Abstract
Objective
Endometriosis is a chronic, estrogen dependent condition that affects 5 – 10 % of reproductive aged women and is associated with pelvic pain and infertility. As the approach to therapy shifts from surgical ablation to pharmacological control, a nonsurgical mode of diagnosis would be desirable. The ENDOmarker study was designed by the NICHD Reproductive Medicine Network (RMN) to obtain well characterized and phenotyped bio specimens in a standardized fashion from women with and without endometriosis.
Design
Development of a diagnostic test
Setting
Academic medical centers
Patients
This study will enroll up to 500 participants, and follow them for up to 5 months. Included subjects are aged 18–44, scheduled to undergo gynecologic surgery (laparoscopy/laparotomy) for clinical reasons.
Interventions
Presence and stage of endometriosis (or its absence) is characterized by visual examination at the time of surgery. Subjects will undergo extensive clinical evaluation pre-operatively and at visits one and four months postoperatively. Endometrial biopsy, blood, urine and disease specific questionnaires will be collected at each visit.
Main outcome
Samples will be placed in a bio-repository to be used to validate and optimize the clinical use of genomic classifiers of the endometrium alone or in combination with serum cytokines as a non-surgical composite marker of endometriosis.
Conclusion
This protocol can serve as a reference for objective collection of high quality bio specimens for discovery or validation of potential nonsurgical diagnosis of presence or severity of disease.
Keywords: Endometriosis, Biomarkers, micro RNA, cytokines, phenotyping
Background
Endometriosis, or ectopic growth of endometrial glands and stroma outside of the uterine cavity, is a common gynecologic disease, found in approximately 5–10 % reproductive aged women and frequently associated with dysmenorrhea, dyspareunia, pelvic pain and infertility(1). The diagnosis of endometriosis is surgical, and prevalence of disease varies by populations (2). Endometriosis is found in 24% of women investigated for pelvic pain and 20% of women undergoing laparoscopic investigation for infertility(3, 4), and 4 % of asymptomatic women undergoing tubal ligation(4). The current clinical opinion is that a surgical procedure such as laparoscopy is required for definitive diagnosis of endometriosis (5). A clinical staging system has been designed to allow clinicians to communicate effectively regarding prognosis and treatment. The American Society for Reproductive Medicine revised classification system for endometriosis (ASRM 1996) is the most widely accepted staging system (6).
Currently, treatment for endometriosis includes 1) surgical ablation of the visible lesions in the pelvis, 2) medical suppression (with oral contraceptives or high dose progestins) or pharmacological induction of a hypo estrogenic endocrine state by stopping a woman’s production of sex steroids (using a GnRH agonist), 3) inhibition of estradiol (E2) biosynthesis with aromatase inhibitors, or 4) use of selective progesterone receptor modulators. Some of these treatments, such as chronic GnRH agonist therapy, are associated with side effects such as hot flashes, genitourinary atrophy, infertility, as well as long-term consequences such as increased bone resorption leading to osteopenia(7, 8). Moreover, these approaches run the risk of hormonal suppression therapy of women who do not have endometriosis associated pelvic pathology. It is hoped that new agents specifically targeting endometriosis may minimize systemic side effects and obviate surgical treatment (9
The ability to diagnose endometriosis and gauge its severity non-invasively would be of great public health and clinical benefit. A marker would potentially minimize the need for diagnostic surgery and identify women best treated medically. A non-surgical marker may identify women with earlier disease, reducing the associated chorionic inflammation, altered immune response and pelvic adhesion formation (10).
Medical management of women with endometriosis is hampered by a lack of a biological biomarker of disease response. Currently, to assess treatment efficacy, either pain control is subjectively determined to be satisfactory, or a repeat surgical procedure is necessary to re-stage the endometriosis and determine the effect of treatment. This adds great expense and complexity to the management of endometriosis as well as to the design of clinical trials of novel therapies. Accurate and early non-invasive diagnosis would decrease morbidity, lower cost, and provide a means of earlier diagnosis for those women who are unable to access surgical care.
