Significance
Understanding the mechanisms that social insects use to communicate their individual status within the colony is vital to understanding the evolution of sociality. This study accomplishes this goal by identifying a royal-recognition pheromone in termites, as well as a king pheromone. Our behavioral assay defines royal-specific responses for one species of termites, which will foster future studies of termite behavior. This study also dates cuticular hydrocarbons as royal pheromones to the rise of termites ∼150 million years ago, suggesting that termites and social Hymenoptera convergently evolved the use of these ubiquitous compounds for communication. In conclusion, we have expanded our understanding of chemically mediated royal recognition in termites and helped to understand the evolution of insect societies.
Keywords: termite, royal pheromone, queen recognition, chemical ecology, cuticular hydrocarbons
Abstract
Chemical communication is fundamental to success in social insect colonies. Species-, colony-, and caste-specific blends of cuticular hydrocarbons (CHCs) and other chemicals have been well documented as pheromones, mediating important behavioral and physiological aspects of social insects. More specifically, royal pheromones used by queens (and kings in termites) enable workers to recognize and care for these vital individuals and maintain the reproductive division of labor. In termites, however, no royal-recognition pheromones have been identified to date. In the current study, solvent extracts of the subterranean termite Reticulitermes flavipes were analyzed to assess differences in cuticular compounds among castes. We identified a royal-specific hydrocarbon—heneicosane—and several previously unreported and highly royal enriched long-chain alkanes. When applied to glass dummies, heneicosane elicited worker behavioral responses identical to those elicited by live termite queens, including increased vibratory shaking and antennation. Further, the behavioral effects of heneicosane were amplified when presented with nestmate termite workers’ cuticular extracts, underscoring the importance of chemical context in termite royal recognition. Thus, heneicosane is a royal-recognition pheromone that is active in both queens and kings of R. flavipes. The use of heneicosane as a queen and king recognition pheromone by termites suggests that CHCs evolved as royal pheromones ∼150 million years ago, ∼50 million years before their first use as queen-recognition pheromones in social Hymenoptera. We therefore infer that termites and social Hymenoptera convergently evolved the use of these ubiquitous compounds in royal recognition.
Social insect societies depend on communication with nestmates to thrive as cohesive groups in challenging environments. Recognition of nestmates helps defend the colony, while recognition of different castes enables proper regulation of colony demography through caste differentiation and maintains the social and reproductive division of labor. Chemical communication is the foundation of nestmate and caste recognition, though other sensory modalities can also be involved (1–3). Most eusocial hymenopterans (ants, bees, and wasps) use colony- or species-specific blends of cuticular hydrocarbons (CHCs) to identify a variety of status conditions, including nestmates, mating status, age, caste, and fertility status (4–9), but blends of carboxylic acids, carboxylic esters, phenols, and alcohols are also used by some taxa (10, 11).
Queen pheromones regulate colony behavior and organization and consolidate reproduction by royal castes, by eliciting two major types of responses: (i) immediate behavioral responses (releaser effects) that consist of aggregation (i.e., retinue response), queen tending, and policing behaviors toward rival reproductives; and (ii) physiological suppression of worker reproduction (primer effects) that consolidates reproduction and maintains harmony within the colony. Queen pheromones have been characterized in a handful of hymenopterans (8, 9, 11–15) but have received scant attention in termites (16).
Termites, nested within the order Blattodea, developed sociality independently from Hymenoptera but share many of the biological features that maintain cooperative and altruistic interactions within the colony. Thus, royal recognition is equally vital to social cohesion and maintaining the division of labor in termites. In social hymenopterans, males are present in the colony only transiently and queens are the only permanent reproductive caste. In subterranean termites, however, colonies are founded by a monogamous queen and king pair (primary reproductives), with an additional developmental pathway that enables secondary or replacement reproductives (neotenics) to differentiate within the colony. Thus, both the king and queen, and the neotenics, are permanent members of the colony, and royal recognition must extend to both sexes.
Living either underground or within wood, termites are generally blind and must rely on chemosensory, vibratory, and tactile signals to communicate. Chemical communication, however, is the primary mediator of recognition in termites. Royal primer pheromones have been identified in only one termite species, Reticulitermes speratus, in which queens release a volatile blend of 2-methyl-1-butanol and n-butyl n-butyrate that inhibits the reproductive differentiation of female workers into supplementary reproductives (16). CHCs have been associated with nestmate recognition and other intracolony interactions in termites (17, 18). Although queens and kings produce a number of different compounds that differentiate them from other castes (18–23), releaser pheromones in royal castes have not been identified in any termite species.
Indeed, few royal-recognition behaviors have been described in termites, impeding progress in this area. In the dampwood termite Zootermopsis nevadensis, the removal of reproductives increased head-butting among workers (24). In the termite Cryptotermes secundus, the suppression of Neofem4, a cytochrome P450 gene highly expressed in queens and involved in CHC biosynthesis, interfered with queen recognition and increased aggression (25, 26). But in neither system have the behaviors been used to isolate queen-recognition pheromones.
Recently, we developed an effective queen-recognition bioassay in the subterranean termite Reticulitermes flavipes that measures rapid lateral (or longitudinal) shaking behavior and increased antennation from workers and soldiers elicited by queens and kings (27). These two behaviors occur frequently throughout the colony but are especially common in close proximity to reproductives (Movie S1). In this study, we used this behavioral assay to identify heneicosane (n-C21) as a reproductive-specific CHC that elicits behavioral royal recognition. Heneicosane is the only known royal-recognition pheromone in termites and the only known king pheromone in insects, offering a unique tool to further understand termite social systems and their chemical mediation.
Results and Discussion
Comparison of Cuticular Extracts: Workers and Royals.
