Abstract
Clinical exome sequencing (CES) is increasingly being used as an effective diagnostic tool in the field of pediatric genetics. We sought to evaluate the parental experience, understanding and psychological impact of CES by conducting a survey study of English-speaking parents of children who had diagnostic CES. Parents of 192 unique patients participated. The parent’s interpretation of the child’s result agreed with the clinician’s interpretation in 79% of cases, with more frequent discordance when the clinician’s interpretation was uncertain. The majority (79%) reported no regret with the decision to have CES. Most (65%) reported complete satisfaction with the genetic counseling experience, and satisfaction was positively associated with years of genetic counselor (GC) experience. The psychological impact of CES was greatest for parents of children with positive results and for parents with anxiety or depression. The results of this study are important for helping clinicians prepare families for the possible results and variable psychological impact of CES. The frequency of parental misinterpretation of test results indicates the need for additional clarity in the communication of results. Finally, while the majority of patients were satisfied with their genetic counseling, satisfaction was lower for new GCs, suggesting a need for targeted GC training for genomic testing.
Keywords: exome sequencing, psychological impact, genomic medicine, genetic counseling, parental experience
Graphical abstract
INTRODUCTION
Clinical exome sequencing (ES) is an effective tool to identify the genes underlying rare, highly penetrant, monogenic and genetically heterogeneous conditions. CES is usually more efficient and cost-effective than traditional single-gene testing [1, 2] and has a yield greater than 30% for many clinical indications across diverse populations [3–5]. While CES is increasingly being integrated into clinical care [2, 6], relatively few studies have examined patient and family understanding and experience of the testing. Improved information about the family experience of CES is critical to guide recommendations for best practices.
Studies of traditional genetic testing have demonstrated that the experience and psychological impact is influenced by multiple factors, including the clinical context (disease and type of test results), patient/parent knowledge of genetics, patient-provider relationship, and psychological state of the patient/parent [7–11]. Genetic results may impact perceived health, healthcare choices, and reproductive decisions [12–15]. Less is known about these factors in the setting of diagnostic CES. Experiences with CES may differ from those of more traditional genetic testing because of its ability to identify novel or incompletely characterized genetic conditions, the possibility of secondary findings (SFs) unrelated to the diagnosis, the high frequency of uncertain results (novel variants in established, clinically well-characterized genes or in novel genes that are not clinically well-characterized) and the potential of re-interpretation of results [16–20].
Early qualitative evaluation of CES indicates the parental experience is mixed. Parents express a duty to pursue testing and feelings of worry and relief regardless of their child’s results; and acceptance, empowerment, and more focused care when results identify a diagnosis [21, 22]. Parents also report isolation and loss of hope about the future, particularly when the diagnosis is novel or rare, but are accepting of uncertain information [22, 23]. While parents endorse the potential of CES as a diagnostic tool and many want to learn secondary results, some are ambivalent about learning uncertain results [24, 25]. These experiences to some degree are shared by parents of children who have had chromosome microarray analysis (CMA), which also has the possibility of uncertain or incidental results [15, 26].
A more complete understanding of the patient and family experience of CES is important for the development of best clinical practices. Guided by the experiences with research on traditional genetic testing and qualitative studies of CMA, we developed a survey to evaluate the experience and psychological impact of clinical CES on parents of children who had testing at a single institution. The aim of the study was to describe parental understanding of results from CES and expand our understanding of the parental experience, including emotional and social effects, satisfaction with genetic counseling and the decision to have CES for their child. We also examined how parental interpretation of the child’s CES results was correlated with these outcomes.
MATERIALS AND METHODS
Participants
Participants were English-speaking parents of a consecutive series of symptomatic patients for whom diagnostic CES was performed, including outpatients in the Division of Clinical Genetics at Columbia University-New York Presbyterian Hospital and inpatients at Morgan Stanley Children’s Hospital. The study was approved by the Columbia University Institutional Review Board. Patients received CES results from April 2012 to June 2015. Parents of outpatients had a consultation with one of 10 genetic counselors (GCs) and one of five medical geneticists. Parents of inpatients met with a medical geneticist at the time of consent and spoke with a GC at the time of results disclosure. The decision to offer CES was made by the clinical team. Factors assessed in the decision included prior negative genetic testing and clinical suspicion of a genetic diagnosis. Genetic education and counseling were conducted in a client-centered manner to promote informed decisions. Parents of minors (<18 years) and of adults (≥18 years) without capacity to consent were invited to participate in the study. The responses of only one parent of each patient (the mother, if both parents responded) were analyzed for this study.
Eligible families were invited to participate via a letter from the treating geneticist and GC, followed by up to five phone calls. A passive decline was registered when no call was returned. Participants completed the survey online or on paper, according to their preference. Participants were provided with a $20 gift card upon completion of the survey. Study enrollment took place from May 2015 through March 2016.
Diagnostic Clinical Exome Sequencing
CES was completed at one of four clinical laboratories. Laboratories reported pathogenic and likely pathogenic variants, and variants of uncertain significance related to the patient’s phenotype. Starting in March 2013, the laboratories offered to return American College of Medical Genetics secondary findings (i.e., medically actionable results unrelated to the primary diagnosis), with the option to opt out [27].
Results were disclosed by the geneticist or GC by phone or in person. Parents were provided a results letter with the clinicians’ interpretation of the results and implications. When a diagnosis was identified, the letter included a description of the diagnosed syndrome, medical recommendations, reproductive risks and support groups. Parents were also given a copy of the laboratory report, which varied in format among laboratories and over time, but categorized results as positive (i.e., pathogenic/likely pathogenic variant [mutation] identified), negative (i.e., no pathogenic variant identified), or uncertain (i.e., variant identified but uncertain clinical significance of the variant or gene).
