Abstract
Aims
While heart failure with preserved and reduced ejection fraction (HFpEF, HFrEF) are well described, determinants and outcomes of HF with mid-range EF (HFmrEF) remain unclear. We sought to examine clinical and biochemical predictors of incident HFmrEF in the community.
Methods and Results
We pooled data from four community-based longitudinal cohorts, with ascertainment of new HF classified into HFmrEF (EF 41–49%), HFpEF (EF ≥50%), and HFrEF (EF ≤40%). Predictors of incident HF subtypes were assessed using multivariable Cox models. Among 28,820 participants free of HF followed for a median of 12 years, there were 200 new HFmrEF cases, compared with 811 HFpEF and 1048 HFrEF. Clinical predictors of HFmrEF included age, male sex, systolic blood pressure, diabetes mellitus, and prior myocardial infarction (multivariable-adjusted P≤0.003 for all). Biomarkers that predicted HFmrEF included natriuretic peptides, cystatin-C, and high-sensitivity troponin (P≤0.0004 for all). Natriuretic peptides were stronger predictors of HFrEF (HR 2.00 per 1-SD increase, 95% CI 1.81–2.20) than of HFmrEF (HR 1.51, 95% CI 1.20–1.90, P for difference 0.01), and did not differ in their association with incident HFmrEF and HFpEF (HR 1.56, 95% CI 1.41–1.73, P for difference 0.68). All-cause mortality following the onset of HFmrEF was worse than that of HFpEF (50 versus 39 events per 1000 person-years, P=0.02), but comparable to that of HFrEF (46 events per 1000 person-years, P=0.78).
Conclusions
We found overlap in predictors of incident HFmrEF with other HF subtypes. By contrast, mortality risk after HFmrEF was worse than HFpEF, and similar to HFrEF.
Keywords: heart failure, risk factor, ejection fraction
The recognition of distinct heart failure (HF) subtypes is important, not only because this classification broadly frames differences in underlying pathophysiology, but also because HF subtypes delineate differential therapeutic approaches.1,2 In general, HF subtypes are classified based on the left ventricular ejection fraction (LVEF), with an LVEF of 50% or more defining HF with preserved ejection fraction (HFpEF), and LVEF of 40% or less defining HF with reduced ejection fraction (HFrEF).1,2 Previous comparisons of HFpEF and HFrEF have often dichotomized the LVEF cut point, and some have omitted a mid-range. These approaches raise the question of whether individuals with HF and an LVEF 41–49%, referred to as mid-range EF (HFmrEF),3 might be a distinct phenotype.
The majority of previous studies focused on HFmrEF have studied samples with existing HF and described cross-sectional associations with clinical characteristics, and describe it as an intermediate phenotype with some features more akin to HFpEF and others to HFrEF.4–7 Potential clinical and biochemical features that precede the development of HFmrEF, however, have not been fully characterized in an inception cohort. Further, few studies have examined outcomes after HFmrEF with mixed results, describing similar outcomes to HFpEF versus HFrEF.7–9
We have previously described differences in clinical predictors for incident HFpEF versus HFrEF among four large community-based samples, however participants with HFmrEF were not examined in this previous study.10 Therefore, in order to better characterize the HFmrEF phenotype, we sought to focus specifically on HFmrEF for this present analysis, and to conduct a comprehensive evaluation not only of risk factors and cardiovascular biomarkers, but also of prognosis after HFmrEF onset. To do so, we leveraged an international collaboration of 4 large community-based cohorts with prospective ascertainment of over 2,500 incident HF events, which were classified into three HF subtypes.10
METHODS
Study Sample
We included participant-level data from four prospective, observational community-based cohorts with prospectively adjudicated HF outcomes.10 For the present investigation, participants attending the following baseline examinations were included: Framingham Heart Study (FHS) original cohort exam 16 (1979–1982) or 24 (1995–1998), FHS offspring cohort exams 2 (1979–1983) or 6 (1995–1998), Cardiovascular Health Study (CHS) exam 1 (1989–1990; 1992–1993 for supplemental African-American cohort), Prevention of Renal and Vascular Endstage Disease (PREVEND) exam 1 (1997–1998), and Multi-Ethnic Study of Atherosclerosis (MESA) exam 1 (2000–2002). From this sample, we excluded participants with prevalent HF (n=472), age <30 years at baseline examination due to extremely low likelihood of developing HF (n=379), and those with missing covariates (n=2177), leaving 28,820 individuals available for the primary analysis.
