Abstract
Background
Biomarkers for assessing osteoarthritis activity necessitate multiple MRI sequences with long acquisition times.
Purpose
To perform 5-minute simultaneous morphometry (thickness/volume measurements) and T2 relaxometry of both cartilage and meniscus, and semi-quantitative MRI Osteoarthritis Knee Scoring (MOAKS).
Study Type
Prospective.
Subjects
15 healthy volunteers for morphometry and T2 measurements, and 15 patients (5 each Kellgren-Lawrence grades 0/2/3) for MOAKS assessment.
Field Strength/Sequence
A 5-minute double-echo steady-state (DESS) sequence was being evaluated for generating quantitative and semi-quantitative osteoarthritis biomarkers at 3T.
Assessment
Flip angle simulations evaluated tissue signals and sensitivity of T2 measurements. Morphometry and T2 reproducibility was compared against morphometry-optimized and relaxometry-optimized sequences. Repeatability was assessed by scanning 5 volunteers twice. MOAKS reproducibility was compared to MOAKS derived from a clinical knee MRI protocol by two readers.
Statistical Tests
Coefficients-of-variation (CVs), concordance confidence intervals (CCI), and Wilcoxon signed-rank tests compared morphometry and relaxometry measurements with their reference standards. DESS MOAKS positive percent agreement (PPA), negative percentage agreement (NPA), and inter-reader agreement was calculated using the clinical protocol as a reference. Biomarker variations between Kellgren-Lawrence groups were evaluated using Wilcoxon rank-sum tests.
Results
Cartilage thickness (p=0.65), cartilage T2 (p=0.69), and meniscus T2 (p=0.06) did not significantly differ from their reference standard (with a 20° DESS flip angle). DESS slightly overestimated meniscus volume (p<0.001). Accuracy and repeatability CVs were <3.3%, except the meniscus T2 accuracy (7.6%). DESS MOAKS had substantial inter-reader agreement and high PPA/NPA values of 87%/90%. Bone marrow lesions and menisci had slightly lower PPAs. Cartilage and meniscus T2, and MOAKS (cartilage surface area, osteophytes, cysts, and total score) was higher in Kellgren-Lawrence groups 2 and 3 than group 0 (p<0.05).
Data Conclusion
The 5-minute DESS sequence permits MOAKS assessment for a majority of tissues, along with repeatable and reproducible simultaneous cartilage and meniscus T2 relaxometry and morphometry measurements.
Keywords: DESS, T2, Morphometry, Cartilage, Meniscus, MOAKS
INTRODUCTION
Knee osteoarthritis (OA) is a multi-factorial whole-joint disease that is one of the leading causes of disability in older populations. Knee OA affects approximately 17 million adults in the United States and has a global prevalence rate of 4% (1, 2). Radiography, the current standard for OA diagnosis, is only sensitive to gross morphological changes that typically occur only in late-stage OA and has poor sensitivity for assessing soft-tissues (3, 4). MRI-based biomarkers such as morphometry (thickness and volume measurements) and T2 relaxation times on the other hand, are promising methods for sensitive detection of early-stage OA. Changes in cartilage and meniscus morphology can indicate early OA progression (5, 6). Similarly, the tissue T2 is sensitive to underlying changes in the collagen matrix, and regional variations of T2 in tissue sub-regions have been used to differentiate between healthy subjects in studies and those with OA (7–9). Moreover, the MRI Osteoarthritis Knee Score (MOAKS) is a semi-quantitative scoring mechanism for assessing radiological features that may be involved in the pathophysiology of OA from a whole-joint perspective (10). MOAKS evaluates features involved in OA progression such as cartilage, menisci, osteophytes, and bone marrow lesions (BMLs), amongst others, on discrete grading scales. Detailed descriptions of MOAKS features and their scoring mechanisms is described by Hunter et al (10). Such MRI features can evaluate the onset of early OA, which is characterized by cartilage lesions and thickness loss, meniscal tears, and bone marrow lesions (11).
Quantitative and semi-quantitative measures are promising for evaluating early OA progression, however acquiring them efficiently, can be challenging. The small volumes of the cartilage and menisci necessitate MRI sequences with high in-plane resolution and thin slices for accurate morphometry measurements (12). T2 relaxometry, on the other hand, requires high signal-to-noise ratios (SNR) for accurate T2 quantification. Performing simultaneous relaxometry and morphometry with a single sequence is difficult since high-resolution and high-SNR are fundamentally at odds with each other. This problem is further exacerbated because the short T2 relaxation time of the meniscus makes it challenging to image and consequently, most OA studies have only investigated cartilage T2 measurements (13, 14). Additionally, semi-quantitative assessment using MOAKS typically requires multiple scan planes and potentially different image contrasts for accurate tissue assessment (10).
Given the need for multiple lengthy sequences, MRI protocols in OA research are often very time consuming. Large clinical studies such as the Osteoarthritis Initiative (OAI) require approximately 30 minutes of scan time for semi-quantitative joint assessment and acquisition of cartilage morphometry and T2 biomarkers alone. Similar scan times to characterize the cartilage and meniscus are reported in other studies (9, 12). Besides patient comfort and imaging costs, long scans and multiple sequences engender problems such as motion artifacts, the need for image registration, and segmentations of multiple image-sets. A single, relatively fast sequence could overcome these problems, potentially leading to increased patient throughput and retention during longitudinal clinical studies. The double-echo in steady-state (DESS) sequence is one potential sequence to achieve such a rapid acquisition.
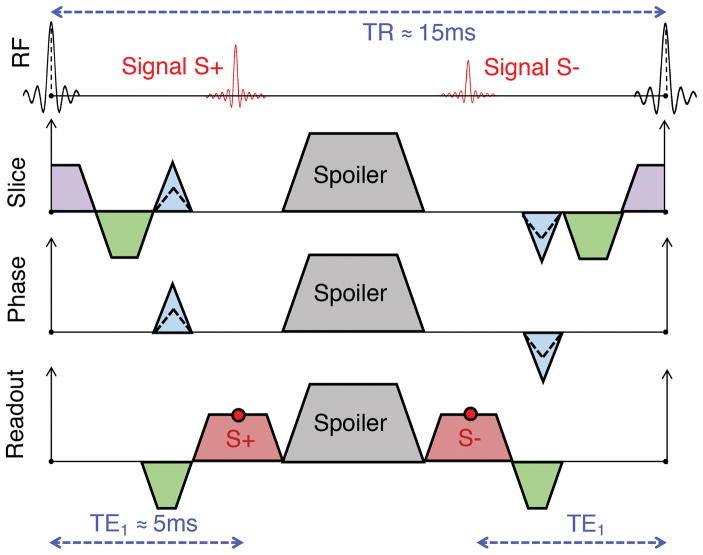
DESS generates two echoes per repetition time (TR) as seen in Fig. 1, separated by a spoiler gradient to avoid banding artifacts (15–18). The first DESS echo (S+) has a T1/T2 contrast, which is similar to a proton-density (PD) contrast in the knee. The second DESS echo (S−) has additional T2 weighting and a controllable diffusion weighting imparted by the spoiler. In the OAI, a variation of DESS (widely available on Siemens scanners) is utilized where cartilage morphometry is performed with a root-mean-square sum of the two echoes (12). However, separating the two echoes can produce two independent diagnostic contrasts and can be used for measuring cartilage T2 through either analytical approaches (19–21) or dictionary-based iterative procedures (22, 23). Example DESS images can be seen in Fig. 2a–c. DESS can produce accurate T2 measurements due to its limited T1 and B1 sensitivity despite only using two echoes (22, 24). Consequently, the purpose of this study was to investigate the feasibility of a using a 5-minute DESS sequence to gather multiple quantitative and semi-quantitative OA biomarkers.
