Abstract
Neural basic helix-loop helix (bHLH) transcription factors promote progenitor cell differentiation by activation of downstream target genes that coordinate neuronal differentiation. Here we characterize a neural bHLH target gene in Xenopus laevis, vexin (vxn; previously sbt1), that is homologous to human c8orf46 and is conserved across vertebrate species. C8orf46 has been implicated in cancer progression, but its function is unknown. Vxn is transiently expressed in differentiating progenitors in the developing central nervous system (CNS), and is required for neurogenesis in the neural plate and retina. Its function is conserved, since overexpression of either Xenopus or mouse vxn expands primary neurogenesis and promotes early retinal cell differentiation in cooperation with neural bHLH factors. Vxn protein is localized to the cell membrane and the nucleus, but functions in the nucleus to promote neural differentiation. Vxn inhibits cell proliferation, and works with the cyclin-dependent kinase inhibitor p27Xic1 (cdkn1b) to enhance neurogenesis and increase levels of the proneural protein Neurog2. We propose that vxn provides a key link between neural bHLH activity and execution of the neurogenic program.
Keywords: neurogenesis, basic helix-loop-helix, p27, cdkn1b, retina, Xenopus laevis
INTRODUCTION
During nervous system development, neural basic helix-loop-helix (bHLH) transcription factors coordinate the commitment of progenitors to the neural fate and promote subsequent neural differentiation (Huang et al., 2014). The regulation of neurogenesis by neural bHLH factors depends upon the transcriptional activation of target genes that function at multiple steps of the neuronal differentiation process to coordinate neurogenesis (Castro and Guillemot, 2011). The regulation of neurogenesis by neural bHLH factors has been extensively studied in Xenopus by monitoring the formation of primary neurons which form in three domains on either side of the midline of the neural plate, as detected by staining for the neuronal marker N-tubulin (Chitnis et al., 1995).
In recent years, significant advances have been made in understanding how neurogenesis is coordinated by these transcription factors. In particular, screens to identify direct transcriptional targets have revealed that neural bHLH factors regulate shared target genes involved in neuronal differentiation and lateral inhibition, as well as unique target genes involved in the development of specific neuronal subtypes (Bertrand et al., 2002; Castro and Guillemot, 2011; Logan et al., 2005; Seo et al., 2007). Some factors required for neurogenesis are not direct targets of neural bHLH activity, such as members of the Cip/Kip cyclin dependent kinase inhibitor (CDKi) family which regulate neurogenesis in concert with neural bHLH factors but are not directly regulated by these factors (Hardwick and Philpott, 2014; Hindley and Philpott, 2012). However, gaps remain in our understanding of how neural differentiation is coordinated. Notably, multiple novel genes of unknown function have been identified in screens for neural bHLH targets (Castro and Guillemot, 2011; Logan et al., 2005; Seo et al., 2007), suggesting that important components of the neuronal differentiation pathway have yet to be characterized.
In a previous screen for direct transcriptional targets of neural bHLH factors we identified a novel gene conserved in vertebrates that is homologous to human c8orf46, and that we now call vexin (vxn, previously sbt1; (Logan et al., 2005)). We show that vxn is expressed in differentiating progenitors, and is required for neurogenesis in the Xenopus neural plate and retina. Overexpression of Xenopus or mouse vxn expands neurogenesis within existing proneural domains, and can cooperate with neural bHLH factors. We further show that vxn and p27Xic1 depend upon each other to increase neurogenesis and that they cooperate to enhance Neurog2 levels post-transcriptionally. We propose that vxn provides a key link between neural bHLH activity and p27Xic1 function during the neurogenic process.
MATERIALS AND METHODS
Isolation of Xenopus laevis vxn sequence
A 258 bp fragment corresponding to Xenopus laevis c8orf46 (sbt1; (Logan et al., 2005)) was labeled with 32P-dCTP using Klenow enzyme and used to screen a stage 28-30 Xenopus head cDNA library (gift of R. Harland). cDNA clones were recovered from positive phage using the Rapid Excision Kit (Stratagene) and sequenced (University of Utah Sequencing Core Facility). Identified clones corresponded to Xenopus laevis c8orf46.L. Candidate protein domains were detected using the NCBI Conserved Domain search tool (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Sequence alignment and phylogenetic conservation analyses
The predicted protein sequence of X. laevis c8orf46.L was used to perform a BLAST search (tblastp) to identify orthologous protein sequences. Multiple sequence alignment was performed using the using the webPRANK program in Jalview2 ((Waterhouse et al., 2009); http://www.jalview.org/) using the following protein sequences: X. tropicalis (XP_002935358.1), Human (NP_689978.2), Rat (NP_001102730.1), Mouse (NP_848486.1), Dog (XP_852534.1), Horse (XP_001494799.1), Cow (NP_001069943.1), Chicken (XP_004939943.2), Quail (XP_015711228.1), Gecko (XP_015264548.1), Sea turtle (XP_007066260.1), Danio rerio (XP_001923735.1), Fugu (XP_011606235), and Stickleback (predicted protein sequence of DT965868). The webPRANK alignment was used to generate a phylogenetic tree using BLOSUM62.
Plasmids, microinjections and in situ hybridizations
The Xenopus and mouse pCS2-vxn expression constructs were made by PCR amplifying the vxn (X. laevis c8orf46.L) coding region and cloning into the pCS2+, pCS2+ HA, pCS2+MYC (5′ and 3′), pCS2+NES and pCS2+NLS vectors. VxnNLSmut mutations were generated by PCR site directed mutagenesis as described (Moore et al., 2002) using Xenopus vxn-3′myc as a template. pCS2-p27Xic1 was obtained from W. Harris at the University of Cambridge (Ohnuma et al., 2002). pCS2-Myc-Neurog2 was made by subcloning the Neurog2 cDNA (Ma et al., 1996) into the EcoRI sites of pCS2+6Myc. All constructs were verified by sequence analysis (University of Utah Core Facility) and by western blot or in vitro translation (Promega, cat #L1170).
Xenopus laevis embryos were obtained by in vitro fertilization, dejellied in 2% cysteine (pH 8.0), grown in 0.1MMR or 0.1MBS (Falk et al., 2007) and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1994). Capped injection mRNA was synthesized using an in vitro mMessage Machine kit (ThermoFisher, cat#AM1340) and were injected into either 1 cell of 2-cell stage embryos, 1 dorsal blastomere at the 4-8-cell stage embryos or into 1 dorsal blastomere at the 32-cell stage as described (Moore et al., 2002). The following vxn constructs were used for mRNA injections. Xenopus vxn, Xenopus vxn-3′myc, Xenopus 5′UTR-vxn-3′myc, Xenopus 5′myc-vxn, Xenopus 5′HA-vxn, Xenopus 5′ NLS-myc-vxn, Xenopus 5′myc-vxn-3′-NES, Xenopus vxn-Nterm (aa1-137), Xenopus vxn-NLSmut-3′myc, mouse vxn, mouse 5′myc-vxn, mouse 5′NLS-vxn. Other constructs used were: Atoh7-ENR (Logan et al., 2005), ccne1 (Vernon and Philpott, 2003), NICD (Coffman et al., 1993), p27Xic (Vernon et al., 2003) and myc-neurog2 (Ma et al., 1996). All mRNAs were coinjected with either β-gal (50-80 pg), or GFP or mCherry CAAX (gift of Kristin Kwan) (400 pg). All constructs were in pCS2+ vectors unless otherwise indicated.
Embryos were collected at the appropriate developmental stages (Nieuwkoop and Faber, 1994), fixed in MEMFA and X-gal staining performed on β-gal injected embryos as described (Turner and Weintraub, 1994b). Embryos injected with GFP mRNA were presorted before in situ hybridization at the open neural plate stage for GFP expression restricted to either the left or right side. In situ hybridization (ISH) with digoxigenin-labeled antisense mRNA probes was performed on whole embryos and retinal sections as previously described (Hutcheson and Vetter, 2001; Van Raay et al., 2005). For fluorescent ISH, fast red (SIGMA F4648) was used as the substrate. The following digoxigenin (DIG)-labeled riboprobes were used for the analysis: Atoh7 (Kanekar et al., 1997), barhl2 (Papalopulu and Kintner, 1996), Delta (Dorsky et al., 1997), Ebf2, Ebf3 (Pozzoli et al., 2001), Elavl3 (Good, 1995), Esr1 (Wettstein et al., 1997), Hermes (Patterson et al., 2000), hes4 (Turner and Weintraub, 1994a), hes5.1 (Wettstein et al., 1997), MyT1 (Bellefroid et al., 1996), neurod1 (Lee et al., 1995), neurog2 (Lamar et al., 2001; Ma et al., 1996), Nrarp (Lamar et al., 2001), N-tub (Richter et al., 1988), p27Xic-1 (Vernon et al., 2003), pax3 (Bang et al., 1997), pou4f2 (Hutcheson and Vetter, 2001), Sox2 (Mizuseki et al., 1998), vsx1 (D'Autilia et al., 2006).
Morpholinos
A translation-blocking antisense morpholino oligonucleotide targeting vxn mRNA (VXNMO-A; 5′; GATCATTTCTCCCTGAGGTAGACTT 3′) was generated (ATG complement underlined; GeneTools, Philomath, OR). For controls, a mismatch morpholino (VXNmisMO; 5′GATgATTTgTCCgTGAcGTAcACTT3′) with 5 base changes (lowercase) and an inverted morpholino (VXNMO-A inverted; 5′ TTCAGATGGAGTCCCTCTTTACTAG3′) were used. A second slightly-overlapping MO, VXNMO-B (5′AGCTCTTTCTGTGTAGCTTGATCAT 3′) was also generated. MO-A and MO-B are predicted to target both the L and S versions of vxn. To test whether vxn MO-A can block translation, we generated a version of vxn that included 19 nucleotides of 5′UTR, subcloned it into pCS2+ 3′myc and sequence verified. Xenopus embryos were injected with Xenopus 5′UTR- vxn-3′myc mRNA (400 pg) alone or co-injected with vxn MO-A or control vxn misMO (40ng). Stage 16 whole embryo lysates were then analyzed by western blot with anti-MYC 9E10 antibody (ThermoFisher Scientific cat#13-2500) to monitor Vxn 3′myc production (Fig. S1A). Protein levels were quantified based on BCA protein assay (Pierce cat#23225) and 30 micrograms of total lysates were loaded per lane.
To ensure rigor and reproducibility of morpholino experiments the following steps were taken: a) two morpholinos targeting vxn were generated and shown to result in identical phenotypes, b) both morpholinos were rescued by co-injection of RNA for mouse vxn, c) MO-B which targets the 5′UTR was rescued with Xenopus 5′myc-vxn which lacks the 5′UTR sequence, d) MO-A was shown to block translation of vxn mRNA in vivo, e) mismatch and inverted control morpholinos were shown to have no effect. In addition, NES-Vxn functions as a dominant negative protein and replicates the phenotype of the vxn morpholinos.
