Abstract
Introduction
We evaluated the association between neighborhood socioeconomic status (NSES) and sleep quality on cognitive decline in the Health and Retirement Study (HRS).
Methods
HRS participants (n=8090), aged 65+ with DNA and multiple biennial cognitive observations (abbreviated Telephone Interview for Cognitive Status) were included. Participants were grouped into quartiles of NSES and sleep quality scores. We adjusted for APOE ε4, demographic, and cardiovascular risk factors. Random effects modeling evaluated cognitive change over time.
Results
NSES and sleep were significantly associated with cognitive decline, and there was a significant interaction between them (p=0.02). Significant differences between high/low NSES and high/low sleep quality (p<.0001) were found.
Conclusions
Sleep and NSES were associated with cognitive decline; the association between sleep and cognition appeared stronger among those with low NSES. The association between low NSES, poor sleep quality, and cognitive decline was roughly equivalent to the association between APOE ε4 and cognitive decline.
Keywords: Sleep hygiene, socioeconomic factors, cognitive dysfunction, cognitive decline, cohort study, APOE
Introduction
The past decade has witnessed growing interest in the effects of sleep on cognitive functioning. A critical factor in overall health and well-being, sleep allows for the consolidation of memory and integration of learning1,2 as well as maintenance of brain plasticity.1 Conversely, sleep disruption or deprivation is associated not only with impaired hippocampal functioning,2 but also greater amyloid-β (Aβ) burden 3–6 and subsequent risk for cognitive decline and dementia.3–7 For instance, one study found that waking after falling asleep and having long episodes of wakefulness were each associated with approximately 40% higher odds of cognitive decline.8 Prospective data show that not obtaining enough sleep increases a person’s odds for dementia or mild cognitive impairment (MCI) by 36%.9 Cognitive decline may be attenuated through better sleep consolidation.10 Sleep disturbance is also a symptom of cognitive impairment, suggesting a bidirectional relationship.11,12
Evidence also documents the relationship between neighborhood socioeconomic status (NSES) and sleep outcomes. NSES describes the relative advantage of a community based on factors like the education, employment status, and financial status of its constituents. Living in a disadvantaged neighborhood versus a privileged one has been associated with increased daytime sleepiness,13 higher odds of restless sleep,14 worse sleep quality,15 and greater likelihood of waking after sleep onset.16
Older adults are more likely to be impacted by their local environments,17–19 and thus are more vulnerable to environmental challenges.17,20 The relationship between neighborhoods and health has been demonstrated in a number of studies21,22 as has the relationship between neighborhood characteristics, cognitive function23 and cognitive decline.24–26
The body of empirical knowledge connects sleep disturbance and neighborhood disadvantage, independently, to cognitive decline. The further association between neighborhood disadvantage and poor sleep outcomes suggests that these factors may act synchronously in the etiology of cognitive decline. To date, no research has tested this compelling argument. The present manuscript uses data from the Health and Retirement Study (HRS) to estimate the combined contribution of neighborhood socioeconomic status and sleep quality to cognitive function.
Methods
The Health and Retirement Study (HRS) 27 was established in 1990 to understand the health-related challenges and successes of Americans aged 50 and older. In 1998, the HRS was merged with the Asset and Health Dynamics Among the Oldest Old (AHEAD) study, which began in 1993. The War Baby Study and the Children of the Depression study were also added to the HRS, producing a large (>37,000 person) cohort representative of the United States population aged 50 and over. The Survey Research Center at the University of Michigan conducts the biennial, in-depth interviews with this cohort. Once participants reach 65 years of age, cognitive tests are included in the biennial interviews. The Survey Research Center obtains informed consent from all participants: oral consent for telephone interviews and written consent for those providing biological samples. Returns of mailed surveys infer consent. The University of Michigan Institutional Review Board approved the HRS study protocol. IRB approvals for the current project was obtained from the Wake Forest School of Medicine and the Duke University Medical Center. The following inclusion criteria were applied, participants must be aged 65 and older, must have cognitive data available, and must have provided DNA samples for genotyping in either 2006 or 2008.