Figure 1.
A spatial-spectral radiofrequency pulse is used for 3D double-echo steady-state (DESS) with water-only excitation (not shown explicitly) which is followed by the sampling of the first echo (S+). The S+ magnetization that is dephased due to the spoiler gradient is rephased by the spoiler in subsequent repetitions and sampled in the S− readout. As a result, the TE2 for the S− echo is defined as 2(TR) – TE1. Spoiler gradients for all DESS sequences were applied in the slice direction. The gradients in red are the readout gradients, in green are the dephasers/rephasers, in blue are the phase encodes, and in purple are the slice-select gradients. All disparate gradients with the same polarity are combined in the actual implementation of the sequence, however, they are separated in this figure for clarity.
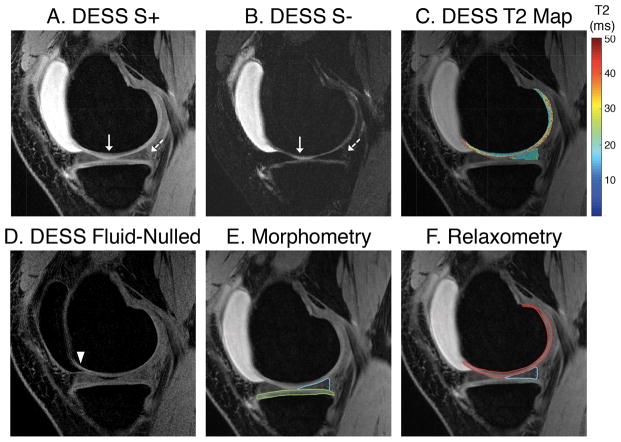
Figure 2.
DESS images with a flip angle of 20° have high signal intensity in the cartilage and meniscus in the S+ echo (a) and adequate contrast between cartilage and the surrounding tissues. The deep layer of cartilage (solid arrow) has higher signal attenuation (b) wherein a combination of the two echoes may underestimate cartilage volume. The posterior end of meniscus is challenging to delineate in the S− echo (dashed arrow) than the S+ echo due to low signal in the surrounding ligamentous structures. Sample T2 maps (c) and fluid nulled images (d) is additional information DESS can generate. The segmented medial tibial cartilage (green) and the medial posterior horn of the meniscus (blue) can be seen in (e) and the T2 maps in the medial femoral cartilage (red) and medial meniscus generated can be seen in (f).
MATERIALS AND METHODS
Simulations
Extended phase graphs (EPG) simulated the impact of flip angles ranging from 1–40° on the 5-minute experimental DESS (DESS-EXP) S+ and S− signal levels for cartilage, meniscus, tendon, muscle, and synovial fluid (25). The full protocol for sequences parameters in this study is listed in Table 1. These simulations were performed to compromise between adequate SNR for T2 relaxometry of meniscus and cartilage, low B1 sensitivity, and sufficient contrast for segmentation. Reference T1 and T2 values for the simulated tissues were obtained from previously established results (21, 26). The simulations were performed with numerical computations using a matrix representation of the EPG formalism with six transverse magnetization states according to methods described previously (20). The T2 values were calculated using the following analytical expression derived previously (where α is the flip angle) (20):
Table 1.
The DESS-MS sequence served as the morphometry reference sequences while the DESS-T2S served as the relaxometry reference sequence. A multi-echo fast-spin-echo sequence with eight echoes was also used for T2 comparisons (*meniscus T2 measurements utilized TEs of 6ms, 13ms, 19ms, and 26ms while cartilage T2 mapping utilized additional TEs of 32ms, 38ms, 45ms, and 51ms). All DESS sequences had a spoiler gradient area of 31.3 mT1m−1ms1 (1.33 cycles of dephasing/mm) and the minimum achievable TE and TR for the specified resolution and bandwidth. Note that for the clinical implementation of DESS-EXP, a readout of 416 samples and a slice thickness of 1.6mm was used to automatically reconstructed 512x512 images and to interpolate 0.8mm thick slices to 2.4mm thicknesses. Abbreviations: RO = readout, PE = phase encodes.
| # | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sequence | DESS | DESS | ME-FSE | DESS |
| Purpose | Morphometry Standard | T2 Standard | T2 Comparison | Test Experimental |
| Shorthand Name | DESS - MS | DESS - T2S | ME-FSE | DESS - EXP |
| Matrix (RO × PE) | 512×512 | 256×256 | 128×128 | 384×512 |
| Resolution (mm2) | 0.31×0.31 | 0.62×0.62 | 1.25×1.25 | 0.42×0.31 |
| Slice Thickness (mm) | 1.5 | 3 | 3 | 1.5 |
| Number of Slices | 80 | 40 | 20 | 80 |
| TE S+ (ms) | 6.5 | 5.1 | N/A* | 5.7 |
| TE S− (ms) | 35.5 | 25.5 | N/A* | 30.1 |
| Number of Echoes | 2 | 2 | 8 | 2 |
| TR (ms) | 21 | 15.3 | 1650 | 17.9 |
| Flip Angle (°) | 20 | 20 | 90 | 20 |
| Bandwidth (± kHz) | 42 | 31 | 31 | 42 |
| Parallel Imaging | None | None | None | 2x1 |
| % Corners Cut | 0 | 0 | 0 | 25 |
| Scan Time (mm:ss) | 14:20 | 2:37 | 2:30 | 4:48 |
The sensitivity of cartilage (nominal T1=1200ms, T2=40ms) and meniscus (nominal T1=1000ms, T2=10ms) T2 measurements to ±5% variations in flip angle were evaluated in order to simulate typical B1 transmit inhomogeneities in the knee. The percent error between the nominal T2 values and the T2 estimates calculated with the B1 inhomogeneities was evaluated. Additionally, for a flip angle of 20°, the percent error of the T2 calculation when the true tissue T1 was different than the assumed tissue T1 (1200ms and 1000ms for cartilage and meniscus respectively) was simulated. Synovial fluid signals in both DESS echoes were used to calculate a weighting factor (β) to perform a dual-echo weighted subtraction for creating fluid-nulled images of the form S+ - (β)S−.
In-vivo Study
The human experiments in this study were divided into two parts. In the first part, the reproducibility and repeatability of the 5-minute DESS-EXP quantitative morphometry and T2 relaxometry measurements of the cartilage and meniscus were assessed in 15 healthy volunteers (seven male, age 27±3; eight female, age 29±3). This cohort included the morphometry-optimized and relaxometry-optimized sequences for comparisons, as described below. In five instances, the volunteers were repositioned mid-protocol and the DESS-EXP scan was repeated to assess intra-subject repeatability. Intra-reader segmentation variability was assessed by measuring the coefficient of variation percentage (CV%) for cartilage and meniscus segmented three times in one volunteer with the DESS-MS sequence on three separate days. CV% was calculated as 100 times the standard deviation of the measurements to the mean.
In the second part of the human study, the 5-minute DESS-EXP MOAKS reproducibility and repeatability was evaluated in OA patients, along with its feasibility in differentiating OA subgroups. The sequence was implemented clinically at our institution and used to scan 15 patients referred for a knee MRI (eight male, age 49±21; seven female, age 42±22), 5 each with Kellgren-Lawrence grades (KLG) OA of 0, 2, and 3 as assessed through radiography. This cohort included the routine clinical knee MRI protocol at our institution for comparisons, as described below. Semi-quantitative MOAKS scoring (with DESS-EXP and the conventional knee MRI protocol) along with quantitative morphometry and T2 relaxometry measurements in the cartilage and meniscus (with only DESS-EXP) were performed in this cohort. MOAKS analysis was only attempted in the clinical cohort as numerous MOAKS features would be absent in healthy volunteers.
