Abstract
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are novel tools for the dissection of circuitry mediating behavior and neural function. Designer receptors based on the muscarinic M3 and M4 subtypes were designed to be activated by clozapine-N-oxide (CNO), a ligand previously shown to be an inert metabolite of clozapine. However, recent work in rats has shown that CNO is reverse metabolized to its parent compound. Furthermore, CNO administration (5 mg/kg IP) attenuates amphetamine-induced locomotion and the evoked dopamine response that accompanies it. As these systems are routinely used to probe the neurocircuitry underlying cocaine-seeking behavior, here we sought to determine whether CNO would have similar effects on cocaine-induced locomotion in rats with a history of cocaine self-administration. In order for muscarinic-based DREADDs to be utilized for the dissection of circuitry underlying behavioral responses to cocaine, the doses of CNO administered to induce DREADD signaling must themselves have no effect on cocaine-induced behavior. Male Sprague-Dawley rats self-administered cocaine (0.35 mg/infusion) for 12 days, followed by 14–21 days of instrumental extinction training. Rats then underwent locomotor testing. CNO (0, 3, or 5 mg/kg) was injected (utilizing a within-subjects design), followed 20 minutes later by cocaine (10 mg/kg IP). Locomotion was monitored for the following 120 minutes. We found that the 5, but not the 3 mg/kg, dose of CNO reduced cocaine-induced locomotion. Thus, studies utilizing DREAADs to probe cocaine-induced behavior should consider these findings when choosing a dose of CNO and include non-DREADD CNO controls.
Keywords: DREADDs, reinstatement, circuitry, CNO, addiction, relapse
Cocaine addiction is a significant public health concern, and no FDA-approved drugs currently exist for its treatment. Identifying the underlying neurocircuitry of cocaine-seeking is necessary in order to determine how cocaine hijacks such circuitry to bias behavior towards drug-seeking. Thus, animal models of various aspects of cocaine addiction are commonly utilized to investigate such circuitry.
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are novel tools for the dissection of circuitry mediating addiction-related behavior. DREADDS rely on viral vectors or transgenic approaches to express designer g-protein coupled receptors to drive the three canonical g-protein signaling cascades. DREADDs that are mutants of the muscarinic M3 or M4 receptor were designed to be exclusively activated by clozapine-N-oxide (CNO) to permit temporally-precise manipulation of specific brain regions [1]. CNO was chosen as a ligand due to reports that it is inert [2]. However, CNO has recently been shown to inhibit amphetamine-induced locomotion and the accompanying evoked dopamine (DA) release in the nucleus accumbens of Long-Evans rats [3]. This effect was dose-dependent, with 5 mg/kg CNO inhibiting both locomotion and evoked DA and 2 mg/kg having no effect on either measure. These effects may have been due to the reverse metabolism of CNO to its parent compound, clozapine, as both clozapine and its metabolite N-desmethylclozapine were found in the plasma of these rats. The finding of reverse metabolism is in agreement with previous reports in humans and Lewis rats [4,5]. In fact, a recent report finds that it is clozapine, and not CNO, that is the ligand driving DREADD activity [6]. Aside from actions as a ligand for muscarinic DREADD receptors, clozapine is an antipsychotic compound that has action at more than two dozen receptors in the CNS, most notably DA2 and serotonin 5-hydroxytryptamine (5-HT) 2a receptors (for review see [7]). The pharmacology of N-Des is not as well studied as clozapine, however, it has been shown to act as an agonist at delta opioid receptors, M1, and 5-HT1a receptors [8,9].
