Abstract
The thalamostriatal system is a major network in the mammalian brain, originating principally from the intralaminar nuclei of thalamus. Its functions remain unclear, but a subset of these projections provides a pathway through which the cerebellum communicates with the basal ganglia. Both the cerebellum and basal ganglia play crucial roles in motor control. Although songbirds have yielded key insights into the neural basis of vocal learning, it is unknown whether a thalamostriatal system exists in the songbird brain. Thalamic nucleus DLM is an important part of the song system, the network of nuclei required for learning and producing song. DLM receives output from song system basal ganglia nucleus Area X and sits within dorsal thalamus, the proposed avian homolog of the mammalian intralaminar nuclei that also receives projections from the cerebellar nuclei. Using a viral vector that specifically labels presynaptic axon segments, we show in Bengalese finches that dorsal thalamus projects to Area X, the basal ganglia nucleus of the song system, and to surrounding medial striatum. To identify the sources of thalamic input to Area X, we map DLM and cerebellar-recipient dorsal thalamus (DTCbN). Surprisingly, we find both DLM and dorsal anterior DTCbN adjacent to DLM project to Area X. In contrast, the ventral medial subregion of DTCbN projects to medial striatum outside Area X. Our results suggest the basal ganglia in the song system, like the mammalian basal ganglia, integrate feedback from the thalamic region to which they project as well as thalamic regions that receive cerebellar output.
Keywords: thalamostriatal, songbird, basal ganglia, cerebellum, RRID: AB_2209751, RRID: AB_2536611, RRID: AB_2534132, RRID: AB_2534134, RRID: AB_2174013, RRID: AB_2340613, RRID: AB_2340675, RRID: AB_2340846, RRID: AB_2313584, RRID: AB_2313581
Graphical Abstract
Introduction
Motor skills learned by imitation and practice, like speaking a language or playing the piano, are under the control of a complex network of neural circuits. The basal ganglia and the cerebellum are key components of these motor systems in the brain that contribute to learning and producing speech (Parrell, Agnew, Nagarajan, Houde, & Ivry, 2017; Watkins, 2011; Wolfram Ziegler, 2016; W Ziegler & Ackermann, 2017) and other complex motor skills (A. J. Bastian, 2006; Dudman & Krakauer, 2016; Manto et al., 2012; Shadmehr & Krakauer, 2008; Shmuelof & Krakauer, 2011). These contributions are thought to take place through basal ganglia and cerebellar output to motor thalamus, which in turn projects to motor cortex (Belyk & Brown, 2017; Bosch-Bouju, Hyland, & Parr-Brownlie, 2013; Ghez & Krakauer, 2000; Jurgens, 2002; Sommer, 2003; W Ziegler & Ackermann, 2017). However, in addition to projecting to cortex, thalamus projects to striatum. Within the motor system, both basal ganglia and cerebellar-recipient thalamus (nuclei VA and VL) have been shown to project to dorsolateral striatum in monkeys (Nikolaus R McFarland & Haber, 2000; N. R. McFarland & Haber, 2001). Basal ganglia-recipient motor thalamus in rats also projects to neostriatum, although cerebellar-recipient does not (Kuramoto et al., 2009). In addition to these connections within the motor system, the entire striatum is massively innervated by a projection that arises mainly from the intralaminar and midline nuclei (Smith et al., 2014). Through a subset of these thalamostriatal projections, output from the cerebellar nuclei can reach the basal ganglia. In rats, intralaminar nucleus CL provides a disynaptic pathway from the dentate nucleus of the cerebellum to dorsolateral striatum, which can influence medium spiny neuron activity in freely-moving animals (Chen, Fremont, Arteaga-Bracho, & Khodakhah, 2014; N. Ichinohe, Mori, & Shoumura, 2000). Transneuronal tracing studies in macaques showed that most of the dentate as well as parts of the interpositus and fastigial nuclei project to the putamen through the intralaminar and ventral thalamic motor nuclei (Hoshi, Tremblay, Feger, Carras, & Strick, 2005). It remains unknown what role is played in motor control by the basal ganglia receiving feedback from thalamus, including the regions that receive input from the cerebellum.
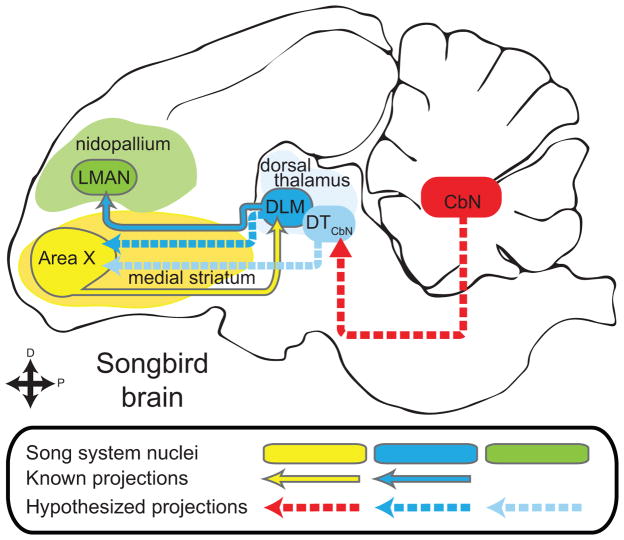
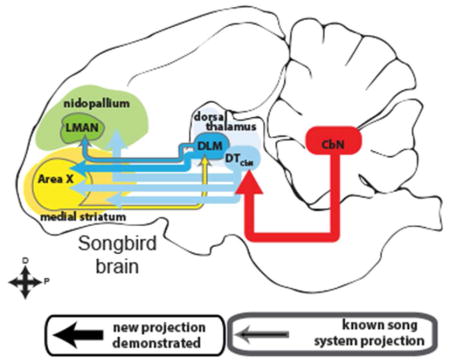
Songbirds represent an ideal model system for investigating vocal learning and motor control, in part because of the structural and functional parallels between speech and birdsong (Bolhuis, Okanoya, & Scharff, 2010; Doupe & Kuhl, 1999; Marler, 1970). The songbird brain contains a network of discrete nuclei for learning and producing song, called the song system (Mooney, 2009), that shares many circuit-level and genetic characteristics with brain areas controlling speech (Konopka & Roberts, 2016). A thalamocortical-basal ganglia loop in the song system known as the anterior forebrain pathway (AFP, see Fig. 1) is required for juveniles to learn song and for adults to modify learned song (Andalman & Fee, 2009; Charlesworth, Tumer, Warren, & Brainard, 2011; Kao, Doupe, & Brainard, 2005). Although the AFP is thought to be homologous to similar loops in the mammalian brain, it is not known if songbirds have a thalamostriatal system similar to that of mammals. Specifically in the song system it is unknown whether in the AFP the basal ganglia nucleus, Area X, receives input from the thalamic nucleus DLM (Fig. 1, dashed dark blue line) or any other region of dorsal thalamus (Gale & Perkel, 2010). Dorsal thalamus is a strong candidate for the source of a thalamostriatal system in songbirds because it is the proposed avian homolog of the intralaminar nuclei in mammals (Veenman, Medina, & Reiner, 1997). Projections from other parts of dorsal thalamus to Area X could also provide a potential neuroanatomical pathway through which cerebellar output might reach the song system (Fig. 1, dashed red line and dashed light blue line) (A. L. Person, Gale, Farries, & Perkel, 2008). This would be particularly interesting given that it remains unknown whether the cerebellum is involved with learning and producing birdsong (Bolhuis et al., 2010; W Ziegler & Ackermann, 2017). We address these anatomical questions (Fig. 1) in Bengalese finches (Lonchura striata domestica). This songbird species is of interest because it depends strongly on auditory feedback (Okanoya & Yamaguchi, 1997; S. M. Woolley & Rubel, 1997) and it has been shown to adapt its song to perturbations of auditory feedback (Sober & Brainard, 2009, 2012) in a manner reminiscent of cerebellar-dependent sensorimotor adaptation (Amy J Bastian, 2008; Parrell et al., 2017).
Figure 1.
The anterior forebrain pathway (AFP) is a thalamocortical-basal ganglia loop in the song system required for learning song. It consists of basal ganglia nucleus Area X (yellow oval), thalamic nucleus DLM (blue oval) and cortical nucleus LMAN (green oval, known song system connections outlined in gray. The rest of the song system is not shown.). Using lentiviral tracing methods, we tested whether thalamus projects to the basal ganglia in a songbird, the Bengalese finch. Specifically we tested whether Area X (yellow oval) receives input from DLM (darker blue dashed arrow) or cerebellar-recipient dorsal thalamus (DTCbN, lighter blue dashed arrows). In order to identify targets of DTCbN, we first mapped out the projections of the cerebellar nuclei (CbN) to dorsal thalamus in detail (red arrow).
The question of whether a thalamostriatal system exists in songbirds has remained unanswered in part because of confounds that arise when using standard neuroanatomical tracers, as recognized previously (Bottjer, Halsema, Brown, & Miesner, 1989; Gale & Perkel, 2010; A. L. Person et al., 2008). Specifically, there is a passing fibers confound when retrograde tracers are used, because thalamocortical axons pass through the striatum. In addition, Area X in songbirds and the surrounding medial striatum (MSt) contain a specialized cell type that projects directly to thalamus, confounding results from standard tracers that travel in both the anterograde and retrograde directions. To avoid these confounds, we used lentiviral vectors that yield only anterograde label, and specifically label presynaptic axon segments. This method allowed us to show that dorsal thalamic neurons form synapses within the basal ganglia, including song system nucleus Area X. Results from these initial experiments, in conjunction with previous work, supported the hypothesis that either DLM or nearby cerebellar-recipient dorsal thalamus project to Area X. To determine which regions of dorsal thalamus project to the Area X, we used standard anatomical tracers to identify and map DLM as well as the regions of dorsal thalamus targeted by projections of the cerebellar nuclei (CbN). The latter region we refer to as cerebellar-recipient dorsal thalamus, DTCbN. We show that Area X receives projections both from DLM and from a dorsal anterior subregion of DTCbN in the same mediolateral plane as DLM. More ventral and posterior subregions of DTCbN, still in medial dorsal thalamus but separate from DLM, project to medial striatum outside of Area X. In the Discussion we relate our results to what is known about cerebellar-recipient thalamus in birds and mammals and propose possible homologs for the region that we refer to as DTCbN. We also briefly review relevant work on the thalamostriatal system in mammals, as we consider the implications of our finding that there is a projection from thalamic nucleus DLM to basal ganglia nucleus Area X in the song system. Our findings suggest that thalamostriatal projections and cerebellar input to the basal ganglia may be general components of vocal motor control across vertebrates.
Methods
All studies were carried out in adult (>90 days post hatch) male Bengalese finches either bought from a supplier or bred in our laboratory. Age of purchased birds was assessed by screening them for adult-like song. Work reported here was approved by the Emory University Institutional Animal Care and Use Committee.
Surgery and tissue collection
We injected neuroanatomical tracers and lentiviral vectors with a stereotaxic apparatus (Leica/MyNeuroLab), using the co-ordinates in Table 1 to target brain regions described in the text. A summary of the injections and which figure they appear in is shown in Table 2. For all surgeries, we induced anesthesia with ketamine-midazolam and when necessary supplemented with isoflurane (0.25–2.5%). After induction, the bird’s scalp was anesthetized locally with ~20uL lidocaine. An incision was made and the top layer of the skull was removed so we coutld localize the landmark “Y0”. We defined Y0 as the most posterior point visible at the junction of the midsagittal sinus and the two sinuses that run on either side of the cerebellum. After moving the pipette to the target co-ordinates on the surface of the skull, we made a craniotomy, opened the dura with a syringe needle, and then lowered the pipette to the target depth.
Table 1.
Stereotaxic co-ordinates for targeting regions studied
| Target | Anterior of y0 (mm) | Lateral of y0 (mm) | Depth (mm) | Beak bar angle below horizontal (°) |
|---|---|---|---|---|
| Dorsal thalamus | 0.9–1.1 | 1.3 | 4.1–4.3 | 45 |
| CbL | −1.2 – −1.6 | 1.3 – 1.4 | 3.25–3.45 | 50 |
| Area X | 5.5–5.7 | 0.9–2.2 | 2.9–3.1 | 20 |
Co-ordinates are given in millimeters, in relation to y0, the bifurcation of the midsagittal sinus, i.e. the point where the cerebellum meets the two cerebral hemispheres. In our birds, y0 sometimes but not always had a triangle shape, where the peak of the triangle is the point where the cerebellum and cerebral hemispheres meet, the sides of the triangle are formed by the cerebellum, and the base crosses the cerebellar folia. To be consistent across birds, we ignored the base of the triangle and chose y0 as the farthest point posterior at the peak of the triangle while the point of the pipette was still within the dark region of the sinus visible through the skull. We give the head angle in terms of the beak bar because we used a MyNeuroLab bird stereotactic instrument (now Leica) where the beak bar angle is by default at 45° below the horizontal but can be rotated in the plane of pitch relative to that 45°.
Table 2.