Clinical Assessment
Participants underwent a detailed medical history, physical examination, fasting blood draw with subsequent laboratory assessment, and electrocardiography. Variables were harmonized across cohorts whenever possible.10 Blood pressure was taken as the average of two seated measurements. Body mass index was calculated as weight divided by height2 and expressed as kg/m2. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or the use of hypoglycemic medications. Electrocardiographic left ventricular (LV) hypertrophy was defined based on accepted voltage and ST-segment criteria, as described previously.10 Prior history of coronary heart disease was ascertained systematically in each parent cohort using a combination of self-report, ECG, review of all available prior medical records, and physician contact.10 Estimated glomerular filtration rate (eGFR) was calculated using baseline creatinine concentrations.11
Laboratory Assessment
Biomarkers were assessed within each cohort, with details summarized in Supplemental Table 1. The following biomarkers were available in at least 2 cohorts and were included in this analysis: natriuretic peptides, high-sensitivity troponin, C-reactive protein, urinary albumin to creatinine ratio (UACR), D-dimer, fibrinogen, soluble ST2, cystatin C, galectin-3, and interleukin-6 (IL6), with the range in coefficients of variation between 2.3 and 12.2%. B-type natriuretic peptide (BNP) was measured in FHS, and its amino terminal cleavage equivalent (NT-proBNP) in the other cohorts. Similarly, high-sensitivity troponin I (hs-TnI) was measured in FHS, and hs-TnT in the remaining cohorts.
Definition of Incident HF Subtypes
Incident HF was prospectively ascertained and adjudicated using established protocols by study investigators within each cohort after review of all available outpatient and hospital records. We reviewed imaging reports at or near the HF onset date to abstract LVEF (92% within 30 days of HF onset), with the majority of LVEF assessments ascertained via echocardiography (> 88% of cases). HF was defined using a combination of signs, symptoms, and/or treatment, as described (5). Each first incident HF event was categorized as HFpEF (LVEF ≥50%), HFmrEF (40% < LVEF <50%), HFrEF (LVEF ≤40%), or unclassified (no LV function assessment available).
Statistical Analysis
Individual-level data were harmonized and pooled for all four cohorts – FHS, PREVEND, CHS, and MESA. Baseline clinical characteristics were summarized by incident HF subtype - HFpEF, HFmrEF, HFrEF, unclassified HF, and no HF. A one-way analysis of variance (ANOVA) was calculated for each baseline characteristic to detect differences amongst HFpEF, HFmrEF, and HFrEF.
We calculated directly standardized incidence rates (sex and age-adjusted with 10-year age strata) of HFpEF, HFmrEF, and HFrEF. Cumulative incidence rates of the three HF subtypes were estimated using a Kaplan-Meier-like method accounting for competing risks of death, other HF subtypes, and unclassified HF. We also examined age- and sex-standardized incidence rates of all-cause mortality after HF onset. A Kaplan-Meier curve was generated for survival after onset of HF and group log-rank and pairwise p-values were estimated. To examine the association of clinical predictors with HF subtype, cause-specific Cox models were fitted separately for HFpEF, HFmrEF, and HFrEF. We accounted for multiple competing risks as above. Covariates known to be associated with HF were entered in the multivariable model 10, including age, sex, race, systolic blood pressure, hypertension treatment, body-mass index, diabetes mellitus, smoking status, and previous myocardial infarction. In secondary analysis, previous myocardial infarction was replaced with previous coronary heart disease. A strata statement was included to specify study cohorts within the pooled analysis.
Cause-specific Cox models were then fitted for each biomarker in each HF subtype separately, after adjusting for the previously mentioned clinical covariates. Cause-specific hazard ratios were calculated per 1-SD increase in each natural log-transformed biomarker. In secondary analyses, we examined whether clinical covariates and biomarkers were associated differentially with risk of HFpEF versus HFmrEF and HFrEF versus HFmrEF. We took all covariates and biomarker models, and compared subtype-specific coefficients using the Lunn-McNeil method.12 All statistical analyses were conducted with SAS version 9.4 for Windows (Cary, NC).
RESULTS
A total of 28,820 participants (mean age of 60±14 years, 54 % women) from four community-based longitudinal cohorts were included in this sample. Over a mean follow-up of 12±4 years, a total of 2749 participants developed incident HF with an average of 11±4 years to HF. A total of 2059 (75%) had LV function assessment at or around the time of HF, permitting subtype classification. Among participants with classified new-onset HF, 811 (39%) had HFpEF, 200 (10%) had HFmrEF, and 1048 (51%) had HFrEF.
Baseline clinical characteristics preceding incident HF are presented by HF subtype in Table 1. Of participants who developed HF, more women were classified as HFpEF vs. HFrEF (59% vs 36%), and the proportion of women among participants developing HFmrEF was intermediate (48%). With respect to clinical risk factors, participants with future HFmrEF shared some baseline similarities with the HFrEF group, including lower BMI’s than HFpEF (27.8 kg/m2 in HFmrEF, 27.9 kg/m2 in HFrEF vs 28.6 kg/m2 in HFpEF), with lower prevalence of obesity (26% in HFmrEF, 29% in HFrEF, 33% in HFpEF), higher prevalence of coronary heart disease than HFpEF (24% in HFmrEF, 25% in HFrEF, and 16% in HFpEF) and lower HDL cholesterol (Table 1). Other clinical characteristics of participants with future HFmrEF were intermediate between those with future HFpEF and HFrEF.
Table 1.
