Significance
Recombinant immunotoxins are anticancer agents consisting of Fv of an antibody that targets a cancer cell and a protein toxin that kills the cell. Despite its short half-life in the circulation, Moxetumomab pasudotox is a recombinant immunotoxin that has produced many complete regressions in patients with leukemia, because the leukemia cells are readily accessible to the agent. Immunotoxins targeting solid tumors are less active, because it takes many hours to enter bulky tumors and by then the blood levels have fallen to ineffective levels. To overcome this deficiency, we have designed and produced a generation of immunotoxins containing albumin-binding domains that dramatically increase their half-life in the circulation and antitumor activity in mice.
Keywords: mesothelin, pancreatic cancer, LMB-100, LMB-164
Abstract
Recombinant immunotoxins (RITs) are chimeric proteins consisting of a Fv that binds to a cancer cell and a portion of a protein toxin. One of these, Moxetumomab pasudotox, was shown to be effective in treating patients with some leukemias, where the cells are readily accessible to the RIT. However, their short half-life limits their efficacy in solid tumors, because penetration into the tumors is slow. Albumin and agents bound to albumin have a long half-life in the circulation. To increase the time tumor cells are exposed to RITs, we have produced and evaluated variants that contain either an albumin-binding domain (ABD) from Streptococcus or single-domain antibodies from Llama. We have inserted these ABDs into RITs targeting mesothelin, between the Fv and the furin cleavage site. We find that these proteins can be produced in large amounts, are very cytotoxic to mesothelin-expressing cancer cell lines, and have a high affinity for human or mouse serum albumin. In mice, the RIT containing an ABD from Streptococcus has a longer half-life and higher antitumor activity than the other two. Its half-life in the circulation of mice ranges from 113 to 194 min compared with 13 min for an RIT with no ABD. Cell uptake studies show the RIT enters the target cell bound to serum albumin. We conclude that RITs with improved half-lives and antitumor activity should be evaluated for the treatment of cancer in humans.
Small proteins often have limited biological activity, because they are rapidly removed from the circulation. Strategies that have been developed to increase half-life and biological activity include modification of the protein by polyethylene glycol (1), attachment to IgG or serum albumin (2), or attachment to small proteins that can bind to albumin (3). Albumin and immunoglobulins have a long serum half-life, because after internalization by pinocytosis they bind to the FcRn receptor and are returned to the circulation (4). Several albumin-binding proteins have been shown to increase half-life; these include proteins from Streptococcus as well as albumin-binding antibody fragments (5). Proteins have also been directly attached to albumin to increase their half-life (6).
Recombinant immunotoxins (RITs) are small chimeric proteins being developed to treat cancer (7). They are composed of the Fv or Fab portion of an antibody or of a cytokine fused to a fragment of a protein toxin. The Fv attaches to a cancer cell, enabling the toxin to enter and kill the cell. Our group focuses on RITs containing portions of Pseudomonas exotoxin A (7). These RITs range in size from 50 to 72 kDa and have half-lives in the circulation that range from 10 to 30 min in mice to several hours in humans. We are developing RITs to treat malignancies expressing CD22, CD25, and mesothelin (7). Moxetumomab pasudotox (Moxe), which targets CD22, has produced many complete and durable remissions in chemorefractory hairy cell leukemia, despite its short half-life in the circulation (8). A major factor in the success of this agent is that the leukemia cells reside in the blood and bone marrow, where they are readily accessible to the RIT.
SS1P is an RIT targeting mesothelin, a protein that is highly expressed on many common cancers (9). The clinical responses to SS1P are much less frequent than to Moxe, although we observed striking antitumor activity in some patients with mesothelioma, who were treated with SS1P combined with pentostatin and cyclophosphamide (10). Because the entry of immunotoxins into solid tumors is a slow process, we hypothesize that the antitumor activity of SS1P would be increased, if we could increase its half-life in the circulation, allowing more time for penetration into tumors. Because RITs are hybrid proteins containing an Fv or Fab fused to a 38-kDa fragment of Pseudomonas exotoxin A, they must be carefully designed so that they fold properly and can be prepared in sufficient quantities for clinical use. Since the toxin kills animal cells, RITs must be produced in Escherichia coli.
The mechanism by which RITs kill target cells is complex, involving at least six distinct biochemical events, which must not be affected by changes in the protein. These steps start with binding of the RIT to a cell-surface antigen, followed by internalization by endocytosis, separation of the Fv from the toxin by furin, retrograde transfer of the toxin to the endoplasmic reticulum (requiring removal of the C-terminal lysine residue, which generates a REDL sequence at the C terminus of the toxin), transfer into the cytosol, and binding of the toxin to NAD and EF2 (11). Thus, determining the location at which to add new protein sequences into the RIT is not straightforward.
Our previous efforts to increase the size and activity of immunotoxins by adding two Fvs in tandem at the amino terminus or inserting an Fv between domain II and III or near the end of domain III usually resulted in proteins that could not be produced or only produced in small amounts without a useful increase in biological activity. In this study, we report the successful production of large amounts of RITs with albumin-binding domains (ABDs) that have long half-lives in the circulation, high cytotoxic activity in vitro, and high antitumor activity in mice.