Injections for each experiment
| Experiment | N | Figure |
|---|---|---|
| Test projection from thalamus to basal ganglia | ||
| Injections of lentivirus in dorsal thalamus | 7* | 3,4,10,11 |
| Injections of AAV in dorsal thalamus | 2 | 12 |
| Test projection from CbN to dorsal thalamus | ||
| Injections of dextran amines in CbL | 3 | 5,6 |
| Injections of dextran amines in dorsal thalamus | 3 | 7,8 |
| * 7 hemispheres from 6 birds | ||
N is the number of injections, given in terms of hemispheres. Figure refers to the figure number(s) in which the results from that experiment appear.
Tracers used included dextran amines, lentiviral vectors, and adeno-associated viral vectors (AAVs). We used iontophoresis to inject fluorophore-tagged dextran amines, 10% in 0.1M phosphate buffer (Life/Invitrogen) and adeno-associated virus expressing either green fluorescent protein (GFP) or mCherry (Penn Vector core. AV-1-PV1963 and AV-1-PV1969). Iontophoretic injections were made with an A&M 2100 stimulator, 7 seconds on, 7 seconds off, 4–10 μA, positive current, with a total injection time of 20–30 minutes. For lentiviral vectors, we made pressure injections with a Nanoject II (Drummond). Lentiviral vectors were a 1:1 solution of mCherry and synapthophysin tagged with GFP. mCherry labeled cell bodies and axons, whereas synaptophysin-GFP specifically labeled synapses. Both the monomeric mCherry construct (Shaner et al., 2004) and the synaptophysin-GFP fusion protein (Grinevich, Brecht, & Osten, 2005) were expressed under control of the Rous sarcoma virus (RSV) promoter. The constructs and the promoter were cloned into a self-inactivating FUGW lentivirus (Lois, Hong, Pease, Brown, & Baltimore, 2002). These vectors were developed for anterograde tracing in songbirds; for more detail see (Bauer et al., 2008; Roberts, Klein, Kubke, Wild, & Mooney, 2008). Injection parameters for lentiviral vector injections with the Nanoject were 32.2 nl per press of the “inject” button, at a speed of 23 nl/second, for a total of 800–1500 nl in dorsal thalamus. We waited 45 seconds between each press of the “inject” button, and left the pipette undisturbed for 5m after injecting the total volume, before raising slowly (~100 μm every 5 seconds). After surgery, birds survived 5–7d when dextran amines and AAV were used, and 20–30d when lentiviral vectors were used. After the appropriate survival time, birds were sacrificed with an overdose of ketamine and midazolam, supplemented with isoflurane when necessary. The perfusate consisted of ~20mL 0.95% saline with heparin, followed by ~50mL 4% paraformaldehyde in 0.1M phosphate buffer (PB). The brain was removed and left in 4% paraformaldehyde, 30% sucrose solution overnight at room temperature, then transferred to 30% sucrose in 0.1M PB, where it was left until it sank in solution. Brains were cut in the parasagittal plane on a sliding freezing microtome and 30–60um sections were stored in 0.1M PB for further processing.
Immunohistochemistry
Immunohistochemistical procedures were performed with antibodies at the dilutions given in Table 3, and according to the following procedure: after an initial rinse in 0.1M PB, we washed the tissue in 2% sodium borohydride in 0.1M PB for 0.5h, followed by three washes for 10m in 0.1M PB, as a form of epitope retrieval. Sections were then placed for 1h in a block solution of 2.5% normal donkey serum, 2.5% normal horse serum, 1% Triton-X 100 in 0.1M PB. Primary antibodies were diluted in 1% NDS, 1% NHS, 0.3% TX-100. Sections were incubated 24–48h at 4°C in primary solutions. Then the sections were rinsed 3×10m in 0.1M PB before incubating with secondary antibodies. Secondaries used with primaries to amplify lentiviral signal were tagged with fluorophores, while the secondary used with the parvalbumin primary was biotinylated so it could be further incubated with Vector labs streptavidin-AMCA (SA-5008) as a tertiary. Both secondary and tertiary solutions consisted of 0.3% TX-100 in 0.1M PB, and incubations in these solutions lasted 1h. In cases where a tertiary was used, the incubation was preceded by 3×10m washes in 0.1m PB. In all cases, sections were washed 3 more times for 10m in 0.1M PB before they were mounted on Fischer Superplus slides, then left overnight to dry. Sections were briefly rehydrated before using Fluoro-Gel with DABCO mounting medium to apply coverslips that were then sealed with clear coat fingernail polish.
Table 3.
Antibodies used
| Name | Immunogen structure | Manufacturer, catalog #, RRID, species, mono/poly | Concentration |
|---|---|---|---|
| Anti-red fluorescent protein | mushroom polyp coral Discosoma | Rockland 600-401-379 RRID: AB_2209751 Rabbit polyclonal |
1:1000 |
| Anti-mCherry | full-length protein mCherry | Life technologies/Invitrogen
M11217 RRID: AB_2536611 Rat monoclonal |
1:1000 |
| GFP tag (clone 3E6) | GFP isolated directly from Aequorea victoria | Life technologies/Invitrogen
A-11120 RRID: AB_2534132 Mouse monoclonal |
1:2000 |
| GFP tag | GFP isolated directly from Aequorea victoria | Life technologies/Invitrogen
A-11122 RRID: AB_2534134 Rabbit polyclonal |
1:2000 |
| Parvalbumin | Parvalbumin purified from frog muscle | EMD Millipore MAB1572 RRID: AB_2174013 Mouse monoclonal |
1:2000 |
| Rhodamine Red X donkey anti rabbit | whole molecule rabbit IgG | Jackson 711-295-152 RRID: AB_2340613 donkey polyclonal |
1:400 |
| Rhodamine Red X donkey anti rat | whole molecule rat IgG | Jackson 712-295-150 RRID: AB_2340675 donkey polyclonal |
1:400 |
| Alexa 488 donkey anti-mouse | whole molecule mouse IgG | Jackson 715-545-150 RRID: AB_2340846 donkey polyclonal |
1:400 |
| Alexa 488 donkey anti-rabbit | whole molecule rabbit IgG | Jackson 711-545-152 RRID: AB_2313584 donkey polyclonal |
1:400 |
| Biotinylated horse anti-mouse IgG (H+L) | whole molecule mouse IgG | Vector laboratories BA-2000 RRID: AB_2313581 horse, polyclonal |
1:400 |
In initial experiments (Fig. 3) we used the Rockland rabbit anti-RFP and Invitrogen mouse anti-GFP antibodies to detect mCherry and GFP-synaptophysin expression. In experiments where we used the Millipore mouse anti-Parvalbumin as a marker for Area X and other song system nuclei (Figs. 4, 10, 11, 12), we could not use the mouse anti-GFP, and so instead we used the Invitrogen rat anti-mCherry and the Invitrogen rabbit anti-GFP.
Production and specificity of antisera
Please see Table 3 for list of antibodies used.
Anti-red fluorescent protein (RFP) antibody, Rockland 600-401-379, RRID:AB_2209751, Rabbit polyclonal, detects RFP but not GFP as shown by Western blot (manufacturer’s datasheet). The antibody has been used previously to amplify mCherry signal expression from viral vectors (De Arcangelis, Liu, Soto, & Xiang, 2009; Dinh & Bernhardt, 2011) including vectors used in neuroscience studies (Redondo et al., 2014; Sreenivasan, Karmakar, Rijli, & Petersen, 2015).
Anti-mCherry, Life technologies/Invitrogen M11217, RRID:AB_2536611, Rat monoclonal, specifically detects mCherry as shown with Western blot and flow cytometry (manufacturer’s datasheet). The antibody has been used previously in virally-mediated neural tracing experiments (Schwarz et al., 2015).
Anti-GFP, Life technologies/Invitrogen A-11120, mouse monoclonal, was raised against GFP isolated from Aequorea Victoria (manufacturer’s datasheet). It has been shown to detect GFP fusion proteins expressed in neurons under genetic control (Busch, Selcho, Ito, & Tanimoto, 2009; Liu, Luo, Carlsson, & Nassel, 2015) and GFP expression resulting from viral vectors (Keen-Rhinehart et al., 2009; Vujovic, Gooley, Jhou, & Saper, 2015)
Anti-GFP, Life technologies/Invitrogen A-11122, rabbit polyclonal, was raised against GFP isolated from Aequorea Victoria (manufacturer’s datasheet). Previous reports show this antibody detects GFP expression induced in neurons by viral vectors (Davis et al., 2011; Lindberg, Chen, & Li, 2013)
Anti-parvalbumin, EMD Millipore MAB1572, mouse monoclonal, was raised against parvalbumin purified from frog muscle, and in Western blots yields a band at 12kDa (the weight of parvalbumin). The antibody shows specific immunoreactivity with parvalbumin expressing interneurons (McKenna et al., 2013) and has been used to label such neurons in songbirds (Li et al., 2013). It has previously been shown that one cell type in Area X expresses parvalbumin and that Area X shows higher immunoreactivity for parvalbumin in its neuropil than the surrounding medial striatum (Braun, Scheich, Schachner, & Heizmann, 1985; Carrillo & Doupe, 2004; Reiner, Laverghetta, Meade, Cuthbertson, & Bottjer, 2004)
Microscopy, digital photography, and image processing
Low-power widefield images were obtained with a Zeiss Axioplan 2 and Olympus IX51. Confocal z-stacks were obtained with Leica SP8 inverted and Olympus FV1000 inverted microscopes. Brightness and contrast were adjusted using ImageJ, Zeiss AxioVision software (for images acquired with the Axioplan 2), or Photoshop (for images acquired with the Olympus IX51). ImageJ was used for all processing of z-stacks, including z projections, adjustment of brightness and threshold, and changing of look-up tables (e.g., to convert red to magenta). We used the following procedure to calculate the distances from the midline shown in figures on parasagittal sections: we chose the zero point to be the middle of the interstitial nucleus of Cajal (InC), which is found at the midline (http://www.zebrafinchatlas.org/) and which we observed usually occurred in two sections in parasagittal sections; we then counted the number of sections including the section in the figure and the section of InC between that section and the zero point; we multiplied that number of sections by the section thickness (e.g. 20 sections × 40 micrometers/section) and lastly we multiplied the total number of micrometers by 1.5, a factor to account for shrinkage that occurred when the brain was fixed. We found this conversion factor by measuring the distance between injection sites and the midline in fixed tissue and solving for the average value that would convert this distance back to the mediolateral distance we used for stereotaxic targeting the injections.
“Drawings” of signal from lentiviral injections
To present results from lentiviral injections, we followed a procedure that yielded figures similar to camera lucida-assisted drawings of light microscopy material. After performing fluorescence immunohistochemistry on sections to amplify signal, we made large tiled scans of the sections with a confocal microscope using a 40× objective. We then used the FIJI distribution of ImageJ (Schindelin et al., 2012) to make z-projections of these tiled scans, compress the z-stack into one x-y plane, and adjust the brightness and contrast. In Adobe Illustrator, we aligned the tiled z-projection of each section with a widefield image taken with a 4× objective of the same section. The 4× objective was used with a DAPI filter to image the Parvalbumin (PV) signal that allowed us to identify the borders of Area X and other areas of interest. To ensure that the 40× images and the 4× images were at the same scale, we placed scale bars of the same size on both images and aligned the scale bars before aligning the images. We then made the 40× images transparent in Illustrator and aligned the actual sections by eye using landmarks, e.g., the edges of th(Schindelin et al., 2012)e sections and cytoarchitectural landmarks that were visible because of slight background autofluorescence. Using a Wacom graphics tablet with a stylus, we outlined regions of interest like Area X based on the PV signal. Next we imported the aligned images and the outlines of regions into Adobe Photoshop as separate layers. On the layers with the 40× tiled z-projections, we used the Lasso tool to outline all areas of signal (either synaptophysin-GFP imaged with Alexa 488 secondaries or mCherry imaged with Rhodamine Red X). We copied these areas with signal to a separate layer and then applied the Invert and Threshold functions. Next we opened the files again in Illustrator and used the Image Trace tool on the inverted and thresholded signal (mode: black and white, threshold: 210–245), paths: 100%, corners: 75%, noise: 15–25px, create: fills, snap curves to lines: no, ignore white: yes). Lastly we selected “Make and Expand” to convert the Image Trace objects to vector art, and then colored the vectors, either magenta for the mCherry signal or green for the synaptophysin-GFP signal. We exported the completed tracing as a .png file.
We show only representative sections from the series of tracings in Figs. 10 and 11. The entire series of tracings is included in the Supporting Information. A link to this data is in the Data Accessibility section.