Baseline characteristics preceding incident clinical outcomes by HF subtype
| Incident HF | P ANOVA | No HF | Unclassified HF | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HFpEF | HFmrEF | HFrEF | ||||
| N=811 | N=200 | N=1048 | N=26071 | N=690 | ||
| Demographics | ||||||
| Age, years | 71 (9) | 72 (8)† | 70 (10) | 0.0003 | 58 (14) | 75 (7) |
| Women, n (%) | 477 (59) | 95 (48)*† | 379 (36) | <0.0001 | 14151 (54) | 366 (53) |
| Race | 0.15 | |||||
| White, n (%) | 692 (85) | 175 (88) | 917 (88) | 21160 (81) | 584 (85) | |
| Black, n (%) | 84 (10) | 21 (11) | 92 (9) | 2394 (9) | 90 (13) | |
| Other, n (%) | 34 (4) | 4 (2) | 37 (4) | 2465 (9) | 16 (2) | |
|
| ||||||
| Clinical covariates | ||||||
| Systolic blood pressure, mmHg | 142 (22) | 142 (22) | 142 (22) | 0.99 | 129 (20) | 142 (22) |
| Diastolic blood pressure, mmHg | 73 (11) | 72 (11)† | 75 (12) | 0.0001 | 74 (10) | 71 (12) |
| Hypertension treatment, n (%) | 435 (54) | 106 (53) | 550 (52) | 0.88 | 6846 (26) | 412 (60) |
| Heart rate, bpm | 68 (11) | 68 (11) | 68 (12) | 0.87 | 67 (11) | 69 (12) |
| Body mass index, kg/m2 | 28.6 (5.5) | 27.8 (4.6)* | 27.9 (4.7) | 0.002 | 26.8 (4.8) | 27.4 (5.2) |
| Diabetes mellitus, n (%) | 156 (19) | 39 (20) | 232 (22) | 0.30 | 1980 (8) | 158 (23) |
| Diabetes medications, n (%) | 97 (12) | 29 (15) | 123 (12) | 0.54 | 1149 (4) | 93 (14) |
| Current smoker, n (%) | 108 (13) | 27 (14) | 206 (20) | 0.0007 | 5800 (22) | 91 (13) |
| Modest alcohol use, n (%) | 130 (16) | 45 (23) | 215 (21) | 0.03 | 6009 (23) | 98 (14) |
| Myocardial infarction, n (%) | 66 (8) | 22 (11) | 184 (18) | <0.0001 | 779 (3) | 87 (13) |
| Coronary heart disease, n (%) | 131 (16) | 47 (24)* | 260 (25) | <0.0001 | 1341 (5) | 190 (28) |
| Cerebrovascular disease, n (%) | 32 (4) | 10 (5) | 71 (7) | 0.03 | 370 (1) | 33 (5) |
| Hyperlipidemia treatment, n (%) | 76 (9) | 16 (8) | 100 (10) | 0.90 | 1897 (7) | 53 (8) |
|
| ||||||
| Laboratory covariates | ||||||
| Total cholesterol | 211 (40) | 209 (38) | 209 (45) | 0.63 | 209 (41) | 209 (39) |
| HDL cholesterol | 50 (15) | 47 (14)* | 47 (14) | <0.0001 | 52 (16) | 51 (16) |
| eGFR, mL/min/1.73m2 | 67 (19) | 63 (19)* | 66 (20) | 0.048 | 76 (18) | 63 (18) |
|
| ||||||
| ECG covariates | ||||||
| Atrial fibrillation, n (%) | 43 (5) | 19 (10) | 61 (6) | 0.07 | 274 (1) | 45 (7) |
| Left ventricular hypertrophy, n (%) | 46 (6) | 13 (7) | 99 (9) | 0.006 | 642 (2) | 56(8) |
| Left bundle branch block, n (%) | 16 (2) | 5 (3) | 59 (6) | 0.0002 | 156 (0.6) | 15 (2) |
P for ANOVA denotes testing for between-group differences among incident HF subtypes (HFpEF, HFmrEF, HFpEF)
P<0.05 for HFmrEF vs HFpEF,
P<0.05 for HFmrEF vs HFrEF
Incidence rates of new-onset HF by subtype
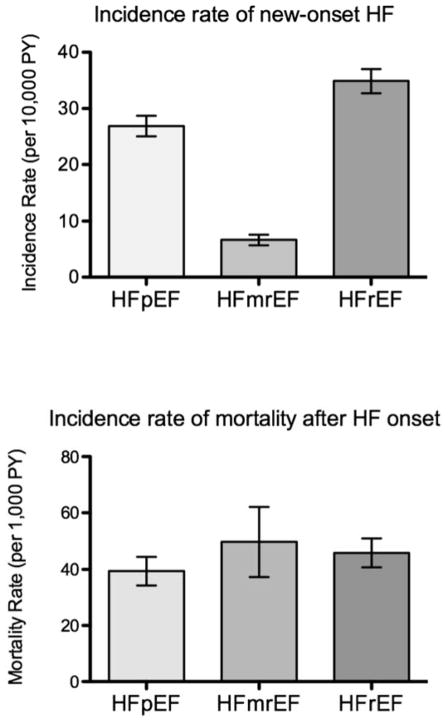
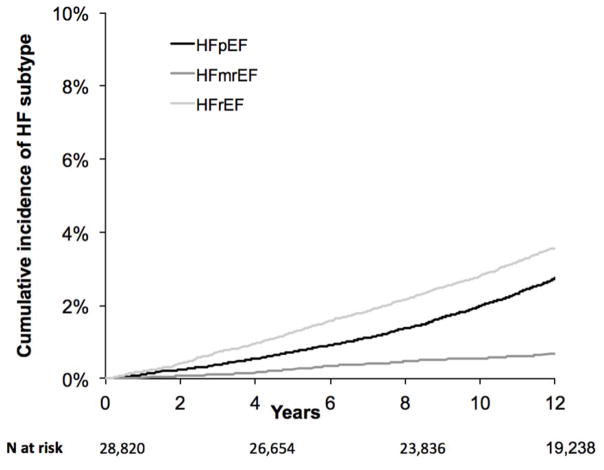
Age- and sex-standardized incidence rates by HF subtype are summarized in Figure 1 and Supplemental Table 1, and demonstrate an incidence rate of 6.7 cases per 10,000 person-years for HFmrEF. Corresponding rates for HFpEF and HFrEF were 26.9 and 34.9 cases per 10,000 person-years. Cumulative incidence plots by HF subtype are shown in Figure 2.
