Abstract
The organization of polarized stereociliary bundles is critical for the function of the inner ear sensory receptor hair cells that detect sound and motion, and these cells present a striking example of Planar Cell Polarity (PCP); the coordinated orientation of polarized structures within the plane of an epithelium. PCP is best understood in Drosophila where the essential genes regulating PCP were first discovered, and functions for the core PCP proteins encoded by these genes have been deciphered through phenotypic analysis of core PCP gene mutants. One illuminating phenotype is the domineering non-autonomy that is observed where abrupt disruptions in PCP signaling impacts the orientation of neighboring wild type cells, because this demonstrates local intercellular signaling mediated by the core PCP proteins. Using Emx2-Cre to generate an analogous mutant boundary in the mouse inner ear, we disrupted vertebrate PCP signaling in Vangl1;Vangl2 conditional knockouts. Due to unique aspects of vestibular anatomy, core PCP protein distribution along the mutant boundary generated in the utricle resembles the proximal side of vang mutant clones in the Drosophila wing, while the boundary in the saccule resembles and the distal side. Consistent with these protein distributions, a domineering non-autonomy phenotype occurs along the Emx2-Cre boundary in the mutant utricle that does not occur in the saccule. These results further support the hypothesis that core PCP function is conserved in vertebrates by demonstrating intercellular PCP signaling in the sensory epithelia of the mouse ear.
Introduction
The core planar cell polarity (PCP) proteins function to orient polarized cellular structures within the plane of an epithelium and to coordinate this polarity with the primary axes of tissues and organs. In Drosophila, PCP organization is evident in epidermal cells of the wing where non-sensory, actin-based hairs called trichomes emerge from the distal side of the cell and point towards the wing tip. In mutants that disrupt PCP signaling, trichomes emerge from the center of apical cell surfaces, fail to project towards the wing tip, and often form distinctive swirling patterns (Goodrich and Strutt, 2011, Vladar et al., 2009). In vertebrates, PCP organization is evident in sensory receptor hair cells of the inner ear that detect sound and motion. Hair cells extend a bundle of actin-rich stereocilia from the apical cell surface that are arranged in rows of increasing height with the tallest adjacent to an elongated primary cilium unique to hair cells called the kinocilium. Together this cytoskeletal ensemble comprises the stereociliary bundle, which also has a distinct intrinsic planar polarization because the kinocilium is always displaced to one side of the stereocilia and adjacent to the tallest stereocilium. Hair cell PCP is evident in the coordinated alignment of stereociliary bundles between neighboring cells in a fashion similar to the coordinated organization of trichomes in Drosophila. This polarized organization contributes to hair cell function because movement of the bundle towards the kinocilium in response to sound or motion triggers the opening of mechanically gated channels, hair cell depolarization and neurotransmission to afferent neurons of the VIIIth nerve (Corey, 2003, Schwander et al., 2010). As a result, the establishment of PCP is an important aspect of inner ear development underlying auditory and vestibular function.
Hair cell PCP in the mouse is dependent upon the vertebrate orthologues of Drosophila PCP genes that include Frizzled3 (Fzd3) and Frizzled6 (Fzd6), Vangl1, Vangl2 and Celsr1 (known as starry night or flamingo in Drosophila). Mutations in these genes result in hair cells with misoriented stereociliary bundles (Curtin et al., 2003, Duncan et al., 2017, Montcouquiol et al., 2003, Torban et al., 2008, Wang et al., 2006). The vertebrate and invertebrate PCP proteins also share similar patterns of polarized asymmetric distributions within cells and at neighboring cell junctions. Moreover in the vertebrate ear, PCP proteins are asymmetrically distributed in both the hair cells and the populations of supporting cells that surround hair cells and separate them from their nearest neighbors (Deans, 2013). Thus for auditory hair cells, FZD3 and FZD6 are enriched near the apical surface and along one side of the cell that is opposite of VANGL2 in the neighboring supporting cell (Giese et al., 2012, Wang et al., 2006). CELSR1 also localizes to both of these cellular locations while none of the PCP proteins are found along the perpendicular boundaries (Duncan et al., 2017). Likewise in Drosophila, Frizzled opposes Vang across intercellular junctions, and within individual cells Frizzled and Vang are always located at opposite poles with Flamingo enriched at both sites (Goodrich and Strutt, 2011, Strutt, 2002). A similar relative distribution of PCP proteins occurs in vestibular hair cells (Deans et al., 2007).
While these similarities are consistent with conservation of function, there are also differences that suggest aspects of PCP development in the vertebrate ear are unique from the Drosophila wing. First hair cells use separate molecular mechanisms for regulating intrinsic planar polarity, which is dependent upon Gαi signaling (Ezan et al., 2013, Tarchini et al., 2013), and coordinating cellular polarity between neighboring cells, which is mediated by the core PCP proteins (Curtin et al., 2003, Duncan et al., 2017, Montcouquiol et al., 2003, Torban et al., 2008, Wang et al., 2006). As a result, individual hair cells still form polarized stereociliary bundles in Fzd3/6 and Vangl2 mutants with the tallest stereocilia asymmetrically positioned next to the kinocilium (Copley et al., 2013, Montcouquiol et al., 2003, Wang et al., 2006). This is in contrast to Drosophila where the trichome emerges from a central location in fz and vang mutant epidermal cells before pointing or leaning in a single direction (Wong and Adler, 1993). Furthermore, while PCP mutant cells are misoriented relative to the body axis in both species, in Drosophila the trichomes retain local coordination and form whirls or other similar patterns (Chae et al., 1999, Taylor et al., 1998, Usui et al., 1999, Vinson and Adler, 1987), while for hair cells the stereociliary bundles are often severely misoriented relative to their nearest neighbors (Curtin et al., 2003, Montcouquiol et al., 2003, Wang et al., 2006). Finally, in two vestibular organs of the vertebrate ear, the utricle and saccule, hair cells are divided between two groups with oppositely oriented stereociliary bundles that meet at an anatomical landmark called the Line of Polarity Reversal (LPR) (Denman-Johnson and Forge, 1999). Despite this abrupt change in the orientation of planar polarized structures, the relative distribution of core PCP proteins is not changed between hair cells separated by the LPR (Deans et al., 2007). This has led to the interpretation that the core PCP proteins do not determine the final orientation of polarized structures in vertebrates, and instead establish a shared polarity axis that coordinates the organization of polarized structures throughout the tissue (Deans, 2013).
Based upon these observations, vertebrate core PCP protein function is likely restricted to relaying intercellular signals between neighboring hair cells to coordinate stereociliary bundle orientation relative to inner ear anatomy. Intercellular PCP signaling in Drosophila is demonstrated genetically by the domineering non-autonomy phenotype that occurs along the borders of frizzled and vang mutant clones. In the case of frizzled, wild type wing cells along the distal border of a clone are inverted and point their trichomes towards the clone, whilst for vang mutant clones it is the wild type cells along the proximal border that are impacted (Adler et al., 2000, Taylor et al., 1998, Vinson and Adler, 1987). These complementary non-autonomous effects correlate with the subcellular enrichments of Frizzled on the distal and Vang on the proximal sides of wing epidermal cells. While it is difficult to generate an analogous clonal boundary in the vertebrate inner ear, several previous studies evaluated the potential for vertebrate PCP proteins to facilitate analogous intercellular signaling. Transplantation studies in developing Xenopus skin, demonstrated that the planar polarity of wild type multiciliated cells is inverted if these cells differentiate adjacent to Vangl2 or Fzd mutant transplants (Mitchell et al., 2009). Similarly in the auditory organ of the developing chick ear, when PCP signaling is disrupted by overexpressing VANGL2 in subsets of cells, adjacent wild type hair cells may be misoriented through a non-autonomous effect (Sienknecht et al., 2011).