Cognitive Assessment
Cognitive function was assessed with a modified version of the Telephone Interview for Cognitive Status (TICS),28 adapted for use in the HRS (range 0–35 points). The TICS correlates strongly with the Mini-Mental State Examination (MMSE);29 and the sensitivity and specificity of the TICS for identifying cases of dementia have been well-documented.30
Neighborhood Socio-Economic Status (NSES)
We used a NSES index that was produced by the RAND Corporation,31 using 6 key neighborhood factors including the percentage of adults aged 25 or older without a high school diploma; percentage of male unemployment; percentage of households with income below the poverty line; percentage of households on public assistance; percentage of female heads of household; and median household income. The index was derived at the level of census tract, generating values from 0 to 100 that are applied to each participant. It has been used in numerous prior studies on the association between NSES and health and cognition.23,32,33
Sleep Assessment
In 2006, the HRS administered a set of questions on sleep quality that were closely aligned with the previously validated Women’s Health Initiative Insomnia Rating Scale34,35 (Table 1). Questions were focused on whether participants had trouble falling asleep, staying asleep, waking too early, and feeling well rested upon waking. These four questions were coded such that higher scores indicated better sleep and lower scores indicated more impaired sleep. Participants who did not complete all four questions were excluded (n=114). Sleep scores and NSES scores were transformed to z-scores so that they were both on a standard deviation unit scale.
Table 1.
Sleep Scale
|
| Responses: Most of time, sometimes, rarely, don’t know/blank |
Item #4 was reverse coded so that higher numbers indicated better sleep.
APOE Genotyping
In 2006 and 2008, HRS investigators requested DNA samples from the cohort. Saliva samples were collected from participants who agreed, and GWAS analyses continued through 2013. Full details of the genotyping methods used have been reported.36 DNA analysis was performed using Human610-Quad BeadChip (Illumina, Inc., San Diego, CA). The database of Genotypes and Phenotypes (dbGaP) houses the study’s genetic data. The corresponding genetic data were processed using PLINK.37 APOE genotypes were imputed from the HRS GWAS data using the 1000 genomes reference dataset to impute gene dosages for the SNPs rs7412 and rs429358. The details of the imputation process are provided in documentation from the HRS study at: http://hrsonline.isr.umich.edu/sitedocs/genetics/candidategene/FileDescription_CognitionBehavior.pdf Subjects with posterior probabilities for imputation < 0.8 for either SNP were excluded.
Demographic Covariates
Demographic variables were considered, including age, sex, and education level in years. We adjusted for both body mass index (BMI) as a continuous, time varying variable and 4 obesity categories at baseline (BMI<=25; 25<BMI<30; 30<=BMI<40; BMI>=40). We combined underweight (n=67) and normal weight participants into one group due to small numbers. This modeling approach was taken, as the effects of weight on cognitive function are complex. Midlife obesity has been demonstrated as a risk factor for cognitive impairment in some studies,38 while others show that weight loss in late life is also a risk factor.39 Severe obesity (BMI>=40) appears to have negative effects on cognitive function even in later life.40 Our approach was designed to capture the effect of obesity at age 65 in addition to the incremental changes in BMI over the course of the study. Race/ethnicity was coded as white vs. other. African-Americans (n=995) were combined with other ethnicities as this group was small (n=161). Cardiovascular health was assessed with age-varying covariates representing the presence or absence of hypertension, diabetes, stroke, and any heart disease (including heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems). In order to be coded as having hypertension or diabetes, participants needed to endorse the condition as diagnosed by a doctor and indicate that they were taking medication for the condition.
Analytic Approach
Each participant’s baseline visit was defined as the first visit where the participant was age 65 or older because HRS participants do not receive serial cognitive assessments until the age of 65. We tested sleep as a simple mediator between NSES and cognitive decline.41 We then derived standardized scores for NSES and Sleep, and each variable was divided into four quartiles, creating four mutually exclusive groups. We grouped those who were in the lowest quartiles for NSES and sleep into group 1; those with low NSES and good sleep formed group 2; participants in the highest quartiles of NSES but with poor sleep were placed in group 3; and group 4 included those with high NSES and good sleep. Group 4 was used as the reference category in the analyses.
We compared participants’ demographic characteristics across the four mutually exclusive NSES (high/low) and Sleep (good/poor) groups using χ2 tests or generalized linear models (SAS PROC GLM). Comparisons were also made between those included in the study and those excluded due to missing data. Logistic regression was used to ascertain the relationship between NSES and sleep. Repeated measures fixed effects models were used to estimate trajectories of cognitive performance based on serial administration of the HRS TICS. This approach captures individual differences in cognitive performance over time while accounting for correlations in repeated measures. The dependent variable was TICS score and independent variables included the four mutually exclusive groups of participants. We adjusted models for age, sex, education level, APOE ε4 carrier status race, BMI, obesity status, and time-varying covariates for hypertension, diabetes, stroke, and any heart disease. Interaction terms between NSES and sleep quality were tested in models with NSES and Sleep as continuous variables. Due to the association between APOE and sleep apnea42 we also tested an interaction term for APOE ε4 carrier status and sleep.
