Abstract
Purpose
Decisions to continue or suspend therapy with immune checkpoint inhibitors are commonly guided by tumor dynamics seen on serial imaging. However, immunotherapy responses are uniquely challenging to interpret because tumors often shrink slowly or can appear transiently enlarged due to inflammation. We hypothesized that monitoring tumor cell death in real-time by quantifying changes in circulating tumor DNA (ctDNA) levels could enable early assessment of immunotherapy efficacy.
Experimental Design
We compared longitudinal changes in ctDNA levels with changes in radiographic tumor size and with survival outcomes in 28 metastatic non-small cell lung cancer patients receiving immune checkpoint inhibitor therapy. CtDNA was quantified by determining the allele fraction of cancer-associated somatic mutations in plasma using a multi-gene next-generation sequencing assay. We defined a ctDNA response as a >50% decrease in mutant allele fraction from baseline, with a second confirmatory measurement.
Results
Strong agreement was observed between ctDNA response and radiographic response (Cohen’s kappa, 0.753). Median time to initial response among patients who achieved responses in both categories was 24.5 days by ctDNA vs. 72.5 days by imaging. Time on treatment was significantly longer for ctDNA responders vs. non-responders (median 205.5 vs. 69 days; P<0.001). A ctDNA response was associated with superior progression-free survival (hazard ratio [HR], 0.29; 95% CI, 0.09–0.89; P=0.03), and superior overall survival (HR, 0.17; 95% CI, 0.05–0.62; P=0.007).
Conclusions
A drop in ctDNA level is an early marker of therapeutic efficacy and predicts prolonged survival in patients treated with immune checkpoint inhibitors for non-small cell lung cancer.
Keywords: Circulating tumor DNA, immunotherapy, immune checkpoint inhibitors, non-small cell lung cancer, cancer biomarker
Introduction
Immune checkpoint inhibitors are known to produce tumor response patterns that are distinct from those of most other systemic anti-cancer therapies (1–7). Delayed tumor shrinkage is frequently observed, and can sometimes be preceded by transient enlargement due to immune cell infiltration (termed pseudo-progression). Thus, during the first few months of treatment, the standard practice of monitoring therapeutic efficacy via serial radiographic scans may not provide clear clinical guidance. Misinterpretation of scans could lead to inappropriate discontinuation of a potentially effective therapy, or conversely, an ineffective treatment could be continued hoping for a delayed response that never comes. A blood biomarker with rapid kinetics could offer an earlier indication of treatment efficacy to help clarify therapeutic management decisions in such cases.
For patients with non-small cell lung cancer (NSCLC), there are currently no blood biomarkers that are routinely used to follow treatment response. Circulating tumor DNA (ctDNA) is emerging as a promising cancer biomarker, and its potential utility in monitoring therapeutic response has been explored for various treatment modalities, including immunotherapy (8–15). Because ctDNA can be distinguished based on the presence of tumor-specific somatic mutations, it is expected to have greater specificity than most serum protein markers. Also, because ctDNA is a byproduct of dying cancer cells and is cleared from the blood with a half-life of ~2 hours (9), its levels provide a real-time snapshot of active tumor cell death rather than simply a measure of tumor burden. Example cases from prior studies (13, 16) have shown that post-treatment ctDNA levels can spike as tumor cells are killed, and can then quickly decline after the initial wave of cell death has subsided.
The present study was undertaken to investigate whether the effectiveness of immunotherapy could be predicted based on early changes in ctDNA levels in patients with metastatic NSCLC. We compared the timing and magnitude of change in ctDNA levels and radiographic tumor size measurements longitudinally during treatment. We examined whether patients with downtrending ctDNA levels were more likely to have a longer duration of treatment benefit. We also examined whether such patients had improved clinical outcomes, including progression-free survival and overall survival.
Materials and Methods
Patients and plasma
In this single-institution study, we collected serial blood samples between October 2014 and May 2016 from patients diagnosed with metastatic NSCLC who were receiving immunotherapy with an anti-PD-1 or anti-PD-L1 drug, alone or in combination with other immunotherapeutic agents. Patients concurrently receiving any other class of systemic cancer therapy were excluded from the study. Treating oncologists were blinded to the results of ctDNA testing, and ctDNA analysis was performed while blinded to clinical data. The study was approved by the Human Investigation Committee of Yale University, and was conducted according to established ethical guidelines as outlined in the Declaration of Helsinki. Informed written consent was obtained from all patients.
Blood samples were collected on the first day of treatment (prior to initiation of therapy), and thereafter at the time of routine clinical blood draws, typically at intervals of 2 or more weeks. Up to 20 mL of blood was collected at each time point in EDTA-containing tubes. Plasma was separated by centrifugation at 1000 × g for 10 minutes within 4 hours of collection, and was stored at −80°C.