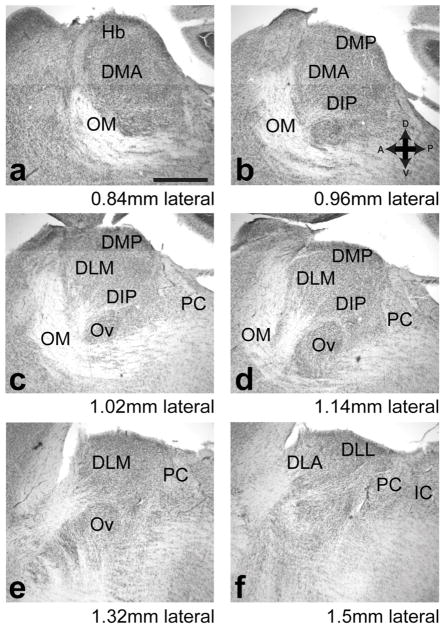
Nomenclature of dorsal thalamus
Regions in dorsal thalamus were named based on previous literature, including the zebra finch brain architecture atlas (H. J. Karten et al., 2013) and the Histological Atlas Browser on the Zebra finch Expression Brain Atlas (http://www.zebrafinchatlas.org/). To guide the reader in our use of this nomenclature, we present in Figure 2 a series of parasagittal sections moving from medial to lateral dorsal thalamus. We emphasize that when we use the terms DLM and DMP we are referring to thalamic nuclei found only in the song system of the songbird which project to LMAN and MMAN, song system nuclei in the nidopallium. Other regions of dorsal thalamus—DMA, DIP, DLL, and DLA—are meant to refer to the subregions of dorsal thalamus that have been delineated for non-songbird avian species such as pigeons. In the discussion we address the question of how our results relate to the subdivisions of avian thalamus, and the homologies between thalamic regions in songbirds, other avian species, and mammals.
Figure 2.
Dorsal thalamus in Bengalese finches as shown in parasagittal series. We adopted nomenclature from atlases and previously published literature as described in the Methods section “Nomenclature of Dorsal Thalamus”. Note that by DLM and DMP we mean the thalamic nuclei found only in songbirds which project to cortical nuclei LMAN and MMAN of the song system, respectively, and not the regions in non-songbird species that share the same names. Series of Nissl-stained parasagittal sections moving from medial (a) to lateral (f). Distance lateral is from midline in this figure is sum of the number of sections from midline (determined as described in Methods) multiplied by section thickness, but not adjusted to account for shrinkage due to fixation. Abbreviations: DIP, dorsointermediate posterior nucleus, DLA, dorsolateral anterior nucleus, DLL, dorsolateral lateral nucleus, DLP, Dorsolateral posterior nucleus, DMP, dorsomedial posterior nucleus, Hb, habenula, IC, inferior colliculus, OM, occipto-mesencephalic tract, PC, posterior commissure. Scale bar in a is 500 μm. Left is anterior, dorsal is up.
Map of DLM and DTCbN
To determine whether song system thalamic nucleus DLM or cerebellar-recipient dorsal thalamus (DTCbN) were the source of projections to Area X or the surrounding medial striatum, we carefully mapped both regions of dorsal thalamus (Figs. 5, 6, and 9). Having done this, we could then superimpose sites of injection into dorsal thalamus on this map and see whether an injection that had produced synaptophysin-GFP label in Area X had been in DLM or DTCbN. As we show, the projections of CbL were consistent across animals (Fig. 6). Likewise we saw that DLM as defined by its input from Area X was consistent across animals (Fig. 9). In addition we could easily recognize DLM even in unstained tissue because of the heavier myelination that showed up as a brighter area when sections were viewed with darkfield through a DIC filter of a 5× objective (Fig. 9). To produce a reference map of dorsal thalamus, we outlined areas in one bird where we injected a GFP expressing viral vector in Area X to label its projection to DLM, and injected dextran amines in CbL to label DTCbN. We used only one bird as a reference so that we could be sure there was no added noise due to slight differences in alignment when sectioning brains and then aligning series of section by eye. Hence in this reference series we could confidently localize DLM and DTCbN in each section relative to each other.
We aligned this map with the injection sites from viral injections in dorsal thalamus by using cytoarchitectural landmarks that were clearly visible when viewing unstained sections at 5× with darkfield. (We combined DIC with darkfield simply because this increased contrast on the microscope used to image injection sites, a widefield Zeiss Axioplan 2). Typical injection sites consisted of neurons expressing either synaptophysin-GFP or mCherry, with a sparse population of cells expressing both. We chose to define injection sites by the mCherry labeled cell bodies because the mCherry label was easier to image at 5x. Comparison of this label with synapthophysin-GFP label in the injection site, as imaged with a confocal, showed no obvious difference between the injection site as defined by mCherry signal or synaptophysin-GFP signal—i.e. injection sites appeared to be mostly homogenous mixture of neurons infected with one or the other viral vector.
Results from the bird that we used as a reference for the map of DLM and DTCbN, as well as raw images of the injection sites from each case in Fig. 10 and 11, and the files showing how they were aligned with this map, are included in the Supporting Information. A link to this data is in the Data Accessibility section.
Results
Dorsal thalamus projects to medial striatum, including Area X
To determine whether dorsal thalamus projects to medial striatum, we used lentiviral vectors for neuroanatomical tracing. There were two advantages of using these lentiviral vectors. The first is that they produce only anterograde label, because they infect only cell bodies at the injection site (Grinevich et al., 2005; Roberts et al., 2008) whereas standard tracers are picked up by the cell body and by axon terminals, traveling to some extent in both the anterograde and retrograde direction (Kobbert et al., 2000; Reiner et al., 2000). If we had used standard tracers, we would not have been able to distinguish between anterograde and retrograde label in Area X, because both Area X and the surrounding medial striatum project directly to dorsal thalamus. The second advantage of the vectors we used is that one contained GFP-tagged synaptophysin, allowing us to specifically label presynaptic axon segments with GFP signal (synaptophysin protein is localized at the presynaptic axon terminal and axon segments proximal to the terminal (Grinevich et al., 2005; Roberts et al., 2008)).
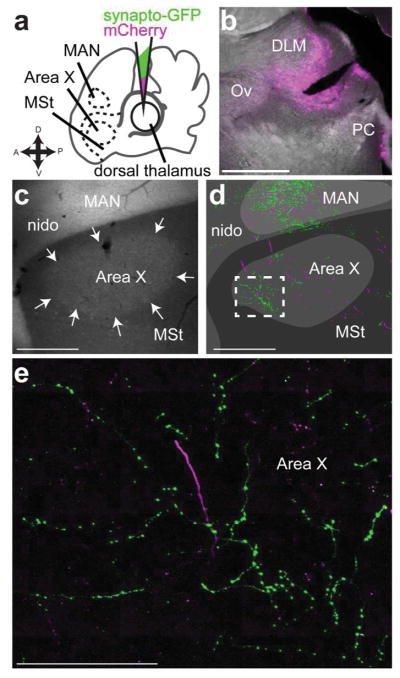
We made an initial series of large injections in dorsal thalamus to simply test whether any part of dorsal thalamus projected to any part of the medial striatum (including Area X). These injections (experiment schematic, Fig. 3a) contained a 1:1 ratio of the synaptophysin-GFP vector and another expressing mCherry (Roberts et al., 2008). The mCherry vector labels cell bodies and axons, allowing us to identify the injection site in dorsal thalamus (Fig. 3b). In no case did we see retrograde label (e.g., label of cell bodies in Area X, medial striatum, or CbL) from injections in dorsal thalamus, making us confident that our results are based exclusively on signal produced by infection of cell bodies local to the injection site and transported in the anterograde direction. We found that injections in dorsal thalamus yielded synaptophysin-GFP label throughout the medial striatum (MSt, Fig. 3c, green arrows). As stated in the introduction, the basal ganglia nucleus of the song system, Area X, sits within MSt of songbirds. We suspected that anterograde label was also present in Area X, based on landmarks, such as the presence of cortical nucleus LMAN within a section. We investigate this using immunohistochemistry to identify the borders of Area X in the next set of experiments described. The axon segments labeled by synaptophysin-GFP had numerous varicosities (Fig. 3d) as seen in the mammalian thalamostriatal system (Berendse & Groenewegen, 1990; Deschenes, Bourassa, & Parent, 1996). Most of the processes we saw labeled by synaptophysin-GFP from this initial set of injections ramified locally with a cluster of boutons (Fig. 3c,d) suggestive of axon terminals, although in some cases we saw axon segments punctuated by varicosities that coursed through striatum for hundreds of micrometers (Fig. 3g). We also saw mCherry labeled processes with varicosities (Fig. 3e, f), indicating that this morphology did not arise as a result of overexpression of synaptophysin. Qualitatively, it appeared that the synaptophysin-GFP (as opposed to mCherry) preferentially labeled the processes that form varicosities within the medial striatum (Fig. 3g, green arrows).
Figure 3.
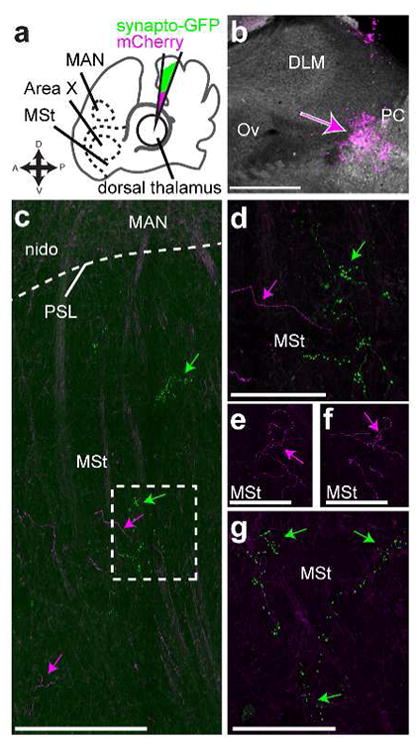
Dorsal thalamus projects to striatum a, Schematic of experiment. We injected into dorsal thalamus (DT) a 1:1 solution of two lentiviral vectors, one expressing synaptophysin-GFP and the other mCherry, and then looked for label in medial striatum (MSt) which contains basal ganglia nucleus of the song system, Area X. One landmark we used to determine whether sections included MSt was the magnocellular nucleus of the anterior nidopallium (MAN) which is obvious even in unstained tissue. b, Widefield image of injection site from representative case. This injection was outside of thalamic nucleus of the song system DLM, just anterior to the posterior commissure (PC) and in the same plane as auditory thalamus nucleus ovoidalis (Ov) c, Image showing mCherry and synaptophysin-GFP labeled processes in MSt ventral to MAN. Magenta arrows, mCherry signal, green arrows, synaptophysin-GFP signal. PSL, pallial-subpallial lamina, nido, nidopallium outside of MAN. d, Synaptophysin-GFP labeled processes in MSt had varicosities, suggesting they were synapses. Higher-power scan of the area outlined in C with a dashed box. e, f. mCherry labeled processes could also be found in MSt with varicosities, indicating this is the actual morphology of the axons and did not result from ectopic (over)expression of synaptophysin-GFP g. Another example of synaptophysin-GFP labeled processes in MSt. e–g are all in the same section within 500 μm of each other but are enlarged to be easily visible. All sections are parasagittal with anterior to the left and dorsal up. Scale bars: b–c, 500 μm; d–g, 100 μm.
Having shown that dorsal thalamus projects to the basal ganglia, we then sought to demonstrate conclusively whether thalamostriatal projections specifically target Area X. We made another series of viral injections in dorsal thalamus (Fig. 4a, b), and for each case we also labeled Area X by performing immunohistochemistry against parvalbumin (PV) (Fig. 4b, c). Neuropil in Area X is more strongly enriched in PV than the surrounding medial striatum (Reiner et al., 2004). This allowed us to clearly identify Area X in each section and determine whether processes labeled by synaptophysin-GFP and mCherry were within its borders. By aligning the PV-stained section with mosaic images of high-powered confocal stacks imaging the GFP and mCherry signal, we could see that some part of dorsal thalamus projected to Area X (Fig. 4d). These processes again had varicosities characteristic of thalamostriatal axon terminals (Fig. 4e). As with the initial set of injections, we could find cases where the synaptophysin-GFP labeled processes appeared to form local clusters of bouton-like structures, but we also saw regions where these processes traveled through striatum for hundreds of micrometers dotted by varicosities.
Figure 4. Dorsal thalamus projects to striatum, including Area X.
a, Schematic of experiment. As in Fig. 3, we injected into dorsal thalamus a 1:1 solution of two lentiviral vectors, one expressing synaptophysin-GFP and the other mCherry, but in these experiments we labeled Area X immunohistochemically with antibodies against parvalbumin, and then determined whether viral label was found in Area X or medial striatum (MSt) as well as the magnocellular nucleus of the anterior nidopallium (MAN). b, Widefield image of injection site from representative bird. c, Parvalbumin stain to outline Area X (white arrows) from the same bird. d, “Camera lucida” style tracing of GFP and mCherry signal from same section shown in (c). e, Confocal image of area shown in white box in d. Note varicosities suggesting synapses. All sections are parasagittal with anterior to the left and dorsal up. Scale bars in a–d, 500 μm. Scale bar in e, 250 μm.
The cerebellar nuclei project to contralateral dorsal thalamus
Although our results showed that dorsal thalamus projects to Area X and the surrounding medial striatum, they did not clearly demonstrate which regions of dorsal thalamus gave rise to the projections to Area X. We noted that signal in Area X resulted from injections that appeared to be in the same mediolateral plane as song system thalamic nucleus DLM (Fig. 3b, Fig. 4b). Based on this result, and on previous work, we hypothesized that the source of projections to Area X would be either DLM or cerebellar-recipient dorsal thalamus (DTCbN) near DLM. To determine whether DLM or DTCbN project to Area X or medial striatum, we first mapped these areas of dorsal thalamus using standard tracers and viral vectors.
