Abstract
We investigated the safety, feasibility, and efficacy of transcranial direct current stimulation (tDCS) combined with constraint-induced movement therapy (CIMT) in children and young adults with unilateral cerebral palsy. Twenty participants were randomized to receive active or sham tDCS. The intervention consisted of 10 consecutive weekday sessions of tDCS applied to the non-lesioned hemisphere (20 minutes) concurrently with CIMT (120 minutes). Participants, caregivers, and interventionists were blinded to group assignment. The primary safety outcome investigated adverse events. The primary behavioral outcome was the Assisting Hand Assessment. All 20 participants (mean age = 12.7 yrs, range = 7.4-21.6 years) were evaluated for the primary outcomes. No serious adverse events occurred, and the most commonly reported minor adverse events were headache and itchiness. Both groups demonstrated a significant improvement in hand function after the intervention, although no significant effect of tDCS was observed (between-group difference = -2.18, 95% CI= [-6.48, 2.12], p = 0.30). Although hand function improved overall, no significant differences between intervention groups were found. Children with preserved corticospinal tract circuitry from the lesioned hemisphere, compared to those without, showed greater improvement in hand function (mean difference = 3.04, 95% CI = [-0.64, 6.72], p = 0.099). Our study demonstrates the safety and feasibility of serial sessions of tDCS, and presents preliminary evidence for the effect of CST circuitry on outcomes following tDCS/CIMT. Future work in children with unilateral cerebral palsy should focus on the optimal dosing and consider individual brain circuitry when describing response to combined interventions.
Clinical Trials Registration
Clinicaltrials.gov NCT 02250092
Keywords: Unilateral cerebral palsy, rehabilitation, Transcranial direct current stimulation, Constraint-induced movement therapy
1. Introduction
Children with unilateral cerebral palsy (UCP) attributed to perinatal stroke or periventricular leukomalacia (PVL) present clinically with hemiparesis, influencing the child's independence in daily life and necessitating rehabilitation to maximize outcomes. Although current rehabilitation interventions can promote recovery, the child's ability to engage and participate in desired activities and roles are impacted throughout their lifetime. Combining interventions such as non-invasive brain stimulation (NIBS) paired with rehabilitation in adult stroke may yield a greater impact on motor skill development as compared to rehabilitation alone. This provides preliminary evidence to support clinical investigations in children with UCP.1-3
In order to investigate the potential for adult stroke protocols to benefit children with stroke, we reported one of the first combined NIBS and rehabilitation studies in children with UCP.4 This study established the framework for safety, feasibility and efficacy of pediatric neuromodulation studies, and a platform upon which future investigations involving brain excitability assessment and neuromodulation could build. One form of neuromodulation is transcranial direct current stimulation (tDCS), which delivers low-level current to modulate brain excitability and can be readily paired with intensive rehabilitation such as constraint-induced movement therapy (CIMT).5, 6 The intended mechanism of cathodal tDCS applied to the non-lesioned hemisphere paired with CIMT aims to facilitate excitability of the lesioned hemisphere and inhibit the exaggerated IHI effects from the non-lesioned hemisphere.7 Based on prior synergistic application of tDCS in adults1, 8, we hypothesized that tDCS combined concurrently with CIMT would result in greater and longer-lasting improvement in hand function. To pursue application of tDCS in children with UCP, we performed a series of methodological studies, including: 1) a modeling study determining current density, electric field, tDCS electrode montage and dosing,9 2) a single-session safety randomized controlled trial of the modeled tDCS dosage,10 and 3) a comparison tDCS methods for electrode location.11 These studies provided preliminary safety evidence to commence a clinical trial of combinatory serial sessions of tDCS+CIMT.
The optimal construct of a combinatory tDCS+CIMT intervention for stroke rehabilitation is not known in adults nor children. One potential factor to consider is the impact of underlying brain circuitry, such as the integrity of the corticospinal tracts (CST), on response to behavioral and neuromodulatory interventions.12-14 Evidence suggests that children with retained CST projections from the lesioned hemisphere show greater improvements in hand function and greater changes in cortical excitability following CIMT compared to those without contralateral projections.15, 16 Examining results by individual circuitry could lead to future analysis of optimal responders to combined interventions, directly contributing to more precise, economic and effective healthcare in the future.
Since the onset of this work, others have explored the effects of combining tDCS and motor training in children with UCP, with potentially positive findings17-19. However, these studies do not report detailed safety outcomes and are potentially confounded by heterogeneous sample of children with unknown CST circuitry. Concerns regarding the safety of NIBS on a developing brain warrant further investigation in studies consisting of serial NIBS assessments and interventions.20 These concerns for children with and without clinical conditions stem from differences in the tDCS peak current density of the electrical field between a child and adult brain4, 21 and unknown long-term effects of neuromodulation in the pediatric population. Detailed reporting of safety outcomes would provide evidence to guide future applications with at-risk clinical populations. Therefore, our aim was to assess the safety, feasibility and preliminary efficacy of a combined tDCS+CIMT intervention on hand function in children with UCP. Based on prior synergistic applications of tDCS in adults1, 8, we hypothesized that tDCS combined concurrently with CIMT would result in the greater and longer-lasting improvement in hand function. Following the intervention, additional analyses explored the relation of CST circuitry to motor outcomes.
2. Material and Method
2.1 Study Design and Setting
This was a randomized, blinded, sham-controlled trial performed at an academic setting at the University of Minnesota (Minneapolis, Minnesota, USA) and a hospital setting at Gillette Children's Specialty Healthcare (St. Paul, Minnesota, USA). Both national and local (Institutional Review Board) regulatory approvals were obtained, including approval from the Food and Drug Administration to secure a pre-approval for an investigational device exemption, for this study.
Participants (children and young adults with UCP) were tested within two weeks prior to intervention (Pre-test), within one week after the intervention (Post-test) and again six months after the intervention (six-month follow-up). During the period from Post-test to six-month follow-up, children had no restrictions from any activity or individual therapies.
