Abstract
Fetal megakaryocytes (Mk) differ from adult Mk in key parameters that affect their capacity for platelet production. However, despite being smaller, more proliferative, and less polyploid, fetal Mk generally mature in the same manner as adult. The phenotypic features unique to fetal Mk predispose patients to several disease conditions: infantile thrombocytopenia, infantile megakaryoblastic leukemias, and poor platelet recovery after umbilical cord blood stem cell transplants. Ontogenic Mk differences also affect new strategies being developed to address global shortages of platelet transfusion units. These donor-independent, ex vivo production platforms are hampered by the limited proliferative capacity of adult-type Mk and the inferior platelet production by fetal-type Mk. Understanding the molecular programs that distinguish fetal versus adult megakaryopoiesis will help in improving approaches to these clinical problems. This review summarizes the phenotypic differences between fetal and adult Mk, the disease states associated with fetal megakaryopoiesis, and recent advances in the understanding of mechanisms that determine ontogenic Mk transitions.
Keywords: ontogeny, megakaryopoiesis, fetal, adult, platelets
Introduction
Mk are specialized mammalian marrow cells responsible for platelet production. They arise from bipotent megakaryocyte-erythroid progenitors [1]. Their differentiation includes a unique program of endomitosis that drives nuclear polyploidization and cellular enlargement. This process is accompanied by lineage consolidation which involves downregulation of erythroid genes and upregulation of Mk surface markers, as well as development of cytoplasmic granules, multivesicular bodies and demarcation membranes. Upon achieving polyploidization and enlargement, they undergo cytoskeletal remodeling to induce formation of proplatelets, preplatelets, and ultimately, platelets [2]. During mammalian embryogenesis, primitive Mk with limited polyploidization capacity appear early in the yolk sac. The first definitive Mk arise from hematopoietic progenitors in the fetal liver (FL) and are then produced in the bone marrow (BM). In humans there are clear phenotypic differences between fetal/neonatal (collectively referred to as fetal) and adult Mk, and these differences have major health implications.
Phenotypic differences and similarities between fetal and adult Mk
The smaller size of fetal Mk has been well-established for several decades (Table 1). Using immunohistochemical staining and light microscope morphometry on normal human tissues, Allen Graeve and de Alarcon estimated diameters of 14.0–15.2 microns for fetal Mk at 12 to 21 weeks gestation as compared to 18.4–20.6 microns for adult Mk [3]. Subsequent studies confirmed the diameters of human fetal Mk at 3 months gestation to range from 12.4–14.8 microns depending on whether marrow or liver was analyzed. Although diameters reached 16.1 microns by 7 months gestation, fetal Mk remained consistently smaller than those produced in human adult BM (21.9 microns in diameter) [4]. A unimodal Gaussian distribution of Mk size persists in neonates and infants until approximately 24 months. At this age, the size distribution becomes bimodal with sub-populations of smaller and larger Mk, and by age 4, most Mk have transitioned into a larger size range characteristic of adulthood [5]. While most studies have focused on normal individuals, the size difference between fetal and adult Mk has also been observed in thrombocytopenic subjects [6].
Table 1.
Phenotypic characteristics of fetal and adult Mk
| Parameter | Fetal/neonatal Mk | Adult Mk | References |
|---|---|---|---|
| Size | Smaller | Larger | [3–6] |
| Polyploidization | Less polyploid | More polyploid | [4, 7, 8] |
| Proliferation | Hyperproliferative in ex vivo culture | Less proliferative in ex vivo culture | [8, 9] |
| Maturation | Express Mk maturation markers | Express Mk maturation markers | [8, 9] |
| Proplatelet formation | Form fewer proplatelets | Form more proplatelets | [8, 16] |
Concurrent with smaller size, fetal Mk also have a lower ploidy that increases with ontogenic stage [7]. Using in situ DNA staining of human FL tissue sections, investigators found the percentage of Mk with 8N ploidy to increase from 16% at 3 months gestation to 33% at 6 months. Mk ≥ 16N were seen only after 7 to 8 months gestation. In BM, only 24% of late fetal Mk had ≥ 64N ploidy, compared to 68% of adult BM Mk [4]. Similar findings have been reported with ex vivo culture derived Mk from cord blood (CB) versus adult peripheral blood (PB) progenitors [8]. Specifically, ~80% of CB derived Mk had 2N ploidy and 2.6% were 8N, while 40% of the PB derived Mk had ≥ 8N ploidy [8].
Fetal and adult Mk also differ in mitotic rates, with multiple experiments showing increased proliferation of fetal Mk. In Mk cultures conducted under standardized conditions, Liu et. al. described a 70-fold expansion of CB CD34+ cells as compared to 5-fold in PB CD34+ cells [9]. In another similarly performed study, CB progenitors expanded 60-fold while PB cells underwent only 10-fold amplification [8]. The ontogenic differences in progenitor expandability inversely reflect their capacity for polyploidization, suggesting that the diminished proliferation of adult progenitors may result from enhanced transition to endomitosis [9].
Although fetal Mk undergo incomplete enlargement and polyploidization, they fully upregulate most lineage-specific factors including the membrane receptors and granule components necessary for platelet formation and function. Thus, fetal and adult Mk express similar levels of membrane proteins integrin alpha-IIb (CD41), integrin beta-3 (CD61), and glycoprotein Ibα (CD42b) [10]. Importantly, the CD42 complex represents a marker of late-stage Mk differentiation [11]. CB progenitor derived Mk also demonstrate abundant expression of the platelet proteins VWF and P-selectin [9]. CB Mk were also observed to have mature ultrastructural characteristics such as an enlarged cytoplasm, abundant granules, and a well-developed demarcation membrane system (DMS). Similar observations have been made with murine neonatal Mk [9].