Results
A total of 12,507 participants who contributed DNA sampled in 2006 to 2008 were eligible for inclusion in the study. Of those participants, a total of 8,709 had APOE genotype data and were age 65 or older, as that is the point at which cognitive data was collected at regular two-year intervals. Of the 8,709, a total of 619 observations were dropped due to missing data (198 missing NSES, 161 missing APOE, 114 missing sleep report, 80 missing BMI, 14 missing hypertension status; 30 missing diabetes, and 22 missing stroke). Comparisons between included and excluded participants can be found in supplemental Table 1. There were no differences between groups in mean sleep ratings, but the excluded group had lower mean NSES scores, was slightly older, and had lower levels of education on average. Fewer excluded participants were APOE ε4 carriers.
Table 2 includes a comparison of 8,090 participants included in the study across four mutually exclusive NSES/Sleep quality groups. Participants with lower NSES scores were slightly older and had a higher frequency of APOE ε4 carriers. The group with low NSES and poor sleep also had the highest frequency of participants with health problems including hypertension, diabetes, stroke, heart disease, and obesity. The higher NSES groups had higher levels of education, higher frequencies of white participants, and lower average BMI levels. Overall there were more female participant than males and specifically, more females in the groups reporting poor sleep.
Table 2.
Demographic Characteristics of 8,090 HRS Participants
| Characteristic | 1 Low NSES Poor Sleep |
2 Low NSES Good Sleep |
3 High NSES Poor Sleep |
4 High NSES Good Sleep |
Total | p-value |
|---|---|---|---|---|---|---|
| n | 1,536 | 1,772 | 2,049 | 2,733 | 8,090 | |
| Age (SD) | 67.9 (3.7) | 67.9 (3.6) | 67.4 (3.5) | 67.1 (3.0) | 67.5 (3.4) | <.0001 |
| Female Sex (%) | 1,014 (66.0) | 984 (55.5) | 1,263 (61.6) | 1,402 (51.3) | 4,663 (57.6) | <.0001 |
| Education (yrs; SD) | 11.1 (3.5) | 11.5 (3.5) | 12.9 (2.7) | 13.3 (2.7) | 12.4 (3.2) | <.0001 |
| Race (White; %) | 1,129 (73.5) | 1,298 (73.3) | 1,934 (94.4) | 2,573 (94.2) | 6,934 (85.7) | <.0001 |
| APOE ε4+ (%) | 425 (27.7) | 511 (28.8) | 505 (24.7) | 726 (26.6) | 2,167 (26.8) | 0.01 |
| BMI (SD) | 28.6 (5.8) | 27.9 (5.2) | 27.5 (5.1) | 27.2 (5.0) | 27.7 (5.3) | <.0001 |
| Hypertension†(%) | 866 (56.4) | 876 (49.4) | 1,050 (51.2) | 1,199 (43.9) | 3,991 (49.3) | <.0001 |
| Diabetes†(%) | 299 (19.5) | 257 (14.5) | 264 (12.9) | 287 (10.5) | 1,107 (13.7) | <.0001 |
| Stroke†(%) | 75 (4.9) | 71 (4.0) | 61 (3.0) | 79 (2.9) | 286 (3.5) | <.0001 |
| Any Heart†*(%) | 415 (27.0) | 390 (22.0) | 519 (25.3) | 569 (20.8) | 1,893 (23.4) | <.0001 |
| Normal weight (BMI<25)(%) | 402 (26.2) | 513 (29.0) | 649 (31.7) | 970 (35.5) | 2534 (31.3) | <.0001 |
| Overweight (25≤BMI<30) (%) | 602 (39.4) | 763 (43.1) | 876 (42.8) | 1088 (39.8) | 3332 (41.2) | |
| Obese (30≤BMI<40) (%) | 467 (30.4) | 451 (25.5) | 471 (23.0) | 615 (22.5) | 2004 (24.8) | |
| Obese (BMI≥40) (%) | 62 (4.0) | 45 (2.5) | 53 (2.6) | 60 (2.2) | 220 (2.7) | |
| Smoking (%) | 850 (55.5) | 1,018 (57.9) | 1,176 (57.7) | 1,571 (57.7) | 4,615 (57.3) | 0.440 |
Abbreviations: APOE= Apolipoprotein E gene; BMI=body mass index; HRS=Health and Retirement Study; NSES=Neighborhood socio-economic status; SD=standard deviation.
Cardiovascular variables are reported here as ever/never having the condition rather than baseline status.