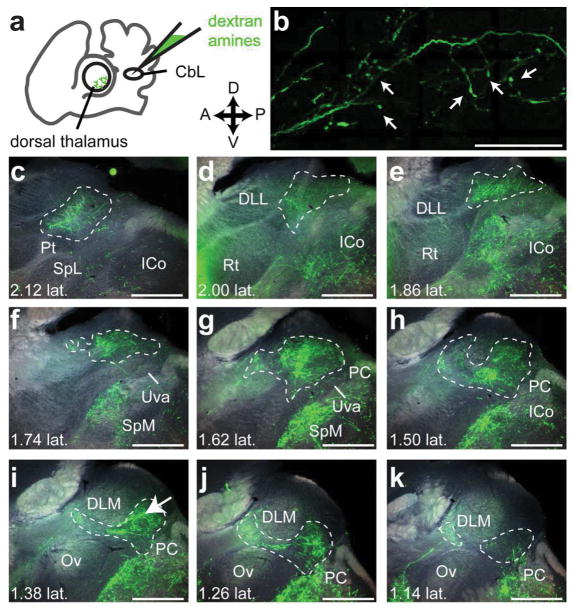
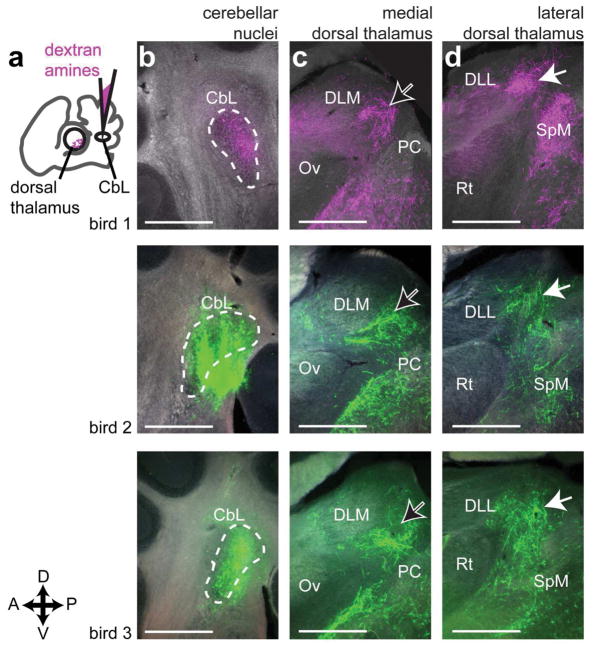
We began by identifying the region of dorsal thalamus targeted by the cerebellar nuclei in Bengalese finches. Our initial set of injections targeted the lateral cerebellar nuclei (CbL) because of the previous work in other songbird species suggesting this was the main source of cerebellar projections to thalamus (A. L. Person et al., 2008; Vates, Vicario, & Nottebohm, 1997). Injections of dextran amines in CbL yielded anterograde label across the mediolateral extent of contralateral dorsal thalamus (Fig. 5). This result was consistent across animals (n=3, Fig. 6). In every case where we successfully targeted CbL, we saw label across the entire mediolateral extent of dorsal thalamus, from near the midline (Fig. 5k) to very laterally near the pretectal nucleus (Pt) (Fig. 5c). We always saw a densely labeled area posterior to song system thalamic nucleus DLM (Fig. 6c, black arrows with white outline), in roughly the same mediolateral plane as the auditory region of thalamus, nucleus ovoidalis (Ov). Results confirming the location of DLM in relation to DTCbN are described in the next section. Cerebellar axon terminals surrounding but not invading DLM were also seen after injections in the cerebellar nuclei (CbN) in zebra finches (A. L. Person et al., 2008). DTCbN was not confined to a region posterior to DLM; the area of dense label extended laterally to the same mediolateral plane as the retinal-recipient nucleus DLL (Fig. 6d, white arrows indicate label from cerebellar injection), posterior to the region that receives retinal output (H. J. Karten et al., 2013). We note that in two of three cases shown in Fig. 6, injections in CbL also yielded some retrograde label of the medial spiriform nucleus (SpM). SpM is known to project to the cerebellum (H. Karten & Finger, 1976; A. L. Person et al., 2008), specifically the cerebellar cortex (J. M. Wild, 1992). In one of the three cases, SpM was retrogradely labeled on both sides of the brain, and on the ipsilateral side where there was no anterograde label from CbL, we saw label of axon collaterals in dorsal thalamus, i.e., it appeared that the tracer traveled retrogradely from the cerebellum to SpM cell bodies and then from SpM traveled anterogradely to label collaterals in dorsal thalamus. However there were few of these collaterals and the amount of signal was very sparse compared to the strong anterograde label seen from all CbL injections (the sparse label of SpM collaterals is shown in the Supporting Information, please see link to those images in the Data Accessibility section). We are therefore confident that the majority of label in contralateral dorsal thalamus in all cases traveled anterograde from CbL. In addition to the label in dorsal thalamus, we also saw widespread signal throughout the midbrain, with dense innervation of the red nucleus, ansa lenticularis, and SpM. These results are consistent with what was reported by Person et al. (2008) and what is seen in other bird species (Arends & Zeigler, 1991) and mammals (Hoshi et al., 2005; Loreta Medina, Veenman, & Reiner, 1997). Since the objective of these experiments was to map the cerebellar-recipient regions of dorsal thalamus, we do not describe these other targets of the cerebellar nuclei further, but we do include the results in the Supporting Information (please see link to those images in the Data Accessibility section). In the Discussion we address how other targets of CbL in the midbrain may relate to the function of the cerebellothalamic projections.
Figure 5.
The lateral cerebellar nucleus (CbL) projects to contralateral dorsal thalamus. a, Schematic (left panel) of experiment showing injection in CbL and widefield image (right) showing injection site. The injection site is shown in Fig. 6, panel b, middle row. b, High-resolution confocal image of axon terminal-like morphology in contralateral dorsal thalamus. This image was taken from the area indicated with a white arrow in i. c–k, representative series of widefield images across dorsal thalamus showing anterograde label from the injection in CbL. c is the most lateral section and k is the most medial. Estimate of distance from midline (e.g. “1.02 lat.”) is given in millimeters, calculated as explained in Methods. Dotted white lines demarcate the areas we considered cerebellar-recipient dorsal thalamus. All sections are parasagittal with anterior to the left and dorsal up. This series is from “bird 2” in Figure 6. All scale bars 500 μm. Abbreviations from c–h: Pt, pretectal nucleus. SpL, lateral spiriform nucleus. ICo, inferior colliculus. DLL, dorsolateral thalamus, lateral part. Rt, nucleus rotundus. Uva, uvaeform nucleus. SpM, medial spiriform nucleus. PC, posterior commissure. Ov, nucleus ovoidalis. DLM, dorsolateral thalamus, medial part.
Figure 6.
CbL axon terminals target medial dorsal thalamus adjacent to song system nucleus DLM, but also target more lateral dorsal thalamus. A, Schematic representation of experiment, showing injection site in lateral cerebellar nuclei, and anterograde label in contralateral dorsal thalamus. B, Injection sites. In one case shown (top row), the fluorophore conjugated to the dextran amines was tetramethylrhodamine. In the other cases, the conjugate was fluorescein. The choice of fluorophore did not affect results. C, More medial site in dorsal thalamus with strong label. DLM, dorsolateral thalamus, medial part. Ov, nucleus ovoidalis. PC, posterior commissure. D, More lateral site in dorsal thalamus with strong label. Abbreviations: DLL, dorsolateral thalamus, lateral part. Rt, nucleus rotundus. SpM, medial spiriform nucleus. All sections are parasagittal, left is anterior and up is dorsal. All scale bars 500 μm.
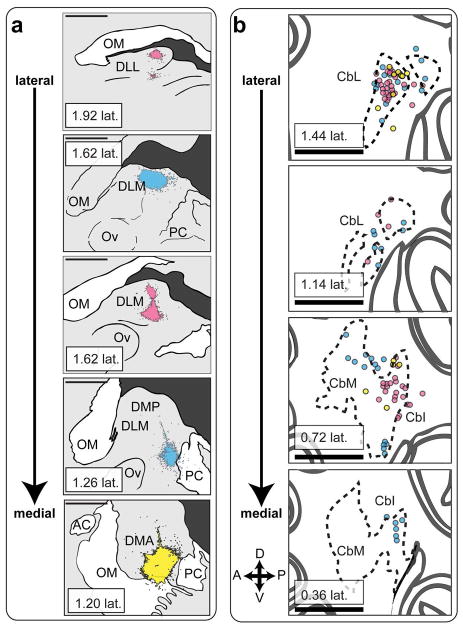
To confirm that CbL projects to dorsal thalamus, we made injections of dextran amines in dorsal thalamus and looked for retrograde label in CbL (Fig. 7a). These injections yielded retrograde label of contralateral CbN (Fig. 7b, 7c). As previously reported for songbirds (Vates et al., 1997), the injections gave strong label in CbL. In addition, we saw retrograde label in intermediate and medial regions of the cerebellar nuclei (CbI and CbM, Fig. 7d), consistent with what has been reported for other bird species (Loreta Medina et al., 1997) and for mammals (Hoshi et al., 2005). We combined results from animals (n=3) in which the mediolateral position of the thalamic injection site varied (Fig. 8) to map of the regions of cerebellar nuclei that project to dorsal thalamus in a songbird. In two of these birds we made multiple injections in the mediolateral plane of dorsal thalamus (Fig. 8, injection sites colored cyan and magenta), which yielded strong retrograde label. A more ventral and medial injection (Fig. 8, injection site colored yellow) yielded less retrograde label. All three injections yielded the strongest retrograde label in CbL, but they also yielded label of many neurons in CbI and a few neurons in CbM as well (Fig. 8b). Hence our results suggest that the strongest projection to dorsal thalamus originates from CbL, but that there is a significant contribution from CbI, and some input from CbM as well.
Figure 7.
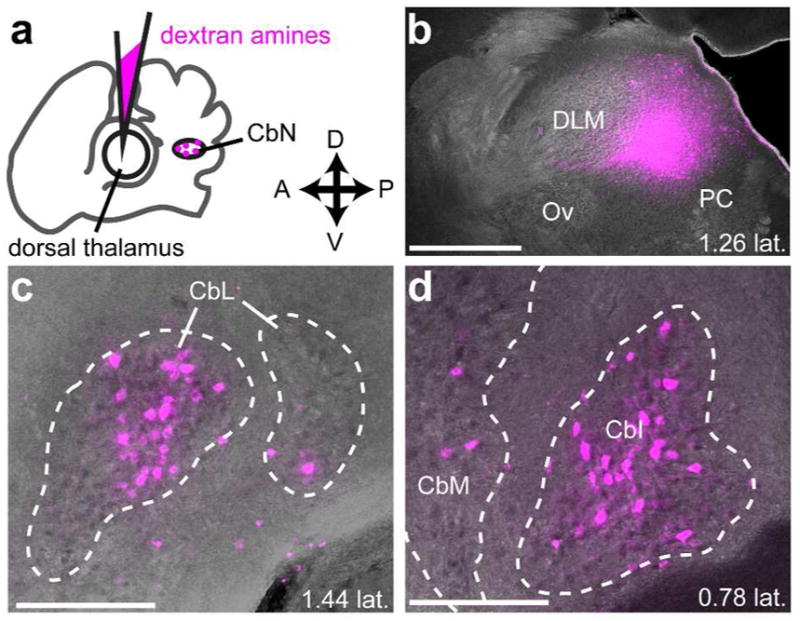
Injections in dorsal thalamus yield retrograde label in the cerebellar nuclei. a, schematic indicating injection site in dorsal thalamus and site of retrograde label in contralateral cerebellar nuclei (CbN) (shown in same plane of cartoon “section”). b, Representative injection site in dorsal thalamus. Parasagittal section. Anterior is left and dorsal is up. DLM, dorsolateral thalamus, medial part. Ov, nucleus ovoidalis. PC, posterior commissure. c, Retrograde label in contralateral lateral cerebellar nucleus (CbL). d, Retrograde label in contralateral intermediate (CbI) and medial (CbM) cerebellar nucleus. All sections are parasagittal with anterior to the left and dorsal up. Scale bar, 500 μm
Figure 8.
All of the cerebellar nuclei project to dorsal thalamus. a, Injection sites in dorsal thalamus from 3 birds (magenta, cyan, yellow) arranged from lateral to medial. Note that the 3rd panel from the top corresponds approximately to the injection site shown in figure 5A. DLL, dorsolateral thalamus, lateral part. OM, occipito-mesencephalic tract. DLM, dorsolateral thalamus, medial part. Ov, nucleus ovoidalis. PC, posterior commissure. b, Schematic of retrograde label in the contralateral cerebellar nuclei arranged from lateral to medial.n Colored circles represent retrogradely filled cell bodies. Color of each circle indicates retrograde label from injection site with the same color in a. CbL, lateral cerebellar nucleus. CbI, intermediate cerebellar nucleus. CbM, medial cerebellar nucleus. All sections are parasagittal, left is anterior and up is dorsal. All scale bars 500 μm.