Results
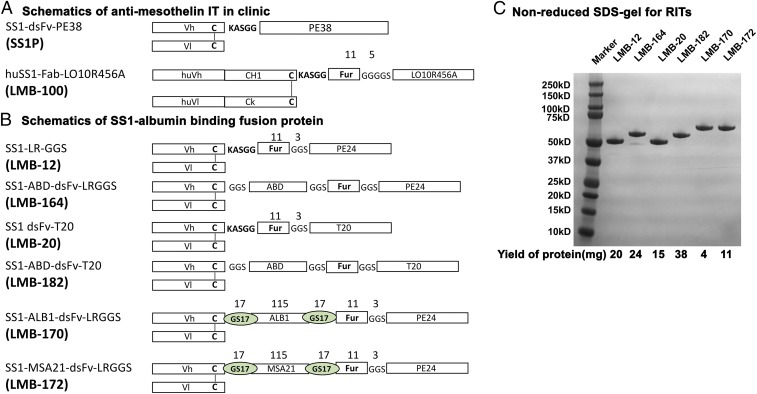
The design of the RITs used in this study is shown in Fig. 1 A and B. LMB-100 is being evaluated in clinical trials (https://clinicaltrials.gov/ct2/show/NCT02810418). LMB-12 contains a Fv targeting mesothelin attached to domain III by a small peptide with a furin cleavage site (12). LMB-164 is a derivative of LMB-12, with insertion of a 54-aa ABD from Streptococcus (ABD-S) (5) between the Fv and the furin peptide and separated from them by GGS linkers. LMB-20 is similar to LMB-12 but has six mutations in domain III designed to remove human T cell epitopes (13). LMB-182 is, in turn, a derivative of LMB-20 with a 54-aa ABD-S. LMB-170 and -172 have the same design as LMB-164 but contain Llama single-domain antibody fragments (nanobodies) in place of the ABD-S and 17-aa GS linkers separating the Vh and the furin site (Fig. 1B).
Fig. 1.
Antimesothelin immunotoxin and albumin-binding fusion proteins. (A) Schematics showing antimesothelin immunotoxins that are in clinic. (B) Schematics showing various SS1–Fv–albumin-binding fusion proteins used in this study. (C) Representative SDS/PAGE analysis showing the purity of the albumin-binding fusion immunotoxin.
The proteins are modular and composed of an Fv linked to the ABD by a short GGS linker. The ABD is followed by a second linker attached to a peptide containing an 11-aa furin cleavage site, which in turn is attached by another GGS linker to domain III. In this modular design, the ABD should not interfere with the binding of the Fv to mesothelin or affect the activity of the toxin. The heavy chain (HC), ABD, peptide, domain III fusion protein, and the light chain (LC) are produced in two separate fermentations, and the resulting proteins are combined into a disulfide-bonded Fv using cys residues introduced into the HC and LC. The SDS gel in Fig. 1C shows each immunotoxin after the final purification step. They are composed of a single band of the expected size, indicating the proteins are of high purity. As indicated at the bottom of the SDS gel, large amounts of protein could be obtained, especially for the ABD-S containing RITs. The final yield of monomeric protein for LMB-164 is 24% and for LMB-182 is 38%.
Cytotoxicity Assays.
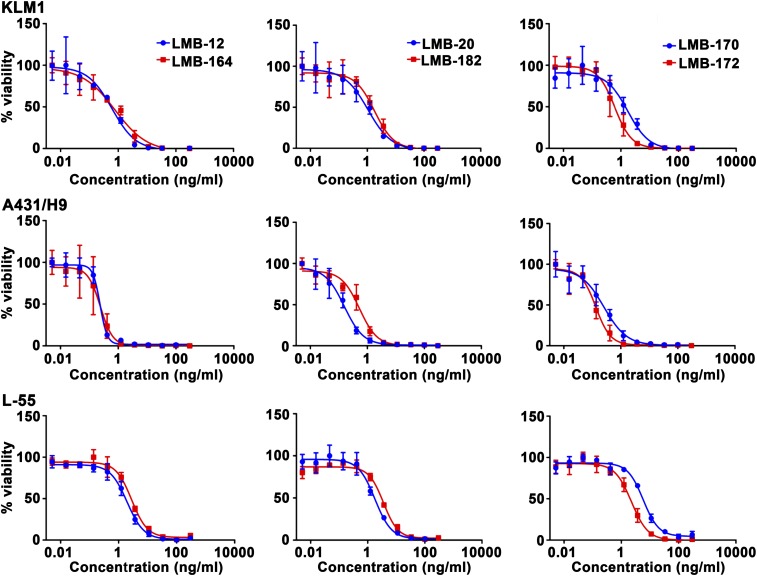
The cytotoxic activity of each RIT was assessed on four cancer cell lines using water-soluble tetrazolium (WST) assays. Three of these—KLM-1 (pancreatic), L55 (lung), and MKN-28 (stomach)—constitutively express mesothelin. A431/H9 is an epidermoid carcinoma transfected with a mesothelin cDNA (14). Representative WST assay data are shown Fig. 2, and the IC50 values are summarized in Table 1. In general, all of the immunotoxins are very active, and the addition of an ABD-S has relatively little effect on cytotoxic activity. All were able to kill cells in the low nanogram per milliliter range. LMB-164 has close to the same activity as LMB-12. LMB-182 is also very active and has similar activity to LMB-20. LMB-170 and LMB-172, with VHH single-domain antibodies from Llama, are also very active.
