Significance
Cooperativity in substrate binding to a protein often involves some kind of interaction between distant binding sites mediated by the protein structure. Experimental studies indicate that the cooperative effect in protein kinase A relies on a shift in conformational equilibrium of the catalytic subunit, but its exact molecular basis remains unclear. Our molecular dynamics simulations provide atomistic insight into conformational states visited by the enzyme and its complexes and suggest a plausible mechanistic model contributing to cooperative substrate binding. Intriguingly, an important role seems to be played by water molecules that occupy two conserved hydration sites within the catalytic subunit.
Keywords: allostery, protein hydration, protein kinases, protein dynamics
Abstract
Substrate binding cooperativity in protein kinase A (PKA) seems to involve allosteric coupling between the two binding sites. It received significant attention, but its molecular basis still remains not entirely clear. Based on long molecular dynamics of PKA and its complexes, we characterized an allosteric pathway that links ATP binding to the redistribution of states adopted by a protein substrate positioning segment in favor of those that warrant correct binding. We demonstrate that the cooperativity mechanism critically depends on the presence of water in two distinct, buried hydration sites. One holds just a single water molecule, which acts as a switchable hydrogen bond bridge along the allosteric pathway. The second, filled with partially disordered solvent, is essential for providing a smooth free energy landscape underlying conformational transitions of the peptide binding region. Our findings remain in agreement with experimental data, also concerning the cooperativity abolishing effect of the Y204A mutation, and indicate a plausible molecular mechanism contributing to experimentally observed binding cooperativity of the two substrates.
Protein kinases (PKs) transfer a phosphate group from nucleoside triphosphate to specific protein substrates, thereby changing their enzymatic activity or propensity to associate with other proteins to exert further effects (1). Thus, they play a role in molecular switches responsible for regulation of various cellular activities. As such, during the evolution process, kinases traded their enzymatic efficiency for the ability to unambiguously switch between “on” and “off” states and to select protein substrates with high specificity (2). Since the tuning of kinase function relies on a complex network of interactions with cofactors and regulatory subunits, it inherently involves an array of subtle allosteric effects (3–9). Malfunction of this regulatory mechanism is associated with a variety of diseases ranging from cancer to immunological and metabolic disorders, motivating the need for its thorough investigation not only to uncover general principles of protein mechanics but also to guide therapeutic and drug design strategies (10).
An interesting functional aspect observed in some kinases is substrate binding cooperativity. Experimental studies of the cyclic AMP-dependent PK (PKA), considered to be a prototypical member of the kinase family, indicate that binding first the ATP increases the affinity toward the protein substrate (11–13). A puzzling fact is that a single point mutation of tyrosine 204, which does not form interactions with any of the substrates, to alanine (Y204A) decouples ligand binding to the catalytic subunit but does not alter its crystal structure (11, 14). Extensive NMR measurements and molecular dynamics (MD) simulations suggest that the allosteric effect relies on changes in relative populations of states that are sampled by the mutant and the wild-type protein (7, 11). The degree of cooperativity apparently varies, depending on the kind of bound nucleotide (13). Being the strongest for ATP, it gradually diminishes with a decreasing number of phosphate groups. Intriguingly, however, an ATP analog, ATPC, in which the oxygen atom linking and phosphate groups is replaced by a methylene group, does not induce binding cooperativity at all (13). This indicates that the cooperative effect not only is triggered by a nucleotide becoming simply sandwiched between the two kinase lobes but also involves more local interactions. Still, however, its exact mechanism remains unclear.
In this study, we use long MD simulations to investigate the interplay between the two ligands binding to the active (phosphorylated) form of PKA. In addition to looking solely at involved biomolecules, we consider also the influence of aqueous solvent. The comparison of crystallographic structures representing members of distinct kinase families reveals the presence of at least several buried water molecules whose positions within the protein core are similarly well preserved as amino acid types in many functionally important locations (15, 16). It remains unknown whether such internal water molecules play any specific role. The participance of water in allosteric networks has been indeed recently demonstrated in the case of Aurora kinase A (17), supporting the idea that specific hydration patterns might have evolved side by side with a macromolecular structure to aid in protein function.
Our results indicate that aside from global structural oscillations of the entire PKA catalytic subunit, the cooperativity in ligand binding may be also mediated by a distinct allosteric pathway. It is supposed to link the nucleotide binding site through the elements of so-called DFG and HRD motifs, named by the triplets of respective amino acids, with the region of the P + 1 loop involved directly in protein substrate positioning for catalysis (2). Furthermore, we demonstrate that an important functional role is played by two conserved hydration sites. One is directly involved in signal transduction by providing a switchable hydrogen bond bridge between the DFG and HRD regions. The second smooths out the free energy landscape that governs P + 1 loop conformations, enabling bimodal conformational transitions whose fine-tuning is the end effect of the entire allosteric mechanism.