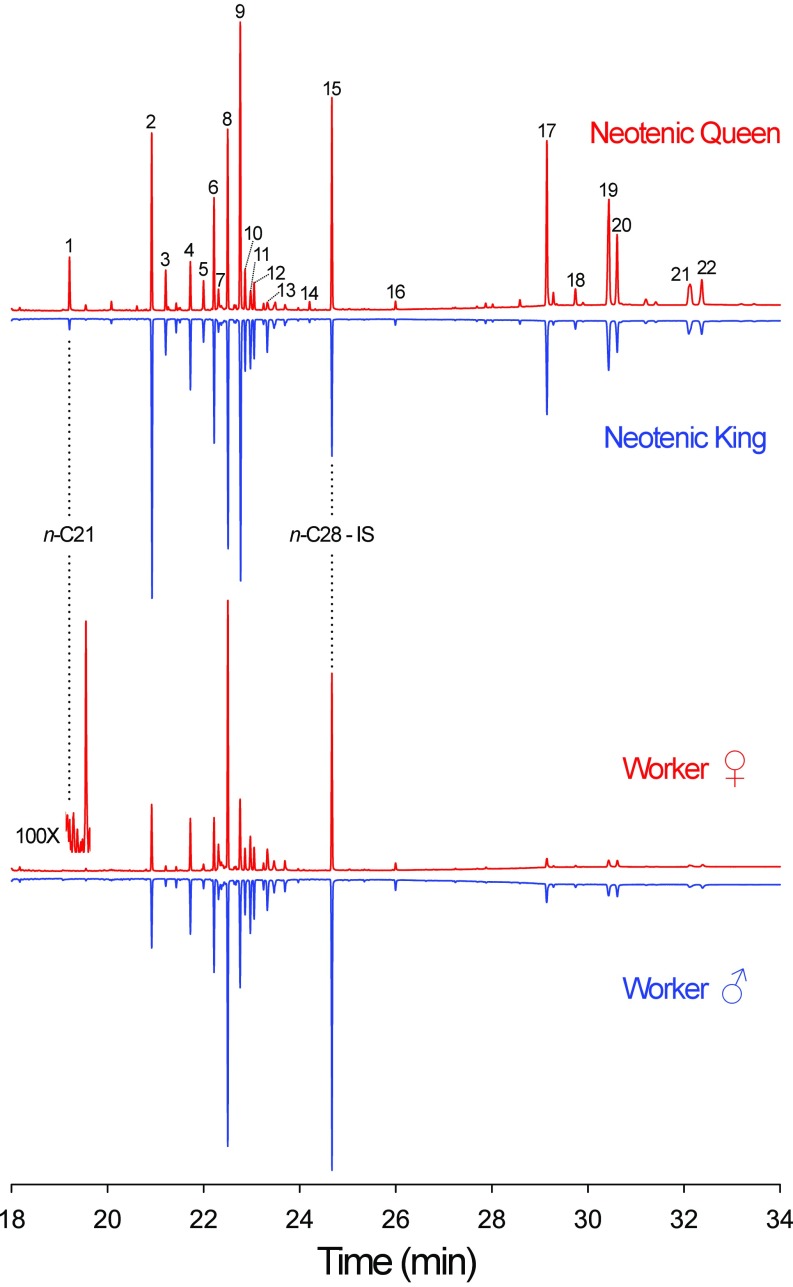
Hexane extracts of neotenic queens and kings and female and male workers were analyzed by GC-MS to discover reproductive-specific compounds that might be involved in royal recognition. The most prominent chemicals in the termite cuticular extracts were normal and monomethyl alkanes ranging from 23 to 25 carbons; they made up >40% of the total mass of CHCs for all termite castes (Figs. 1 and 2 and Table S1). Although our results largely support previous reports of CHCs in R. flavipes (18, 28–31), no tricosenes or tetracosenes were found in detectable amounts in our samples. These compounds were present in low levels in previous studies as well, and typical variation in CHC profiles across populations likely explains these discrepancies.
Fig. 1.
Gas chromatograms of reproductive and worker castes in R. flavipes. Females are marked in red and males in blue. The reproductive-specific compound heneicosane (n-C21) and the internal standard (IS) octacosane (n-C28) are marked. Numbered peaks correspond with peaks listed in Table S1. The Inset (100X, lower left) shows an approximate 100× magnification of the n-C21 region in the chromatogram of a female worker, showing no n-C21.
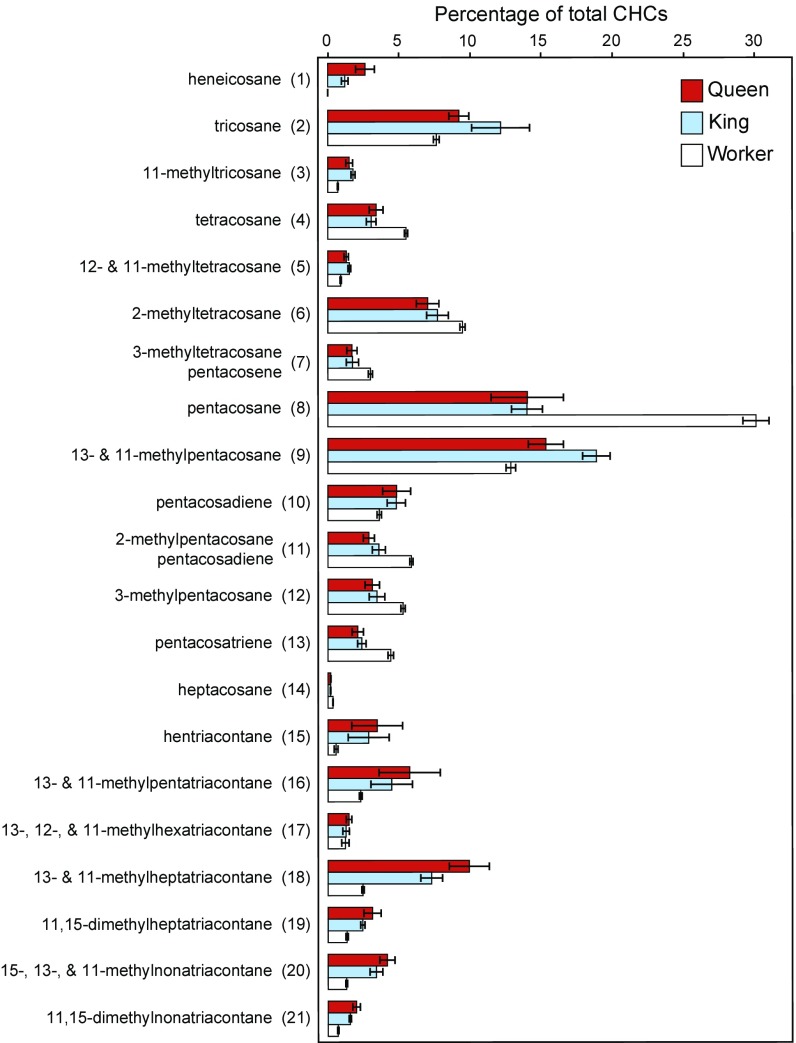
Fig. 2.