Figure 1.
Incidence rates of new-onset HF (A) and mortality after HF onset (B) for HF subtypes.
Figure 2.
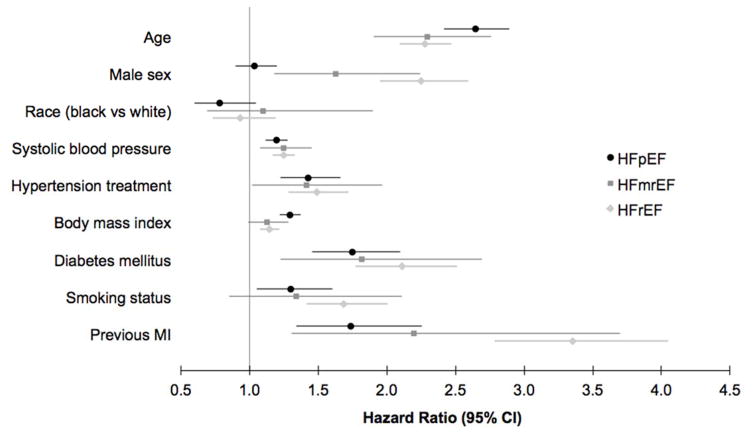
Clinical predictors of HF subtype. Incident HF outcomes are denoted by colors, with black representing HFpEF, medium gray representing HFmrEF, and light gray representing HFrEF. Point estimate represents multivariable-adjusted hazard ratio (for the presence vs absence of dichotomous traits, and per 10 year increase in age, and per 4 kg/m2 increase in body mass index), and whiskers denote 95% confidence intervals.
Clinical predictors of incident HFmrEF
In multivariable-adjusted analyses, older age, male sex, higher systolic blood pressure, hypertension treatment, diabetes mellitus, and prior myocardial infarction predicted incident HFmrEF (P<0.05 for all, Table 2). The effect of clinical predictors on risk of future HFpEF, HFmrEF, and HFrEF are summarized in Figure 3. In secondary analyses, we examined prevalent coronary heart disease in place of previous myocardial infarction, and found an independent association with HFpEF (HR 1.45, 95% CI 1.19–1.77), HFmrEF (HR 2.04, 95% CI 1.39–3.02), and HFrEF (HR 2.42, 95% CI 2.04–2.87). When added to the multivariable model, interim myocardial infarction had a nearly three-fold increased hazard for HFrEF, over two-fold increased hazard of HFmrEF, and 34% increased hazard of HFpEF (HR 2.91, 95% CI 2.37–3.57 for HFrEF, HR 2.23, 95% CI 1.36–3.65 for HFmEF, and HR 1.34, 95% CI 1.02–1.77 for HFpEF). The median time between interim MI and HF onset was 0.7 years (25th percentile 0.02 years, 75th percentile 3.4 years).
Table 2.