2.2 Participants
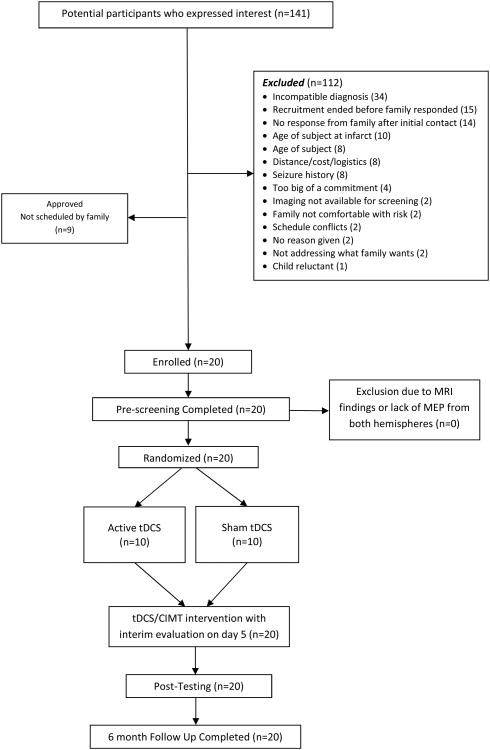
Of 141 interested families and participants, twenty children with UCP (14% of interested families) due to perinatal stroke or PVL met the eligibility criteria, enrolled, and participated in the study (Figure 1). Baseline characteristics are shown in Table 1. The sample was 50% male, with a mean age of years 12 years, 9 months (median age 11 years, 3 months; SD = 4 years, 2 months; range = 7 years, 5 months – 21 years, 7 months). The criteria for inclusion and exclusion are described in an earlier protocol publication.22 In brief, we included participants, between 7-21 year of age, with a radiologically-confirmed diagnosis of hemispheric stroke or PVL and without seizure within the two years prior to study participation. Children with bilateral and asymmetric PVL (n=1) were included if they had a clinical presentation of UCP. After meeting diagnostic criteria, participants were subsequently included if a motor evoked potential (MEP) from the less-affected hand was elicited while transcranial magnetic stimulation (TMS) testing of the non-lesioned hemisphere. Children with other neurological diagnoses, recent treatments with injections for spasticity management, or contraindications for magnetic resonance imaging (MRI) or NIBS procedures were excluded. All participants ages 7-17 provided written informed assent with accompanying legal guardian written consent. Participants ages 18 and older provided written consent.
Figure 1.
CONSORT diagram and study flow.
Table 1. Participant Demographics.
| Sham+CIMT (n=10) | Active+CIMT (n=10) | |
|---|---|---|
| Sex | ||
| Male | 4 (40%) | 5 (50%) |
| Female | 6 (60%) | 5 (50%) |
| Median age | 13y 2m | 12y 4m |
| Age range (min, max) | 8y 2m, 21y 7m | 7y 5m, 16y 11m |
| Affected Side | ||
| Right | 8 (80%) | 7 (70%) |
| Left | 2 (20%) | 3 (30%) |
| CST Circuitry | ||
| Ipsilateral | 4 (40%) | 4 (40%) |
| Contralateral | 6 (60%) | 6 (60%) |
| MACS | ||
| Level I | 1 (10%) | 1 (10%) |
| Level II | 8 (80%) | 8 (80%) |
| Level III | 0 (0%) | 1 (10%) |
| Level IV | 1 (1%) | 0 (0%) |
| Lesion Location | ||
| Cortical | 0 (0%) | 1 (10%) |
| Subcortical | 1 (10%) | 1 (10%) |
| Both Cortical and Subcortical | 9 (90%) | 8 (80%) |
Data are No. (%), CIMT: Constraint-induced movement therapy, CST: Corticospinal tract, MACS: Manual Ability Classification System, m: months, y: years
2.3 Intervention
Participants completed ten tDCS (1×1 LTE, Soterix Medical, New York, NY) sessions consisting of 20 minutes of combined stimulation and CIMT, followed by 100 minutes of CIMT alone in small groups (up to three children). TDCS electrodes were configured with the cathode positioned on the non-lesioned hemisphere primary motor cortex (M1) as identified by the TMS motor hotspot and the anode on the contralateral supraorbital prominence (SO). As cathodal tDCS may decrease cortical excitability, our study design included a M1-SO tDCS montage to rebalance the hypothesized exaggerated interhemispheric inhibition upon the lesioned hemisphere, as reported in adults with stroke.23 Children in the active tDCS group (Active+CIMT), received 0.7 mA for 20 minutes, which was gradually introduced during a 30-second ramp-up phase and extinguished during a 30-second ramp-down phase. The procedures were identical for participants in the sham tDCS group (Sham+CIMT), however the tDCS device was set to a built-in sham setting, which simulated the ramp-up and ramp-down phases of stimulation. CIMT was structured with the less-affected arm placed in a sling during each two-hour intervention session. Therapy was administered to each participant by a trained interventionist engaging the child in shaping activities for function and motor skill development. Additionally, all participants were instructed on a daily home program.
2.4 Safety and Medical Assessment
2.4.1 Questionnaires
Participants were asked systematic questions before and after all brain stimulation sessions (TMS testing and tDCS intervention) related to potential minor adverse event (MAE), which was our primary safety outcome. 24, 25 (Gillick et al. 2017, Front Pediatr, In Review) The child was asked to report if a symptom was present (e.g. tingling, itchiness, headache, etc.) and then rate the “severity” of the symptom. The relation of the symptom to the intervention was determined by the investigator.
2.4.2 Child and Caregiver Feedback
In addition to the symptom-specific questionnaire, the child and their caregiver were asked an open-ended question regarding well-being. Following the brain stimulation session, at least three check-in phone calls were made during the study timeframe to identify any persistent symptoms and seek caregiver input as to how the child was tolerating the study experience.
2.4.3 Physician Evaluations
Physician evaluations were conducted with a caregiver present at all testing time points and following the 5th tDCS session. Evaluations consisted of the Modified Pediatric Stroke Outcome Measure-Short neuro Exam (PSOM-SNE) to monitor medical stability during participation.26
2.4.4 Vital Sign Assessment
Blood pressure and heart rate were monitored daily for each child using an automatic blood pressure machine with an appropriately sized cuff for each participant. The same equipment was used for the pre/post measurements. All children were encouraged to drink 8-12 ounces of water prior to daily participation.
2.4.5 Grip Strength
Grip strength was measured in both hands. As the cathodal tDCS intervention was targeting down-regulation of the non-lesioned hemisphere, the potential exists that the less-affected hand could have a decrease in function such as grip strength. Grip strength was measured with a handheld dynamometer. Three trials per hand were recorded at all testing points and additionally on the fifth day of the intervention (Day 5) as an interim safety assessment.