Results
Substrate Binding.
Kinase–peptide interactions.
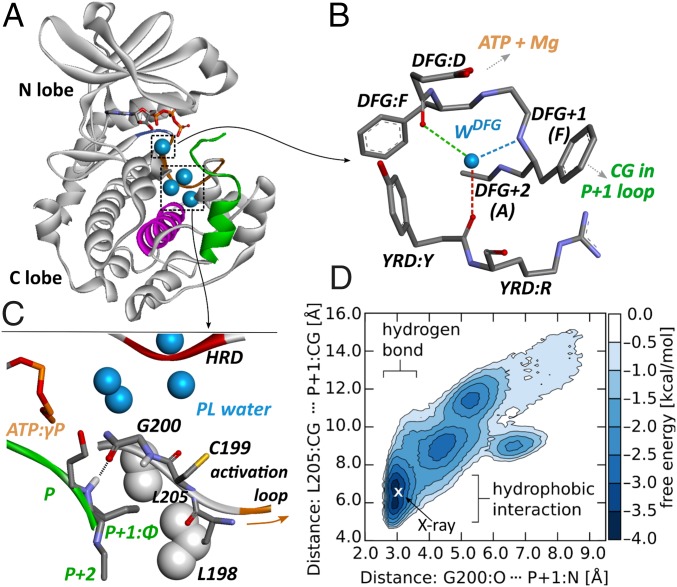
Peptide binding to the catalytic subunit is mediated by residues located predominantly in the large, C-lobe (Fig. 1A) (18). Typically, they interact with up to four peptide amino acids on either side of the phosphate-accepting residue (P-site) (19), including the region responsible for specific recognition (R-R-X-S-, where “S” is the phosphate accepting serine and “X” and “” represent “any” and “large hydrophobic” amino acids, respectively) (20). Precise positioning of the peptide P-site is achieved through a hydrogen bond of the P + 1 main chain amide nitrogen with the G200 carbonyl group and further stabilized by hydrophobic interactions of the P + 1 side chain with a nonpolar cavity formed by L198, P202, and L205 (Fig. 1C). Notably, as indicated by NMR data, the C-terminal side of the peptide recognition sequence retains a significant degree of mobility upon kinase binding, especially in the slow, microsecond to millisecond, time scale (12). This observation is further supported by conformational variability of the P-site and disorder of P + 2 and P + 3 peptide residues reported in crystal structures (20, 21).
Fig. 1.
PKA - peptide binding. (A) Peptide binding and hydration sites within the catalytic subunit of PKA, (B) DFG hydration site, (C) water pocket and details of peptide binding, and (D) free energy landscape for SP20 interactions with the P + 1 loop.
Our simulations of a ternary complex comprising PKA, ATP, and either SP20 or kemptide-like (KL) peptide substrates confirm the dynamic nature of kinase–peptide interactions within the P + 1 loop region. In the most probable configuration, both interactions observed in crystal structures, that is the hydrogen bond between the P + 1 site and G200 together with hydrophobic contact maintained by the P + 1 side chain, are indeed present. They are observed to repeatedly break and reestablish, however, subject to conformational variations of the peptide and kinase P + 1 loop (Fig. 1D).
P + 1 loop fluctuations.
To characterize structural variability of the kinase P + 1 loop, we performed an rmsd-based cluster analysis of its conformations (see Supporting Information for details). Independent clustering of trajectories obtained for ternary complexes including SP20 or KL peptide substrates indicated that in each case two clusters gathered more than 99% of simulation frames (Fig. 2A). Notably, the centroids of those two clusters were equivalent for both simulation sets (with mutual rmsd well below the 1.7 Å cutoff limit used for clustering; Fig. 2B). Consequently, the P + 1 kinase loop can be considered to switch bimodally between two major states. The dominant one, representing a crystal-like conformation (rmsd 1.1 Å with respect to the 3FJQ structure; Fig. 2B), is present for, on average, simulation time ( and for SP20 and KL simulations, respectively). Projection of individual trajectories on those two conformations reveals that the switching occurs in the time scale of tens to hundreds of nanoseconds (Fig. 2C).
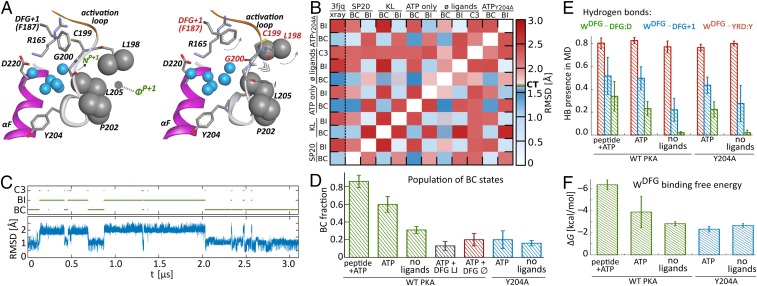
Fig. 2.
