrazides to yield peptide-αacyl azides (46) is enjoying widespread use, we decided to adapt this protocol for the oxidative hydrolysis of hydroxamic acids. Clean conversion of Asp(β-HA) to Asp was observed upon treatment of substrates 5a through 5g with NaNO2 in pH 1 phosphate buffer at 0 °C, for 1–2 h (Fig. 4, Table 2, and SI Appendix, section 12).
To confirm the covalent structure of the ligation products, we prepared authentic peptides corresponding to products 7a and 7d. These compounds were indistinguishable from 7a and 7d prepared by chemical ligation and subsequent conversion of Asp(β-HA) to Asp (SI Appendix, Figs. S65 and S66). To investigate the chiral integrity of ligation products 7a and 7d, we prepared diastereomeric epi-7a and epi-7d, containing either a d-Leu or d-Ser residue, respectively, at the site of ligation. Diastereomer epi-7a was not detected in the crude product 7a, suggesting that ligation proceeded with negligible epimerization of the O-acyl hydroxamic acid isopeptide intermediate (SI Appendix, Fig. S65). Crude product 7d, on the other hand, contained as much as 2% of diastereomer epi-7d (SI Appendix, Fig. S66). The propensity of peptide-Ser-αthioesters to epimerize has been noted (47), and may be responsible for the comparatively worse outcome observed in this case.
Chemical Synthesis of the Z Domain.
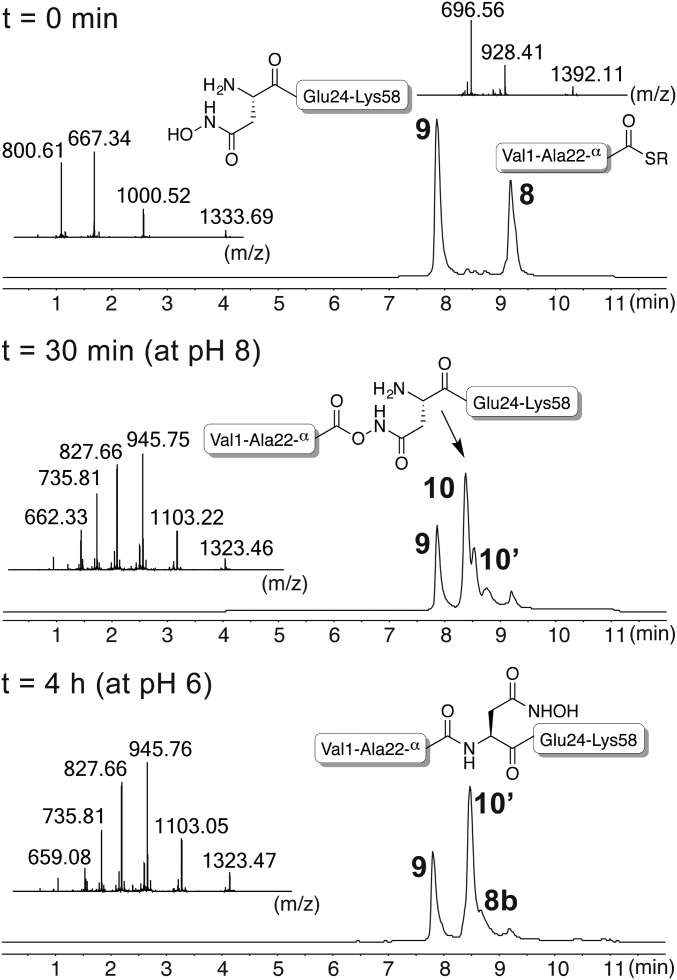
We investigated the utility of Asp(β-HA)-mediated chemical ligation in the synthesis of the Z domain (48). The most successful approach involved a single chemical ligation to form an Ala-Asp(β-HA) peptide bond (a Leu-Asn bond in the wild-type sequence). LC-MS data showing the reaction of peptide-αthioester 8 with N-terminal Asp(β-HA)-peptide 9 in a two-step ligation are shown in Fig. 5. After 30 min at pH 8, complete conversion to O-acyl hydroxamic acid ligation product 10 was confirmed, and the pH was adjusted to 6. After 4 h, LC-MS analysis showed conversion of 10 to an isomeric species 10′, presumed to be the desired amide product. As for our model studies, formal hydrolysis of thioester 8 occurred to the extent of ∼10% (product 8b). Desired product 10′ was isolated by preparative HPLC in 49% yield.