Multivariable-adjusted clinical predictors of incident HFmrEF and other HF subtypes
| HfpEF N=811 |
HFmrEF N=200 |
HFrEF N=1048 |
P for equality | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | All groups | HFmEF vs HFpEF | HFmEF vs HFrEF | |
| Clinical covariates | |||||||||
| Age (per 10 years) | 2.65 | 2.42, 2.89 | 2.29 | 1.91, 2.76 | 2.28 | 2.09, 2.47 | 0.03 | 0.12 | 0.93 |
| Men | 1.03 | 0.89, 1.20 | 1.63 | 1.18, 2.24 | 2.25 | 1.95, 2.59 | <0.0001 | 0.005 | 0.046 |
| Race | 0.78 | 0.60, 1.00 | 1.14 | 0.69, 1.89 | 0.93 | 0.73, 1.19 | 0.81 | ||
| Systolic BP (per 20mmHg) | 1.20 | 1.12, 1.28 | 1.25 | 1.07, 1.45 | 1.25 | 1.17, 1.33 | 0.66 | ||
| Hypertension treatment | 1.49 | 1.24, 1.79 | 1.41 | 1.02, 1.95 | 1.49 | 1.29, 1.72 | 0.91 | ||
| Body-mass index (per 4 kg/m2) | 1.30 | 1.23, 1.38 | 1.12 | 0.99, 1.28 | 1.14 | 1.07, 1.21 | 0.003 | 0.03 | 0.86 |
| Diabetes mellitus | 1.75 | 1.46, 2.11 | 1.81 | 1.22, 2.68 | 2.10 | 1.77, 2.50 | 0.31 | ||
| Smoking status | 1.31 | 1.07, 1.62 | 1.33 | 0.85, 2.09 | 1.68 | 1.41, 1.99 | 0.16 | ||
| Previous myocardial infarction | 1.74 | 1.34, 2.26 | 2.20 | 1.31, 3.71 | 3.36 | 2.78, 4.05 | 0.0001 | 0.39 | 0.10 |
| eGFR (per 30 mL/min/1.73m2) | 1.02 | 0.85, 1.21 | 0.71 | 0.48, 1.04 | 0.84 | 0.71, 0.99 | 0.11 | ||
|
| |||||||||
| Biomarkers | |||||||||
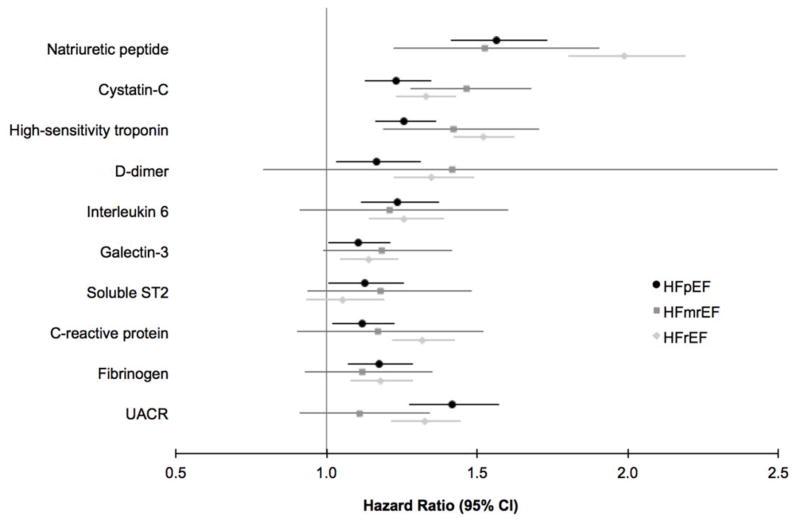
| Natriuretic peptide | 1.56 | 1.41, 1.73 | 1.51 | 1.20, 1.90 | 2.00 | 1.81, 2.20 | 0.0003 | 0.68 | 0.01 |
| Cystatin-C | 1.23 | 1.12, 1.35 | 1.49 | 1.30, 1.70 | 1.33 | 1.24, 1.43 | 0.10 | ||
| High-sensitivity troponin | 1.26 | 1.16, 1.37 | 1.41 | 1.17, 1.70 | 1.52 | 1.43, 1.63 | 0.003 | 0.24 | 0.40 |
| D-dimer | 1.18 | 1.05, 1.34 | 1.21 | 0.91, 1.62 | 1.34 | 1.21, 1.48 | 0.30 | ||
| IL 6 | 1.24 | 1.12, 1.38 | 1.19 | 1.00, 1.42 | 1.25 | 1.13, 1.38 | 0.92 | ||
| Galectin-3 | 1.10 | 1.01, 1.21 | 1.18 | 0.93, 1.48 | 1.14 | 1.05, 1.24 | 0.82 | ||
| Soluble ST2 | 1.13 | 1.01, 1.26 | 1.18 | 0.90, 1.53 | 1.05 | 0.93, 1.19 | 0.58 | ||
| C-reactive protein | 1.12 | 1.02, 1.23 | 1.12 | 0.93, 1.35 | 1.31 | 1.21, 1.42 | 0.02 | 0.95 | 0.10 |
| Fibrinogen | 1.18 | 1.08, 1.30 | 1.11 | 0.91, 1.34 | 1.17 | 1.07, 1.28 | 0.83 | ||
| Urinary albumin to creatinine ratio | 1.42 | 1.28, 1.58 | 1.00 | 0.74, 1.35 | 1.32 | 1.21, 1.48 | 0.06 | 0.03 | 0.06 |
HR represent hazard ratios of HF subtype associated with the presence vs absence of a dichotomous variable, or per increment in continuous variable as denoted in the table. HR for race is comparison of black vs white race. Multivariable-adjusted models include age, sex, race, systolic blood pressure, hypertension treatment, body-mass index, diabetes mellitus, smoking status, previous myocardial infarction. Biomarker models include all clinical covariates plus individual biomarkers.