Any Heart Condition includes: heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems
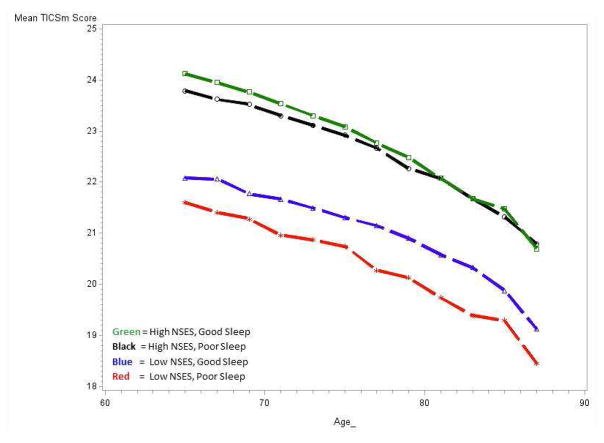
Simple mediation was tested using the methods of Baron and Kenny.41 NSES and sleep were nominally but significantly associated with each other in an unadjusted model (Odds Ratio: 1.07, 95% CI: 1.02, 1.11) but did not remain significant when covariates were added. In a mixed effects model testing interaction terms with sleep scale and NSES modeled as continuous variables, the interaction term was significant (−0.08, p-value=0.02) as was the APOE by sleep interaction term (−0.17, p-value=0.03). Model fit statistics (-2LL, AIC, BIC) indicated that our decision to include both BMI and obesity in the models provided the best fit when compared to either term alone. The results of models with four mutually exclusive NSES by sleep groups are shown in Table 3 and plotted Figure 1. Compared to participants with high NSES and good sleep, those with the lowest NSES and poor sleep showed significantly greater cognitive decline over time (−0.53, 95% CI: −0.73, −0.32). Figure 1 illustrates that high and low NSES appear to drive the distinctions between the four groups. Models stratified by APOE ε4 carrier status, shown in Figures 2a and b further demonstrate that the association was also driven by individuals who were not APOE ε4 carriers. Among APOE ε4 carriers, there were no statistically significant differences between the four groups. Model results by APOE ε4 carrier status are shown in Supplemental Table 2.
Table 3.
Estimates from Mixed Effects Models with NSES/Sleep in 4 Mutually Exclusive Groups (n=8090).
| Parameter | Estimate | Confidence Interval | p-Value |
|---|---|---|---|
| Intercept | 24.90 | (23.42, 26.38) | <0.0001 |
| Age | −0.22 | (−0.24, −0.20) | <0.0001 |
| Sex (female) | 0.83 | (0.69, 0.97) | <0.0001 |
| Education | 0.58 | (0.56, 0.60) | <0.0001 |
| Race (white) | 2.53 | (2.32, 2.74) | <0.0001 |
| BMI | 0.11 | (0.10, 0.13) | <0.0001 |
| Diabetes | −0.87 | (−1.02, −0.73) | <0.0001 |
| Hypertension | −0.98 | (−1.09, −0.88) | <0.0001 |
| Any Heart Condition* | −1.07 | (−1.18, −0.95) | <0.0001 |
| Stroke | −2.09 | (−2.29, −1.90) | <0.0001 |
| Normal Weight (BMI<25) | Reference | - | <0.0001 |
| Overweight (25≤BMI<30) | −0.06 | (−0.24, 0.12) | 0.50 |
| Obese (30≤BMI<40) | −0.56 | (−0.81, −0.32) | <0.0001 |
| Obese (BMI≥40) | −1.92 | (−2.47, −1.37) | <0.0001 |
| APOE ε4+ | −0.57 | (−0.73, −0.42) | <0.0001 |
| Sleep NSES 1 | −0.53 | (−0.73, −0.32) | <0.0001 |
| Sleep NSES 2 | −0.27 | (−0.46, −0.08) | 0.006 |
| Sleep NSES 3 | 0.02 | (−0.16, 0.20) | 0.806 |
| Sleep NSES 4 | Reference | - | - |
Abbreviations: BMI= body mass index; Sleep NSES 1= poor sleep and low NSES; Sleep NSES 2= good sleep and low NSES; Sleep NSES 3= poor sleep and high NSES; Sleep NSES 4= good sleep and high NSES.
Any heart condition includes: heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems
Figure 1.
Cognitive Trajectories Plotted by Age and NSES/Sleep Group Status
Figure 2.