The most important phenotypic difference between fetal and adult Mk concerns platelet producing efficiency. Despite their mature marker expression, CB derived Mk produce 3-fold fewer proplatelets and platelets on a per-cell basis as compared with PB derived Mk [8]. This difference likely results from the fetal polyploidization deficit, which limits enlargement and ultimately restricts the allocation of cell mass toward platelet formation. Whether fetal Mk have additional proplatelet formation defects independent of their size remains to be determined.
Clinical significance of the infantile megakaryocyte phenotype
The distinct phenotypic features of fetal Mk may have a variety of clinical consequences (Table 2). Considered below are four problems in which this phenotype has been implicated as a major contributing factor. The first of these problems, neonatal thrombocytopenia, occurs in ~5% of all neonates, 22–35% of NICU admissions, and ~73% of low birth weight infants <1000 g [12]. The propensity for thrombocytopenia is inversely proportional to gestational age and results from cell-intrinsic defects in Mk morphogenesis (i.e. enlargement and polyploidization) [12]. One of the most common inciting features consists of sepsis, most likely due to an increased demand placed on platelet production. In a recent study in the Netherlands, sepsis was identified in 7% of all hospitalized neonates, and severe thrombocytopenia (<50,000 platelets/µl) occurred in 20% of septic patients [13]. The presence of thrombocytopenia in this cohort increased risk of mortality almost 4-fold. Management of neonatal thrombocytopenia remains controversial, with platelet transfusions frequently provided to prevent intra-ventricular hemorrhage (IVH). A recent clinical trial confirmed that neonatal thrombocytopenia predisposes to IVH but found no correlation between the degree of risk and the degree of thrombocytopenia and could identify no benefit associated with platelet transfusion [14]. Against such a tenuous benefit must be weighed the risks of platelet transfusion, which include bacterial infection, transfusion mediated lung injury, alloimmunization, and financial cost. Thrombopoietin (Tpo) receptor agonists have gained widespread clinical use in enhancing platelet production in adults. However, compelling in vitro data predict that these agents will lack efficacy in neonates, as Tpo stimulation paradoxically exacerbates defects in infantile Mk morphogenesis [15].
Table 2.
Clinical significance of fetal Mk and challenges of ex vivo platelet formation
| Clinical and therapeutic impact of Mk ontogeny | References |
|---|---|
| Neonatal thrombocytopenia | [12, 13] |
| Premature neonates | |
| Neonates with sepsis | |
|
| |
| Delayed platelet recovery after CB-HSC transplantation | [16, 18] |
| The need for more frequent platelet transfusions after CB-SC transplants | |
|
| |
| Megakaryoblastic neoplasia | [19, 20, 22] |
| Down syndrome transient myeloproliferative disorder | |
| Acute megakaryoblastic leukemia with the RBM15-MKL1 fusion | |
|
| |
| Challenges of ex vivo platelet production as a source for platelets | [25, 26] |
| Poor proliferative capacity of adult Mk | |
| Infantile nature of CB derived and iPSC derived Mk | |
| Inefficient platelet formation ex vivo | |
The second problem consists of delayed platelet recovery in umbilical cord blood hematopoietic stem cell (CB-HSC) transplant recipients [16]. For transplantation, CB-HSC offer several advantages over adult HSC and may represent the only curative option for hard-to-match patients with lethal diseases [17]. A major drawback of CB-HSC transplantation has been inferior platelet recovery. In a study of adult leukemia/lymphoma patients, CB-HSC recipients experienced a 3-fold delay in the time to platelet independence as compared with recipients of adult PB HSC [18]. This delay translated into a 2-fold increase in the number of platelet transfusions required. In pediatric transplant recipients, CB-HSC were associated with a 2.3-fold delay in platelet recovery, and marrow morphometry documented equivalent Mk numbers but decreased Mk size in CB-HSC recipients as compared with adult HSC recipients [16]. In fact, the differences in Mk size correlated directly with the differences in platelet recovery.
The third clinical problem concerns the leukemic propensity of fetal Mk progenitors. Two distinct Mk neoplasms occur almost exclusively in neonates: 1) Down syndrome associated transient myeloproliferative disorder (DS-TMD), and 2) acute megakaryoblastic leukemia with the RBM15-MKL1 gene fusion (AMKL R-M) [19]. Epidemiologic profiles suggest that fetal status may constitute an oncogenic “hit” for these entities. Such a concept is supported in DS-TMD by the spontaneous disease regression as neonates age over several months [19]. Recent whole exome sequencing studies provide further support. One such study has shown that the majority of DS-TMD cases carry no secondary genetic abnormalities beyond the hallmark GATA1s mutations coupled with trisomy 21 [20]. A subsequent study demonstrated that DS-AMKL arises from a TMD clone that acquires additional mutations in multiple genes including cohesin components, CTCF, epigenetic regulators like EZH2 and KANSL1 and members of signaling pathways such as JAK family and RAS pathways [21]. Similarly, AMKL R-M displays a strikingly sparse mutational landscape as compared with other classes of non-Down syndrome AMKL [22]. Murine models also highlight the importance of ontogenic stage, with knockins for both GATA1s and RBM15-MKL1 displaying Mk abnormalities that are largely restricted to the fetal liver period [23, 24].