Fig. 2.
Cell-killing curve of LMB-12, LMB-164, LMB-20, LMB-182, LMB-170, and LMB-172 on KLM1, A431/H9, and L-55. Representative cytotoxicity assay on mesothelin-positive cancer cell lines using WST8 assay after 3 d incubation with RIT. KLM1, pancreatic cancer cell line; A431/H9, epidermoid carcinoma cell line transfected with MSLN cDNA; L-55, lung carcinoma.
Table 1.
Summary of 1C50s (ng/mL) on different cancer cell lines
| Cell line | LMB-12 | LMB-164 | LMB-20 | LMB-182 | LMB-170 | LMB-172 |
| KLM1 | 0.71 | 0.77 | 1.06 | 1.8 | 1.7 | 0.6 |
| A431/H9 | 0.23 | 0.24 | 0.17 | 0.54 | 0.26 | 0.13 |
| L-55 | 1.96 | 2.8 | 1.8 | 3.5 | 5.8 | 2.2 |
| MKN-28 | 0.2 | 0.2 | 0.27 | 0.54 | 0.63 | 0.18 |
Albumin Binding.
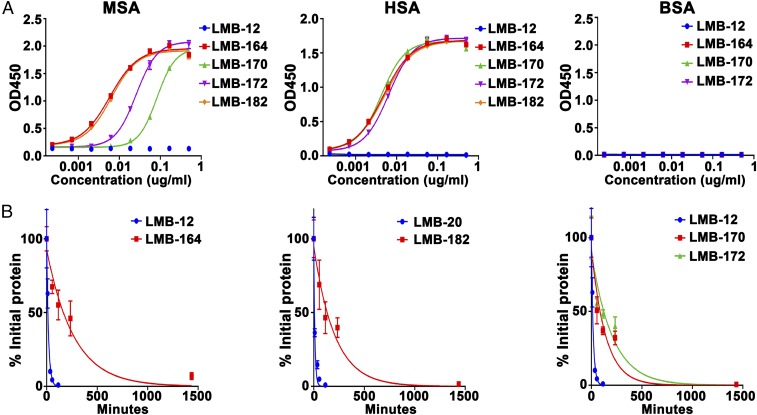
To analyze the albumin-binding affinity, we used ELISAs in which 96-well plates were coated with mouse serum albumin (MSA), human serum albumin (HSA), or BSA. The RITs were added at increasing concentrations and an antibody to domain III (IP12) used to measure how much RIT was bound to the albumin. As shown in Fig. 3, LMB-164, LMB-170, and LMB-172 all bind to HSA with high affinity. As expected, LMB-12, which has no ABD-S, did not bind. The binding to MSA is different from binding to HSA. LMB-164 and LMB-182 are the best binders, followed by LMB-172 and LMB-170. None of the proteins bind to BSA. The concentrations that give half-maximal binding are shown in Table 2. The apparent affinity for HSA is very high, 80–120 pM. The affinity for MSA is a little lower, 0.1–2.15 nM.
Fig. 3.
Properties of ABD–immunotoxin fusion protein. (A) Binding of LMB-12, LMB-164, LMB-20, LMB-182, LMB-170, and LMB-172 proteins to MSA, HSA, and BSA. Microtiter plates were coated with MSA, HSA, or BSA in PBS at 4 °C; diluted fusion immunotoxins were added; and bound immunotoxin were measured by ELISA. (B) Pharmacokinetics of LMB-12, LMB-164, LMB-20, LMB-182, LMB-170, and LMB-172 in mice. Nu/nu mice were injected i.v. with 25 μg of fusion protein. Blood samples were drawn at different times. The levels of immunotoxin in mouse blood were measured by ELISA.
Table 2.
Summary of mouse serum half-life and albumin-binding properties of ABD–immunotoxin fusion proteins
| LMB no. | Half-life, min | SE | AUC | SE | MSA half-maximum binding, nM | HSA half-maximum binding, nM |
| LMB-12 | 12.7 | 0.8 | 255.2 | 38.0 | None | None |
| LMB-164 | 193.8 | 60.2 | 11,255.0 | 2,434.0 | 0.10 | 0.08 |
| LMB-20 | 7.9 | 0.7 | 248.0 | 22.0 | None | None |
| LMB-182 | 142.0 | 16.3 | 8,073.5 | 1,338.3 | 0.11 | 0.09 |
| LMB-170 | 101.3 | 22.9 | 7,609.3 | 647.0 | 2.15 | 0.12 |
| LMB-172 | 144.1 | 20.8 | 9,017.8 | 1,100.6 | 0.40 | 0.09 |
Effect of Albumin on Cytotoxicity and LMB-164 Entry.