Study Instrument
A GC and a medical geneticist designed the survey using previously validated measures (Table S1) and questions developed for this study (Appendix 1). The survey design is described in the supplemental materials.
Statistical analysis
The statistical analysis is described in the supplemental materials. Briefly, binary variables were assessed through chi-squared tests and continuous variables were assessed by ANOVA. Statistical analysis was completed in SAS [28].
RESULTS
Enrollment
Three hundred sixty-seven families met the study eligibility criteria. Families without working contact information (n=30) were excluded, and the remaining 337 families were invited to participate. Sixty-three families declined, including 37 who failed to return any of the invitation calls and 26 families who actively declined for reasons including: no interest (n=7), no time (n=3), privacy concerns (n=2), child deceased or too sick (n=4), raised bad memories (n=3), and no reason (n=7). Surveys were sent to 274 families. Twelve families declined participation after receiving the survey because of discomfort with the questions (n=8), privacy concerns (n=2) or no time (n=2). A total of 192 (57%) unique families completed the survey (Figure S1). Comparison of demographics of the 192 enrolled and 145 unenrolled families showed an over-representation of white, non-Hispanic individuals and younger age of children in the enrolled group (Table S2).
Demographics
Mothers were the most frequent responders (68% of all responders). When only one parent completed the survey, 83% were mothers. For the analysis presented, fathers’ responses were excluded for the 54 families in which both parents completed the survey, to eliminate biases created by correlated data. Seventy-seven percent of the participants reported here are mothers and 91% completed the survey online. Most parents identified as white, non-Hispanic (67%), had a bachelor’s degree or higher (71%), and were employed (64%). The mean time from receiving results to completing the survey was 16 months (Table 1). For the parents who had the option to receive secondary results, most (95%) elected to receive them and 2% (n=3) had secondary results. The parents were knowledgeable about genetics: with a mean score of 86% (95% CI: 84-88%) on the genomic knowledge scale, 30% correctly answered all eight true/false genetics questions and over half (58%) correctly answered the questions specific to CES.
Table 1.
Demographics of 192 participant parents and their children
| Parent Demographics | Total | N | % | |
|---|---|---|---|---|
| Gender | Male | 192 | 24 | 13% |
| Age in years* | 192 | 40 | 8.35 (20-67) | |
| Marital Status | Never married | 192 | 15 | 8% |
| Married or living as married | 192 | 158 | 82% | |
| Separated/ Divorced | 192 | 18 | 9% | |
| Race | White, Non-Hispanic | 192 | 128 | 67% |
| White, Hispanic | 192 | 33 | 17% | |
| Black | 192 | 11 | 6% | |
| Asian | 192 | 16 | 8% | |
| Other or Not reported | 192 | 4 | 2% | |
| Education | ≤ High School | 192 | 31 | 16% |
| Some College | 192 | 26 | 14% | |
| College Degree | 192 | 67 | 35% | |
| Graduate Degree | 192 | 66 | 34% | |
| Job | Employed full or part time | 192 | 122 | 64% |
| Keeping House/ Raising Children | 192 | 50 | 26% | |
| Unemployed/ Retired/ Disabled | 192 | 20 | 10% | |
| Genetic Knowledge | All correct | 192 | 58 | 30% |
| CES Knowledge | All correct | 192 | 112 | 58% |
| Child demographics | N | % | ||
| Gender | Male | 192 | 105 | 55% |
| Deceased/ Fetus | 192 | 14 | 7% | |
| Additional family member(s) affected | 192 | 33 | 17% | |
| Age of child in years* | 192 | 6.8 | 5.6 (.2-23.6) | |
| Inpatient | 192 | 45 | 23% | |
| Medical Geneticist | MD1 | 192 | 92 | 48% |
| MD2 | 69 | 36% | ||
| MD3 | 31 | 16% | ||
| Genetic Counselor | <5yr | 147 | 68 | 46% |
| experience*** | ≥5yr | 79 | 54% | |
| Indication | Single System | 192 | 32 | 17% |
| Multiple Systems | 192 | 38 | 20% | |
| Neurological | 192 | 122 | 64% | |
| Re-analysis | 192 | 3 | 2% | |
| Opt In for Secondary Findings (n=150)** | 150 | 143 | 95% | |
| Received Secondary Findings | 150 | 2 | 1% | |
| Time in months between results returned and survey* | 16 | 9 (1-42) | ||
Abbreviations: month (m), Secondary Findings (SF): results unrelated to child’s diagnosis, clinical exome sequencing (CEM)
Mean, standard deviation, range
42 had testing prior to SF offered
excludes in patients
The mean age of the child who had CES was 6.8 years at the time of testing. A quarter were seen as inpatient consults. The majority (64%) of the children had a neurological component to their illness such as development delay, intellectual disability, or seizures. Fewer (20%) had involvement of more than one system but no neurological component (e.g., a kidney and heart defect) or an isolated condition (e.g., a heart defect) (Table 1).
Parental recall of testing process
When making the decision to have CES for their child, parents indicated they received advice from a variety of specialists and used other resources, the most frequent being educational websites (53%). Most parents (65%) reported discussing CES with the provider for an hour or less before making the decision to have CES. Over half (61%) reported that their child had prior genetic testing. (Table S3).
Interpretation of child’s CES results
Clinician interpretation of results (which may have differed from the laboratory interpretation of results) included 79 positive, 35 uncertain, and 78 negative results. The 79 positive cases included four children who had a genetic diagnosis identified by CMA testing performed concurrently with CES. Parents of these four children all interpreted their child’s CES results as positive on the survey, and for the purposes of this study, the clinician interpretation of results was documented as positive. Nearly half of the results interpreted as uncertain by the laboratory were re-interpreted by the clinicians based on the child’s clinical profile (31% as positive, 18% as negative) (Table 2).