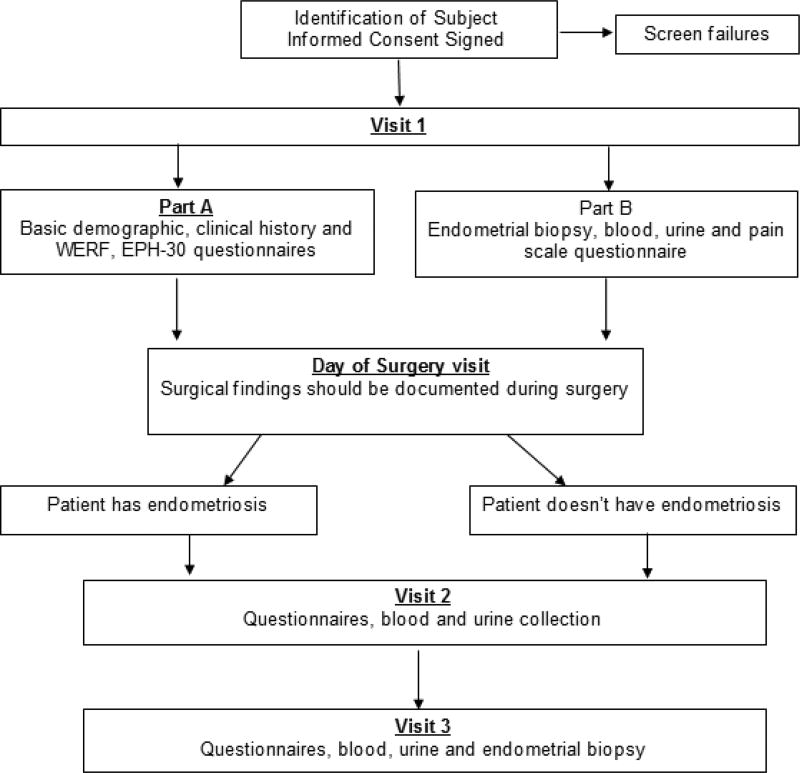
Identification of biomarker of disease has many pitfalls (11). Biomarker development requires specific phases of development, extensive testing, and validation (12). Of paramount importance is the proper collecting, storage, and phenotyping of clinical specimens(11). The purpose of this manuscript is to present the methods, design, and analysis plan of the Reproductive Medicine Network (RMN) study entitled Evaluation, Validation and Refinement of Noninvasive Diagnostic Biomarkers for Endometriosis (ENDOmarker) study (NCT03161704) (Fig. 1). This protocol may serve as a model for future studies to evaluate diagnostic tests for women with endometriosis.
Figure 1.
ENDOmarker study flowchart, the flowchart below summarizes study visits
Materials and Methods
The ENDOmarker study is a multi-center longitudinal prospective cohort study designed to evaluate diagnostic test characteristic of novel methods to diagnose women with endometriosis. Study participants will be recruited from the clinics of the Reproductive Medicine Network and affiliated entities after obtaining written informed consent from the subjects. The protocol was approved by the University of Pennsylvania IRB which served as a central IRB (IRB number 821891). Recruited subjects will meet the inclusion and exclusion criteria detailed below. The primary objective of this study is to validate and optimize the clinical use of genomic classifiers obtained by endometrial biopsy alone (13) or in combination with serum cytokines as a non-surgical composite marker of endometriosis presence, stage and absence or presence of endometriosis associated uterine/pelvic pathology.
The secondary objective is to correlate change in a panel of serum markers with change in endometriosis specific health related quality of life at one month and three-four months following surgery. In addition, we plan to develop a biobank of plasma, serum, DNA, RNA, urine and endometrial tissue from the study participants, with and without the confirmed diagnosed endometriosis, to support future discovery of novel biomarkers for the noninvasive diagnostic modalities. Standards for specimen collection and data collection were modified from standards for the World Endometriosis Research Foundation (WERF) (14–16).
The overall goal of the inclusion/exclusion criteria is to identify women aged 18–44 who are scheduled to undergo gynecologic surgery (laparoscopy/laparotomy) for clinical reasons. Study will exclude women if they are pregnant, having current or past diagnosis of any malignancy, HIV-positive, having clinical evidence of active cervical infection or pelvic inflammatory disease. Subjects will undergo extensive clinical evaluation at visit one, visit two and visit three. Endometrial biopsy, blood, urine and questionnaires will be collected.