Measurement and Monitoring of ctDNA
Cell-free DNA was extracted from 1 mL aliquots of thawed plasma using a QIAamp MinElute Virus Vacuum Kit (Qiagen). Tumor-derived somatic mutations within cell-free DNA were identified and quantified using an enhanced version of the Error-Suppressed Deep Sequencing method previously published by our group (Fig. 1A; detailed in the Supplementary Methods) (17, 18). The assay simultaneously queried thousands of possible point mutations and insertions/deletions within 43 mutation-prone regions of 24 cancer-associated genes (Supplementary Table S1). High-throughput DNA sequencing was performed in 75 base-pair, paired-end mode on an Illumina HiSeq2500 instrument. Mutations found in plasma were compared to those identified in tumor tissue when available from routine clinical evaluation by a CLIA-certified clinical laboratory. To quantify ctDNA, we calculated the allelic fraction of mutant tumor-derived DNA within total cell-free DNA in plasma based on mutant and wild-type sequence counts obtained from next-generation sequencing data. If more than one mutation was identified in a baseline sample, we used the mutation having the highest allelic fraction to track ctDNA levels over time. Data evaluating the detection sensitivity and reproducibility of ctDNA measurements are provided in Supplementary Figures S2, S3, and S4.
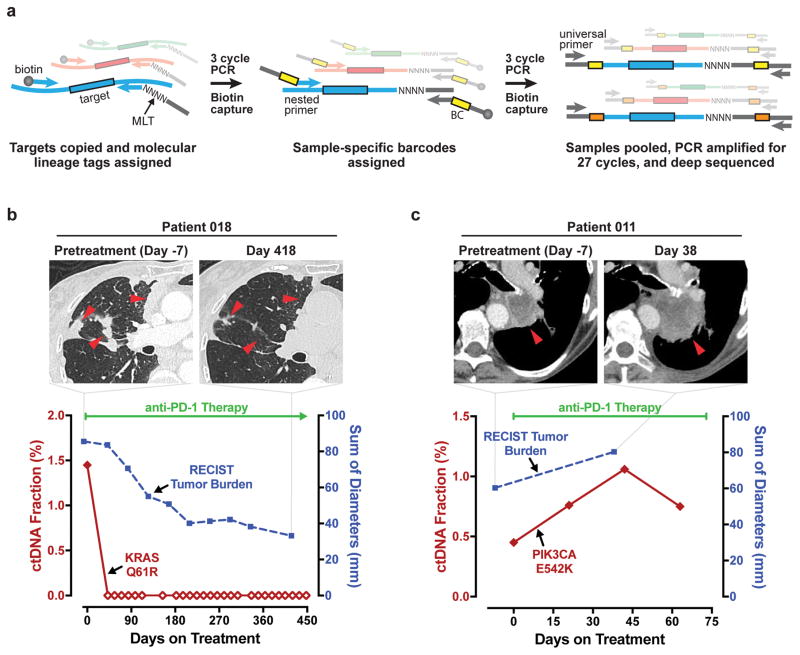
Fig. 1. Schematic of ctDNA assay and representative patient cases.
A, Schematic illustration of the enhanced Error Suppressed Deep Sequencing assay for circulating tumor DNA (ctDNA) quantitation. MLT, molecular lineage tag; BC, barcode. B and C, Plasma levels of ctDNA and measurements of radiographic tumor burden are plotted for two representative patients with metastatic NSCLC: a patient with treatment response and a patient with progressive disease. B, An 89 year-old woman who received anti-PD-1 immunotherapy as first-line treatment achieved undetectable ctDNA on day 42, and met radiographic response criteria on day 125. The patient received 27 cycles of immunotherapy, with treatment continuing as of the data cutoff date. Undetectable ctDNA is indicated by open diamonds. C, A 73 year-old woman who received first-line anti-PD-1 immunotherapy failed to meet criteria for radiographic or ctDNA response. Radiographic progression was noted on day 38 and therapy was stopped on day 73 (date of death). Radiographic and ctDNA measurements for the remaining 26 patients in the study are presented in Supplementary Fig. S1.
Radiographic Data and Clinical Outcomes
Patients underwent computed tomographic (CT) scans within 30 days prior to their first immunotherapy treatment, and then typically at intervals of 6–12 weeks thereafter or when clinically indicated. Scans were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 (19), by a radiologist blinded to ctDNA data. Radiographic tumor burden was quantified as the sum of longest unidimensional diameters of target tumor lesions. Radiographic responses were recorded as partial response if the tumor burden decreased by at least 30%, as progressive disease if the tumor burden increased by at least 20% or if new lesions appeared, or as stable disease if neither criterion was met. Treatment duration was determined based on the off-treatment date designated by the treating oncologist. Progression-free survival was defined as the interval between treatment initiation and the date of disease progression or death, whichever occurred earlier. Overall survival was defined as the time interval from treatment initiation to death. A censor date of May 15, 2016 was applied if no endpoint was met.