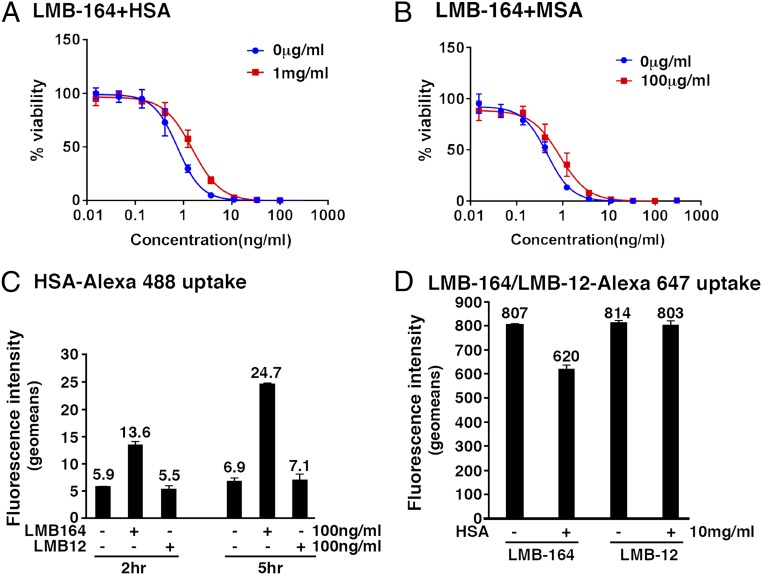
Before carrying out mouse experiments, we determined if binding to MSA or HSA affected immunotoxin activity. For these studies, we used LMB-164, whose ABD binds tightly to HSA and MSA. The data in Fig. 4 A and B and Table 3 show that there is a concentration-dependent inhibition of activity by both HSA and MSA, with a threefold inhibition at 10 mg/mL HSA and a twofold inhibition at 1 mg/mL MSA. As expected HSA and MSA had no effect on LMB-12, and MSA inhibited the activity of LMB-172, which binds to MSA.
Fig. 4.
Effect of albumin on activity of LMB164: cell-killing curve of LMB164 in the presence of HSA (A) and in the presence of MSA (B). (C) HSA uptake: KLM1 cells were treated with 10 μg/mL HSA-alexa488 with or without 100 ng/mL of LMB-164 for the indicated time. Relative fluorescence intensity was measured by flow and expressed as geomean subtracted background without HSA–Alexa-488. (D) LMB-164 uptake: KLM1 cells incubated with LMB164–alexa-647 at indicated concentration with or without 10 mg/mL of HSA for 3 h. Fluorescence intensity used geomean subtracted from the background cells without LMB164–Alexa-647.
Table 3.
IC50s (ng/mL) of immunotoxins on KLM1 cells with albumin
| Albumin, μg/mL | LMB no. | 0 | 1 | 10 | 102 | 103 | 104 |
| HSA | LMB-12 | 0.57 | 0.53 | 0.77 | 0.9 | 0.71 | 0.44 |
| LMB-164 | 0.75 | 1.64 | 2.22 | 1.95 | 1.53 | 2.06 | |
| MSA | LMB-12 | 0.42 | 0.67 | 0.47 | 0.38 | 0.24 | |
| LMB-164 | 0.46 | 0.85 | 0.91 | 0.86 | 0.87 | ||
| LMB-172 | 0.31 | 0.9 | 1.1 | 0.59 | 1.55 |
Because of the very high affinity of LMB-164 for HSA and because of the high concentrations of HSA in the blood (40 mg/mL), we assumed that LMB-164 would enter cells bound to HSA. To investigate entry, we used fluorescently labeled proteins. Fig. 4C shows that LMB-164 enhances the uptake of Alexa-HSA by 2–3-fold, indicating HSA is entering cells bound to LMB-164. Fig. 4D shows that HSA inhibits the uptake of Alexa–LMB-164 by only 20%, perhaps by interfering with binding of LMB-164 to mesothelin.
Half-Life Studies.
To determine the effect of the ABDs on the half-life of the new RITs in the blood of mice, we injected nu/nu mice with 25 μg of each RIT. We collected blood at 5, 15, 40, 60, and 120 min for LMB-12 and for LMB-20, which have short half-lives, and at 5 min and 1, 2, 4, and 24 h for the ABD-containing immunotoxins. The relative blood levels and half-lives are shown in Fig. 3 and Table 2. The blood levels are normalized by plotting the data as the percent of the 5-min value. As expected, the half-life for LMB-12 is short, 13 min (12). The half-life of LMB-20 is even shorter (8 min). In marked contrast, the half-lives of the ABD-containing immunotoxins are much longer. LMB-164 has a half-life of 194 min. The others range from 101 to 144 min. We used the PK data to calculate area under the curve (AUCs) and found that the AUC for LMB-164 was increased 44-fold and the AUCs for LMB-170, LMB-172, and LMB-182 were also greatly increased (Table 2).