Rationale
Eutopic endometrium differs at the mRNA, microRNA, and protein levels and responsiveness to progesterone, in women with endometriosis compared to women without disease(1, 17). Giudice and colleagues assessed if high fidelity classifiers could be developed to diagnose and stage endometriosis using margin tree analysis of genomic data derived from endometrium. They analyzed eutopic endometrium from 148 women without/with endometriosis and/or other uterine/pelvic pathologies. A tree-like sequence of binary decisions, using specific genes, distinguished: 1) absence or presence of uterine/pelvic pathology; 2) endometriosis or no endometriosis; and 3) minimal/mild or moderate/severe disease. Best-performing classifiers diagnosed endometriosis with 90–100% accuracy, some using relatively few genes, which have high value for developing diagnostic and therapeutic targets(13).
Serum cytokines have been evaluated as biomarker for endometriosis (18, 19). While the individual diagnostic performance of specific markers was poor, and often contradictory, there may be promise in using markers in combination (18). One example is the use of classification tree analysis, and a two-tiered strategy to maximize both sensitivity and specificity, resulting in good diagnostic test performance. For examples, a three-marker panel of CA-125, macrophage chemotactic protein-1, and leptin could diagnose 51% of subjects as to the presence of endometriosis with 89% accuracy. A four-marker panel of CA-125, macrophage chemotactic protein-1, leptin, and macrophage migration inhibitory factor could diagnose 48% of subjects with 93% accuracy. To be assigned a diagnosis, a subject needed to be classified as having endometriosis (or being disease free) consistently by the two classification trees. If a diagnosis can be accurately obtained with a genomic profiler (alone, or in combination with serum cytokines), then cytokines may also serve as a marker(s) of response or progression of disease.
The goal of this protocol is to obtain bio specimens for multiple concomitant purposes: 1) to validate the preliminary findings of the use of both a genomic profiler and serum cytokines in a new independent sample, 2) assess if there is increased accuracy in a combined test, and 3) to develop a bank of specimens for future studies of potential novel serum biomarkers. This proposal will both validate previous biomarkers and collect specimens for discovery of new markers.
Study population
Recruitment goal is to recruit up to 500 healthy women scheduled to undergo a gynecologic surgical procedure. Subjects will be phenotyped at the time of surgery (see below). Bio-specimens will be obtained from 150 women with diagnosed endometriosis and 150 with the absence of endometriosis. Of the women with endometriosis, the target is to have 75 women enrolled with minimal/mild disease and 75 women with moderate/ severe disease. Because the presence or absence of endometriosis (and the severity) will be determined at the time of their surgery, the plan is to recruit greater than 300 women to ensure that there are the desired numbers in each of the outlined sub groups. It is possible that all anticipated sub-population will be identified before 500 subjects are enrolled.
Procedures
Endometrial biopsy tissue, serum, plasma, whole blood and urine will be collected (figure 1). A disease-specific questionnaire regarding health-related quality of life will be completed by women who will be diagnosed as having (or not having) endometriosis at the time of their scheduled surgery. Bio specimens will be collected prior to, or at the time of surgery (visit 1). The patients will then undergo planned surgical procedure when the presence or absence of endometriosis is confirmed. Collection of health-related quality of life and bio specimens will then be repeated one month (visit 2) and three to four months post-surgery (visit 3).
Informed consent can be obtained in a separate visit, or in conjunction with visit 1. Visit 1 can be conducted as a standalone outpatient visit or at the time of scheduled surgery. Visit 1 consists of two components, completion of questionnaires (part A) and, collection of bio specimens including endometrial sampling (part B). If not conducted concomitantly, Part A may occur up to 60 days prior to Part B. Part B can occur up to 90 days prior to the Day of Surgery visit (Table 2).
Table 2.