To evaluate the potential for intercellular PCP signaling to coordinate the stereociliary bundle polarity of vestibular hair cells we used a knock-in line in which the Emx2 coding sequence is replaced by that of Cre recombinase (Kimura et al., 2005) to produce Vangl1;Vangl2 conditional knockouts (V1/V2 CKOs). In these mice a genetic boundary between wild type and mutant hair cells is generated by the unique pattern of Emx2-Cre mediated recombination occurring in the utricular and saccular maculae. While this boundary lies in close proximity to the LPR (Jiang et al., 2017), this does not pose a limitation since in vertebrates intercellular PCP signaling and the intrinsic polarity of the stereociliary bundle are regulated by distinct mechanisms (Deans, 2013). More importantly, because of the anatomic organization of the vestibular system, the mutant boundaries generated in the utricle and saccule are complementary, and resemble the proximal and distal sides of Drosophila vang mutant clones respectively. Using this unique genetic model, we demonstrate domineering non-autonomy in the mouse inner ear and further demonstrate the conservation of core PCP proteins as intercellular signals that coordinate intracellular and tissue polarity.
Results
Vangl1 and Vangl2 are required to establish Planar Cell Polarity throughout the vestibular maculae
The conservation of core PCP gene function between the Drosophila wing and mouse inner ear hair cells was first demonstrated in the Looptail mutant mouse line which has a point mutation in the vang orthologue Vangl2 (Montcouquiol et al., 2003), and the spin cycle and crash mutant mice which have independent point mutations in the flamingo/stan orthologue Celsr1 (Curtin et al., 2003). The salient phenotype of these mutants is craniorachischisis, a severe neural tube defect in which the neural tube fails to close along the length of the embryo, and is a distinguishing phenotype of core PCP mutants in mice (Copp et al., 2003, Tissir and Goffinet, 2013). Despite this profound impact on the neural tube, mutations in the core PCP genes only affect stereociliary bundle orientation in subsets of hair cells. For example in Vangl2 mutant cochlea, misoriented stereociliary bundles are found throughout the third row of outer hair cells (OHCs) while OHCs in the first row remain properly aligned (Montcouquiol et al., 2003, Yin et al., 2012). Similarly, vestibular hair cell phenotypes are restricted to a specialized central region of the utricle called the striola in Vangl2 KOs, with very little impact on hair cells located in extrastriolar regions (Yin et al., 2012). One explanation could be genetic redundancy and compensation from the related gene Vangl1, as has been proposed for the auditory system (Torban et al., 2008). It is also possible that there are additional signaling pathways regulating planar polarity in the vestibular maculae that are not found in Drosophila because they are necessary for formation of the LPR, and might compensate for the loss of Vangl2 (Deans, 2013, Deans et al., 2007).
To distinguish between these alternatives, vestibular hair cell stereociliary bundle orientation was evaluated in postnatal day 2 (P2) utricles from Vangl1 KOs and Pax2-Cre; V1/V2 CKOs. While Vangl1 KOs are viable, mice lacking Vangl1 and Vangl2 are not, and so Pax2-Cre was used to restrict gene deletion to a subset of tissues including the inner ear and kidney (Ohyama and Groves, 2004). Pax2-Cre; V1/V2 CKO mice are born at predicted Mendelian ratios but do not survive beyond weaning presumably due to the formation of kidney cysts and related renal deficiencies (data not shown). To simplify the analysis of planar polarity phenotypes, β2-SPECTRIN immunofluorescence was used to label the actin-rich cuticular plate that anchors the stereocilia at the apical cell surface. A salient feature of β2-SPECTRIN labeling is the fonticulus, a gap in labeling located at one edge of the cuticular plate where the kinocilium emerges from the cell surface and where the basal body associated with the kinocilium is located (Fig. 1A–C). Since the kinocilium is always located adjacent to the tallest stereocilium, the lateral position of the basal body and fonticulus are a reliable readout for the polarization and orientation of the stereociliary bundle. Moreover, β2-SPECTRIN labeling is sufficient to map hair cell planar polarity throughout the utricle and saccule and locate the position of the LPR in either sensory epithelium (Fig. 1D,E).
Figure 1. Vestibular hair cell PCP visualized via β2-SPECTRIN immunolabeling.
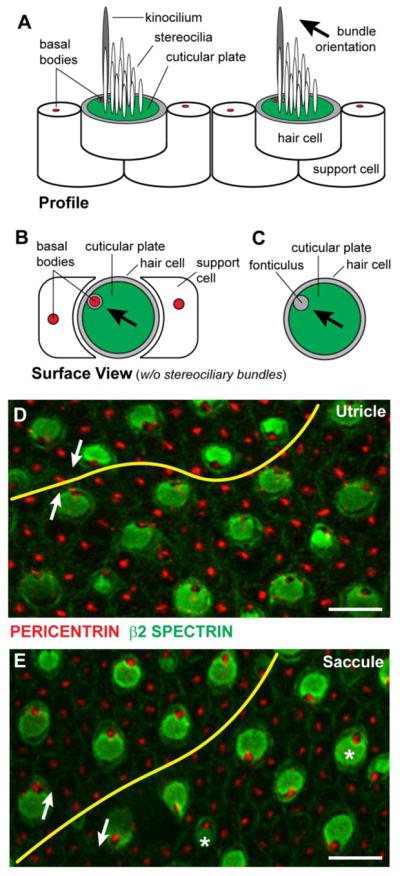
(A) Lateral profile of vestibular hair cells and supporting cells. Hair cells are readily distinguished by a polarized bundle of stereocilia projecting from the apical cell surface that is organized in a staircase fashion with the tallest stereocilia adjacent to a single tubulin based kinocilium. Stereocilia are anchored in the actin-rich cuticular plate (green) and the kinocilium is associated with a basal body (red). (B) Surface view of a vestibular hair cell without the stereociliary bundle and kinocilium illustrating the lateral position of the basal body. Hair cells are surrounded by supporting cells that also contain a single basal body however the polarized distribution of the basal bodies in these cells is not obvious. (C) In hair cells the basal body is located within a gap in the cuticular plate called the fonticulus, and the position of the fonticulus can be used as a readout of stereociliary bundle polarization and orientation. (D) β2-SPECTRIN and PERICENTRIN labeling of cuticular plates and basal bodies respectively shows the planar polarity of the utricular maculae and can be used to map the LPR (yellow line). (E) β2-SPECTRIN and PERICENTRIN labeling of the saccular maculae and the position of the LPR. The orientation of stereociliary bundles (white arrows) for pairs of cells separated by the LPR, and occasional cells that are misplaced (asters) relative to the LPR are indicated. Labeled samples were collected at P0. Scale bars are 10 μm.