Table 2.
Interpretation of exome sequencing results. Clinician interpretation compared to laboratory test report. Parental interpretation of child’s results compared to clinician interpretation. N and column %.
| Laboratory Report of Exome Sequencing Results | |||||||
|---|---|---|---|---|---|---|---|
| Clinician Interpretation | Positive | Uncertain | Negative | Total | |||
| N | % | N | % | N | % | N | |
| Positive | 63 | 95% | 16 | 31% | 0 | 0% | 79 |
| Uncertain | 3 | 5% | 26 | 51% | 6 | 8% | 35 |
| Negative | 0 | 0% | 9 | 18% | 69 | 92% | 78 |
| Total | 66 | 51 | 75 | 192 | |||
| Clinician Interpretation of Exome Sequencing Results | |||||||
|---|---|---|---|---|---|---|---|
| Parental Interpretation | Positive | Uncertain | Negative | Total | |||
| N | % | N | % | N | % | N | |
| Positive | 70 | 89% | 4 | 11% | 2 | 3% | 76 |
| Uncertain | 8 | 10% | 25 | 71% | 13 | 17% | 46 |
| Negative | 1 | 1% | 5 | 14% | 56 | 72% | 62 |
| Blank | 0 | 0% | 1 | 3% | 7 | 9% | 8 |
| Total | 79 | 35 | 78 | 192 | |||
Overall, the parental interpretation of the child’s result agreed with the clinician interpretation in 79% of cases. The frequency of parental concordance was somewhat greater for results interpreted by the clinician as positive (89%) than for those interpreted as negative (72%) or uncertain (71%) (p=0.02) (Table S4a). Parents not infrequently interpreted negative results as uncertain (17%) or uncertain results as negative (14%) (Table 2). In 73% of the instances where the parental interpretation disagreed with the clinician, the clinician had re-interpreted the laboratory report. For most of these differences in interpretation, the parent’s interpretation was either consistent with the laboratory report or the child had multiple variants reported. Parents whose interpretation agreed with the clinician’s interpretation reported more positive experiences from the testing and had more healthy behaviors and lower health locus of control. Time from results disclosure to survey completion was not associated with correct parental interpretation (Table S4ab).
Parental Experience of CES
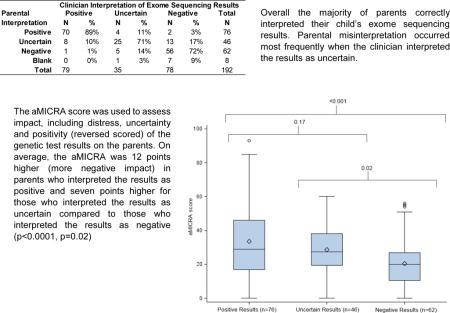
The most common emotions parents reported at the time of learning their child’s CES results were frustration (33%), worry (30%), and curiosity (30%). Among parents who interpreted their child’s results as positive, worry was the most common emotion, whereas among those who interpreted the results as uncertain or negative, frustration was most common (Figure 1).
Figure 1.
Parental reported emotions upon learning of child’s CES results for all types of results and stratified by parental interpretation of child’s CES results. Significant difference between positive and negative results (*), uncertain and negative results (#), positive and uncertain results (^) (Chi square test, p <0.05).
All but five parents reported sharing their child’s CES results, and all parents who interpreted the result as positive shared the results. The majority reported sharing with family (94%) and a non-geneticist physician (83%), while fewer shared results with friends (56%), employers (16%) or insurance companies (10%).
Sixty percent of parents who interpreted their child’s results as positive responded that the CES results affected their child’s medical care, including medical appointments or additional testing. This was less frequently reported when parents interpreted the results as uncertain (35%) (p=0.01) or negative (13%) (p<0.0001). Nearly half (49%) of parents who interpreted their child’s results as positive reported that the results affected non-medical care for their child, such as participating in or starting a support group, meeting other affected individuals or participating in research. Parents of children with negative results were less likely to report that results affected non-medical care (19%) (p<0.0005), but parents who interpreted the results as uncertain reported that results affected non-medical care almost as often (39%) as the positive results group (p=0.3).
When provided with a list of 11 positive experiences (e.g., provided closure or answers about diagnosis, prognosis, recurrence risk; gave reassurance about cause or more control or hope; helped to identify research, treatment or specialists) and 10 negative experiences (e.g., not helpful; increased worry; led to more questions, uncertainty or loss of privacy; negative effects on relationships, insurance or job) (appendix pages 6–7), parents reported on average 3 positive experiences and 1.7 negative experiences with the testing. Parents who interpreted the child’s results as positive reported more positive experiences (4.8) than those with uncertain (1.7) (p <0.0001) or negative results (1.9) (p <0.0001). The number of negative experiences reported by parents who interpreted the results as positive (1.7) was greater than in the negative results group (0.9) (p=0.008) and similar in the the uncertain results group (1.3) (p=0.2).
In the analysis restricted to the 147 parents whose child was evaluated in clinic and therefore had a pre-test GC session, the majority of parents (65%) reported being satisfied with the genetic counseling experience, as measured by the genetic counseling satisfaction scale, and this did not differ by the parental interpretation of the child’s results. The proportion of parents reporting satisfaction was somewhat greater when the GC had ≥ 5 years of experience (76%) than when the GC had less experience (58%) (p=0.02). Parental depression and anxiety and time from results disclosure to survey completion were not associated with genetic counseling satisfaction. Parents satisfied with genetic counseling reported more personal healthy behaviors (p= 0.005) than parents who had some dissatisfaction (Table S5ab).