Vxn localization in animal caps and the neural plate
Embryos were injected with 800pg-1ng of Xenopus 5′myc–vxn, vxn-3′myc, vxn-NLSmut-3′myc, or mouse 5′myc-vxn mRNA at the 1-cell stage. Animal cap explants were dissected at stage 9, fixed in MEMFA and stained with primary anti-MYC antibody 9E10 (1:500) followed by secondary goat anti-mouse Alexa 488 (1:2000). Nuclei were stained with Hoechst dye before mounting animal caps onto slides using Fluoromount (ThermoFisher Scientific cat#00-4958-02). Images were captured as confocal Z-series on a Nikon A1. To analyze Vxn localization in the neural plate, embryos were injected as described above, allowed to grow until stage 15, fixed in MEMFA and stained with anti-Myc (1:500) or anti-Vxn (1:400) as described above.
Anti-VXN Antibody generation and affinity purification
A rabbit polyclonal antibody was generated directed against a peptide (EYIGASNCAFEDD) in the conserved C-terminus of the protein. The antibody was affinity purified using a SulfoLink™ column (ThermoFisher Scientific cat# 20401) according to manufacturer’s protocols. To confirm that the affinity purified antibody recognizes both Xenopus and mouse VXN protein, western blot analysis of in vitro translated Xenopus vxn-3′myc and mouse 5′myc-vxn was performed using 1:400 of anti-Vxn antibody and confirmed by using anti-MYC 9E10 antibody to monitor Vxn 3′myc levels (Fig. S1B). Protein levels were quantified based on BCA protein assay (ThermoFisher Scientific) and 30 μg of total lysates were loaded per lane.
Neural plate expansion, cell density and pH3 antibody staining
Sox2, hes4, and pax3 expression domains were determined by measuring the distance from the midline to lateral edge of expression and the average per cent change relative to the uninjected side ± s.e.m determined.
Capped mRNA for Xenopus 5′myc-vxn (1 ng), p27Xic, ccne1 + GFP (400 pg), or GFP alone (1.4 ng) was injected into 1 cell of 2-cell stage embryos. For other experiments, 5′myc-vxn (1 ng) + GFP (400pg), GFP alone (1.4ng) or 5′myc-vxn (1 ng) + ccne1 (50pg) or 5′myc-vxn (1 ng) + p27XicMO (10ng) was used. Embryos were fixed in MEMFA at the open neural plate stage and then immunostained with 1:500 anti-phosphorylated histone3 (pH3) (Upstate) and secondary goat anti-rabbit Alexa Fluor-488, 568 or alkaline phosphatase (1:2000, Thermo Fisher Scientific) using previously described methods (Burns and Vetter, 2002). pH3 density was calculated using the ImageJ program to analyze microscope images of each embryo. First, the number of pH3- positive cells were scored in the GFP-positive area on the injected side and then an area of exact size and corresponding location was selected on the uninjected side for pH3 scoring. All graphs and statistical analysis was performed using Graph Pad Prism version 6.0c (Graph Pad Software, La Jolla, CA). A p27XicMO was also generated (5′GCAFFFCGATGTGGAAAGCAGCCAT 3′; Vernon and Philpott, 2003). Two tailed Student’s t-tests were used and p≤0.05 was considered significant.
Retinal injections, BrdU labeling, and immunostaining staining
For retinal cell fate analysis, either one dorsal blastomere (D1.1.1 or D1.2.2) of 32-cell stage embryos was injected with mRNA or plasmid DNA was lipofected at stages 15-18 into the presumptive retina as previously described (Agathocleous et al., 2009; Moore et al., 2002). For clone size analysis, the V1.2.1 cell of 32-cell stage embryos was injected to generate smaller clones of cells (Huang and Moody, 1993). At stage 41, embryos were fixed, embedded and sectioned and imaged for retinal cell type analysis as previously described (Kanekar et al., 1997). Immunostaining was performed using primary antibodies: Müller glia were assessed using rabbit anti-CRALBP [1:1000; a gift from Dr J. Saari (Bunt-Milam and Saari, 1983), and cones with rabbit anti-calbindin (1:100, Sigma), followed by secondary goat-anti rabbit Alexa Fluor 488 or 568 (1:2000, ThermoFisher Scientific). Statistical significance was assessed as described above. BrdU labeling and paraffin sectioning was performed as previously described (Perron et al., 1998). Sections of stage 41 BrdU-injected embryos were stained with rabbit anti-GFP (1:500, Torrey Pines) and mouse-anti BrdU (1:100; BD Biosciences) as previously described (Van Raay et al., 2005).
Western blot analysis
One-cell Xenopus embryos were injected with capped mRNA for Xenopus transcripts: 5′ myc-neurog2 (50 pg per embryo), vxn (1 ng), and p27Xic1 (50 pg). Embryos were lysed at stage 15 in lysis buffer (20mM Tris-HCl pH 7.4, 140mM NaCl, 1% NP-40, 10% glycerol, 2mM EDTA, 1mM MgCl2, Halt protease inhibitor (Thermo Scientific)), and cleared of debris by centrifugation at 12,000 rpm for 15 minutes. Lysates were run on a western blot and Myc-Neurog2 protein levels were detected with goat anti-c-myc (1:1000, Santa Cruz Biotechnology, sc-789-G), followed by donkey anti-goat HRP (Jackson ImmunoResearch Laboratories) and developed with Clarity Western ECL substrate (BioRad). The membrane was stripped and probed with Rabbit anti-β-actin (1:1000, Abcam), followed by donkey anti-rabbit HRP (Jackson ImmunoResearch Laboratories) as a loading control. Band intensities were quantified with ImageJ, and Myc-Neurog2 protein levels were normalized to β-actin.
RESULTS
Xenopus laevis c8orf46.L encodes a predicted protein conserved across vertebrates
In a previous screen for shared direct transcriptional targets of the neural bHLH factors Atoh7 and Neurod1 we identified a novel gene fragment ((Logan et al., 2005); previously referred to as sbt1). We identified full-length clones from a stage 28-30 head cDNA library corresponding to Xenopus laevis c8orf46.L, containing an open reading frame that encodes a predicted 190 amino acid protein. BLAST analysis identified related sequences encoding predicted uncharacterized protein c8orf46 homologs in multiple vertebrate species including birds, reptiles, fish, amphibians and mammals (Fig. 1), with no identified orthologs in non-vertebrate species. We confirmed that orthologous genes flank the Xenopus tropicalis, mouse, and human c8orf46 genes (Mybl1 and Adhfe1). The amino acid interval 47-190 encodes a domain of unknown function (DUF4648) belonging to Src Homology 3 (SH3) superfamily (1.71e-06; NCBI Conserved Domain Database) but no other conserved functional domains were detected. We have named the gene vexin (vxn).
Figure 1. Alignment and phylogenetic tree of c8orf46 orthologs.
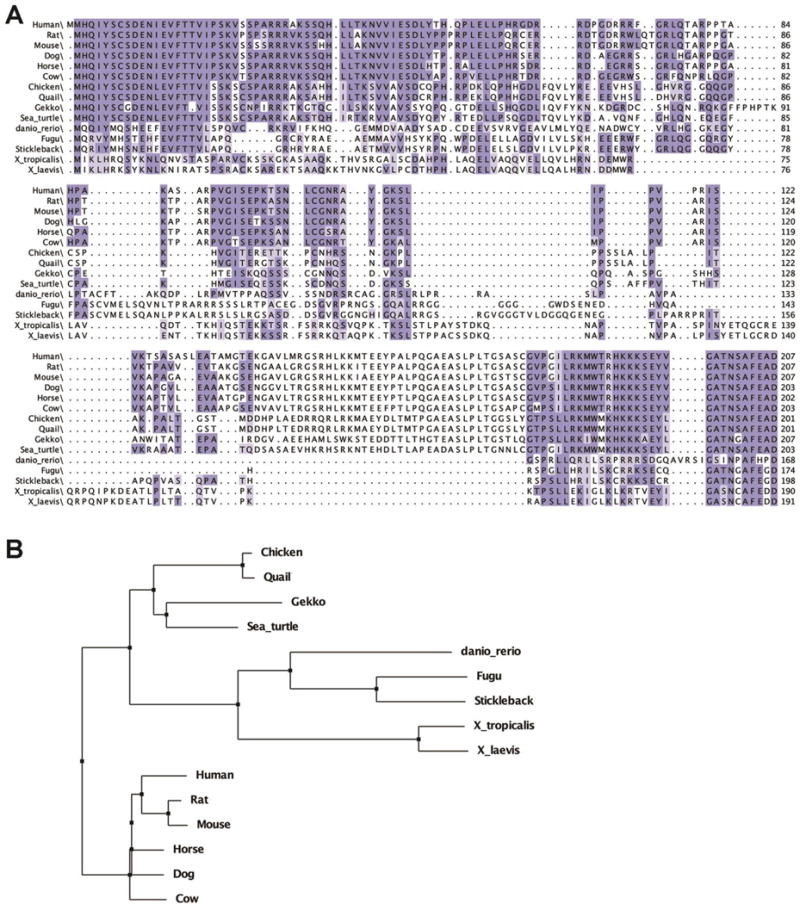
(A) The predicted protein sequence encoded by the Xenopus laevis gene c8orf46.L (vxn) was aligned to predicted protein sequences encoded by c8orf46 orthologs from other vertebrate species (see methods for accession numbers) using the webPRANK program in Jalview2 (Waterhouse et al., 2009). Residues conserved in at least 70% of the aligned sequences are indicated with dark blue shading while light blue shading indicates residues that are 50-69% conserved. (B) The webPRANK alignment was used to generate a phylogenetic tree using BLOSUM62.
Vxn is expressed in the developing nervous system
Vxn is expressed during the course of nervous system (CNS) development (Fig. 2A), with expression in differentiating primary neurons (stage 15; Fig. 2B), in the neural tube and olfactory placodes at stage 19 (Fig. 2C), and in the developing eye and other anterior neural structures by stage 27 (Fig. 2D, E). Sections showed that vxn was excluded from the ventricular zone, and was concentrated in lateral domains where cells are differentiating (Fig. 2F, G). Vxn was expressed in the optic vesicle at stage 25, and increased in the eye at later stages (Fig. 2H, I). In stage 41 retina, vxn was localized to the central ciliary marginal zone (CMZ; Fig. 2J, K), and excluded from the peripheral stem cell zone (Perron et al., 1998). BrdU co-labeling showed that vxn is expressed in differentiating progenitors, and is downregulated in differentiated cells of the central retina (Fig. 2L, M). Thus, vxn is transiently expressed in the CNS as progenitors undergo neuronal differentiation.