For most of our studies, we diluted the immunotoxin in PBS with 0.2% HSA to stabilize the protein. To investigate if the HSA has any effect on half-life of LMB-164, we performed PK studies with and without 0.2% HSA. Although the half-life value varied between 2 and 3 h, the presence of 0.2% HSA had a very small effect (Table S1).
Antitumor Experiments.
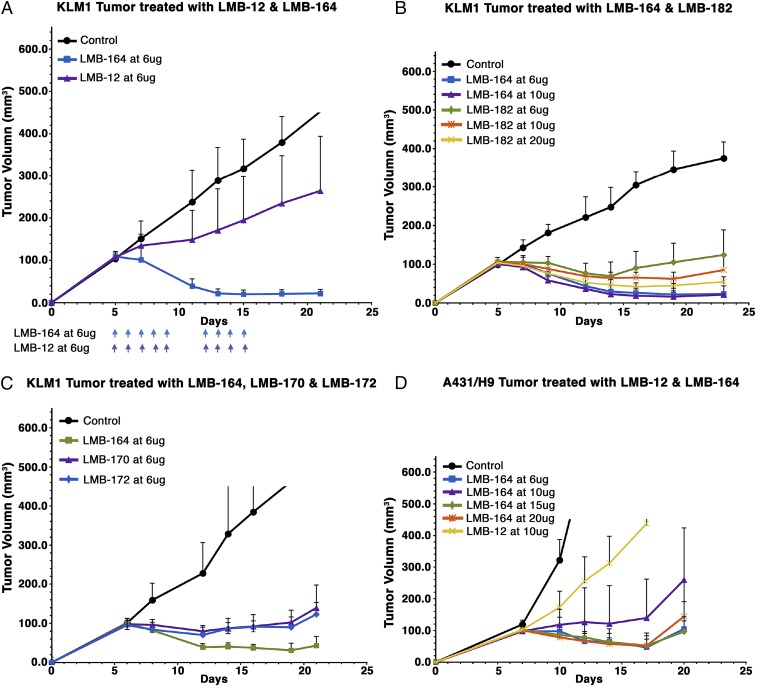
To determine the usefulness of treating tumors with an ABD-contacting immunotoxin, we treated mice with daily doses of LMB-12 or LMB-164. Fig. 5A shows an experiment in which mice with KLM-1 tumors ∼100 mm3 in size were given daily doses of 6 μg of LMB-164 or LMB-12. All tumors treated with LMB-164 responded and decreased to a very small size. On day 10, two of the seven were undetectable. The responses lasted for more than 30 d before the tumors started to regrow. In contrast, LMB-12 at 6 μg/d was much less effective; it slowed tumor growth but did not produce tumor regressions. At this dose, schedule LMB-164 was safe and only caused a slight decrease in the weight of treated mice (Fig. S1).
Fig. 5.
Antitumor activity of ABD–immunotoxin fusion protein in nude mice. (A) Antitumor activity of LMB-164 in mesothelin expressing KLM1 xenograft. (B) Antitumor activity of LMB-164 and LMB-182 in mesothelin expressing KLM1 xenograft. (C) Antitumor activity of LMB-164, LMB-170, and LMB-172 in KLM1 xenograft. (D) Activity of LMB-12 and LMB-164 in A431/H9 xenograft.
We next compared the antitumor activity of LMB-164 with LMB-182 that resembles LMB-164 but has deimmunizing mutations in T cell epitopes in domain III. LMB-182 has about 40% of the cytotoxic activity of LMB-164 in cell culture (Table 1). Toxicity studies showed LMB-182 could safely be given at higher doses than LMB-164. The data in Fig. 5B show that daily doses of 10 or 20 μg of LMB-182 caused tumor regressions, although these regressions were smaller than regressions produced by a daily dose of 6 or 10 μg of LMB-164.
LMB-170 and LMB-172 contain albumin-binding nanobodies from Llama antibodies (Fig. 1). LMB-172 has the same activity in cell culture as LMB-164; LMB-170 is about twofold less active (Table 1). To compare their antitumor activity with LMB-164, mice were treated with daily doses each of 6 μg. The data in Fig. 5C show that LMB-164 is more active than LMB-170 and LMB-172. Factors contributing to a lower antitumor activity include a shorter half-life in mice and the lower activity cytotoxic activity of LMB-170.
To determine if other tumors would respond to LMB-164, we examined the effect of LMB-164 on A431/H9 tumors. The data in Fig. 5D show that LMB-164 at 6 μg per day causes regressions on A431/H9 tumors, whereas LMB-12 only slows growth.