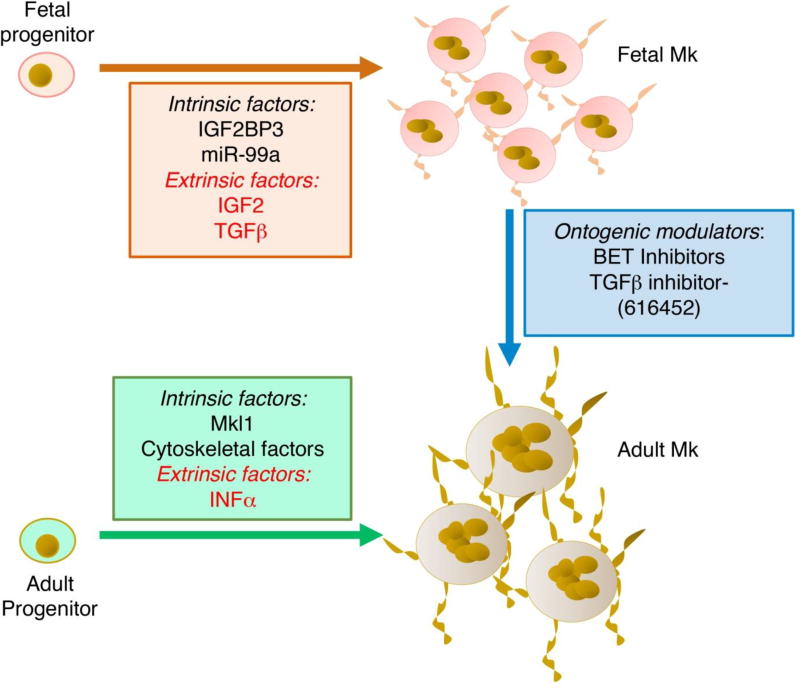
The fourth problem relates to recent initiatives to develop donor-independent sources of platelets to treat patients with thrombocytopenia. The need for such sources is emerging in developed countries due to steadily rising platelet demands coupled with restricted donor supplies [25]. Recent refinements in ex vivo culture of Mk derived from a variety of sources have enhanced feasibility of producing bioactive platelets or Mk for transfusion [25, 26]. A major limiting factor in this process is the poor proliferative capacity of adult type Mk in culture, despite efficient platelet biogenesis. By contrast, CB Mk proliferate extensively in culture but show limited platelet production. New technology has permitted Mk generation from iPSC, raising the possibility of personalized platelet cultivation [27]. However, the infantile nature of iPSC-derived Mk greatly restricts the efficiency of platelet production in this system [28]. Thus, efficient scale-up will require a biphasic system in which Mk expansion in accomplished in fetal mode, followed by induction of an adult program to maximize platelet production (Figure 1).
Figure 1.
Schematic model of fetal versus adult Mk generated in ex-vivo culture. The graph depicts some of the features of Mk derived from fetal progenitors such as enhanced proliferation and impaired morphogenesis. In contrast, Mk derived from adult progenitors show enhanced morphogenesis and diminished proliferation. Listed are intrinsic and extrinsic factors that determine these differences. Ontogenic modulators represent pharmacologic agents that promote phenotypic switching through targeting of intrinsic and extrinsic determinants.
Molecular differences between fetal and adult megakaryopoiesis
Multiple signaling and transcriptional programs control megakaryopoiesis. The phenotypic differences between fetal and adult Mk likely arise from ontogenic differences in these programs. Studies from the past three decades have revealed several molecular differences between fetal and adult stage megakaryopoiesis (Table 3). Most of these differences are cell-intrinsic, but the microenvironment may also contribute to Mk ontogenic transitions. In addition, recent work suggests that developmental origin may also distinguish fetal from adult Mk progenitors [29]. In particular, adult Mk progenitors appear to originate from the HSC compartment while fetal Mk progenitors arise as well as from committed progenitors downstream of HSC. These differences in cell of origin could also contribute to the distinct phenotypic features of fetal and adult Mk.
Table 3.
Molecular and signaling differences between fetal and adult Mk
| Parameter | Predicted effects in megakaryopoiesis | References |
|---|---|---|
| Progenitor origin | ||
| Fetal Mk arise from HSC and committed progenitors downstream of HSC | Heterogeneous origin | [29] |
| Adult Mk originate directly from HSC | Homogenous origin | [29] |
|
| ||
| Erythroid genes expression | ||
| Fetal Mk express erythroid antigens | Incomplete lineage consolidation | [30, 31] |
| Adult Mk devoid of erythroid antigens | Complete lineage consolidation | [30, 31] |
|
| ||
| Cytokines and cytokine receptors expression | ||
| TPO higher in neonates than adults | Positively regulates megakaryopoiesis | [9, 33] |
| TPO receptor (C-MPL) upregulated in fetal Mk | Positively regulates megakaryopoiesis | [9] |
| SDF1 receptor (CXCR-4) downregulated in fetal Mk | Negatively regulates megakaryopoiesis | [28, 38–40] |
| Negatively regulates megakaryopoiesis | ||
| TGFβ receptor upregulated in fetal Mk | [28] | |
|
| ||
| Signaling Pathways | ||
| IGF/mTOR pathway hyperactivated in fetal Mk | Increases proliferation of Mk | [9, 65] |
| JAK2 pathway hyperactivated in fetal Mk | Increases proliferation of Mk | [9] |
| Cell cycle, DNA replication and mitosis components are enriched in adult Mk | Increases Mk polyploidization | [28] |
| p21 is downregulated in fetal Mk | Increases proliferation and reduces polyploidization | [9, 34] |
| TGFβ signaling hyperactivated in fetal Mk | Negatively regulates Mk polyploidization | [28] |
|
| ||
| Transcription factors and transcription | ||
| GATA-1 upregulated in fetal Mk | Permits differentiation | [9] |
| P-TEFb is less active in fetal Mk | Decreases Mk morphogenesis and suppression of erythroid genes | [31] |
|
| ||
| RNA Binding factors | ||
| IGF2BP3 expressed in fetal Mk | Decreases P-TEFb activity, Mk morphogenesis, and suppression of erythroid genes | [31] |
| Lin28B expressed in fetal Mk | Positively regulates the expression of erythroid genes | [28, 31] |
| HMGA1 expressed in fetal Mk | Enhances megakaryopoiesis | [31] |
|
| ||
| microRNA (miR) | ||
| miR-9 and miR-224 are upregulated in fetal Mk | Predicted to inhibit megakaryopoiesis | [28. 40] |
| miR-99a is upregulated in fetal Mk | Predicted to enhance Mk proliferation | [45] |
| miR-181a is downregulated in fetal Mk | Predicted to enhance erythroid gene expression | [46] |
| Let-7 miRs are downregulated in fetal Mk | Predicted to enhance erythroid gene expression | [28] |
Despite complete execution of most aspects of the lineage program, fetal Mk do show a tendency toward “leaky” erythroid gene expression. This feature was initially identified by Woo et al. in comparing gene expression profiles of purified Mk progenitors from murine fetal liver versus adult marrow: 7 erythroid transcripts were in the top 122 fetal-upregulated transcripts [30]. Recently, we confirmed these findings in human progenitors, with Mk derived from CB but not adult progenitors showing partial expression of the erythroid antigen glycophorin A (GPA) [31]. The concurrent expression of CD41 and GPA suggests a diminished ability of CB derived Mk to undergo complete lineage consolidation. Although CD41 expression on progenitors does not exclude the possibility for erythroid development, Psaila et al. observed that CD42 expression does represent full commitment to the Mk lineage with loss of erythroid potential [32].