Institutional review board approval and informed consent was obtained for all 30 participants in this study. All scans were performed on identical Discovery MR750 3.0T MRI scanners (GE Healthcare, Waukesha, WI). Healthy volunteers were scanned with a flexible 16-channel receive-only knee coil array (Neocoil, Pewaukee, WI) on a research scanner while clinical scans were performed on a clinical scanner with an 8-channel transmit-receive knee coil (InVivo, Gainesville, Florida), according to institutional guidelines.
Morphometry
Cartilage thickness and meniscal volume and locations are morphological parameters sensitive to changes in early-OA, and consequently, were analyzed in this study (6, 27). Average cartilage thickness over the total area of subchondral bone on the tibial plateau in the medial femorotibial compartment was measured using a manual, quality-controlled segmentation using custom software (Chondrometrics GmbH, Ainring, Germany). Meniscus volume measurements in the posterior horn of the medial meniscus were performed manually using Amira FEI (Hillsboro, Oregon, USA). Cartilage and meniscus segmentation was performed using the DESS S+ echo. Cartilage thickness was computed from the 3D reconstructions of the cartilage surfaces while meniscus volume was computed as the total volume of the segmented voxels.
DESS-EXP was accelerated using 2x1 parallel imaging and by zero-filling the outer elliptical edges of k-space to reach a scan time of 5 minutes (28). Reference morphometry measurements to evaluate DESS-EXP reproducibility were obtained from a 14-minute DESS-MS sequence (scan parameters in Table 1). DESS-MS had no acceleration in order to decouple imaging acceleration (parallel imaging and corner cutting) artifacts from physiological artifacts. It also had a higher in-plane resolution than the OAI DESS in order to assist in separating the femoral and patellar cartilage, which has been shown to be challenging (12, 29). Both acquisitions (DESS-MS and DESS-EXP) of one knee were analyzed in parallel but with blinding to the acquisition type or the MRI sequence parameters. To qualitatively assess image quality, one reader (S.M.) with 13 years of experience in cartilage segmentation, compared the overall appearance of the two morphometry scans for segmentation purposes.
T2 Relaxometry
The T2 relaxation times in the medial femoral cartilage and the posterior horn of the medial meniscus were calculated in the most medial slice in the medial femoral condyle, where the anterior and posterior horns of the meniscus were separated triangles in the anterior/posterior direction (Fig. 2f). DESS fluid-nulled images were used to help guide segmentation in regions with large synovial fluid signal. Reference T2 measurements to evaluate DESS-EXP reproducibility were obtained from a previously-optimized Cartesian DESS sequence (scan parameters in Table 1) achieving high SNR through thick slices, low in-plane resolution, and a low sampling bandwidth (21). DESS-EXP images were downsampled to a 0.6×0.6×3.0mm3 resolution to increase SNR and to match the DESS-T2S resolution. Reference T1 values for cartilage were 1.2s and for the meniscus were 1.0s (21). The reported T2 values were averages of all pixel T2 values in the entire segmented region of interest. The T2 measurements were also qualitatively compared to T2 measured using a monoexponential fit from a multi-echo fast-spin-echo sequence (ME-FSE) due to its pervasive use, especially in the OAI. However, since ME-FSE is prone to over-estimating T2 due to stimulated echoes, statistical comparisons for T2 measurements were only performed in relation to DESS-T2S (24, 30).
Coil-combination for T2 measurement sequences was performed using R=1 sensitivity encoding (SENSE) due to its reduced sensitivity to noise biases, compared to sum-of-squares (21, 31). SENSE allows noise from different channels to cancel, and the use of coil sensitivities attenuates noise contributions in regions of limited coil sensitivity. Coil-sensitivity maps were approximated by low-pass filtering the lowest TE image according to methods described previously (21). Since DESS T2 fits are sensitive to the S− SNR, this SNR was estimated using the average signal from the segmented tissues and the standard deviation of the background signal from the first slice of the acquisition in order to minimize structured anatomical noise and possible aliasing. The DESS-EXP readout bandwidth (±42kHz, 4.6ms readout) was higher than the bandwidth of DESS-T2S (±31kHz, 4.1ms readout) to reduce the readout duration while simultaneously achieving a higher resolution. Similarly, DESS-EXP had 384×512 samples (in the readout and phase encode directions respectively) to maintain a short TE and TR in order to achieve high SNR for T2 measurements, while simultaneously generating high in-plane resolution images.
MOAKS
Semi-quantitative assessment of the knee using MOAKS was performed independently by two readers, one an experienced radiologist with 13 years of experience (E.O.) and the other an orthopedic surgeon with 1 year of experience in MOAKS scoring (S.E.). Reference MOAKS measurements were obtained using the clinical knee MRI protocol at our institution (detailed parameters listed in Supplementary Table 1). As MOAKS assesses 182 features, not all of which that can be evaluated through an invasive gold-standard reference such as arthroscopy, the conventional imaging protocol was used as a reference standard. Moreover, the MOAKS method has been designed and validated using MR imaging methodologies (10). Consequently, the metrics of positive percentage agreement (PPA) and negative percentage agreement (NPA) for DESS-EXP MOAKS were used as surrogates for the more-typical sensitivity and specificity calculations.
The readers performed the MOAKS scoring first using the 5-minute DESS-EXP sequence (with S+ and S− multiplanar reformatting) and subsequently, using the routine clinical knee MRI protocol, which served as the comparison metric. The MOAKS scoring was sub-divided into fifteen different joint features (listed in Table 3) as described in detail in previous guidelines (32). Sagittal DESS slices were reviewed with a slice thickness of 2.4mm while the axial and coronal reformations were reviewed with slice thicknesses of 2.5mm. The number of occasions where DESS-EXP underestimated BML size was calculated. Following the independent readings, a consensus review was performed amongst the two readers to assess reader discrepancies.
Table 3.
Statistical analysis of the MOAKS assessment comparing the DESS-EXP only scan to that of the conventional clinical protocol. The number of findings (prior to the consensus review) for both the DESS-EXP and conventional protocols, the total number of instances scored, and the prevalence rate can be seen in the table. In addition, positive percentage agreement (PPA) and negative percentage agreement (NPA) for both readers (separated by commas), and the corresponding inter-reader Cohen’s κ percentage (with a 95% confidence interval) can also be seen. The ‘Cysts’ category includes bone marrow lesion cysts, Baker cysts, and ganglion cysts. The ‘Other’ category includes the features of the patellar tendon, iliotibial band, and loose bodies. Abbreviations: Conv. = Conventional clinical protocol, Cartilage SA = cartilage surface area, BML = bone marrow lesion.