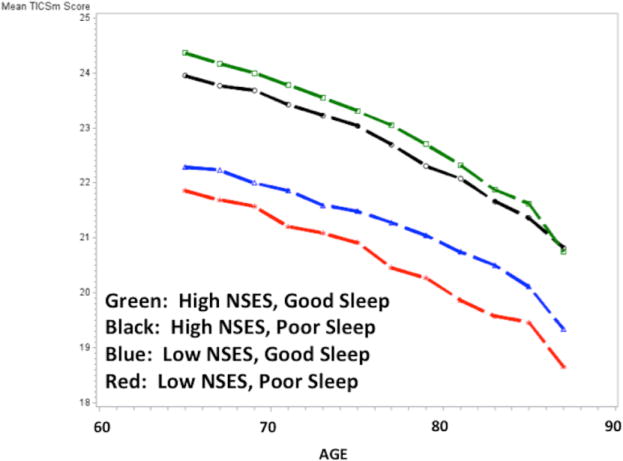
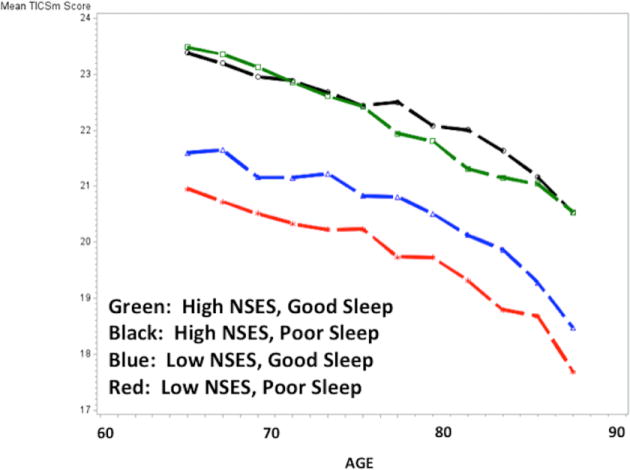
Figure 2a. Cognitive Trajectories Plotted by Age and NSES/Sleep Group Status; Participants without the APOE ε4 Allele.
Figure 2b. Cognitive Trajectories Plotted by Age and NSES/Sleep Group Status; Participants with One or More APOE ε4 Alleles.
Discussion
The prevalence of sleep-disordered breathing (SDB) is higher in older adults43 and has increased over the last two decades.44 Sleep quality is commonly affected by the surrounding environment. Air quality, noise pollution, proximity to busy roads, and neighborhood characteristics all factor into the sleep environment. In the current study, we have shown that the combined effects of sleep quality and neighborhood characteristics as measured by the NSES are associated with poorer cognitive function over time. Individuals who lived in more impoverished neighborhoods but reported good sleep quality demonstrated better cognitive function than those in impoverished neighborhoods who reported poor sleep quality. The groups with the highest cognitive function were those who were in neighborhoods with higher NSES regardless of reported sleep quality. The low NSES and poor sleep quality group experienced the greatest cognitive decline over time, and the combined effects of poor sleep quality and low NSES on cognition was roughly equivalent to the magnitude of carrying one or more APOE ε4 alleles.
APOE ε4 carrier status is associated not only with Alzheimer’s disease,45 but also with cardiovascular disease46 and sleep apnea.42 Our findings of stronger effects of subjective complaints among APOE ε4 non-carriers appear counter to emerging data on the association between APOE ε4 carrier status and objective measures of sleep.47,48 However, they are in line with one report that included both subjective sleep and objective sleep measures in APOE ε4 carriers.49 Associations were found between objective sleep measures and APOE ε4, but subjective complaints were not associated. The authors suggest that it is possible that objectively measured sleep changes may be detected prior to the onset of subjective complaints. If we had objective measures of sleep in the current study, perhaps our results would have agreed with this report. This discrepancy should be addressed in future prospective studies that have both objective and subjective sleep assessments.
Modifiable Risk Factors for Cognitive Decline
Participants in the lower NSES groups had higher frequencies of obesity at baseline; those in the higher NSES groups had a lower frequency of obesity at baseline and generally lower BMIs over the course of the study. Our findings show a higher prevalence of cardiovascular disease and other cardiovascular risk factors among those with the lowest levels of NSES and those with the poorest sleep reports. This study was not designed to address the temporality of these associations; however previous reports corroborate the association between NSES, sleep and health.15,50 One might speculate that the segment of the population with low NSES also has less access to health care and limited resources to address health problems.