The ability of CNO administration to produce neurochemical and behavioral effects independent of binding to DREADDs is a potential concern for studies intending to use these systems to probe cocaine-induced behavior. We and others utilize the extinction-reinstatement model of cocaine relapse. This model involves the operant intravenous self-administration of cocaine, followed by a period of extinction training in which the previously reinforced response is no longer reinforced. As the operant response declines in frequency, animals can be tested for reinstatement of the drug-seeking response, which is akin to a relapse event in humans. A number of stimuli induce the reinstatement of the cocaine-seeking response (e.g. cue, stress), including a non-contingent injection of cocaine itself. In order for muscarinic receptor-based DREADDs to be utilized for the dissection of circuitry underlying cocaine-primed reinstatement, doses of CNO administered to induce DREADD signaling must themselves have no effect on cocaine-induced behavior.
Here we tested the ability of CNO administration (3 and 5 mg/kg, IP) to attenuate cocaine-induced locomotion in male Sprague-Dawley rats, a strain commonly utilized for preclinical reinstatement studies. These doses were chosen based on their use in such studies previously [10,11]. While it was recently shown that 5 mg/kg CNO did not alter cocaine-induced locomotion in cocaine-naïve male Sprague-Dawley rats [10], here we utilized rats with a history of cocaine self-administration and extinction training, as this would be the history of rats tested for cocaine-primed reinstatement using DREADD technology.
Methods
Subjects
Eight adult male Sprague Dawley rats (Charles River, 300–350 g; Raleigh, NC, USA) were housed in a temperature-controlled vivarium. Rats were maintained on a 12 hr reverse-light cycle and all procedures were carried out during the dark phase of the cycle. Animals were provided 20 grams of standard lab chow daily and water was available ad libitum. All work was approved by the Institutional Animal Care and Use Committees of the University of Florida.
Drugs
Both cocaine and CNO were provided by the NIDA Controlled Substances Program (Research Triangle Institute, NC, USA). Cocaine was dissolved in 0.9% physiological saline at a concentration of 4 mg/mL. CNO was dissolved in 0.5% DMSO in saline at 3 or 5 mg/mL concentrations.
Surgery
Ketamine (87.5 mg/ kg, i.p.) and xylazine (5 mg/kg, i.p.) were utilized as anesthetics and were administered in a volume of 1 mL/kg. Catheters (SILASTIC silicon tubing, ID 0.51 mm, OD 0.94 mm, Dow Corning, Midland, MI) were implanted and secured into the jugular vein with suture thread (Surgical Specialties Corp., Wyomissing, PA). The catheter tubing passed subcutaneously and exited from the back where it was connected to a cannula (Plastics One, Roanoke, VA, USA) embedded in a rubber harness (Instech, Plymouth Meeting, PA, USA) worn by the rat for the duration of the self-administration. Keterolac was administered on the day surgery and for the following three days to provide analgesia. Heparin (100 units/mL in 0.1 mL) was administered for the duration of self-administration.
Cocaine self-administration and extinction training
After a 5–7 day period of recovery, all rats were trained to self-administer cocaine in standard 2-lever operant chamber (Med Associates, St. Albans, VT). Self-administration occurred under a fixed ratio-1 (FR-1) schedule of reinforcement during daily 2 hr sessions. Presses on the active lever delivered intravenous cocaine infusion (0.35 mg/infusion in 0.1 mL). Infusions were paired with a tone (2900 Hz tone) and illumination of a stimulus light above the lever. Infusions were followed by a 20 second time out period, during which time lever presses were recorded, but did not result in delivery of cocaine or cues. Presses on the inactive lever were not reinforced but were recorded. Upon meeting self-administration criteria (9 or more infusions for 12 days), rats began extinction training during which time levers were presented but presses resulted in no programmed consequences. Following 14–21 days of extinction training, rats began locomotor testing. This timing was chosen, as it is when reinstatement testing frequently occurs (e.g. [12,13]).