Representation of 21 analyzed GC peaks across caste. Average percent (±SEM) of the total cuticular mass of numbered peaks corresponding with peaks listed in Fig. 1 and Table S1. Workers of both sexes are averaged together in this figure. Sample sizes are 57 workers, 7 queens, and 7 kings.
Our GC-MS analyses revealed 20 GC peaks shared across all castes and 1 reproductive-specific compound (Figs. 1 and 2 and Table S1). Nine of the shared peaks were enriched in workers relative to queens and kings, including tetracosane, pentacosane, and pentacosatriene, and 11 were enriched in kings and queens, including tricosane, hentriacontane, and 13- and 11-methylheptatriacontane (Fig. 2 and Table S1). Heneicosane was found to be a unique royal compound, whereas long-chain ≥C35 mono- and dimethyl alkanes, although present in workers, were much more represented in royals (Figs. 1 and 2 and Table S1). In earlier work, n-C21 was found in only trace and unquantifiable amounts in every caste of R. flavipes and in workers of Reticulitermes lucifugus and Reticulitermes banyulensis (17, 31), and was not reported in a more recent study on Reticulitermes (18). Although some variation in CHC profiles is to be expected across populations, strong differences in a component with caste-specific patterns like n-C21 are unexpected. Early publications reporting trace amounts of n-C21 in workers and soldiers used pooled extractions of multiple termites and, therefore, could have resulted in cross-contamination of castes. More recent studies do not mention n-C21 at all, suggesting that there may be regional differences in R. flavipes CHCs, or perhaps that the neotenics evaluated in these studies were not reproductively active. Interestingly, in the drywood termite Cryptotermes secundus, n-C21 appears to be unique to workers (22).
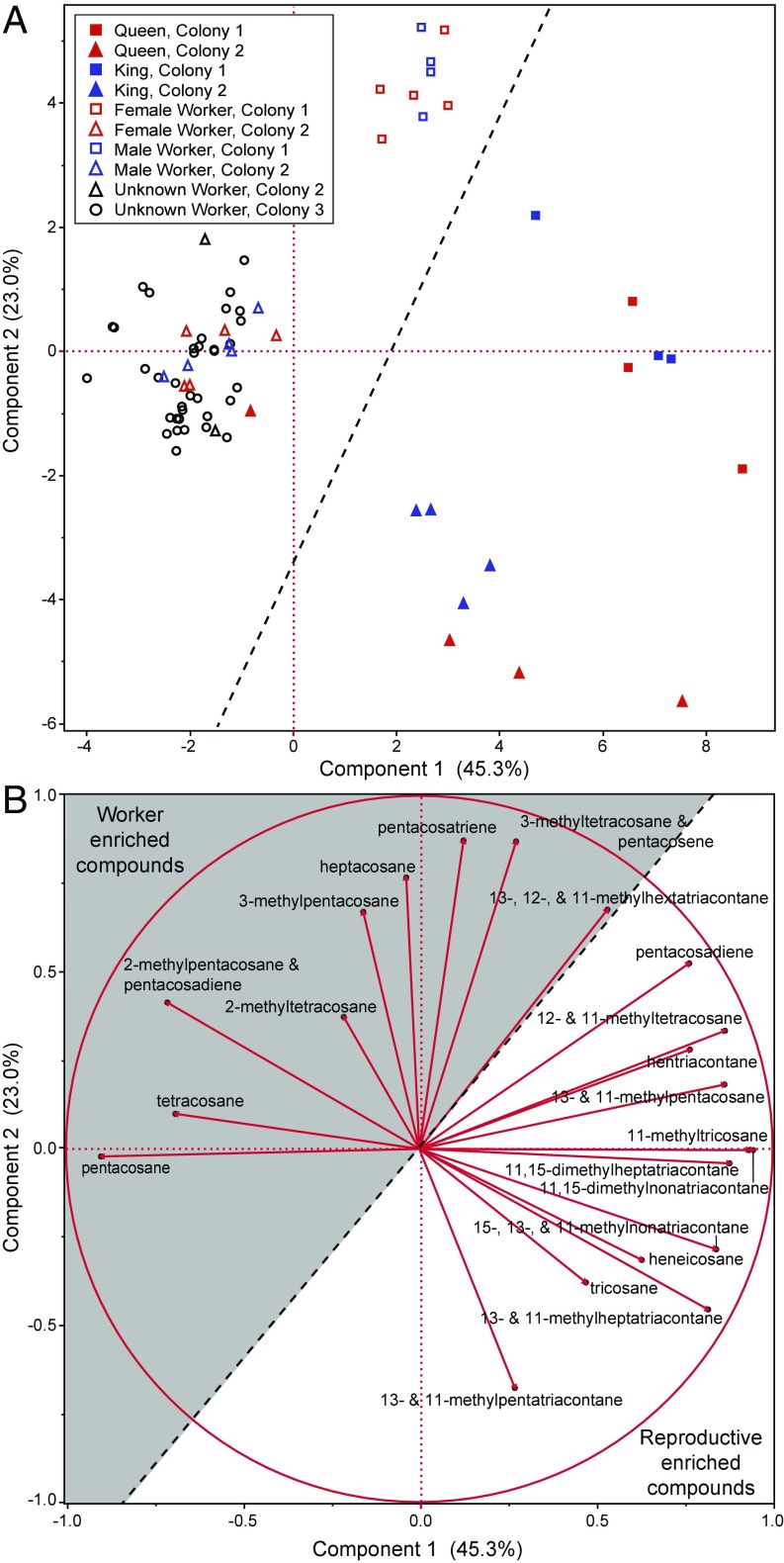
Principal component analysis (PCA) of 21 CHC peaks from termites revealed a strong separation across reproductive and nonreproductive castes (Fig. 3A). The first two principal components accounted for 68.3% of total chemical variation. The eigenvectors that contributed most to separation between workers and reproductives suggested that n-C21, tricosane, and the suite of compounds with a chain length ≥35 carbons were primary indicators of reproductive status, while tetracosane, 2-methyltetracosane, pentacosane, 2-methylpentacosane, and 3-methylpentacosane were the primary indicators of a worker-type CHC profile (Fig. 3B). One queen sample was situated within the worker group (Fig. 3A), but given the overall similarity of other queens across colonies and the sometimes difficult task of identifying newly emerged neotenic queens, it is likely that this was a misidentified worker labeled as a queen.
Fig. 3.