Supplemental Table 2 shows that the association between obesity, diabetes, hypertension, and cognitive decline are stronger among those who carry the APOE ε4 allele, confirming numerous prior reports.51–53 All of these factors: sleep quality,54 neighborhood characteristics,55 and cardiovascular risk factors56 are potentially modifiable and can be intervened upon. In the absence of pharmacological treatments to forestall cognitive decline and dementia onset, interventions on modifiable risk factors have the potential to delay the onset of symptoms and may thereby reduce the prevalence of disease as previous projections suggest.57 Whether such interventions have an impact on specific Alzheimer’s pathologies is an area that requires more research, although a recent report suggests that midlife vascular risk factors are associated with increased Aβ deposition.58 Even if such interventions acted independently of AD pathology, there is still a potential for reduction in prevalence of dementia as a projection study suggested that even elimination of AD pathology would only reduce the number of dementia cases by 50%.59
Limitations
There are several limitations that should be noted. NSES was considered at only one time point and it is possible that participants may have moved during the follow-up period. The sleep scale we used to evaluate sleep quality is not a validated instrument, although the questions are nearly identical to the Women’s Health Initiative Insomnia Rating Scale34 which has been validated.35 We used the sleep assessment from the 2006 exam to maximize the number of responding participants. This limits our ability to assess temporality of these relationships. Using the limited data available, we evaluated correlations between the 2006 sleep reports and earlier interviews (2002, 2004). We found that among those who provided several self-reported sleep measures, their ratings were highly correlated, suggesting stability among sleep quality ratings. Cardiovascular health and disease status were self-reported, although when possible, we corroborated the self-report with consistent self-report of medication use to treat each condition. There were relatively few instances where a participant reported a condition without the use of medication to treat the condition. If an individual reported a condition with corroborating medication usage, we counted the condition as present for the remainder of the follow-up period. This was done as participants may fail to report conditions consistently during later follow-up due to onset of cognitive problems or perhaps in some cases, conditions resolved. In either case, the presence of a previously reported midlife cardiovascular condition was considered for our purposes, as a risk factor for later cognitive problems. Several medications are known to impact sleep including drugs for blood pressure, high cholesterol, heart disease, and memory medications. These data are available for a subset of HRS participants in a sensitive data supplement to which we did not have access. We were therefore unable to control for these factors. Finally, there were a number of significant differences in demographic characteristics across groups. While we adjusted for these differences, it is possible that there were residual effects for which we cannot adjust.
Conclusions
We know of few reports examining the association between neighborhood environments, sleep, and cognitive function although these relationships have considerable face validity. How and when over the life course NSES or sleep problems have their most powerful impact is unknown. More detailed and longitudinal characterization of environments may help resolve this issue. Evidence for the association between sleep and cognitive function is growing but little is known about factors that impact sleep. Objective evaluation of sleep including measures of sleep disturbance, apnea, early waking, and hypoxia are needed as these factors may be differentially associated with specific elements of individuals’ environment.
More detailed characterization of the environment beyond NSES is needed as well. Light pollution can interfere with sleep; artificial light sources have been associated with increased apnea-hypopnea index scores.60 Future studies should consider noise pollution, proximity to busy roads, and air pollution. Noise from these sources has been tied to changes in sleep structure and continuity.61,62 Air pollution may impact sleep via respiratory disturbances.63 Other factors to consider include usage of medications that may interfere with sleep and those that promote sleep. Dietary factors may also contribute to sleep quality. Numerous recent reports have associated dietary quality with cognitive decline and dementia incidence64–66 but few have evaluated the association between diet and sleep. Diet is relevant in the context of neighborhood environs as access to healthy food is a key feature of one’s residential surroundings.
This initial report on the association between neighborhood and sleep highlights an interaction between two potentially modifiable risk factors for AD. The combined effects of these factors was roughly equivalent in magnitude and direction to the effect of APOE ε4 carrier status on cognitive decline in this cohort. We acknowledge that “modifiable” risk factors may not be easy to change. However, we encourage further study to determine the mechanisms behind these associations and to identify the most malleable and impactful factors for risk reduction.
Supplementary Material
Research In Context.
Systematic Review
We searched Pubmed for articles pertaining to neighborhood socioeconomic status, sleep, and cognitive decline and found no articles matching our search terms. We were able to find research on neighborhood and sleep; neighborhood and cognitive decline; as well as sleep and cognitive decline. Associations between lower neighborhood socioeconomic status and cognitive decline have been shown previously, as have associations between objectively and subjectively measured sleep quality and cognition.
Interpretation
In this preliminary evaluation, we have shown that the association between sleep and cognitive decline may vary depending upon environmental conditions such as neighborhood socioeconomic status. Among individuals with high socioeconomic status, self-reported sleep quality does not appear to influence cognitive decline significantly. Among individuals with low neighborhood socioeconomic status, sleep quality appears to make a significant difference in cognitive function over time.