Multiple secreted factors regulate megakaryopoiesis at various stages. The main megakaryopoietic cytokine, which acts both at early and late stages, is Tpo. In humans, circulating Tpo levels are significantly higher in neonates than in adults [33]. Tpo engagement of its receptor (TpoR, encoded by MPL) activates multiple signaling cascades including JAK/STAT, MEK/MAPK, and PI3K/Akt/mTOR pathways. In studies by Liu et al., Tpo-stimulated CB derived Mk activated MAPK/ERK to a similar degree as adult counterparts, but showed hyperactivation of JAK2 and mTOR pathways [9]. Consistent with their enhanced Tpo sensitivity, CB Mk expressed elevated levels of TpoR and of mTOR downstream targets S6K and p-4E-BP-1 [9]. As a functional corollary, inhibition of mTOR by rapamycin reduced proliferation and maturation of CB Mk without affecting polyploidization. By contrast, mTOR inhibition in adult Mk affected all parameters including polyploidization, a difference attributable to higher adult levels of Cyclin-dependent kinase inhibitor 1 (p21) which is required for mTOR regulation of polyploidization [9, 34]. Thus, CB Mk hyperactivation of mTOR combined with p21 deficiency might uncouple Mk maturation from polyploidization and promote proliferative expansion during development [9]. In this context, the recent implication of mTORC1 in activating erythroid genes (through inducing mitochondrial biogenesis [35]) might partially explain the leaky expression of erythroid genes in CB Mk.
An additional megakaryopoietic cytokine consists of stromal cell derived factor 1 (SDF-1). SDF-1 has been shown to enhance polyploidization of human Mk and to mediate migration of murine Mk to the marrow vascular niche, a milieu promoting terminal differentiation and platelet release [36, 37]. Interestingly, the SDF-1 receptor CXCR4 was found to be deficient in human fetal Mk [28, 38–40]. This deficiency correlated with fetal-specific expression of microRNAs, miR-9 and miR-224, that target CXCR4 transcripts [40]. Thus, differential expression of CXCR4 may also contribute to ontogenic differences in Mk phenotype.
Characterization of the Mk transcriptome at fetal, neonatal and adult stages revealed enrichment in adult Mk of transcripts related to differentiation, platelet formation, and cell cycle [28]. In fetal Mk, the most highly enriched transcripts were related to angiogenesis, integrins, extracellular matrix, and transforming growth factor β receptor (TGFβR)/bone morphogenetic protein (BMP) signaling. Comparison of mRNAs encoding transcription factors essential for megakaryopoiesis (GABPα, FLI1, RUNX1, GATA1, FOG1 and NF-E2) showed differences only in GATA1 expression, which was highly enhanced in fetal Mk [9].
Several microRNAs (miRs) have been implicated in regulating megakaryopoiesis [41–44]. 32 miRs have been found to differ between fetal-like Mk (from embryonic stem cells) and adult Mk [28]. Among those enriched in the fetal-like Mk were miR-9 and miR-224, both of which target CXCR4 [40]. Also enriched in fetal Mk is miR99a [45], predicted to target CTDSPL, encoding a retinoblastoma protein (Rb) phosphatase that promotes E2F binding by Rb. Increased miR-99a in fetal Mk correlates with decreased CTDSPL, increased hyperphosphorylated Rb, and E2F-mediated induction of D-type cyclins [45]. These data thus suggest that miR-99a contributes to the hyperproliferative phenotype in fetal Mk by promoting cell cycle transition through the classic G1-S checkpoint.
A miR found to be downregulated in fetal Mk is miR-181a which putatively targets LIN28B, encoding an oncofetal RNA binding factor [46]. Lin28b displays selective expression in fetal HSC and Mk and likely contributes to the decreased levels of Let-7 miRs found in fetal progenitors [28, 31, 47]. Lin28b has been implicated in ontogenic programming of HSC, erythroid, and lymphoid lineages [47–49], but its influence on megakaryopoiesis remains undetermined. One potential effect of Lin28b on megakaryopoiesis could be through alteration of Wnt signaling. Thus, Lin28b suppression of Let-7b could potentially induce expression of the receptor Frizzled4, as the corresponding transcript is a putative target of this miR [50]. Let-7b suppression also causes downregulation of Wnt3a and upregulation of Wnt5b. Notably, Wnt3a has been found to enhance Mk maturation and proplatelet formation, while Wnt5a appears to inhibit these processes [51]. Thus, Lin28b could theoretically contribute to Mk ontogenic regulation through a Let-7-Wnt pathway, but experimental verification of such a pathway is needed.