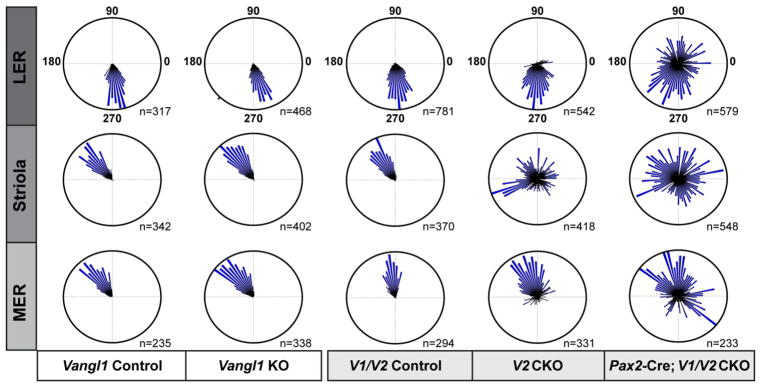
In Pax2-Cre; V1V2 CKOs, the loss of both VANGL proteins significantly disrupts planar polarity throughout the utricular maculae (Figs. 2,3). While individual hair cells retain intracellular polarity based upon the lateral position of the fonticulus, mutant cells are frequently misoriented relative to their nearest neighbors as illustrated in the lateral extrastriolar region (Fig. 2A). Quantification of the orientations of individual stereociliary bundles using circular histograms demonstrates that there is no impact when only Vangl1 is removed (Fig. 3). Consistent with this result, auditory PCP phenotypes were not reported for Vangl1 mutants generated through an independent genetrap approach (Song et al., 2010). In contrast and as previously described, in Vangl2 KOs and CKOs the vestibular PCP phenotype is limited to the striola region of the utricle and saccule (Fig. 3 and (Copley et al., 2013, Yin et al., 2012)). However, in Pax2-Cre; V1/V2 CKOs the penetrance of the vestibular PCP phenotype is greater than in a Pax2-Cre; Vangl2 CKO control genotype demonstrating genetic redundancy between Vangl1 and Vangl2, and an important contribution of VANGL1 to the polarized orientation of a sub-population of vestibular hair cells located in the lateral and medial extrastriolar regions. A prominent planar polarity phenotype was also observed in the Pax2-Cre; V1/V2 CKO saccule (Fig. 4), however while the orientation of individual hair cells was generally different from their neighbors (Fig. 4D,E) the population as a whole retained some organization along a common axis (Fig. 4F). Thus, in the saccule additional patterning cues may also still contribute while in the utricle absence of VANGL1 and VANGL2 is sufficient to completely disrupt hair cell patterning.
Figure 2. Vangl1 and Vangl2 are required for proper stereociliary bundle orientation in the utricular maculae.
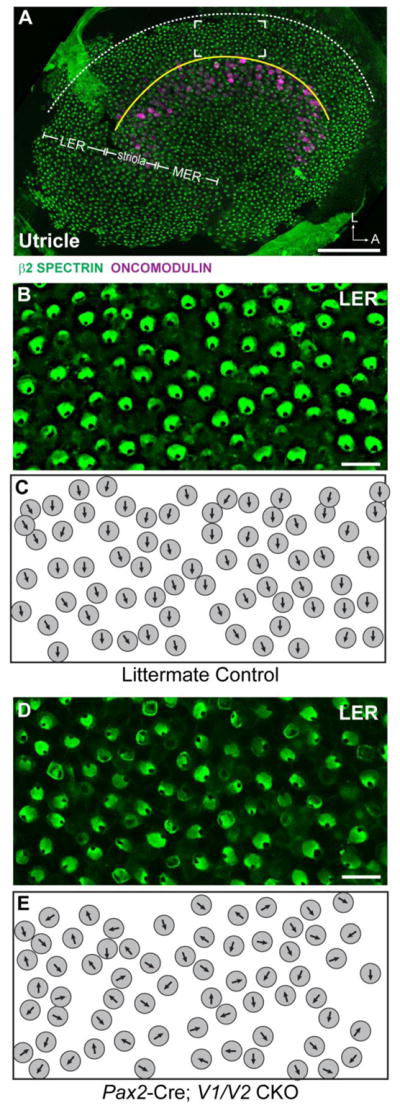
(A) An overview of the mouse utricular maculae at P2 showing the relative positions of β2-SPECTRIN labeled hair cells analyzed in this study. The utricle was broadly divided into three domains, the Striola which contains ONCOMODULIN-expressing hair cells, and the lateral extrastriolar region (LER) and medial extrastriolar region which flank the Striola. In the utricle the approximate location of the LPR (yellow line) corresponds to the boundary between the LER and the Striola. The lateral boundary of the sensory epithelia (dashed white line) serves as a reference for stereociliary bundle orientation measures as described in the methods, and the framed region is the approximate location of images presented in B&C. (B) Vestibular hair cells from the LER of littermate control mice labeled with β2-SPECTRIN. (C) Schematic illustration of stereociliary bundle orientations for hair cells imaged in ‘B’. (D) Vestibular hair cells from the LER of Pax2-Cre; V1/V2 CKO mice labeled with β2-SPECTRIN. (E) Schematic illustration of stereociliary bundle orientations for hair cells imaged in ‘D’. Scale bars are 100μm (A) or 10μm (B,D).
Figure 3. Quantification of utricular stereociliary bundle orientations following Vangl1 and Vangl2 gene deletion.
The orientation of individual stereociliary bundles graphed on circular histograms for all hair cells analyzed in the LER, Striola and MER regions of the P2 utricular maculae from mice of each indicated genotype. For these histograms, 90° is oriented toward the lateral border of the sensory epithelia (Fig. 2A, dashed white line) and 270° is oriented away from the lateral border. The total number of hair cells represented by each histogram (n) is shown, and bin width is 5°.
Figure 4. Vangl1 and Vangl2 are required for proper stereociliary bundle orientation in the saccular maculae.
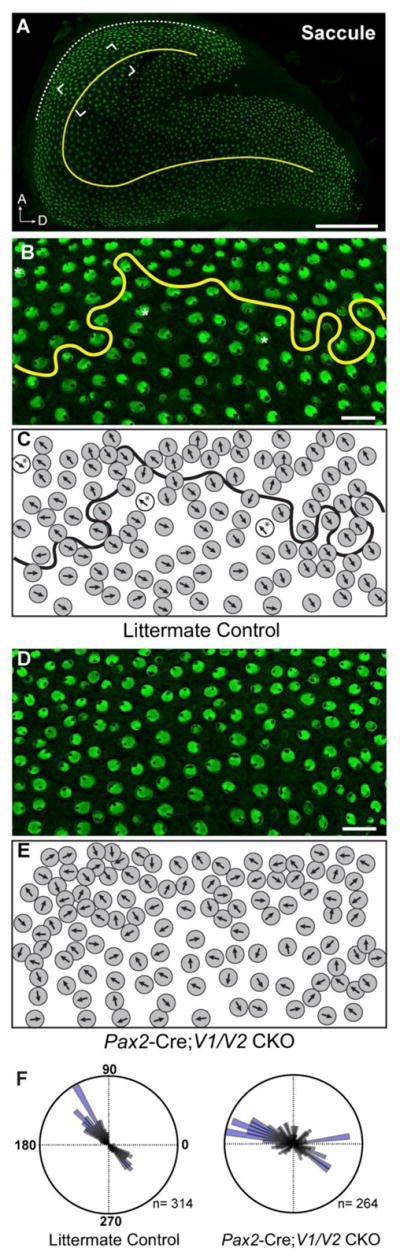
(A) An overview of the P2 mouse saccular maculae showing the relative position (framed region) of β2-SPECTRIN labeled hair cells analyzed in this study and the approximate location of the LPR (yellow line). Analysis was restricted to this region because the LPR is less circuitous here relative to other parts of the sensory epithelia. The adjacent lateral boundary of the sensory epithelia (dashed white line) serves as a reference for stereociliary bundle orientation measures. (B) Vestibular hair cells from the saccule of littermate control mice labeled with β2-SPECTRIN and flanking the LPR (yellow line). (C) Schematic illustration of stereociliary bundle orientations for hair cells imaged in ‘B’. Occasional hair cells with stereociliary bundle orientations that do not match their neighbors are indicated (asters). (D) Vestibular hair cells from the saccule of Pax2-Cre; V1/V2 CKO mice labeled with β2-SPECTRIN. (E) Schematic illustration of stereociliary bundle orientations for hair cells imaged in ‘D’. (F) The orientation of individual stereociliary bundles graphed on circular histograms for all hair cells analyzed in the saccule Pax2-Cre; V1/V2 CKO and littermate control mice. For these histograms, 90° is oriented toward the lateral border of the sensory epithelia as illustrated in ‘A’ and 270° is oriented away from this border. The total number of hair cells represented by each histogram (n) is shown, and bin width is 5°.