Future Directions
There are a number of factors that may influence these associations that should be addressed in future studies. Sleep should be evaluated using objective measures of hypoxia, apnea, sleep duration, or sleep disturbances. The influence of neighborhood socioeconomic status on cognitive health should be studied in greater detail as well. Low neighborhood socioeconomic status has a number of additional environmental implications such as air pollution, noise pollution, and proximity to busy roads. These factors have not been studied in conjunction with one another to evaluate their combined associations with cognitive decline. Another key limitation in studies of neighborhood factors is a lack of research into the effect of timing and duration of such exposures. The associations between where we live and how well we sleep are complex and offer potential for intervention. More work needs to be done to better understand these relationships.
Acknowledgments
This work was funded by NIA grant R01 AG042633. The HRS is supported jointly by the National Institute on Aging (NIA U01AG009740) and the Social Security Administration. M.K. is a statistical consultant for Scion NeuroStim, LLC. M.W.L. receives consulting fees from Zinfandel Pharmaceutics and Cabernet Pharmaceutics.
Footnotes
Disclosures:
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gorgoni M, D’Atri A, Lauri G, Rossini PM, Ferlazzo F, De Gennaro L. Is sleep essential for neural plasticity in humans, and how does it affect motor and cognitive recovery? Neural Plast. 2013;2013:103949. doi: 10.1155/2013/103949. Epub 102013 Jun 103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S29–34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 3.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–483. doi: 10.1097/YCO.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, et al. Objectively Measured Sleep and beta-amyloid Burden in Older Adults: A Pilot Study. SAGE Open Med. 2014;2 doi: 10.1177/2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37(4):655–663. doi: 10.5665/sleep.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JC, Espeland MA, Brunner RL, Lovato LC, Wallace RB, Leng X, et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12(1):21–33. doi: 10.1016/j.jalz.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36(7):1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 12.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nature reviews Neurology. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desantis AS, Diez Roux AV, Moore K, Baron KG, Mujahid MS, Nieto FJ. Associations of neighborhood characteristics with sleep timing and quality: the Multi-Ethnic Study Of Atherosclerosis. Sleep. 2013;36(10):1543–1551. doi: 10.5665/sleep.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassett E, Moore S. Neighbourhood disadvantage, network capital and restless sleep: is the association moderated by gender in urban-dwelling adults? Soc Sci Med. 2014;108:185–93. doi: 10.1016/j.socscimed.2014.1002.1029. Epub 2014 Feb 1019. [DOI] [PubMed] [Google Scholar]
- 15.Hale L, Hill TD, Friedman E, Nieto FJ, Galvao LW, Engelman CD, et al. Perceived neighborhood quality, sleep quality, and health status: evidence from the Survey of the Health of Wisconsin. Soc Sci Med. 2013;79:16–22. doi: 10.1016/j.socscimed.2012.07.021. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller-Rowell TE, Curtis DS, El-Sheikh M, Chae DH, Boylan JM, Ryff CD. Racial disparities in sleep: the role of neighborhood disadvantage. Sleep Med. 2016;27–28:1–8. doi: 10.1016/j.sleep.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glass TA, Rasmussen MD, Schwartz BS. Neighborhoods and Obesity in Older Adults: The Baltimore Memory Study. American journal of preventive medicine. 2006;31(6):455–463. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke P, Nieuwenhuijsen ER. Environments for healthy ageing: A critical review. Maturitas. 2009;64(1):14–19. doi: 10.1016/j.maturitas.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Yen IH, Michael YL, Perdue L. Neighborhood Environment in Studies of Health of Older Adults: A Systematic Review. American Journal of Preventive Medicine. 2009;37(5):455–463. doi: 10.1016/j.amepre.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawton MP. Environment and other determinants of well-being in older people. Gerontologist. 1983;23(4):349–357. doi: 10.1093/geront/23.4.349. [DOI] [PubMed] [Google Scholar]
- 21.Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York: Oxford University Press; 2003. [Google Scholar]
- 22.Yen IH, Michael YL, Perdue L. Neighborhood environment in studies of health of older adults: a systematic review. Am J Prev Med. 2009;37(5):455–463. doi: 10.1016/j.amepre.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih RA, Ghosh-Dastidar B, Margolis KL, Slaughter ME, Jewell A, Bird CE, et al. Neighborhood socioeconomic status and cognitive function in women. American journal of public health. 2011;101(9):1721–1728. doi: 10.2105/AJPH.2011.300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke PJ, Weuve J, Barnes L, Evans DA, Mendes de Leon CF. Cognitive decline and the neighborhood environment. Ann Epidemiol. 2015;25(11):849–854. doi: 10.1016/j.annepidem.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffield KM, Peek MK. Neighborhood context and cognitive decline in older Mexican Americans: results from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. American journal of epidemiology. 2009;169(9):1092–1101. doi: 10.1093/aje/kwp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeki Al Hazzouri A, Haan MN, Osypuk T, Abdou C, Hinton L, Aiello AE. Neighborhood socioeconomic context and cognitive decline among older Mexican Americans: results from the Sacramento Area Latino Study on Aging. Am J Epidemiol. 2011;174(4):423–431. doi: 10.1093/aje/kwr095. doi:410.1093/aje/kwr1095. Epub 2011 Jun 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juster FT, Suzman R. An overview of the health and retirement study. Journal of Human Resources. 1995;30:S7–S56. [Google Scholar]