Parental opinions about timing and decision to have CES
Twenty-one percent of parents expressed some regret over the decision to have CES for their child; regret was similar across result types (Table S6a). There was a modest increase in reported regret when their GC had fewer than 5 years of experience than when the GC had greater experience (32% vs 16%, p=0.02). Parental depression was modestly associated (p=0.02) with regret, while anxiety and the time from results disclosure to survey completion were not. The number of reported positive experiences related to CES was greater for parents without regret (p=0.002) (Table S6b). Genetic counseling satisfaction and regret were moderately associated; the proportion with regret was 32% among parents who were dissatisfied with the genetic counseling process, but only 17% among those who were satisfied (p= 0.01).
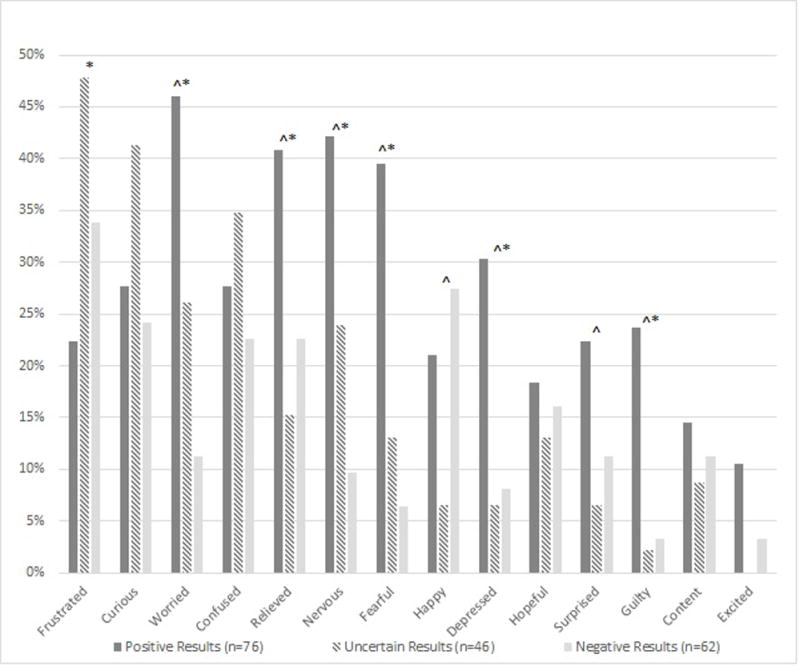
When asked whether they would have changed the timing of when they learned the CES results, over half (51%) of parents indicated they would have had testing sooner if it were possible (Figure 2). A quarter (24%) would have liked to have had results before they had children and 22% would have liked to have had results sooner or at the time of their child’s symptom onset. One parent whose child was diagnosed with Emery Dreifuss muscular dystrophy by CES indicated she would have preferred never to learn the results.
Figure 2.
Parental responses to “If you could have chosen when to learn about the results, when would that have been?” stratified by parental interpretation of child’s CES results.
Associations of parental psychological experience
Thirteen percent of parents had a Generalized Anxiety Disorder-7 (GAD-7) score ≥10, indicating moderate to severe anxiety, which is similar to the general population prevalence of 18.1% [29]. Twelve percent of parents had a Personalized Health Questionnaire-9 (PHQ-9) score ≥10 indicating moderate or severe depression, which is higher than the prevalence of the general population (7.6%), but more similar to the prevalence in women (9.5%) [30]. Parental depression and anxiety were not associated with time from results disclosure. The presence of depression and anxiety were greater in parents who interpreted their children’s results as positive (14% and 15%, respectively) than negative (8% and 8%, respectively) or uncertain (4% and 7%, respectively), but the differences were not significant (p=0.25 and p=0.22, respectively).
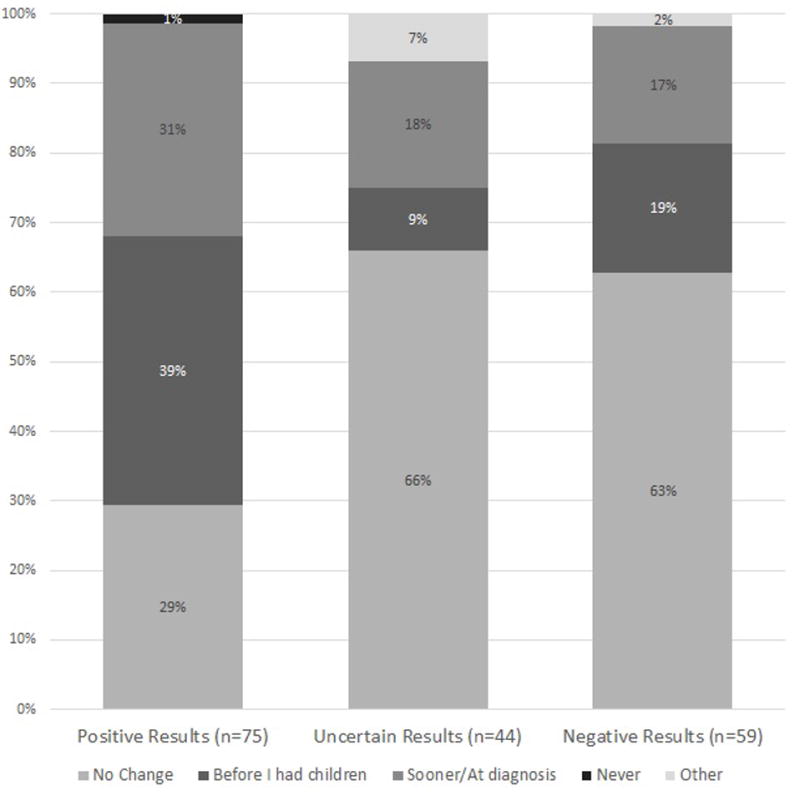
The aMICRA score was used to assess impact, including distress, uncertainty and positivity (reverse scored) of the genetic test results on the parents. On average, the aMICRA was 12 points higher (more negative impact) in parents who interpreted the results as positive compared those who interpreted results as negative, after adjusting for confounders (p<0.0001) (Figure 3, Table 3). The same pattern was observed for the aMICRA distress and uncertainty subdomains but not the positivity subdomain (Table S6ab). Parents who were not anxious or depressed, or who had more healthy behaviors, had significantly lower overall aMICRA scores and lower scores in the uncertainty and distress subdomains. The time from results disclosure to completion of the study survey was not associated with the aMICRA scores (Table 3).