P + 1 loop states and DFG water binding. (A) Centroids of clusters representing P + 1 loop conformations in BC and BI states. (B) Rmsd matrix illustrating the similarity of BC and BI clusters in all systems (CT, 1.7 Å cutoff threshold used for clustering). (C) Time evolution of P + 1 loop rmsd with respect to the 3FJQ structure, illustrating bimodal switching between clusters during a representative MD trajectory. (D) Population of BC states in all considered cases. (E) Fraction of hydrogen bonds to DFG water. (F) Binding free energy of DFG water to PKA.
The two P + 1 loop conformations apparently differ in their ability to maintain stable interactions with the peptide substrate (Fig. 2A). In the dominant conformation, in the following referred to as “binding competent” (BC), the hydrogen bond between peptide P + 1 site and kinase G200 is formed in cases, whereas in the less populated state—“binding incompetent” (BI)—its fraction is only . This difference in peptide binding competence can be explained by structural analysis of BC and BI cluster centroids (Fig. 2A). In the BI configuration, the ability to form two important contacts between the P + 1 loop region and peptide substrate is severely impaired. Compared with the BC state, the DFG + 1 phenylalanine is shifted toward the HRD arginine and displaces C199 side chain in the direction of peptide P + 1 residue. The reorientation of C199 is accompanied by the rotation of the L198–G200 main chain, which turns the carbonyl oxygen of G200 in a direction less favorable for accepting a hydrogen bond from the peptide P + 1 amide nitrogen. Furthermore, this rotation leads to the disruption of the hydrophobic pocket involved in P + 1 side chain binding.
Shifts in P + 1 loop populations.
Similar, essentially bimodal P + 1 loop behavior can be identified also in the absence of peptide substrate—that is, in simulations of PKA with only ATP and Mg2+ ions bound—or of the phosphorylated apo structure (Fig. 2B). In the latter case, an additional, third nonnegligible cluster of P + 1 loop conformations accounted for 0.26 of total accumulated simulation time. It represents a configuration in which the C199–G200 main chain is flipped with respect to the BC structure even further than in the BI case, making the formation of the putative hydrogen bond with peptide substrate rather improbable.
Intriguingly, the population of BC configuration appears to be sensitive to ligand binding. In simulation frames, in which the peptide P + 1 residue maintains binding contacts with kinase P + 1 loop, BC configuration is observed with the probability of (Fig. 2D). In the absence of peptide substrate, but with ATP and two Mg2+ ions still present in the ATP binding site, BC fraction is —that is, similar to the one observed for the ternary complex—irrespective of whether P + 1 loop–peptide interaction is formed. Remarkably, however, upon the removal of ATP and Mg2+, the BC population drops to only , despite the fact that the P + 1 loop does not have any direct contacts with the ATP binding region.
These findings indicate a kind of “conformational preselection” mechanism, in which binding of ATP shifts the population of the P + 1 binding segment toward conformations preferably interacting with the not-yet-bound peptide substrate. As we point out in the following, this mechanism seems to critically rely on the presence of local hydration structures.
Water Influence.
Involved hydration structures.
Structural alignment of crystal structures representing active catalytic subunits of diverse kinases reveals several highly conserved hydration sites (15, 16). Two of them are found between the two substrate binding regions. The first one, called the “DFG site,” holds just a single water molecule that is tightly embedded between the DFG and HRD kinase motifs and forms up to four hydrogen bonds with surrounding amino acids (Fig. 1B), depending on the type of DFG + 2 residue. The second, called in the following “PL pocket,” is localized between the F-helix, HRD motif, and P + 1 loop (Fig. 1C) and holds several water molecules.
The DFG site in the active PKA offers three hydrogen-bonding possibilities to its centrally located water molecule (Fig. 1B). The most stable interaction is maintained with the main chain oxygen atom of tyrosine in the YRD motif (note that in PKA histidine, H, in the HRD motif is replaced by tyrosine, Y) and is present during % of the simulation time (Fig. 2E). Strikingly, the stability of the two remaining hydrogen bonds turns out to depend on binding of ATP and, to a lesser degree, peptide substrate. ATP and Mg2+ presence, which is directly sensed by the side chain of the DFG aspartic acid, D184, significantly increases the population of hydrogen bond between its main chain carbonyl group and water molecule. This, in turn, apparently leads to further stabilization of water interaction with the main chain nitrogen of the DFG + 1 residue—that is, phenylalanine in PKA. The strengthening of hydrogen bonding upon ligand binding is reflected by an increase in water affinity to the DFG hydration site by almost 4 kcal/mol, as measured between the apo structure and the ternary complex (Fig. 2F). Presumably, this considerable thermodynamic effect is necessary to stabilize the DFG + 1 residue in a conformation in which the interaction of its bulky, hydrophobic side chain with C199 from the P + 1 loop shifts the P + 1 loop population toward the BC rather than the BI configuration.