2.6 Behavioral Outcomes
The primary behavioral outcome was the Assisting Hand Assessment (AHA), a measure of two-handed, or bimanual, function in a novel play or functional task for children with UCP.27 The secondary outcome measures was the assessment of performance and satisfaction in goals using the Canadian Occupational Performance Measure (COPM) with recent evidence suggesting children can identify and rate progress towards goals in clinical trials.28, 29
2.7 TMS and circuitry assessment
Methods for TMS testing are described in a previous publication.11 Briefly, electromyography (EMG) activity from the first dorsal interosseous (FDI) muscles was recorded bilaterally. EMG signals were band-pass filtered (15-2000Hz) and digitized. All data were collected and stored using a custom data acquisition program written with LabVIEW (V2012, National Instruments, Austin, TX) on a laptop computer which was also used to monitor real-time EMG activity. TMS pulses were delivered to each hemisphere using a 70-mm figure of eight coil connected to the Bistim2 and 2002 stimulator set (MagStim Inc., Dyfed, UK). Individual reconstructions of head and brain tissue were created from T1-anatomical images and used in a frameless stereotactic neuronavigation system (Brainsight, Rogue Research Inc., Montreal, Quebec, Canada) to ensure accurate coil location and position on the scalp. First, we confirmed presence of a non-lesioned hemisphere MEP to guide tDCS electrode placement. We characterized CST circuitry by lesioned hemisphere assessment. We stratified our sample by presence (contralateral circuitry) or absence (ipsilateral circuitry) of MEPs from TMS testing of the lesioned hemisphere.
2.8 Statistical and Power Analysis
Power was calculated through an a priori analysis for the primary outcome (AHA) using an estimated scaled score difference between the Pre-test and Post-test measurements.22 Power was computed using a formula for normally distributed statistics with 10 participants per intervention group across a range of values for the correlation between Pre-test and Post-test measurements and Type I error equal to 0.05. In a previous study using the AHA as the primary outcome measure, a treatment effect of 5.4 AHA units was found and was considered a clinically meaningful change for this tDCS exploratory study.4 Using a conservative standard deviation (7.1 units) and correlation between Pre-test and Post-test AHA (0.8) based on our previous work, this study was designed with 80% power to detect the difference of 5.4 AHA units between Active+CIMT and Sham+CIMT groups.
Using a random number generator and envelopes sealed by the study biostatistician, each participant was randomized to the Active or the Sham tDCS group. Randomization was blocked and stratified by presence or absence of a lesioned hemisphere MEP with randomly permuted blocks of two and four. Participants, caregivers, and investigators, with the exception of the Principal Investigator, were blinded to group assignment. Specifically, blinded investigators conducted all assessments and interventions, with the principal investigator (PI- BTG) blinded to the behavioral outcome assessment (e.g. AHA, COPM). As was IRB-required, the PI provided supervision during neuromodulation sessions (TMS and tDCS)..
The Fisher's Exact test was used for comparing proportions of children reporting MAEs between groups and a relative risk was calculated, with exact unconditional 95% confidence intervals using the score statistic. Within-group analyses of behavioral outcomes comparing Pre-test to Post-test and Pre-test to six-month follow-up were performed with paired t-tests. Between-group analysis of outcomes compared mean changes from Pre-test to each time point between intervention groups and was adjusted for baseline differences for added precision. The t-distribution with corresponding model degrees of freedom was used for confidence intervals and P-values. Analyses to examine treatment effects within the pre-specified CST circuitry (contralateral or ipsilateral) subgroups were conducted similarly. P-values less than 0.05 were considered statistically significant. Given the preliminary nature of this study, we did not correct for multiple comparisons. All statistics were computed using SPSS v21 (IBM, Armonk, NY) and R.30
3. Results
Recruitment began December 2014, and the study was conducted between April 2015 and December 2016. All 20 participants were assessed for the primary outcome measures.
3.1 Safety Outcomes
A total of 60 TMS testing sessions were completed (100% participation) across all 20 children and time points. During TMS testing, 11 children reported MAE during TMS testing sessions. The proportion of the group with MAE reports included nausea (1/20), tactile symptoms at the site of stimulation (1/20), headache (1/20), dizziness (3/20), and sleepiness (7/20) with sleepiness as the most common.
A total of 199 of 200 tDCS/CIMT sessions (99.5%) were completed across 20 children as one child missed one combined intervention session due to a medical appointment. During tDCS intervention, children in both the real and sham tDCS groups reported MAE. The most common reported MAEs were headache (5/20 children), tingling (4/20 children) and itchiness (4/20 children) (Table 2). Although there was a greater risk of headache in the Active+CIMT group as evidenced by the relative risk of 4.0, there were no significant differences in the proportion of participants reporting MAEs between groups for any symptom. A minority of participants in both groups reported symptoms. Mild erythema occurred at the site of the anode placed on the SO prominence during one session in two children. All reported and observed symptoms resolved within the 20-minute session.
Table 2. tDCS-Related MAE.
| Active+CIMT (n=10) | Sham+CIMT (n=10) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Reported MAE | Proportion of group with MAE reports | Total reports (n) | Proportion of group with MAE reports | Total reports (n) | Relative Risk | p-value* | 95% CI |
| Headache | 0.4 | 9 | 0.1 | 1 | 4.0 | 0.303 | 0.61, 104.2 |
| Unusual feelings on the skin of the head | 0.2 | 5 | 0.1 | 1 | 2.0 | >0.999 | 0.19, 54.0 |
| Itchiness | 0.1 | 5 | 0.3 | 6 | 0.33 | 0.582 | 0.01, 2.86 |
| Unusual feelings or emotions | 0.1 | 2 | 0 | 0 | -- | >0.999 | 0.07, Inf |
| Dizziness | 0 | 0 | 0.3 | 3 | -- | 0.211 | 0, 1.28 |
| Nausea | 0 | 0 | 0.1 | 1 | -- | >0.999 | 0, 14.0 |
| Tingling | 0.2 | 10 | 0.2 | 2 | 1.0 | >0.999 | 0.07, 14.2 |
| Difficulty paying attention | 0 | 0 | 0.1 | 1 | -- | >0.999 | 0, 14.0 |
CIMT: Constraint-induced movement therapy; Inf: Infinity; MAE: Minor adverse events; CI: Confidence Interval.