Statistical Analysis
Agreement between radiographic response and ctDNA response was assessed with Cohen’s kappa coefficient. Wilcoxon rank sum tests were used to assess the difference in magnitude of early ctDNA drop between radiographic responders and non-responders and the difference in days on treatment between ctDNA responders and non-responders. A Wilcoxon signed rank test was used to evaluate the difference in timing of ctDNA response vs. radiographic response. Association of time-varying ctDNA response status with progression-free survival and overall survival were estimated separately by Cox proportional hazards regression models. Extended Kaplan-Meier survival curves (20) were provided to illustrate the resulting hazard ratios. All patients were classified as non-responders by ctDNA at baseline. Subjects with at least 50% reduction of ctDNA from baseline with a second consecutive confirmatory value were considered ctDNA responders. Responders with subsequent measurements above the 50% threshold with a second consecutive confirmatory value were re-classified as non-responders. Measurements of ctDNA that occurred >2 weeks after the off-treatment date were excluded. Tests were two-sided and P-values <0.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patients and clinical samples
We enrolled 49 patients with metastatic NSCLC who were receiving immune checkpoint inhibitor therapy. The study focused on 28 patients in whom somatic mutations were identified in baseline plasma. We analyzed a total of 182 serial plasma samples that were obtained at baseline, during treatment, or up to 2 weeks after termination of immunotherapy (provided that another line of treatment had not yet been initiated). Patients had a minimum of 2 and a maximum of 27 serial samples. Among the 28 patients, 22 were treated with single-agent anti-PD-1 or anti-PD-L1 therapy and 6 received combination immunotherapy (detailed in Supplementary Fig. S1). Patient characteristics of the 28 subjects with trackable ctDNA are summarized in Supplementary Table S2. Individual characteristics of all 49 enrolled patients are provided in Supplementary Table S3. No significant difference was found in overall survival between the 28 patients who had detectable ctDNA mutations at baseline and the 21 patients who did not (HR, 1.86; 95% CI, 0.76 to 4.52; P=0.17; Supplementary Fig. S5). Comparison of baseline characteristics between these two populations yielded no significant differences other than gender (for which we have no reasonable physiological explanation; Supplementary Table S4).
Longitudinal Quantification of Somatic Mutations in Plasma
ctDNA was quantified by determining the allelic fraction of cell-free DNA fragments that harbored cancer-associated somatic mutations. We used an assay in which multiplexed PCR amplification products of 43 mutation-prone regions in 24 genes were subjected to ultra-deep next-generation sequencing (Fig. 1A). True mutant sequences were distinguished from sequencer misreads and PCR polymerase misincorporations using molecular and computational error-suppression techniques (described in the Supplementary Methods). The mutant allele fraction was determined by comparing read counts of variant and wild-type sequences (Supplementary Table S5). More than one mutation was detected in the baseline plasma of 8 out of 28 patients; in these cases we used the mutation with the highest allele fraction at baseline for longitudinal monitoring. Routine clinical testing of available tumor tissue by an independent laboratory identified somatic mutations in 14 patients (Supplementary Table S6). We identified the identical mutation in the plasma DNA of all but one of these 14 cases. Among the 49 total enrolled patients in the study, 20 patients had mutations found in tissue which were also assessed by the ctDNA assay; a concordant mutation was found in the plasma of 13 of these patients. One patient had a mutation identified in ctDNA which was tested for, but was not identified in tumor tissue. Based on these results (summarized in Supplementary Table S3), the calculated concordance was 65% (13 True Positive/[13 True Positive + 1 False Positive + 6 False Negative]). Detection rates of ctDNA are known to be lower in non-treatment-naïve cohorts.
Figure 1 provides illustrative trajectories of serial ctDNA measurements and radiographic tumor burden for two patients in our study; one who had a robust, durable immunotherapy response (Fig. 1B) and one who had rapid disease progression (Fig. 1C). For the treatment responder, we noted a rapid decline in mutant ctDNA to undetectable levels at the first blood draw. The corresponding change in tumor size was more gradual, with radiographic partial response achieved at the third scan. This patient was continuing to receive immunotherapy as of the data cutoff date. In contrast, the patient without treatment response was observed to have immediate increases in both ctDNA levels and radiographic tumor burden, and therapy was stopped shortly thereafter. Radiographic and ctDNA trajectories are provided in Supplementary Figure S1 for the remaining 26 study patients.
Treatment response by ctDNA correlates with radiographic response
To formally investigate whether ctDNA could be used to monitor immunotherapy response, we first tested for agreement between radiographic response and change in ctDNA level. Although a transient increase in ctDNA levels can occur when tumor cells die in the initial phases of therapy, we predicted that the short duration and variable timing of such a spike would be difficult to consistently measure. Instead, we examined whether radiographic response was associated with a drop in ctDNA levels, which we expected would be sustained for a longer duration as the number of actively dying tumor cells diminished. We defined a “ctDNA response” as a drop in ctDNA level to <50% of baseline, with a second successive confirmatory measurement (modeled after response criteria for prostate-specific antigen) (21).