To test this hypothesis, we carried out additional simulations of PKA in complex with ATP and Mg2+ ions, in which the hydrogen bonding of DFG water was artificially switched off, or the DFG hydration site was forced to remain empty. In the first scenario, the DFG water molecule was replaced by a neutral, spherical particle that interacted with the rest of the system only by water-like Lennard–Jones (LJ) potential. In the second scenario, the vacancy of the DFG site was enforced by placing there a water-sized dummy atom that was impenetrable to solvent molecules but otherwise did not interact with any other atoms (except for relative position restraints with respect to selected protein atoms; see Materials and Methods for details).
Intriguingly, despite the presence of ATP and Mg2+ ions, in both scenarios the BC population of the P + 1 loop turned out to be even lower than in the case of unperturbed apo structure (Fig. 2D). This drop in BC fraction was more pronounced for LJ-bound rather than the completely vacated DFG hydration site. Altogether, it indicates that the water molecule in the DFG site acts not merely as a space-filling object but rather as a hydrogen bond bridge connecting the DFG and HRD motifs. Its specific, directional interactions seem to be an integral part of the communication pathway that links the ATP/Mg2+-sensing DFG aspartic acid with distant C199, which is involved in peptide positioning.
PL pocket.
If the DFG water can be thought of as an actuator, solvent in the PL pocket seems to act as a lubricant, ensuring the mobility of the entire mechanism. Viewed in crystal structures, the PL pocket appears to contain typically six water molecules. Contrary to this static picture, MD simulations reveal a pronounced dynamics of water content and structure within this area. The actual number of observed water molecules varies between 2 and 11 (Fig. S1), reflecting large conformational fluctuations of the neighboring P + 1 loop. Those fluctuations are justified by the fact that the region of the polypeptide chain that includes the P + 1 loop and extends from phosphorylable residue in the activation loop until the residue preceding conserved tyrosine 204 does not form any stable direct hydrogen bonds with the kinase core (Fig. 3C). Taking this into account, it appears that, owing to its localization between the flexible P + 1 loop and the rigid kinase core, water inside the PL pocket serves (i) as a cushion allowing for reversible attachment/detachment of the P + 1 and activation loops upon kinase activation/desactivation and (ii) as a lubricant facilitating conformational mobility of the peptide recognition segment. The latter is necessary for kinase ability to maintain interactions with the flexible substrate polypeptide chain and apparently also serves as a mechanism for the modulation of binding affinity.
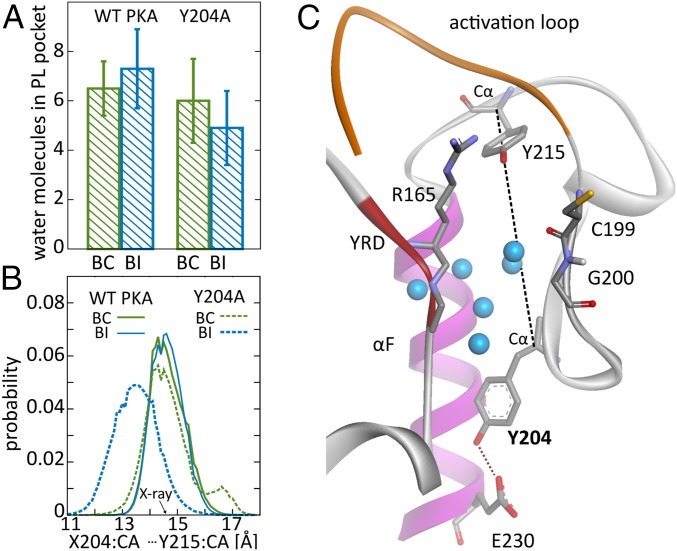
Fig. 3.
PL pocket in WT and Y204A PKA. (A) Water content in the PL pocket for BC and BI states in WT and mutant PKA. (B) Distributions of distance between Cα atoms of residues 204 and 215 for BC and BI states in WT and Y204A PKA. (C) Position of Y204 with respect to PL pocket.
The role of PL pocket hydration in conformational preselection mechanism outlined above is reflected by the observation that the transition of the P + 1 loop from the BC to BI state leads to an increase in average water population from to —that is, roughly by a single water molecule (Fig. 3A). This increase in water content is likely a response to the shift of C199 and L198 residues outwards from the kinase core upon the transition from BC to BI states, which increases the PL pocket volume (Fig. 2A).