Figure 2. Vexin is expressed in the developing Xenopus nervous system.
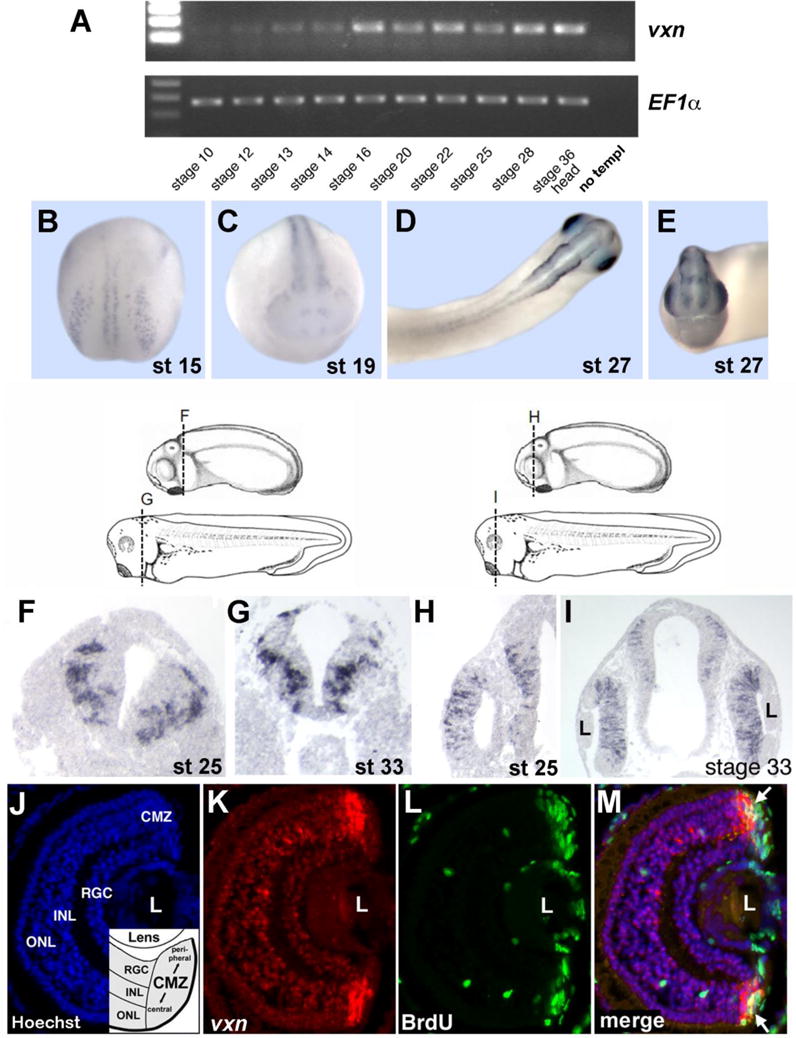
(A) RT-PCR analysis of vxn expression using Xenopus whole embryo cDNA. EF1α was used as a loading control. Control PCR was performed using no cDNA template (no templ). (B-E) Whole-mount in situ hybridization analysis of vxn expression at stage 15 (B), stage 19 (C), and at stage 27 (D, dorsal view; E, anterior view).
(F-I) Shown are stage 25 and stage 33 d with axial levels for the sections in F-I (Nieuwkoop and Faber, 1994). In situ hybridization analysis of vxn expression on transverse sections of the neural tube at stage 25 (F), and 33 (G), and of the head including the optic vesicle at stage 25 (H) and the developing retina at stage 33 (I).
(J-M) Analysis of vxn expression in the stage 41 retina. (J) Nuclear Hoechst staining shows the three layers of post-mitotic neurons (RGC, INL, ONL) within the central retina, as well as the peripheral ciliary marginal zone (CMZ) that contains retinal progenitors. (K) Fluorescent in situ hybridization shows vxn expression (red) in the CMZ. (L) Anti-BrdU immunostaining (green) labels proliferating retinal progenitors in the CMZ. (J) A merged image of (J-L) shows that vxn expression overlaps with BrdU-positive progenitors (arrows), and is also detected in early differentiating post-mitotic cells more centrally. Abbreviations: L, lens; RGC, retinal ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Vxn protein localizes to the nucleus and cell membrane
Since vxn encodes a novel protein with unknown subcellular distribution, we investigated its localization by injecting mRNA for 5′ myc epitope-tagged Xenopus Vxn, then immunostaining isolated animal cap tissue using anti-myc antibodies. Myc-Vxn localized to the nucleus and to the cell membrane of animal cap cells (Fig. 3A), with similar subcellular localization using 5′ HA-tagged Vxn (Fig. S2A). The localization of 5′ myc-tagged mouse Vxn was identical (Fig. S2B). We confirmed that endogenous Vxn in the neural plate is similarly localized to the nucleus and cell membrane using a rabbit polyclonal antibody directed against a peptide in the conserved C-terminus of the protein (Fig. 3B). Within the nucleus, high magnification confocal imaging of both myc-tagged and endogenous Vxn showed the protein in distinct subnuclear domains that stain weakly with Hoechst (Fig. 3C). We confirmed co-localization of membrane-localized mCherry-CAAX with Xenopus 5′ myc-Vxn (Fig. 3D).
Figure 3. Vxn protein localizes to the nucleus and the cell membrane.
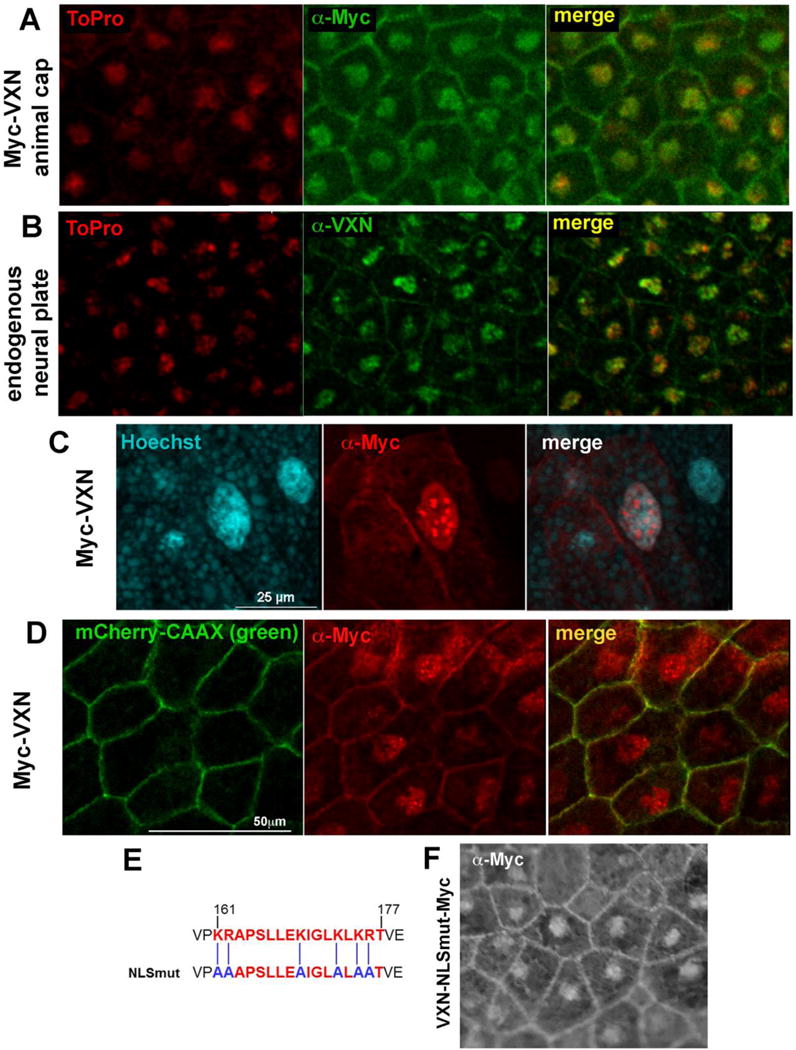
(A,B) Anti-myc immunostaining for myc-tagged Vxn (Myc-Vxn, green) in isolated animal cap explants (A) or anti-Vxn (green) staining of endogenous protein in the neural plate (B) shows localization in the nucleus (labeled by ToPro, red), and at the cell membrane.
C) Myc-Vxn (red) within the nucleus is localized to subnuclear domains that stain weakly with Hoechst (blue).
(D) At the membrane, Myc-Vxn (red) colocalizes with mCherry-CAAX (green).
(E) Mutation of critical lysine residues to alanine within the candidate nuclear localization sequence (NLSmut).
(F) Vxn-NLSmut-Myc detected by immunostaining with α-myc localizes to both the nucleus and the cell membrane.
Since Vxn is localized to the nucleus, we sought to identify putative nuclear localization sequences (NLS). We used the program SeqNLS (Lin and Hu, 2013), which predicted that residues 161-177 of Xenopus vxn encoded an NLS (score: 0.953; Fig. 3E). To test this, we performed targeted mutagenesis, converting six K/R amino acids to A to generate vxnNLSmut-3′myc (Fig. 3E). Immunostaining for Vxn-NLSmut expressed in animal cap tissue showed that the protein was still localized to both the nucleus and the cell membrane (Fig. 3F). Notably, the protein at the cell membrane was associated with loops or blebs of various sizes (Fig. S2C), although the significance of this is unclear. A truncated version of Vxn consisting of amino acids 1-137 and lacking the C-terminus similarly localized to both the nucleus and cell membrane (Fig. S2D). Together, we conclude that nuclear localization of Vxn does not strictly depend upon the C-terminal region containing the candidate NLS.
Vxn is required for neuronal differentiation
To test whether vxn is required for neuronal differentiation, we designed two translation-blocking antisense oligonucleotide morpholinos (MO-A, MO-B). We confirmed by western blot that co-injection of MO-A blocked translation of 3′ myc-tagged Vxn (Fig. S1A). We injected from 10ng to 60ng of vxn MO-A into 1 cell of 2-4 cell stage embryos and found reduced N-tubulin expression in stage 15 embryos (Fig 4A, 20ng at 4 cell stage), with greater penetrance at increasing doses (Fig. 4D). A control inverted morpholino (Inv MO-A) had no effect (Fig. 4B, E, 88% no change, n=60 embryos), nor did a 5bp mismatch MO-A (Fig. 4D, 95% no change, n=21). The block on neurogenesis by MO-A could be overcome by co-injection of 1ng mouse vxn mRNA (Fig 4C, E; 84% rescued, n=112 embryos), indicating conservation of function. Injection of MO-B reduced N-tubulin expression at even lower doses (Fig. 4E, G), and this could be rescued by co-injection of 1 ng of either Xenopus vxn (Fig 4F, G; 82% rescued, n=20 embryos) or mouse vxn mRNA (Fig 4G; 92% rescued, n=20 embryos). Injection of 200pg of neurod1 mRNA results in expanded or ectopic neurogenesis (Fig S3, n=106; Lee et al., 1995), and this effect was reduced by co-injection of 30ng of MO-B (Fig. S3 n=98), indicating that vxn functions downstream of neurod1.