Fig. 5.
Synthesis of a 58-mer peptide by Asp(β-HA)-mediated chemical ligation. Thioester VDNKFNKEQQ10NAFYEILHLP20NA-αCO-S-C6H4-CH2-CO2H 8 and hydroxamic acid D(β-HA)EEQRNAF30IQSLKDDPSQ40SANILLAEAKK50LNDAQAPK58-αCONH2 9 were first combined at pH 8 for 30 min, to generate isopeptide 10. Then, the reaction mixture was acidified to pH 6; formation of amide product 10′ was confirmed after 4 h. The MS insets correspond to isopeptide 10 (30-min timepoint) and amide product 10′ (4-h timepoint), respectively. Product 10′ was obtained in 49% yield after isolation by preparative HPLC.
To prepare Z domain molecules with entirely natural side chains, the Asp(β-HA) residue of ligation product 10′ was converted to either Asn or Asp. Treatment of 10′ with either Zn in 1 M ascorbic acid (SI Appendix, Fig. S69) or NaNO2 (SI Appendix, Fig. S70) furnished the desired reaction products 10a and 10b in 99% and 71% yield, respectively, corresponding to overall yields of 49% and 35% for the ligation and Asp(β-HA) transformation sequences.
Significance.
In this work, we have documented the facile rearrangement, via O,N-acyl shift, of O-acyl isopeptides derived from Asp(β-HA). When combined with the ability of Asp(β-HA) to form O-acyl isopeptides by reaction with peptide-αthioesters under mild aqueous conditions, this observation forms the basis of an amide-forming chemical ligation reaction. Conversion of the Asp(β-HA) residue to either aspartic acid or asparagine postligation enables the synthesis of native peptides by this strategy. This reaction sequence is analogous to the ligation/desulfurization approach to protein synthesis by native chemical ligation, and is complementary to native chemical ligation with β-thiol-Asn (49, 50), β-thiol-Asp (51), or auxiliaries (52, 53) which enable ligation at the same Xaa-Asp and Xaa-Asn sites.
Before this work, cysteine, selenocysteine, and the variety of nonnatural amino acids functionalized with thiols and selenols were the only amino acids known to form isopeptides with acyl donors under similarly mild conditions. A variety of isopeptides defined by attachment of a peptide C terminus to the side chain of serine, threonine, tyrosine, or tryptophan have been reported (33). But, synthesis of these compounds requires the use of highly activated esters and appropriate side-chain protection, rendering them of limited utility for chemical ligation.
A number of considerations should be made when applying Asp(β-HA)-mediated chemical ligations in a new context. Although practical reaction times have been demonstrated (hours), the reaction is ∼30-fold slower than native chemical ligation, and requires the use of peptide-αarylthioesters. The reaction appears compatible with a range of peptide-Xaa-αthioesters, with the exception of β-branched residues (unacceptably slow reaction rate) and Lys (lactam formation). Suitable Xaa-Asp or Xaa-Asn sites should be chosen accordingly, to maximize the chance of success.
A side reaction observed consistently in chemical ligations involving N-terminal Asp(β-HA) and peptide-αthioesters was apparent hydrolysis of the peptide-αthioester reaction partner, to the extent of ∼10%. The hydrolytic stabilities of peptide-αthioesters and peptide-αO-acyl hydroxamic acids employed in this work were similar at mildly alkaline pH (SI Appendix, sections 17 and 18); therefore, the origin of this side product is unclear. Formation of peptide-αcarboxylate to some degree may be an inherent limitation of chemical ligations involving N-terminal Asp(β-HA), but further studies are required to resolve this issue.