Discussion
We show here that we can construct RITS with a 14-fold increase in half-life and greatly enhanced antitumor activity by inserting small protein domains that bind to serum albumin. RITs kill cells by a complex process that requires that many regions of the protein interact productively with components of the target cell. Therefore, the ABD must not prevent binding of the Fv to mesothelin; decrease the rate of RIT internalization; decrease hydrolysis by furin (needed to separate the Fv from the domain III of the toxin); prevent binding of the C-terminal REDL of the toxin to the KDEL receptor, which transports it to the endoplasmic reticulum; decrease transport across the endoplasmic reticulum membrane; and finally decrease binding to NAD and to the diphthamide residue on EF2 (11). Our previous efforts to add additional functional domains containing Fvs to RITs were generally unsuccessful, mainly due to protein aggregation (15, 16). Fig. S2 lists proteins with additional Fvs that we have previously investigated. It is likely that single-domain proteins fold more efficiently than larger Fvs that are composed of two chains and do not interfere with the folding of other protein domains in the RIT.
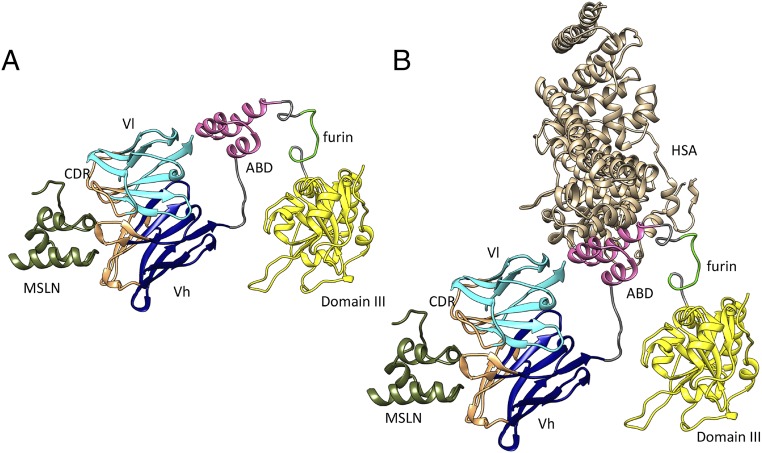
The ribbon structure in Fig. 6A shows the location of the ABD in the immunotoxins. It is inserted after the Fv and before the furin peptide. We used GGS linkers to connect the ABD to the Fv and to the furin peptide to allow the various domains to freely rotate. This strategy allows the Fv to bind to mesothelin. In addition, furin can cleave the peptide connecting the ABD to domain III so that domain III is released and can reach the endoplasmic reticulum, enter the cytosol, and inactivate EF2. We know this strategy is successful, because in cytotoxicity assays LMB-164 is just as active as LMB-12 that does not have an ABD (Table 1).
Fig. 6.
Ribbon drawing of LMB164 with MSLN (A) and with HSA and MSLN (B). The LC is in cyan, and the HC is in blue. ABD domain is in magenta, and domain III of PE is in yellow. Also marked are bound MSLN and HAS as well as furin cleavage site.
Fig. 6B depicts how HSA is expected to bind to LMB-164. The model emphasizes the modular nature of the RIT and indicates that albumin can rotate away from the Fv, allowing it to bind to mesothelin on the cell surface. To determine if LMB-164 entered the cell as a complex with albumin, we labeled albumin with Alexa-488 and found that albumin entry was enhanced by the addition of LMB-164 (Fig. 4C).
LMB-164 has a half-life of 194 min in the mouse circulation; this is about 15-fold greater than the half-life of 13 min for LMB-12 with no ABD. We expected that LMB-164 would have even a longer half-life, because MSA has a half-life in mice of 35–39 h (4), and LMB-164 has a very high affinity for MSA and the concentration of MSA in the blood is very high so any RIT that dissociated from it would quickly rebind to MSA. The 194-min half-life indicates that LMB-164 is being degraded while still bound to MSA. To investigate the possibility that LMB-164 would dissociate from albumin at pH 5 and not be recycled, we compared binding to albumin at pH 7.4 and pH 5.0 and found no difference (Fig. S3). We have previously studied the degradation of immunotoxin anti-TacFv–PE-38 in mice and found that large amounts are degraded by kidney and liver (17).
LMB-172 and LMB-170 have weaker binding to MSA than LMB-164 (Fig. 3A). PK studies show that these proteins have extended half-lives (101 min for LMB-170 and 144 min for LMB-172), but these half-lives are shorter than LMB-164 (194 min), showing that affinity contributes to half-life.
LMB-164 was analyzed in several cancer xenograft models. When given daily at 6 μg per day, it produced complete or near-complete and long-lasting regressions of KLM1 pancreatic cancers, while LMB-12 with no ABD only caused tumor growth to slow (Fig. 5). Surprisingly, LMB-182, which is similar in structure to LMB-164, except for mutations in domain III designed to remove human T cell epitopes, and is about 2.5-fold less active than LMB-164 in vitro, had lower antitumor activity in mice, even when given at 20 μg per mouse. Its half-life is about 25% shorter than that of LMB-164, which may contribute to the decrease in efficacy.