Emx2-Cre mediated recombination creates a genetic boundary in the utricle and saccule
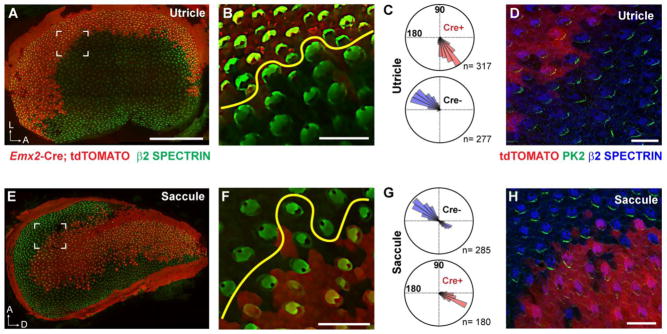
The utricular and saccular maculae are patterned into distinct functional domains that can be distinguished based upon their patterns of afferent innervation and the opposing orientations of stereociliary bundles in domains separated by the LPR (Denman-Johnson and Forge, 1999, Desai et al., 2005, Maklad et al., 2010). In the utricle, hair cells are oriented with the kinocilium of their stereociliary bundles directed towards the LPR while in the saccule the stereociliary bundles point away (Fig. 1D,E). This regional organization is established in part through the restricted expression of transcription factors like EMX2 which is found in hair cells and supporting cells of the lateral utricle and the inner region of the saccule (Jiang et al., 2017). Cre-mediated lineage tracing using Emx2-Cre knock-in mice (Kimura et al., 2005) and Cre-dependent reporters (Madisen et al., 2010) reveals a similar pattern of expression (Fig. 5A,E and (Jiang et al., 2017)). Thus in Emx2 Cre/WT; R26RtdTomato/WT mice, a border of reporter expression runs parallel to the LPR in both vestibular organs (Fig. 5B,F). While the boundary of Cre-dependent reporter expression and the LPR are highly correlated in the postnatal utricle, in the postnatal saccule there is not an exact match, and many hair cells along this boundary have stereociliary bundle orientations corresponding to the inner region that are Cre-negative and do not express tdTomato (Fig. 5F,G). Nonetheless, and more significant, reporter activation demonstrates a sharp boundary of Cre-mediated recombination generated by Emx2-Cre (Fig. 5A,E) that can be used to study PCP signaling along a genetic boundary between mutant and wild type cells.
Figure 5. Emx2-Cre generates a genetic boundary that parallels the LPR.
(A) Emx2-Cre mediated recombination and activation of the LoxP-Stop-LoxP reporter tdTomato is restricted to the lateral region of the P2 mouse utricle. (B) In the utricle tdTomato expression is tightly correlated with the LPR (yellow line). (C) The orientation of individual stereociliary bundles from cells that express (Cre+) or do not express (Cre−) Emx2-Cre. (D) PRICKLE2 (Pk2) immunolabeling of the utricular maculae at E18.5 demonstrates enrichment of the VANGL/PRICKLE complex at the hair cell to supporting cell boundary facing away from the Emx2-Cre expression domain. (E) Emx2-Cre mediated recombination and activation of tdTomato is restricted to the inner region of the P2 mouse saccule. (F) In the saccule tdTomato expression occurs within the vicinity of the LPR (yellow line) but this correlation is not as precise as for the utricle. (G) The orientation of individual stereociliary bundles from cells that express (Cre+) or do not express (Cre−) Emx2-Cre. Many Cre− cells share the same bundle orientation as cells in the inner region. (H) Pk2 immunolabeling of the saccular maculae at E18.5 demonstrates enrichment of the VANGL/PRICKLE complex at the hair cell boundary facing towards the Emx2-Cre expression domain.
The core PCP proteins are asymmetrically distributed in the hair cells and supporting cells of the vestibular maculae similar to the distribution of the orthologous proteins in the Drosophila wing (Deans et al., 2007, Montcouquiol et al., 2006, Wang et al., 2006). Furthermore, while the orientation of stereociliary bundles changes at the LPR, the relative subcellular distribution of the core PCP proteins is constant throughout the vestibular maculae (Deans et al., 2007). As a result, and due to the unique anatomy of the vestibular maculae, there is a complementary distribution of core PCP proteins relative to the boundary of Emx2-Cre mediated recombination between the utricle and the saccule (Fig. 5D,H). The side of hair cells containing VANGL and PRICKLE proteins, as visualized by immunofluorescent labeling of PRICKLE2 in embryonic day 18.5 (E18.5) tissue, is opposite of the Emx2-Cre boundary in the utricle (Fig. 5D), while in the saccule PRICKLE2 is adjacent to the Emx2-Cre boundary (Fig. 5H). This complementary distribution is reminiscent of the organization of core PCP proteins in cells adjacent to mutant clones in the Drosophila wing (Goodrich and Strutt, 2011). For wild type cells located distal to a mutant clone, the Vang complex is enriched along the cell boundary adjacent to the clone, similar to the Cre boundary in saccule (Fig. 5H), whilst for wild type cells located on the proximal side of a mutant clone, the Vang complex is enriched along the opposite cell boundary similar to the Cre boundary in the utricle (Fig. 5D). While a unique feature of the vestibular maculae distinguishes it from the fly wing is the LPR, the proximity of the Emx2-Cre boundary to the LPR does not impede experimental analyses of intercellular PCP signaling because the asymmetric distribution of PCP signaling complexes remain constant across the LPR. As a result, it is possible to use the vestibular maculae of CKOs generated with Emx2-Cre as a vertebrate rendition of a Drosophila mutant clone to assay the conservation and divergence of PCP signaling between the two organisms.
Vangl proteins contribute to intercellular PCP signaling
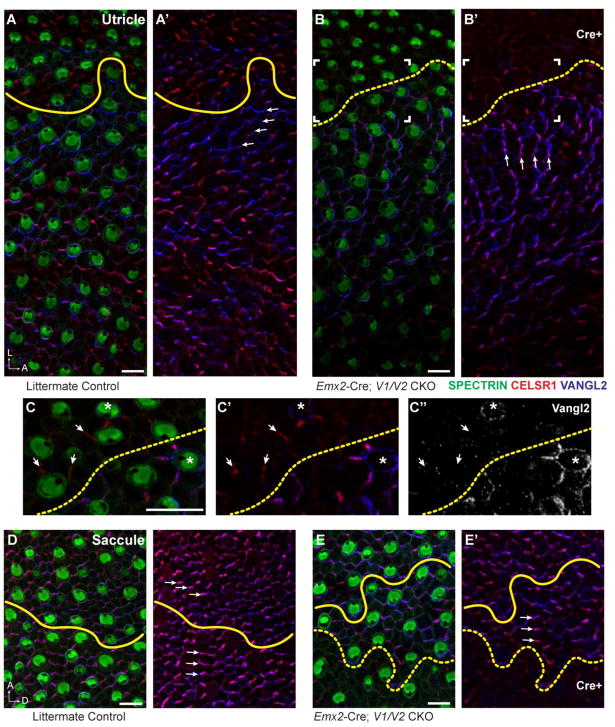
Since Vangl1 and Vangl2 are required for vestibular hair cell PCP throughout the utricle and saccule, the potential for VANGL1/2 proteins to relay intercellular PCP signals between neighboring cells was evaluated using Emx2-Cre; V1/V2 CKOs. For these experiments the border between wild type and mutant cells was mapped by immunofluorescent labeling for VANGL1 or VANGL2 (Fig. 6), and the stereociliary bundle orientation of hair cells in the vicinity of this boundary were measured based upon β2-SPECTRIN labeling of the cuticular plate (Fig. 7). For littermate controls in which VANGL1/2 protein expression is not disrupted, stereociliary bundle polarity was used to identify the LPR which was used a proxy of the Emx2-Cre boundary for quantitative and qualitative analyses. As predicted based upon the Pax2-Cre; V1/V2 CKO phenotype (Fig. 2), the lateral region of the utricle expressing Emx2-Cre contains hair cells with randomized stereociliary bundles that are misoriented relative to their neighbors (Fig. 6B,B′). In contrast, for Cre-negative regions of the utricle, the planar polarity axis and hence stereociliary bundle orientation remains coordinated between neighboring cells. However, despite this local coordination, wild type hair cells adjacent to the Emx2-Cre mutant boundary of the utricle are noticeably impacted, and their stereociliary bundles are rotated and reorient along the anterior-posterior axis of the maculae (Fig. 6B). Moreover, the distribution of VANGL1/2 and CELSR1 proteins also rotated laterally in hair cells and supporting cells in this region, so that they are aligned with the modified stereociliary bundle polarity axis (Fig. 6A′,B′, arrows). In some extreme cases the polarized distribution of VANGL2 is lost and it surrounds individual Cre-negative cells located near the border of Emx2-Cre expression (Fig. 6C–C″, asters). Despite this change in VANGL2 distribution, intracellular polarity remained intact in these cells as demonstrated by the lateral position of the fonticulus. Lastly, in some areas CELSR1 is still present at cellular boundaries between adjacent supporting cells in the Cre-positive domain where it retains an asymmetric distribution at intercellular junctions (Fig. 6C–C″, arrows).