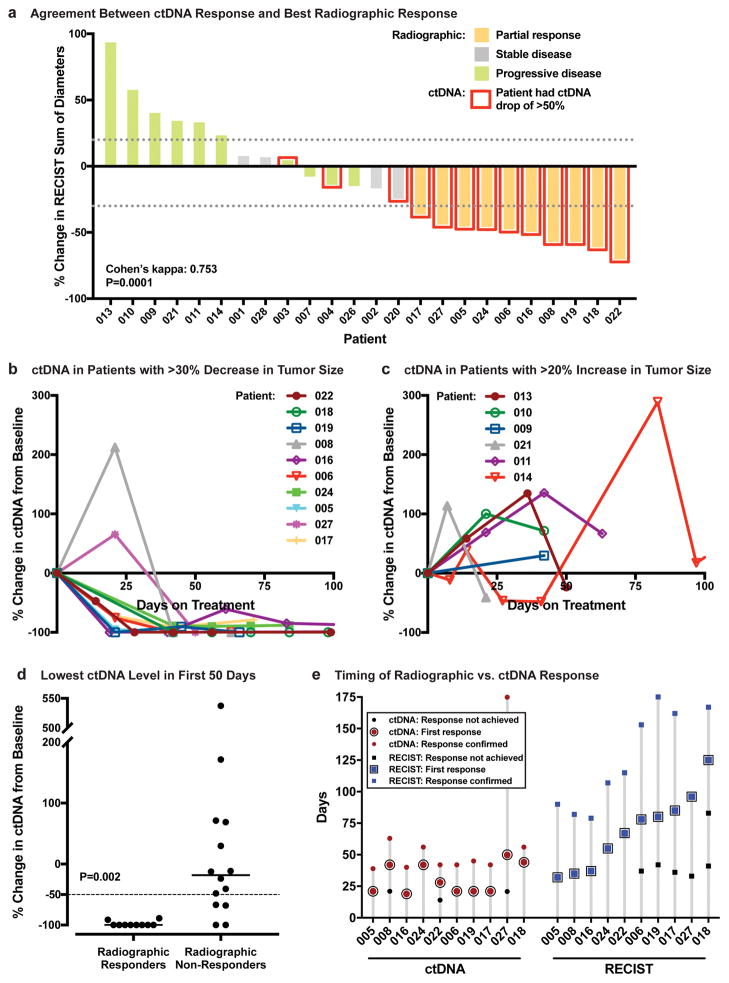
We found strong agreement between ctDNA response and best radiographic response using the Cohen’s kappa statistic (κ=0.753; 95% confidence interval [CI], 0.501–1.000; P<0.001) among the 24 patients in the study who were evaluable for radiographic response by RECIST criteria (Fig. 2A). Ten patients had a radiographic partial response (PR), all of whom also had a ctDNA response. Eleven patients failed to achieve PR and also had no ctDNA response. The three remaining patients with discordant responses all had ctDNA responses without achieving radiographic PR: two had radiographic progressive disease (PD), and one had stable disease (SD). This analysis excluded 3 patients who lacked a post-treatment scan (patients 012, 023, and 025) and 1 patient with an unevaluable target lesion due to surrounding lung atelectasis (patient 015).
Figure 2. Concordance, Magnitude, and Timing of ctDNA and Radiographic Response to Immunotherapy.
A, Agreement of ctDNA response and best radiographic response, defined as the lowest ratio of [tumor burden on any post-baseline scan] to [tumor burden at baseline] (26) (n = 24 patients). Tumor burden was measured according to RECIST, version 1.1 (19). Red outline indicates patients who achieved a ctDNA response. Dotted lines indicate a 30% decrease or 20% increase in RECIST sum of diameters. B and C, Percentage change in ctDNA level from baseline during the first 100 days of immunotherapy among patients with at least a 30% decrease (B, n = 10) or a 20% increase (C, n = 6) in RECIST-defined tumor burden. D, Lowest ctDNA level (percentage change from baseline) measured within the first 50 days after initiation of immunotherapy, for patients who achieved radiographic partial response vs. those who did not. Each dot represents one patient (n = 24). The median value for each group is indicated by a horizontal line. A dashed line indicates a 50% decrease in ctDNA level, which is the threshold for ctDNA response. P=0.002 by Wilcoxon rank sum test. E, Time to radiographic vs. ctDNA response among patients who achieved both types of response (n = 10). Dates of ctDNA and radiographic measurements meeting response criteria are shown. Also shown are preceding time points that failed to meet response criteria as well as confirmatory measurements for both ctDNA and imaging.
Early patterns of change in ctDNA level are compared in Figures 2B and 2C for patients whose best radiographic response was PR with >30% tumor shrinkage vs. PD with >20% tumor growth, respectively. Patients who achieved radiographic response all showed a substantial drop in ctDNA level, with 2 patients showing a temporary spike preceding the drop. In contrast, patients with >20% increase in tumor size showed a more variable ctDNA trend, and none met ctDNA response criteria.