Study visits
| Consent | Visit 1 | Day of Surgery |
Visit 2 | Visit 3 | ||
|---|---|---|---|---|---|---|
| Part A | Part B | |||||
| Date of Visit | Must occur prior to start of Visit 1, but can be same day as Visit 1 | Any time after consent is signed | Must occur no more than 60 days after Part A | Must occur no more than 90 days after Part B (can occur same day as Visit 1) | 1 month +/− 14 days after surgery | 14 weeks +/− 4 weeks |
| Cycle Timing of Visit | Can occur any day | Can occur any day | Must occur on cycle days 6–12 or 18–25 | Can occur any day (unless combined with Visit 1, Part B) | Preferably on cycle day 6–12 | Must occur on cycle days 6–12 or 18–25 |
| CRFs | ||||||
| Registration | x | |||||
| Eligibility | x | |||||
| WERF EPHect Standard Questionnaire | x | |||||
| EHP-30 | x | x | x | |||
| Pain Scale | x | x | x | |||
| Biospecimen Collection | x | x | x | |||
| Prior Medical History | x | |||||
| Concomitant Medications | x (or on Day of Surgery | X (or at Visit 1 Part B) | x | x | ||
| Surgical Form | x | |||||
| Surgical Worksheet | x | |||||
| Post-Operative Form | x | |||||
| End of Study | x | |||||
| Protocol Deviation | ||||||
| Adverse Event Log | ||||||
| Serious Adverse Event | ||||||
Collection of historical data (part A) consists of a medical history, including a detailed gynecological history pain scale and quality of life (QOL). Questionnaires were modified from the WERF Endometriosis Phenome and Biobanking Harmonization Project (EPHect): EPHect Patient Questionnaire - Standard (EPQ-S).
The biospecimen collection is preferably collected on Day 6–12 of the participant’s menstrual cycle, ideally closer to Day 12 as there will be more tissue the later in the range the biopsy occurs. If the biospecimens can’t be reasonably collected in that time window, the samples may be collected on cycle days 18–25. The collection must occur no more than 90 days before the phenotyping at the time of planned surgery.
Phenotyping
All women will be characterized with regard to stage of menstrual cycle and LMP. Hormonal assays and endometrial histology will be used to determine specimen phenotype.
At the time of the planned surgery, all women will be characterized as to the presence or absence of endometriosis (using the revised ASRM staging system (6) by visual inspection (documented with photographs). Concomitant pathology (fibroids, ovarian cyst, adenomyosis, hydrosalpinx, or other) will also be documented based on the findings at the time of surgery. The locations, extent, and severity of adhesions will also be documented. Any therapy (ablation, resection or other) will be documented. This study will have no influence on the planned surgical procedure or future therapy. Visit 2 and visit 3 are scheduled based on the date of the surgery. These visits will preferably occur on day 6–12 of the participant’s menstrual cycle. Visit 2 is scheduled 4 weeks (plus or minus 14 days) after the Day of Surgery Visit. Visit 3 is scheduled 12 – 16 weeks, plus or minus 4 weeks after the Day of Surgery Visit. Bio specimens, (Serum, plasma, urine and endometrial sampling), pain scale and quality of life questionnaire (QOL) will be administered in the same fashion as visit 1. Endometrial sampling is only repeated at visit 3 (figure 1). If the bio specimens can’t be reasonably collected within the time frame of cycle day (CD) 6–12, the samples may be collected on CD 18–25.
Sample collection
Endometrial tissue will be collected by SOPs(14, 20, 21) and will be divided into up to three specimens depending on amount. A portion is snap frozen in liquid nitrogen, and a portion will be placed in formalin and embedded in paraffin for histologic evaluation and dating, and harmonization with serum progesterone levels. Phlebotomy is preformed to obtain samples of serum and plasma (total of 30 cc of blood). Urine (40–50 mL) is obtained with clean-catch technique
Study questionnaires
Standards recommended by the World Endometriosis Research Foundation (WERF) Endometriosis Phenome and Biobanking Harmonization Project (EPHect) will be applied to standardize surgical phenotype(20), clinical phenotype(22) as well as tissue and fluid collection and processing(20). Participants will complete the Endometriosis Health Profile (EHP-30), which was developed in the UK(23, 24) and validated in the USA(25).
Sample size
The sample size requirements for identifying important biomarkers should proceed by evaluating precision of the true positive rate (TPR) and false positive rate (FPR). Assuming the sensitivity of the marker cutoff (individually or in combination) is p = # test positive/n, where the sample size (n), number of women diagnosed with endometriosis i.e. cases, necessary to estimate p with precision +/− L is given by the formula n=Z*Zp(1-p)/(L*L), (22, 26). Here Z corresponds to the correct percentile of the standard normal distribution, here we assume Z=1.96 to correspond to a 95% confidence interval(CI). Therefore, assuming a 90% sensitivity of the diagnostic test, and a very precise 95% confidence interval of ± approximately 5.0% (i.e. 90% with a 95% CI of 85% – 95%) we would need 139 cases of endometriosis and the same number of controls. We will round up to obtain 150 in each group.