PCA of gas chromatograms from worker, queen, and king termites of R. flavipes. (A) Principal component (PC) ordination of 21 GC peaks. Red markers represent females, blue represent males, and black markers are workers that were not sexed. Shapes correspond to colonies 1 (squares), 2 (triangles), and 3 (circles). Filled shapes are active reproductives (i.e., neotenic kings or queens), while empty shapes represent termite workers. The dotted diagonal black line separates all royal individuals from workers. The percent of variance explained by each PC is indicated on the x and y axes. (B) PC1 and PC2 eigenvectors, showing in the unshaded section that compounds enriched in reproductives such as n-C21, n-C23, and ≥35 carbon alkanes, weigh toward the reproductive phenotype. Worker-enriched compounds present in the shaded portion of the figure weigh toward the worker phenotype.
Queens and kings were not distinguished from each other in the PCA, but they do possess several compounds with differential proportions (Fig. 2 and Table S1). Compounds with ≤25 carbons represented a higher percentage of the total CHCs in kings (except for n-C21), while queens had higher proportions of compounds with ≥35 carbons. Lastly, there was a clear separation of colonies in the PCA. Tricosane, tetracosane, 2-methyltetracosane, pentacosane, 2-methylpentacosane, and 13- and 11-methylpentatriacontane appeared to be compounds linking colonies 2 and 3 while separating them from colony 1. Differences across colonies are to be expected, as termites differentiate nestmates from nonnestmates based on their CHC profiles. Importantly, colony differences are represented by a different group of compounds in the PCA, and the differences between castes are consistent for each colony analyzed.
Cuticular Extracts Elicit Caste-Specific Behaviors.
Behavioral observations identified royal-recognition behaviors as shaking (or lateral oscillatory movements) and antennation, both of which were elevated in the presence of royal castes. Although both behaviors are reported and, in general, both indicate royal recognition, shaking was a more reliable and informative metric in our experiments. Across our assays involving live termites, workers and soldiers elicited 5.8 ± 0.98 (SEM, n =19) and 4.1 ± 0.90 (n = 14) shaking events in a single session, respectively, while neotenic queens (Movies S2 and S3) and kings elicited 23.3 ± 2.60 (n = 9) and 41.5 ± 7.94 (n = 8) shaking events. Nonreproductive individuals elicited significantly lower shaking responses than queens and kings [P < 0.05, Tukey’s honest significant difference (HSD)]. Similarly, workers and soldiers elicited 25.8 ± 1.77 and 19.8 ± 1.07 antennations in a single session, respectively, while queens and kings elicited 65.4 ± 10.01 and 39.6 ± 6.56 antennations, which demonstrates a significantly stronger antennation response to reproductive individuals (P < 0.05, Tukey’s HSD).
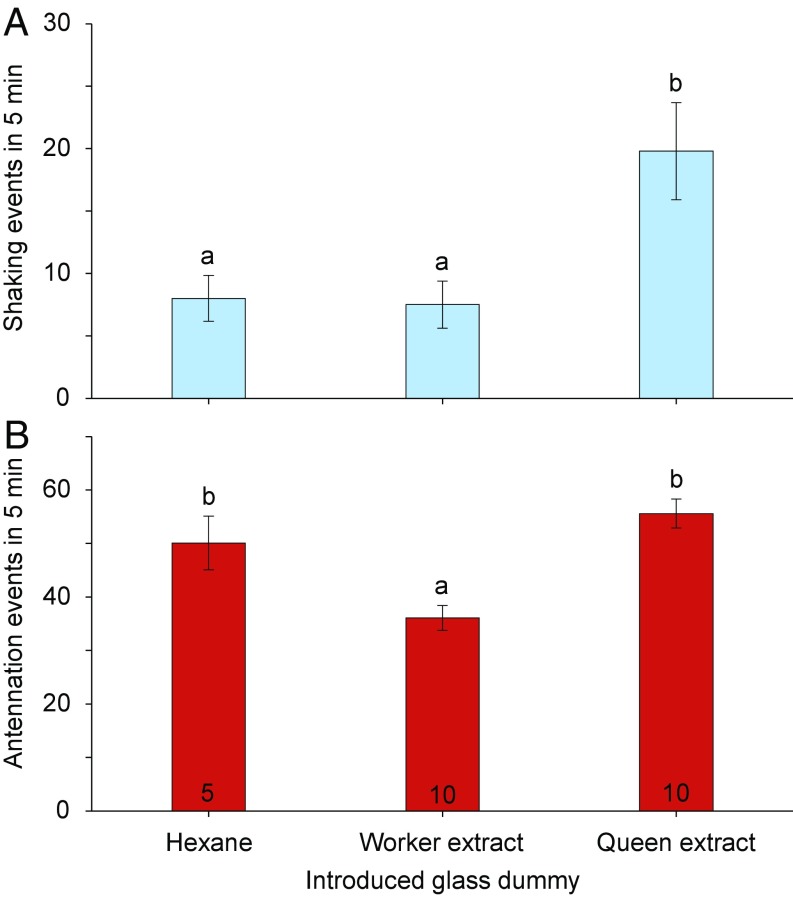
Bioassays using hexane extracts from neotenic queens and workers applied to glass dummies also showed a significant difference in shaking behavior, but not antennation, between reproductive and nonreproductive extracts (Fig. 4), demonstrating that cuticular compounds mediate the royal-recognition response.
Fig. 4.
Termite responses to glass dummies treated with extracts of workers and neotenic queens. Lateral shaking (A) and antennation (B) were measured during 5-min assays for each treatment. Glass dummies were treated with 20 μL of each solution. Worker extracts were created by pooling six workers (six termite equivalents), with mass approximately equal to three neotenic queens to match the queen extract of three queens. Each assay dish consisted of 30 workers, 2 soldiers, and an introduced glass dummy. Letters indicate significance using one-way ANOVA and Tukey’s HSD. Error bars are SEM. Number of replicate assays is indicated within each bar.
Heneicosane: Royal-Recognition Pheromone.