Figure 4. Vxn is required for primary neuron differentiation.
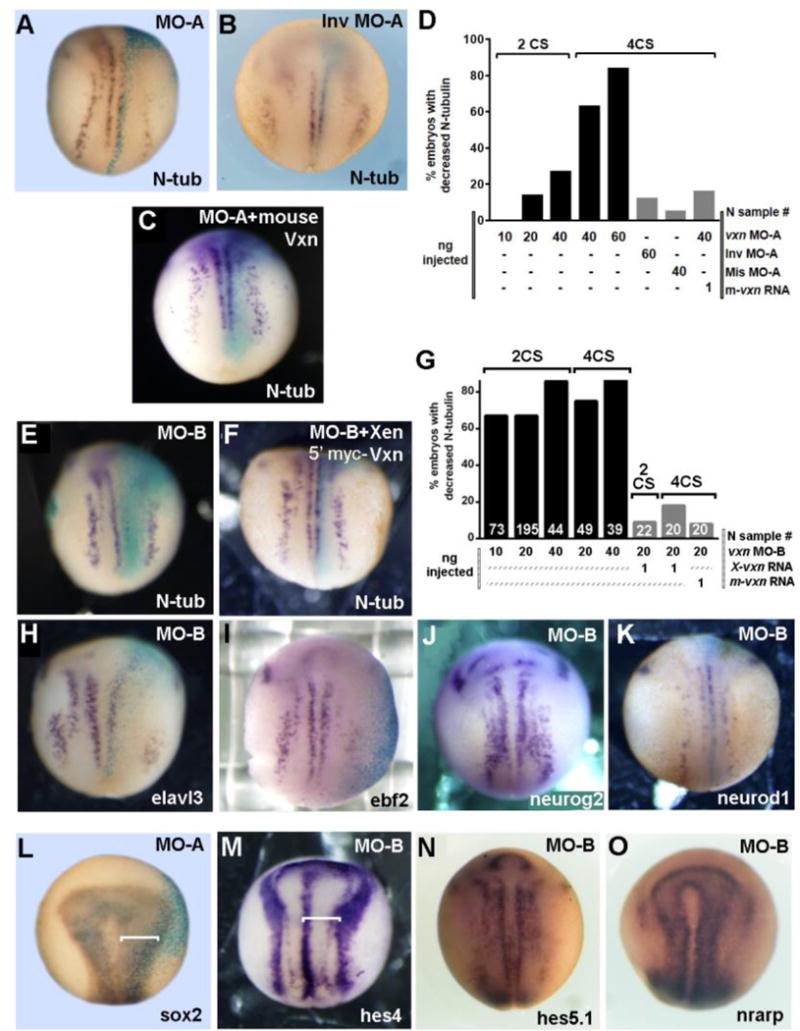
(A-E) Injection 20ng of vxn translation-blocking morpholino (MO-A) at the 4-cell stage (4CS) causes reduced N-tubulin (N-tub) positive primary neurons at stage 14 (A), with increased inhibition at increasing doses of MO-A (D), while injection of 20ng of inverted morpholino (Inv MO-A) does not (B). Primary neurogenesis is rescued by co-injection of vxn MO-A with 1ng of mouse vxn mRNA (C, D).
(E-G) Primary neurogenesis is inhibited by injection of 20ng of MO-B at the 4CS (E), with efficient inhibition with injection of 10-40ng of vxn MO-B at the 2 or 4 CS (G). Loss of N-tub can be rescued by co-injection of 1ng of Xenopus 5′myc-vxn (F) or mouse (G) vxn mRNA. (H-O) Injection of vxn MO-B inhibits expression of other markers of differentiating primary neurons, including elavl3 (H) and ebf2 (I), but has no effects on expression of neurogenic bHLH genes such as neurog2 (J) or neurod1 (K).
(L, M) Injection of vxn MO-A or MO-B causes expansion of progenitors in the neural plate as revealed by expansion of sox2 (L) or the neural plate domain bounded by hes4 (M).
(N, O) Injection of vxn MO-B causes no change in the expression of Notch pathway genes such as hes5.1 (N) and nrarp (O).
For all experiments, mRNA encoding either GFP or β-galactosidase was co-injected to label the injected side (right). X-gal staining is light blue.
Consistent with a block in primary neurogenesis, injection of 20ng MO-B caused reduced expression of the neural bHLH target genes elavl3 (Fig. 4H; 64%, n=74) and ebf2 (Fig. 4I; 58%, N=5), but no decrease in the expression of neurog2 (Fig. 4J; 85% no change, n=84) or neurod1 (Fig. 4K; 82% no change, n=50), indicating that vxn is required downstream of the neural bHLH factors. 20ng MO-B injection also caused expansion of neural progenitors in the neural plate as labeled by sox2 (Fig. 4L; 89%, n=47), or hes4 (Fig. 4M; 74%, n=23) at the neural plate border, indicating failure of neural progenitors to exit the cell cycle and differentiate. Activation of Notch signaling can prevent neurogenesis (Pierfelice et al., 2011), however we saw no increase in expression of the Notch target genes hes5.1 (Fig. 4N; 100% no change, n=12) or nrarp (Fig. 4O; 100% no change, n=28) after morpholino injection, suggesting that the Notch pathway is not activated. Taken together, these results indicate that vxn is required downstream of proneural bHLH factors for the differentiation of primary neurons.
Overexpression of vxn enhances primary neurogenesis and cooperates with proneural bHLH factors
To test whether vxn is sufficient to promote neuronal differentiation, we overexpressed Xenopus vxn by injection of 1 ng of mRNA at the 2-cell stage and observed expanded expression of N-tubulin confined to the three domains that normally give rise to primary neurons at stage 15, with no ectopic expression in non-neural ectoderm (Fig 5A; 65%, n=238 embryos). There was also expanded expression of several other neuronal differentiation genes that are expressed downstream of proneural bHLH factors such as ebf3 (Fig. 5B; 70%, n=23 embryos) and elavl3 (Fig 5C; 80%, n=44 embryos). This was coupled with reduction in the width of the neural plate as labeled by sox2 (Fig. 5D; 77%, n=151), and by hes4 (77%, n=13) at the neural plate border (Fig. 5E), suggesting depletion of progenitors in the neural plate.
Figure 5. Vxn overexpression enhances primary neuron differentiation.
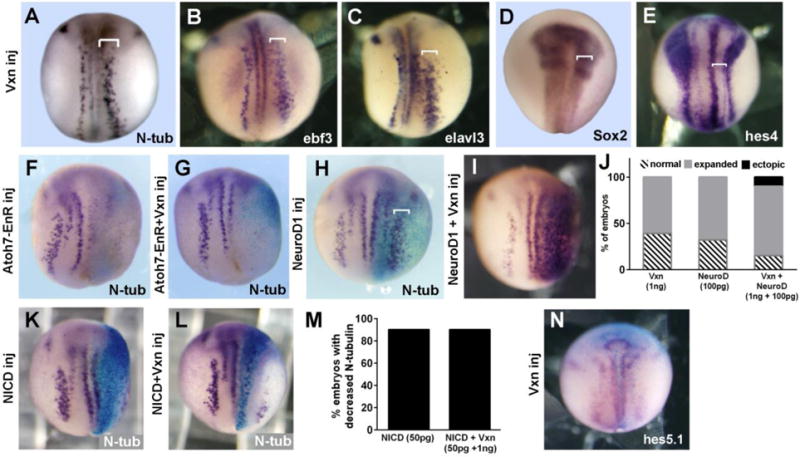
(A-C) Injection of 1ng vxn mRNA at the 2-cell stage (2CS) causes expansion of primary neurons (brackets) labeled by N-tub (A), ebf3 (B) and elavl3 (C).
(D-E) Injection of 1 ng vxn mRNA causes reduction of progenitors in the neural plate as revealed by narrowing of the sox2 domain (D) or the neural plate domain bounded by hes4 (E).
(F,G) Differentiation of N-tub labeled primary neurons is blocked by injecting mRNA encoding Atoh7-EnR (F), and this cannot be overcome by co-injection of vxn mRNA.
(H-J) Injection of low doses of neurod1 mRNA (100pg) causes expansion of N-tub labeled primary neurons (H, bracket), which is enhanced by co-injection of 1ng vxn mRNA (bracket in I, J).
(K-N) Injection of 50pg mRNA encoding the intracellular domain of Notch (NICD) blocks differentiation of N-tub labeled primary neurons (K, M), and this is not rescued by co-injection of 1ng vxn mRNA (L, M). Injection of 1ng vxn mRNA does not change expression of the Notch target gene hes5.1 (N).
For all experiments, mRNA encoding either GFP or β-galactosidase was co-injected to label the injected side (right). X-gal staining is light blue.
Vxn expanded neurogenesis within the existing domains of primary neurons, suggesting that it may depend upon proneural gene function. To test this, we injected 250pg mRNA encoding Atoh7-EnR (Xath5EnR; (Logan et al., 2005)), which dominantly blocks the ability of neurogenin- and atonal-family bHLH factors to promote neurogenesis (Logan et al., 2005). We confirmed loss of N-tubulin expression in stage 15 embryos (Fig 5F; 80% reduced expression, n=20 embryos), which was not rescued by co-injection of 1ng vxn mRNA (Fig 5G, 83% reduced expression, n=18 embryos). Since vxn expands the stripe of primary neurons, it may cooperate with neural bHLH factors within these domains. To test this, we injected a low dose of neurod1 mRNA (50pg) at the 2-cell stage, which results in expansion of the stripes of primary neurons with no ectopic neurogenesis (Fig. 5H, J; 68%, n=174). Injection of 1ng vxn mRNA similarly widened the stripes of primary neurons (Fig. 5J; 61%, n=51), while co-injection of neurod1 together with vxn mRNA caused an increase in the percentage of embryos with expanded primary neurogenesis (85%, n=82) and 12% of embryos showing ectopic neurogenesis (Fig. 5I, J).