| MOAKS Feature | Positive Finds | Total Reads | Positive % | PPA | NPA | Cohen’s κ % |
|---|---|---|---|---|---|---|
| Cartilage Thickness | 35 | 420 | 8.3 | 100, 94 | 100, 100 | 85 (78–99) |
| Cartilage SA | 50 | 420 | 11.9 | 96, 96 | 100, 100 | 85 (74–97) |
| Osteophytes | 164 | 360 | 45.6 | 96, 97 | 99, 99 | 65 (54–76) |
| Meniscus Tears | 5 | 1080 | 0.5 | 67, 50 | 100, 100 | 0 (−99–99) |
| Meniscus Signal | 23 | 720 | 3.2 | 33, 57 | 99, 97 | 63 (39–87) |
| Meniscus Extrusion | 14 | 120 | 11.7 | 100, 88 | 100, 100 | 81 (56–99) |
| BML Subregions | 82 | 450 | 18.2 | 88, 79 | 100, 100 | 72 (58–85) |
| BML Sizes | N/A | N/A | N/A | 73, 79 | 100, 100 | 80 (69–91) |
| Hoffa Synovitis | 10 | 30 | 33.3 | 67, 50 | 89, 100 | 19 (−49–87) |
| Effusion Synovitis | 14 | 30 | 46.7 | 100, 100 | 100, 100 | 86 (61–99) |
| Bursa | 2 | 90 | 2.2 | 100, 100 | 100, 100 | 99 (99–99) |
| Cysts | 57 | 570 | 10.0 | 92, 94 | 99, 100 | 74 (60–88) |
| Ligaments | 3 | 180 | 1.7 | 100, 100 | 100, 100 | 66 (0–99) |
| Other | 4 | 90 | 4.4 | 0, 100 | 100, 100 | 0 (−99–99) |
|
| ||||||
| Total | 545 | 5010 | 10.9 | 88, 88 | 99, 99 | 76 (72–81) |
Statistical Analysis
Accuracy CV% for DESS-EXP in cartilage and meniscus was calculated for the morphometry and relaxometry measurements by comparing to DESS-MS and DESS-T2S respectively. Precision CV% was calculated for the quantitative morphometry and T2 relaxometry measurements in the meniscus and cartilage within the two repeated scans in five volunteers. Statistical significance between accuracy and repeatability values was assessed with Wilcoxon signed-rank tests. Bland-Altman plots were used to evaluate the agreement between DESS-EXP and the comparison sequences, as well as the repeatability of the measurements. Bland-Altman plots with 95% limits of agreement (LOA) were computed by calculating the average difference between the two measurements and ±1.96 times the standard deviation of the differences (33). Concordance correlation coefficients (CCC) and their confidence intervals (CCC-CI) were also calculated to assess agreement and repeatability (34). Differences in S− cartilage and meniscus SNR between the DESS-T2S sequence and DESS-EXP were assessed with Wilcoxon signed-rank tests.
The PPA and NPA of DESS-EXP MOAKS, prior to and following the consensus review was calculated (35). Inter-reader reliability and the corresponding 95% confidence intervals (CI) for DESS-EXP MOAKS scores were calculated using Cohen’s unweighted Kappa percentage (κ%). Statistical differences in morphometry, T2 relaxometry, and semi-quantitative MOAKS measurements (averaged between both readers) between Kellgren-Lawrence (KL) groups were assessed using Wilcoxon rank-sum tests for the unpaired observations. Non-parametric analysis was chosen for this study due to the small sample size and the positive skew of patient data, which violates the assumption of a normal distribution. All statistical significance was assessed at a significance level of p<0.05.
RESULTS
Simulations
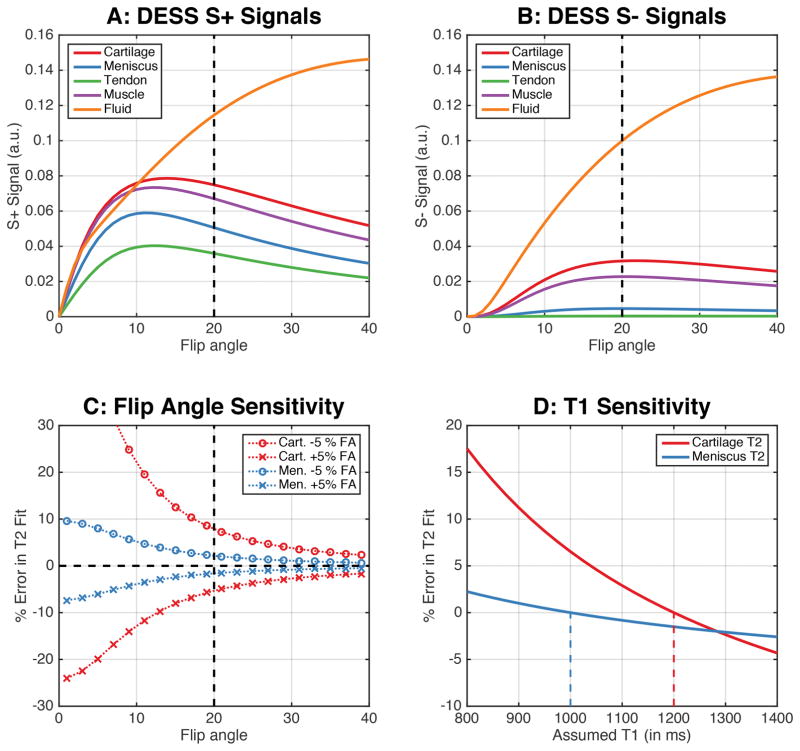
Given the tradeoffs between SNR, contrast, and B1 sensitivity, a flip angle of 20° was chosen for this study (dashed vertical line in Fig. 3a–c). The maximum signal for cartilage and meniscus in the S− echo occurred closer to 20° (Fig. 3a–b). At flip angles closer to 20° there was a considerable S+ signal difference between cartilage and the surrounding tissues such as the meniscus, muscle, and synovial fluid, which provided adequate contrast for cartilage and meniscus segmentation. Due to the limited B1 sensitivity at a flip angle of 20°, ±5% B1 variations induced a minimal ±7% change in cartilage T2 and a ±2% change in meniscus T2 values (Fig. 3c). Similarly, the error propagation in T2 values due to ±10% variation in cartilage and meniscus T1 values from assumed values was only <3%. DESS images acquired with a 20° flip angle, along with overlaid cartilage and meniscus segmentations and T2 maps show that the S− image (Fig. 2b) had lower signal in the deep layer of cartilage (solid arrow) and lower meniscus-ligament contrast (dotted arrow). Consequently, the S+ image was used for segmentation (Fig. 2c). A β of 1.2 was used to create fluid-nulled images (Fig. 2d).
Figure 3.
Simulating the DESS sequence for different musculoskeletal tissue shows that a flip angle of 20° can maintain high signal levels in both the S+ and S− echoes (a–b) while maintaining an adequate contrast between the cartilage, meniscus, and other surrounding tissues (a). At higher flip angles, T2 measurements are less sensitive to B1 variations (c). Increasing flip angles have lower sensitivity to B1 variations and errors in T2 fits. For the T2 calculation in this study, a T1 of 1200ms and 1000ms was assumed for cartilage and meniscus (vertical dashed lines in panel D). The impact of true T1 values being different from those assumed show that ±10% T1 variations only engender <±3% variations in T2 values (d). The T1 values used for simulating the cartilage, meniscus, tendon, muscle, and synovial fluid were 1.2s, 1.0s, 1.1s, 1.4s, and 2.5s respectively while the T2 values were 40ms, 10ms, 5ms, 30ms, and 600ms, respectively.
Morphometry
The accuracy CVs under 3.3% showed high agreement with the DESS-MS scan while the precision CVs under 2.8% showed excellent repeatability of DESS-EXP (Table 2). The intra-reader variability CVs for the three repeated segmentations were 1.1% and 2.6% for the cartilage and meniscus respectively. There was no statistically significant difference (p=0.65) in cartilage thickness measurements between DESS-MS (1.72 ± 0.22mm) and DESS-EXP (1.72 ± 0.21mm). Although there was a slight meniscus volume overestimation with DESS-EXP (727 ± 203mm3) compared to DESS-MS (698 ± 208mm3), the 3% accuracy CV suggests that this variation was minimal. The morphometry concordance measurements over 94% showed excellent agreement with the comparison standards and a high repeatability (Table 2). In all fifteen scans, the reader ranked the 14-minute DESS-MS scan worse in terms of image quality than the DESS-EXP scan due to slight motion artifacts.
Table 2.