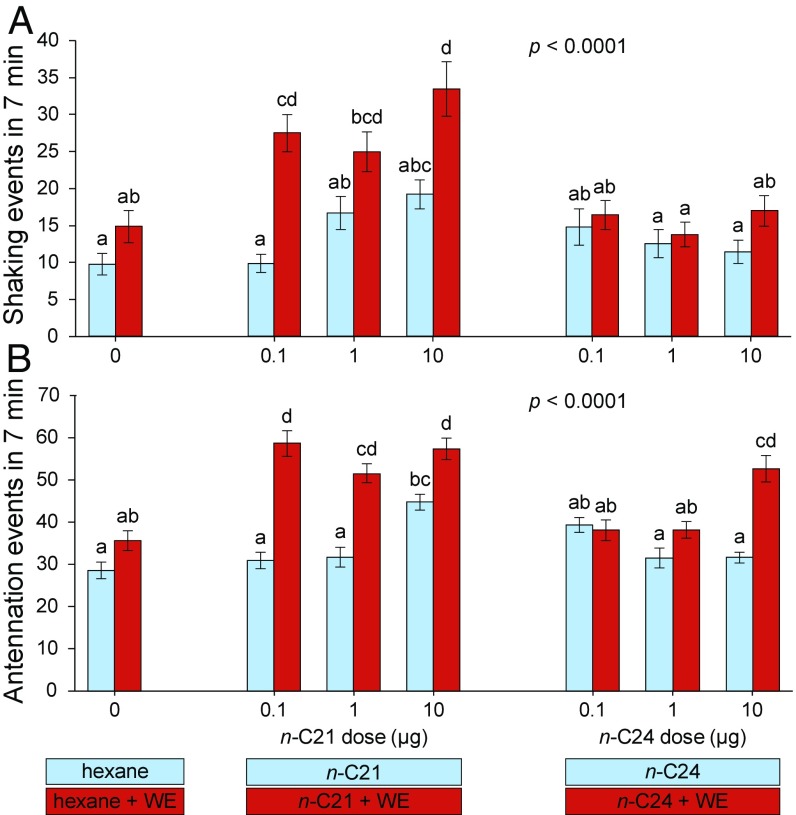
We tested the ability of n-C21 to elicit royal-recognition behavior when placed on glass dummies at three treatment doses (0.1, 1, and 10 μg). Tetracosane was also included to control for a generalized response to high doses of straight-chain alkanes. Glass dummies coated with nestmate worker extracts in combination with one of the two hydrocarbons served to control for general nestmate recognition signals whose absence might induce aggression, policing, or other alarm behaviors. The bioassays demonstrated significant elevations of both shaking and antennation in the presence of n-C21 (Fig. 5). To begin, n-C21 applied alone to dummies stimulated a clear positive dose response, with shaking events doubling between 0.1 and 10 μg of n-C21 (Fig. 5A). The 1 and 10 μg treatments of n-C21 alone were also significantly different from the control hexane extract (P = 0.0187 for 1 μg and P = 0.0008 for 10 μg, Dunnett’s test) (Movies S4 and S5). Shaking responses elicited by tetracosane were not significantly different from responses to hexane (control), and there was no dose-dependent response to tetracosane.
Fig. 5.
Termite responses to glass dummies treated with heneicosane or tetracosane alone and together with worker extracts. Lateral shaking (A) and antennation (B) in response to glass dummies treated with heneicosane (n-C21) or tetracosane (n-C24) alone (blue bars) and with worker extracts (red bars) were measured during 5-min assays for each treatment. Glass dummies were coated with 20 μL of each solution. Worker extracts were created by pooling six workers with mass approximately equal to three neotenic queens to approximate the queen concentration. Each assay dish consisted of 30 workers, 2 soldiers, and an introduced glass dummy. Letters indicate significance using one-way ANOVA and Tukey’s HSD. Error bars are SEM. Ten replicate assays were conducted for each treatment.
Stimulation with n-C21 in the presence of worker cuticular extracts significantly strengthened the shaking and antennation responses compared with n-C21 alone (P < 0.05, Tukey’s HSD). However, both shaking and antennation events appeared to be at their maximum levels, even at 0.1 μg, as evidenced by the lack of dose-response patterns. The average mass ± SEM of heneicosane on a neotenic queen was 32.0 ± 8.1 ng (Table S1), so our treatments with n-C21 represented the extracts of ∼3, 30, and 300 queens, respectively. It is important to note however, that higher concentrations of pheromones are often required on synthetic vs. natural substrates. Some insects are less responsive to pheromones placed on artificial substrates (e.g., ref. 32), and the use of solvent to apply the pheromone to the substrate may disrupt its natural stratification within the CHC layer (e.g., ref. 33).
Nevertheless, the combination of worker extract with any of the three doses of n-C21 stimulated a significantly stronger shaking response than the worker extract control (P = 0.0063 for 0.1 μg, P = 0.0351 for 1 μg, and P < 0.0001 for 10 μg; Dunnett’s test). Conversely, stimulation with tetracosane in combination with worker extract failed to stimulate greater shaking or antennation, except at a high dose, where we observed only increased antennation, a royal-specific response measure less reliable than shaking (27). Across all treatments, the shaking responses elicited by n-C21 within a worker chemical background were comparable to responses to live termite queens (27) and queen extracts (Fig. 4).
These findings strongly support the conclusion that n-C21 is a royal-recognition pheromone in R. flavipes, and that chemical context is important for effective communication of the recognition signal. Chemical context was previously reported as a major factor in queen recognition for the trap-jaw ant Odontomachus brunneus, in which queen-specific compounds elicit submissive postures in workers, but only when a blend of familiar ant cuticular compounds is included (34). As in R. flavipes, the ant queen-recognition signal is conserved across populations, but otherwise the ant and termite systems appear quite divergent. In R. flavipes, n-C21 is unique to queens and kings and, by itself, can elicit royal-recognition responses, whereas in the ant, (Z)-9-nonacosene occurs in both workers and queens, is relatively more abundant in queens, and, by itself, does not stimulate any behaviors. Ants also appear to readily distinguish between this queen-enriched alkene on a native worker vs. a nonnestmate cuticular chemistry background. In R. flavipes, it remains unknown whether the chemical context requires a colony-specific odor, or if more-general species-level profiles might be as effective. However, our demonstration that workers respond equally to native and foreign queens (27) would suggest that n-C21 and related royal-specific pheromones might suppress the aggressive responses that are normally elicited by foreign-colony CHCs. Further exploration of the ability of extracts of unrelated termites to elicit recognition behaviors could help us to understand how intercolony or population-level variation in cuticular profiles may affect royal recognition, colony fusion, and other competitive interactions in termites.