Locomotor Testing
Locomotor testing was conducted in plexiglass automated activity chambers (16 × 16 × 15 inches) with Photobeam Activity Software for automated locomotor behavior rating (San Diego Instruments, San Diego, CA). Rats were habituated to the chambers for 1 hour on the day prior to beginning the locomotor testing. Subsequently, rats were tested for cocaine-induced locomotion following an injection of CNO (0, 3 or 5 mg/kg IP, administered in a volume of 1 mL/kg), using a within-subjects design. Rats were injected with each dose in a counterbalanced manner; tests were separated by 2–3 days. For each test, the CNO was administered and rats were placed into the locomotor chamber and behavior was recorded. Twenty minutes later, cocaine (10 mg/kg IP administered in a volume of 1 mL/kg) was injected and rats were placed back into the chamber for 120 minutes.
Statistical Analysis
Data was analyzed using GraphPad Prism (version 6.00, GraphPad Software, La Jolla, CA). An alpha level of p≤0.05 was set for all statistical analyses. Repeated Measure (RM) analyses of variance (ANOVA) was used to compare locomotor between Doses, with both Time and Dose as within-subjects factors. Tukey post-hoc analyses that controlled for multiple comparisons were used. G*power3.1 was used to conduct a post hoc assessment of power.
Results
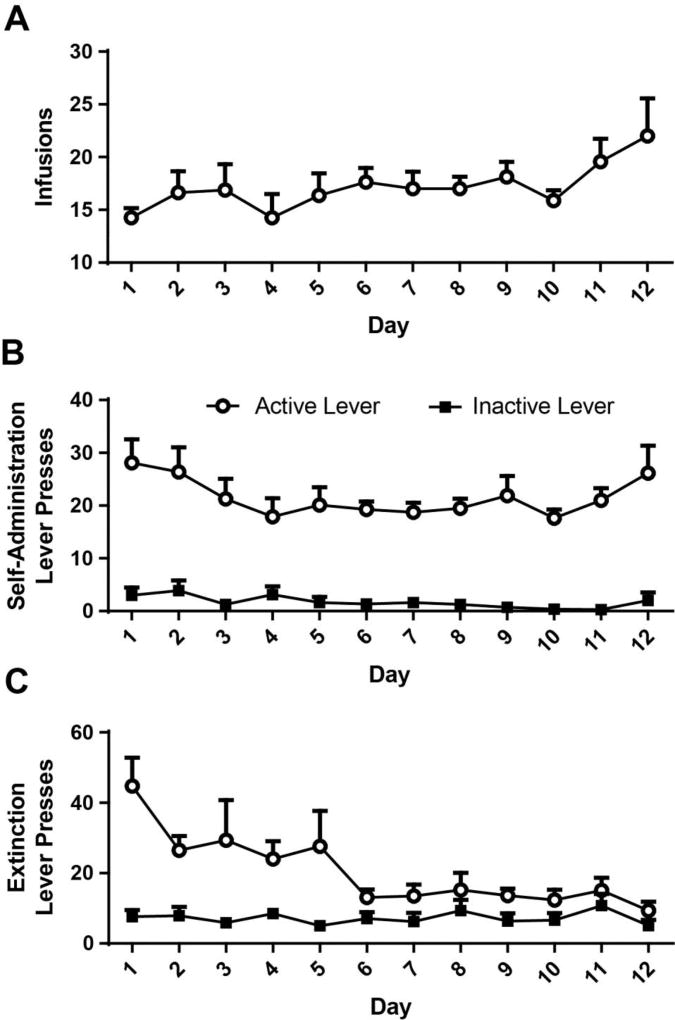
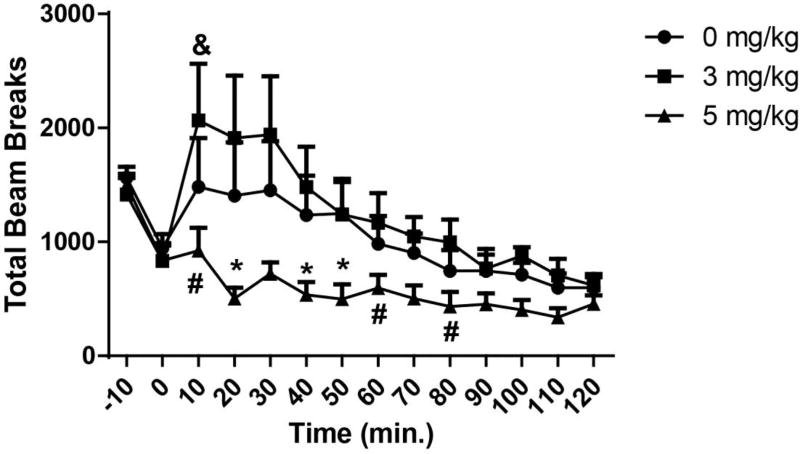
All eight rats met self-administration criteria for infusions (Fig. 1A), and discriminated between active and inactive levers during self-administration (Fig. 1B) and extinction training (Fig. 1C). The dependent variable for locomotor behavior was total number of beam crossings, summed in 10 minute intervals. Data sets from one rat, for the 3 and 5 mg/kg doses of CNO, contained values more than two standard deviations from the mean and thus this rats’ data were eliminated from further data analysis. A significant Dose×Time interaction was detected [F (26, 156) = 2.130, p=0.0025]. A significant main effect of Time was also detected [F (13, 78) = 11.78, p<0.0001]. A trend towards a significant effect of Dose was found [F (2, 12) = 3.227 p=0.0756]. Post-hoc tests found that at 20, 40 and 50 minutes post-cocaine injection, locomotor behavior was reduced by the 5 mg/kg dose relative to both 0 and 3 mg/kg CNO (p<0.05). At 10, 60, and 80 minutes post-cocaine, 5 mg/kg CNO reduced