Radiographic responders show a substantial reduction in ctDNA levels
Next, we evaluated the magnitude of change in ctDNA level among radiographic responders and non-responders. We found that the lowest ctDNA measurement relative to baseline within the first 50 days of treatment was significantly lower for patients who achieved radiographic PR than for those who did not (P=0.002; Fig. 2D). Although we set the threshold for ctDNA response at −50%, the actual drop that we observed in patients who achieved a radiographic PR was much greater. Of the 10 radiographic responders, 8 patients achieved undetectable ctDNA and 2 patients had changes of −89% and −91% (median ctDNA change, −100%; interquartile range [IQR], −100% to −100%). Among the 14 radiographic non-responders, the change in ctDNA level was much more variable (median ctDNA change, −18%; IQR, −70% to 69%). Of note, there were two radiographic non-responders who achieved undetectable ctDNA, and both appeared to derive long-term clinical benefit from immunotherapy as independently judged by their treating oncologists. One of these patients remained on therapy for 386 days until death from a bowel perforation, and the other patient continued to receive immunotherapy as of the data cutoff date (at least 152 days). We therefore compared overall survival between patients who achieved undetectable levels of ctDNA at any post-treatment time point vs. those who did not and found the former group had a superior overall survival (HR, 0.11; 95% CI, 0.02 to 0.88; P=0.037).
ctDNA response is seen more rapidly than radiographic response
To evaluate our hypothesis that treatment efficacy can be more rapidly assessed by ctDNA than by imaging, we compared the timing of ctDNA and radiographic responses among the 10 patients who achieved a response in both categories (Fig. 2E). The median time to initial ctDNA response was 24.5 days from the start of treatment, whereas the median time to initial radiographic PR was 72.5 days (confirmation of response with a second measurement was obtained at a median of 43.5 days for ctDNA and 115 days for imaging, respectively). Recognizing that such a comparison could be inherently biased because ctDNA was generally measured earlier, we compared the timing of first ctDNA or radiographic assessment to the timing of initial response. In this group of 10 patients, the first ctDNA measurement occurred a median of only 14.5 days earlier than the first scan. However, initial ctDNA response occurred a median of 42.5 days earlier than initial radiographic response (P=0.004). We note that because the cohort evaluable for this analysis was only 10 patients, it is uncertain whether a similar pattern would be observed in a larger cohort of patients with imaging available at earlier time points.
Longer-term treatment benefit is seen among ctDNA responders
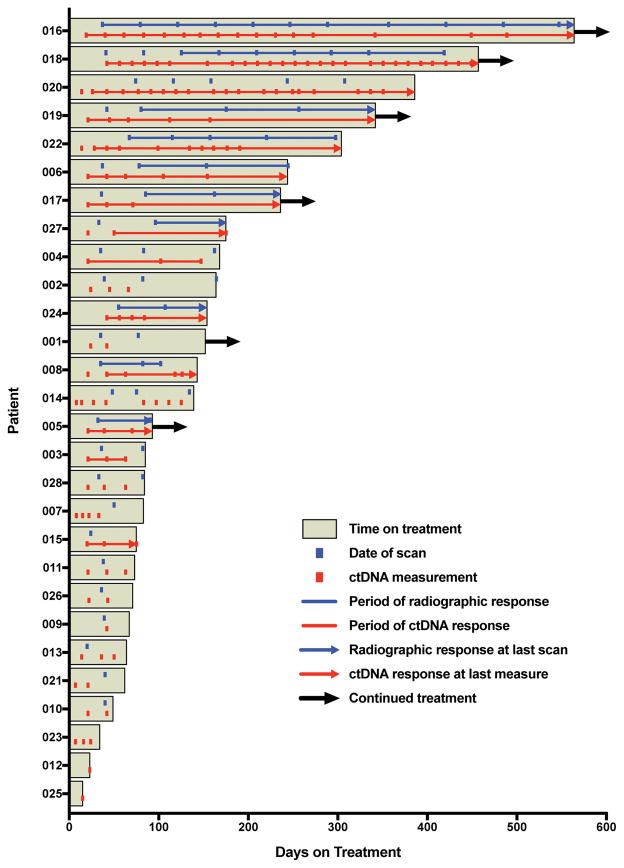
Figure 3 shows the duration of immunotherapy treatment and the periods of radiographic and ctDNA response for each of the 28 patients in the study. Because an oncologist’s decision to continue or terminate therapy was based on clinical factors beyond just the radiographic response (but without knowing ctDNA results), the duration of therapy offers an additional, clinically relevant gauge of treatment efficacy. The median duration on therapy was significantly longer for the 14 ctDNA responders compared to the 14 patients without ctDNA response (205.5 vs. 69 days; P<0.001). As 5 of the patients with ctDNA response were continuing immunotherapy at last follow-up (vs. only 1 non-responding patient), this difference in therapy duration is likely underestimated.
Figure 3. Duration of Treatment and Intervals of Radiographic and ctDNA Response.
The relationship between duration of treatment benefit and achievement of radiographic or ctDNA response is shown (n = 28 patients). Immunotherapy treatment durations are plotted as horizontal bars, with arrows indicating ongoing therapy as of the data cutoff date. Overlying lines depict periods of radiographic and ctDNA response for each patient, and hash marks indicate measurement time points. Arrows denote ongoing ctDNA or radiographic response based on the last available measurement.