One other feature of ligations involving N-terminal Asp(β-HA) should be mentioned: addition of the thiol reagent 4-mercaptophenylacetic acid (a catalyst for native chemical ligation), at a concentration of 50 mM, stopped productive reaction, with appearance of peptide-αcarboxylate and conversion of N-terminal Asp(β-HA) to Asn [potentially from reduction of the O-acyl hydroxamate, by a mechanism described recently (44); SI Appendix, Fig. S81]. Addition of tris(2-carboxyethyl)phosphine at 20 mM was tolerated, but approximately doubled the extent of peptide-αcarboxylate formation (again, presumably via reduction of the O-acyl isopeptide intermediate; SI Appendix, Fig. S82).
Conclusion
The unique, mutual reactivity of peptide-αthioesters and N-terminal cysteine-peptides forms a basis for the total synthesis of proteins by native chemical ligation. With continued development, we anticipate that the reaction of N-terminal Asp(β-HA)-peptides (or structural variants) with suitable acyl derivatives may become a useful complementary method, and potentially form the basis of an “orthogonal” amide-forming ligation that can proceed in the presence of an N-terminal cysteine-peptide and/or a thioester. This would represent a powerful advance, enabling, for example, fully convergent protein synthesis without the need for protecting groups or intermediate functional group conversions (e.g., conversion of a peptide-αhydrazide ligation product to a peptide-αthioester) (2).
Methods
Ligations.
In a one-step ligation procedure, peptide-αthioester and Asp(β-HA)-peptide were dissolved in 6 M guanidine hydrochloride, 200 mM phosphate, pH 7 buffer, and combined in a plastic tube (final concentrations of 5 and 6.5 mM, respectively). The reaction mixture was allowed to stand at ambient temperature, and reaction mixture aliquots were periodically withdrawn for LC-MS analysis. Upon completion, the product was isolated by preparative HPLC, as described in SI Appendix.
In a two-step ligation procedure, peptide-αthioester and Asp(β-HA)-peptide were combined as above, but at pH 8. After 30 min, a reaction mixture aliquot was analyzed by LC-MS, to confirm complete consumption of thioester. Once confirmed, the reaction mixture was adjusted to pH 6, by dilution into 10 volumes of 6 M guanidine hydrochloride, 200 mM phosphate, pH 6 buffer. Upon completion, the product was isolated by HPLC.
Conversion to Asn.
An Asp(β-HA)-containing peptide was dissolved to 1 mg/mL in 1.0 M ascorbic acid, and transferred to 3-mL glass vials in 1-mL portions (the reduction was most effective when the reaction mixture was less than 1 mL). Zinc powder (100 mg) and a magnetic stir bar were added to each vial. The vials were suspended in a temperature-controlled water bath (37 °C), and the suspensions were stirred vigorously for 20 h. Upon completion, the reaction mixtures were pooled, diluted with 95/5 water/acetonitrile, and filtered through a 0.2-μm polytetrafluoroethylene syringe filter. The product was isolated by HPLC.
Conversion to Asp.
An Asp(β-HA)-containing peptide was dissolved in 6 M guanidine hydrochloride, 200 mM phosphate, pH 3 buffer (2 mM), in a glass vial. The solution was adjusted to pH 1, and the vial was suspended in an ice/water bath. Next, 0.2 M NaNO2 in deionized water was added, to bring the concentration to 10 mM. Upon completion, residual oxidant was quenched by addition of 0.5 M ascorbic acid. The product was isolated by HPLC.
Supplementary Material
Acknowledgments
We thank Ethan Evans, Alex Vinogradov, and Prof. JoAnne Stubbe for insightful discussions, and Alex Mijalis for technical assistance with automated flow-based peptide synthesis. This work was supported by Defense Advanced Research Projects Agency Award 023504-001 (to B.L.P.), a Bristol-Myers Squibb unrestricted grant in Synthetic Organic Chemistry (B.L.P.), a Novartis Early Career Award (B.L.P.), and Novo Nordisk STAR Programme.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718356115/-/DCSupplemental.