locomotion relative only to the 3 mg/kg dose. At 10 minutes, the 3 mg/kg dose increased locomotion relative to the 0 mg/kg dose. As the n for this study was only 7, we determined effect size and conducted a post-hoc power analysis with G*power. The effect size for the Dose×Time interaction was 0.5446, which is considered to be a large effect size. We next used our effect size to calculate power for this comparison, finding that our power was sufficient, at 0.9999.
Fig. 1. Rat self-administration and extinction data.
Panel A. Mean number of infusions attained across the 12 day self-administration period. Panel B. Mean number of active and inactive lever presses during cocaine self-administration. Panel C. Mean number of active and inactive lever presses during the first 12 days of extinction training.
Discussion
Here we report that 5 mg/kg CNO attenuated cocaine-induced locomotion in rats with a history of cocaine self-administration (Fig. 2). Our findings agree with those of MacLaren et al., [3], who found that this dose, but not a lower one, attenuated amphetamine-induced locomotion. However, this is in contrast with a recent report that conducted a similar experiment in cocaine-naïve Sprague-Dawley rats, finding no change in cocaine-induced locomotion after 5 mg/kg CNO [10]. This is likely explained by the well-established effect of prior history of cocaine exposure on the glutamatergic and dopaminergic systems responsible for cocaine-induced locomotion [14]. Furthermore, based on the previous report that the ability of CNO to attenuate amphetamine-induced locomotion was due to suppression of evoked NAc DA, we propose that this is likely the neurochemical effect underlying the behavioral result observed here. It is also possible that the back metabolism of CNO to clozapine occurred. Clozapine is a D2 antagonist, and recently a D2 agonist was shown to enhance cocaine-induced locomotion through action in the VTA [15]. Thus, clozapine’s actions at D2 receptors may be responsible for the reduction in locomotion observed here. Neither our results, nor those of MacLaren et al. [3], have found that CNO doses between 2 and 5 mg/kg alter spontaneous locomotion in the absence of DREADD expression. Thus, the current evidence suggests that CNO alters psychostimulant–induced hyperlocomotion only.
Fig. 2. CNO at 5 mg/kg but not 3 mg/kg (IP) attenuated cocaine-induced locomotion.