Figure 6. Domineering non-autonomy phenotype of Emx2-Cre; V1/V2 CKOs occurs in the utricular maculae.
(A,A′) Hair cell PCP, as evident in the polarized displacement of the fonticulus following β2-SPECTRIN labeling, and the asymmetric distribution of CELSR1 and VANGL2 (arrows) are aligned along a common axis that spans the LPR (yellow line) in the P0 mouse utricle. (B,B′) In Emx2-Cre; V1/V2 CKOs stereociliary bundle orientation and VANGL2 expression is disrupted in the lateral Cre-positive region of the utricular maculae. The stereociliary bundle orientation of wild type cells in the Cre-negative region are also misoriented, and adopt a new polarity axis that remains aligned with a similarly reoriented distribution of CELSR1 and VANGL2 (arrows). The framed region corresponds to panels ‘C-C” and the boundary of Cre-mediated recombination based upon VANGL2 fluorescence is indicated (dashed yellow line). (C–C″) Higher magnification image of cells at the Cre expression boundary (dashed yellow line) reveals changes in VANGL2 distribution which encircles some cells near the boundary or encircles isolated wild type cells remaining in the Emx2-Cre expressing domain (asters). CELSR1 persists at some intercellular boundaries (arrows) despite the loss of VANGL1 and VANGL2. (D,D′) In the P0 saccule hair cell PCP and the asymmetric distribution of CELSR1 and VANGL2 are similarly aligned along a common axis that spans the LPR (yellow line). (E,E′) In Emx2-Cre; V1/V2 CKOs stereociliary bundle orientation and core PCP protein distribution is not altered in wild type cells abutting the Cre-recombination boundary (dashed yellow line). Note that unlike the utricle, in the saccule the LPR and the Emx2-Cre recombination boundary are in close proximity but do not always coincide. Scale bars are 10μm.
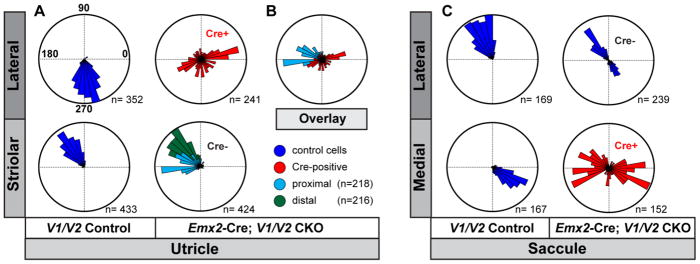
Figure 7. Quantification of domineering non-autonomy in Emx2-Cre; V1/V2 CKOs.
(A) The orientation of individual stereociliary bundles from P2 littermate controls and Emx2-Cre; V1/V2 CKO utricles graphed on circular histograms for all hair cells analyzed in the lateral and striolar regions of the utricular maculae. For controls these regions were distinguished based upon the position of the LPR while for CKOs the Cre-boundary was determined based upon remaining VANGL2 expression. (B) An overlay of histograms corresponding to a subset of wild type cells located proximal to the Cre-boundary and Cre-positive cells from the lateral region suggest that some hair cells are coordinated along a shared axis. (C) The orientation of individual stereociliary bundles for all cells analyzed in the lateral and medial regions of the saccular maculae. For controls these regions were distinguished based upon the position of the LPR while for CKOs the Cre-boundary was determined based upon remaining VANGL2 expression. Since the LPR and the Cre-boundary do not overlap in the saccule, this approach results in a bimodal distribution of bundle orientations aligned along a common axis for the Cre-negative lateral region of V1/V2 CKOs, and a unimodal distribution for the lateral region of littermate controls. For these histograms, 90° is oriented toward the lateral border of the sensory epithelia (Fig.2A,4A dashed white line) and 270° is oriented away from the lateral border. The total number of hair cells represented by each histogram (n) is shown, and bin width is 10°.
These changes in stereociliary bundle orientation and core PCP protein distribution are restricted to the Emx2-Cre; V1/V2 CKO utricle and corresponding changes do not occur in the mutant saccule (Fig. 6D–E). While in the saccule the Emx2-Cre boundary and the LPR do not overlap and the LPR is located on the Cre-negative side of this boundary, a phenotypic evaluation of domineering non-autonomy is still possible because saccular hair cells remain aligned along a common polarity axis regardless of their bundle orientation. Unlike the case for the utricle, in the Emx2-Cre; V1/V2 CKO saccule, the orientation of Cre-negative hair cells located adjacent to the Emx2-Cre boundary is not impacted by the loss of PCP in the neighboring Cre-positive region (Fig. 6E). Similarly, the polarized distribution of VANGL2 and CELSR1 are not altered in wild type cells along the Emx2-Cre boundary (Fig. 6E′).
The Emx2-Cre; V1/V2 CKO phenotype was quantified by graphing stereociliary bundle orientation on circular histograms. For this evaluation the mutant boundary was identified based upon VANGL1 or VANGL2 immunolabeling in CKOs while for littermate controls the LPR was used to approximate this boundary. In addition, since the Emx2-Cre boundary follows a tortuous path throughout most of the saccule, analysis of this sensory organ was restricted to an antero-ventral region of the sensory epithelia where the boundary is consistently more linear (Fig. 4A). Quantification of stereociliary bundle orientations for the utricle revealed disorganization in the Cre-positive lateral extrastriolar region compared to controls (Fig. 7A) although the range of bundle orientations is not as broad or as random as seen in this region of Pax2-Cre; V1/V2 CKOs. The range of stereociliary bundle orientations measured in the Cre-negative striolar region was also expanded and differed from controls. For analysis of the striolar region stereociliary bundle orientation was quantified separately for two groups of hair cells based upon their position relative to the Emx2-Cre boundary. A ‘proximal’ group consisted of the first three rows of hair cells located nearest the Cre boundary while a second ‘distal’ group was located farther away from the boundary and consisted of hair cell rows four through six. In general, and consistent with qualitative analysis (Fig. 6), these two groups were differentially impacted by the loss of Vangl1 and Vangl2 in the adjacent lateral extrastriolar region. Thus the majority of cells in the proximal group were rotated approximately 45 degrees (Fig. 7). Remarkably when the orientation of stereociliary bundles in the Cre-negative proximal group are compared to the Cre-positive cells in the LER, many are aligned along a common axis (Fig. 7B) despite the reorientation of Cre-negative cells through domineering non-autonomy. One possibility is that CELSR1 remaining at cell boundaries in the lateral extrastriolar region might contribute to coordinating the polarity axis across the Cre-boundary though this hypothesis is based solely upon correlative observations (Fig. 6C). In contrast for the saccule, the stereociliary bundle orientation of wild type cells adjacent to Emx2-Cre is normal (Fig. 7C) and are oriented along a common axis regardless of their position relative to the LPR (Fig. 7). Together the behaviors of wild type cells along the Cre boundary in the utricle and saccule resembles domineering non-autonomy phenotypes described for the Drosophila wing, with wild type hair cells in the utricle behaving like Drosophila cells located proximal to a vang clone and wild type cells in the saccule behaving like Drosophila cells located distal to a vang clone (Fig. 8).