Fisher exact test for the proportion of children reporting MAE between groups. Total reports represents the cumulative number of MAEs for the 10 day intervention
3.2 Behavioral Outcomes
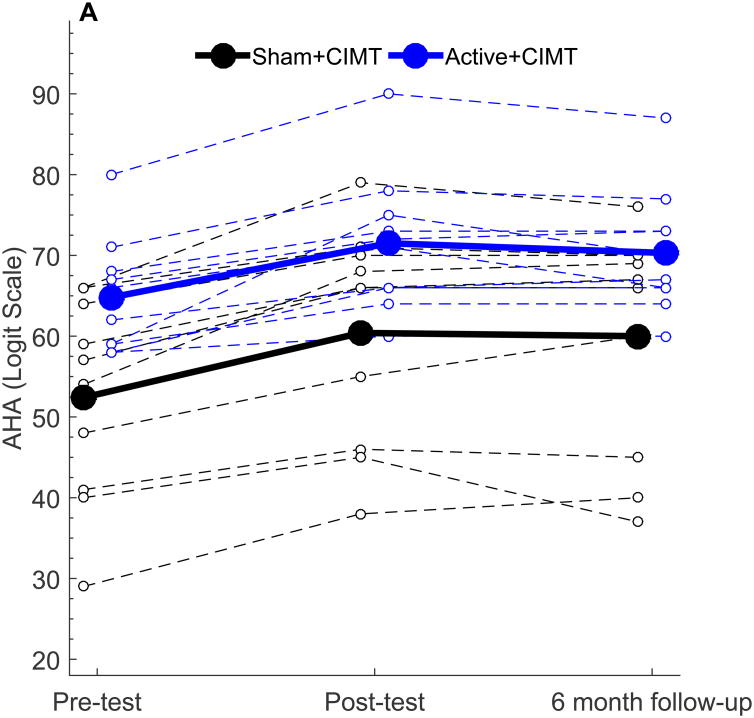
The mean and standard deviation of AHA scores at Pre-Test, Post-Test and Follow-up were 52.4 AHA units ± 12.6, 60.4 ± 13.5, and 60.0 AHA units ± 14.0 in the Sham+CIMT group and 64.8 AHA units ± 7.10, 71.2 ± 8.56, and 70.3 AHA units ± 7.69 in the Active+CIMT group. Within-and between-group changes in behavioral outcomes are summarized in Table 3. The AHA score at Pre-test, Post-test, and six-month follow-up is shown in Figure 2A, plotted in 0-100 AHA logit units. Both groups demonstrated a significant increase in AHA score following the intervention (p < 0.001). A significant increase in AHA from Pre-test to six-month follow-up was also noted (both groups, p < 0.001). No significant difference was observed in AHA score change between groups at Post-test (p = 0.30) or at six-month follow-up (p = 0.31). While no minimal clinically important difference has been established for the AHA, the smallest detectable difference for this measure is five 0-100 logit-based AHA units.27, 31 All ten participants in the Sham+CIMT group and seven of ten participants in the Active+CIMT group met or exceeded this amount. While there is a potential for a ceiling effect, the highest Pre-test AHA score was 80 AHA units out of a maximum 100 AHA units. This participant's Post-test AHA score was 90, which was still 10 units from the maximum AHA score, suggesting that a ceiling effect was not present in our sample.
Table 3. Within-group and between-group differences in motor outcomes from Pre-test.
| Post-test | Six-month Follow-Up | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sham+CIMT (n=10) | Active+CIMT (n=10) | Between-group difference | Sham+CIMT (n=10) | Active+CIMT (n=10) | Between-group difference | |
| AHA (0-100 Logit Scale) | 8.00 [5.66, 10.34] | 6.40 [3.40, 9.40] | -2.18 [-6.48, 2.12] | 7.60 [3.92, 11.28] | 5.50 [3.23, 7.77] | -2.44 [-7.36, 2.48] |
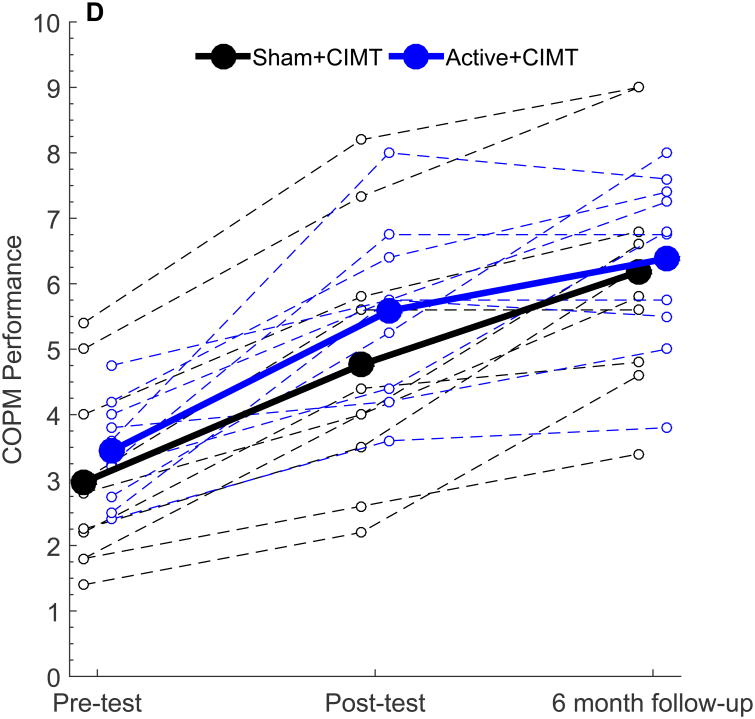
| COPM Performance | 1.80 [1.27, 2.33] | 2.14 [1.23, 3.05] | 0.26 [-0.76, 1.28] | 3.22 [2.57, 3.87] | 2.94 [2.04, 3.84] | -0.29 [-1.38, 0.81] |
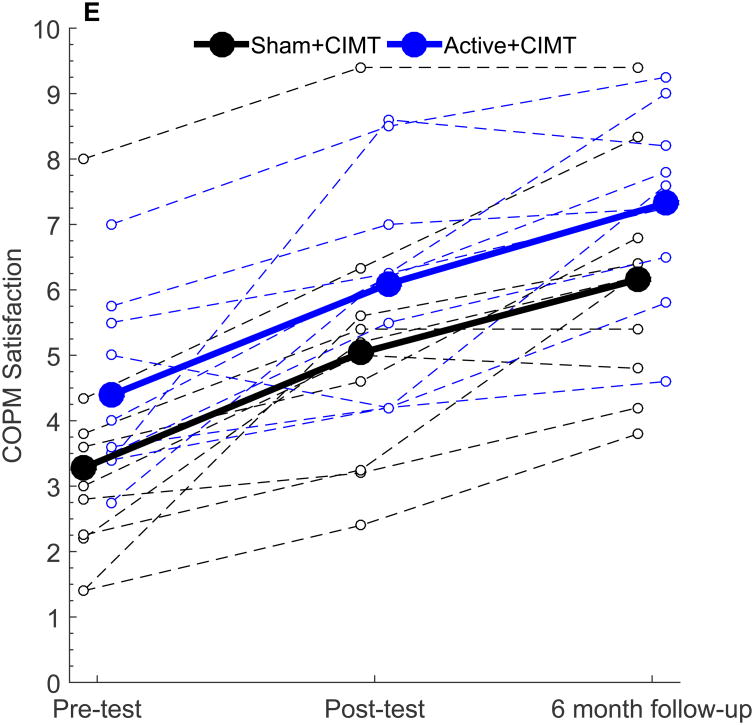