Figure 3.
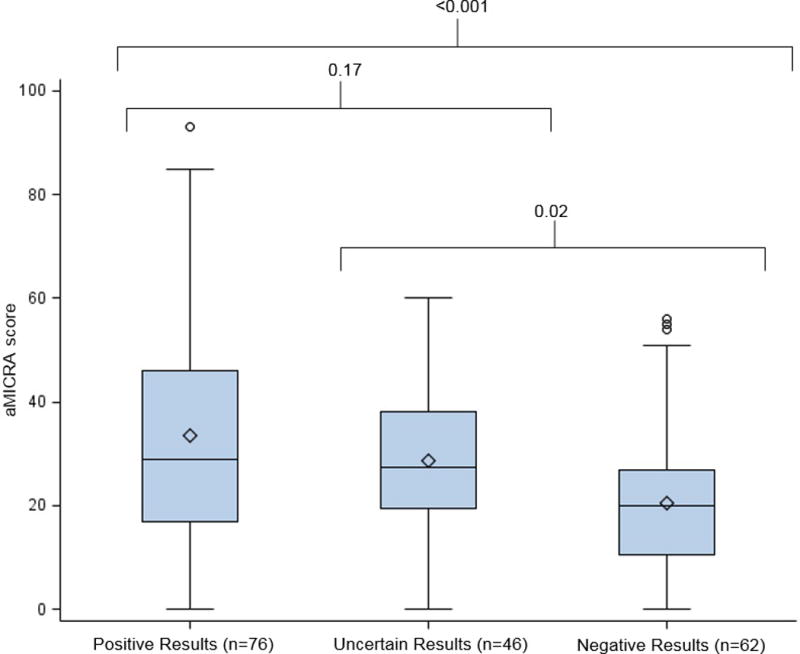
Box plot of aMICRA scores by parental interpretation of child’s exome sequencing results. Two sample t-tests with pair wise analysis.
Table 3.
Association of aMICRA scores (outcome) and parental interpretation of child CES results (primary predictor) and other demographic and parental experience variables.
| Variable | Reference | Estimate | 95% CI U | 95% CI L | p-value | N |
|---|---|---|---|---|---|---|
| Parental Intp. of CES Results | Pos vs Neg | 13.0 | 6.8 | 19.3 | <.0001 | 175 |
| Unc vs Neg | 8.2 | 1.1 | 15.3 | 0.023 | ||
| Pos vs Unc | 4.8 | −2.0 | 11.7 | 0.16 | ||
| Parental Intp. of CES Results* | Pos vs Neg | 12.1 | 5.8 | 18.3 | 0.0002 | 175 |
| Unc vs Neg | 7.3 | 0.5 | 14.0 | 0.035 | ||
| Pos vs Unc | 4.8 | −1.8 | 11.4 | 0.15 | ||
| Parental Misinterpreation of CES Results | Incorrect vs Correct | 3.8 | −3.1 | 10.8 | 0.28 | 181 |
| Parental Gender | Male vs Female | 1.0 | −7.4 | 9.4 | 0.82 | 181 |
| Child Gender | Male vs Female | 4.1 | −1.5 | 9.7 | 0.15 | 181 |
| Parental Age (years) | 0.2 | −0.2 | 0.5 | 0.29 | 181 | |
| Child Age (years) | −0.3 | −0.8 | 0.3 | 0.32 | 181 | |
| Marital status | Married vs Unmarried | 4.1 | −4.3 | 12.6 | 0.34 | 171 |
| Race/ Ethnicity | Non-White vs White | −0.3 | −6.3 | 5.7 | 0.91 | 181 |
| Education | < College vs ≤College | −0.8 | −6.9 | 5.4 | 0.81 | 181 |
| Employment | Employed vs Unemployed | 0.5 | −5.4 | 6.3 | 0.87 | 181 |
| Time in months between results returned and survey | 0.0 | −0.3 | 0.3 | 0.97 | 181 | |
| Medical Geneticist | MD1 vs All | 1.9 | −6.7 | 10.5 | 0.66 | 174 |
| MD2 vs All | 3.7 | −5.2 | 12.6 | 0.41 | ||
| Genetic Counselor experience** | ≥ 5yr vs <5yr | −2.4 | −9.0 | 4.3 | 0.49 | 141 |
| Reported cost | No cost vs cost | −3.0 | −9.0 | 3.1 | 0.33 | 181 |
| Reported method of results disclosure | Phone vs other | −3.8 | −9.4 | 1.8 | 0.18 | 181 |
| Location | In Patient vs Clinic | −2.3 | −9.0 | 4.5 | 0.51 | 181 |
| Indication | Single system vs Neurological | −0.6 | −8.3 | 7.2 | 0.89 | 181 |
| Multiple systems vs Neurological | −2.1 | −9.4 | 5.1 | 0.56 | ||
| Single system vs Multiple systems | 1.6 | −7.8 | 10.9 | 0.74 | ||
| Decision Regret Scale (DRS) | No Regret vs Regret | −5.8 | −12.8 | 1.2 | 0.10 | 181 |
| Genetic Counseling Satisfaction Scale (GCSS)** | Not Satisfied vs Satisfied | 1.9 | −5.3 | 9.0 | 0.61 | 137 |
| Personal Health Questionnaire (PHQ-9) | Not Depressed vs Depressed | −28.9 | −36.3 | −21.5 | <.0001 | 181 |
| Generalized Anxiety Disorder 7 Item (GAD-7) | No Anxiety vs Anxiety | −24.8 | −32.4 | −17.1 | <.0001 | 181 |
| Genetic Knowledge | Not All Correct vs All Correct | −0.9 | −7.1 | 5.3 | 0.77 | 180 |
| Exome Sequencing Knowledge | Not All Correct vs All Correct | −1.4 | −7.0 | 4.3 | 0.63 | 180 |
| Internal Health Locus of Control (IHLC) | higher=more control | 0.4 | −0.5 | 1.3 | 0.38 | 181 |
| Healthy Behaviors (HB) | higher= more healthy behaviors | −1.5 | −2.1 | −0.9 | <.0001 | 181 |
Abbreviations: Adapted Multidimensional Impact of Cancer Risk Assessment (aMICRA), Clinical Exome Sequencing (CES), 95% Confidence Interval Upper and Lower (95% CI U, L).