Thalamic nucleus of the song system DLM is adjacent to but separate from cerebellar-recipient regions of dorsal thalamus
In addition to mapping DTCbN, we used similar methods to define the borders of thalamic song system nucleus DLM, so that we could show whether injections of lentiviral vector in either region of dorsal thalamus resulted in labeled axon terminals within Area X. We mapped DLM by injecting tracer in Area X (Fig. 9), since the main target of Area X projection neurons is DLM (Luo & Perkel, 1999a, 1999b). Injections of AAV-GFP in Area X (Fig. 9a) produced anterograde label of the calyceal terminals formed by projection neurons of Area X in DLM (Figure 9b–d). We noticed that anterograde label from Area X injections was always confined to an area of dorsal thalamus that appeared as a bright oval when viewed with a DIC objective, presumably due to heavier myelination (Fig. 9b–d). Based on this observation, we concluded that this bright area can serve as a marker for the borders of DLM across animals. We never saw anterograde label within DLM, as defined by this brighter oval, from injections in CbL. We cannot rule out the possibility that CbI or CbM might project to DLM, but as we showed these projections to dorsal thalamus are relatively small compared to the projection from CbL (Fig. 7 and 8). Therefore our results suggest that DLM and DTCbN are two adjacent but distinct areas.
Figure 9.
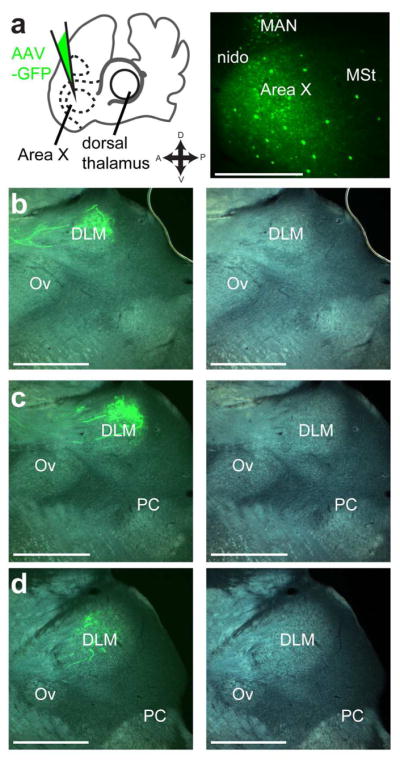
Thalamic song system nucleus DLM is adjacent to but separate from DTCbN. A, Iontophoretic injection of AAV-GFP vector in Area X. Left panel, schematic of injection site. Right panel, representative section showing injection. nido, nidopallium. MAN, magnocellular nucleus of anterior nidopallium. MSt, medial striatum. B–D, Resulting anterograde label of calyceal-like terminals in DLM (dashed white line). Note that label is confined to bright oval as seen in tissue when viewed with DIC filter. DLM, dorsolateral thalamus, medial part. Ov, nucleus ovoidalis. PC, posterior commissure. All sections are parasagittal, left is anterior and up is dorsal. All scale bars: 500 μm
DLM and a dorsal anterior subregion of medial DTCbN project to Area X
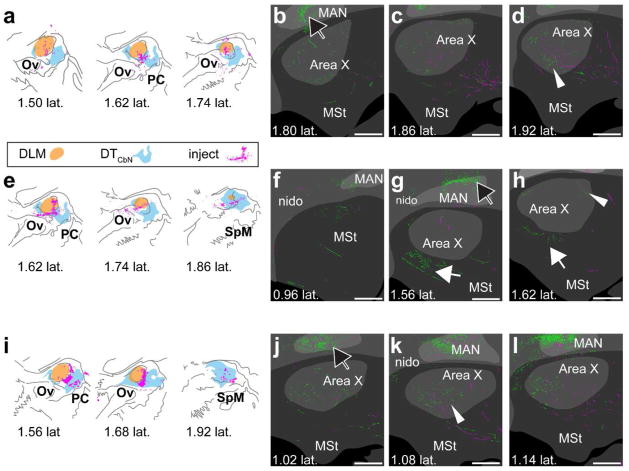
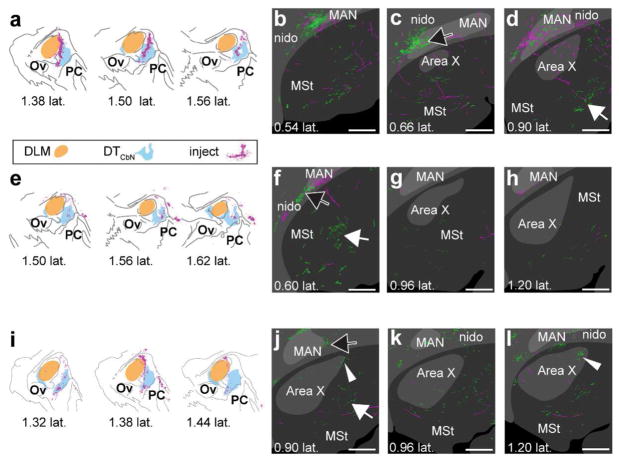
Having produced a map of DLM and DTCbN, we next determined whether either region projects to the medial striatum. We made a series of smaller viral injections targeting either DLM or the medial regions of DTCbN that sits posterior to DLM. For each case, we aligned the injection site with a reference map of DLM and DTCbN (see Methods). We found that whenever the injection site included DLM or the immediately adjacent dorsal anterior subregion of medial DTCbN (n=3 hemispheres from two birds, Fig. 10a, e, i), it produced anterograde label in Area X (Fig. 10b–d, f–h, j–l). In two of the three cases, there was strong label of processes with varicosities, similar to what is shown in Fig. 3e, across Area X (Fig. 10d and k, arrow with white outline and no fill). In the remaining case, there was less label in Area X (Fig. 10h, arrow with white outline and no fill) and there was also label of processes with varicosities just ventral to Area X (Fig. 10g, solid white arrow). There was no obvious topography in the dorsoventral or anteroposterior planes, although we did often see that label was strongest in the same mediolateral plane as the injection site. The entire series from each case shown in Figs. 10 and 11 can be accessed in the Supporting Information (please see link in Data Accessibility section). We made another set of injections (n=3), which transfected neurons in the more posterior and ventral subregion of medial DTCbN while either partially (Fig. 11a, i) or entirely avoiding DLM (Fig. 11e). Results from these injections indicated that this ventral posterior subregion of medial DTCbN does not target Area X. Instead it projects to MSt posterior and ventral to Area X (Fig.11d, f, j, solid white arrows). We did occasionally see small areas of GFPsignal within Area X (Fig. 11j and l, arrow with white outline and no fill) from these injections but strongly-labeled processes with varicosities were posterior and ventral to Area X, and they were much more medial (as in Fig.11f and j). Again label was usually strongest in the same mediolateral plane as the injection site.
Figure 10.
DLM and adjacent DTCbN project to Area X. a–d, case where injection was mostly in DLM. Synaptophysin-GFP label was evident in Area X (for example see white arrowhead in d) e–h, case where injection was in DLM and surrounding DTCbN. Synaptophysin-GFP label was evident in medial striatum (G and H, white arrow) and in Area X (h, white arrowhead). i–l, case where injection was mostly in DTCbN. Although the injection was mainly in DTCbN, we again saw strong synaptophysin-GFP label in Area X (for example k, white arrowhead). a,e,y, Injection sites. Orange region, DLM; cyan region, DTCbN; magenta, cell bodies expressing mCherry. b–d, f–h, j–l. Series of sections from lateral to medial showing Area X, surrounding medial striatum, and overlying nidopallium. Green, GFP signal. Magenta, mCherry signal. Dark gray, no parvalbumin label; gray, some parvalbumin label; light gray, strong parvalbumin label. White arrowheads, examples of GFP signal in Area X; solid white arrow, GFP signal outside Area X in MSt; black arrow with white outline, GFP signal in nidopallium. DLM, dorsolateral thalamus, medial part. MAN, magnocellular nucleus of anterior nidopallium. MSt, medial striatum. nido, nidopallium. Ov, nucleus ovoidalis. PC, posterior commissure. SpM, medial spiriform nucleus. To facilitate comparison across cases, distances from midline for injection sites are based on the map of DLM and DTCbN we created as described in Methods. All sections are parasagittal, left is anterior and up is dorsal. All scale bars 500 μm.
Figure 11.
More medial and posterior regions of DTCbN project to medial striatum. a–d, case where injection was mostly in DTCbN and more medial. Strong synaptophysin-GFP label was posterior and/or medial of Area X (d, white arrow). There was almost no GFP label in Area X in this case. e–h, case where injection was in DTCbN but posterior of DLM. Again the strongest Synaptophysin-GFP label was medial of Area X (f, white arrow). i–l, Area X, arrow with white outline in l. a,e,i, Injection sites in DLM and DTCbN for 3 birds. Sections are arranged from medial to lateral reading from left to right. Orange region, DLM; cyan region, DTCbN; magenta, cell bodies expressing mCherry. b–d, f–h, j–l, series of sections from lateral to medial showing Area X, surrounding medial striatum, and overlying nidopallium. Green, GFP signal. Magenta, mCherry signal. Dark gray, no parvalbumin label; gray, some parvalbumin label; light gray, strong parvalbumin label. Arrow with white outline and no fill, GFP signal in Area X; solid white arrow, GFP signal outside Area X in MSt; black arrow with white outline, GFP signal in nidopallium. DLM, dorsolateral thalamus, medial part. MAN, magnocellular nucleus of anterior nidopallium. MSt, medial striatum. nido, nidopallium. Ov, nucleus ovoidalis. PC, posterior commissure. SpM, medial spiriform nucleus. To facilitate comparison across cases, distances from midline for injection sites are based on the map of DLM and DTCbN we created as described in Methods. All sections are parasagittal, left is anterior and up is dorsal. All scale bars 500 μm.
Regardless of injection site we saw strong label of synaptophysin-GFP and mCherry processes in nidopallium, the cortical layer overlaying the basal ganglia. Injections that included DLM produced label in cortical song system nucleus LMAN as expected, given it is the known target of DLM (for example Fig. 10g and j, black arrow with white outline), and injections in DTCbN separate from DLM tended to produce label in nidopallium outside of LMAN but still within an area of somewhat stronger labeling for parvalbumin (Fig. 11c, f, and j, black arrow with white outline). Since the injections produced label both in cortex as just described, and in the basal ganglia, these results suggest that either individual neurons in dorsal thalamus project to both striatum and cortex, or that thalamic neurons that project to striatum are intermingled with those that project to cortex.
DLM sends a substantial projection to Area X
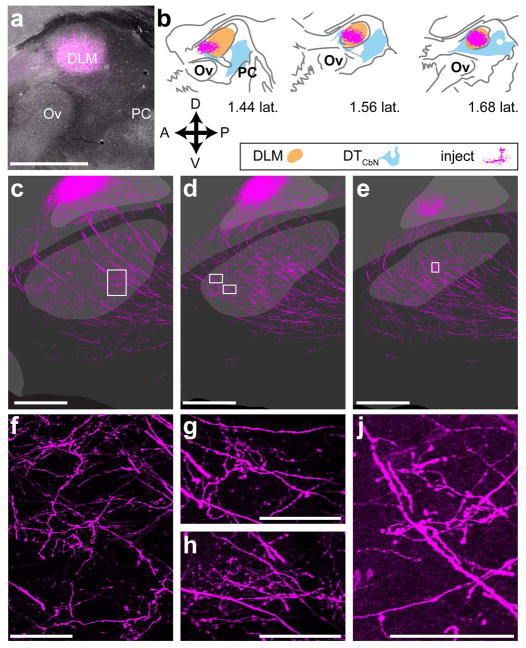
The results from lentiviral injections suggested that Area X receives input from both DLM and the anterior dorsal region of medial DTCbN. The projection from Area X to DLM plays a crucial function in the anterior forebrain pathway (Goldberg & Fee, 2012; Kojima, Kao, & Doupe, 2013; Luo & Perkel, 1999a, 1999b; Abigail L Person & Perkel, 2005). Therefore finding that a reciprocal projection exists from DLM to Area X would significantly change our understanding of the song system. To verify that DLM alone projects to Area X, we carried out a separate set of injections targeting DLM, using AAV that encoded mCherry. This allowed us to achieve three things: (1) produce strictly anterograde label, as we had done with the lentivirus, (2) make smaller injections with iontophoresis so that they were confined to DLM, and (3) show that the varicosities and axon terminal-like morphologies we saw after lentiviral injections did not arise due to ectopic expression of synaptophysin.
Injections of AAV-mCherry in DLM (n=2) yielded significant amounts of label throughout Area X. As shown in a representative case (Fig. 12), iontophoretic injection of the virus allowed us to contain the vector almost entirely within DLM (Fig. 12a, b). Injections of AAV-mCherry in DLM yielded strong label of axons passing through striatum on their way to cortical nucleus of the song system nucleus LMAN, but also revealed a dense network of processes that blanketed Area X (Fig. 12, c–e). These processes included lengthy axon segments dotted with varicosities as well as local ramifications that appeared to bear numerous boutons (Fig. 12, f–j).