To test whether vxn enhances neurogenesis by interfering with Notch signaling, we co-injected 50pg of mRNA for NICD (Notch intracellular domain; (Coffman et al., 1993)). Vxn was unable to interfere with NICD-mediated suppression of N-tubulin expression (79% reduced expression n=76 compared to 77% reduced expression, n=30, Fig. 5K-M). In addition, injection of 1ng vxn mRNA at the 2-cell stage did not reduce expression of the Notch target genes hes5.1 (Fig. 5N, 87% no change, n=30) or nrarp (100% no change, n=70; not shown), indicating that the Notch signaling pathway was not suppressed. Overall, we conclude that vxn enhances the ability of neural bHLH factors to promote neurogenesis.
Vxn is required for retinal neuron differentiation
Since vxn is expressed in the developing retina, we asked whether it plays a role in retinal neurogenesis. We injected mRNA for GFP (300pg) either alone or together with 3ng of vxn mismatch MO-A or vxn MO-A into 1 dorsal blastomere at the 32-cell stage resulting in clones of GFP-labeled cells in stage 41/42 retina (Moore et al., 2002). Injection of control 5bp vxn mismatch MO had no effect on retinal histogenesis, however, injection of vxn MO-A resulted in approximately a 10-fold increase in the production of Muller glia (MG) and/or neuroepithelial (NEP) cells at the expense of virtually all neuronal cell types (Fig. 6A, B; *= p<0.001, #= p<0.01; n= 11 retinas, 2188 cells for GFP; n=5 retinas, 1244 cells for GFP + vxn mismatch MO-A; n= 14 retinas, 1927 cells for GFP + vxn MO-A). Immunostaining for CRALBP to distinguish MG from NEP cells showed that both populations increased significantly (*= p<0.01), although the majority were undifferentiated NEP cells (Fig. 6C; n= 5 retinas, 703 cells for GFP; n=6 retinas, 1009 cells for GFP + Vxn MO-A). Thus, we conclude that vxn is required for retinal neurogenesis.
Figure 6. Vxn is required for retinal neuron differentiation.
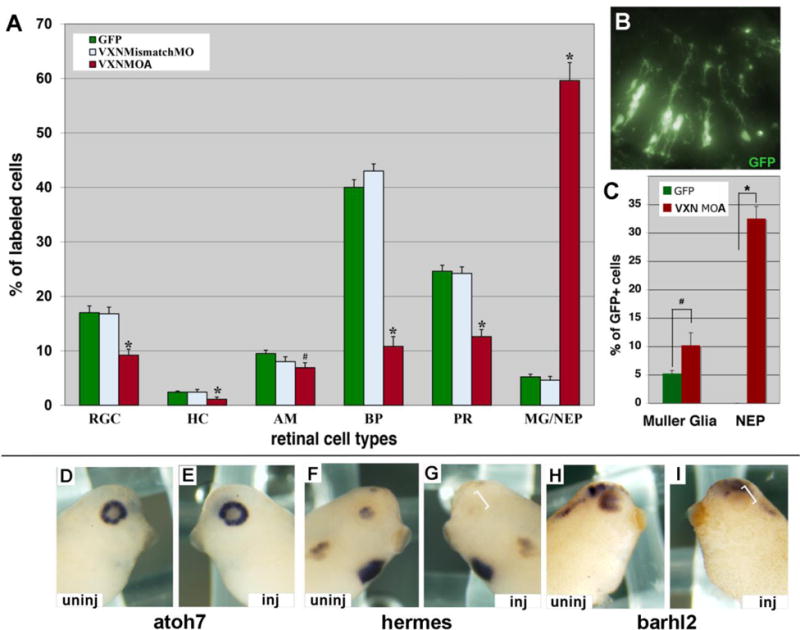
(A-C) mRNA for GFP (300pg) was injected alone or together with 3ng of vxn mismatch MO-A or vxn MO-A into 1 dorsal blastomere at the 32-cell stage. Embryos were cryosectioned at stage 41, and GFP-labeled retinal cell types were counted. vxn MO-A caused a significant decrease in all retinal neuron types, and a significant increase in Muller glia (MG) and/or neuroepithelial cells (NEP) (A). A section of stage 41 retina showing many GFP-labeled retinal cells with the morphology of Muller glia and/or NEPs (B). Immunostaining for CRALBP to distinguish MG from NEPs showed that both populations increased significantly (C).
(D-I) Injection of 20ng of vxn MO-B at 8CS did not alter atoh7 expression in stage 34-35 embryos (D,E), but prevented expression of hermes (F,G) and barhl2 (H,I) on the injected (inj) side (brackets; E,G,I) when compared to the uninjected (uninj) side (D, F, H).
RGC, retinal ganglion cells; HC, horizontal cells; AC, amacrine cells; BP, bipolar cells; PR, photoreceptor cells; MG, Muller glial cells; NEP, neuroepithelial cells. *p<0.001 and #p<0.01 by Student’s t-test.
To determine whether genes involved in retinal neurogenesis were altered we injected 20ng of vxn MO-B into a dorsal animal blastomere D1 at the 8-cell stage, which will target most of the eye on one side of the embryo. At stage 34-35 vxn MO-B did not alter expression of retinal progenitor genes such as Sox2, Vsx1 or atoh7 (Fig. 6D, E; Table S1). However, vxn MO-B did reduce expression of differentiation markers for retinal neurons, including hermes, barhl2, ebf3, elavl3, and pou4f2 (Fig. 6F-I; Table S1). Similar effects on retinal differentiation markers were observed with MO-A (Table S1). Thus, we conclude that vxn is not required for retinal progenitor specification, but is required for retinal neuron differentiation. Consistent with our findings in the neural plate, vxn MO-A did not alter expression of the Notch target genes esr1 and hes5.1 (Table S1), indicating that the block on neural differentiation is not due to enhanced Notch signaling.
Vxn promotes neural differentiation in the retina
To determine whether vxn can influence retinal neuron differentiation we performed in vivo transfection of vxn and GFP cDNA into the optic vesicles of stage 18 embryos. At stage 41/42, retinal cells transfected with vxn showed a significant bias towards early born cell types such as retinal ganglion cells (RGCs) and photoreceptors, at the expense of bipolar cells and Mueller glia, which are late born cell types (Fig. 7A; *= p<0.001; n= 5 retinas, 747 cells for GFP; n=8 retinas, 772 cells for GFP + vxn). For comparison, retinal cells were also injected with atoh7 cDNA and transfected cells showed a significant bias toward RGCs (Fig. 7A*= p<0.001; n= 4 retinas, 411 cells), consistent with published data (Kanekar et al., 1997). Calbindin immunostaining showed that early-born cones are significantly increased (Fig. 7B; *= p<0.001; n=5 retinas, 63/159 photoreceptors for GFP; n= 7 retinas, 137/190 photoreceptors for GFP + vxn). The increase in differentiation of early born cell types is almost identical to that seen with overexpression of neural bHLH factors (Kanekar et al., 1997; Moore et al., 2002), suggesting that vxn may be a key mediator of their function in the retina.
Figure 7. Targeted retinal overexpression of vxn promotes early retinal cell differentiation.
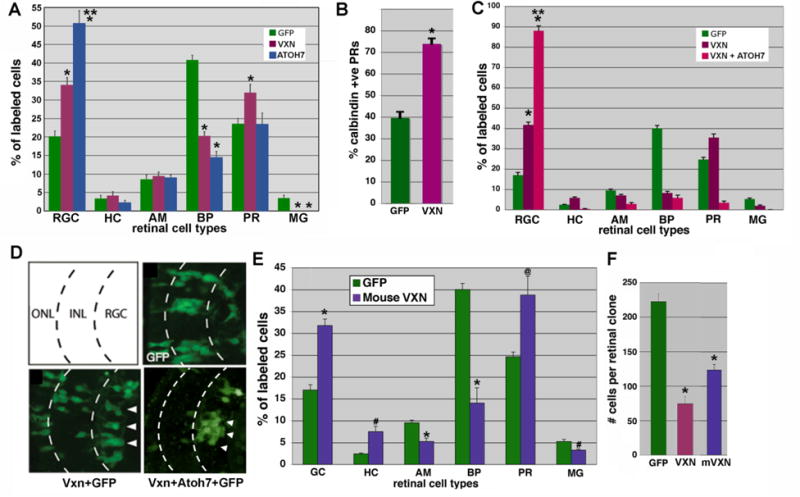
(A,B) GFP (300pg) was lipofected alone or together with Xenopus vxn (250 pg) or Xenopus atoh7 into the optic vesicle of stage 18 embryos. Embryos were cryosectioned at stage 41, and GFP-labeled retinal cell types were counted. Atoh7 expression caused a significant increase in RGCs. Vxn expression caused a significant increase in RGCs and photoreceptors, that were confirmed to be calbindin-positive cones, which are born early (B). Later born bipolar cells and Mueller glia were significantly reduced. *p<0.001 relative to GFP,** p<0.001 relative to vxn + GFP.
(C) Injection of vxn mRNA into 1 cell of a 32- cell embryo produced a 2.5-fold increase in the number of GFP-labeled RGCs as compared to injection of GFP mRNA control alone, while vxn + atoh7 caused almost 90% of labeled cells to differentiate as RGCs. *p<0.001 relative to GFP alone, ** p<0.001 relative to vxn + GFP.
(D) Schematic of 3 retinal layers: the outer nuclear layer (ONL), the inner nuclear layer (ONL), and the retinal ganglion cell (RGC) layer. Injection of GFP mRNA alone reproducibly labeled neuronal subtypes within all 3 layers. Vxn + GFP mRNA injection resulted in an increased number of GFP-positive cells in the RGC layer (arrowheads). Vxn + atoh7 + GFP mRNA caused almost all labeled cells to differentiate as RGCs.
(E) Injection of mRNA for mouse vxn had a similar effect, causing a significant increase in early born cell types (GCs, HCs and PRs) at the expense of later born cell types (BPs, AMs). * p<0.001, # p<0.01, @p<0.02.
(F) Injection of mRNA for either Xenopus or mouse vxn together with GFP mRNA into blastomere V1.2.1 at the 32-cell stage resulted in reduced labeled retinal clone size in stage 41 retina, consistent with reduced retinal progenitor proliferation. *p < 0.01, as compared to GFP alone.
RGC, retinal ganglion cells; HC, horizontal cells; AC, amacrine cells; BP, bipolar cells; PR, photoreceptor cells; MG, Muller glial cells.