References
- 1.Schnölzer M, Kent SBH. Constructing proteins by dovetailing unprotected synthetic peptides: Backbone-engineered HIV protease. Science. 1992;256:221–225. doi: 10.1126/science.1566069. [DOI] [PubMed] [Google Scholar]
- 2.Kent SBH. Total chemical synthesis of proteins. Chem Soc Rev. 2009;38:338–351. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson BL, Soellner MB, Raines RT. Chemical synthesis of proteins. Annu Rev Biophys Biomol Struct. 2005;34:91–118. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 5.Rose K. Facile synthesis of homogeneous artificial proteins. J Am Chem Soc. 1994;116:30–33. [Google Scholar]
- 6.Dirksen A, Hackeng TM, Dawson PE. Nucleophilic catalysis of oxime ligation. Angew Chem Int Ed Engl. 2006;45:7581–7584. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson BL, Kiessling LL, Raines RT. Staudinger ligation: A peptide from a thioester and azide. Org Lett. 2000;2:1939–1941. doi: 10.1021/ol0060174. [DOI] [PubMed] [Google Scholar]
- 8.Saxon E, Armstrong JI, Bertozzi CR. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org Lett. 2000;2:2141–2143. doi: 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]
- 9.Bode JW, Fox RM, Baucom KD. Chemoselective amide ligations by decarboxylative condensations of N-alkylhydroxylamines and α-ketoacids. Angew Chem Int Ed Engl. 2006;45:1248–1252. doi: 10.1002/anie.200503991. [DOI] [PubMed] [Google Scholar]
- 10.Harmand TJ, Murar CE, Bode JW. Protein chemical synthesis by α-ketoacid-hydroxylamine ligation. Nat Protoc. 2016;11:1130–1147. doi: 10.1038/nprot.2016.052. [DOI] [PubMed] [Google Scholar]
- 11.Tam JP, Miao Z. Stereospecific pseudoproline ligation of N-terminal serine, threonine, or cysteine-containing unprotected peptides. J Am Chem Soc. 1999;121:9013–9022. [Google Scholar]
- 12.Li X, Lam HY, Zhang Y, Chan CK. Salicylaldehyde ester-induced chemoselective peptide ligations: Enabling generation of natural peptidic linkages at the serine/threonine sites. Org Lett. 2010;12:1724–1727. doi: 10.1021/ol1003109. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Xu C, Lam HY, Lee CL, Li X. Protein chemical synthesis by serine and threonine ligation. Proc Natl Acad Sci USA. 2013;110:6657–6662. doi: 10.1073/pnas.1221012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp DS, Carey RI. Synthesis of a 39-peptide and a 25-peptide by thiol capture ligations: Observation of a 40-fold rate acceleration of the intramolecular O,N-Acyl-Transfer reaction between peptide-fragments bearing only cysteine protective groups. J Org Chem. 1993;58:2216–2222. [Google Scholar]
- 15.Liu CF, Tam JP. Chemical ligation approach to form a peptide bond between unprotected peptide segments: Concepts and model study. J Am Chem Soc. 1994;116:4149–4153. [Google Scholar]
- 16.Liu CF, Tam JP. Peptide segment ligation strategy without use of protecting groups. Proc Natl Acad Sci USA. 1994;91:6584–6588. doi: 10.1073/pnas.91.14.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson PE. Native chemical ligation combined with desulfurization and deselenization: A general strategy for chemical protein synthesis. Isr J Chem. 2011;51:862–867. [Google Scholar]
- 18.Malins LR, Payne RJ. Modern extensions of native chemical ligation for chemical protein synthesis. In: Liu L, editor. Protein Ligation and Total Synthesis I. Springer; Berlin: 2015. pp. 27–87. [DOI] [PubMed] [Google Scholar]