ctDNA response is associated with improved progression-free and overall survival
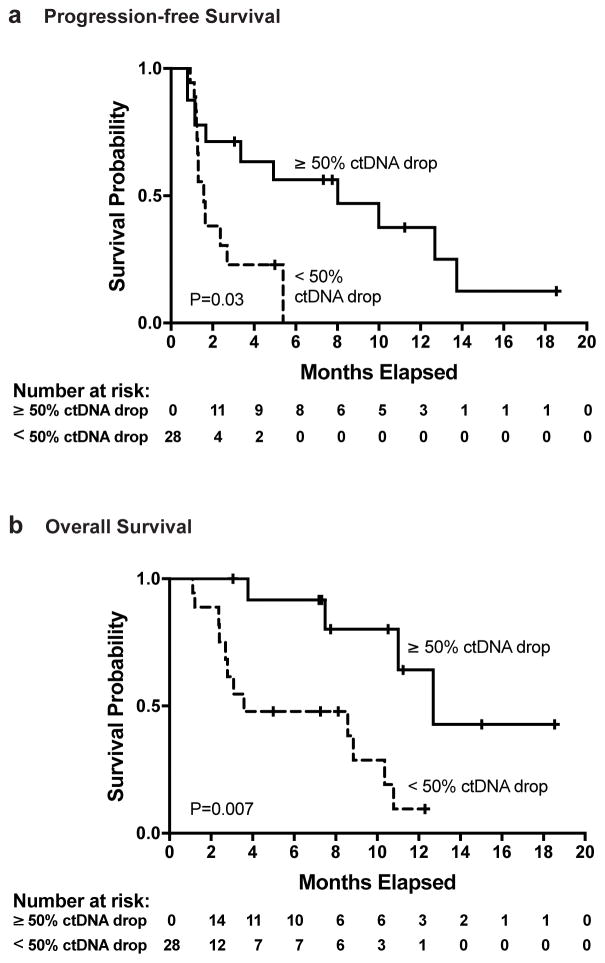
Finally, we evaluated the association between ctDNA response and survival outcomes. Because by definition, ctDNA response could not be assessed until after starting treatment, we used an extended Kaplan-Meier estimator(20) to incorporate the time-varying categorization of patients as ctDNA responders or non-responders. We found that achievement of a ctDNA response was associated with a significantly lower risk of disease progression or death (hazard ratio [HR], 0.29; 95% CI, 0.09 to 0.89; P=0.03; Fig. 4A). Analysis of overall survival (Fig. 4B) showed that ctDNA response was associated with a significantly lower risk of death (HR, 0.17; 95% CI, 0.05 to 0.62; P=0.007). In comparison, patients who achieved radiographic response appeared to have a lower risk of death, but this association failed to reach statistical significance (HR 0.22; 95% CI, 0.05 to 1.02; P=0.053). An additional landmark analysis also demonstrated a superior overall survival among those with a ctDNA response (HR 0.13; 95% CI 0.03 to 0.51; P=0.0034; Supplementary Fig. S6). A similar landmark analysis of progression-free survival failed to achieve statistical significance, likely because 12 of 28 patients were excluded due to death or censoring prior to determination of landmark status, leaving only 4 patients in the non-responder group (HR 0.29; 95% CI 0.06 to 1.45; P=0.13; Supplementary Fig. S6).
Figure 4. Progression-free and Overall Survival According to ctDNA Response.
Extended Kaplan-Meier curves (20) provide estimates of (A) progression-free survival and (B) overall survival for all patients (n = 28). A time-varying categorization was used to designate patients as ctDNA responders or ctDNA non-responders. All patients were initially classified as non-responders because response could not be assessed until after treatment initiation. Patients were considered ctDNA responders if a ≥50% reduction of ctDNA was observed from baseline with a second consecutive confirmatory value. Patients were subsequently re-classified as non-responders if two consecutive measurements rose above the 50% threshold.
Discussion
Our study demonstrates that circulating tumor DNA can be a clinically informative biomarker to complement radiographic monitoring of response in patients receiving immune checkpoint inhibitor therapy for non-small cell lung cancer. We found strong agreement between radiographic response and ctDNA response, which we defined as a drop in ctDNA level to less than half of the baseline value. CtDNA responses were seen significantly sooner than radiographic responses, indicating that ctDNA monitoring could provide an early measure of therapeutic efficacy. Our data also show that patients who achieve a ctDNA response are more likely to have a longer duration of treatment benefit, and superior progression-free and overall survival.
While 21 of 24 radiographically evaluable patients had concordant ctDNA and radiographic responses, the ctDNA findings for the three patients with discordant responses nevertheless appeared to reflect their clinical course. Two patients had short-lived ctDNA responses without achieving radiographic PR, and both had relatively short treatment durations with the downward ctDNA trend reversing before therapy was discontinued. The third patient nearly met RECIST criteria for PR and his ctDNA quickly became undetectable and remained so for over a year on treatment until he died from an unrelated cause. In fact, most patients who had a long-term benefit from immunotherapy rapidly achieved a dramatic and persistent drop in ctDNA (~90–100%). Although we used a decline of >50% as a threshold to define ctDNA response, achievement of undetectable ctDNA may prove to be a stronger predictor of long-term response, and may identify patients who comprise the “tail” of the survival curve (22). This could be explored in future studies with larger patient cohorts.