P for difference between HFmEF vs HFpEF and HFmEF vs HFrEF listed if P for equality between all groups < 0.10.
Figure 3.
Biomarker predictors of HF subtype. Incident HF outcomes are denoted by colors, with black representing HFpEF, medium gray representing HFmrEF, and light gray representing HFrEF. Point estimate represents multivariable-adjusted hazard ratio (per 1-standard deviation increase in log-transformed biomarker), and whiskers denote 95% confidence intervals.
We tested whether a given clinical predictor had differential effects on risk of HFmrEF, HFpEF, and HFrEF using the Lunn-McNeil method (Table 2).12 The impact of male sex on risk of HFmrEF (HR 1.63, 95% CI 1.18–2.24) was significantly different compared both with HFpEF (HR 1.03, 95% CI 0.89–1.20) and HFrEF (HR 2.25, 95% CI 1.95–2.59, P=0.005 for HFmrEF vs HFpEF and P=0.046 for HFmrEF vs HFrEF comparisons). We also observed a stronger association of BMI with HFpEF than HFmrEF (HR 1.30, 95% CI 1.23–1.38 for HFpEF than HR 1.12, 95% CI 0.99–1.28 for HFmrEF, P=0.03 for comparison). By contrast, the association of BMI with HFmrEF was similar to that with HFrEF (P=0.86 for comparison).
Biomarker predictors of incident HFmrEF
The associations of individual biomarker analyses (adjusting for clinical variables) with incident HFpEF, HFmrEF, and HFrEF are summarized in Table 2 and Figure 4. Biomarker predictors of HFmrEF included natriuretic peptides, with each 1-standard deviation increase in log-transformed natriuretic peptide associated with a 1.5-fold increased hazard of HFmrEF (HR 1.51, 95% CI 1.20–1.90). Similarly, higher cystatin-C and hs-troponin were associated with higher risk of HFmrEF (HR 1.49, 95% CI 1.30–1.70, and HR 1.41, 95% CI 1.17–1.70, respectively). In analyses adjusting for both clinical variables and natriuretic peptides, hs-troponin and cystatin-C remained significant predictors of HFmrEF (HR 1.34, 95% CI 1.02–1.56, and HR 1.36, 95% CI 1.15–1.60, respectively).
Figure 4.
Cumulative incidence of HF subtype. Incident HF outcomes are denoted by colors, with black representing HFpEF, medium gray representing HFmrEF, and light gray representing HFrEF.
We directly compared the effect of a single biomarker on HFmrEF, HFpEF, and HFrEF to examine whether differential effects exist (Table 2). We found that natriuretic peptides had similar effects on risk of HFmrEF and HFpEF, whereas the risk of HFrEF associated with a 1-standard deviation change in biomarker was greater than for HFmrEF (HR 2.00, 95% CI 1.81–2.20 versus HR 1.51, 95% CI 1.20–1.90, P=0.01 for comparison).
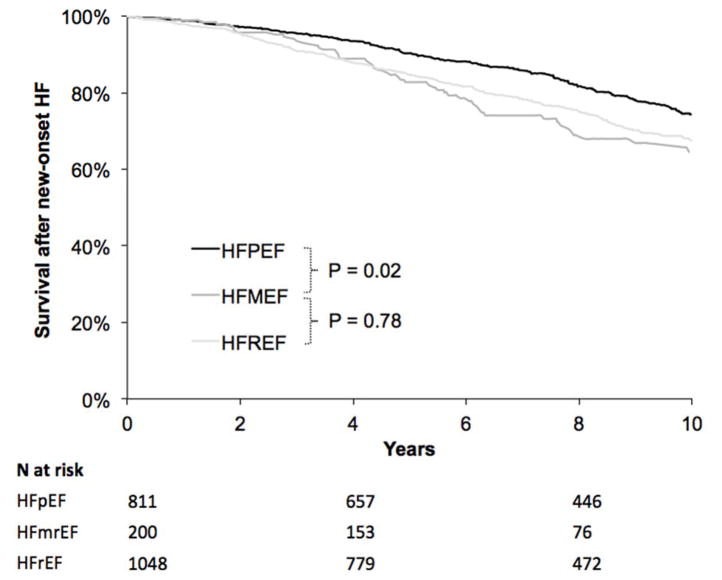
All-cause mortality rates after HF onset
After the onset of HF, there were 32 deaths among 200 participants with HFmrEF, 231 deaths among 811with HFpEF, and 312 deaths among 1048 with HFrEF. The all-cause mortality rate was 497 events per 10,000 person years among participants with HFmrEF, 394 per 10,000 person-years in those with HFpEF, and 459 per 10,000 person years in those with HFrEF. As shown in the survival curves in Figure 5, survival was lower among participants with HFmrEF than in those with HFpEF (log-rank P=0.02) and similar to those with HFrEF (log-rank P=0.78).