LMB-164 was also tested in mice against one other cancer, A431/H9. LMB-164 produced regressions of these tumors, but the responses were less robust than for KLM-1 tumors (Fig. 5D). A431/H9 cells are transfected with a human mesothelin cDNA and shed large amounts of mesothelin, which could constitute a significant barrier to the entry of LMB-164, when small amounts of an immunotoxin are administered to mice.
Guo et al. (18) have reported the production of a cytotoxic protein made up of an affibody targeting Her2/neu with an ABD from Streptococcus fused directly to a fragment of Pseudomonas exotoxin A. Affibodies are nonimmunoglobulin-based affinity ligands and are small (58 amino acids) and have no cysteines. Therefore, they fold more easily than Fv portions of antibodies that are larger and disulfide bonded and in which the two subunits must pair properly to orient the LCs and HCs so they can bind to antigen. Also, the affibody construct described contains a His tag for purification, which is not suitable for use in humans, and the yields of pure protein are not described. In the proteins reported here, tags were not used for purification, and the yields ranged up to 38% of the starting protein.
A major obstacle to the use of protein-based therapeutics that contain nonhuman proteins such as LMB-164 is the developments of antidrug antibodies. We have recently reported an approach to prevent the antibody response to immunotoxins, which is the coadministration of nanoparticles containing rapamycin with the immunotoxin. This therapy not only prevents antibody formation but also induces specific immune tolerance (19, 20).
Summary and Conclusion
We have prepared RITs targeting mesothelin that contain different ABDs that increase their half-life in the circulation and their antitumor activity in mice. The most potent of these (LMB-164) contains a 54-aa polypeptide from Streptococcus. These data support further preclinical development of these agents.
Materials and Methods
Construction, Expression, and Purification of RIT.
Double-stranded gene fragments (g Blocks; Integrated DNA Technologies) corresponding to the desired sequences were ligated into our standard laboratory immunotoxin production vector as described previously (21) using the Gibson Assembly Master Mix (New England Biolabs) according to the manufacturer’s instructions. All RITs were expressed and purified from E. coli inclusion bodies as described with minor modifications. Briefly, inclusion bodies from E. coli were dissolved in GTE buffer (6 M guanidine–HCl, 100 mM Tris⋅HCl, 2 mM EDTA). The denatured proteins were refolded in 1,000 mL 100 mM Tris⋅HCl, 1 mM EDTA, 0.5 M arginine, and 0.5 M NDSB-201, pH 10, for 31 h and then dialyzed against 30 mM Tris⋅HCl, 0.1 M urea for 17 h. The refolded proteins were purified using Q-Sepharose, Mon-Q ion-exchange, and size exclusion chromatography as described (21). The ABD-S sequence is described in Zygren (22) and the Llama nano-body sequences in patents (US 20070269422 A1 and US 20060228355 A1).
Cell Culture.
Human cell lines KLM1 (23), MKN28 (24), L55 (25), and A431/H9 (14) were described previously. Cells were grown in RPMI and supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C. Cell identities were confirmed by short tandem repeat analysis. The cytotoxic activity of the immunotoxins was evaluated by WST8 assay after 3 d of treatment according to manufacturer instructions (Dojindo Molecular Technologies). A431/H9 cells were plated at 2.5 × 103 cells per well and other cells at 4 × 103 cells per well. For detecting the effects of albumin on cytotoxic activity, MSA (Sigma-Aldrich Co.) or HSA (Grifols Therapeutics Inc.) was added 24 h before addition of immunotoxin. After 24 h of immunotoxin exposure, the cells were washed and incubated with fresh medium for an additional 48 h followed by WST assays. All assays were run with three replicates and repeated at least twice.
Albumin-Binding Assay.
Microtiter plates were coated with 50 µL of MSA, HSA, or BSA (2 µg/mL) in PBS at 4 °C overnight. Immunotoxins diluted by casein blocking buffer (0.5% casein in PBS) were added at room temperate for 2 h, followed by incubation with anti-PE antibody, IP12 (26). After washing, HRP-conjugated goat anti-mouse IgG was added, followed by TMB substrate kit (Thermo Scientific). Data were analyzed by sigmoidal 4PL and in Graphpad Prism vs. 7.01 (GraphPad Software, Inc.).
HSA and LMB164 Uptake.
KLM1 cells were seeded in six-well plates and treated with HSA-Alexa-488 or LMB-164-Alexa-647 for indicated times. Cells were then removed by trypsin, washed with FACS buffer (PBS with 5% FBS, 0.1% NaN3), and analyzed by flow cytometry using a FACS Calibur (Becton Dickinson).
In Vivo Half-Life.