Statistical results and concordance correlation coefficient (CCC) and the corresponding confidence interval (CCC-CI) from the comparisons performed between morphometry and T2 measurements performed with the different DESS sequences. P-values in bold refer to the rejection of the null hypothesis that median of the differences of the two comparison quantities is zero. Accuracy coefficient of variation percentage (CV%) for morphometry was determined by comparing cartilage thickness and meniscus volume with the measurements obtained from DESS-MS. Accuracy CV% for T2 relaxometry was determined by comparing the T2 values to those obtained from DESS-T2S. Precision CV% was calculated using the morphometry and relaxometry measurements obtained from the two pairs of repeated scans in five volunteers. Abbreviations: Comp. = Comparison.
| Parameter | Tissue | Comp. 1 | Comp. 2 | CV% | CCC | CCC-CI | P-Value |
|---|---|---|---|---|---|---|---|
| Accuracy | |||||||
|
| |||||||
| Thickness | Cartilage | DESS-MS | DESS-EXP | 1.5 | 98% | 94–99% | 0.65 |
| Volume | Meniscus | DESS-MS | DESS-EXP | 3.3 | 99% | 97–99% | <0.001 |
| T2 | Cartilage | DESS-T2S | DESS-EXP | 3.3 | 84% | 63–94% | 0.69 |
| T2 | Meniscus | DESS-T2S | DESS-EXP | 7.6 | 46% | 11–70% | 0.06 |
|
| |||||||
| Precision | |||||||
|
| |||||||
| Thickness | Cartilage | Scan 1 | Scan 2 | 0.7 | 99% | 95–100% | 1.00 |
| Volume | Meniscus | Scan 1 | Scan 2 | 2.8 | 94% | 56–99% | 0.82 |
| T2 | Cartilage | Scan 1 | Scan 2 | 1.8 | 86% | 13–98% | 0.61 |
| T2 | Meniscus | Scan 1 | Scan 2 | 2.1 | 83% | 20–97% | 1.00 |
T2 Relaxometry
There were no significant differences between cartilage T2 (p=0.69) and meniscus T2 (p=0.06) measurements obtained with DESS-EXP (36.2 ± 2.1ms and 11.5 ± 1.0ms respectively) compared with DESS-T2S (37.2 ± 2.8ms and 10.6 ± 1.0ms respectively). Cartilage and meniscus T2 values for DESS-EXP were highly repeatable, as seen through a concordance of over 83% and repeatability CV% of under 3% (Table 2). DESS-T2S SNR for cartilage and meniscus (50±9 and 14±2 respectively) was significantly higher (p<0.01) than DESS-EXP SNR (32±8 and 11±2 respectively). Analogously, the T2 concordances with DESS-T2S were higher for cartilage than for meniscus. The ME-FSE T2 values for cartilage and meniscus (39.6 ± 4.4ms and 11.6 ± 1.4ms respectively) were 8% and 10% higher, respectively, than those from DESS-T2S.
Bland-Altman Analysis
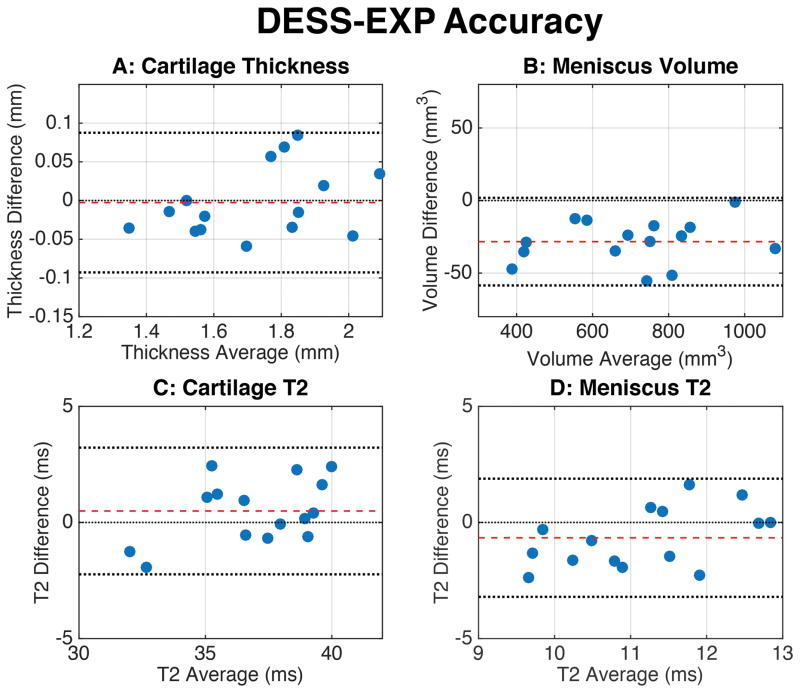
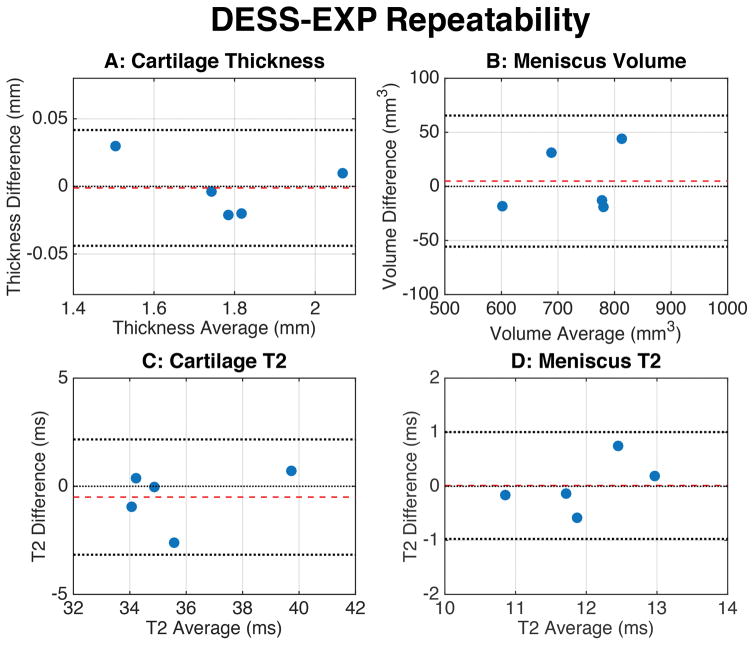
The Bland-Altman accuracy measurements (Fig. 4) showed that all measurements lay within the 95% LOA. The distribution of the values was randomly oriented around the zero-difference line, except for the meniscus, where DESS-EXP slightly overestimated meniscal volume. Similarly, Bland-Altman precision measurements (Fig. 5) show that the mean difference between the repeated scans was very close to the zero-difference line and that the spread of values was symmetric around this line, within the 95% LOA.
Figure 4.
Bland-Altman plots showing the accuracy of the morphometry and T2 measurements for the DESS-EXP sequence. Bland-Altman differences were computed by subtracting the DESS-EXP measurements from their corresponding standard measurements (i.e. DESS-MS or DESS-T2S). The dashed red line indicates the mean of the differences while the dotted black lines represent the upper and lower limits of agreement (LOA). All of the measurements were contained within the 95% LOA lines, which suggested comparable results between DESS-EXP and the standard sequences. The meniscus had a minimal overestimation of volume in DESS-EXP.
Figure 5.
Bland-Altman plots for evaluating repeatability metrics for the DESS-EXP sequence. The plots showed the difference between the repeated DESS-EXP measurements, against an average of the two measurements. The dashed red line indicates the mean of the differences while the dotted black lines represent the upper and lower limits of agreement (LOA). The mean value of the differences was close to zero and the measurements were scattered above and below the zero-difference line. There was no systematic error observed, suggesting that repeated iterations of the DESS-EXP sequence can provide comparable quantitative measurements.