The use of circulating tumor DNA as a quantitative biomarker for assessment of immunotherapy response has been explored in prior studies (12–15), most of which have focused on tracking of driver mutations in patients with melanoma using digital PCR or allele-specific PCR. Interestingly, some of these studies report observing a transient spike preceding a decline in ctDNA levels in a subset of patients, likely reflecting DNA release as tumor cells are killed. We observed such a spike in patients 008 and 027, both of whom went on to have durable responses. It would be important to avoid misinterpreting such a spike as disease progression. In fact, Xi et al. (13) found that an early spike in ctDNA level during the first month on treatment was correlated with an objective response to T-cell transfer therapy. In the present study, we focused on measuring a drop in ctDNA because we predicted that a transient spike would be difficult to consistently measure due to variability in its timing and magnitude.
There are several limitations of our study that are important to note. One potential limitation is that all patients were not treated with a uniform immunotherapy regimen. We took this approach because we had no reason to expect that the mechanism of ctDNA release or interpretation of ctDNA changes would differ among various immune checkpoint inhibitors. While our findings appear generally applicable across the entire therapeutic class, our study population was not large enough to formally evaluate ctDNA response patterns for each agent individually. Another limitation of our study comes from the variability in the timing of blood collection. To avoid excess venipuncture, we collected plasma when patients were undergoing blood draws for clinical testing; collection at pre-specified time points would have allowed us to evaluate the ctDNA trends more consistently. Additionally, our study population may be biased because we excluded patients who did not have detectable mutant ctDNA at baseline (detectable ctDNA may be associated with worse prognosis). Although our assay covered a broad panel of 43 mutation-prone regions, even broader mutation coverage may enable tracking of ctDNA in a higher proportion of patients (23–25). If DNA were extracted from a larger volume of plasma, we might also have increased the probability of finding mutant copies in patients with very low-abundance ctDNA. Moreover, mutations in different genomic targets could have variable detection sensitivities. However, there will likely remain cases where mutant ctDNA copies are below detection limits at baseline. We found that a high proportion of patients in our study had mutations in the KRAS oncogene. This is likely because patients with mutations in other driver oncogenes (e.g. EGFR) often do not receive immunotherapy because they are less likely to benefit, or they may receive a combination of immunotherapy and targeted therapy (which is an exclusion criterion for the study). However, we believe that our findings should be generalizable beyond KRAS-mutant lung cancer because the ability to quantify changes in ctDNA levels should not depend on the presence of mutations in a particular gene. We should also note that the reliability of prediction of clinical endpoints is dependent on the reproducibility of ctDNA measurements. The coefficients of variation for our assay ranged from 7.8–25% when measuring mutant allele fraction of technical replicate spike-in samples, and 25.5–35.0% when measuring total read counts of purification replicates (Supplementary Figs. S3 and S4). Finally, because our study had a relatively small sample size and lacked a validation cohort, the results must be reproduced in an independent population before they can be used to guide clinical practice.
An important category of patients that our study did not directly address are those who failed to achieve radiographic response criteria, but may have had long-term disease stability while on immunotherapy, indicating a durable clinical benefit. We categorized patients in this study as “responders” or “non-responders”, but perhaps separately evaluating a third category of patients with stable disease might help to identify some patients who may be benefiting from therapy by avoiding progression of disease. Our sample size is too small to permit such an analysis in the present study, but perhaps it could be explored in a larger, future study.
We chose to quantify ctDNA trends based on changes in mutant allele fraction rather than the number of mutant molecules per mL of plasma. A legitimate concern with our approach is that the allele fraction can be affected by changes in the levels of background wild-type DNA, which could be caused by various factors such as inflammation, trauma, physical activity, or infection. Indeed, some patients in our study did show fluctuations in ctDNA allele fraction without having corresponding changes in radiographic tumor burden (e.g. patients 014 and 028). This could be partly explained by the rapid clearance of ctDNA which makes its steady-state levels especially sensitive to changes in the rate of release; but it could also be explained by changes in the levels of background wild-type DNA. However, we have found that the alternative approach of quantifying mutant molecules per mL of plasma can produce inconsistent results because of variability in purification yield and efficiency of converting plasma DNA into sequencing libraries.
The rapid and profound ctDNA changes observed among treatment responders in this study were in clear contrast to the typically more modest radiographic reductions in tumor bulk seen within the same time-frame. A likely explanation is that ctDNA levels reflect the rate of active tumor cell death, rather than total tumor mass. Such rapid response kinetics may be an advantage of ctDNA over protein biomarkers, which are generally secreted from live tumor cells and track more closely with overall tumor burden. Although our study was focused on lung cancer, a disease for which reliable protein markers do not exist, we anticipate that ctDNA could also find similar utility as an early marker of immunotherapy response in other malignancies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Immune checkpoint inhibitors have changed the landscape of lung cancer treatment by offering long-term disease control with fewer side effects than traditional chemotherapy. However, because most patients do not benefit from this powerful new treatment class, there is a critical need to develop biomarkers that quickly evaluate the efficacy of immunotherapy treatment. In this study, we tested whether circulating tumor DNA (ctDNA) obtained from peripheral blood could be used to predict immunotherapy response in patients with metastatic non-small cell lung cancer. We observed strong agreement between radiographic response to immunotherapy and a reduction in ctDNA level to half of its pre-treatment value. Such a ctDNA response was seen significantly earlier than radiographic response, and was associated with improved patient survival. These findings provide rationale for use of ctDNA in conjunction with standard imaging to provide an earlier and more comprehensive assessment of immunotherapy efficacy.