| COPM Satisfaction | 1.76 [0.96, 2.56] | 1.70 [0.50, 2.90] | 0.25 [-1.14, 1.64] | 2.88 [1.93, 3.83] | 2.94 [1.68, 4.19] | 0.59 [-0.79, 1.96] |
| More-affected hand grip strength (kg)* | 0.18 [-1.20, 1.57] | 0.14 [-1.79, 2.06] | 0.56 [-1.90, 3.02] | 0.79 [-0.55, 2.13] | 0.15 [-1.49, 1.79] | -0.32 [-2.56, 1.93] |
Data are mean [95% CI] %; Bolded values are significantly different from Pre-test (p<0.001);
One participant (Sham+CIMT) excluded;
CST: Corticospinal tract, AHA: Assisting Hand Assessment, COPM: Canadian Occupational Performance Measure; CIMT: Constraint-induced movement therapy
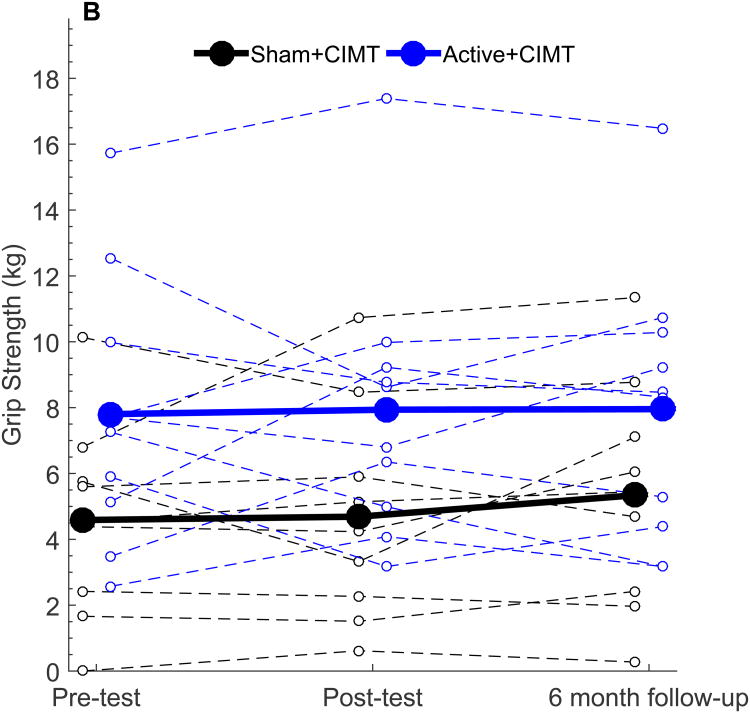
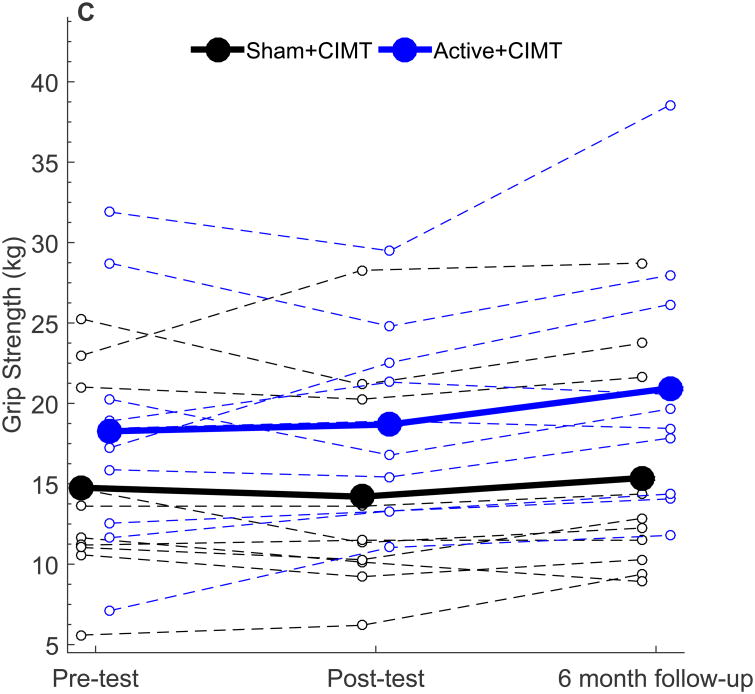
Figure 2.
Behavioral outcomes following tDCS+CIMT intervention. All plots show individual (dashed lines) and group mean (solid line) data. (A) AHA (n=20), (B) Grip Strength in More-affected hand (n=19), (C) Grip Strength in Less-affected hand (n=19), (D) COPM Performance (n=20), (E) COPM Satisfaction (n=20). AHA: Assisting Hand Assessment, COPM: Canadian Occupational Performance Measure.
Similar patterns of change were noted in the COPM. Both groups showed significant increases in COPM Performance at Post-test (both groups p < 0.001) and at six-month follow (both groups p < 0.001). No significant differences in the change in COPM performance scale were noted between groups (p = 0.59). For the COPM Satisfaction scale, similar within-group improvements were noted at Post-test and at six-month follow-up. Between-group differences were also similar to COPM performance (Post-test, p = 0.71; follow-up, p = 0.38).
For the measure of grip strength, one participant (Sham+CIMT) could not elicit a measurable grip strength reading due to difficulty positioning the dynamometer and was excluded from analysis. For the remaining 19 participants, no significant changes in grip strength in the more-or less-affected hand were observed at Post-test (Fig 2B; all p > 0.05) or at six-month follow-up (all p > 0.05). Furthermore, no significant differences in change in grip strength were noted between groups at any time point (all p > 0.05). Grip strength measurements of the less-affected hand remained stable with no between group differences observed from Pre-test to Day 5 (1.08 kg, [-1.30, 3.45], p = 0.35) and Day 5 to Post-test (-0.09 kg, [-2.60, 2.42], p = 0.94). Participants were tested with the same device and tester on all occasions.