Figure 12. DLM sends a significant projection to Area X.
a, representative image of iontophoretic injection in DLM. b, injection site mapped onto borders of DLM and DTCbN as in Figs. 10 and 11, showing that it injection was confined to DLM. c–e, series of consecutive sections showing label from injection throughout Area X and in LMAN f–j, Axon segments dotted with varicosities as well as locally-ramifying axon-terminal like morphologies in Area X. Each panel is a confocal image of the area(s) surrounded by a white box in the panel above it. Ov, nucleus ovoidalis, PC, posterior commissure. Scale bars: a, c–e 500 μm. f–h, 50 μm. j, 100 μm.
Discussion
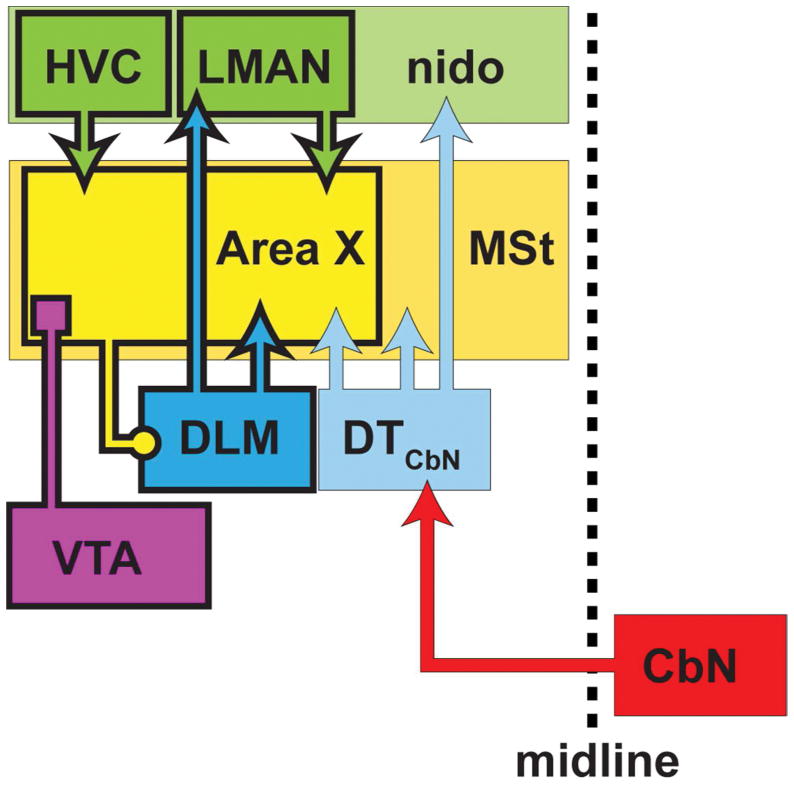
The main result of this paper is that, in Bengalese finches, the basal ganglia nucleus Area X of the song system receives input from two regions of dorsal thalamus: the thalamic nucleus of the anterior forebrain pathway, DLM, and the dorsal anterior subregion of cerebellar-recipient thalamus(DTCbN) that lies adjacent to DLM (Fig. 13). Below we address technical considerations and relate our results to previous work. Then we discuss possible functions of the pathways we identified and how future studies might test for these functions.
Figure 13. Summary of results.
We showed that, in the Anterior Forebrain Pathway (AFP) of the song system, the basal ganglia nucleus Area X receives input from the thalamic nucleus, DLM, as well as adjacent subregions of cerebellar-recipient dorsal thalamus (DTCbN) (blue arrows). Hence DLM provides feedback to Area X similar to projections from thalamus to the striatum in mammals, and DTCbN provides a route for output from the cerebellar nuclei (CbN, red arrow) to reach the basal ganglia in the song system through thalamus. More posterior and medial regions of DTCbN project to medial striatum outside of Area X (lighter blue arrow). We also found that DTCbN projects to nidopallium outside of cortical song system nucleus LMAN (lighter blue arrow), implying that DTCbN may communicate both with the song system and with general motor areas outside the song system. Canonical nuclei of the song system are outlined in heavy black lines.
Thalamostriatal projections
We provide strong evidence that dorsal thalamus projects to the medial striatum in a songbird (Fig. 3, Fig. 4). Although this was suggested by retrograde label in prior anatomical studies of songbirds (Castelino, Diekamp, & Ball, 2007; Lewis, Ryan, Arnold, & Butcher, 1981; A. L. Person et al., 2008), as well as anterograde and retrograde label from studies in pigeons (Kitt & Brauth, 1982; Veenman, Karle, Anderson, & Reiner, 1995; J. Wild, 1987), several methodological confounds have prevented a definitive demonstration, as recognized previously (Bottjer et al., 1989; Gale & Perkel, 2010; A. L. Person et al., 2008). Briefly, the confounds are: (1) passing fibers in the basal ganglia en route from thalamus to cortex could pick up tracer that would retrogradely label thalamic neurons, and this would be indistinguishable from retrograde label due to actual thalomstriatal synapses; (2) Area X and surrounding medial striatum project directly to dorsal thalamus, and so it is not clear whether label in Area X from standard tracers injected in dorsal thalamus has traveled anterogradely or retrogradely. Therefore, to demonstrate whether these projections exist, a method is needed to anterogradely label presynaptic segments of axons of thalamic neurons. The lentiviral vectors we used infected the cell body, yielding signal that only traveled in the anterograde direction (Grinevich et al., 2005; Roberts et al., 2008), and we specifically labeled presynaptic terminals in the basal ganglia using a vector encoding synaptophysin tagged with GFP (Fig. 3, Fig. 4). The synaptophysin-GFP labeled processes had varicosities suggestive of synapses (Fig. 3, Fig. 4), as reported for the thalamostriatal system in mammals (Berendse & Groenewegen, 1990; Deschenes, Bourassa, & Parent, 1996). There were also mCherry-labeled axons with the same morphology, implying that these processes occur naturally and did not arise because of the ectopic expression of synaptophysin (Fig. 3). The approach we have taken here therefore complements previous reports of possible thalamostriatal projections and addresses confounds faced by those previous reports. Another approach, commonly used to identify thalamostriatal synapses in mammals, is to stain for glutamate receptors vGlut1 and vGlut2, since it is known that thalamostriatal axon terminals in mammals preferentially express vGlut2 (while corticostriatal terminals express vGlut1) (D. V. Raju, Shah, Wright, Hall, & Smith, 2006; Dinesh V Raju & Smith, 2005). Future studies could test whether this approach would work in songbirds (Karim, Pervin, & Atoji, 2015; Karim, Saito, & Atoji, 2014), as suggested previously (Gale & Perkel, 2010).
Cerebellothalamic projections
We carried out detailed studies of the cerebellothalamic projection in a songbird, presenting a map of the regions of dorsal thalamus targeted by CbL in Bengalese finches (Fig. 5). Notably, anterograde label from injections in CbL was always outside of song system thalamic nucleus DLM (Fig. 5, Fig. 6, Fig. 9), as reported previously for zebra finches (A. L. Person et al., 2008), although there were heavily-labeled regions immediately posterior to DLM (Fig. 5, Fig. 6). We noted that in some cases there was label in dorsal thalamus due to “retrograde” label of collaterals from SpM (H. Karten & Finger, 1976) but based on the very sparse label seen ipsilaterally even when SpM was strongly labeled (see Supporting Information via the link in Data Accessibility section), we are confident the majority of label traveled anterogradely from CbL. To confirm our results showing anterograde label in DTCbN, we made injections in dorsal thalamus and showed retrograde label of CbL, consistent with previous work (Vates et al., 1997). We also demonstrated retrograde label in CbI and CbM (Fig. 7, Fig. 8), a finding that as far as we know has not been been reported previously for songbirds, although it has been reported that neurons in all regions of CbN project to to thalamus in pigeons (Korzeniewska & Gunturkun, 1990; L. Medina & Reiner, 1997; J Martin Wild, 1988; Wylie, Glover, & Lau, 1998) and mammals (Asanuma, Thach, & Jones, 1983; Haroian, Massopust, & Young, 1978; Hoshi et al., 2005; Sugimoto, Mizuno, & Itoh, 1981; Tracey, Asanuma, Jones, & Porter, 1980).
Projections to Area X from DLM and DTCbN
We mapped DTCbN as well as DLM so that we could determine which region of dorsal thalamus gives rise to projections to Area X. We were surprised to find that both DLM and a subregion of DTCbN adjacent to DLM project to Area X (Fig. 10). We do not think this finding can be explained by spillover of viral vector from DLM into DTCbN or vice versa; note that we saw strong synaptophysin-GFP label in Area X when the injection was mostly contained within DLM (Fig. 10a) and when it was mostly contained to DTCbN(Fig. 10i). We also note that injection sites in dorsal thalamus as defined by mCherry signal were not noticeably different from sites defined by synaptophysin-GFP signal (which did not fill cell bodies enough to image with the widefield microscope used to record injection sites, but was obvious when imaged with a confocal). Future studies could expand on our results using trans-synaptic tracers, although our understanding is that such methods do not currently work in songbirds (Mundell et al., 2015).
Comparative considerations
Our results raise several questions about homologies between the Bengalese finch and other species, which we address here.
First we turn to the question of which regions of thalamus in birds and mammals are homologous to the cerebellar-recipient region of dorsal thalamus in songbirds, which we have referred to as DTCbN. Recall that as we showed and other authors have reported (A. L. Person et al., 2008; Vates et al., 1997), in songbirds this region occurs throughout almost the entire mediolateral axis of posterior dorsal thalamus, and is targeted mainly by the lateral cerebellar nuclei. Our study of the projections of this region of dorsal thalamus focused mainly on a more medial region in the same mediolateral plane as DLM. We showed that this region is separate from DLM (Figs. 5, 6, 9). In addition to its projections to the striatum, we showed that it projects to anterior nidopallium surrounding song system nucleus LMAN (Figs. 10, 11).
Most studies identifying cerebellar-recipient dorsal thalamus in other (non-songbird) avian species have been carried out with pigeons. The only detailed study based on anterograde label from tracer injections in CbN concluded that a rostral region of dorsal thalamic nucleus DLP was the principal target of these projections (Arends & Zeigler, 1991), and that this region was distinct from a neighboring region targeted by the vestibular nuclei. However both DLP and neighboring dorsal thalamic region DIP have been reported as targets of CbN based on degeneration studies (H. J. Karten & Dubbeldam, 1973) and on retrograde label from tracer injections in dorsal thalamus (Korzeniewska & Gunturkun, 1990; J Martin Wild, 1988; Wylie et al., 1998). Separate studies considered the projections of these dorsal thalamic regions to the forebrain, and found that DIP and DLP target adjacent regions of nidopallium, with rostral regions of DLP targeting more rostral regions of nidopallium (Gamlin & Cohen, 1986; Kitt & Brauth, 1982). In summary, the region of songbird dorsal thalamus that we studied receives projections from CbN, as do DLP and possibly DIP in pigeons, but it does not receive input from globus pallidus, while DIP in pigeons does. The region we studied projects to the anterior nidopallium as both DIP and rostral DLP have been reported to do in pigeons. We tentatively suggest that the dorsal anterior subregion of medial DTCbN, which sits immediately posterior to song system thalamic nucleus DLM, is most like the region in pigeons identified as rostral DLP in terms of its afferents and efferents.
How DTCbN relates to cerebellar-recipient thalamus in mammals is less clear. As is well known, in mammals this region is commonly divided into two parts, one belonging to motor thalamus and another belonging to the intralaminar nuclei (Asanuma et al., 1983; Aumann, Rawson, Finkelstein, & Horne, 1994; Sugimoto et al., 1981). The dorsal thalamus in birds has been proposed as the homolog to the intralaminar nuclei in mammals (Veenman et al., 1997). However, there are to our knowledge no reports that dorsal thalamus in birds receives strong ascending inputs from the same nuclei in the midbrain and brainstem that target the intralaminar nuclei in mammals, such as the acetylcholinergic groups or the superior colliculi (Van der Werf, Witter, & Groenewegen, 2002). If there truly is little or no input to dorsal thalamus from the same nuclei in lower regions that innervate the intralaminar nuclei of mammals, it would imply that dorsal thalamus is more like mammalian motor thalamus. Although cerebellar-recipient motor thalamus in primates is reported to give rise to striatal projections (Nikolaus R McFarland & Haber, 2000; N. R. McFarland & Haber, 2001), at least one study reports that cerebellar-recipient regions of motor thalamus in rats does not give rise to such projections (Kuramoto et al., 2009), while intralaminar nucleus CL does project to dorsolateral striatum (N. Ichinohe et al., 2000). Conversely, if avian dorsal thalamus is homologous to the intralaminar nuclei, it raises the possiblity that a separate region of thalamus in birds, homologous to mammalian motor thalamus, would also receive input from the cerebellar nuclei. A separate region homologous to the ventral tier motor nuclei has been proposed (Loreta Medina et al., 1997) but not studied extensively in songbirds. At this time we feel that is unclear whether the region of DTCbN we studied is more related to cerebellar-recipient motor thalamus or the intralaminar nuclei in mammals.