A total of 150 cases and 150 controls is a relatively large number of potential participants, and this sample size will allow us to estimate the sensitivity and specificity of a potential diagnostic test for endometriosis with good precision. In the event that the sensitivity was lower, the precision of our estimate would still be within 10%, L=0.1. (Table 1)
Table 1.
Sample size per group (cases or controls) required to estimate the proportion p, with the desired width, L, of a 95% confidence interval.
| L=0.025 | L=0.05 | L=0.06 | L=0.07 | L=0.10 | |
|---|---|---|---|---|---|
| p=0.7 | 1,291 | 323 | 224 | 165 | 42 |
| p=0.8 | 984 | 246 | 171 | 126 | 62 |
| p=0.9 | 554 | 139 | 97 | 71 | 35 |
| p=0.95 | 292 | 73 | 51 | 38 | 18 |
Statistical Analysis
The putative molecular markers will be evaluated as potential diagnostic markers with a focus on discriminative ability (sensitivity, specificity and predictive value). Several candidate markers can be examined simultaneously by a variety of methods, such as Classification and Regression Trees (CART) analysis(27, 28). Change in markers will be correlated with change in score of health-related Quality of Life (QOL) questionnaire at baseline, 1 month and 3–4 months after surgery.
Expected Recruitment
Preliminary data demonstrate that >90% of patients undergoing laparoscopy for pain and/or infertility and 75% of patients scheduled to undergo laparoscopic tubal ligation will agree to participate. From past experience of women undergoing laparoscopy for infertility or pelvic pain 70% had evidence of endometriosis and 25% definitively did not. Past experience has also suggested that the division in terms of severity of endometriosis will not be equal. In the one study 66% had minimal/mild disease and 34% were diagnosed with moderate/severe disease(18). In the second study 33% had minimal/mild disease and 67% were diagnosed with moderate/severe endometriosis (13).
A 1/3, 2/3 distribution (in either direction) would require enrollment of 225 women with endometriosis (75 and 150 in each group). Assuming 50% of women approached have endometriosis and 5% cannot be classified as to disease status, we estimate the need to enroll approximately 474 subjects. For convenience, the estimated total number of women has been rounded to up to 500. Actual enrollment will be monitored in real time and we will stop enrollment when desired strata are filled with the desired sample size.
Discussion
The noninvasive diagnosis of endometriosis is an optimal use of a biomarker. A predictive biomarker would minimize the need for a surgical diagnosis and its inherit morbidity. Availability of a biomarker would also reduce the number of women without endometriosis who might be empirically treated with medical therapy with associated side effects. Additionally, a marker that would indicate disease severity or response to treatment could potentially aid in titration of dose and duration of therapy. The identification and development of a biomarker is complex and has distinct phases (12, 13, 29, 30). The first phase is that of a preclinical exploration to identify promising markers. The second phase is the establishment of a clinical assay to be used on a larger scale study. Phase III is testing the utility of the biomarker often with a longitudinal or retrospective cohort (12, 29, 31). Important components of biomarker development are standardized bio-specimen collection and handling, accurate phenotyping, transparent reporting of data and validation of results. Complete and accurate reporting is necessary to enable readers to assess the potential for bias in the study and to evaluate the generalizability of the results. This study was designed to fulfill the STARD (Standards for Reporting of Diagnostic Accuracy) statement to improve reporting the quality of studies of diagnostic accuracy (32).
This protocol was informed by the WERF (EPHect): which was designed to harmonize and standardize the collection of data relevant to large scale collaborative for research in endometriosis. This project gave guidance to collection of clinical data, phenotyping, as well as standard operating procedures (SOPs) for bio specimen collection, processing and storage(14–16, 20, 33). The protocol attempts to balance the key components of comprehensive data collection, while maintaining feasibility and minimizing the time commitment required by the participant and the study team. The WERF short forms were used to document clinical data, medication use and quality of life. SOPS were modified to minimize complexity and included collection of endometrium and serum to be used for pre-specified analysis while also aliquoting and banking endometrium, serum, plasma and urine for future use.