- 28.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614. doi: 10.1001/archneurol.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RAND. Neighborhood SES Index Data Core User’s Documentation Series 2010, 15. 2010. [Google Scholar]
- 32.Glymour MM, Mujahid M, Wu Q, White K, Tchetgen Tchetgen EJ. Neighborhood disadvantage and self-assessed health, disability, and depressive symptoms: longitudinal results from the health and retirement study. Ann Epidemiol. 2010;20(11):856–861. doi: 10.1016/j.annepidem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosso AL, Flatt JD, Carlson MC, Lovasi GS, Rosano C, Brown AF, et al. Neighborhood Socioeconomic Status and Cognitive Function in Late Life. American journal of epidemiology. 2016;183(12):1088–1097. doi: 10.1093/aje/kwv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 35.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychological assessment. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 36.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, et al. Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2010;6(3):265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer’s disease. Curr Opin Clin Nutr Metab Care. 2009;12(1):15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 40.Espeland MA, Luchsinger JA, Baker LD, Neiberg R, Kahn SE, Arnold SE, et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88(21):2026–2035. doi: 10.1212/WNL.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb DJ, DeStefano AL, Foley DJ, Mignot E, Redline S, Givelber RJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63(4):664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Current neurology and neuroscience reports. 2012;12(5):537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 46.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. American journal of epidemiology. 2002;155(6):487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 47.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE epsilon4 carriers. Sleep. 2013;36(6):873–880. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56(1):45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 49.Drogos LL, Gill SJ, Tyndall AV, Raneri JK, Parboosingh JS, Naef A, et al. Evidence of association between sleep quality and APOE epsilon4 in healthy older adults: A pilot study. Neurology. 2016;87(17):1836–1842. doi: 10.1212/WNL.0000000000003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Prev Med. 2010;51(3–4):275–278. doi: 10.1016/j.ypmed.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, et al. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis. 2013;22(8):1361–1369. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. Jama. 1999;282(1):40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 53.Peila R, White LR, Petrovich H, Masaki K, Ross GW, Havlik RJ, et al. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: the Honolulu-Asia aging study. Stroke. 2001;32(12):2882–2889. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- 54.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig J, Duncan GJ, Gennetian LA, Katz LF, Kessler RC, Kling JR, et al. Neighborhood effects on the long-term well-being of low-income adults. Science. 2012;337(6101):1505–1510. doi: 10.1126/science.1224648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2013;9(6):657–665. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American journal of public health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. Jama. 2017;317(14):1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brookmeyer R, Kawas CH, Abdallah N, Paganini-Hill A, Kim RC, Corrada MM. Impact of interventions to reduce Alzheimer’s disease pathology on the prevalence of dementia in the oldest-old. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2016;12(3):225–232. doi: 10.1016/j.jalz.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamauchi M, Jacono FJ, Fujita Y, Kumamoto M, Yoshikawa M, Campanaro CK, et al. Effects of environment light during sleep on autonomic functions of heart rate and breathing. Sleep Breath. 2014;18(4):829–835. doi: 10.1007/s11325-014-0951-7. doi:810.1007/s11325-11014-10951-11327. Epub 12014 Feb 11313. [DOI] [PubMed] [Google Scholar]
- 61.Basner M, Muller U, Elmenhorst EM. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34(1):11–23. doi: 10.1093/sleep/34.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evandt J, Oftedal B, Krog NH, Nafstad P, Schwarze P, Aasvang GM. A population-based study on nighttime road traffic noiseand insomnia. Sleep. 2016;14:00441–00416. doi: 10.1093/sleep/zsw055. [DOI] [PubMed] [Google Scholar]
- 63.Zanobetti A, Redline S, Schwartz J, Rosen D, Patel S, O’Connor GT, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182(6):819–825. doi: 10.1164/rccm.200912-1797OC. doi:810.1164/rccm.200912-201797OC. Epub 202010 May 200927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.