Figure 8. Domineering non-autonomy in Emx2-Cre; V1/V2 mutants resembles Drosophila mutant clones.
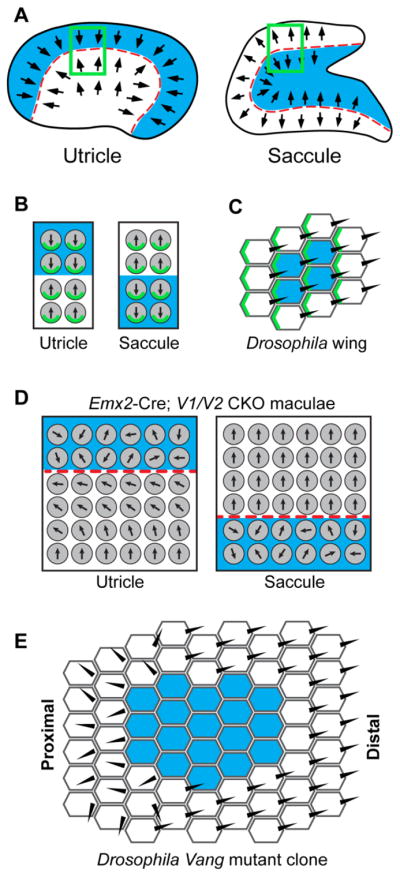
(A) Emx2-Cre (blue shading) is expressed in the lateral region of the utricular maculae and the inner region of the saccular maculae, and creates a genetic boundary of Cre-mediated recombination that parallels the LPR (red dashed line) found in both organs. (B) In the utricle, the VANGL/PRICKLE PCP complex (green) is enriched at the hair cell boundary opposite of the Emx2-Cre boundary while in the saccule this complex is at the adjacent hair cell boundary. (C) For genetically labeled clones in the Drosophila wing, VANG (green) is enriched at the cell boundary opposite of the clone in proximal locations and enriched at the cell boundary adjacent to the clone in distal locations. (D) When PCP signaling is disrupted along the mutant boundary in Emx2-Cre; V1/V2 CKOs, a domineering non-autonomy phenotype is revealed by the reorientation of neighboring wild type cells in the utricle. Similar non-autonomous phenotype does not occur in the saccule. (C) Domineering non-autonomy phenotypes are similarly restricted to the proximal side of Vang mutant clones in the Drosophila wing.
Discussion
In this study we have disrupted PCP signaling in the mouse using Vangl1/Vangl2 conditional knockouts to evaluate the conservation of intercellular PCP signaling between sensory receptor hair cells of the developing inner ear and epithelia cells of the Drosophila wing. We show that these two genes are partially redundant and required throughout the vestibular maculae to coordinate stereociliary bundle orientation using Pax2-Cre to promote gene deletion at the earliest stages of inner ear development. We also demonstrate the utility of Emx2-Cre as a research tool and show that it can be used to generate a genetic boundary to specifically enable assays of intercellular PCP signaling within the inner ear sensory epithelia (Fig. 8A). Moreover, due to the unique anatomy of the vestibular maculae in the mouse, the relative organization of core PCP proteins along the boundaries of Emx2-Cre recombination are complementary between the utricle and saccule. In the saccule a protein complex including the VANGL2 associated protein PRICKLE2 is enriched at the cell boundary facing Emx2-Cre expression, while in the utricle PRICKLE2 is enriched at the boundary opposite of Emx2-Cre (Fig. 8B). This relative distribution is similar to that of PCP proteins along the distal and proximal sides respectively of PCP mutant clones in the Drosophila wing (Fig. 8C). Furthermore, as would be predicted from this parallel, a domineering non-autonomy phenotype altering the orientation of wild type hair cells occurs in the Emx2-Cre; V1/V2 CKO utricle that does not occur in the saccule (Fig. 8D–E).
The vertebrate core PCP proteins form asymmetric intercellular bridges in which VANGL proteins enriched along one cellular boundary oppose FZD proteins from the neighboring cell in a molecular complex stabilized by CELSR. In Drosophila the analogous molecular assembly is established by intercellular and intracellular interactions between the core PCP proteins (Goodrich and Strutt, 2011, Strutt, 2002). As a result, since Vang is enriched on the proximal side of wing epithelial cells, when a vang mutant clone is generated this leads the neighboring wild type cells along the proximal boundary to relocate their normal distribution of Vang. This results in a corresponding Fz redistribution to the opposite side of those cells, and local intercellular propagation of this PCP protein arrangement explains why wild type cells located a few cell diameters away from the clonal boundary are also affected. This also accounts for why reorientation depends on the cell position relative to the clone, and why vang and fz mutants have complementary non-autonomous affects. Similar redistributions of vertebrate PCP proteins have not been documented at mutant boundaries, however the general conservation of PCP function suggests that this would also be the case. This interpretation is further supported by our observation that CELSR1 and VANGL2are also redistributed in cells adjacent to the Emx2-Cre boundary. However, unlike the wild type cells neighboring vang clones, wild type hair cells in the mutant utricle are not fully inverted as might be expected and instead are only reoriented approximately 45 degrees. One possibility is that this modest change in orientation is sufficient for hair cells and supporting cells to maintain a continuous PCP axis with wild type cells adjacent to the Cre-positive domain. Regardless, the domineering non-autonomy phenotype of Emx2-Cre; V1/V2 CKOs further demonstrates the conservation of intercellular PCP signaling mechanisms between the Drosophila wing epithelia and the sensory epithelia of the mouse inner ear.
The domineering non-autonomy phenotype in the Emx2-Cre; V1/V2 CKO utricle is also consistent with two previous studies that also employed vertebrate models. In one set of experiments intercellular core PCP signaling across the developing auditory organ of the chick was disrupted through viral-mediated overexpression of VANGL2 in subsets of hair cells and supporting cells (Sienknecht et al., 2011). This manipulation had a non-autonomous effect and altered the orientation of stereociliary bundles in neighboring non-transfected cells, and similar to our observations in the mouse showed that signaling could be conveyed between hair cells via the intervening supporting cells. The non-autonomous effect was also more frequent along one side of transfected cells suggesting that vertebrate PCP signaling is directional similar to Drosophila. This was further demonstrated by a separate study focused on multi-ciliated epidermal cells of the developing Xenopus embryos. These cells develop translational planar polarity upon differentiation when they establish motile cilia beating in a common direction (Mitchell et al., 2009). Polarization is determined by signals received from neighboring cells, and wild type cells will become misoriented if forced to differentiate adjacent to transplanted cells missing Vang or Frizzled. In addition to being non-autonomous, this effect was similar to Drosophila Vang and Frizzled mutant clones because the non-autonomous effect occurred on the opposite side of transplanted tissue lacking Vang compared to tissue lacking Frizzled (Taylor et al., 1998, Vinson and Adler, 1987). By using Emx2-Cre to make a mutant border we have extended these two studies to the mouse inner ear by modeling the domineering non-autonomy that occurs at the proximal side of a Drosophila Vang clone in the Emx2-Cre; V1/V2 CKO utricle (Fig. 8). We anticipate that Emx2-Cre should enable further comparisons to Drosophila clones, including conditional mutants for other core PCP proteins. An exciting prospect would be the generation of Frizzled CKOs using Emx2-Cre with the prediction that domineering non-autonomy phenotypes would be spatial complementary to V1/V2 CKOs and thus be restricted to the saccule.