The study was also designed to minimize burden to the participant. Endometrial sampling was offered as part of a separate pre-operative visit, or as part of the planned surgical procedure. Allowing collection of the endometrial tissue at preoperative visit aided in collation of the specimen within the desired proliferate phase of the cycle if the surgery cannot be scheduled within the desired menstrual cycle phase. Collection at the time of the planned surgery minimizes discomfort related to the endometrial samples, as it is performed after induction of anesthesia but before the start of the planned operative procedure.
Diagnosis of endometriosis will be made with visual inspection using ASRM staging and does not require biopsy of affected areas, so that standard of care will not be affected by requiring surgical procedures outside the scope of the planned procedure. Confirmation of endometriosis, or its absence, will be documented with represented photographs. Phenotyping will be performed in a standardized fashion at the time of the surgery or based on operative report with assistance of the surgical team. This study is designed to be pragmatic. There are very few exclusion criteria so that a large number of women may be included and phenotyped based on presence or absence of disease while collecting information regarding prior history and medication use. Therapy of endometriosis will not be dictated in the protocol, will be left to the discretion of the clinical team, and will be documented.
This study design allows post hoc stratification of samples based on phenotype rather than only including women with desired disease characteristics. A larger number of women will be enrolled than the desired sample size as all subjects may not contribute a “usable” bio specimen. For example, some women may have equivocal finding of disease and would not be chosen as a case or a control. Additionally, the overall sample size is sufficiently large so subsets might be evaluated, such as women naive to hormonal therapy or those with (or without) concomitant gynecologic pathology such as ovarian cyst or uterine leiomyoma.
It is recognized that finding from this protocol will be preliminary and will need to be validated in other populations as novel biomarkers may perform differently in other populations. Moreover, care must be taken to account for the effect of confounding factors such as result difference between assay batches, the effect from concomitant gynecologic pathology or prevalence of endometrioses in the subpopulations analyzed as well as Type I error (11).
Endometrial samples and serum will be collected to validate genomic classifiers and cytokine concentrations in the nonsurgical diagnosis of women with endometriosis. However, samples of serum, plasma, DNA, RNA, urine and endometrial tissue will be stored in a bio repository for potential use in the future in nonbiased proteomic or genomic approaches for discovery. This bio-repository will serve as a valuable resource for future collaboration with other scientists.
Conclusion
Diagnosis and treatment of endometriosis have always been primarily surgical. However, laparoscopic diagnosis of endometriosis is still inaccurate, The ENDOmarker study is designed to obtain well characterized and phenotyped biospecimens in a standardized fashion from reproductive aged women with and without endometriosis to be used to validate and optimize the clinical use of genomic classifiers obtained by endometrial biopsy alone or in combination with serum cytokines as a non-surgical composite marker of endometriosis.
Acknowledgments
The authors express their thanks to other members of the Cooperative Reproductive Medicine Network (RMN), Serdar Bulun, Richard Burney, Hugh Taylor and the Endometrial Research Focus Group of the National Cooperative Centers for Translational Research in Reproduction and Infertility (NCTRI) as well as the RMN Advisory Committee and Data Safety Monitoring Board for their clinical review of the ENDOmarker protocol.