Heneicosane is conserved as a unique signal in both kings and queens and thus encodes “royal-status”. Although we have not thoroughly explored all differences in king and queen chemical profiles, the presence of a shared pheromone in both castes is nonetheless striking. In social insects in general, queen pheromones serve two related functions: as releasers of attendant and other behaviors by workers, and as primers that suppress reproductive activation in workers. We have only established that n-C21 releases royal-recognition behaviors. In eusocial hymenopterans, the presence of only a queen and not a king enables the same signal to function as both releaser and primer pheromone, as evident across a range of bees, ants, and wasps. Interestingly however, in the trap-jaw ant, (Z)-9-nonacosene elicits queen recognition but fails to suppress worker reproduction (34). In termites, on the other hand, the presence of queens and kings might have forced a functional differentiation of primer and releaser pheromones. Termite queens and kings could share releaser pheromones because they elicit similar behavioral responses from workers and soldiers. Heneicosane appears to encode this shared royal-recognition function, and, at least in theory, no other royal-specific components are necessary to effect its releaser function. The primer function in termites, however, requires the differential suppression of separate developmental pathways for neotenic males and females, which would presumably require unique queen and king primer pheromones. Indeed, in the only primer pheromone that has been reported in termites, the pheromone is produced by R. speratus queens and specifically suppresses queen differentiation; the respective king pheromone has not been elucidated and this primer pheromone appears to have no releaser effects (16). Although the primer effects of n-C21 have not been evaluated, we suspect that it might function exclusively as a releaser because it is shared by both queens and kings.
Sociality in insects has evolved independently multiple times. The eusocial hymenopterans evolved ∼100 Mya, and the conserved use of CHCs in nestmate recognition and as queen pheromones presumably evolved around this time as well. Our finding of a CHC as a royal-recognition pheromone in termites not only contributes evidence to the discussion that CHCs are a conserved class of social-recognition pheromones (4, 9) but also pushes their emergence in eusocial communication to ∼150 Mya, when eusocial termites evolved from within the cockroaches. CHCs have evolved multiple functions, primary among them is to prevent water loss and pathogen attack (4). The shared use of CHCs as recognition and/or fertility signals in eusocial insects appears to represent a striking example of the convergent cooption of specific CHCs to encode communication signals. Cockroaches, which are solitary relatives of termites, coopted CHCs well before social insects by using CHCs and their derivatives as sex pheromones (35). Eusocial insects presumably redirected these signals for royal recognition and to suppress reproduction in workers.
Distinguishing reproductives from other castes is of pivotal importance to the success of social insect colonies, and delineating which chemicals mediate these interactions will help to understand how these complex societies evolved (9). Future work should focus on investigating this shared pheromone and differentiating king and queen recognition, perhaps by discovering sex-specific cuticular pheromones or volatile components. This is a unique opportunity in social insect research to explore the evolution and mechanistic pathways of royal pheromones in both male and female backgrounds. These efforts would go hand in hand with exploring other cuticular chemicals that might constitute the royal pheromone blend, as well as possible primer effects of n-C21and the neural and olfactory basis for detecting the n-C21 signal. The function of the shaking response also merits further exploration, as a deeper understanding of this behavior could uncover a cornerstone of termite behavioral patterns. Shaking behaviors are widely seen throughout the colony and are documented across most termite lineages (36–38). With its connection to reproductive recognition, evaluating the context-dependent signals encoded in the shaking response should be an important next step in termite behavioral research.
Materials and Methods
Termite Collection.
For GC-MS, we collected termites from two colonies (colonies 1 and 2 in Fig. 3A) in Carl Alwin Schenck Memorial Forest (Raleigh, NC) in 2014 and 2015, and one colony (colony 3 in Fig. 3A) from Lake Johnson Park (Raleigh, NC) in 2015. For assays testing cuticular extracts and hydrocarbons, three colonies were collected from Lake Johnson Park in 2015. Termite colonies were maintained in laboratory conditions for ∼6 mo before use. Whole tree limbs or logs with termites were split into smaller pieces and set out in shallow pans to dry. Using either plastic container lids with moist paper towels underneath or ∼10-cm PVC pipes containing coils of moistened corrugated cardboard, the termites passively moved out of the drying wood and into the moist substrate. Fully separated colonies were kept either in clear plastic boxes lined with moist sand and pine shims for food or in 9-cm Petri dishes with an autoclaved substrate consisting of 70% sawdust and 30% α-cellulose. Colonies were maintained in opaque plastic containers in a ∼24 °C incubator under a 14:10 light:dark cycle with lights-on at 0600 hours.
Production of Secondary Reproductives.
Royal recognition was initially observed in primary, colony-founding queens and kings. We chose not to use these individuals in our experiments because they were difficult to find and R. flavipes readily generates replacement, or neotenic, reproductives. To produce these individuals, ∼2,000 to 5,000 termites were subdivided into 5-cm Petri dishes without reproductives. Newly emerged neotenics typically appeared within 2 to 3 wk and were removed to prevent any inhibition of subsequent queen or king differentiation in the neotenic-generating dishes. We maintained newly emerged neotenics in 9-cm dishes containing ∼500 workers and 20 to 50 soldiers until extracted or used in experiments and neotenic queens were confirmed to be reproductively active. Most of the emerging neotenics were ergatoid, or worker-derived, and were wingless. On rare occasions, we observed nymphoid neotenics emerging, which were identified by their wing buds; they elicited recognition responses and possessed similar chemical profiles to ergatoid neotenics in our analysis. Additionally, neotenic kings typically differentiated one at a time, while multiple female neotenics differentiated simultaneously in one dish, which limited the number of kings available to analyze and led to the use of queen extracts alone in some behavioral assays.
Cuticular Extracts and GC-MS.
Individual termites from every caste were sexed (females possess an enlarged seventh tergite which obscures the eighth and ninth tergite, while males have a seventh tergite with a visible eighth and ninth tergite) and freeze-killed for 15 min at −20 °C, followed by extraction in 200 µL of hexane containing 100 ng of octacosane (n-C28) as an internal standard. Extraction lasted for 2 min with intermittent gentle mixing. Extracts were removed to a new vial, evaporated under a gentle stream of high-purity nitrogen, redissolved in 50 µL of hexane, and transferred to a 100-µL glass insert in a 1.5-mL GC autoinjection vial. A volume of 2 μl was injected in splitless mode using a 7683B Agilent autosampler into a DB-5 column (20 m × 0.18 mm internal diameter × 0.18 µm film thickness; J&W Scientific) in an Agilent 7890 series GC (Agilent Technologies) connected to a flame ionization detector with ultrahigh-purity hydrogen as carrier gas (0.75 mL/min constant flow rate). The column was held at 50 °C for 1 min, increased to 320 °C at 10 °C/min, and held at 320 °C for 10 min.