Figure 5.
Survival after new HF onset by HF subtype. Incident HF outcomes are denoted by colors, with black representing HFpEF, medium gray representing HFmrEF, and light gray representing HFrEF. P-value is pairwise log-rank test as indicated.
DISCUSSION
We examined clinical and biochemical predictors of new-onset HFmrEF, and outcomes after diagnosis of HFmrEF within the context of a unique international collaboration of four large community-based cohorts. Our principal findings were as follows: (1) clinical predictors are shared among HF subtypes, with a few notable differences; (2) biochemical predictors of HFmrEF include natriuretic peptides, cystatin-C, and high-sensitivity troponin; and (3) all-cause mortality after new-onset HF is similar among those classified as HFmrEF and HFrEF, but worse than in those classified as HFpEF.
Previous studies have noted similarities among clinical profiles of patients with HFmrEF and HFpEF, including older age, and higher prevalences of hypertension, atrial fibrillation, and diabetes mellitus.4,7,13,14 The consistent exception is a higher frequency of coronary artery disease among those with HFmrEF compared with HFpEF.4,6,7,14 We now extend previous observations to examine predictors of new onset HF. Our findings demonstrate that age, sex, blood pressure, diabetes mellitus, and previous myocardial infarction all predict incident HFmrEF. When comparing the effect of a given clinical covariate on the risk of HFmrEF versus other HF subtypes, we note that men had a risk of HFmrEF, which was lower than risk of HFrEF, but more pronounced than the risk of future HFpEF. Further, BMI was more strongly related to HFpEF than HFmrEF or HFrEF.
Biochemical profiles of patients with HFmrEF have demonstrated natriuretic peptide concentrations that are largely intermediate between those with HFrEF, who have the highest neurohormonal activation, and the group with HFpEF with lowest natriuretic peptide levels.13,14 Our study demonstrates that natriuretic peptide concentrations among generally healthy adults help predict future risk of HFmrEF. Interestingly, the magnitude of the risk estimate for natriuretic peptides was similar for HFmrEF and HFpEF, and greatest for HFrEF. By contrast, we find that cystatin C and high-sensitivity troponin predict HFmrEF with similar effect sizes as HFpEF and HFrEF. We find that eGFR is not associated with future HFpEF or HFmrEF, with a borderline association for HFrEF. The difference between cystatin C and creatinine-based eGFR is consistent with prior studies demonstrating greater sensitivity of cystatin C as a marker for future risk of adverse outcomes.15
Among patients with existing HF enrolled in cross-sectional registries or clinical trials, the prevalence of HFmrEF has ranged between 13–24%.4,6–8,13 We now estimate incidence rates in an inception cohort, which suggest that the incidence rate of HFmrEF is about a tenth of total HF. Data on outcomes for patients with HFmrEF have been discrepant, with some studies showing a clear association of lower LVEF with worse outcomes, including a recent analysis of the TOPCAT trial demonstrating lower survival among those with LVEF 44–50% than those with LVEF > 50%.9,14,16,17 Other studies have shown no significant differences in mortality among HF subtypes parsed by LVEF.7,8,13,18 Certainly, among population-based cohorts in the absence of HF, an asymptomatically reduced LVEF in the same mid-range of 40–50% bears a worse prognosis than normal LVEF,19,20 which appears to extend even into the 50–55% LVEF range.21 One important note is that, unlike our study, no prior studies were inception cohorts, which may have contributed to mixed results. Among participants with new-onset HF in the community, we found that those with incident HFmrEF have similarly poor survival to those with incident HFrEF, and slightly better survival than those with incident HFpEF.
Our study had a number of limitations. While our findings show that HFmrEF shares antecedent clinical and biomarker predictors with HFpEF (BMI and natriuretic peptides), as well as HFrEF (coronary artery disease), and a clinical course similar to HFrEF, we were not able to ascertain whether HFmrEF is a phenotype in transition,22 given lack of serial LVEF data after HF onset. A previous study in patients with HFmrEF undergoing exercise testing shows a favorable prognosis among those with previously low LVEF.5 This highlights the importance of understanding LVEF longitudinally among patients with HF, as LVEF is known to be dynamic over time, with longitudinal increases in LVEF among those with HFrEF, and decreases in LVEF among those with HFpEF.23 HF subtypes were classified based on LV function assessment performed as part of clinical care at the time of HF presentation, thus echocardiographic imaging was not standardized, and the narrow range of LVEF defining HFmrEF may have resulted in misclassification. This also left 27% of cases as unclassified, which may have led to differential bias. Participants under age 30 and those with missing key covariates were excluded, resulting in potential bias. Clinical information after HF onset was limited, including the use of HF-specific therapies and devices potentially influencing mortality analyses after HF onset. Lastly, we were not able to determine the exact pathogenesis of HF.