- 19.Burke HM, McSweeney L, Scanlan EM. Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology. Nat Commun. 2017;8:15655. doi: 10.1038/ncomms15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Tam JP. Orthogonal coupling of unprotected peptide segments through histidyl amino terminus. Tetrahedron Lett. 1997;38:3–6. [Google Scholar]
- 21.Payne RJ, Ficht S, Greenberg WA, Wong CH. Cysteine-free peptide and glycopeptide ligation by direct aminolysis. Angew Chem Int Ed Engl. 2008;47:4411–4415. doi: 10.1002/anie.200705298. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GL, et al. Peptide ligations accelerated by N-terminal aspartate and glutamate residues. Org Lett. 2011;13:4770–4773. doi: 10.1021/ol2017356. [DOI] [PubMed] [Google Scholar]
- 23.Hondal RJ, Nilsson BL, Raines RT. Selenocysteine in native chemical ligation and expressed protein ligation. J Am Chem Soc. 2001;123:5140–5141. doi: 10.1021/ja005885t. [DOI] [PubMed] [Google Scholar]
- 24.Gieselman MD, Xie L, van Der Donk WA. Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org Lett. 2001;3:1331–1334. doi: 10.1021/ol015712o. [DOI] [PubMed] [Google Scholar]
- 25.Metanis N, Keinan E, Dawson PE. Traceless ligation of cysteine peptides using selective deselenization. Angew Chem Int Ed Engl. 2010;49:7049–7053. doi: 10.1002/anie.201001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malins LR, Mitchell NJ, McGowan S, Payne RJ. Oxidative deselenization of selenocysteine: Applications for programmed ligation at serine. Angew Chem Int Ed Engl. 2015;54:12716–12721. doi: 10.1002/anie.201504639. [DOI] [PubMed] [Google Scholar]
- 27.Durek T, Alewood PF. Preformed selenoesters enable rapid native chemical ligation at intractable sites. Angew Chem Int Ed Engl. 2011;50:12042–12045. doi: 10.1002/anie.201105512. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell NJ, et al. Rapid additive-free selenocystine-selenoester peptide ligation. J Am Chem Soc. 2015;137:14011–14014. doi: 10.1021/jacs.5b07237. [DOI] [PubMed] [Google Scholar]
- 29.Bauer L, Exner O. The chemistry of hydroxamic acids and N-hydroxyimides. Angew Chem Int Ed Engl. 1974;13:376–384. [Google Scholar]
- 30.Illuminati G, Mandolini L. Ring closure reactions of bifunctional chain molecules. Acc Chem Res. 1981;14:95–102. [Google Scholar]
- 31.Kemp DS, et al. Peptide synthesis by prior thiol capture. 4. Amide bond formation: The effect of a side-chain substituent on the rates of intramolecular O,N-acyl transfer. J Org Chem. 1986;51:3320–3324. [Google Scholar]
- 32.Rojas CM, Rebek J. Convergent functional groups: Intramolecular acyl transfer through a 34-membered ring. J Am Chem Soc. 1998;120:5120–5121. [Google Scholar]
- 33.Panda SS, Hall CD, Oliferenko AA, Katritzky AR. Traceless chemical ligation from S-, O-, and N-acyl isopeptides. Acc Chem Res. 2014;47:1076–1087. doi: 10.1021/ar400242q. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara S, Shin KH, Hess GP. An approach to the specific cleavage of peptide bonds. I. The acyl migration in dipeptides containing hydroxyamino acids in anhydrous hydrogen fluoride. J Am Chem Soc. 1962;84:4921–4928. [Google Scholar]
- 35.Shao Y, Paulus H. Protein splicing: Estimation of the rate of O-N and S-N acyl rearrangements, the last step of the splicing process. J Pept Res. 1997;50:193–198. doi: 10.1111/j.1399-3011.1997.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 36.Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Novel and efficient synthesis of difficult sequence-containing peptides through O-N intramolecular acyl migration reaction of O-acyl isopeptides. Chem Commun (Camb) 2004:124–125. doi: 10.1039/b312129a. [DOI] [PubMed] [Google Scholar]