Rats were administered CNO (0, 3, 5 mg/kg IP) utilizing a within-subjects, counterbalanced design. Each dose was tested on a different day, with at least two days separating tests. Rats were injected with CNO and placed into the locomotor chamber for 20 min prior to receiving cocaine (10 mg/kg IP). * = p<0.05 compared to both 0)0 and 3 mg/kg CNO doses. # = p<0.05 relative to 3 mg/kg CNO. & = p<0.05 relative to 0 mg/kg only. N=7.
The ability of CNO to attenuate cocaine-primed reinstatement of cocaine-seeking in rats with hM4Di expressed in the caudal ventral pallidum is dose-dependent, with 20 but not 10 mg/kg CNO attenuating reinstatement [16]. The present results would indicate that this effect on reinstatement may have been due to the ability of CNO to inhibit locomotion. Importantly, the ability of 5 mg/kg CNO to attenuate cocaine-induced locomotion is dissociable from its ability to attenuate cocaine-primed reinstatement. Wunsch et al., (2017) demonstrated that 5 mg/kg CNO in combination with AAV-hM4Di expressed in the midline thalamic nuclei (MTN) attenuates cocaine-primed reinstatement. However, 5 mg/kg CNO administered to rats in which either the posterior MTN-NAc projection or MTN-basolateral amygdala were targeted with cre-dependent AAV-hM4Di resulted in no change in cocaine-primed reinstatement relative to vehicle-injected rats. Thus, it seems that motivation to seek cocaine can overcome the locomotor-suppressing effects of 5 mg/kg CNO.
It is possible that age, sex, strain and species may influence CNO effects, and the results obtained here may only apply to adult male Sprague-Dawley rats. However, adult, male Sprague-Dawley rats are typically used for studies of cocaine-primed reinstatement. When selecting the optimal CNO dose for the study of cocaine-induced behavior, we recommend obtaining a physiological readout of whether a lower dose of CNO (3 mg/kg or less) works in the particular DREADD system via electrophysiology or fos expression prior to beginning behavioral studies. For example, Nation et al., (2016) recently confirmed that 3 mg/kg CNO was effective at inducing fos expression following HM3D(Gq) overexpression in the subfornical organ [16]. Finally, as suggested previously [3], the use of non-DREADD-expressing, CNO-injected controls are necessary when utilizing such systems to investigate cocaine-induced behavior. Alternatively, the use of another DREADD approach may be chosen, such as the modified kappa opioid receptor (KORD) that is activated by the ligand salvinorin B (SALB), which is currently believed to be inert [17,18]. Additional ligands for M3- and M4-based DREADDs are also in development and include compound 21 and perlapine [19]. However, more work is needed to understand the effects of these compounds on behavior. Finally, in addition to DREADDs, other chemogenetic approaches are available, although DREADDS remain the most widely used approach [20].
Highlights.
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are utilized to probe neurocircuitry of behavior and are activated by the ligand clozapine-N-oxide (CNO).