In summary, we found overlap in clinical and biochemical predictors of incident HFmrEF with other HF subtypes. Age, male sex, blood pressure, diabetes mellitus, and previous myocardial infarction predicted HFmrEF, as did natriuretic peptides, cystatin-C, and high-sensitivity troponin. Despite shared features, we found a few notable differences – higher BMI was a predictor of HFpEF but not HFmrEF, and natriuretic peptides were stronger predictors of HFrEF than of HFmrEF. While predictors of HFmrEF had some shared features with HFpEF vs HFrEF, all-cause mortality after new-onset HF was worse for HFmrEF than HFpEF, but similar to HFrEF. The fact that outcomes after HFmrEF mirror those after HFrEF suggests that HFmrEF may be more akin to HFrEF with respect to clinical course. This raises the question of whether potential therapies thus far reserved for patients with HFrEF may be of benefit in those with intermediate LVEF.
Supplementary Material
Acknowledgments
Funding sources: This work was partially supported by the National Heart, Lung and Blood Institute, including the Framingham Heart Study (contract N01-HC25195 and HHSN268201500001I), the Cardiovascular Health Study (HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org). MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. Funding support for the MESA Renal Function dataset was provided by grant DK083538-01. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study has been made possible by grants from the Dutch Kidney Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. de Boer is supported by the Netherlands Heart Foundation (CVON-DOSIS, grant 2014-40) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350, to Dr. de Boer). Dr. Nayor received support from the Clinical Skills Development Core Training Grant U10HL110337 from the NHLBI. Dr. Ho is supported by K23-HL116780 and a Hassenfeld Research Scholar Award (Massachusetts General Hospital, Boston, MA). Dr. Lee is supported by a mid-career award from the Heart and Stroke Foundation of Canada, and is the Ted Rogers Chair in Heart Function Outcomes. In FHS samples, measurement of sST2 was performed by Critical Diagnostics, Inc, and measurement of hsTnI was performed by Singulex, Inc. Dr. Ho had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Psaty serves on a DSMB for a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. Dr. DeFilippi has received research support from Roche Diagnostics, consulting fees from Roche, Siemens Healthcare Diagnostics, Alere, Metanomics, and Ortho Diagnostics, has served on the endpoint committee for Radiometer and Quintiles, and has received royalties from UpToDate. Dr. Januzzi has received research grant funding from Roche Diagnostics and Critical Diagnostics, and has served on the advisory board for Critical Diagnostics.
References
- 1.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular …. Journal of the American College of \ldots. 2013 doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-327–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Heart Fail. 2016;4:464–472. doi: 10.1016/j.jchf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Nadruz W, West E, Santos M, Skali H, Groarke JD, Forman DE, Shah AM. Heart Failure and Midrange Ejection Fraction: Implications of Recovered Ejection Fraction for Exercise Tolerance and Outcomes. Circ Heart Fail. 2016;9:e002826. doi: 10.1161/CIRCHEARTFAILURE.115.002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced ( Am J Cardiol. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, Oconnor CM, Sun JL, Yancy CW, Young JB OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 8.Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721–730. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O’Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–462. doi: 10.1093/eurheartj/ehv464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016:9. doi: 10.1161/CIRCHEARTFAILURE.115.003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 13.Delepaul B, Robin G, Delmas C, Moine T, Blanc A, Fournier P, Roger-Rollé A, Domain G, Delon C, Uzan C, Boudjellil R, Carrié D, Roncalli J, Galinier M, Lairez O. Who are patients classified within the new terminology of heart failure from the 2016 ESC guidelines? ESC Heart Fail. 2017;4:99–104. doi: 10.1002/ehf2.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toma M, Ezekowitz JA, Bakal JA, Oconnor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF Trial. Eur J Heart Fail. 2014;16:334–341. doi: 10.1002/ejhf.19. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG, Matsushita K, Arnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA Candesartan in Heart Failure Reduction in Mortality CHARM Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 17.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 18.Rickenbacher P, Kaufmann BA, Maeder MT, Bernheim A, Goetschalckx K, Pfister O, Pfisterer M, Brunner-La Rocca H-P TIME-CHF Investigators. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) Eur J Heart Fail. 2017 doi: 10.1002/ejhf.798. [DOI] [PubMed] [Google Scholar]
- 19.Pandhi J, Gottdiener JS, Bartz TM, Kop WJ, Mehra MR. Comparison of characteristics and outcomes of asymptomatic versus symptomatic left ventricular dysfunction in subjects 65 years old or older (from the Cardiovascular Health Study) Am J Cardiol. 2011;107:1667–1674. doi: 10.1016/j.amjcard.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 21.Tsao CW, Lyass A, Larson MG, Cheng S, Lam CSP, Aragam JR, Benjamin EJ, Vasan RS. Prognosis of Adults With Borderline Left Ventricular Ejection Fraction. JACC Heart Fail. 2016;4:502–510. doi: 10.1016/j.jchf.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam CSP, Solomon SD. Fussing Over the Middle Child: Heart Failure With Mid-Range Ejection Fraction. Circulation. 2017;135:1279–1280. doi: 10.1161/CIRCULATIONAHA.117.027324. [DOI] [PubMed] [Google Scholar]
- 23.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.