p-values from ANOVA for categorical predictors, dummy reference variable created for predictor variables with three categories, two sample t-test for continuous predictors.
Adjusted for confounders of child gender and method of result disclosure, other significant variables were not associated with parental interpretation of results (primary predictor)
Analysis excludes in patients
DISCUSSION
This study provides a description of the reported parental understanding and experience of diagnostic CES in minor children or adult children lacking capacity to consent. These results demonstrate that while the majority of parents correctly interpret their child’s CES results, there is some misunderstanding, particularly when results are reinterpreted by the clinician or are uncertain. The years of experience of the treating GC is positively associated with parental satisfaction with the decision to have CES and with the genetic counseling experience. Consistent with prior studies, the results highlight that the experience of parents is varied and is associated with the child’s CES results and parental anxiety or depression.
Parental Interpretation of Results
Although overall the parent’s interpretation of their child’s test results was consistent with the clinician’s interpretation, interpretations were discordant nearly a quarter of the time, more frequently when the clinician re-interpreted the laboratory report. While misinterpretation was not associated with satisfaction about the decision to have testing, genetic counseling experience or psychological impact of the results, it was associated with fewer positive experiences attributed to testing. Our results highlight both the importance and the challenge of effective communication of the clinician’s interpretation. Clinician and laboratory interpretations may differ because of the clinicians’ greater familiarity with the patient’s phenotype or expert knowledge of the gene or condition, particularly in cases of novel or rare conditions. Not infrequently, CES reports list multiple uncertain variants. Although the number of uncertain variants reported differs by laboratory and clinical indication and will decrease as variant interpretation improves, our results, which found misunderstanding occurred when multiple variants were reported, indicate that reporting a large number of variants may be negatively impacting parental understanding of their child’s CES results. Anticipatory guidance regarding the potential for uncertain results is part of the informed consent process [31], and pre-test counseling about the potential for discrepancies between the laboratory and clinician interpretation may also be beneficial.
The multifaceted issue of uncertainty is recognized in genomic testing. There may be uncertainty about pathogenicity of identified variants. Even when a pathogenic variant is identified there maybe uncertainty about the implications for the child’s diagnosis and prognosis [15]. Furthermore, genomic testing is complicated by the “nuanced negative,” or the possibility that negative CES results today may change with re-analysis in the future [19]. When a patient is strongly suspected to have an underlying genetic cause but no molecular causes are identified by CES,, the results may be communicated to the patient as “negative at this time.” The results disclosure includes the discussion of the possibility that as genomic knowledge improves a cause could be identified in future re-analysis. These complexities likely play a role in some of the discordance between parental and clinician interpretations, as well as the relatively high frequency of parental confusion reported about the results. A critical component of obtaining consent for CES and disclosing CES results is preparing a patient for these uncertainties.
Timing of testing
Over half (61%) of the parents recalled that their child had other genetic testing prior to undergoing CES, and for many of these families, CES was the next step in their child’s diagnostic odyssey. While the average age of 6.8 years at the time of testing was relatively young, older child age was associated with a lower positive impact of the results, as measured by the aMICRA positivity domain. Additionally, many parents wanted to learn of their child’s results sooner, e.g., at the time of the child’s symptom onset, or to learn of the risk before they had children, even when this was not realistic (such as in de novo conditions). These results suggest, that at least for some parents, an aggressive approach to genetic diagnosis, potentially with CES as a first-tier option, even in the prenatal or neonatal periods, should be offered. Further research into the most effective timing for CES to maximize medical and familial benefits is needed.
Experience
Similar to studies on reactions to CMA and CES testing that have reported parental feelings of relief, better ability to cope with guilt, but also worry and a loss of hope for the future [22–24, 26], our results indicate that CES elicits many different emotions in parents. While frustration, worry and fear were some of the most frequent emotions reported upon learning results, curiosity and relief were reported nearly as often. Additionally, consistent with published experience with traditional genetic testing, the overall impact of genetic testing, as measured by the aMICRA, was greatest in parents who interpreted their child’s CES results as positive, especially with regard to the uncertainty and distress subdomains [11, 13]. At the same time, parents of children with positive results reported more positive experiences with the results than those with other types of results. It is important to consider that many of the parents are caring for critically or chronically ill children. Studies have previously documented how parents’ experience caring for a sick child increases their capacity to manage additional negative health information [24]. This resilience may be reflected in our observation of lower aMICRA scores and higher satisfaction with genetic counseling in parents who reported more personal healthy behaviors.