These results are consistent with PCP signaling acting as part of a three tiered mechanism regulating planar polarity in the developing maculae that consists of subcellular, intercellular (or PCP), and tissue polarity signals (Deans, 2013, Lewis and Davies, 2002). In this model the core PCP proteins facilitate intercellular signaling that coordinates the orientation of stereociliary bundles relative their neighbors, and along a common polarity axis that spans the LPR. In contrast, subcellular polarity is cell intrinsic and can be established independent of PCP signaling with each cell coordinated along a common polarity axis by the core PCP proteins. This distinction between intracellular and intercellular planar polarity may be unique to vertebrates because in Drosophila intracellular polarity is also impacted in PCP mutants since the pre-hair structure that precedes the trichome is centrally located (Wong and Adler, 1993). Moreover, subcellular planar polarity in hair cells is organized through a Gαi signaling pathway that includes LGN and mInsc which patterns the apical cell surface of hair cells (Ezan et al., 2013, Tarchini et al., 2013) and does not have a comparable function in Drosophila. Lastly, tissue polarity signals acting upstream of PCP signaling (1) orient the PCP axis relative to the inner ear and (2) establish the position of the LPR. For tissue-wide polarity the PCP axis is generally thought to be established by non-canonical Wnt signaling (Gao et al., 2011, Qian et al., 2007, Yang, 2012), while formation of the LPR is dependent upon the transcription factor Emx2 (Holley et al., 2010, Jiang et al., 2017).
This three tiered model is dependent upon the capacity of core PCP proteins to facilitate intercellular signaling between neighboring hair cells throughout the vestibular maculae and to relay these signals via the intervening supporting cells to establish a common polarity axis. Consistent with this we demonstrate that Vangl1 and Vangl2 are required throughout the utricle and that in their absence stereociliary bundle orientation is random. The coordinated alignment of neighboring hair cells is also disrupted throughout the saccule though the orientation hair cells remain generally associated with a common planar polarity axis. During development the membraneous labyrinth undergoes significant morphogenesis (Koo et al., 2009, Morsli et al., 1998, Nichols et al., 2008) and the remaining organization in the saccule may be a consequence of PCP-independent cellular rearrangements associated with this growth. More significant however is the demonstration that VANGL1 and VANGL2 contribute to intercellular signaling that coordinates the planar polarity axis, and that this aspect of core PCP function is conserved between Drosophila and vertebrate systems despite the morphological and cellular specializations of inner ear hair cells. Altogether these data further demonstrate that PCP signaling is an essential aspect of vestibular hair cell development and aptly positioned to coordinate stereociliary bundle orientation with the overall anatomy and organization of the sensory epithelia of the inner ear.
Materials and Methods
Mouse husbandry and genotyping
Pax2-Cre; V1V2 CKO mice were produced by intercrossing Pax2-Cre; Vangl1 ΔTMs/WT; Vangl2 ΔTMs/WT male mice with Vangl1 Floxed; Vangl2 Floxed female mice. The Pax2-Cre line (Ohyama and Groves, 2004) was provided by Andrew Groves (Baylor College of Medicine). The Vangl1 Floxed mouse line (Wang et al., 2016) was provided by Jeremy Nathans (Johns Hopkins University School of Medicine) and was used to derive the Vangl1 ΔTMs/WT allele through intercross with CMV-Cre (JAX #006054). Emx2-Cre; V1/V2 CKO mice were produced by intercrossing Emx2 Cre/WT; Vangl1 ΔTMs/WT; Vangl2 ΔTMs/WT male mice with Vangl1 Floxed; Vangl2 Floxed female mice. Emx2-Cre knockin mice (Kimura et al., 2005) were provided by Shin-Ichi Aizawa (Riken CDB) and lineage tracing was conducted by crossing Emx2 Cre/WT males with Cre-dependent tdTomato reporter mice (JAX #007909). For colony preservation all mouse lines were back-crossed with hybrid B6129SF1/J females (JAX #101043). For timed breeding and tissue staging, noon on the day of vaginal plug visualization was considered embryonic day 0.5 (E0.5), and postnatal day 0 (P0) was the day mice were born. All mice were maintained at the University of Utah under IACUC approved guidelines and genotyped by PCR amplification (reaction details available on request).
Antibodies and Immunofluorescence
For immunofluorescent labeling, inner ears were fixed for 2 hours in a solution of 4% paraformaldehyde prepared in 67mM Sorensons’ phosphate buffer (pH 7.4), and inner ear sensory organs were micro-dissected to expose the sensory epithelia and permeabilized using a blocking solution (5% donkey serum, 1% BSA, and PBS) supplemented with 0.5% Triton X-100. Primary antibodies were diluted in blocking solution supplemented with 0.1% Tween-20 and incubated with the tissue overnight at 4°C. Tissue was washed thoroughly with PBS-T (PBS and 0.05% Tween-20), followed by incubation with species-specific, Alexa Fluor-conjugated (Invitrogen) or DyLight-conjugated (Jackson ImmunoResearch) secondary antibodies. Tissue was subsequently washed with PBS-T, mounted using Prolong Gold (Molecular Probes, P36930), and imaged via structured illumination microscopy using a Zeiss Axio Imager M.2 with ApoTome.2 attachment. Images were collected with Zeiss Zen software, and figures were prepared using Adobe Illustrator. The following commercial antibodies were used in this study: β2-SPECTRIN (BD Biosciences 612562); ONCOMODULIN (Santa Cruz Sc7446); PERICENTRIN (Covance PRB-432C); VANGL1 (Sigma HPA025235). The following antibodies were generously provided by the following individuals: CELSR1 (Danelle Devenport, Princeton Univ.), PRICKLE2 (Jeffery Axelrod, Stanford Univ.), VANGL2 (Mireille Montcouquiol, Univ. Bordeaux).
Quantification of hair cell orientation
For quantification stereociliary bundle orientations on circular histograms, utricular and saccular maculae were immunolabeled using antibodies against β2-SPECTRIN and imaged at 63× magnification as previously described. Stereociliary bundle orientation was determined based upon the polarized position of the fonticulus (Fig. 1B,C), with orientation vectors extending from the center of the cuticular plate to the fonticulus, and a user defined reference-line drawn along the lateral border of the sensory epithelia (Figs. 2A&4A). Measurements and graphing were conducted using customized software developed in Python (Duncan et al., 2017), or Oriana circular graphing software (Kovach Computing Services). For Pax2-Cre; V1/V2 CKOs and associated control genotypes, measurements were obtained from utricular hair cells located in three distinct regions (LER, Striola and MER) and separate circular histograms were created for each (Fig. 2A). Regions were identified based upon immunolabeling for the striolar marker ONCOMODULIN, and measurements were pooled within each region using 3 utricles from each genotype. Saccular hair cell measurements were obtained from a region of the antero-ventral portion of the saccule spanning the LPR (Fig. 4A). Measurements were pooled from 2 saccules obtained from each genotype. Emx2-Cre; V1/V2 CKOs were subject to the same quantification methods and software as above and regions selected for analysis were identified by VANGL1 or VANGL2 immunolabeling to identify the extent of Cre-mediated gene deletion. The first row of lateral hair cells adjacent to the boundary were excluded from analysis. Orientation measurements were pooled from 3 utricles or saccules from each genotype.
Highlights.