The bimodal free energy landscape underlying those conformational transitions becomes severely affected by the removal of water lubricant by placing a solvent-repelling probe into the PL pocket. The P + 1 loop becomes trapped in multiple local minima, corresponding to nonstandard configurations. Even more importantly, its state is no longer coupled to the state of the DFG + 1 residue, which abrogates communication with the second substrate binding site (Fig. S2).
Y204A Mutation.
The mutation of Y204 to alanine is known to suppress substrate binding cooperativity (7, 11, 18). To assess whether it interferes with the proposed conformational preselection mechanism, we conducted simulations of the mutant with and without ATP. Generally, the population of BC states in the Y204A mutant is observed to be somewhat smaller than in the WT PKA. Remarkably, however, it does not show a significant shift in response to ATP binding (Fig. 2D), which is consistent with the expected loss of cooperativity. The analysis of hydrogen bonding to DFG water (Fig. 2E) indicates similar enhancement in the presence of nucleotide as in the WT structure, but with no subsequent increase of water affinity to the mutant (Fig. 2F). It suggests that the mutated kinase more easily adapts to the vacated DFG site, likely in agreement with its originally increased preference for the BI state, whose stabilization in the absence of DFG water has been discussed for the WT protein above (Fig. 2D). This points to changes in the P + 1 loop region as the likely reason for cooperativity suppression. Indeed, even though the P + 1 loop in Y204A PKA apparently oscillates between the same states as in the WT protein (Fig. 2B), the underlying mechanism involving PL water fluctuations is altered. While the number of PL water molecules in the BC state for the mutant (6.0 1.7) is roughly the same as in the WT protein, the transition to the BI state is associated with a decrease of water content (to 4.9 1.5 molecules) as opposed to an increase observed in the WT (Fig. 3A). The role of additional water that fills the increased PL pocket volume in the BI state turns out to be taken by A204, which shifts Å into the pocket interior, as quantified by its distance to Y215, which is at the opposite side of the pocket (Fig. 3B). It highlights an important role of well-preserved polar contact between Y204 side chain and E230 (Fig. 3C), which is lost in the Y204A mutant. Its existence allegedly prevents the movement of the residue 204 and rigidifies the wall of the PL pocket, allowing water to accommodate the changes in the pocket volume upon transitions between BC and BI states without excessive stabilization of the BI conformation.
Discussion
PKA studies based on X-ray crystallography and NMR spectroscopy indicate that catalytic function and regulation of this enzyme rely on nontrivial dynamics (12). Typically considered in this context, global structural transitions of the catalytic subunit between closed, intermediate, and open forms happen within milliseconds and are beyond the reach of standard computational methods. Still, however, local conformational dynamics occurring on the nanosecond to microsecond time scale probed by our simulations conforms to the picture of dynamically regulated enzyme.
Focusing on the P + 1 loop, which is involved in peptide substrate positioning, we observe that, although its most frequently encountered “ground” state corresponds to geometry revealed by X-ray crystallography, the entire region repeatedly visits alternative conformations. By presenting less favorable interactions to the peptide substrate, they provide the means for modulating its overall binding affinity. Subtle shifts in P + 1 loop populations seem to be governed by the nucleotide-dependent state of the DFG/DFG + 1 motif, contributing together to a conformational preselection mechanism that, we believe, participates in the experimentally characterized substrate binding cooperativity.
A natural question arises as to what extent the mechanism revealed by our computer simulations meets reality. A number of experimental findings support or at least do not contradict the picture presented here. A recent thorough analysis of binding cooperativity between peptide substrate and a series of nucleotide analogs (13) led to the conclusion that binding synergy is triggered by polar interactions involving a nucleotide substrate region that corresponds to the oxygen atom linking and ATP phosphate groups. Its close proximity to the carboxyl group of the DFG aspartic acid, D184, (3.5 Å) suggests that this residue may indeed act as a sensor of nucleotide presence that initiates the allosteric effect. It is in line with our observation concerning the role of D184 in modulation of DFG water hydrogen bonding in a substrate-dependent manner (Fig. 2E). It seems that the presence of the Mg2+ ion, whose coordination is typically ascribed to the D184 side chain, is not critical in this context, since the cooperativity effect was also experimentally confirmed for a nonnucleotide ligand, balanol, which binds without the aid of divalent cations but still exposes its polar oxygen atom toward the D184 to less than 3 Å distance [Protein Data Bank (PDB) ID code 1BX6] (22). Interestingly, the central role of the DFG aspartic acid in the regulation of substrate binding cooperativity in a nucleotide-dependent manner has been also pointed out in the case of tyrosine kinase SRC (4). Given the fact that the water molecule in the DFG site is actually found in most active kinase structures, it raises a question concerning its general role as a part of a central allosteric hub.