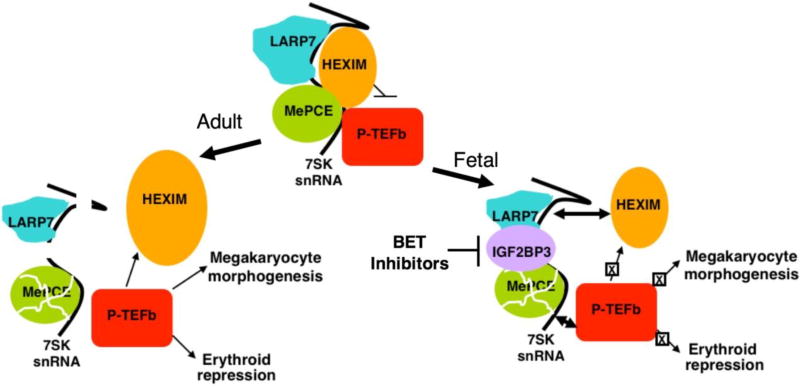
Molecular differences in the core transcriptional machinery also distinguish adult and fetal Mk. Adult Mk morphogenesis imposes massive transcriptional demands, which are met through a unique program of RNA polymerase II (RNAPII) activation [52]. This program involves global and irreversible activation of the P-TEFb kinase complex (Cdk9/Cyclin T) whose functions are to release RNAPII from sites of promoter-proximal stalling and accelerate transcriptional elongation [52, 53]. In most cell types, P-TEFb predominantly resides in an inactive reservoir, ensconced in a large ribonucleoprotein complex that contains its inhibitor HEXIM1/2 (Figure 2). This complex, known as the 7SK snRNP, contains the RNA scaffold 7SK as well as the 7SK stabilizing proteins MePCE and LARP7. P-TEFb activation in non-Mk occurs through a release mechanism that is target gene-localized, reversible, and controlled by feedback inhibition. A unique aspect of P-TEFb activation in Mk consists of its irreversible release due to destruction of the 7SK snRNP (Figure 2). A key step initiating this process is the upregulation of the active protease calpain 2, which directly degrades MePCE. Inhibition or knockdown of calpain 2 blocks adult Mk morphogenesis. The other 7SK stabilizing factor LARP7 also undergoes downregulation during Mk differentiation, by a calpain independent mechanism involving transcript modulation. Loss of MePCE and LARP7 cause 7SK degradation and release of active P-TEFb. This mode of P-TEFb activation is particularly important for induction of a cohort of cytoskeletal factors that drive adult Mk morphogenesis. These factors include Mkl1, Filamin A (FlnA), Hic-5, and α-actinin-1 (ACTN1).
Figure 2.
Model of ontogenic regulation of megakaryocyte morphogenesis. In adult megakaryopoiesis (left arrow), downregulation of LARP7 and proteolysis of MePCE destabilize 7SK snRNA, leading to unopposed P-TEFb activation. This mode of P-TEFb activation promotes upregulation of megakaryocyte morphogenesis factors, most notably MKL1, as well as upregulation of HEXIM1 and lineage consolidation via erythroid repression. In fetal megakaryopoiesis (right arrow), expression of IGF2BP3 stabilizes 7SK snRNA despite downregulation of LARP7 and MePCE. Persistence of 7SK allows for feedback inhibition of P-TEFb, dampening both the upregulation of megakaryocyte morphogenesis factors such as MKL1 and lineage consolidation via erythroid repression.
NB: Permission was obtained from JCI to republish this figure.
Fetal Mk manifest several molecular defects symptomatic of impaired P-TEFb activation: 1) failure to induce the P-TEFb-dependent cytoskeletal factors, 2) deficiency in phosphorylation of P-TEFb substrates RNAPII and Spt5, and 3) global decrease in histone H2B K120 monoubiquitination, a P-TEFb-driven epigenetic mark [31]. The failure to induce the P-TEFb-dependent cytoskeletal factors most likely explains the diminished morphogenesis of fetal Mk. Thus, Mkl1 has been identified as a master regulator of adult Mk morphogenesis, with deficiency in mice compromising polyploidization and platelet production [54–56]. ACTN1 knockdown blocks adult Mk morphogenesis [52], and germline human mutations have been identified in rare cases of autosomal dominant macrothrombocytopenia [57, 58]. Similarly, FlnA loss of function impairs Mk polyploidization in culture and causes macrothrombocytopenia in vivo in mice and humans [59, 60].
Despite manifesting a failure in the Mk pathway of P-TEFb activation, fetal Mk do execute the key initiating steps in this pathway, i.e downregulation of MePCE and LARP7 [31]. These steps normally suffice for destruction of the 7SK RNA and dissolution of the kinase-repressive complex. However, 7SK levels remain high in fetal Mk despite downregulation of stabilizing proteins, suggesting the existence of fetal-specific 7SK stabilizing factor(s) that block morphogenesis. Functional screening of candidates through enforced expression in adult Mk has ruled out several fetal RNA-binding proteins including HMGA1 and HMGA2 but has implicated IGF2BP3, an oncofetal mRNA binding factor [31, 61]. Thus, ectopic expression of IGF2BP3 in adult progenitors induces a fetal phenotypic shift of Mk both in human cell culture and murine marrow transplant models [31]. These findings may partly explain the diminished platelet counts observed in mice stably engrafted with HSC expressing IGF2BP3 [62]. Further support for involvement of IGF2BP3 comes from loss of function studies in which knockdown shifts fetal Mk toward an adult phenotype with regard to morphogenesis and platelet formation. Molecular findings validating IGF2BP3 as a fetal-specific 7SK stabilizer include its direct interaction with this RNA target, its regulation of 7SK levels, and its regulation of P-TEFb signaling. Specifically, IGF2BP3 knockdown in fetal Mk decreases 7SK levels, increases phosphorylation of P-TEFb substrates, and upregulates expression of P-TEFb-dependent cytoskeletal factors. These findings indicate that IGF2BP3 serves as a master switch for the Mk ontogenic phenotype (Figure 2).