We investigated whether vxn could cooperate with atoh7 to promote retinal neurogenesis. We confirmed by injecting 400 pg of GFP and 250 pg of vxn mRNA into 1 dorsal blastomere at the 32-cell stage that vxn promoted a significant increase in RGCs at the expense of later born bipolar cells and Mueller glia in stage 41/42 retina, as compared to GFP alone (Fig. 7C, D; *= p<0.001; n=9 retinas, 1706 cells for GFP; n= 15 retinas, 3389 cells for GFP + vxn). Strikingly, co-injection of 250pg of atoh7 mRNA with 250pg of vxn mRNA resulted in almost 90% of labeled cells differentiating into RGCs, the earliest born cell type, which is significantly more than with vxn alone, and more than we previously observed after expression of atoh7 alone (Fig. 7C, D; **= p<0.001; (Kanekar et al., 1997; Moore et al., 2002)). We also injected 300 pg mouse vxn mRNA at the 32-cell stage and observed a similar increase in differentiation of RGCs and photoreceptors in stage 41/42 retina (Fig. 7E; *= p<0.001, #= p<0.01, @= p<0.02; n= 9 retinas, 1706 cells for GFP; n= 5 retinas, 650 cells for GFP + mouse vxn). Thus, we conclude that vxn has a conserved ability to enhance the differentiation of early born retinal cell types at the expense of later born cell types.
To determine if vxn overexpression inhibits proliferation of retinal progenitors, we performed quantitative clonal analysis by injecting GFP mRNA alone or together with vxn mRNA into the V1.2.1 blastomere of 32-cell stage embryos (Moore et al., 2002). The progeny of this blastomere contribute a small but reproducible number of cells to the retina (Huang and Moody, 1993). We found that vxn mRNA injection reduced retinal clone size by 65% (Fig. 7F; *= p<0.001; n=6 retinas, 1334 cells for GFP; n= 6 retinas, 446 cells for vxn + GFP), suggesting that proliferation is inhibited in vxn-expressing retinal progenitors as compared to those expressing only GFP. Mouse vxn had a similar effect on retinal progenitor proliferation, resulting in 45% reduction in retinal clone size (Fig. 7F; *= p<0.001; n= 6 retinas, 493 cells for mouse vxn + GFP). Thus, vxn enhances cell cycle exit and differentiation of retinal progenitors.
Vxn functions in the nucleus to enhance neurogenesis
We sought to determine whether Vxn functions in the nucleus or at the cell membrane to promote neurogenesis. To preferentially drive Vxn into the nucleus, we added the NLS sequence from SV40 T antigen (PKKKRKV; (Kalderon et al., 1984)) to the 5′ end of myc-tagged Xenopus vxn cDNA (Fig. 8A). We injected 1ng of mRNA at the 1 cell stage, and found by anti-myc immunostaining of animal cap tissue that the protein was localized exclusively to the nucleus (Fig. 8B). Overexpression of NLS-myc-vxn by injection of 1ng mRNA at the 2-cell stage expanded expression of N-tubulin in stage 15 embryos (Fig. 8C; 70%, n=22 embryos). As we observed following injection of vxn, there was no change in the levels of expression of Notch target genes hes5.1 (n=18) or nrarp (n=20). In addition, we observed inhibition of N-tubulin expression in 69% of embryos after injection of 40ng MO-B at the 2 cell stage (n=42) and this inhibition could be rescued by co-injection of 1 ng of mRNA encoding 5′ NLS-myc-vxn (11% reduced N-tubulin, n=53). To determine whether nuclear localized Vxn could influence retinal neurogenesis, we injected 400 pg of GFP and 250 pg of NLS-myc-vxn mRNA into 1 dorsal blastomere at the 32-cell stage and scored GFP labeled cells in retinal sections of stage 41/42 embryos. 5′ NLS-myc-vxn promoted a significant increase in RGCs, which is the earliest born cell type, as compared to GFP alone (Fig. 8D; *= p<0.001; n=5 retinas, 577 cells for GFP; n= 12 retinas, 2287 cells for GFP + NLS-myc-vxn). Thus, nuclear localized Vxn mimics the function of unmodified Vxn, suggesting that it can function in the nucleus to regulate neurogenesis.
Figure 8. Vxn functions within the nucleus to promote neural differentiation.
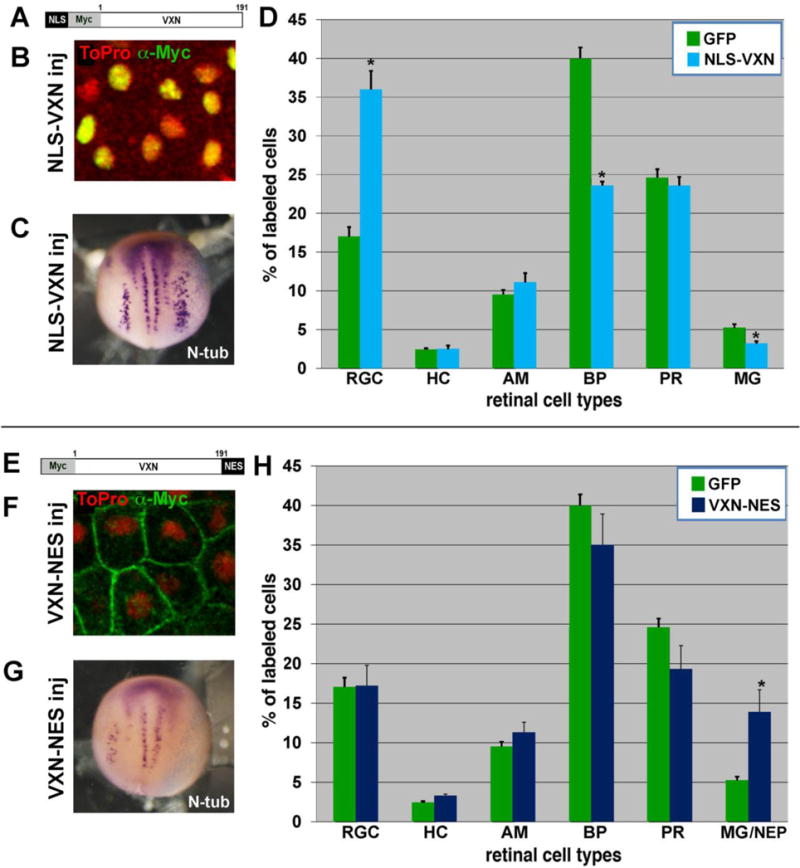
(A) Addition of NLS sequence at the N-terminus of Myc-Vxn to create NLS-myc-vxn.
(B) Anti-myc immunostaining for myc-tagged NLS-Vxn (green) shows localization exclusively in the nucleus (labeled by ToPro, red).
(C) Overexpression of NLS-myc-vxn mRNA promotes expansion of N-tubulin labeled primary neurons in the neural plate on the injected side (right).
(D) mRNA for GFP (300pg) was injected alone or together with mRNA for NLS-myc-vxn (250 pg) into 1 dorsal blastomere at the 32-cell stage. Embryos were cryosectioned at stage 41, and GFP-labeled retinal cell types were counted. NLS-myc-vxn expression caused a significant increase in RGCs. *p<0.001.
(E) Addition of NES sequence at the C-terminus of 5′ myc-vxn to create 5′myc-vxn-NES.
(F) Anti-myc immunostaining for myc-tagged Vxn-NES (green) shows localization exclusively at the membrane and excluded from the nucleus (labeled by ToPro, red).
(G) Overexpression of 5′myc-vxn-NES mRNA inhibits differentiation of N-tubulin labeled primary neurons in the neural plate on the injected side (right).
(H) mRNA for GFP (300pg) was injected alone or together with mRNA for 5′myc-vxn-NES (250 pg) into 1 dorsal blastomere at the 32-cell stage. Embryos were cryosectioned at stage 41, and GFP-labeled retinal cell types were counted. Vxn-NES expression caused a significant increase in Mueller glia/NEPs. *p<0.001.
To test whether nuclear localization is required for Vxn function, we added the nuclear export sequence (NES) from MAPKK (LQKKLEELEL; (Kutay and Guttinger, 2005)) to the 3′ end of myc-tagged Xenopus vxn cDNA (Fig. 8E). Anti-myc immunostaining of animal cap tissue after injection of 1ng of mRNA at the 1-cell stage showed that the protein was excluded from the nucleus and localized exclusively at the cell membrane (Fig. 8F). To test whether myc-vxn-NES could influence neurogenesis, we injected 1 ng mRNA into 1 cell of 2-cell stage embryos. We observed reduced expression of N-tubulin on the injected side in stage 15 embryos, similar to what we saw after injection of vxn MO, suggesting that myc-Vxn-NES may function as a dominant-negative protein (Fig. 8G; 54%, N= 170 embryos). Consistent with this, we also observed expansion of Sox2-labeled neural progenitors in the neural plate (data not shown; 72%, n=22). Furthermore, we saw no change in the levels of expression of Notch target genes hes5.1 (n=16) or nrarp (n=13), confirming that myc-vxn-NES does not inhibit neurogenesis by increasing Notch signaling. We observed gastrulation and neural tube closure defects in embryos injected with myc-vxn-NES mRNA (Fig. S4). Thus, Vxn localized exclusively to the membrane may interfere with morphogenetic movements in the embryo.
To determine whether Vxn that is excluded from the nucleus can alter retinal neurogenesis, we injected 400 pg of GFP and 250 pg of myc-vxn-NES mRNA into 1 dorsal blastomere at the 32-cell stage. In stage 41/42 retina, we observed a significant increase in the production of MG/NEP cells, as compared to GFP alone (Fig. 8H; *= p<0.001; n=5 retinas, 577 cells for GFP; n= 8 retinas, 1854 cells for GFP + myc-vxn-NES). This inhibition of retinal neurogenesis is similar to what was observed following injection of vxn MO, further indicating that myc-Vxn-NES may antagonize normal Vxn function. Thus, nuclear localization is both necessary and sufficient for Vxn to enhance neurogenesis.
Vxn and p27Xic1 require each other to regulate neurogenesis
Since overexpression of vxn reduced progenitors in the neural plate and clone size in the retina, we asked whether it increases cell cycle exit. We co-injected 1 ng of vxn mRNA with 400 pg of GFP mRNA with into 1 cell of 2-cell stage embryos and performed immunostaining at stage 14 for phosphorylated histone 3 (pH3), which marks dividing cells at the G2/M transition. We found that vxn reduced the number of pH3-labeled cells on the injected side by 50% (Fig. 9C-E; *= p<0.01, n=10 embryos), while control GFP injection had no significant effect (Fig. 9A, B, E; n=10 embryos). pH3 staining was reduced in both the neural plate region and also in lateral ectoderm (bracket in Fig. 9D), suggesting that vxn inhibits proliferation in the absence of endogenous neuronal differentiation factors. Co-injection of 50pg of mRNA for cyclin E (ccnde), which can promote cell cycle progression from G1 arrest (Ohnuma et al., 2002), reversed the decrease in pH3 on the injected side (Fig. S5A; *= p<0.001, n=13 embryos).
Figure 9. Vxn and p27Xic1 work together to regulate cell cycle exit and neurogenesis.