A subset of termite CHC samples were run on an Agilent 5975 mass selective detector coupled to an Agilent 6890 GC for GC-MS analyses. The GC was operated in splitless injection mode and fitted with a DB-5MS column (30 m × 0.25 mm × 0.25 μm; Agilent). The oven was programmed from 50 to 310 °C at 15 °C/min after an initial delay of 2 min and held at 310 °C for 10 min. Injector temperature was 280 °C, MS quadrupole temperature was 150 °C, MS source temperature was 230 °C, and transfer line temperature was 300 °C. CHCs were identified primarily based on their electron ionization mass spectra and Kovats indices on the DB-5 column. Methyl branch positions of mono- and dimethylalkanes were determined from characteristic even- and odd-mass fragments of their respective mass spectra (39) as well as by their calculated retention indices (40). We did not determine the positions of double bonds.
PCA.
Chromatograms of queens, kings, male and female workers, and male and female soldiers were exported from Agilent ChemStation (OpenLAB Chromatography Data System Edition C.01.06). Using queen and worker chromatograms, 21 relevant peaks were selected to discriminate among castes. Excluded peaks were typically <1% of total chromatogram area for all samples. To include samples from multiple GC runs, retention times were converted to Kovats retention indices using the formula for temperature-programmed chromatography. All peaks were normalized to the n-C28 internal standard, and area percentages were input into the PCA matrix. PCA was conducted in JMP (41).
Transfer of Queen Extracts to Glass Dummies.
A colony from Lake Johnson Park was divided into groups of 30 workers and 2 soldiers in 5-cm Petri dishes and allowed to acclimate for 7 d. Individual workers and neotenic queens were extracted in hexane (200 µL per individual), and extracts were reduced and applied to glass dummies formed from melted Pasteur pipettes (∼2 mm × ∼6 mm). Before every assay, dummies were rinsed in hexane and dried, and 20 µL of extract was applied onto each dummy in a glass Petri dish and allowed to dry for 5 min before introduction into assay dishes. Final concentrations of extracts applied to dummies were three queen equivalents per 20 µL, six worker equivalents per 20 μL for worker controls, and hexane as a negative control. Dummies were added and observations began 2 min after introduction to settle the termites. Lateral shaking and antennation events performed by resident termites in response to coated glass dummies were recorded during a 5 min period. The bioassays tested one dummy per Petri dish from one colony (n = 5 dishes for queens and 10 dishes for controls). Each dish was observed with a queen extract, and then with worker and hexane controls, with a rest period of at least 24 h between assays.
Application of Heneicosane to Glass Dummies.
Two colonies of termites were collected from Lake Johnson Park for use in this assay. We tested whether n-C21 or n-C24 applied to glass dummies could elicit shaking behavior and antennation. Tetracosane was included as a control because it is present at similar relative amounts in the cuticular extracts of all castes (Fig. 2). We hypothesized that worker extracts might enhance behavioral responses by providing a familiar termite or nestmate chemical context to termites in the assay. Therefore, each of the two hydrocarbons was tested alone and in combination with termite worker extracts, and we tested worker extract alone and hexane as negative controls. Extracts were created as described above and applied to dummies for the assay. For n-C21 and n-C24, we applied 0.1, 1, and 10 μg per dummy, and in treatments with worker extracts, we used two worker equivalents per dummy to match the mass of CHCs typically found on a neotenic queen. A treated dummy was introduced, and after 2 min we recorded termite behavior for 5 min. These bioassays tested one dummy per Petri dish with two colonies (n = 10 dishes) for each treatment. Experimental groups were observed in multiple assays, with a rest period of at least 24 h between assays.
Supplementary Material
Acknowledgments
We thank Paul Labadie for help collecting termites and the administrative staff of Historic Yates Mill County Park, Lake Johnson Park, and Carl Alwin Schenck Memorial Forest for their support during our project. This investigation was supported, in part, by the Blanton J. Whitmire endowment at North Carolina State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721419115/-/DCSupplemental.
References
- 1.Liebig J, Peeters C, Hölldobler B. Worker policing limits the number of reproductives in a ponerine ant. Proc R Soc Lond B Biol Sci. 1999;266:1865–1870. [Google Scholar]
- 2.Tibbetts EA, Dale J. A socially enforced signal of quality in a paper wasp. Nature. 2004;432:218–222. doi: 10.1038/nature02949. [DOI] [PubMed] [Google Scholar]
- 3.West MJ. Foundress associations in polistine wasps: Dominance hierarchies and the evolution of social behavior. Science. 1967;157:1584–1585. doi: 10.1126/science.157.3796.1584. [DOI] [PubMed] [Google Scholar]
- 4.Blomquist GJ, Bagneres AG, editors. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 5.Penick CA, Liebig J. A larval ‘princess pheromone’ identifies future ant queens based on their juvenile hormone content. Anim Behav. 2017;128:33–40. [Google Scholar]
- 6.Polidori C, et al. Post-mating shift towards longer-chain cuticular hydrocarbons drastically reduces female attractiveness to males in a digger wasp. J Insect Physiol. 2017;100:119–127. doi: 10.1016/j.jinsphys.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Richard F-J, Hunt JH. Intracolony chemical communication in social insects. Insectes Soc. 2013;60:275–291. [Google Scholar]
- 8.Smith AA, Millar JG, Hanks LM, Suarez AV. A conserved fertility signal despite population variation in the cuticular chemical profile of the trap-jaw ant Odontomachus brunneus. J Exp Biol. 2013;216:3917–3924. doi: 10.1242/jeb.089482. [DOI] [PubMed] [Google Scholar]
- 9.Van Oystaeyen A, et al. Conserved class of queen pheromones stops social insect workers from reproducing. Science. 2014;343:287–290. doi: 10.1126/science.1244899. [DOI] [PubMed] [Google Scholar]