Therapeutic targeting of IGF2BP3 activity may prove to be challenging, but its expression in fetal Mk can be modulated by inhibitors of bromo and extra-terminal domain (BET) factors [31]. Specifically, BET inhibitors downregulate IGF2BP3 in fetal Mk and promote the phenotypic and molecular features of an adult program, including enhancement of platelet release. Enforced expression of IGF2BP3 blunts the drug effects, supporting it as a relevant target. These findings provide proof of principle for pharmacologic manipulation of Mk ontogenic phenotype by circumventing a fetal-specific blockade in P-TEFb signaling.
Microenvironmental influences
A role for the microenvironment in influencing Mk ontogenic phenotype has been suggested in mouse transplant experiments [63]. Engraftment of fetal stem cells in adult mice yields fetal-type Mk one week post-transplant, but after a month donor-derived Mk assume adult size and ploidy. A clue to the nature of this influence came from gene expression profiling of fetal and adult Mk progenitors, showing type I interferon response genes to be significantly upregulated in adult versus fetal cells [30]. Furthermore, ex vivo treatment of fetal Mk progenitors with IFN-α significantly blunted their proliferation. Thus, interferon-producing myeloid cells in the adult marrow may provide an extrinsic cue to modulate the Mk ontogenic phenotype.
An important fetal microenvironmental cue likely comprises insulin-like growth factors (IGF), which drive tissue growth during embryogenesis and are known to induce HSC proliferation [64]. Klusmann et al. demonstrated that stromal IGF2 promotes proliferation of fetal but not adult Mk through the activation of an IGFR1/mTOR/E2F signaling pathway [65]. Notably, this pathway is repressed by wild type GATA1 but not the leukemogenic GATA1s mutant associated with DS-TMD. Molecular determinants of fetal IGF2 expression are the IGF2BP family members, which bind and stabilize IGF transcripts. The abundant expression of IGF2BP3 in human neonatal hematopoietic progenitors [31] suggests these cells could serve as autocrine and juxtacrine sources of IGF2 within the fetal liver. An additional fetal influence consists of TGFβ1, abundantly produced by fetal liver hepatoblasts and sinusoidal endothelium [66, 67]. Fetal Mk show enhanced expression of TGFβR/BMP and extracellular matrix factors induced by TGFβ [28]. Phenotypic consequences of Mk exposure to TGFβ include impaired enlargement and polyploidization [68, 69], implicating this cytokine as a morphogenic modulator. However, TGFβ signaling also mediates features of adult-type megakaryopoiesis by promoting extension of proplatelet processes [70]. Thus, the effects of this pathway are complex and may depend on differentiation stage.
Conclusion
Multiple intrinsic and extrinsic factors contribute to Mk ontogenic differences (Table 3). Fetal factors promote cellular proliferation through a standard mitotic cell cycle, while adult factors promote a morphogenesis program that employs an endomitotic cell cycle. A complete understanding of these factors will permit therapeutic manipulation of the ontogenic phenotype. Potential applications include novel treatments for neonatal thrombocytopenia and Mk neoplasms, as well as optimized approaches for ex vivo platelet or Mk production. To optimize ex vivo yield and quality, the hyperproliferative pathways of fetal Mk can be exploited for initial expansion, and the morphogenetic circuitry of adult Mk can be harnessed for subsequent platelet release (Figure 1). Feasibility has already been demonstrated for pharmacologic targeting of fetal factors, e.g. IGF2BP3 and TGFβ, to elicit adult properties [31, 69]. Future studies will pave the way toward enjoying the best of both ontogenic worlds: scalability and productivity.
Highlights.
Fetal Mk are phenotypically different than adult Mk
Fetal Mk are matured as adult Mk
Fetal Mk phenotypic features predispose to certain Mk related diseases
Cell intrinsic and extrinsic factors contribute to Mk ontogenic differences
Fetal Mk can be established in adult mode to enhance ex vivo platelets production
Acknowledgments
This work was supported by NIH grants DK090926 and HL130550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Machlus KR, Italiano JE., Jr The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol. 2013;201:785–796. doi: 10.1083/jcb.201304054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen Graeve JL, de Alarcon PA. Megakaryocytopoiesis in the human fetus. Arch Dis Child. 1989;64:481–484. doi: 10.1136/adc.64.4_spec_no.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma DC, Sun YH, Chang KZ, Zuo W. Developmental change of megakaryocyte maturation and DNA ploidy in human fetus. Eur J Haematol. 1996;57:121–127. doi: 10.1111/j.1600-0609.1996.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs DA, McGinn SG, Cantu CL, Klein RR, Sola-Visner MC, Rimsza LM. Developmental differences in megakaryocyte size in infants and children. Am J Clin Pathol. 2012;138:140–145. doi: 10.1309/AJCP4EMTJYA0VGYE. [DOI] [PubMed] [Google Scholar]
- 6.Sola-Visner MC, Christensen RD, Hutson AD, Rimsza LM. Megakaryocyte size and concentration in the bone marrow of thrombocytopenic and nonthrombocytopenic neonates. Pediatr Res. 2007;61:479–484. doi: 10.1203/pdr.0b013e3180332c18. [DOI] [PubMed] [Google Scholar]
- 7.de Alarcon PA, Graeve JL. Analysis of megakaryocyte ploidy in fetal bone marrow biopsies using a new adaptation of the feulgen technique to measure DNA content and estimate megakaryocyte ploidy from biopsy specimens. Pediatr Res. 1996;39:166–170. doi: 10.1203/00006450-199601000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZJ, Italiano J, Jr, Ferrer-Marin F, et al. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood. 2011;117:4106–4117. doi: 10.1182/blood-2010-07-293092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato A, Kawamata N, Tamayose K, et al. Ancient ubiquitous protein 1 binds to the conserved membrane-proximal sequence of the cytoplasmic tail of the integrin alpha subunits that plays a crucial role in the inside-out signaling of alpha IIbbeta 3. J Biol Chem. 2002;277:28934–28941. doi: 10.1074/jbc.M204340200. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri ZM, De Marco L, Gatti L, Bader R, Montgomery RR. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983;72:1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M. Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion. 2013;53:2814–21. doi: 10.1111/trf.12343. quiz 2813. [DOI] [PubMed] [Google Scholar]