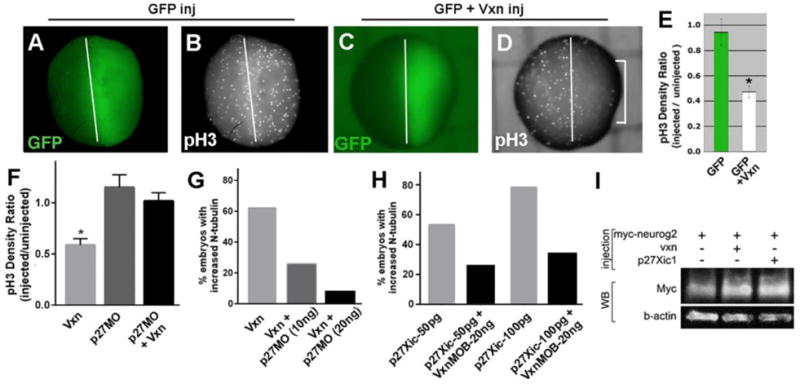
(A-E) Injection of 1ng GFP mRNA at 2CS (A) does not change pH3 labeling (B), while co-injection of 1ng vxn mRNA causes significant reduction of pH3 labeling on the injected side (bracket in D; E). * = p<0.01.
(F, G) Co-injection of p27Xic1 morpholino (p27MO) with vxn mRNA rescues the pH3 levels (F, *p< 0.001), and prevents expanded N-tubulin (G) on the injected side.
(H) Injection of p27Xic1 mRNA at 2CS promotes expanded N-tubulin, which is reversed by co-injection of 20ng vxn MO-B.
(I) Western blot (WB) to detect Myc-Neurog2 when expressed alone or together with vxn or p27Xic1. β-actin was detected as a loading control.
For all experiments, mRNA encoding GFP was co-injected to label the injected side (right).
A key regulator of both cell cycle exit and neurogenesis during Xenopus neural development is the cyclin-dependent kinase inhibitor p27Xic1 (Vernon et al., 2003), so we tested whether vxn function depends upon p27Xic1. The reduction of pH3 labeling caused by injection of 1ng vxn mRNA (*= p<0.01), was blocked when it was injected together with 10ng p27Xic1 MO (Fig. 9F; n=16), indicating that vxn requires p27Xic1 to inhibit proliferation. Furthermore, expansion of N-tubulin expression following 1ng vxn mRNA injection (62%, n=35) was suppressed by co-injection of 10ng or 20ng 27Xic1 MO (Fig. 9G, 25%, n=35 and 7%, n=125 respectively). Conversely, injection of 50pg or 100pg of p27Xic1 mRNA at the 2-cell stage results in expansion of the stripes of N-tubulin labeled primary neurons at stage 15 (Fig. 9H; 53%, n= 49 and 78%, n=49), and this was reduced by co-injection 20ng vxn MO-B (Fig. 9H; 25%, n=32 and 33%, n=53). Injection of vxn mRNA into one cell at the 2-cell stage resulted in upregulation of p27Xic1 on the injected side (Fig. S5B; 72%, n=65 embryos), but injection of vxn MO did not result in loss of p27Xic1 expression, thus this effect may be indirect. Thus, we conclude that vxn and p27Xic1 functionally depend upon each other for their ability to enhance neurogenesis.
We assessed whether vxn may enhance levels of proneural protein, similar to p27Xic1 (Vernon et al., 2003) and p27Kip1 (Nguyen et al., 2006). We injected mRNA at the 1-cell stage for myc-neurog2 either alone or together with vxn or p27Xic1, and performed western blot of lysates from stage 14 embryos. We found that co-expression of either vxn or p27Xic1 enhanced the levels of exogenously expressed Neurog2 protein (2-fold and 2.7-fold respectively), indicating that both Vxn and p27Xic1 can promote post-transcriptional accumulation of this protein (Fig 9I; average fold increase for Vxn + Myc-Neurog2 = 3.3±1.4, n=3).
DISCUSSION
Vxn encodes a novel protein conserved in vertebrates
We describe the expression and function of a novel gene that is required for neurogenesis during Xenopus development. Vxn was originally identified as a fragment in a screen for direct shared targets of neural bHLH factors Atoh7 and Neurod1 (sbt1; (Logan et al., 2005)), and was also identified in an independent screen in Xenopus for direct targets of Neurog2 and Neurod1 (Seo et al., 2007). The gene is orthologous to human c8orf46, and encodes a protein that is conserved across vertebrate species. The only conserved domain identified is a domain of unknown function (DUF4648), a member of the SH3 superfamily which is known to bind proline-rich sequences. SH3 domains are involved in mediating protein-protein interactions, particularly of proteins involved in cytoplasmic signaling. Since Vxn lacks any apparent catalytic activity, it likely functions as a component of a protein signaling network (Pawson and Schlessingert, 1993). There likely remain additional unidentified genes that are involved in coordinating the process of neural differentiation. Our findings, and other studies (Martinez-De Luna et al., 2013), highlight the value of using Xenopus for analyzing the function of novel genes potentially involved in regulating neurogenesis.
Vxn is transiently expressed in the developing Xenopus CNS as progenitors initiate differentiation, consistent with its role as a neural bHLH target gene. There is a high degree of functional conservation for this protein across divergent species since mouse vxn can enhance neural differentiation in the Xenopus neural plate and the retina. In mouse, the vxn ortholog is expressed during neurogenesis, but is also highly enriched in the adult brain, particularly in the cerebral cortex (unpublished, and http://mouse.brain-map.org/). C8orf46 orthologue was highlighted as a member of the brain “ignorome”, which are genes that are highly enriched in brain but that have little to no associated scientific literature (Pandey et al., 2014). In post-mitotic neurons of the mouse brain vxn may have a different function than in progenitors, or it may have species-specific or stage–dependent roles depending upon the availability of partner proteins.
Vxn functions in the nucleus to regulate neurogenesis
Vxn protein has an unusual subcellular distribution, being localized to both the cell membrane and the nucleus in the Xenopus neural plate. Within the nucleus, we observed subnuclear localization in domains that stain weakly with Hoechst. This nuclear localization is conserved in human cells, potentially in nuclear speckles suggesting a possible role in pre-mRNA splicing (SK-MEL-30 human melanoma cells; proteinatlas.org). Nuclear localization did not depend upon the candidate NLS sequence in the C-terminus of the protein, suggesting association with other proteins to achieve this localization.
Vxn must function in the nucleus to enhance neurogenesis, and it acts as a dominant negative when excluded from the nucleus, suggesting that it may relocalize critical partner proteins out of the nucleus. One important mode of regulation for p27 is regulation of nuclear localization (Hnit et al., 2015), however we found no evidence that coexpression of NES-Vxn altered the localization of tagged p27Xic1. It remains to be determined whether Vxn shuttles between the nucleus and cell membrane, although Vxn that is strictly localized to the nucleus functions normally to promote neurogenesis. It is unclear whether Vxn has a function at the cell membrane. Versions of Vxn with mutation of the NLS sequence, or truncation of the C-terminus caused unusual loops at the membrane when expressed in animal cap explants. In addition, overexpression of NES-Vxn caused defects in convergent extension. This could be due to a direct role in morphogenesis, or due to disruption of normal cell cycle kinetics, since disruption of cell cycle exit can cause defects in convergent extension of the paraxial mesoderm (Leise and Mueller, 2004).
Vxn is required for neural differentiation
We found that knockdown of vxn prevents neural differentiation in the neural plate and retina, which results in expansion of progenitors. There was no reduction in the expression of the neural bHLH factors themselves, but expression of target genes as well as downstream differentiation genes was inhibited, suggesting that vxn regulates aspects of proneural factor function. Overexpression of vxn did not promote ectopic neurogenesis in non-neural ectoderm, but rather expanded neural differentiation within existing domains of proneural gene expression, and we found that vxn strongly cooperates with neural bHLH factors to promote neurogenesis. Thus, the ability of vxn to regulate neurogenesis depends upon the activity of neural bHLH factors. Since vxn is a direct proneural target gene (Logan et al., 2005), we propose that once expressed it reinforces neural bHLH factor activity to consolidate the neural differentiation process. We found that vxn could enhance levels of exogenously expressed Neurog2 protein, suggesting that it may enhance proneural protein stability. One important mode for regulation of neural bHLH factor function and neurogenesis is through multisite phosphorylation on serine-proline residues by cyclin-dependent kinases (cdks) and potentially other proline-directed kinases, which reduces protein stability and DNA binding and thus constrains differentiation (Ali et al., 2011; Hardwick and Philpott, 2014, 2015; Hindley et al., 2012; McDowell et al., 2014; Philpott, 2015). It is therefore possible that Vxn functions to regulate neural bHLH activity by modulating multi-site phosphorylation or other post-translational modifications.
Vxn and p27Xic1 depend upon each other to enhance neurogenesis
The ability of Vxn to regulate neurogenesis bears striking similarity to what has been reported for p27Xic1 (cdkn1b), the lone member of the Cip/Kip cyclin dependent kinase inhibitor (CDKi) family in Xenopus (Vernon et al., 2003). p27Xic1 is not a direct target for neural bHLH factors, but like vxn it is required for the differentiation of primary neurons, and when overexpressed can enhance neurogenesis within existing domains of proneural gene expression (Vernon et al., 2003). In addition, co-expression of p27Xic1 and atoh7 significantly potentiates the generation of retinal ganglion cells as compared to atoh7 alone, similar to what we observed with co-expression of vxn (Ohnuma et al., 2002). The ability of p27Xic1 to enhance neural bHLH activity and neurogenesis can be uncoupled from cell cycle regulation (Vernon et al., 2003).
We find evidence that p27Xic1 and vxn work together. Vxn inhibits proliferation throughout the injected side of the embryo, consistent with the broad expression of p27Xic1 in the neural plate and myotome, and vxn requires p27Xic1 to inhibit proliferation. The ability of vxn to increase neurogenesis is limited to the domains of primary neuron formation, consistent with the additional requirement for neural bHLH gene expression, and both vxn and p27Xic1 depend upon each other to enhance neurogenesis. Furthermore, they both promote accumulation of Neurog2 protein. We propose that vxn provides a key link between neural bHLH activity and execution of the neurogenic program in cooperation with p27Xic1.
Since vxn was required for p27Xic1 to expand primary neurogenesis, vxn likely modulates p27Xic1 function, potentially through post-translational modification, protein stability or subcellular localization (Hnit et al., 2015). We did not find evidence for direct interaction between Vxn and p27Xic1, although this cannot be ruled out. Vxn may be acting on regulators of p27Xic1, such as the E3 ubiquitin ligase SCFskp2, which targets p27Xic1 for proteolysis, and negatively regulates neurogenesis independent of effects on cell cycle regulation (Boix-Perales et al., 2007). Another protein NM23-X4 interacts with and negatively regulates p27Xic1 during Xenopus retinal gliogenesis, but its pattern of expression is quite distinct from vxn, so they likely have independent roles (Mochizuki et al., 2009). Overall, we suggest that vxn may promote neurogenesis by facilitating p27Xic1 function, and together they enhance levels and/or activity of neural bHLH factors.