3.3 Comparison of CST circuitry
The relationship between CST circuitry and changes in hand function from Pre-test to Post-test and six-month follow-up is shown in Table 3. For the 12 participants with contralateral circuitry, the mean difference in AHA score between the Active+CIMT and Sham+CIMT groups was -1.29 (95% CI = [-8.58, 6.00], p = 0.70). For the eight participants with ipsilateral CST circuitry, the mean difference in AHA score between the Active+CIMT and Sham+CIMT groups was -2.29 ([-5.90, 1.32], p = 0.16). When examining the overall effect of CST laterality on changes in motor function adjusted for intervention group, those participants with contralateral CST circuitry had an 8.42 unit increase in the AHA compared to a 5.38 unit increase in those with ipsilateral circuitry (mean difference = 3.04, [-0.64, 6.72], p = 0.099). This potential effect of laterality was also present at six-month follow-up, but was smaller in magnitude between groups (mean difference = 1.75, [-2.75, 6.25], p = 0.42). There were no significant differences in grip strength or COPM performance and satisfaction ratings between Active+CIMT and Sham+CIMT within each CST circuitry subgroup (all other p > 0.05).
4. Discussion
In this clinical trial, we examined the safety, feasibility, and preliminary efficacy of a combined neuromodulatory and behavioral intervention in participants with UCP. No serious adverse events occurred and a combined tDCS+CIMT group-format intervention proved feasible with all enrolled participants completing the study. Similar to the safety of other pediatric tDCS studies, no participants experienced any serious adverse events, adding to a growing body of evidence that tDCS, with the reported dosing parameters, has been found to be safe and feasible for application in pediatric populations.32, 33
The lack of significant difference between intervention groups in our study could be attributed to the low intensity of tDCS dosing and timing of motor training and stimulation. It is possible that 0.7 mA was an insufficient intensity to produce a measureable behavioral effect beyond the motor learning related to CIMT aspect of the intervention. Inconsistent effects of tDCS on motor performance are evident in adults with stroke, and in children and young adults with and without UCP. 1, 32, 34 However, others have found positive effects of 1.0 mA anodal tDCS on gait and balance outcomes with the electrode placed on the lesioned motor cortex.17-19 Although no adverse events were reported in these studies, applying stimulation on the ipsilesional side may be less efficacious without a thorough understanding of circuitry and corticospinal tract integrity as relates to more-affected hand control. Additionally, recent modeling studies demonstrate that age and the integrity of underlying brain anatomy (i.e. lesions) influence the distribution of current across the scalp potentially, which may contribute to the variable efficacy of different montages of tDCS in children with brain lesions.21, 35-37 The effects of tDCS may also be dependent on the sequence with which it is paired with rehabilitation. Timing-dependent effects have been studied only in adults. Based on previous synergistic applications of tDCS,1, 8 we paired tDCS simultaneously with CIMT. However, others have shown that priming, applying tDCS before training, also results in improved outcomes.38, 39 The observed neuroplastic effects reported by others as measured with changes in neurophysiologic measures are likely related to the individual's baseline level of brain excitability40 and response to intervention (e.g. excitability changes resulting from rehabilitation and/or neuromodulation). Overall, these factors indicate that the optimal tDCS montage, dosing, and timing deserve further exploration in neurorehabilitation settings.
Another potential explanation for the lack of significant tDCS effects in our study is that the intensity of CIMT negated any observable effects of tDCS. As we did not assess daily changes in outcomes, the benefit of tDCS may have been detectable early in the intervention, before being obscured by the cumulative effect of CIMT. Despite the low intensity of CIMT in our study, the average change in AHA score was comparable to studies using higher intensity CIMT.41, 42 Overall, the dose and timing of the intervention deserves further investigation to provide higher-level evidence of efficacy of such synergistic interventions in children and young adults with UCP.
Importantly, although we report no primary effect of tDCS when combined with CIMT, the magnitude of change in motor function following intervention was associated with CST circuitry. Participants with contralateral circuitry showed greater improvement in hand function compared to participants with ipsilateral organization, adjusted for group assignment. Evidence suggests that behavioral and neurophysiologic changes may differ between children with contralateral and ipsilateral CST projections. Following CIMT, children with retained CST projections from the lesioned hemisphere show improvements in hand function, specifically quality of movements and speed,16 and increased excitability of the primary motor cortex.15 In contrast, children with absent CST projections from the lesioned hemisphere may demonstrate improvements in the quality of hand function only16 and a decrease in primary motor cortex excitability.15 Altogether, this suggests that CIMT has a differing influence on behavioral and neurophysiologic parameters. When comparing the effect of tDCS within circuitry subgroups, we found similar improvements in children with contralateral circuitry between Active+CIMT and Sham+CIMT groups. Interestingly, children with ipsilateral circuitry in the Active+CIMT group showed smaller yet non-significant improvements compared to those in the Sham+CIMT (Table 3). In the ipsilateral circuitry subgroup, the non-lesioned hemisphere is primarily controlling the more-affected hand. Inhibitory tDCS applied to the non-lesioned hemisphere, as employed in our study, may have offset any improvements gained from CIMT resulting in the small improvement following the intervention compared to Sham+CIMT. Children with ipsilateral circuitry may therefore require a longer intervention period (e.g. additional sessions or longer duration of sessions) to achieve the same magnitude of change as observed in children with contralateral circuitry. Different montages, such as anodal of tDCS applied to the non-lesioned hemisphere, may be more efficacious for participants with ipsilateral circuitry, however such interventions have yet to be tested in a controlled study. Altogether, these results support the evidence indicating that responsivity to therapy is likely related to underlying brain organization, suggesting incorporation of CST circuitry as a potential biomarker to individualize therapies for children and young adults with UCP.
Our findings of the association between circuitry and a combined tDCS+CIMT intervention gains expands upon prior findings examining the association between circuitry and CIMT alone for children with UCP.12, 15, 43-46 Although it appears that all children with UCP demonstrate change following rehabilitation regardless of circuitry, knowledge of circuitry patterns and the relationship to change following intervention will aid in future efforts toward precision medicine16, 47. The potential to individualize intervention reinforces the need for future studies in this population with early brain injuries to consider CST circuitry as relates to outcomes and development.
This study has several limitations. First, this was an exploratory study and included a sample of 20 participants with UCP. Therefore, replication in larger cohorts is warranted. The findings related to differences in motor outcomes between CST circuitry groups likewise indicates a need for separate trials to investigate different stimulation parameters for each sub-group. Despite the preliminary nature of this study, the variability in age and lesion presentation among participants reflects a clinically relevant population of children and young adults with UCP. Second, our tools to assess hand function, while valid and reliable, may not have been sensitive enough to detect changes in behavior following a combined tDCS+CIMT intervention. Still, changes in all outcomes were observed regardless of the age of participants, signifying that continued change in motor abilities and goal attainment is possible. Finally, our study did not include a CIMT-only control group. Due to the sham setting of the tDCS device, each participant reported minimal, but sensible, stimulation which may have caused placebo effects in the Sham+CIMT group.