- 13.Ree IMC, Fustolo-Gunnink SF, Bekker V, Fijnvandraat KJ, Steggerda SJ, Lopriore E. Thrombocytopenia in neonatal sepsis: Incidence, severity and risk factors. PLoS One. 2017;12:e0185581. doi: 10.1371/journal.pone.0185581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparger KA, Assmann SF, Granger S, et al. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr. 2016;170:687–694. doi: 10.1001/jamapediatrics.2016.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastos KM, Slayton WB, Rimsza LM, Young L, Sola-Visner MC. Differential effects of recombinant thrombopoietin and bone marrow stromal-conditioned media on neonatal versus adult megakaryocytes. Blood. 2006;108:3360–3362. doi: 10.1182/blood-2006-04-018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignatz M, Sola-Visner M, Rimsza LM, et al. Umbilical cord blood produces small megakaryocytes after transplantation. Biol Blood Marrow Transplant. 2007;13:145–150. doi: 10.1016/j.bbmt.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. 2009;44:621–626. doi: 10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- 18.Solh M, Brunstein C, Morgan S, Weisdorf D. Platelet and red blood cell utilization and transfusion independence in umbilical cord blood and allogeneic peripheral blood hematopoietic cell transplants. Biol Blood Marrow Transplant. 2011;17:710–716. doi: 10.1016/j.bbmt.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arber DA, Brunning RD, Orazi A, Porwit A, Peterson L, Thiele J. Acute myeloid leukaemia with recurrent genetic abnormalities. In: Swerdlow SH, Camp E, Harris NL, et al., editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2017. pp. 130–171. [Google Scholar]
- 20.Nikolaev SI, Santoni F, Vannier A, et al. Exome sequencing identifies putative drivers of progression of transient myeloproliferative disorder to AMKL in infants with Down syndrome. Blood. 2013;122:554–561. doi: 10.1182/blood-2013-03-491936. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Toki T, Okuno Y, et al. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nat Genet. 2013;45:1293–1299. doi: 10.1038/ng.2759. [DOI] [PubMed] [Google Scholar]
- 22.de Rooij JD, Branstetter C, Ma J, et al. Pediatric non-Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat Genet. 2017;49:451–456. doi: 10.1038/ng.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet. 2005;37:613–619. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 24.Mercher T, Raffel GD, Moore SA, et al. The OTT-MAL fusion oncogene activates RBPJ-mediated transcription and induces acute megakaryoblastic leukemia in a knockin mouse model. J Clin Invest. 2009;119:852–864. doi: 10.1172/JCI35901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gollomp K, Lambert MP, Poncz M. Current status of blood 'pharming': megakaryoctye transfusions as a source of platelets. Curr Opin Hematol. 2017;24:565–571. doi: 10.1097/MOH.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thon JN, Mazutis L, Wu S, et al. Platelet bioreactor-on-a-chip. Blood. 2014;124:1857–1867. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takayama N, Nishimura S, Nakamura S, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bluteau O, Langlois T, Rivera-Munoz P, et al. Developmental changes in human megakaryopoiesis. J Thromb Haemost. 2013;11:1730–1741. doi: 10.1111/jth.12326. [DOI] [PubMed] [Google Scholar]
- 29.Notta F, Zandi S, Takayama N, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351:aab2116. doi: 10.1126/science.aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woo AJ, Wieland K, Huang H, et al. Developmental differences in IFN signaling affect GATA1s-induced megakaryocyte hyperproliferation. J Clin Invest. 2013 doi: 10.1172/JCI40609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elagib KE, Lu CH, Mosoyan G, et al. Neonatal expression of RNA-binding protein IGF2BP3 regulates the human fetal-adult megakaryocyte transition. J Clin Invest. 2017;127:2365–2377. doi: 10.1172/JCI88936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psaila B, Barkas N, Iskander D, et al. Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol. 2016;17:83-016-0939-7. doi: 10.1186/s13059-016-0939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walka MM, Sonntag J, Dudenhausen JW, Obladen M. Thrombopoietin concentration in umbilical cord blood of healthy term newborns is higher than in adult controls. Biol Neonate. 1999;75:54–58. doi: 10.1159/000014077. [DOI] [PubMed] [Google Scholar]
- 34.Raslova H, Baccini V, Loussaief L, et al. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107:2303–2310. doi: 10.1182/blood-2005-07-3005. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Zhang Y, Ni M, et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat Cell Biol. 2017;19:626–638. doi: 10.1038/ncb3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerriero R, Mattia G, Testa U, et al. Stromal cell-derived factor 1alpha increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood. 2001;97:2587–2595. doi: 10.1182/blood.v97.9.2587. [DOI] [PubMed] [Google Scholar]
- 37.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 38.Riviere C, Subra F, Cohen-Solal K, et al. Phenotypic and functional evidence for the expression of CXCR4 receptor during megakaryocytopoiesis. Blood. 1999;93:1511–1523. [PubMed] [Google Scholar]
- 39.Mazharian A, Watson SP, Severin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37:1238–1249.e5. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrer-Marin F, Gutti R, Liu ZJ, Sola-Visner M. MiR-9 contributes to the developmental differences in CXCR-4 expression in human megakaryocytes. J Thromb Haemost. 2014;12:282–285. doi: 10.1111/jth.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garzon R, Pichiorri F, Palumbo T, et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proc Natl Acad Sci U S A. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edelstein LC, Bray PF. MicroRNAs in platelet production and activation. Blood. 2011;117:5289–5296. doi: 10.1182/blood-2011-01-292011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmrich S, Henke K, Hegermann J, Ochs M, Reinhardt D, Klusmann JH. miRNAs can increase the efficiency of ex vivo platelet generation. Ann Hematol. 2012;91:1673–1684. doi: 10.1007/s00277-012-1517-z. [DOI] [PubMed] [Google Scholar]