Funding
This study is supported by National Institute of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360–5. doi: 10.1016/j.fertnstert.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenken RS, Guzick DS. Revised endometriosis classification: 1996. Fertil Steril. 1997;67(5):815–6. doi: 10.1016/s0015-0282(97)81390-8. [DOI] [PubMed] [Google Scholar]
- 4.Wellbery C. Diagnosis and treatment of endometriosis. Am Fam Physician. 1999;60(6):1753–62. 67–8. [PubMed] [Google Scholar]
- 5.Endometriosis and infertility. Fertility and Sterility. 82:40–5. doi: 10.1016/j.fertnstert.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 7.Kiesel L, Schweppe KW, Sillem M, Siebzehnrubl E. Should add-back therapy for endometriosis be deferred for optimal results? Br J Obstet Gynaecol. 1996;103(Suppl 14):15–7. [PubMed] [Google Scholar]
- 8.Moghissi KS. Add-back therapy in the treatment of endometriosis: the North American experience. Br J Obstet Gynaecol. 1996;103(Suppl 14):14. [PubMed] [Google Scholar]
- 9.Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med. 2017;377(1):28–40. doi: 10.1056/NEJMoa1700089. [DOI] [PubMed] [Google Scholar]
- 10.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83(1–5):149–55. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 11.Palmer SS, Barnhart KT. Biomarkers in reproductive medicine: the promise, and can it be fulfilled? Fertil Steril. 2013;99(4):954–62. doi: 10.1016/j.fertnstert.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 13.Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–99. doi: 10.1210/en.2014-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassbender A, Rahmioglu N, Vitonis AF, Vigano P, Giudice LC, D'Hooghe TM, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil Steril. 2014;102(5):1244–53. doi: 10.1016/j.fertnstert.2014.07.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahmioglu N, Fassbender A, Vitonis AF, Tworoger SS, Hummelshoj L, D'Hooghe TM, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril. 2014;102(5):1233–43. doi: 10.1016/j.fertnstert.2014.07.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker CM, Laufer MR, Stratton P, Hummelshoj L, Missmer SA, Zondervan KT, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil Steril. 2014;102(5):1213–22. doi: 10.1016/j.fertnstert.2014.07.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–98. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, Chittams J, et al. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril. 2008;89(5):1073–81. doi: 10.1016/j.fertnstert.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De Smet F, et al. Noninvasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010;25(3):654–64. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 20.Vitonis AF, Vincent K, Rahmioglu N, Fassbender A, Buck Louis GM, Hummelshoj L, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil Steril. 2014;102(5):1223–32. doi: 10.1016/j.fertnstert.2014.07.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon E, Vo KC, McIntire RA, Aghajanova L, Zelenko Z, Irwin JC, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 2011;95(6):2120–2. 2.e1–12. doi: 10.1016/j.fertnstert.2011.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran WG. Methodological problems in the study of human populations. Ann N Y Acad Sci. 1963;107:476–89. doi: 10.1111/j.1749-6632.1963.tb13293.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Jenkinson C, Kennedy S. Development of the Short Form Endometriosis Health Profile Questionnaire: the EHP-5. Qual Life Res. 2004;13(3):695–704. doi: 10.1023/B:QURE.0000021321.48041.0e. [DOI] [PubMed] [Google Scholar]
- 24.Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: The Endometriosis Health Profile-30. Obstet Gynecol. 2001;98(2):258–64. doi: 10.1016/s0029-7844(01)01433-8. [DOI] [PubMed] [Google Scholar]
- 25.Jones G, Jenkinson C, Taylor N, Mills A, Kennedy S. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the Endometriosis Health Profile Questionnaire. Hum Reprod. 2006;21(10):2686–93. doi: 10.1093/humrep/del231. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson SC, Felinger A, Guiochon G. Optimizing the sample size and the retention parameters to achieve maximum production rates for enantiomers in chiral chromatography. Biotechnol Bioeng. 1992;40(10):1210–7. doi: 10.1002/bit.260401011. [DOI] [PubMed] [Google Scholar]
- 27.Breiman LFJ, Stone C, Olshen A. Classification and Regression Trees. New York: Wadsworth; 1984. [Google Scholar]
- 28.B ZHaS. Recursive Partitioning and Its Applications. New York: Springer; 2010. [Google Scholar]
- 29.Hall JA, Brown R, Paul J. An exploration into study design for biomarker identification: issues and recommendations. Cancer Genomics Proteomics. 2007;4(3):111–9. [PubMed] [Google Scholar]
- 30.Rothman N, Stewart WF, Schulte PA. Incorporating biomarkers into cancer epidemiology: a matrix of biomarker and study design categories. Cancer Epidemiol Biomarkers Prev. 1995;4(4):301–11. [PubMed] [Google Scholar]
- 31.Bonassi S, Neri M, Puntoni R. Validation of biomarkers as early predictors of disease. Mutat Res. 2001;480–481:349–58. doi: 10.1016/s0027-5107(01)00194-4. [DOI] [PubMed] [Google Scholar]
- 32.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138(1):W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 33.G CW. Sampling Techniques. 2. New York: John Wiley and Sons, Inc.; 1963. [Google Scholar]