5. Conclusions
With recent advances in neuromodulatory research in pediatric populations, NIBS may complement current intensive upper-extremity therapies that promote recovery in children and young adults with UCP. Our study demonstrates the safety and feasibility of such group interventions using tDCS with further indications for exploration of optimal dosing. Future studies may identify individualization of NIBS (placement, intensity, duration) and therapies (CIMT, bimanual, traditional approaches) based upon underlying CST circuitry. Understanding these factors may alter the dosing parameters currently reported, such as exploration of higher intensity of stimulation, with the overarching goal of improved long-term outcomes for this population.
Table 4. Changes in motor outcomes from Pre-test stratified by CST circuitry.
| Contralateral (n=12) | Ipsilateral (n=8) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Post-test | Six-month follow-up | Post-test | Six-month follow-up | |||||
|
| ||||||||
| Sham+CIMT (n=6) | Active+CIMT (n=6) | Sham+CIMT (n=6) | Active+CIMT (n=6) | Sham+CIMT (n=4) | Active+CIMT (n=4) | Sham+CIMT (n=4) | Active+CIMT (n=4) | |
| AHA (0-100 Logit Scale) | 8.83 [4.71, 12.95] | 8.00 [2.98, 13.0] | 8.00 [1.20, 14.8] | 6.5 [2.59, 10.4] | 6.75 [4.03, 9.47] | 4.00 [1.75, 6.25] | 7.00 [2.32, 11.7] | 4.00 [1.75, 6.25] |
| COPM Performance | 1.73 [0.95, 2.50] | 2.33 [0.83, 3.82] | 2.97 [2.00, 3.94] | 3.01 [1.39, 4.63] | 1.90 [0.56, 3.24] | 1.86 [0.05, 3.67] | 3.60 [2.23, 4.97] | 2.84 [1.44, 4.24] |
| COPM Satisfaction | 1.33 [0.66, 2.01] | 2.07 [-0.14, 4.27] | 2.67 [1.40, 3.94] | 3.18 [0.94, 5.43] | 2.40 [0.05, 4.75] | 1.15 [0.15, 2.15] | 3.20 [0.64, 5.76] | 2.56 [0.71, 4.41] |
| More-affected hand grip strength (kg)* | 0.45 [-2.13, 3.04] | 1.01 [-1.45, 3.46] | 0.70 [-2.33, 3.72] | 0.25 [-2.57, 3.08] | -0.15 [-2.75, 2.45] | -1.17 [-5.90, 3.55] | 0.91 [0.39, 1.43] | 0.00 [-3.07, 3.07] |
Data are mean [95% CI] %;
One participant (Sham+CIMT and ipsilateral) excluded;
CST: Corticospinal tract, AHA: Assisting Hand Assessment, COPM: Canadian Occupational Performance Measure; CIMT: Constraint-induced movement therapy
Highlights.
tDCS and motor training in children with cerebral palsy is safe and feasible
All children improved hand function, regardless of intervention group
Children with contralateral circuitry showed greater motor improvement
Expanded research on dosing and brain circuitry in this population is indicated
Acknowledgments
This study was funded the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institutes of Child Health and Development K01 Award (HD078484-01A1), the Cerebral Palsy Foundation, the Foundation for Physical Therapy Magistro Family Grant, and Minnesota's Discovery, Research, and Innovation Economy (MnDRIVE). The project described was also supported in part by Award UL1 TR000114 and KL2 TR000113.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The PI had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors thank the Center for Neurobehavioral Development and Center for Magnetic Resonance Imaging (P41 EB015894) at the University of Minnesota. We also thank the mentors, collaborators, therapists, therapy supervisors, study coordinators, and research administration personnel from Gillette Children's Specialty Healthcare. Most importantly, we are grateful for the energies of the families, caregivers and participants involved in this study.
Abbreviations
- tDCS
Transcranial direct current stimulation
- TMS
Transcranial magnetic stimulation]
- NIBS
Non-invasive brain stimulation
- UCP
Unilateral cerebral palsy
- CIMT
Constraint-induced movement therapy
- MEP
Motor evoked potential
- CST
Corticospinal tract
- M1
Primary motor cortex
- SO
Supraorbital
- AHA
Assisting Hand Assessment
- COPM
Canadian Occupational Performance Measure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–29. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- 2.Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial Direct Current Stimulation Potentiates Improvements in Functional Ability in Patients With Chronic Stroke Receiving Constraint-Induced Movement Therapy. Stroke. 2017;48(1):229–32. doi: 10.1161/STROKEAHA.116.014988. [DOI] [PubMed] [Google Scholar]
- 3.Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90(3):398–410. doi: 10.2522/ptj.20090075. [DOI] [PubMed] [Google Scholar]
- 4.Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Thomas W, et al. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: a randomized controlled trial. Dev Med Child Neurol. 2014;56(1):44–52. doi: 10.1111/dmcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19(1):41–7. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–5. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillick BT, Kirton A, Carmel JB, Minhas P, Bikson M. Pediatric stroke and transcranial direct current stimulation: methods for rational individualized dose optimization. Front Hum Neurosci. 2014;8:739. doi: 10.3389/fnhum.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillick BT, Feyma T, Menk J, Usset M, Vaith A, Wood TJ, et al. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys Ther. 2015;95(3):337–49. doi: 10.2522/ptj.20130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich TL, Menk JS, Rudser KD, Chen M, Meekins GD, Pena E, et al. Determining Electrode Placement for Transcranial Direct Current Stimulation: A Comparison of EEG- Versus TMS-Guided Methods. Clin EEG Neurosci. 2017 doi: 10.1177/1550059417709177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackey A, Stinear C, Stott S, Byblow WD. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Front Neurol. 2014;5:117. doi: 10.3389/fneur.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staudt M. Reorganization after pre- and perinatal brain lesions. J Anat. 2010;217(4):469–74. doi: 10.1111/j.1469-7580.2010.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staudt M, Gordon AM. Combining rTMS and CIMT: A “one-size-fits-all” therapy for congenital hemiparesis? Neurology. 2016;86(18):1652–4. doi: 10.1212/WNL.0000000000002645. [DOI] [PubMed] [Google Scholar]
- 15.Juenger H, Kuhnke N, Braun C, Ummenhofer F, Wilke M, Walther M, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: a transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Dev Med Child Neurol. 2013;55(10):941–51. doi: 10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- 16.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 17.Collange Grecco LA, de Almeida Carvalho Duarte N, Mendonca ME, Galli M, Fregni F, Oliveira CS. Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: a pilot, randomized, controlled, double-blind, clinical trial. Clin Rehabil. 2015;29(12):1212–23. doi: 10.1177/0269215514566997. [DOI] [PubMed] [Google Scholar]