- 44.Qu M, Fang F, Zou X, et al. miR-125b modulates megakaryocyte maturation by targeting the cell-cycle inhibitor p19INK4D. Cell Death Dis. 2016;7:e2430. doi: 10.1038/cddis.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandi R, Gutti U, Undi R, Sahu I, Gutti RK. Understanding thrombocytopenia: physiological role of microRNA in survival of neonatal megakaryocytes. J Thromb Thrombolysis. 2015;40:310–316. doi: 10.1007/s11239-015-1238-y. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Zhang J, Gao L, et al. MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death Differ. 2012;19:378–386. doi: 10.1038/cdd.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copley MR, Babovic S, Benz C, et al. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat Cell Biol. 2013;15:916–925. doi: 10.1038/ncb2783. [DOI] [PubMed] [Google Scholar]
- 48.Lee YT, de Vasconcellos JF, Yuan J, et al. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013;122:1034–1041. doi: 10.1182/blood-2012-12-472308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Undi RB, Gutti U, Gutti RK. Role of let-7b/Fzd4 axis in mitochondrial biogenesis through wnt signaling: In neonatal and adult megakaryocytes. Int J Biochem Cell Biol. 2016;79:61–68. doi: 10.1016/j.biocel.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Macaulay IC, Thon JN, Tijssen MR, et al. Canonical Wnt signaling in megakaryocytes regulates proplatelet formation. Blood. 2013;121:188–196. doi: 10.1182/blood-2012-03-416875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elagib KE, Rubinstein JD, Delehanty LL, et al. Calpain 2 activation of P-TEFb drives megakaryocyte morphogenesis and is disrupted by leukemogenic GATA1 mutation. Dev Cell. 2013;27:607–620. doi: 10.1016/j.devcel.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elagib KE, Goldfarb AN. Megakaryocytic irreversible P-TEFb activation. Cell cycle (Georgetown, Tex.) 2014;13:1827–1828. doi: 10.4161/cc.29324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng EC, Luo Q, Bruscia EM, et al. Role for MKL1 in megakaryocytic maturation. Blood. 2009;113:2826–2834. doi: 10.1182/blood-2008-09-180596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halene S, Gao Y, Hahn K, et al. Serum response factor is an essential transcription factor in megakaryocytic maturation. Blood. 2010;116:1942–1950. doi: 10.1182/blood-2010-01-261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith EC, Thon JN, Devine MT, et al. MKL1 and MKL2 play redundant and crucial roles in megakaryocyte maturation and platelet formation. Blood. 2012;120:2317–2329. doi: 10.1182/blood-2012-04-420828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunishima S, Okuno Y, Yoshida K, et al. ACTN1 mutations cause congenital macrothrombocytopenia. Am J Hum Genet. 2013;92:431–438. doi: 10.1016/j.ajhg.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gueguen P, Rouault K, Chen JM, et al. A missense mutation in the alpha-actinin 1 gene (ACTN1) is the cause of autosomal dominant macrothrombocytopenia in a large French family. PLoS One. 2013;8:e74728. doi: 10.1371/journal.pone.0074728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nurden P, Debili N, Coupry I, et al. Thrombocytopenia resulting from mutations in filamin A can be expressed as an isolated syndrome. Blood. 2011;118:5928–5937. doi: 10.1182/blood-2011-07-365601. [DOI] [PubMed] [Google Scholar]
- 60.Jurak Begonja A, Hoffmeister KM, Hartwig JH, Falet H. FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood. 2011;118:2285–2295. doi: 10.1182/blood-2011-04-348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dutta A, Hutchison RE, Mohi G. Hmga2 promotes the development of myelofibrosis in Jak2V617F knockin mice by enhancing TGF-beta1 and Cxcl12 pathways. Blood. 2017;130:920–932. doi: 10.1182/blood-2016-12-757344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palanichamy JK, Tran TM, Howard JM, et al. RNA-binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J Clin Invest. 2016;126:1495–1511. doi: 10.1172/JCI80046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slayton WB, Wainman DA, Li XM, et al. Developmental differences in megakaryocyte maturation are determined by the microenvironment. Stem Cells. 2005;23:1400–1408. doi: 10.1634/stemcells.2004-0373. [DOI] [PubMed] [Google Scholar]
- 64.Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, Nilsson SK. Megakaryocytes colocalise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 2013;11:782–792. doi: 10.1016/j.scr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Klusmann JH, Godinho FJ, Heitmann K, et al. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev. 2010;24:1659–1672. doi: 10.1101/gad.1903410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson NL, Flanders KC, Smith JM, Ellingsworth LR, Roberts AB, Sporn MB. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989;108:661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugiyama D, Kulkeaw K, Mizuochi C. TGF-beta-1 up-regulates extra-cellular matrix production in mouse hepatoblasts. Mech Dev. 2013;130:195–206. doi: 10.1016/j.mod.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Kuter DJ, Gminski DM, Rosenberg RD. Transforming growth factor beta inhibits megakaryocyte growth and endomitosis. Blood. 1992;79:619–626. [PubMed] [Google Scholar]
- 69.Huang N, Lou M, Liu H, Avila C, Ma Y. Identification of a potent small molecule capable of regulating polyploidization, megakaryocyte maturation, and platelet production. J Hematol Oncol. 2016;9:136. doi: 10.1186/s13045-016-0358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badalucco S, Di Buduo CA, Campanelli R, et al. Involvement of TGFbeta1 in autocrine regulation of proplatelet formation in healthy subjects and patients with primary myelofibrosis. Haematologica. 2013;98:514–517. doi: 10.3324/haematol.2012.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]