- 37.Sohma Y, et al. The ‘O-acyl isopeptide method’ for the synthesis of difficult sequence-containing peptides: Application to the synthesis of Alzheimer’s disease-related amyloid beta peptide (Abeta) 1-42. J Pept Sci. 2005;11:441–451. doi: 10.1002/psc.649. [DOI] [PubMed] [Google Scholar]
- 38.Yoshiya T, Kawashima H, Sohma Y, Kimura T, Kiso Y. O-acyl isopeptide method: Efficient synthesis of isopeptide segment and application to racemization-free segment condensation. Org Biomol Chem. 2009;7:2894–2904. doi: 10.1039/b903624e. [DOI] [PubMed] [Google Scholar]
- 39.Raj M, Wu H, Blosser SL, Vittoria MA, Arora PS. Aldehyde capture ligation for synthesis of native peptide bonds. J Am Chem Soc. 2015;137:6932–6940. doi: 10.1021/jacs.5b03538. [DOI] [PubMed] [Google Scholar]
- 40.Um IH, Kim GR, Kwon DS. The effects of solvation and polarizability on the reaction of S-para-nitrophenyl thiobenzoate with various anionic nucleophiles. Bull Korean Chem Soc. 1994;15:585–589. [Google Scholar]
- 41.Johnson ECB, Kent SBH. Insights into the mechanism and catalysis of the native chemical ligation reaction. J Am Chem Soc. 2006;128:6640–6646. doi: 10.1021/ja058344i. [DOI] [PubMed] [Google Scholar]
- 42.Canne LE, Bark SJ, Kent SBH. Extending the applicability of native chemical ligation. J Am Chem Soc. 1996;118:5891–5896. [Google Scholar]
- 43.Weller CE, Huang W, Chatterjee C. Facile synthesis of native and protease-resistant ubiquitylated peptides. ChemBioChem. 2014;15:1263–1267. doi: 10.1002/cbic.201402135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weller CE, et al. Aromatic thiol-mediated cleavage of N-O bonds enables chemical ubiquitylation of folded proteins. Nat Commun. 2016;7:12979. doi: 10.1038/ncomms12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirota FN, DeMaster EG, Lee MJC, Nagasawa HT. Generation of nitric oxide and possibly nitroxyl by nitrosation of sulfohydroxamic acids and hydroxamic acids. Nitric Oxide. 1999;3:445–453. doi: 10.1006/niox.1999.0257. [DOI] [PubMed] [Google Scholar]
- 46.Fang GM, et al. Protein chemical synthesis by ligation of peptide hydrazides. Angew Chem Int Ed Engl. 2011;50:7645–7649. doi: 10.1002/anie.201100996. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami T, Aimoto S. The use of a cysteinyl prolyl ester (CPE) autoactivating unit in peptide ligation reactions. Tetrahedron. 2009;65:3871–3877. [Google Scholar]
- 48.Nilsson B, et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 49.Sato K, et al. The total chemical synthesis of the monoglycosylated GM2 ganglioside activator using a novel cysteine surrogate. Chem Commun (Camb) 2015;51:9946–9948. doi: 10.1039/c5cc02967h. [DOI] [PubMed] [Google Scholar]
- 50.Sayers J, Thompson RE, Perry KJ, Malins LR, Payne RJ. Thiazolidine-protected β-thiol asparagine: Applications in one-pot ligation-desulfurization chemistry. Org Lett. 2015;17:4902–4905. doi: 10.1021/acs.orglett.5b02468. [DOI] [PubMed] [Google Scholar]
- 51.Thompson RE, Chan B, Radom L, Jolliffe KA, Payne RJ. Chemoselective peptide ligation-desulfurization at aspartate. Angew Chem Int Ed Engl. 2013;52:9723–9727. doi: 10.1002/anie.201304793. [DOI] [PubMed] [Google Scholar]
- 52.Brik A, Ficht S, Yang YY, Bennett CS, Wong CH. Sugar-assisted ligation of N-linked glycopeptides with broad sequence tolerance at the ligation junction. J Am Chem Soc. 2006;128:15026–15033. doi: 10.1021/ja065601q. [DOI] [PubMed] [Google Scholar]
- 53.Lutsky M-Y, Nepomniaschiy N, Brik A. Peptide ligation via side-chain auxiliary. Chem Commun (Camb) 2008:1229–1231. doi: 10.1039/b718945a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.