Although the homology of DTCbN with thalamic nuclei in mammals is unclear, we point out that, like the thalamostriatal system in mammals, the regions of thalamus we studied have specific projections to distinct regions of the basal ganglia. Thalamic nucleus of the song system DLM projects specifically to Area X, as does the dorsal anterior subregion of medial DTCbN (Fig. 10), but the ventral posterior subregion of medial DTCbN projects to medial MSt outside Area X (Fig. 11). This specificity of the projections of different thalamic regions is reminiscent of the specificity seen in the projections of the of the CM-PF complex of the intralaminar nuclei in mammals (Smith, Raju, Pare, & Sidibe, 2004).
One final comparative question concerns whether single neurons in dorsal thalamus of the songbird project to both cortex and striatum. In mammals, the question of to what extent thalamic neurons target cortex and striatum has been addressed with single-neuron axon tracing studies. These studies find that neurons in the CMPf complex of the intralaminar nuclei send only sparse axon terminals to cortex, while sending off several collaterals within striatum that ramify locally to form dense clusters of axon terminals (Deschenes, Bourassa, Doan, & Parent, 1996; Parent & Parent, 2005). In contrast, neurons in in other parts of the intralaminar nuclei or in motor thalamus terminate heavily in cortex, and when present their thalamostriatal collaterals form long branches throughout striatum with varicosities that synapse with several medium spiny neurons en passant (Deschenes, Bourassa, & Parent, 1996; Noritaka Ichinohe, Iwatsuki, & Shoumura, 2001; Lacey, Bolam, & Magill, 2007). We saw processes with morphologies similar to both types of thalamostriatal projections in Area X after our injections (Figs. 3, 4, Fig. 12), and these injections also always labeled axons passing through Area X to form a thick cloud of relatively small axon terminals in LMAN (Figs. 3, 4, 10, 12). We never saw obvious cases where the thicker axon segments passing through Area X extended collaterals locally. This implies either that the thalamostriatal projections arose from a separate set of neurons, or that the collaterals arose as the axons entered the striatum at the base of the forebrain. Based on the previous literature in mammals, it seems that single-axon tracing studies would also be the best approach to address the question of striatal versus cortical targeting of dorsal thalamic neurons in songbirds.
Functional considerations
One proposed function of thalamostriatal projection in mammals is to convey surprising stimuli that can “rebias” ongoing action selection (Bradfield, Hart, & Balleine, 2013; Minamimoto, Hori, & Kimura, 2009; Smith et al., 2014). Another proposed function is to provide a motor efference copy from lower motor centers (Fee, 2012). However, for DLM to perform either of these functions it would require ascending inputs from lower brain areas, which have not been reported. Current models of the songbird AFP do not include a thalamostriatal projection (Fee & Goldberg, 2011), but they propose that a function of the AFP is to modulate the variability of song; the models posit that the brain uses this variability to improve and maintain the ability of the bird to sing (Dhawale, Smith, & Olveczky, 2017; Fee & Goldberg, 2011; Kuebrich & Sober, 2015; S. Woolley & Kao, 2015). With respect to these models, an alternate hypothesis for the function of the projection from DLM to Area X (Figs. 10, 12) would be that synapses that DLM neurons form with multiple neurons in Area X allow DLM to contribute to correlated activity across neurons in the AFP. If for example single DLM neurons contact multiple medium spiny neurons (MSNs) in Area X, similar to the way that thalamic neurons contact multiple MSNs in the mammalian striatum (Deschenes, Bourassa, Doan, et al., 1996; Deschenes, Bourassa, & Parent, 1996; Kuramoto et al., 2009; N. R. McFarland & Haber, 2001; Parent & Parent, 2005), this would position them to correlate activity across the multiple neurons with which they form synapses. Modeling studies suggest correlations in activity enable neural networks to produce behavioral variability such as that generated by the AFP (Darshan, Wood, Peters, Leblois, & Hansel, 2017). One way to test for this function would be to reversibly and specifically inactivate thalamostriatal synapses in Area X and measure the effects on vocal variability.
Physiological studies would also be required to address the question of how the cerebellum interacts with the song system via output to dorsal thalamus. A previous physiological study in rats found mixed effects on firing activity of MSNs when optogenetically stimulating regions of CbN that project to thalamus (Chen et al., 2014). Future studies in songbirds should determine at the physiological level how input from the cerebellum via DTCbN modulates activity of target neurons in Area X. Work in mammals suggests the most likely target would be MSNs (Dube, Smith, & Bolam, 1988) and cholinergic interneurons (Lapper & Bolam, 1992)—both cell types are found in Area X (Carrillo & Doupe, 2004; Reiner et al., 2004).
Although we showed that a subregion of DTCbN projects to Area X, our other results on the projections of CbL and DTCbN raise questions about what the function of the cerebellum’s interactions with the song system might be. We found, as previous papers have (Arends & Zeigler, 1991; A. L. Person et al., 2008), that CbL targets not just DTCbN but also many sites throughout the midbrain (see Appendices). Because CbL targets these other areas, it seems very unlikely that there is a song-system specific region of CbN. It could be possible that there are single neurons in CbL that synapse only with neurons in DTCbN projecting to Area X, although in mammals it is thought that single neurons in CbN that project to thalamus also send collaterals to the red nucleus (Shinoda, Futami, Mitoma, & Yokota, 1988). Based on the other regions it targets, it is likely that CbL interacts with previously described descending pathways in the avian brain (J. Wild & Williams, 2000; J. M. Wild, 1992) with connections similar to the pyramidal tract in mammals. To our knowledge, the connections of CbL with these pyramidal tract-like pathways in birds have not been studied behaviorally, and even in mammals the functions of these pathways remain an open question (Horne & Butler, 1995; Houk, Keifer, & Barto, 1993; Shmuelof & Krakauer, 2011). Our results also raise the possiblity that the dorsal anterior subregion of medial DTCbN that projects to Area X also projects to nidopallium just outside of LMAN (Fig. 10, Fig. 11). Future studies should test this using some technique that allows labeling single neurons (Deschenes, Bourassa, Doan, et al., 1996; Kuramoto et al., 2009). If it were the case that single dorsal thalamic neurons project to both Area X and nidopallium outside of the song system, it could be that the function of these projections is to help co-ordinates neural activity in the song system with activity in motor systems that control muscles involved in both song and non-song behaviors. By the same token, we emphasize that we also showed that the ventral posterior part of medial DTCbN projects to medial striatum outside of Area X (Fig. 11). Assuming that the song system evolved from already existing motor pathways (Farries, 2001; Feenders et al., 2008), then, our findings imply that a pathway from the cerebellum to the basal ganglia through thalamus may have existed before the song system evolved. It remains unclear if these projections contribute directly to motor control in general or if they have some other function such as task-level control of behavior (Bradfield et al., 2013; DeLong & Wichmann, 2009; Minamimoto et al., 2009; Smith et al., 2014) but our results suggest these pathways may be a general feature of motor systems in vertebrate brains.
Acknowledgments
This work was supported by NIH grants R01NS084844 (SJS) and R01DC014364 (TFR) and NSF grants IOS-1456912 (SJS), IOS-1457206 (TFR), and IOS-1451034 (TFR). Imaging acquisition was supported by the Emory University Integrated Cellular Imaging Microscopy Core of the Emory Neuroscience NINDS Core Facilities grant, 5P30NS055077. We are grateful to the laboratories of Donna Maney and Dieter Jaeger at Emory University for material support.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
Data accessibility:
Supporting information is in a repository on the Biolucida server provided by MBF biosciences. https://wiley.biolucida.net/images/?page=images&selectionType=collection&selectionId=71
Literature cited
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proceedings of the National Academy of Sciences. 2009;106(30):12518–12523. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends J, Zeigler HP. Organization of the cerebellum in the pigeon (Columba livia): II. Projections of the cerebellar nuclei. J Comp Neurol. 1991;306(2):245–272. doi: 10.1002/cne.903060204. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Distribution of Cerebellar Terminations and Their Relation to Other Afferent Terminations in the Ventral Lateral Thalamic Region of the Monkey. Brain Research Reviews. 1983;5(3):237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- Aumann T, Rawson J, Finkelstein D, Horne M. Projections from the lateral and interposed cerebellar nuclei to the thalamus of the rat: a light and electron microscopic study using single and double anterograde labelling. Journal of Comparative Neurology. 1994;349(2):165–181. doi: 10.1002/cne.903490202. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16(6):645–649. doi: 10.1016/j.conb.2006.08.016. S0959-4388(06)00139-5 [pii] [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Current opinion in neurology. 2008;21(6):628. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory–vocal integration in the songbird. The Journal of Neuroscience. 2008;28(6):1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyk M, Brown S. The origins of the vocal brain in humans. Neurosci Biobehav Rev. 2017;77:177–193. doi: 10.1016/j.neubiorev.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. Journal of Comparative Neurology. 1990;299(2):187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nature Reviews Neuroscience. 2010;11(11):747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Bosch-Bouju C, Hyland BI, Parr-Brownlie LC. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. 2013 doi: 10.3389/fncom.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bradfield LA, Hart G, Balleine BW. The role of the anterior, mediodorsal, and parafascicular thalamus in instrumental conditioning. 2013 doi: 10.3389/fnsys.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Scheich H, Schachner M, Heizmann CW. Distribution of parvalbumin, cytochrome oxidase activity and 14C-2-deoxyglucose uptake in the brain of the zebra finch. Cell and tissue research. 1985;240(1):101–115. [Google Scholar]
- Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. Journal of Comparative Neurology. 2009;513(6):643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- Carrillo GD, Doupe AJ. Is the songbird area X striatal, pallidal, or both? An anatomical study. Journal of Comparative Neurology. 2004;473(3):415–437. doi: 10.1002/Cne.20099. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus area X of the medial striatum in male zebra finches (Taeniopygia guttata) J Comp Neurol. 2007;502(4):544–562. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- Charlesworth JD, Tumer EC, Warren TL, Brainard MS. Learning the microstructure of successful behavior. Nature neuroscience. 2011;14(3):373–380. doi: 10.1038/nn.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nature neuroscience. 2014;17(12):1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darshan R, Wood WE, Peters S, Leblois A, Hansel D. A canonical neural mechanism for behavioral variability. Nature Communications. 2017;8:15415. doi: 10.1038/ncomms15415. https://www.nature.com/articles/ncomms15415#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, … Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biological psychiatry. 2011;69(7):668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis V, Liu R, Soto D, Xiang Y. Differential association of phosphodiesterase 4D isoforms with β2-adrenoceptor in cardiac myocytes. Journal of Biological Chemistry. 2009;284(49):33824–33832. doi: 10.1074/jbc.M109.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M, Wichmann T. Update on models of basal ganglia function and dysfunction. Parkinsonism & related disorders. 2009;15:S237–S240. doi: 10.1016/S1353-8020(09)70822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. European Journal of Neuroscience. 1996;8(2):329–343. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Bourassa J, Parent A. Striatal and cortical projections of single neurons from the central lateral thalamic nucleus in the rat. Neuroscience. 1996;72(3):679–687. doi: 10.1016/0306-4522(96)00001-2. [DOI] [PubMed] [Google Scholar]
- Dhawale AK, Smith MA, Olveczky BP. The Role of Variability in Motor Learning. Annual review of neuroscience. 2017 doi: 10.1146/annurev-neuro-072116-031548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh T, Bernhardt TG. Using superfolder green fluorescent protein for periplasmic protein localization studies. Journal of bacteriology. 2011;193(18):4984–4987. doi: 10.1128/JB.00315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual review of neuroscience. 1999;22(1):567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Dube L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267(4):455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Krakauer JW. The basal ganglia: from motor commands to the control of vigor. Current opinion in neurobiology. 2016;37:158–166. doi: 10.1016/j.conb.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Farries MA. The oscine song system considered in the context of the avian brain: lessons learned from comparative neurobiology. Brain, behavior and evolution. 2001;58(2):80–100. doi: 10.1159/000047263. [DOI] [PubMed] [Google Scholar]
- Fee MS. Oculomotor learning revisited: a model of reinforcement learning in the basal ganglia incorporating an efference copy of motor actions. Frontiers in Neural Circuits. 2012:6. doi: 10.3389/Fncir.2012.00038. Artn38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–170. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, … Jarvis ED. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PloS one. 2008;3(3):e1768. doi: 10.1371/journal.pone.0001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Anatomy of a songbird basal ganglia circuit essential for vocal learning and plasticity. J Chem Neuroanat. 2010;39(2):124–131. doi: 10.1016/j.jchemneu.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Cohen DH. A second ascending visual pathway from the optic tectum to the telencephalon in the pigeon (Columba livia) Journal of Comparative Neurology. 1986;250(3):296–310. doi: 10.1002/cne.902500304. [DOI] [PubMed] [Google Scholar]