Conformational changes further down the allosteric pathway outlined in our study also remain in agreement with NMR measurements based on 2D1H/15N spectra (11, 13). The most pronounced changes in chemical shifts that occur upon ATP binding involve, aside from PKA regions directly interacting with the nucleotide, the region of A188 (DFG + 2) residue together with G200 and T201 residues from the distant P + 1 loop. The amide group of A188 is in the direct vicinity of the DFG water molecule (3 Å distance; Fig. 1B). According to our simulations, the reorientation of this water molecule, likely influencing chemical shifts, is supposed to be involved in allosteric signal transduction. In turn, the shift of conformational equilibrium within the P + 1 loop, affecting the local environment of residues G200 and T201, is postulated to be the major factor that modulates peptide binding affinity. We also observe that both of the above mentioned effects occur predominantly upon nucleotide rather than subsequent peptide substrate binding. This observation remains in agreement with larger magnitudes of chemical shifts between apo and ATP-bound structure than between the latter and the full ternary complex (11, 13, 23).
An important link in the proposed communication chain relies on the interplay between the DFG + 1 residue and P + 1 loop. A role of the DFG + 1 residue for peptide substrate selectivity in serine–threonine kinases was recently noted (24). It was attributed to a conformational selection mechanism in which the DFG + 1 side chain imposed more or less catalytically competent conformation on the phosphate-accepting residue of the protein substrate depending on its identity. Here, we observe a similar effect involving the DFG + 1 phenylalanine, albeit directed toward C199 and influencing the P + 1 loop conformational equilibrium before peptide binding. Remarkably, the DFG + 1 and residue 199 maintain close contact in crystal structures (3.5 to 5 Å distance), and their character of large hydrophobic amino acids is well conserved within serine–threonine kinases (they are typically leucine or phenylalanine at the DFG + 1 position and valine or cysteine at position 199). Furthermore, the mutation of C199 to Ala (C199 A) was shown to abrogate PKA activity (25), indicating the importance of its side chain. Given that this side chain does not form direct interactions with the protein substrate nor is involved in catalysis, this observation further supports its potential role in linking the P + 1 loop with the DFG + 1 side chain.
Our simulations attribute an important function to water bound within the catalytic subunit. A single water molecule occupying the DFG site provides a switchable hydrogen bond bridge whose state depends on the arrangement of the kinase backbone. In turn, the solvent enclosed in a large pocket by the P + 1 loop constitutes an adaptable cushion that smooths out the free energy landscape governing structural oscillations of the peptide positioning segment. Fine-tuning of this landscape that involves a shift in the pocket water content seems to be the key for proper implementation of the signal transmitted by the DFG water. Perturbation of this mechanism by Y204A mutation, which breaks an important contact of the residue 204 with E230 and destabilizes one of the pocket walls, impairs the modulation of the P + 1 loop conformational equilibrium. This result is in agreement with experimental findings indicating that the Y204A mutation eliminates substrate binding cooperativity (18). It also provides a viable explanation as to why the mutation of Y204 to phenylanine (Y204F) has a much weaker disturbing effect than Y204A on catalytic activity (18), if one assumes that owing to its size the phenylalanine side chain is better anchored within the protein environment than alanine.
Conclusions
Based on long MD simulations, we characterized a putative mechanism contributing to experimentally detected substrate binding cooperativity in PKA. The observed allosteric pathway that links nucleotide and protein binding sites starts at the nucleotide-sensing side chain of the DFG aspartic acid, D184. The next step is provided by an isolated, conserved water molecule that is tightly embedded between the DFG and HRD motifs. Upon conformational change of D184 caused by nucleotide binding, its hydrogen bonding interactions become enhanced and engage the main chain of phenylalanine at the DFG + 1 position. The resulting shift in conformational equilibrium of the DFG + 1 side chain influences its interactions with C199 from the P + 1 loop. This loop is involved in positioning of the protein substrate for catalysis, however it remains highly dynamic owing to the presence of a water pocket, separating it from the rigid kinase core. The interplay between the DFG + 1 residue and C199 shifts the equilibrium of spontaneously sampled P + 1 loop conformations in favor to configurations typically observed in crystal structures that are capable of maintaining proper interactions with the protein substrate. Accordingly, the outlined mechanism connects the presence of a ligand in the nucleotide binding site with an increase in a functionally competent population of protein positioning segments. Since the change of its conformational equilibrium occurs even before the protein substrate is present, we term the observed effect a conformational preselection mechanism.
Materials and Methods
PKA Structures.