Acknowledgments
We thank Kaya Bilguvar and Christopher Castaldi for help with next-generation sequencing; Joseph DeLuca, Scott Daniska, and Brian Dagliere for assistance with oligonucleotide synthesis; Maheen Zakaria and Rofina Johnkennedy for help with compiling ctDNA measurement data; and Kira Pavlik and Stephanie Buonfiglio for help with compiling radiographic response data. This research was supported by grants to A.A. Patel from the National Cancer Institute (RO1-CA197486-01A1), the LUNGevity Foundation, the Honorable Tina Brozman Foundation, and the Yale Cancer Center; to R.S. Herbst from the Yale SPORE in Lung Cancer (P50-CA196530); and to L.D. Wilson from the Kalimeris Fund.
Footnotes
Conflicts of Interest: Patent applications have been filed covering aspects of the described ctDNA assay technology, with A.A.P. listed as an inventor.
References
- 1.Langer CJ. Emerging immunotherapies in the treatment of non-small cell lung cancer (NSCLC): the role of immune checkpoint inhibitors. Am J Clin Oncol. 2015;38:422–30. doi: 10.1097/COC.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, D’Angelo SP, Wolchok JD. Rapid eradication of a bulky melanoma mass with one dose of immunotherapy. N Engl J Med. 2015;372:2073–4. doi: 10.1056/NEJMc1501894. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of Immune-Related Response Criteria and RECIST v1. 1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510–7. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoos A, Wolchok JD, Humphrey RW, Hodi FS. CCR 20th Anniversary Commentary: Immune-Related Response Criteria--Capturing Clinical Activity in Immuno-Oncology. Clin Cancer Res. 2015;21:4989–91. doi: 10.1158/1078-0432.CCR-14-3128. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116–8. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 8.Pereira E, Camacho-Vanegas O, Anand S, Sebra R, Catalina Camacho S, Garnar-Wortzel L, et al. Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers. PLoS One. 2015;10:e0145754. doi: 10.1371/journal.pone.0145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 11.Shinozaki M, O’Day SJ, Kitago M, Amersi F, Kuo C, Kim J, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007;13:2068–74. doi: 10.1158/1078-0432.CCR-06-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2:42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi L, Pham TH, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. Circulating Tumor DNA as an Early Indicator of Response to T-cell Transfer Immunotherapy in Metastatic Melanoma. Clin Cancer Res. 2016;22:5480–6. doi: 10.1158/1078-0432.CCR-16-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28:1996–2001. doi: 10.1093/annonc/mdx212. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Long GV, Boyd S, Lo S, Menzies AM, Tembe V, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol. 2017;28:1130–6. doi: 10.1093/annonc/mdx026. [DOI] [PubMed] [Google Scholar]
- 16.Girotti MR, Gremel G, Lee R, Galvani E, Rothwell D, Viros A, et al. Application of Sequencing, Liquid Biopsies, and Patient-Derived Xenografts for Personalized Medicine in Melanoma. Cancer discovery. 2016;6:286–99. doi: 10.1158/2159-8290.CD-15-1336. [DOI] [PubMed] [Google Scholar]
- 17.Narayan A, Carriero NJ, Gettinger SN, Kluytenaar J, Kozak KR, Yock TI, et al. Ultrasensitive measurement of hotspot mutations in tumor DNA in blood using error-suppressed multiplexed deep sequencing. Cancer Res. 2012;72:3492–8. doi: 10.1158/0008-5472.CAN-11-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan A, Bommakanti A, Patel AA. High-throughput RNA profiling via up-front sample parallelization. Nat Methods. 2015;12:343–6. doi: 10.1038/nmeth.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) European journal of cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the Impact of a Time-Varying Covariate with an Extended Kaplan-Meier Estimator. The American Statistician. 2005;59:301–7. [Google Scholar]
- 21.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 22.Hellmann MD, Kris MG, Rudin CM. Medians and Milestones in Describing the Path to Cancer Cures: Telling “Tails”. JAMA Oncol. 2016;2:167–8. doi: 10.1001/jamaoncol.2015.4345. [DOI] [PubMed] [Google Scholar]
- 23.Song C, Liu Y, Fontana R, Makrigiorgos A, Mamon H, Kulke MH, et al. Elimination of unaltered DNA in mixed clinical samples via nuclease-assisted minor-allele enrichment. Nucleic Acids Res. 2016;44:e146. doi: 10.1093/nar/gkw650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, et al. Analytical and Clinical Validation of a Digital Sequencing Panel for Quantitative, Highly Accurate Evaluation of Cell-Free Circulating Tumor DNA. PLoS One. 2015;10:e0140712. doi: 10.1371/journal.pone.0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–54. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK, Lee JJ, Ng C, Hong D, Gong J, Naing A, et al. Change in tumor size by RECIST correlates linearly with overall survival in phase I oncology studies. J Clin Oncol. 2012;30:2684–90. doi: 10.1200/JCO.2011.36.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.