- 18.Duarte NAC, Grecco LA, Galli M, Fregni F, Oliveira CS. Effect of transcranial direct-current stimulation combined with treadmill training on balance and functional performance in children with cerebral palsy: a double-blind randomized controlled trial. PLoS One. 2014;9(8):e105777. doi: 10.1371/journal.pone.0105777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grecco LA, de Almeida Carvalho Duarte N, Mendonca ME, Cimolin V, Galli M, Fregni F, et al. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: a randomized controlled double-blind clinical trial. Res Dev Disabil. 2014;35(11):2840–8. doi: 10.1016/j.ridd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci. 2014;8:600. doi: 10.3389/fnhum.2014.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moliadze V, Schmanke T, Andreas S, Lyzhko E, Freitag CM, Siniatchkin M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin Neurophysiol. 2015;126(7):1392–9. doi: 10.1016/j.clinph.2014.10.142. [DOI] [PubMed] [Google Scholar]
- 22.Gillick BT, Menk J, Mueller B, Meekins G, Krach LE, Feyma T, et al. Synergistic effect of combined transcranial direct current stimulation/constraint-induced movement therapy in children and young adults with hemiparesis: study protocol. BMC Pediatr. 2015;15:178. doi: 10.1186/s12887-015-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–9. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Garvey MA, Kaczynski KJ, Becker DA, Bartko JJ. Subjective reactions of children to single-pulse transcranial magnetic stimulation. J Child Neurol. 2001;16(12):891–4. doi: 10.1177/088307380101601205. [DOI] [PubMed] [Google Scholar]
- 25.Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, et al. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Arch Phys Med Rehabil. 2015;96(4 Suppl):S104–13. doi: 10.1016/j.apmr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchen L, Westmacott R, Friefeld S, MacGregor D, Curtis R, Allen A, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43(6):1602–8. doi: 10.1161/STROKEAHA.111.639583. [DOI] [PubMed] [Google Scholar]
- 27.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol. 2007;49(4):259–64. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Cusick A, Lannin NA, Lowe K. Adapting the Canadian Occupational Performance Measure for use in a paediatric clinical trial. Disabil Rehabil. 2007;29(10):761–6. doi: 10.1080/09638280600929201. [DOI] [PubMed] [Google Scholar]
- 29.Vroland-Nordstrand K, Eliasson AC, Jacobsson H, Johansson U, Krumlinde-Sundholm L. Can children identify and achieve goals for intervention? A randomized trial comparing two goal-setting approaches. Dev Med Child Neurol. 2016;58(6):589–96. doi: 10.1111/dmcn.12925. [DOI] [PubMed] [Google Scholar]
- 30.Team RC. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria.: 2016. [Google Scholar]
- 31.Krumlinde-Sundholm L. Reporting outcomes of the Assisting Hand Assessment: what scale should be used? Dev Med Child Neurol. 2012;54(9):807–8. doi: 10.1111/j.1469-8749.2012.04361.x. [DOI] [PubMed] [Google Scholar]
- 32.Kirton A, Ciechanski P, Zewdie E, Andersen J, Nettel-Aguirre A, Carlson H, et al. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology. 2017;88(3):259–67. doi: 10.1212/WNL.0000000000003518. [DOI] [PubMed] [Google Scholar]
- 33.Gillick BT, Friel KM, Menk J, Rudser K. Therapeutic Brian Stimulation Trials in Children with Cerebral Palsy. In: Gilbert D, editor. Pediatric Brain Stimulation. Oxford: Academic Press; 2016. pp. 209–36. [Google Scholar]
- 34.Ciechanski P, Kirton A. Transcranial Direct-Current Stimulation Can Enhance Motor Learning in Children. Cereb Cortex. 2017;27(5):2758–67. doi: 10.1093/cercor/bhw114. [DOI] [PubMed] [Google Scholar]
- 35.Opitz A, Falchier A, Yan CG, Yeagle EM, Linn GS, Megevand P, et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep. 2016;6:31236. doi: 10.1038/srep31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opitz A, Paulus W, Will S, Antunes A, Thielscher A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015;109:140–50. doi: 10.1016/j.neuroimage.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Minjoli S, Saturnino GB, Blicher JU, Stagg CJ, Siebner HR, Antunes A, et al. The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. NeuroImage: Clinical. 2017;15:106–17. doi: 10.1016/j.nicl.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeiarati G, et al. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. NeuroRehabilitation. 2013;33(1):49–56. doi: 10.3233/NRE-130927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(3 Suppl):S79–87. doi: 10.1016/j.apmr.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy JM, Gillick BT, Carey JR. Priming the brain to capitalize on metaplasticity in stroke rehabilitation. Phys Ther. 2014;94(1):139–50. doi: 10.2522/ptj.20130027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eliasson AC, Krumlinde-sundholm L, Shaw K, Wang C. Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: an adapted model. Dev Med Child Neurol. 2005;47(4):266–75. doi: 10.1017/s0012162205000502. [DOI] [PubMed] [Google Scholar]
- 42.Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Repair. 2011;25(8):692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- 43.Holmstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52(2):145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 45.Staudt M, Berweck S. Is constraint-induced movement therapy harmful in unilateral spastic cerebral palsy with ipsilateral cortico-spinal projections? Dev Med Child Neurol. 2014;56(3):202–3. doi: 10.1111/dmcn.12372. [DOI] [PubMed] [Google Scholar]
- 46.Smorenburg AR, Gordon AM, Kuo HC, Ferre CL, Brandao M, Bleyenheuft Y, et al. Does Corticospinal Tract Connectivity Influence the Response to Intensive Bimanual Therapy in Children With Unilateral Cerebral Palsy? Neurorehabil Neural Repair. 2017;31(3):250–60. doi: 10.1177/1545968316675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam M, Nordstrand L, Holmstrom L, Kits A, Forssberg H, Eliasson AC. Is outcome of constraint-induced movement therapy in unilateral cerebral palsy dependent on corticomotor projection pattern and brain lesion characteristics? Dev Med Child Neurol. 2014;56(3):252–8. doi: 10.1111/dmcn.12353. [DOI] [PubMed] [Google Scholar]