Coordinates for the phosphorylated apo form were taken from the corresponding crystal structure deposited in PDB (26) (PDB ID code 4NTS) (25). All ligand-bound forms were based on the crystal structure of PKA in complex with ATP, two Mn2+ ions, and peptide inhibitor PKI alpha (PDB ID code 3FJQ) (27). A missing N-terminal, helical fragment of 3FJQ with respect to 4NTS (residues 8 to 14) was added based on homology to obtain identical PKA sequences for all simulated states, and Mn2+ ions were exchanged to Mg2+. All structures were prepared with two phosphorylated residues (T197 and S338), with protonation states of titratable groups assigned for pH 7.0, by the Propka server (28). Four C-terminal residues of PKI (sequence: NAIH) were mutated to obtain ternary complexes with (i) substrate peptide SP20, with ASIHD C terminus and (ii) KL substrate with ASLG C terminus. The latter peptide was designed to combine the high-affinity N-terminal part of PKI with the alternative to the SP20 version of the region recognized by the PKA P + 1 loop. All modeling was performed with the Discovery Studio Visualizer 4.5 program (Dassault Systèmes BIOVIA). The structures were parametrized with an Amber99SB-ILDN force field (29), with additional parameters for ATP (30) and phosphorylated residues (31). Simulations were conducted using periodic boundary conditions with rhombic dodecahedron boxes providing at least a 13 Å space margin with respect to protein atoms, filled with TIP3P water molecules (in addition to crystallographic water molecules) (32) and NaCl salt atoms necessary to neutralize the system and achieve 150 mmol/L salt concentration.
MD simulations were carried out with the Gromacs 2016 program (33), using a standard explicit solvent simulation protocol whose details are described in Supporting Information.
Nonphysical particles (NPs) used in some simulations corresponded to (i) an LJ-like particle, corresponding to a neutral atom with LJ parameters identical as in the TIP3P water oxygen atom type and (ii) a dummy particle with nonbonded interactions only toward water oxygen atoms described by a repulsive potential. With kcal/mol, the parameter was chosen empirically to warrant water repulsion only from designated regions, resulting in Å for simulations with an empty DFG site and Å for simulations with an empty PL pocket. The particles were placed within a protein structure by using a restraining potential attached to neighboring heavy atoms. In the case of simulations probing the effect of water displacement from the DFG site, we considered two different, independent setups to make sure that the results were not affected by putative protein structure perturbation induced by the restraining potential: (i) restraints of the type proposed by Boresch (34) involving bond, angle, and dihedral potentials relative to three C atoms from residues 166 to 168, with equilibrium values and force constants determined based on averages and variances of the respective terms, evaluated based on standard simulations, and (ii) a flat-bottom potential relative to the center of mass (COM) of D166 amide nitrogen and F185 C atom. In simulations involving water repulsion from the PL pocket, we used a harmonic potential relative to the COM of atoms from R165, D166, and L205. Further details concerning the restraining potentials are given in Supporting Information.
Free energy calculations for water binding to the DFG site were conducted using the double decoupling method (DDM) (35), as in our previous study (36) (see Supporting Information for details).
The final results and error estimates were based on five independent calculations with different starting structures, generated from snapshots of standard simulations.
Supplementary Material
Acknowledgments
This work was supported by Narodowe Centrum Nauki Sonata UMO-2013/11/D/ST4/02821 and European Molecular Biology Organization Installation Grant 3051/2015 (to P.S.). Simulations were in part conducted within Interdisciplinary Centre for Mathematical and Computational Modeling University of Warsaw Computational Grant GB66-18.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720024115/-/DCSupplemental.
References
- 1.Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 2.Taylor SS, Kornev AP. Protein kinases: Evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011;36:65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornev AP, Taylor SS. Dynamics-driven allostery in protein kinases. Trends Biochem Sci. 2015;40:628–647. doi: 10.1016/j.tibs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foda ZH, Shan Y, Kim ET, Shaw DE, Seeliger MA. A dynamically coupled allosteric network underlies binding cooperativity in Src kinase. Nat Commun. 2015;6:5939. doi: 10.1038/ncomms6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marino KA, Sutto L, Gervasio FL. The effect of a widespread cancer-causing mutation on the inactive to active dynamics of the B-Raf kinase. J Am Chem Soc. 2015;137:5280–5283. doi: 10.1021/jacs.5b01421. [DOI] [PubMed] [Google Scholar]