MOAKS
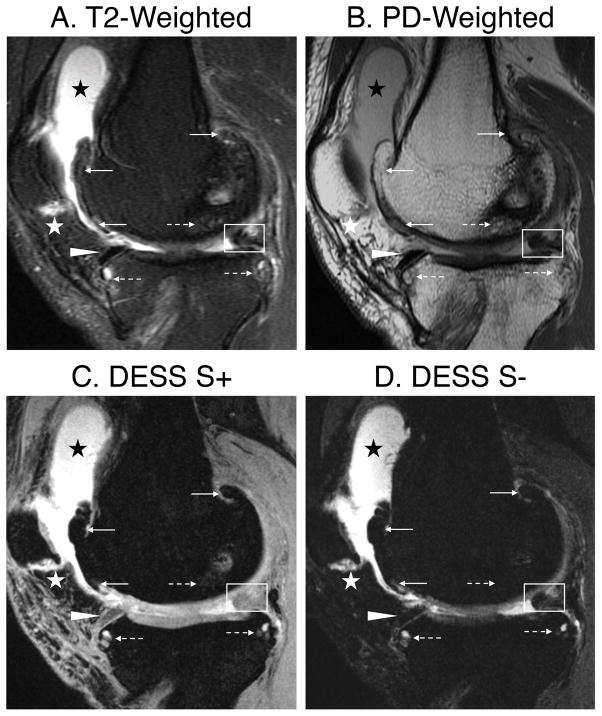
The average MOAKS PPA and NPA for both readers were 87% (83–91% CI) and 99% (99–99% CI) respectively (detailed breakdown in Table 3). Following consensus review, PPA increased slightly 88% (83–91% CI) but the near-perfect NPA remained the same. The PPA was lowest in assessing meniscus signal and tears, however, this was also driven by the low prevalence rate. There was substantial concordance amongst the two readers with a κ of 76% (CI of 72–81%). In comparison to sagittal T2-weighted and PD-weighted sequences, the DESS-EXP echoes enabled visualization of several features scored with MOAKS (Fig. 6). Cartilage loss, osteophytes, meniscal maceration, and synovitis were visualized equivalently between the two protocols, however, the femoral BML (dotted arrow) appeared with a diminished size with DESS-EXP. Overall, DESS-EXP underestimated BML sizes in 11 out of 60 (18%) of instances. There were 12 instances of false-negatives for evaluating increased meniscus signal with DESS-EXP.
Figure 6.
Comparing fast-spin-echo acquisitions and the DESS-EXP acquisition shows that DESS-EXP was successful in visualizing several MOAKS features. The contrast of DESS S+ was useful in identifying femoral osteophytes (solid arrows), large effusion synovitis (black star), and mild Hoffa’s synovitis (white star). The S− echo was especially helpful in identifying extrusion of the anterior horn of the medial meniscus (solid triangle), and increased signal and partial maceration of the posterior horn of the medial meniscus (white box). Bone marrow lesions (dashed arrow) as well as associated cysts were identified in both the femoral and tibial compartment. DESS did however, underestimate the bone marrow lesion grade in the central femoral compartment.
OA Subgroup Discrimination
Despite being a small pilot study, the 5-minute DESS-EXP accurately differentiated between patients with no OA and those with KLG2 and KLG3 OA (Table 4). Cartilage and meniscus T2 varied significantly in KLG2 and KLG3 patients, as compared to baseline KLG0 patients (p<0.02). KLG2 and KLG3 cohorts varied significantly for MOAKS features of surface area of cartilage loss, osteophytes, cysts, and total MOAKS score compared to KLG0 (p<0.05). In addition, KLG3 also had significantly different MOAKS features of cartilage thickness loss and number of BML subregions (p<0.05). There were no instances where significant differences compared to KLG0 were demonstrated with DESS-EXP but not the conventional protocol, and vice-versa.
Table 4.
A comparison of the quantitative DESS-EXP morphometry and T2 relaxometry measurements and the semi-quantitative MOAKS measurements for patients with osteoarthritis of Kellgren-Lawrence grades (KLG) of 0, 2, and 3. Entries in bold represent a significant difference as compared to the KLG0 cohort.
| KL Grade | Quantitative Parameters | ||
|---|---|---|---|
| 0 | 2 | 3 | |
| Cartilage Thickness (mm) | 2.09 ± 0.24 | 1.83 ± 0.23 (p = 0.31) | 1.74 ± 0.32 (p = 0.69) |
| Meniscus Volume (mm3) | 562 ± 105 | 842 ± 405 (p = 0.31) | 642 ± 279 (p = 0.69) |
| Cartilage T2 (ms) | 33.8 ± 2.7 | 41.8 ± 5.7 (p = 0.02) | 46.5 ± 6.4 (p < 0.01) |
| Meniscus T2 (ms) | 12.7 ± 1.0 | 16.6 ± 3.1 (p = 0.02) | 16.8 ± 4.0 (p < 0.01) |
|
| |||
| KL Grade | Semi-Quantitative MOAKS Parameters | ||
| 0 | 2 | 3 | |
|
| |||
| Cartilage Thickness | 0 ± 0 | 1.5 ± 1.7 (p = 0.17) | 5.2 ± 3.9 (p = 0.04) |
| Cartilage SA | 0.2 ± 0.4 | 2.4 ± 1.5 (p = 0.04) | 6.8 ± 4.1 (p = 0.04) |
| Osteophytes | 0 ± 0 | 11.8 ± 15.4 (p = 0.01) | 20.1 ± 7.6 (p = 0.01) |
| Meniscus Tears | 0 ± 0 | 0 ± 0 (p = 1.00) | 0.3 ± 0.7 (p = 1.00) |
| Meniscus Signal | 0.3 ± 0.7 | 1.2 ± 2.2 (p = 0.72) | 1.0 ± 1.3 (p = 0.40) |
| Meniscus Extrusion | 0 ± 0 | 0.5 ± 0.5 (p = 0.17) | 0.9 ± 0.9 (p = 0.17) |
| BML Subregions | 1.2 ± 0.8 | 2.4 ± 1.5 (p = 0.20) | 8.3 ± 3.8 (p = 0.01) |
| BML Sizes | 2.0 ± 2.2 | 1.7 ± 0.8 (p = 0.79) | 4.7 ± 2.0 (p = 0.07) |
| Hoffa Synovitis | 0.3 ± 0.4 | 0.1 ± 0.2 (p = 0.72) | 0.5 ± 0.7 (p = 0.84) |
| Effusion Synovitis | 0.8 ± 1.3 | 0 ± 0 (p = 0.44) | 2.1 ± 1.0 (p = 0.14) |
| Bursa | 0 ± 0 | 0 ± 0 (p = 1.00) | 0.2 ± 0.4 (p = 1.00) |
| Cysts | 0.4 ± 0.4 | 1.5 ± 0.5 (p = 0.02) | 3.6± 1.7 (p = 0.02) |
| Ligaments | 0.1 ± 0.2 | 0 ± 0 (p = 1.00) | 0.2 ± 0.4 (p = 1.00) |
| Other | 0 ± 0 | 0 ± 0 (p = 1.00) | 0.3 ± 0.3 (p = 0.17) |
|
| |||
| Total | 5.0 ± 4.8 | 17.3 ± 8.9 (p = 0.03) | 55.0 ± 20.7 (p < 0.01) |
DISCUSSION
In this study, DESS-EXP provided accurate and repeatable quantitative morphometry and T2 relaxometry measurements of cartilage and meniscus, along with semi-quantitative MOAKS assessment for the whole knee, using a single 5-minute 3D DESS acquisition. The morphometry measurements of cartilage and meniscus with DESS-EXP showed no significant differences to the morphometry measured with the gold-standard DESS-MS sequence. The morphometry repeatability was high and the inter-sequence variability was similar to the intra-reader variability. The T2 relaxation times generated by DESS-EXP showed high precision and high accuracy based on comparisons with a lower-resolution DESS-T2S scan in the cartilage and meniscus, enabled by adequate SNR in the S− echo. The MOAKS measurements performed with DESS-EXP compared well with the measurements performed with the conventional clinical protocol in several tissues. Despite being a small pilot study, the DESS-EXP quantitative and semi-quantitative measurements could differentiate between clinical OA subgroups.