Here we found that CNO itself, in the absence of DREADD expression, attenuated locomotor behavior induced by cocaine
Only a higher dose (5 mg/kg) of CNO suppressed cocaine-induced locomotion and not a lower dose (3 mg/kg)
Acknowledgments
Funding: This work was funded by the National Institute of Drug Abuse DA033436.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Dong S, Allen JA, Farrell M, Roth BL. A chemical-genetic approach for precise spatio-temporal control of cellular signaling. Mol. Biosyst. 2010;6:1376–1380. doi: 10.1039/c002568m. [DOI] [PubMed] [Google Scholar]
- 2.Alves-Rodrigues A, Leurs R, Willems E, Timmerman H. Binding of clozapine metabolites and analogues to the histamine H3 receptor in rat brain cortex. Arch. Pharm. (Weinheim) 1996;329:413–416. doi: 10.1002/ardp.19963290808. [DOI] [PubMed] [Google Scholar]
- 3.MacLaren DAA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, et al. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. Eneuro. 2016;3 doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin G, McKay G, Midha KK. Characterization of metabolites of clozapine N-oxide in the rat by micro-column high performance liquid chromatography/mass spectrometry with electrospray interface. J. Pharm. Biomed. Anal. 1996;14:1561–1577. doi: 10.1016/0731-7085(96)01738-4. [DOI] [PubMed] [Google Scholar]
- 5.Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328:243–250. [PubMed] [Google Scholar]
- 6.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science (80-.) 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nucifora FC, Mihaljevic M, Lee BJ, Sawa A. Clozapine as a model for antipsychotic development. Neurotherapeutics. 2017;14:750–761. doi: 10.1007/s13311-017-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olianas MC, Dedoni S, Ambu R, Onali P. Agonist activity of N-desmethylclozapine at delta-opioid receptors of human frontal cortex. Eur. J. Pharmacol. 2009;607:96–101. doi: 10.1016/j.ejphar.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Odagaki Y, Kinoshita M, Ota T. Comparative analysis of pharmacological properties of xanomeline and N-desmethylclozapine in rat brain membranes. J Psychopharmacol (Oxford) 2016;30:896–912. doi: 10.1177/0269881116658989. [DOI] [PubMed] [Google Scholar]
- 10.Wunsch AM, Yager LM, Donckels EA, Le CT, Neumaier JF, Ferguson SM. Chemogenetic inhibition reveals midline thalamic nuclei and thalamo-accumbens projections mediate cocaine-seeking in rats. Eur. J. Neurosci. 2017;46:1850–1862. doi: 10.1111/ejn.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM. Corticostriatal Afferents Modulate Responsiveness to Psychostimulant Drugs and Drug-Associated Stimuli. Neuropsychopharmacology. 2016;41:1128–1137. doi: 10.1038/npp.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaCrosse AL, O’Donovan SM, Sepulveda-Orengo MT, McCullumsmith RE, Reissner KJ, Schwendt M, et al. Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression. J. Neurosci. 2017;37:5809–5821. doi: 10.1523/JNEUROSCI.3717-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaCrosse AL, Hill K, Knackstedt LA. Ceftriaxone attenuates cocaine relapse after abstinence through modulation of nucleus accumbens AMPA subunit expression. Eur. Neuropsychopharmacol. 2016;26:186–194. doi: 10.1016/j.euroneuro.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalivas PW, Striplin CD, Steketee JD, Klitenick MA, Duffy P. Cellular mechanisms of behavioral sensitization to drugs of abuse. Ann. N. Y. Acad. Sci. 1992;654:128–135. doi: 10.1111/j.1749-6632.1992.tb25961.x. [DOI] [PubMed] [Google Scholar]
- 15.Koulchitsky S, Delairesse C, Beeken T, Monteforte A, Dethier J, Quertemont E, et al. Activation of D2 autoreceptors alters cocaine-induced locomotion and slows down local field oscillations in the rat ventral tegmental area. Neuropharmacology. 2016;108:120–127. doi: 10.1016/j.neuropharm.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Nation HL, Nicoleau M, Kinsman BJ, Browning KN, Stocker SD. DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite. J. Neurophysiol. 2016;115:3123–3129. doi: 10.1152/jn.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vardy E, Robinson JE, Li C, Olsen RHJ, DiBerto JF, Giguere PM, et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron. 2015;86:936–946. doi: 10.1016/j.neuron.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K, et al. Behavioral and Physiological Effects of a Novel Kappa-Opioid Receptor-Based DREADD in Rats. Neuropsychopharmacology. 2016;41:402–409. doi: 10.1038/npp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Choo H, Huang X-P, Yang X, Stone O, Roth BL, et al. The first structure-activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem. Neurosci. 2015;6:476–484. doi: 10.1021/cn500325v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]