- 6.Pucheta-Martínez E, et al. An allosteric cross-talk between the activation loop and the ATP binding site regulates the activation of Src kinase. Sci Rep. 2016;6:24235. doi: 10.1038/srep24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja LG, Kornev AP, McClendon CL, Veglia G, Taylor SS. Mutation of a kinase allosteric node uncouples dynamics linked to phosphotransfer. Proc Natl Acad Sci USA. 2017;114:E931–E940. doi: 10.1073/pnas.1620667114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Y, Pond MP, Roux B. Tyrosine kinase activation and conformational flexibility: Lessons from Src-family tyrosine kinases. Acc Chem Res. 2017;50:1193–1201. doi: 10.1021/acs.accounts.7b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, et al. A dynamic hydrophobic core orchestrates allostery in protein kinases. Sci Adv. 2017;3:e1600663. doi: 10.1126/sciadv.1600663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight ZA, Lin H, Shokat KM. Targeting the cancer kinome through polypharmacology. Nat Rev Cancer. 2010;10:130–137. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masterson LR, Mascioni A, Traaseth NJ, Taylor SS, Veglia G. Allosteric cooperativity in protein kinase A. Proc Natl Acad Sci USA. 2008;105:506–511. doi: 10.1073/pnas.0709214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masterson LR, et al. Dynamics connect substrate recognition to catalysis in protein kinase A. Nat Chem Biol. 2010;6:821–828. doi: 10.1038/nchembio.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Li G, Walters MA, Taylor SS, Veglia G. Uncoupling catalytic and binding functions in the cyclic AMP-dependent protein kinase A. Structure. 2016;24:353–363. doi: 10.1016/j.str.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Ten Eyck LF, Xuong NH, Taylor SS. Crystal structure of a cAMP-dependent protein kinase mutant at 1.26 Å: New insights into the catalytic mechanism. J Mol Biol. 2004;336:473–487. doi: 10.1016/j.jmb.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Shaltiel S, Cox S, Taylor SS. Conserved water molecules contribute to the extensive network of interactions at the active site of protein kinase A. Proc Natl Acad Sci USA. 1998;95:484–491. doi: 10.1073/pnas.95.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight JDR, Hamelberg D, McCammon JA, Kothary R. The role of conserved water molecules in the catalytic domain of protein kinases. Proteins. 2009;76:527–535. doi: 10.1002/prot.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cyphers S, Ruff EF, Behr JM, Chodera JD, Levinson NM. A water-mediated allosteric network governs activation of aurora kinase A. Nat Chem Biol. 2017;13:402–408. doi: 10.1038/nchembio.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MJ, Adams JA, Taylor SS. Structural basis for peptide binding in protein kinase A. J Biol Chem. 2003;278:10613–10618. doi: 10.1074/jbc.M210807200. [DOI] [PubMed] [Google Scholar]
- 19.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 20. Madhusudan, et al.(1994) cAMP-dependent protein kinase: Crystallographic insights into substrate recognition and phosphotransfer. Protein Sci 3:176–187. [DOI] [PMC free article] [PubMed]
- 21.Das A, et al. Protein kinase A catalytic subunit primed for action: Time-lapse crystallography of Michaelis complex formation. Structure. 2015;23:2331–2340. doi: 10.1016/j.str.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Narayana N, et al. Crystal structure of the potent natural product inhibitor balanol in complex with the catalytic subunit of cAMP-dependent protein kinase. Biochemistry. 1999;38:2367–2376. doi: 10.1021/bi9820659. [DOI] [PubMed] [Google Scholar]
- 23.Masterson LR, et al. Dynamically committed, uncommitted, and quenched states encoded in protein kinase A revealed by NMR spectroscopy. Proc Natl Acad Sci USA. 2011;108:6969–6974. doi: 10.1073/pnas.1102701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, et al. Identification of a major determinant for serine-threonine kinase phosphoacceptor specificity. Mol Cell. 2014;53:140–147. doi: 10.1016/j.molcel.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastidas AC, Wu J, Taylor SS. Molecular features of product release for the PKA catalytic cycle. Biochemistry. 2015;54:2–10. doi: 10.1021/bi500684c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berman HM. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson EE, et al. Comparative surface geometry of the protein kinase family. Protein Sci. 2009;18:2016–2026. doi: 10.1002/pro.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theor Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 29.Lindorff-Larsen K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meagher KL, Redman LT, Carlson HA. Development of polyphosphate parameters for use with the AMBER force field. J Comput Chem. 2003;24:1016–1025. doi: 10.1002/jcc.10262. [DOI] [PubMed] [Google Scholar]
- 31.Craft JW, Legge GB. An AMBER/DYANA/MOLMOL phosphorylated amino acid library set and incorporation into NMR structure calculations. J Biomol NMR. 2005;33:15–24. doi: 10.1007/s10858-005-1199-0. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 33.Abraham MJ, et al. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 34.Boresch S, et al. Absolute binding free energies: A quantitative approach for their calculation. J Phys Chem B. 2003;107:9535–9551. [Google Scholar]
- 35.Gilson MK, Given JA, Bush BL, McCammon JA. The statistical-thermodynamic basis for computation of binding affinities: A critical review. Biophys J. 1997;72:1047–1069. doi: 10.1016/S0006-3495(97)78756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setny P. Prediction of water binding to protein hydration sites with discrete, semi-explicit solvent model. J Chem Theory Comput. 2015;11:5961–5972. doi: 10.1021/acs.jctc.5b00839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.