For steady-state sequences such as DESS, the signal intensity in the two echoes is sensitive to flip angle. By simulating the DESS-EXP signal for common musculoskeletal tissues, a 20° flip angle balanced the need for high SNR, adequate contrast, and limited B1 sensitivity. While the maximum S+ signal expectedly occurred close to the Ernst angles for cartilage and meniscus (approximately 10–12°), such flip angles produced lower S− signal, which is known to affect T2 quantification (22). Moreover, smaller flip angles also had considerably lower cartilage-muscle and cartilage-fluid contrast, which would make cartilage segmentation challenging. Additionally, smaller flip angles created higher B1 sensitivity for the S−/S+ signal ratio. With a flip angle of 20°, there was a limited sensitivity to B1 variations, suggesting that higher flip angles may be more robust for T2 relaxometry and may obviate the need for a B1 mapping sequence. Similarly, the T2 measurements for cartilage and meniscus assumed certain T1 values based on previous measurements, however, due to the limited T1 sensitivity of DESS, even considerable deviations in the actual T1 only cause a minimal T2 change.
DESS-EXP had excellent accuracy and precision as seen through CVs under 3.3%, especially compared to the intra-reader repeatability of 3%. Although there was no significant difference between DESS-MS and DESS-EXP for cartilage thickness quantification, DESS-EXP had higher measured meniscus volumes. One possible explanation for this might an underestimation of meniscal volume in DESS-MS induced by subject motion. Since DESS-MS was a 14-minute scan, some amount of motion was expected, as was corroborated in the qualitative assessment of image quality. As the meniscus is primarily bordered by cartilage, any motion in the superior-inferior or anterior-posterior direction would blur the cartilage-meniscus boundaries and lead to meniscus volume underestimation in DESS-MS. Despite this variation, the extent of this overestimation produced a systematic accuracy CV of only 3%. This minimal systematic bias and the high precision suggests that the faster DESS-EXP sequence could be successfully used for monitoring longitudinal morphometry variations.
Besides the higher resolution, the separation of the two DESS-EXP echoes offered an additional advantage over the OAI DESS sequence, wherein the dual-echoes are combined. The deeper layers of cartilage have higher attenuation in the S− echo, so a root-mean-square sum of the two echoes may obfuscate the boundary of the deep cartilage and the subchondral bone, leading to cartilage thickness underestimations. Similarly, the S− echo has minimal contrast between the meniscus and the surrounding ligamentous structures, which makes delineating the posterior boundary of the meniscus challenging. Thus, only using the higher-SNR and contrast of the S+ signal makes cartilage and meniscus segmentation easier. In future studies, using a linear combination of the two echoes could also be explored for segmentation.
The accuracy and concordance of the DESS-EXP sequence for meniscus T2 values were lower than those for cartilage. Since the TE and TR of DESS-EXP were higher than those of DESS-T2S it is not unreasonable to expect some noise-induced T2 overestimation, even with DESS-EXP. However, while the DESS-EXP meniscus T2 measurements were approaching significance, they were not statistically significantly higher than those from DESS-T2S (p=0.06). Moreover, due to the short-T2 nature of the meniscus, even small variations in measured T2 values result in larger CV measurements, compared to cartilage. Overall, the relaxometry accuracy in this study compares very well with previous studies (9, 21, 36) and also has a comparable repeatability (13, 21). The ME-FSE T2 comparisons were included in this study as a qualitative reference due to the use of the sequence in the OAI. The resultant T2 estimations for both cartilage and menisci seemed to be overestimations, which agrees with previous studies (24, 30).
DESS-EXP had good overall PPA for MOAKS features, and near-perfect NPA. The contrast, resolution, and SNR offered by DESS for assessing these tissues helped maintain similar MOAKS scoring as compared to the clinical protocol. The ability to create multi-planar reformations for both echoes of DESS-EXP was especially useful in assessing the multitude of features associated with MOAKS. Of all the features assessed, DESS had high diagnostic accuracy for scoring cartilage and osteophytes, as well as fluid-sensitive features such as cysts, synovitis, and bursitis. The overall Cohen’s κ percentage showed substantial agreement between the two readers and is promising for future semi-quantitative scoring.
There was slightly lower MOAKS accuracy for BMLs and meniscal degeneration. It has been reported that DESS underestimates the size of BMLs due to T2* susceptibility effects, however, separating the two DESS echoes may help minimize this effect (12). By lowering the TE, the duration for susceptibility accrual decreases and the S+ echo can be more sensitive to BMLs. PPA for Hoffa’s synovitis was also somewhat low, however, it is often challenging to resolve without gadolinium-enhanced MRI, although a DESS acquisition with high-diffusion weighting has shown promising results (37, 38). On the other hand, the decreased PPA for meniscal degeneration was not intrinsic to the sequence and could potentially be improved in future studies. Meniscal tear and signal discrepancies were associated with increased signal in the tissue that likely occurred due to the TE of DESS-EXP, which was kept low to generate accurate meniscus T2 values.
For future studies, the readers could be calibrated using pairwise comparisons between DESS and the clinical protocol to evaluate if the increased meniscal signal is due to sequence effects or physiological effects. Overall however, the lower PPA for MOAKS meniscus signal is not a concern because the MOAKS measurements relies entirely on a binary (yes/no) qualitative observation. The availability of quantitative pixel-by-pixel meniscus T2 measurements afforded by DESS-EXP will enable readers to ascertain the cause of the increased meniscal signal and may help characterize meniscus tears as well as subtle meniscal degeneration.
Even if the BML and meniscal limitations with DESS cannot be improved and explicit semi-quantitative MOAKS measurements are required, DESS-EXP can be paired with a single additional coronal PD-weighted fat-saturated (PDFS) sequence. In this pairing, DESS will be able to generate the quantitative T2 and morphometry parameters, as well as MOAKS features relating to cartilage, osteophytes, synovitis, bursas, and cysts, while the coronal PDFS method could be used to generate MOAKS features relating to BMLs and the menisci. Such a two-sequence quantitative and semi-quantitative protocol would only require 7–8 minutes of scan time and would still be able to considerably increase the efficiency of current OA research protocols. Future studies could evaluate the performance of such a paired-protocol in order to evaluate overall MOAKS performance.
The inclusion of the DESS-EXP clinically showcases its robustness for multi-site clinical studies as there was no need for trained researchers conducting the scans or custom reconstruction methods. Despite the small number of sub-groups of KL patients used in this study, there were still significant differences in the quantitative measurements and several MOAKS features. As has been shown previously, the cartilage and meniscus T2 measurements differentiated OA patients with different KL grades (14). The morphometry measurements were not significantly different between subgroups, however, morphometry is generally associated with longitudinal changes rather than cross-sectional changes due to large inter-subject physical variability. The MOAKS findings from this study were also able to differentiate between OA subgroups based on hallmark OA features such as cartilage loss, osteophyte formation, and presence of BMLs (10). While beyond the scope of this work, further studies with larger subgroups will be essential to evaluate the physiological validity of all the potential biomarkers measured in this study. Additionally, future studies could also assess the strengths and weaknesses of 3D-FSE methods for quantitative and semi-quantitative imaging.