- Ghez C, Krakauer J. The organization of movement. Principles of neural science. 2000;656:668. [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nature Neuroscience. 2012;15(4):620. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. The Journal of Neuroscience. 2005;25(36):8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroian AJ, Massopust LC, Young PA. Topographical Organization of Cerebellothalamic Projections in Rat. Anatomical Record. 1978;190(2):414–414. [Google Scholar]
- Horne MK, Butler EG. The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog Neurobiol. 1995;46(2):199–213. [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature neuroscience. 2005;8(11):1491–1493. doi: 10.1038/Nn1544. [DOI] [PubMed] [Google Scholar]
- Houk JC, Keifer J, Barto AG. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993;16(1):27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Iwatsuki H, Shoumura K. Intrastriatal targets of projection fibers from the central lateral nucleus of the rat thalamus. Neuroscience letters. 2001;302(2):105–108. doi: 10.1016/s0304-3940(01)01666-4. [DOI] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain research. 2000;880(1):191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neuroscience & Biobehavioral Reviews. 2002;26(2):235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433(7026):638–643. doi: 10.1038/Nature03127. [DOI] [PubMed] [Google Scholar]
- Karim MR, Pervin M, Atoji Y. Glutamatergic circuits in the song system of Zebra Finch brain determined by gene expression of Vglut2 and Glutamate receptors. Research in Agriculture Livestock and Fisheries. 2015;1(1):61–70. [Google Scholar]
- Karim MR, Saito S, Atoji Y. Distribution of vesicular glutamate transporter 2 in auditory and song control brain regions in the adult zebra finch (Taeniopygia guttata) Journal of Comparative Neurology. 2014;522(9):2129–2151. doi: 10.1002/cne.23522. [DOI] [PubMed] [Google Scholar]
- Karten H, Finger TE. A direct thalamo-cerebellar pathway in pigeon and catfish. Brain research. 1976;102(2):335–338. doi: 10.1016/0006-8993(76)90888-x. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Brzozowska-Prechtl A, Lovell PV, Tang DD, Mello CV, Wang H, Mitra PP. Digital atlas of the zebra finch (Taeniopygia guttata) brain: A high-resolution photo atlas. Journal of Comparative Neurology. 2013;521(16):3702–3715. doi: 10.1002/cne.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Dubbeldam JL. The organization and projections of the paleostriatal complex in the pigeon (Columba livia) Journal of Comparative Neurology. 1973;148(1):61–89. doi: 10.1002/cne.901480105. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis D, Martin E, Nair H, Ressler K, … Wilson M. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Molecular psychiatry. 2009;14(1):37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitt C, Brauth S. A paleostriatal-thalamic-telencephalic path in pigeons. Neuroscience. 1982;7(11):2735–2751. doi: 10.1016/0306-4522(82)90097-5. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S. Current concepts in neuroanatomical tracing. Prog Neurobiol. 2000;62(4):327–351. doi: 10.1016/s0301-0082(00)00019-8. [DOI] [PubMed] [Google Scholar]
- Kojima S, Kao MH, Doupe AJ. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proceedings of the National Academy of Sciences. 2013;110(12):4756–4761. doi: 10.1073/pnas.1216308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka G, Roberts TF. Insights into the neural and genetic basis of vocal communication. Cell. 2016;164(6):1269–1276. doi: 10.1016/j.cell.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska E, Gunturkun O. Sensory properties and afferents of the N. dorsolateralis posterior thalami of the pigeon. Journal of Comparative Neurology. 1990;292(3):457–479. doi: 10.1002/cne.902920311. [DOI] [PubMed] [Google Scholar]
- Kuebrich B, Sober S. Variations on a theme: Songbirds, variability, and sensorimotor error correction. Neuroscience. 2015;296:48–54. doi: 10.1016/j.neuroscience.2014.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto E, Furuta T, Nakamura KC, Unzai T, Hioki H, Kaneko T. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cerebral Cortex. 2009;19(9):2065–2077. doi: 10.1093/cercor/bhn231. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. Journal of Neuroscience. 2007;27(16):4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51(3):533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. Journal of Comparative Neurology. 1981;196(2):347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Li J, Zhou X, Huang L, Fu X, Liu J, Zhang X, … Zuo M. Alteration of CaBP Expression Pattern in the Nucleus Magnocellularis following Unilateral Cochlear Ablation in Adult Zebra Finches. PloS one. 2013;8(11):e79297. doi: 10.1371/journal.pone.0079297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. Journal of Comparative Neurology. 2013;521(14):3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo J, Carlsson MA, Nassel DR. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. Journal of Comparative Neurology. 2015;523(12):1840–1863. doi: 10.1002/cne.23768. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Luo M, Perkel DJ. A GABAergic, strongly inhibitory projection to a thalamic nucleus in the zebra finch song system. Journal of Neuroscience. 1999a;19(15):6700–6711. doi: 10.1523/JNEUROSCI.19-15-06700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Perkel DJ. Long-range GABAergic projection in a circuit essential for vocal learning. The Journal of comparative neurology. 1999b;403(1):68–84. [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SNF, Gerwig M, … Timmann D. Consensus Paper: Roles of the Cerebellum in Motor Control-The Diversity of Ideas on Cerebellar Involvement in Movement. Cerebellum. 2012;11(2):457–487. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Birdsong and speech development: Could there be parallels? There may be basic rules governing vocal learning to which many species conform, including man. American scientist. 1970;58(6):669–673. [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. Journal of Neuroscience. 2000;20(10):3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. Journal of Comparative Neurology. 2001;429(2):321–336. doi: 10.1002/1096-9861(20000108)429:2<321::Aid-Cne11>3.0.Co;2-A. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, … Brown RE. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. Journal of Comparative Neurology. 2013;521(6):1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina L, Reiner A. The efferent projections of the dorsal and ventral pallidal parts of the pigeon basal ganglia, studied with biotinylated dextran amine. Neuroscience. 1997;81(3):773–802. doi: 10.1016/s0306-4522(97)00204-2. [DOI] [PubMed] [Google Scholar]
- Medina L, Veenman CL, Reiner A. Evidence for a possible avian dorsal thalamic region comparable to the mammalian ventral anterior, ventral lateral, and oral ventroposterolateral nuclei. J Comp Neurol. 1997;384(1):86–108. [PubMed] [Google Scholar]
- Minamimoto T, Hori Y, Kimura M. Roles of the thalamic CM–PF complex—basal ganglia circuit in externally driven rebias of action. Brain research bulletin. 2009;78(2):75–79. doi: 10.1016/j.brainresbull.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neurobiology of song learning. Curr Opin Neurobiol. 2009;19(6):654–660. doi: 10.1016/j.conb.2009.10.004. S0959-4388(09)00141-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell NA, Beier KT, Pan YA, Lapan SW, Goz Ayturk D, Berezovskii VK, … Cepko CL. Vesicular stomatitis virus enables gene transfer and transsynaptic tracing in a wide range of organisms. Journal of Comparative Neurology. 2015;523(11):1639–1663. doi: 10.1002/cne.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require realtime auditory feedback to produce normal song syntax. J Neurobiol. 1997;33(4):343–356. doi: 10.1002/(SICI)1097-4695(199710)33:4<343::AID-NEU1>3.0.CO;2-A. [pii] [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. Journal of Comparative Neurology. 2005;481(1):127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- Parrell B, Agnew Z, Nagarajan S, Houde J, Ivry RB. Impaired feedforward control and enhanced feedback control of speech in patients with cerebellar degeneration. Journal of Neuroscience. 2017:3363–3316. doi: 10.1523/JNEUROSCI.3363-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including Area X. Journal of Comparative Neurology. 2008;508(5):840–866. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46(1):129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. Journal of Comparative Neurology. 2006;499(2):231–243. doi: 10.1002/Cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Smith Y. The Basal Ganglia VIII. Springer; 2005. Differential localization of vesicular glutamate transporters 1 and 2 in the rat striatum; pp. 601–610. [Google Scholar]
- Redondo RL, Kim J, Arons AL, Ramirez S, Liu X, Tonegawa S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature. 2014;513(7518):426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. Journal of Comparative Neurology. 2004;469(2):239–261. doi: 10.1002/Cne.11012. [DOI] [PubMed] [Google Scholar]
- Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG. Pathway tracing using biotinylated dextran amines. J Neurosci Methods. 2000;103(1):23–37. doi: 10.1016/s0165-0270(00)00293-4. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. The Journal of Neuroscience. 2008;28(13):3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, … Schmid B. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, … Kremer EJ. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature. 2015 doi: 10.1038/nature14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Experimental Brain Research. 2008;185(3):359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature biotechnology. 2004;22(12):1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Futami T, Mitoma H, Yokota J. Morphology of single neurones in the cerebellorubrospinal system. Behav Brain Res. 1988;28(1–2):59–64. doi: 10.1016/0166-4328(88)90076-9. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron. 2011;72(3):469–476. doi: 10.1016/j.neuron.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, … Bolam JP. The thalamostriatal system in normal and diseased states. 2014 doi: 10.3389/fnsys.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nature neuroscience. 2009;12(7):927–U144. doi: 10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Brainard MS. Vocal learning is constrained by the statistics of sensorimotor experience. Proc Natl Acad Sci U S A. 2012;109(51):21099–21103. doi: 10.1073/pnas.12136221091213622109. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Curr Opin Neurobiol. 2003;13(6):663–670. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Sreenivasan V, Karmakar K, Rijli FM, Petersen CC. Parallel pathways from motor and somatosensory cortex for controlling whisker movements in mice. European Journal of Neuroscience. 2015;41(3):354–367. doi: 10.1111/ejn.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Mizuno N, Itoh K. An autoradiographic study on the terminal distribution of cerebellothalamic fibers in the cat. Brain Res. 1981;215(1–2):29–47. doi: 10.1016/0006-8993(81)90489-3. [DOI] [PubMed] [Google Scholar]
- Tracey DJ, Asanuma C, Jones EG, Porter R. Thalamic Relay to Motor Cortex - Afferent Pathways from Brain-Stem, Cerebellum, and Spinal-Cord in Monkeys. Journal of Neurophysiology. 1980;44(3):532–554. doi: 10.1152/jn.1980.44.3.532. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Research Reviews. 2002;39(2):107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-”cortical” loops in the song system of oscine songbirds. Journal of Comparative Neurology. 1997;380(2):275–290. doi: 10.1002/(sici)1096-9861(19970407)380:2<275::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Karle EJ, Anderson KD, Reiner A. Thalamostriatal projection neurons in birds utilize LANT6 and neurotensin: a light and electron microscopic double-labeling study. J Chem Neuroanat. 1995;9(1):1–16. doi: 10.1016/0891-0618(95)00057-e. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Medina L, Reiner A. Avian homologues of mammalian intralaminar, mediodorsal and midline thalamic nuclei: Immunohistochemical and hodological evidence. Brain Behavior and Evolution. 1997;49(2):78–98. doi: 10.1159/000112983. [DOI] [PubMed] [Google Scholar]
- Vujovic N, Gooley JJ, Jhou TC, Saper CB. Projections from the subparaventricular zone define four channels of output from the circadian timing system. Journal of Comparative Neurology. 2015;523(18):2714–2737. doi: 10.1002/cne.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins K. Developmental disorders of speech and language: from genes to brain structure and function. In: Braddick O, Atkinson J, Innocenti G, editors. Gene Expression to Neurobiology and Behaviour: Human Brain Development and Developmental Disorders. Vol. 189. 2011. p. 225. [DOI] [PubMed] [Google Scholar]
- Wild J. Thalamic projections to the paleostriatum and neostriatum in the pigeon (Columba livia) Neuroscience. 1987;20(1):305–327. doi: 10.1016/0306-4522(87)90022-4. [DOI] [PubMed] [Google Scholar]
- Wild J, Williams M. Rostral wulst in passerine birds. I. Origin, course, and terminations of an avian pyramidal tract. Journal of Comparative Neurology. 2000;416(4):429–450. [PubMed] [Google Scholar]
- Wild JM. Vestibular projections to the thalamus of the pigeon: an anatomical study. Journal of Comparative Neurology. 1988;271(3):451–460. doi: 10.1002/cne.902710312. [DOI] [PubMed] [Google Scholar]
- Wild JM. Direct and indirect “cortico”-rubral and rubro-cerebellar cortical projections in the pigeon. J Comp Neurol. 1992;326(4):623–636. doi: 10.1002/cne.903260409. [DOI] [PubMed] [Google Scholar]
- Woolley S, Kao M. Variability in action: contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control. Neuroscience. 2015;296:39–47. doi: 10.1016/j.neuroscience.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Rubel EW. Bengalese finches Lonchura Striata domestica depend upon auditory feedback for the maintenance of adult song. J Neurosci. 1997;17(16):6380–6390. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie DR, Glover R, Lau K. Projections from the accessory optic system and pretectum to the dorsolateral thalamus in the pigeon (Columbia livia): a study using both anterograde and retrograde tracers. J Comp Neurol. 1998;391(4):456–469. [PubMed] [Google Scholar]
- Ziegler W. The phonetic cerebellum: cerebellar involvement in speech sound production. Elsevier; Boston: 2016. [Google Scholar]
- Ziegler W, Ackermann H. Subcortical Contributions to Motor Speech: Phylogenetic, Developmental, Clinical. 2017 doi: 10.1016/j.tins.2017.06.005. [DOI] [PubMed] [Google Scholar]