An ongoing challenge of genetic testing is evaluating medical utility [32]. There are still relatively few genetic diagnoses with effective treatments or cures. Despite this, over a third of parents in our study reported some perceived medical or non-medical utility, and this was more frequent when the parental interpretation of results was positive. Our findings are consistent with other studies that have documented patients’ perceived utilities of genetic testing for providing closure, focusing clinical care, guiding reproductive decisions, and increasing access to research and social supports [22, 23, 33]. This is an important component to genetic testing that should not be overlooked.
There were several factors that somewhat surprisingly were not associated with parental experience. Transient psychological experiences of higher levels of anxiety and distress within 6-12 months of testing have been reported with traditional genetic testing [34]. Time since receiving results and completing the survey did not show any differences in parental interpretation of results, satisfaction with testing or genetic counseling experience, psychological impact, anxiety or depression. When we repeated the analyses excluding the 77 parents who completed the survey within 12 months of receiving results, the relationships between result type, parental anxiety and depression and psychological impact remained significant, as did the relationships between GC experience and parent satisfaction with the decision to have testing and with genetic counseling. The frequency of misinterpretation of test results also did not change. The lack of an effect of time may be due to the small number of parents reporting within one month of test disclosure; our data likely represent the long-term rather than acute impact of results on parents, arguably the most important component of the impact to understand.
Limitations
There are limits to the generalizability of our findings given the participation rate of 57%, with an overrepresentation of white, non-Hispanic participants. Several parents chose not to respond because they found the questions brought up difficult thoughts or they were concerned about privacy, and their experiences may have differed. Only the mothers’ responses were included in the analysis when both parents responded, however when the analysis was repeated with the excluded fathers’ responses, results were similar. (Paired parental experiences will be reported separately.) The study was available only in English and respondents had high levels of employment and education. Additionally, most parents had prior experience with genetic and other medical testing and high knowledge of genetics. Our study sample came from a single institution, but five geneticists and 10 GCs were involved in care. Although counseling and disclosure sessions were not formally standardized, our division uses peer training and promotes client-centered counseling, which foster similarities across providers’ practices. Additionally, the sample included both outpatients and patients evaluated during a hospitalization. While we did not observe associations with these variables, variation in medical acuity may have affected patient experiences in ways not measured by this study. Our study was retrospective and therefore expectations before testing and baseline psychological state could not be assessed. The measures of aMICRA, GCSS and DRS were validated in adult patients who themselves were tested; validity in our study of parents responding to the testing of their children is unknown. However, the pattern of aMICRA scores stratified by type of result is consistent with results in published studies of adult patients [11, 13]. Because this was an exploratory analysis, we did not correct for multiple testing and some observed associations may have occurred by chance.
Practice Implications
Our results highlight some of the challenges of CES, including the complexity of nuanced results disclosures and managing expectations [35]. Introducing the potential for difference in laboratory and clinician interpretation in the informed consent process as well as effective communication of the clinician’s interpretation of the child’s results, along with an explanation of any differences in interpretation in the disclosure, may be helpful. There was a trend towards a higher frequency of regret when the parent interpreted the child’s results as negative and of frustration with uncertain results. These experiences demonstrate the importance of setting realistic expectations and discussing the potential uncertainty in the pretest session.
Although modest, the positive association between GCs’ years of experience and parents’ satisfaction with the decision to have CES and their genetic counseling experience underscore the potential impact of the GC. Despite this association, there was no association between parental misinterpretation of results and GC experience. The ability to educate patients is similar across counselors, but more experienced GCs likely have greater comfort with changing technology and more advanced counseling skills, allowing them to engage the patient/family emotionally in a session and address psychosocial issues. The psychosocial component of a session is potentially more important than the educational component and requires tailoring to the family. Patients who feel emotionally supported rather than passive receivers of information more frequently report the genetic counseling session to be positive and have better retention of information [36, 37]. Continued emphasis on the importance of psychosocial counseling to foster a relationship that includes responding to emotional and medical concerns, providing support and validation, assistance with coping, and facilitating empowerment is essential, especially when new technologies are introduced into practice. Effective of methods of pre-session education = might also help to minimize the educational time and maximize the tailored counseling time in a session. This finding also has important implications for future research – to include assessment of how GCs’ approaches and techniques vary by experience.
The results of this survey of parents of children who received diagnostic CES suggest that parental experiences vary and are influenced by many factors, including the child’s CES results and parental understanding, the genetic counseling process, and parental psychology. The results of this study have important implications for clinicians to help prepare families for the experience of CES and recognize families who would benefit from different strategies for pre-test education, counseling, and results disclosure. Finally, it is important to recognize that we are in an era of genomic medicine, and clinical whole genome sequencing is increasingly available. Given the parallels of WGS and CES, our results should also be considered by clinicians offering WGS.
Supplementary Material
Acknowledgments
We thank the families who participated in this research. Funding was provided by the 2016 Jane Engelberg Memorial Fellowship, an annual grant from the Engelberg Foundation to the National Society of Genetic Counselors, Inc, and NIH grants HG006600 and P50HG007257
Footnotes
CONFLICT OF INTEREST: Megan Cho is a current employee of GeneDx but was not at the time that she delivered patient care for those included in this publication. The other authors have no other conflict of interests.