Notably, misregulation of c8orf46 is associated with human cancer, particularly breast cancer, where it is part of a 45-gene signature that is highly predictive of recurrence in metastatic triple-negative breast cancer (Kuo et al., 2012). In addition, the estrogen-responsive enhancer region of c8orf46 becomes hypermethylated in endocrine-resistant cancer cells from patients in which cancer has relapsed (Guan et al., 2010; He et al., 2012; Stone et al., 2015). Since misregulation of p27 is also associated with breast cancer, it will be interesting to investigate the relationship of these two genes in cancer (Guan et al., 2010; He et al., 2012).
Supplementary Material
Highlights.
Vexin (vxn) is a c8orf46 homolog that is a neural bHLH target gene in Xenopus.
Neurogenesis in the neural plate and retina requires vxn.
Vxn cooperates with neural bHLH factors to promote neurogenesis.
p27Xic1 (cdkn1b) and vxn depend upon each other to enhance neurogenesis
Vxn protein functions in the nucleus to regulate neurogenesis
Acknowledgments
We acknowledge Constance Dooley, Jianmin Zhang, and Kaitlyn Phuong Le for technical contributions and Ella Maricq for naming the gene. This research was supported by the National Institutes of Health, through the National Eye Institute under Ruth L. Kirschstein National Research Service Award T32 EY024234 to JMR, and EY012274 and EY017650 to MLV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS:
No competing interests declared.
References
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Hindley C, McDowell G, Deibler R, Jones A, Kirschner M, Guillemot F, Philpott A. Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development. 2011;138:4267–4277. doi: 10.1242/dev.067900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang AG, Papalopulu N, Kintner C, Goulding MD. Expression of Pax-3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development. 1997;124:2075–2085. doi: 10.1242/dev.124.10.2075. [DOI] [PubMed] [Google Scholar]
- Bellefroid EJ, Bourguignon C, Hollemann T, Ma Q, Anderson DJ, Kintner C, Pieler T. X-MyT1, a Xenopus C2HC-type zinc finger protein with a regulatory function in neuronal differentiation. Cell. 1996;87:1191–1202. doi: 10.1016/s0092-8674(00)81815-2. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Boix-Perales H, Horan I, Wise H, Lin HR, Chuang LC, Yew PR, Philpott A. The E3 ubiquitin ligase skp2 regulates neural differentiation independent from the cell cycle. Neural development. 2007;2:27. doi: 10.1186/1749-8104-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. The Journal of cell biology. 1983;97:703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Vetter ML. Xath5 regulates neurogenesis in the Xenopus olfactory placode. Dev Dyn. 2002;225:536–543. doi: 10.1002/dvdy.10189. [DOI] [PubMed] [Google Scholar]
- Castro DS, Guillemot F. Old and new functions of proneural factors revealed by the genome-wide characterization of their transcriptional targets. Cell cycle. 2011;10:4026–4031. doi: 10.4161/cc.10.23.18578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- D'Autilia S, Decembrini S, Casarosa S, He RQ, Barsacchi G, Cremisi F, Andreazzoli M. Cloning and developmental expression of the Xenopus homeobox gene Xvsx1. Dev Genes Evol. 2006;216:829–834. doi: 10.1007/s00427-006-0109-0. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Good PJ. A conserved family of elav-like genes in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Wang Y, Xie R, Chen L, Bai J, Lu J, Kuo MT. p27(Kip1) as a prognostic factor in breast cancer: a systematic review and meta-analysis. J Cell Mol Med. 2010;14:944–953. doi: 10.1111/j.1582-4934.2009.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick LJ, Philpott A. Nervous decision-making: to divide or differentiate. Trends Genet. 2014;30:254–261. doi: 10.1016/j.tig.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick LJ, Philpott A. Multi-site phosphorylation regulates NeuroD4 activity during primary neurogenesis: a conserved mechanism amongst proneural proteins. Neural development. 2015;10:15. doi: 10.1186/s13064-015-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Wang X, Chen L, Guan X. A crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother Radiopharm. 2012;27:399–402. doi: 10.1089/cbr.2010.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley C, Ali F, McDowell G, Cheng K, Jones A, Guillemot F, Philpott A. Post-translational modification of Ngn2 differentially affects transcription of distinct targets to regulate the balance between progenitor maintenance and differentiation. Development. 2012;139:1718–1723. doi: 10.1242/dev.077552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley C, Philpott A. Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem J. 2012;444:375–382. doi: 10.1042/BJ20112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnit SS, Xie C, Yao M, Holst J, Bensoussan A, De Souza P, Li Z, Dong Q. p27(Kip1) signaling: Transcriptional and post-translational regulation. Int J Biochem Cell Biol. 2015;68:9–14. doi: 10.1016/j.biocel.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Huang C, Chan JA, Schuurmans C. Proneural bHLH genes in development and disease. Current topics in developmental biology. 2014;110:75–127. doi: 10.1016/B978-0-12-405943-6.00002-6. [DOI] [PubMed] [Google Scholar]
- Huang S, Moody SA. The retinal fate of Xenopus cleavage stage progenitors is dependent upon blastomere position and competence: studies of normal and regulated clones. J Neurosci. 1993;13:3193–3210. doi: 10.1523/JNEUROSCI.13-08-03193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Developmental biology. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Chang YY, Lai LC, Tsai MH, Hsiao CK, Chang KJ, Chuang EY. Molecular characteristics and metastasis predictor genes of triple-negative breast cancer: a clinical study of triple-negative breast carcinomas. PloS one. 2012;7:e45831. doi: 10.1371/journal.pone.0045831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Guttinger S. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 2005;15:121–124. doi: 10.1016/j.tcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lamar E, Deblandre G, Wettstein D, Gawantka V, Pollet N, Niehrs C, Kintner C. Nrarp is a novel intracellular component of the Notch signaling pathway. Genes & development. 2001;15:1885–1899. doi: 10.1101/gad.908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Leise WF, Mueller PR., 3rd Inhibition of the cell cycle is required for convergent extension of the paraxial mesoderm during Xenopus neurulation. Development. 2004;131:1703–1715. doi: 10.1242/dev.01054. [DOI] [PubMed] [Google Scholar]
- Lin JR, Hu J. SeqNLS: nuclear localization signal prediction based on frequent pattern mining and linear motif scoring. PloS one. 2013;8:e76864. doi: 10.1371/journal.pone.0076864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MA, Steele MR, Van Raay TJ, Vetter ML. Identification of shared transcriptional targets for the proneural bHLH factors Xath5 and XNeuroD. Developmental biology. 2005;285:570–583. doi: 10.1016/j.ydbio.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Martinez-De Luna RI, Ku RY, Lyou Y, Zuber ME. Maturin is a novel protein required for differentiation during primary neurogenesis. Developmental biology. 2013;384:26–40. doi: 10.1016/j.ydbio.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell GS, Hindley CJ, Lippens G, Landrieu I, Philpott A. Phosphorylation in intrinsically disordered regions regulates the activity of Neurogenin2. BMC Biochem. 2014;15:24. doi: 10.1186/s12858-014-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Bilitou A, Waters CT, Hussain K, Zollo M, Ohnuma S. Xenopus NM23-X4 regulates retinal gliogenesis through interaction with p27Xic1. Neural development. 2009;4:1. doi: 10.1186/1749-8104-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KB, Schneider ML, Vetter ML. Posttranslational mechanisms control the timing of bHLH function and regulate retinal cell fate. Neuron. 2002;34:183–195. doi: 10.1016/s0896-6273(02)00666-9. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes & development. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Pub; New York: 1994. [Google Scholar]
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–2446. doi: 10.1242/dev.129.10.2435. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Lu L, Wang X, Homayouni R, Williams RW. Functionally enigmatic genes: a case study of the brain ignorome. PloS one. 2014;9:e88889. doi: 10.1371/journal.pone.0088889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalopulu N, Kintner C. A Xenopus gene, Xbr-1, defines a novel class of homeobox genes and is expressed in the dorsal ciliary margin of the eye. Developmental biology. 1996;174:104–114. doi: 10.1006/dbio.1996.0055. [DOI] [PubMed] [Google Scholar]
- Patterson KD, Cleaver O, Gerber WV, White FG, Krieg PA. Distinct expression patterns for two Xenopus Bar homeobox genes. Dev Genes Evol. 2000;210:140–144. doi: 10.1007/s004270050020. [DOI] [PubMed] [Google Scholar]
- Pawson T, Schlessingert J. SH2 and SH3 domains. Current biology : CB. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Developmental biology. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Philpott A. Multi-site phospho-regulation of proneural transcription factors controls proliferation versus differentiation in development and reprogramming. Neurogenesis (Austin) 2015;2:e1049733. doi: 10.1080/23262133.2015.1049733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron. 2011;69:840–855. doi: 10.1016/j.neuron.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Pozzoli O, Bosetti A, Croci L, Consalez GG, Vetter ML. Xebf3 is a regulator of neuronal differentiation during primary neurogenesis in Xenopus. Developmental biology. 2001;233:495–512. doi: 10.1006/dbio.2001.0230. [DOI] [PubMed] [Google Scholar]
- Richter K, Grunz H, Dawid IB. Gene expression in the embryonic nervous system of Xenopus laevis. Proc Natl Acad Sci U S A. 1988;85:8086–8090. doi: 10.1073/pnas.85.21.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souopgui J, Solter M, Pieler T. XPak3 promotes cell cycle withdrawal during primary neurogenesis in Xenopus laevis. EMBO J. 2002;21:6429–6439. doi: 10.1093/emboj/cdf644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A, Zotenko E, Locke WJ, Korbie D, Millar EK, Pidsley R, Stirzaker C, Graham P, Trau M, Musgrove EA, Nicholson RI, Gee JM, Clark SJ. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nat Commun. 2015;6:7758. doi: 10.1038/ncomms8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes & development. 1994a;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994b;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Vernon AE, Devine C, Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development. 2003;130:85–92. doi: 10.1242/dev.00193. [DOI] [PubMed] [Google Scholar]
- Vernon AE, Philpott A. A single cdk inhibitor, p27Xic1, functions beyond cell cycle regulation to promote muscle differentiation in Xenopus. Development. 2003;130:71–83. doi: 10.1242/dev.00180. [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein DA, Turner DL, Kintner C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