Mice (6–8 wk, 23–25 g) were injected i.v. with 25 µg of immunotoxin (n = 4). Blood was drawn at 5, 15, 40, 60, and 120 min for LMB-12 and LMB-20 and at 5 min and 1, 2, 4, and 24 h for LMB-164, LMB-182, LMB-170, and LMB-172. The concentration of immunotoxins was measured by ELISA using a standard curve for each immunotoxin (26). Briefly, microtiter plates were coated with 50 µL of 1 µg/mL MSLN-hFc (a fusion protein consisting of human IgG Fc fused to the mesothelin protein) in PBS at 4 °C overnight. Diluted standards or serum samples were applied, followed by incubation with anti-PE antibody (IP12) and HRP-labeled goat anti-mouse IgG. After washing, plates were developed using the TMB substrate kit (Thermo Scientific). Serum half-life was calculated using a “one-phase decay” model in Graphpad Prism version 7.01. The 5-min sample was used as the first value and set at 100%. The animal protocol (LMB-014) was approved by the National Cancer Institute Animal Care and Use Committee, and the animals were handled according to institute guidelines.
Antitumor Experiments.
Female (5–10 per group) nu/nu mice (6-wk-old, 18–22 g) were injected s.c. in the flank with 5 × 106 KLM1 or 2 × 106 A431/H9 cells (23, 14). After 7 d, when the tumors reached ∼100 mm3, groups of mice with similar average weight and tumor size were injected i.v. with immunotoxins in PBS containing 0.2% HSA on days indicated in results. Body weight and tumor size were measured every 2 or 3 d. Mice were euthanized if they lost more than 2 g of weight or when the tumors grew to more than 600 mm3. No animals were excluded from statistical analysis.
Graphs and Statistics.
Data were plotted and analyzed using GraphPad Prism vs. 7.01 (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest statement: I.P. is an inventor on several patents on immunotoxins that have all been assigned to the NIH. All other authors declare no potential conflicts of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721780115/-/DCSupplemental.
References
- 1.Jevševar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–128. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JT, et al. Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain. J Biol Chem. 2011;286:5234–5241. doi: 10.1074/jbc.M110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor-Semmes RL, et al. GSK2374697, a novel albumin-binding domain antibody (AlbudAb), extends systemic exposure of exendin-4: First study in humans–PK/PD and safety. Clin Pharmacol Ther. 2014;96:704–712. doi: 10.1038/clpt.2014.187. [DOI] [PubMed] [Google Scholar]
- 4.Strohl WR. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs. 2015;29:215–239. doi: 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygren PA, Eliasson M, Abrahmsén L, Uhlén M, Palmcrantz E. Analysis and use of the serum albumin binding domains of streptococcal protein G. J Mol Recognit. 1988;1:69–74. doi: 10.1002/jmr.300010204. [DOI] [PubMed] [Google Scholar]
- 6.Lim SI, Hahn YS, Kwon I. Site-specific albumination of a therapeutic protein with multi-subunit to prolong activity in vivo. J Control Release. 2015;207:93–100. doi: 10.1016/j.jconrel.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan R, Bera T, Pastan I. Mesothelin: A new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 10.Hassan R, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weldon JE, Pastan I. A guide to taming a toxin–Recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–4700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weldon JE, et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazor R, et al. Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc Natl Acad Sci USA. 2012;109:E3597–E3603. doi: 10.1073/pnas.1218138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho M, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 15.Bera TK, Onda M, Brinkmann U, Pastan I. A bivalent disulfide-stabilized Fv with improved antigen binding to erbB2. J Mol Biol. 1998;281:475–483. doi: 10.1006/jmbi.1998.1948. [DOI] [PubMed] [Google Scholar]
- 16.Bera TK, Williams-Gould J, Beers R, Chowdhury P, Pastan I. Bivalent disulfide-stabilized fragment variable immunotoxin directed against mesotheliomas and ovarian cancer. Mol Cancer Ther. 2001;1:79–84. [PubMed] [Google Scholar]
- 17.Kobayashi H, et al. Pharmacokinetics of 111In- and 125I-labeled antiTac single-chain Fv recombinant immunotoxin. J Nucl Med. 2000;41:755–762. [PubMed] [Google Scholar]
- 18.Guo R, et al. Fusion of an albumin-binding domain extends the half-life of immunotoxins. Int J Pharm. 2016;511:538–549. doi: 10.1016/j.ijpharm.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto TK, et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nat Nanotechnol. 2016;11:890–899. doi: 10.1038/nnano.2016.135. [DOI] [PubMed] [Google Scholar]
- 20.Mazor R, et al. Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity. Proc Natl Acad Sci USA. 2018;115:E733–E742. doi: 10.1073/pnas.1717063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 22.Nygren PA. Alternative binding proteins: Affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008;275:2668–2676. doi: 10.1111/j.1742-4658.2008.06438.x. [DOI] [PubMed] [Google Scholar]
- 23.Hollevoet K, et al. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–2049. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alewine C, et al. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–2661. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu XF, Zhou Q, Hassan R, Pastan I. Panbinostat decreases cFLIP and enhances killing of cancer cells by immunotoxin LMB-100 by stimulating the extrinsic apoptotic pathway. Oncotarget. 2017;8:87307–87316. doi: 10.18632/oncotarget.20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan G, et al. Protection of the furin cleavage site in low-toxicity immunotoxins based on Pseudomonas exotoxin A. Toxins (Basel) 2016;8:217. doi: 10.3390/toxins8080217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.