The experimental 5-minute DESS-EXP sequence may help mitigate some of the problems faced in clinical studies such as the OAI. For example, there is no additional need for image registration between the echoes for correlating relaxometry and morphometry variations and there is no need for segmentation of multiple image sets. While the sequence in this study was currently not perfect for evaluating BMLs and menisci, DESS-EXP could easily be paired with PDFS sequences to still enable rapid quantitative protocols for OA studies. By consolidating the scan time required to generate the quantitative and semi-quantitative parameters listed in this study, the DESS-EXP method makes it possible to reduce total protocol duration or to augment the protocols with sequences that can probe into additional promising biomarkers. Overall, the speed, accuracy, precision, and ease-of-use of this method is encouraging for its use in clinical research studies in order to gain insights into large clinical populations.
Some of the limitations of this study were the small sample sizes used to compare the DESS-EXP quantitative and semi-quantitative parameters to their corresponding standards. It should be noted that despite not being entirely accurate due to the use of multi-channel coils, the background-ROI method for estimating SNR serves as a useful comparison metric for signal quality, especially for intra-subject comparisons and in a limited spatial region such as the meniscus (39). This study also only analyzed cartilage thickness in the medial tibial compartment however, cartilage thickness measurements in femoral and patellar sub-regions along within different tissue layers could be measured, since different regions of the tissue have varied sensitivities to changes in OA (40). The MOAKS readers in this study could not be blinded to the two protocols offered due to the inherent contrasts and the number of image sets provided. Additionally, since arthroscopy cannot assess all MOAKS features, it was not utilized as validation metric, however, there may have been instances of errors generated by the routine clinical protocol.
In conclusion, we have demonstrated the feasibility of a 5-minute MRI method to simultaneously assess a majority of MOAKS features, along with accurate and repeatable quantitative morphometry and T2 relaxometry measurements in both cartilage and meniscus.
Supplementary Material
Acknowledgments
We thank Dr. Jarrett Rosenberg for providing assistance with statistics and Drs. Dushyant Thakur and Jeff Wood for assistance with assessing radiography images.
Grant Support:
Contract grant sponsor: National Institutes of Health (NIH); contract grant numbers NIH R01 AR063643, R01 EB002524, K24 AR062068, and P41 EB015891. Contract grant sponsor: GE Healthcare (research support).
Footnotes
Disclosures:
The authors wish to disclose the following, with respect to potential conflicts of interest relevant for this study. S.M. and W.W. are part time employees and co-owners of Chondrometrics GmbH. F.E. is CEO/CMO and co-owner of Chondrometrics GmbH. He has received consulting fees from Merck KGaA as well as honoraria from Medtronic (less than $10,000 each). Neither S.M., W.W., nor F.E. were involved in the design of the study.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14:212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. 2011;13:247. doi: 10.1186/ar3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloecker K, Wirth W, Guermazi A, Hitzl W, Hunter DJ, Eckstein F. Longitudinal change in quantitative meniscus measurements in knee osteoarthritis--data from the Osteoarthritis Initiative. Eur Radiol. 2015;25:2960–8. doi: 10.1007/s00330-015-3710-7. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 7.Welsch GH, Mamisch TC, Domayer SE, et al. Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures--initial experience. Radiology. 2008;247:154–161. doi: 10.1148/radiol.2471070688. [DOI] [PubMed] [Google Scholar]
- 8.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 9.Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthr Cartil. 2010;18:1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA. MOAKS (MRI Osteoarthritis Knee Score) Osteoarthr Cartil. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyten FP, Denti M, Filardo G, Kon E, Engebretsen L. Definition and classification of early osteoarthritis of the knee. Knee Surgery, Sport Traumatol Arthrosc. 2012;20:401–406. doi: 10.1007/s00167-011-1743-2. [DOI] [PubMed] [Google Scholar]
- 12.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauscher I, Stahl R, Cheng J, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21:1474–84. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruder H, Fischer H, Graumann R, Deimling M. A new steady-state imaging sequence for simultaneous acquisition of two MR images with clearly different contrasts. Magn Reson Med. 1988;42:35–42. doi: 10.1002/mrm.1910070105. [DOI] [PubMed] [Google Scholar]
- 16.Redpath TW, Jones RA. FADE--a new fast imaging sequence. Magn Reson Med. 1988;6:224–234. doi: 10.1002/mrm.1910060211. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Cho ZH. Fast SSFP gradient echo sequence for simultaneous acquisitions of FID and echo signals. Magn Reson Med. 1988;8:142–150. doi: 10.1002/mrm.1910080204. [DOI] [PubMed] [Google Scholar]
- 18.Hargreaves B. Rapid gradient-echo imaging. J Magn Reson Imaging. 2012;36:1300–1313. doi: 10.1002/jmri.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsch GH, Scheffler K, Mamisch TC, et al. Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn Reson Med. 2009;62:544–9. doi: 10.1002/mrm.22036. [DOI] [PubMed] [Google Scholar]
- 20.Sveinsson B, Chaudhari A, Gold G, Hargreaves B. A simple analytic method for estimating T2 in the knee from DESS. Magn Reson Imaging. 2017;38:63–70. doi: 10.1016/j.mri.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhari AS, Sveinsson B, Moran CJ, et al. Imaging and T2 relaxometry of short-T2 connective tissues in the knee using ultrashort echo-time double-echo steady-state (UTEDESS) Magn Reson Med. 2017 doi: 10.1002/mrm.26577. Early View. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3 T. Magn Reson Med. 2012;67:1086–96. doi: 10.1002/mrm.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng C-C, Mei C-S, Duryea J, et al. Dual-pathway multi-echo sequence for simultaneous frequency and T2 mapping. J Magn Reson. 2016;265:177–187. doi: 10.1016/j.jmr.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE. T2 Relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging. 2014;0:105–113. doi: 10.1002/jmri.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigel M. Extended phase graphs: dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging. 2015;41:266–95. doi: 10.1002/jmri.24619. [DOI] [PubMed] [Google Scholar]
- 26.Jordan CD, Saranathan M, Bangerter NK, Hargreaves BA, Gold GE. Musculoskeletal MRI at 3.0 T and 7. 0 T: a comparison of relaxation times and image contrast. Eur J Radiol. 2013;82:734–739. doi: 10.1016/j.ejrad.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emmanuel K, Quinn E, Niu J, et al. Quantitative measures of meniscus extrusion predict incident radiographic knee osteoarthritis - data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2016;24:262–269. doi: 10.1016/j.joca.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein MA, Fain SB, Riederer SJ. Effect of windowing and zero-filled reconstruction of MRI data on spatial resolution and acquisition strategy. J Magn Reson Imaging. 2001;14:270–280. doi: 10.1002/jmri.1183. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein F, Hudelmaier M, Wirth W, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–1220. doi: 10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 32.Roemer FW, Guermazi A, Collins JE, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort − Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17:466. doi: 10.1186/s12891-016-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1986;1:307–10. [PubMed] [Google Scholar]
- 34.I-Kuei Lin L. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 35.Collins JE, Losina E, Nevitt MC, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68:2422–2431. doi: 10.1002/art.39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauscher I, Stahl R, Cheng J, et al. Meniscal Measurements of T1ρ and T2 at MR Imaging in Healthy Subjects and Patients with Osteoarthritis 1. Radiology. 2008;249:591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McWalter E, Sveinnson B, Oei E, et al. Detecting Synovitis without Intravenous Contrast using a Modified DESS MRI Sequence. Proc 8th Annu Int Work Osteoarthritis; Asilomar. 2015. [Google Scholar]
- 38.Guermazi A, Roemer FW, Hayashi D, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011;70:805–11. doi: 10.1136/ard.2010.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26:375–385. doi: 10.1002/jmri.20969. [DOI] [PubMed] [Google Scholar]
- 40.Hunter DJ, Niu J, Zhang Y, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. AnnRheum Dis. 2009;68:349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.