- 10.Keeling CI, Slessor KN, Higo HA, Winston ML. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci USA. 2003;100:4486–4491. doi: 10.1073/pnas.0836984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slessor KN, Kaminski L-A, King GGS, Borden JH, Winston ML. Semiochemical basis of the retinue response to queen honey bees. Nature. 1988;332:354–356. [Google Scholar]
- 12.Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc Natl Acad Sci USA. 2003;100:10341–10346. doi: 10.1073/pnas.1834281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holman L, Hanley B, Millar JG. Highly specific responses to queen pheromone in three Lasius ant species. Behav Ecol Sociobiol. 2016;70:387–392. [Google Scholar]
- 14.Holman L, Jørgensen CG, Nielsen J, d’Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc Biol Sci. 2010;277:3793–3800. doi: 10.1098/rspb.2010.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocher SD, Grozinger CM. Cooperation, conflict, and the evolution of queen pheromones. J Chem Ecol. 2011;37:1263–1275. doi: 10.1007/s10886-011-0036-z. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura K, et al. Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA. 2010;107:12963–12968. doi: 10.1073/pnas.1004675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagneres A-G, Killian A, Clement J-L, Lange C. Interspecific recognition among termites of the genus Reticulitermes: Evidence for a role for the cuticular hydrocarbons. J Chem Ecol. 1991;17:2397–2420. doi: 10.1007/BF00994590. [DOI] [PubMed] [Google Scholar]
- 18.Darrouzet E, et al. Endocrine control of cuticular hydrocarbon profiles during worker-to-soldier differentiation in the termite Reticulitermes flavipes. J Insect Physiol. 2014;61:25–33. doi: 10.1016/j.jinsphys.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Hanus R, Vrkoslav V, Hrdý I, Cvacka J, Sobotník J. Beyond cuticular hydrocarbons: Evidence of proteinaceous secretion specific to termite kings and queens. Proc Biol Sci. 2010;277:995–1002. doi: 10.1098/rspb.2009.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himuro C, Yokoi T, Matsuura K. Queen-specific volatile in a higher termite Nasutitermes takasagoensis (Isoptera: Termitidae) J Insect Physiol. 2011;57:962–965. doi: 10.1016/j.jinsphys.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Liebig J, Eliyahu D, Brent CS. Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol. 2009;63:1799–1807. [Google Scholar]
- 22.Weil T, Hoffmann K, Kroiss J, Strohm E, Korb J. Scent of a queen-cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften. 2009;96:315–319. doi: 10.1007/s00114-008-0475-8. [DOI] [PubMed] [Google Scholar]
- 23.Howard RW, et al. Cuticular hydrocarbons of Reticulitermes virginicus (Banks) (Isoptera, Rhinotermitidae) and their role as potential species-recognition and caste-recognition cues. J Chem Ecol. 1982;8:1227–1239. doi: 10.1007/BF00990755. [DOI] [PubMed] [Google Scholar]
- 24.Penick CA, Trobaugh B, Brent CS, Liebig J. Head-butting as an early indicator of reproductive disinhibition in the termite Zootermopsis nevadensis. J Insect Behav. 2013;26:23–34. [Google Scholar]
- 25.Hoffmann K, Gowin J, Hartfelder K, Korb J. The scent of royalty: A p450 gene signals reproductive status in a social insect. Mol Biol Evol. 2014;31:2689–2696. doi: 10.1093/molbev/msu214. [DOI] [PubMed] [Google Scholar]
- 26.Korb J, Weil T, Hoffmann K, Foster KR, Rehli M. A gene necessary for reproductive suppression in termites. Science. 2009;324:758. doi: 10.1126/science.1170660. [DOI] [PubMed] [Google Scholar]
- 27.Funaro C, Schal C, Vargo EL. 2018. Queen and king recognition in the subterranean termite, Reticulitermes flavipes: Evidence for royal recognition pheromones. PLoS One, in press.
- 28.Bagnères AG, et al. Cuticular hydrocarbons and defensive compounds of Reticulitermes flavipes (Kollar) and R. santonensis (feytaud): Polymorphism and chemotaxonomy. J Chem Ecol. 1990;16:3213–3244. doi: 10.1007/BF00982094. [DOI] [PubMed] [Google Scholar]
- 29.Clément J-L, et al. Biosystematics of Reticulitermes termites in Europe: Morphological, chemical and molecular data. Insectes Soc. 2001;48:202–215. [Google Scholar]
- 30.Dronnet S, Lohou C, Christides J-P, Bagnères A-G. Cuticular hydrocarbon composition reflects genetic relationship among colonies of the introduced termite Reticulitermes santonensis feytaud. J Chem Ecol. 2006;32:1027–1042. doi: 10.1007/s10886-006-9043-x. [DOI] [PubMed] [Google Scholar]
- 31.Howard RW, McDaniel CA, Blomquist GJ. Cuticular hydrocarbons of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae) J Chem Ecol. 1978;4:233–245. [Google Scholar]
- 32.Nishida R, Fukami H. 1983. Female sex pheromone of the German cockroach, Blattella germanica. Mem Coll Agric Kyoto Univ 122:1–24.
- 33.Hughes GP, Spikes AE, Holland JD, Ginzel MD. Evidence for the stratification of hydrocarbons in the epicuticular wax layer of female Megacyllene robiniae (Coleoptera: Cerambycidae) Chemoecology. 2011;21:99–105. [Google Scholar]
- 34.Smith AA, Millar JG, Suarez AV. A social insect fertility signal is dependent on chemical context. Biol Lett. 2015;11:20140947. doi: 10.1098/rsbl.2014.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eliyahu D, Nojima S, Mori K, Schal C. New contact sex pheromone components of the German cockroach, Blattella germanica, predicted from the proposed biosynthetic pathway. J Chem Ecol. 2008;34:229–237. doi: 10.1007/s10886-007-9409-8. [DOI] [PubMed] [Google Scholar]
- 36.Howse PE. On the significance of certain oscillatory movements of termites. Insectes Soc. 1965;12:335–345. [Google Scholar]
- 37.Ohmura W, Takanashi T, Suzuki Y. Behavioral analysis of tremulation and tapping of termites (Isoptera) Sociobiology. 2009;54:269–274. [Google Scholar]
- 38.Whitman JG, Forschler BT. Observational notes on short-lived and infrequent behaviors displayed by Reticulitermes flavipes (Isoptera: Rhinotermitidae) Ann Entomol Soc Am. 2007;100:763–771. [Google Scholar]
- 39.Nelson DR. Discovery of novel trimethylalkanes in the internal hydrocarbons of developing pupae of Heliothis virescens and Helicoverpa zea. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:647–659. doi: 10.1016/s1096-4959(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 40.Carlson DA, Bernier UR, Sutton BD. Elution patterns from capillary GC for methyl-branched alkanes. J Chem Ecol. 1998;24:1845–1865. [Google Scholar]
- 41.JMP, A Business Unit of SAS 2015. JMP Pro 12 (SAS Institute, Inc., Cary, NC)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.