References
- 1.Valencia CA, et al. Clinical Impact and Cost-Effectiveness of Whole Exome Sequencing as a Diagnostic Tool: A Pediatric Center’s Experience. Front Pediatr. 2015;3:67. doi: 10.3389/fped.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shashi V, et al. Practical considerations in the clinical application of whole-exome sequencing. Clin Genet. 2016;89(2):173–81. doi: 10.1111/cge.12569. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312(18):1870–9. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312(18):1880–7. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farwell KD, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med. 2014;17:587–595. doi: 10.1038/gim.2014.154. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias A, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014;16(12):922–31. doi: 10.1038/gim.2014.58. [DOI] [PubMed] [Google Scholar]
- 7.Ashida S, et al. The role of disease perceptions and results sharing in psychological adaptation after genetic susceptibility testing: the REVEAL Study. Eur J Hum Genet. 2010;18(12):1296–301. doi: 10.1038/ejhg.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves KD, et al. Behavioral and psychosocial responses to genomic testing for colorectal cancer risk. Genomics. 2013;102(2):123–30. doi: 10.1016/j.ygeno.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritz ER, et al. Psychological impact of genetic testing for hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2005;23(9):1902–10. doi: 10.1200/JCO.2005.07.102. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg AM, Chan-Smutko G, Pirl WF. Psychiatric implications of cancer genetic testing. Cancer. 2015;121(3):341–60. doi: 10.1002/cncr.28879. [DOI] [PubMed] [Google Scholar]
- 11.Lumish HS, et al. Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns. 2017 doi: 10.1007/s10897-017-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao S, et al. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94–7. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella D, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21(6):564–72. [PubMed] [Google Scholar]
- 14.Palmer CG, et al. A prospective, longitudinal study of the impact of GJB2/GJB6 genetic testing on the beliefs and attitudes of parents of deaf and hard-of-hearing infants. Am J Med Genet A. 2009;149a(6):1169–82. doi: 10.1002/ajmg.a.32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayeems RZ, et al. Parents’ Experience with Pediatric Microarray: Transferrable Lessons in the Era of Genomic Counseling. J Genet Couns. 2016;25(2):298–304. doi: 10.1007/s10897-015-9871-3. [DOI] [PubMed] [Google Scholar]
- 16.Dorschner MO, et al. Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am J Hum Genet. 2013;93(4):631–40. doi: 10.1016/j.ajhg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer SL, et al. Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89(3):275–84. doi: 10.1111/cge.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biesecker BB, et al. How do research participants perceive “uncertainty” in genome sequencing? Genet Med. 2014;16(12):977–80. doi: 10.1038/gim.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner D, Raspberry KA, King M. The nuanced negative: Meanings of a negative diagnostic result in clinical exome sequencing. Sociol Health Illn. 2016;38(8):1303–1317. doi: 10.1111/1467-9566.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalia SS, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genetics in Medicine. 2016;19:249–255. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- 21.Lewis KL, et al. Participant use and communication of findings from exome sequencing: a mixed-methods study. Genet Med. 2016;18(6):577–83. doi: 10.1038/gim.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell AM, et al. Not the end of the odyssey: parental perceptions of whole exome sequencing (CES) in pediatric undiagnosed disorders. J Genet Couns. 2016;25(5):1019–31. doi: 10.1007/s10897-016-9933-1. [DOI] [PubMed] [Google Scholar]
- 23.Krabbenborg L, et al. Understanding the psychosocial effects of CES test results on parents of children with rare diseases. J Genet Couns. 2016;25(6):1207–1214. doi: 10.1007/s10897-016-9958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapp JC, et al. Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clin Genet. 2014;85(2):120–6. doi: 10.1111/cge.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabor HK, et al. Informed consent for whole genome sequencing: a qualitative analysis of participant expectations and perceptions of risks, benefits, and harms. Am J Med Genet A. 2012;158a(6):1310–9. doi: 10.1002/ajmg.a.35328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiff M, et al. “What does it mean?”: uncertainties in understanding results of chromosomal microarray testing. Genet Med. 2012;14(2):250–8. doi: 10.1038/gim.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green RC, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–74. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. SAS 9.4 [computer program] Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- 29.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt LA, Brody DJ. Depression in the U.S. household population, 2009–2012. NCHS data brief. 2014;172 [PubMed] [Google Scholar]
- 31.Bernhardt BA, et al. Experiences with obtaining informed consent for genomic sequencing. Am J Med Genet A. 2015;167a(11):2635–46. doi: 10.1002/ajmg.a.37256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke W, Laberge AM, Press N. Debating clinical utility. Public Health Genomics. 2010;13(4):215–23. doi: 10.1159/000279623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiff M, et al. Parents’ perceptions of the usefulness of chromosomal microarray analysis for children with autism spectrum disorders. J Autism Dev Disord. 2015;45(10):3262–75. doi: 10.1007/s10803-015-2489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beran TM, et al. The trajectory of psychological impact in BRCA1/2 genetic testing: does time heal? Ann Behav Med. 2008;36(2):107–16. doi: 10.1007/s12160-008-9060-9. [DOI] [PubMed] [Google Scholar]
- 35.Amendola LM, et al. Illustrative case studies in the return of exome and genome sequencing results. Per Med. 2015;12(3):283–295. doi: 10.2217/pme.14.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashtiani S, et al. Parents’ experiences of receiving their child’s genetic diagnosis: a qualitative study to inform clinical genetics practice. Am J Med Genet A. 2014;164a(6):1496–502. doi: 10.1002/ajmg.a.36525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin J, Semaka A, Hadjipavlou G. Conceptualizing genetic counseling as psychotherapy in the era of genomic medicine. J Genet Couns. 2014;23(6):903–9. doi: 10.1007/s10897-014-9728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