PCP proteins Vangl1 and Vangl2 share overlapping functions in vestibular hair cells
Intervening support cells relay PCP signaling between neighboring hair cells
Core features of PCP signaling are conserved between vertebrates and invertebrates
Domineering Non-autonomy in PCP signaling occurs in the mouse inner ear
Acknowledgments
This work was supported by NIH/NIDCD grant R01 DC013066 (MRD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADLER PN, TAYLOR J, CHARLTON J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- CHAE J, KIM MJ, GOO JH, COLLIER S, GUBB D, CHARLTON J, ADLER PN, PARK WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–9. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- COPLEY CO, DUNCAN JS, LIU C, CHENG H, DEANS MR. Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2. J Neurosci. 2013;33:14001–16. doi: 10.1523/JNEUROSCI.1307-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPP AJ, GREENE ND, MURDOCH JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- COREY D. Sensory transduction in the ear. J Cell Sci. 2003;116:1–3. doi: 10.1242/jcs.00101. [DOI] [PubMed] [Google Scholar]
- CURTIN JA, QUINT E, TSIPOURI V, ARKELL RM, CATTANACH B, COPP AJ, HENDERSON DJ, SPURR N, STANIER P, FISHER EM, NOLAN PM, STEEL KP, BROWN SDM, GRAY IC, MURDOCH JN. Mutation of Celsr1 Disrupts Planar Polarity of Inner Ear Hair Cells and Causes Severe Neural Tube Defects in the Mouse. Current Biology. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- DEANS MR. A balance of form and function: planar polarity and development of the vestibular maculae. Semin Cell Dev Biol. 2013;24:490–8. doi: 10.1016/j.semcdb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEANS MR, ANTIC D, SUYAMA K, SCOTT MP, AXELROD JD, GOODRICH LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci. 2007;27:3139–47. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENMAN-JOHNSON K, FORGE A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–35. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- DESAI SS, ZEH C, LYSAKOWSKI A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005;93:251–66. doi: 10.1152/jn.00746.2003. [DOI] [PubMed] [Google Scholar]
- DUNCAN JS, STOLLER ML, FRANCL AF, TISSIR F, DEVENPORT D, DEANS MR. Celsr1 coordinates the planar polarity of vestibular hair cells during inner ear development. Dev Biol. 2017;423:126–137. doi: 10.1016/j.ydbio.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZAN J, LASVAUX L, GEZER A, NOVAKOVIC A, MAY-SIMERA H, BELOTTI E, LHOUMEAU AC, BIRNBAUMER L, BEER-HAMMER S, BORG JP, LE BIVIC A, NURNBERG B, SANS N, MONTCOUQUIOL M. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat Cell Biol. 2013;15:1107–15. doi: 10.1038/ncb2819. [DOI] [PubMed] [Google Scholar]
- GAO B, SONG H, BISHOP K, ELLIOT G, GARRETT L, ENGLISH MA, ANDRE P, ROBINSON J, SOOD R, MINAMI Y, ECONOMIDES AN, YANG Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–76. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIESE AP, EZAN J, WANG L, LASVAUX L, LEMBO F, MAZZOCCO C, RICHARD E, REBOUL J, BORG JP, KELLEY MW, SANS N, BRIGANDE J, MONTCOUQUIOL M. Gipc1 has a dual role in Vangl2 trafficking and hair bundle integrity in the inner ear. Development. 2012;139:3775–85. doi: 10.1242/dev.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODRICH LV, STRUTT D. Principles of planar polarity in animal development. Development. 2011;138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLEY M, RHODES C, KNEEBONE A, HERDE MK, FLEMING M, STEEL KP. Emx2 and early hair cell development in the mouse inner ear. Dev Biol. 2010;340:547–56. doi: 10.1016/j.ydbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG T, KINDT K, WU DK. Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. Elife. 2017:6. doi: 10.7554/eLife.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA J, SUDA Y, KUROKAWA D, HOSSAIN ZM, NAKAMURA M, TAKAHASHI M, HARA A, AIZAWA S. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J Neurosci. 2005;25:5097–108. doi: 10.1523/JNEUROSCI.0239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOO SK, HILL JK, HWANG CH, LIN ZS, MILLEN KJ, WU DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS J, DAVIES A. Planar cell polarity in the inner ear: how do hair cells acquire their oriented structure? J Neurobiol. 2002;53:190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- MADISEN L, ZWINGMAN TA, SUNKIN SM, OH SW, ZARIWALA HA, GU H, NG LL, PALMITER RD, HAWRYLYCZ MJ, JONES AR, LEIN ES, ZENG H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKLAD A, KAMEL S, WONG E, FRITZSCH B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340:303–21. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL B, STUBBS JL, HUISMAN F, TABOREK P, YU C, KINTNER C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–9. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTCOUQUIOL M, RACHEL RA, LANFORD PJ, COPELAND NG, JENKINS NA, KELLEY MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- MONTCOUQUIOL M, SANS N, HUSS D, KACH J, DICKMAN JD, FORGE A, RACHEL RA, COPELAND NG, JENKINS NA, BOGANI D, MURDOCH J, WARCHOL ME, WENTHOLD RJ, KELLEY MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSLI H, CHOO D, RYAN A, JOHNSON R, WU DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–35. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS DH, PAULEY S, JAHAN I, BEISEL KW, MILLEN KJ, FRITZSCH B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–58. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYAMA T, GROVES AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–9. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- QIAN D, JONES C, RZADZINSKA A, MARK S, ZHANG X, STEEL KP, DAI X, CHEN P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWANDER M, KACHAR B, MULLER U. Review series: The cell biology of hearing. J Cell Biol. 2010;190:9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIENKNECHT UJ, ANDERSON BK, PARODI RM, FANTETTI KN, FEKETE DM. Non-cell-autonomous planar cell polarity propagation in the auditory sensory epithelium of vertebrates. Dev Biol. 2011;352:27–39. doi: 10.1016/j.ydbio.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG H, HU J, CHEN W, ELLIOTT G, ANDRE P, GAO B, YANG Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRUTT DI. The asymmetric subcellular localisation of components of the planar polarity pathway. Seminars in Cell & Developmental Biology. 2002;13:225–231. doi: 10.1016/s1084-9521(02)00041-1. [DOI] [PubMed] [Google Scholar]
- TARCHINI B, JOLICOEUR C, CAYOUETTE M. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Dev Cell. 2013;27:88–102. doi: 10.1016/j.devcel.2013.09.011. [DOI] [PubMed] [Google Scholar]
- TAYLOR J, ABRAMOVA N, CHARLTON J, ADLER PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIR F, GOFFINET AM. Shaping the nervous system: role of the core planar cell polarity genes. Nat Rev Neurosci. 2013;14:525–35. doi: 10.1038/nrn3525. [DOI] [PubMed] [Google Scholar]
- TORBAN E, PATENAUDE AM, LECLERC S, RAKOWIECKI S, GAUTHIER S, ANDELFINGER G, EPSTEIN DJ, GROS P. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci U S A. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USUI T, SHIMA Y, SHIMADA Y, HIRANO S, BURGESS RW, SCHWARZ TL, TAKEICHI M, UEMURA T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–95. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- VINSON CR, ADLER PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–51. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- VLADAR EK, ANTIC D, AXELROD JD. Planar cell polarity signaling: the developing cell’s compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, GUO N, NATHANS J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, WILLIAMS J, RATTNER A, WU S, BASSUK AG, GOFFINET AM, NATHANS J. Patterning of papillae on the mouse tongue: A system for the quantitative assessment of planar cell polarity signaling. Dev Biol. 2016;419:298–310. doi: 10.1016/j.ydbio.2016.09.004. [DOI] [PubMed] [Google Scholar]
- WONG LL, ADLER PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–21. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Y. Wnt signaling in development and disease. Cell Biosci. 2012;2:14. doi: 10.1186/2045-3701-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN H, COPLEY CO, GOODRICH LV, DEANS MR. Comparison of phenotypes between different vangl2 mutants demonstrates dominant effects of the Looptail mutation during hair cell development. PLoS One. 2012;7:e31988. doi: 10.1371/journal.pone.0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]