Abstract
An understanding of ion–protein interactions is key to a better understanding of the molecular mechanisms of proteins, such as enzymes, ion channels, and ion pumps. A potassium ion channel, KcsA, has been extensively studied in terms of ion selectivity. Alkali metal cations in the selectivity filter were visualized by X-ray crystallography. Infrared spectroscopy has an intrinsically higher structural sensitivity due to frequency changes in molecular vibrations interacting with different ions. In this review article, I attempt to summarize ion-exchange-induced differences in Fourier transform infrared spectroscopy, as applied to KcsA, to explain how this method can be utilized to study ion–protein interactions in the KcsA selectivity filter. A band at 1680 cm−1 in the amide I region would be a marker band for the ion occupancy of K+, Rb+, and Cs+ in the filter. The band at 1627 cm−1 observed in both Na+ and Li+ conditions suggests that the selectivity filter similarly interacts with these ions. In addition to the structural information, the results show that the titration of K+ ions provides quantitative information on the ion affinity of the selectivity filter.
Keywords: Infrared spectroscopy, Membrane proteins, Ion–protein interactions, Ion channel
Introduction
A biological membrane is basically composed of a lipid bilayer which acts as an insulator for various kinds of electrically charged materials, such as Na+, K+, and Cl−ions. Membrane proteins embedded in the lipid bilayer are important for the permeation or transportation of these ions. Channel proteins form a pore that is permeable to specific ion types, with a gate that controls the current. Ion pumps and transporters possess ion binding sites, and their affinities are smoothly controlled by coupling with the conformational changes that are induced by some input of energy.
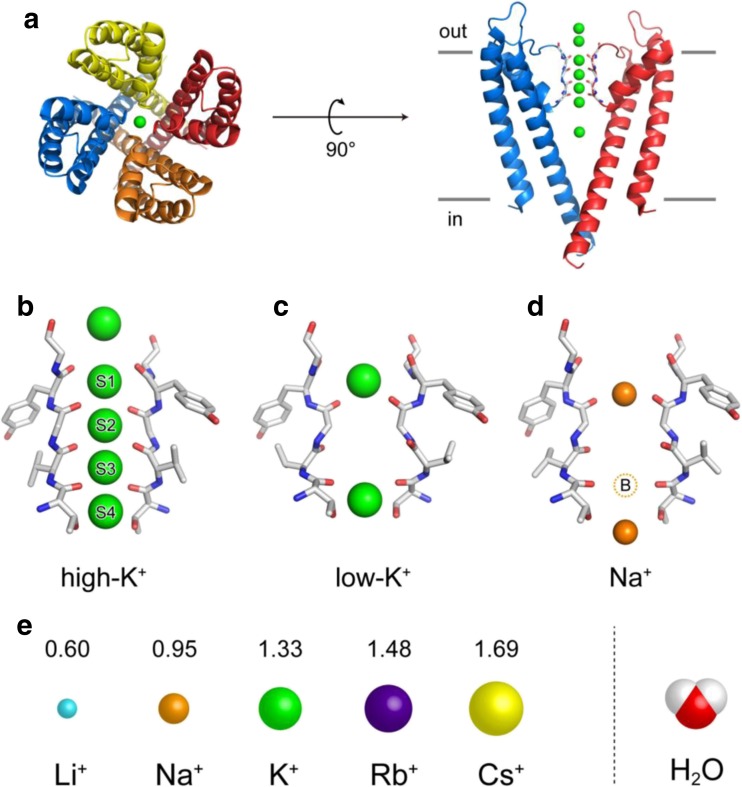
Ion selectivity is a key factor that characterizes ion channels and pumps. For example, K+ channels are selectively permeable to K+ over Na+ ions, typically at a ratio of 1000:1 (Heginbotham and MacKinnon 1993; LeMasurier et al. 2001). Typical K+ channels form tetrameric structures that surround the central pore (Fig. 1a). The high selectivity to K+ is realized by the distinctive backbone structure formed in the pore, where the TVGYG sequence is highly conserved in K+ channels; this structure is known as the “selectivity filter”. The C = O groups of the main chains at the selectivity filter point inwards and are optimally arranged to accommodate K+ ions (Fig. 1b, c) (Doyle et al. 1998; Zhou et al. 2001). Therefore, the dehydration of K+ upon entering the filter is almost an energetically barrierless process. In contrast, Na+ is not stably coordinated by the C = O groups in the filter without conformational changes. The collapse of the filter structure has been captured as an X-ray crystal structure image (Fig. 1d) (Lockless et al. 2007). The collapse was also induced by a low concentration of K+ ions, which means that K+ alone can stabilize the filter structure. In principle, it is accepted that the selective filter structure is important for explaining high K+ selectivity.
Fig. 1.
X-ray crystal structure of KcsA. a Top (left) and side (right) view of the entire KcsA, a K+ ion channel, transmembrane region (PDB: 1K4C). Four monomers are colored in red, yellow, blue, and orange, respectively. The green spheres represent K+ ions in the pore. b–d The selectivity filter region (TVGYG) was excised from two monomers with K+ ions (b high concentration, c low concentration) or Na+ ions (d). PDB codes of the structural models in b, c, and d: 1K4C, 1K4D, and 2ITC, respectively. e Schematic illustrations of ionic radii of alkali metal cations (Li+, Na+, K+, Rb+, and Cs+) scaled against a water molecule [the values are ångströms (Å) obtained from the Pauling estimation; Hille 2001] and van der Waals radii for H and O
Perfusion-induced difference attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy was first applied to study the structural changes of an acetylcholine receptor upon ligand binding (Baenziger et al. 1992). The method has been well reviewed by several groups (Lorenz-Fonfria et al. 2012; Nyquist et al. 2001; Rich and Iwaki 2007). Here it can be confirmed that the method is also applicable to a K+ ion channel and is a potential tool for studying ion selectivity among alkali metal cations.
Ion-exchange-induced difference ATR-FTIR spectrum measured upon replacement of Na+ with K+
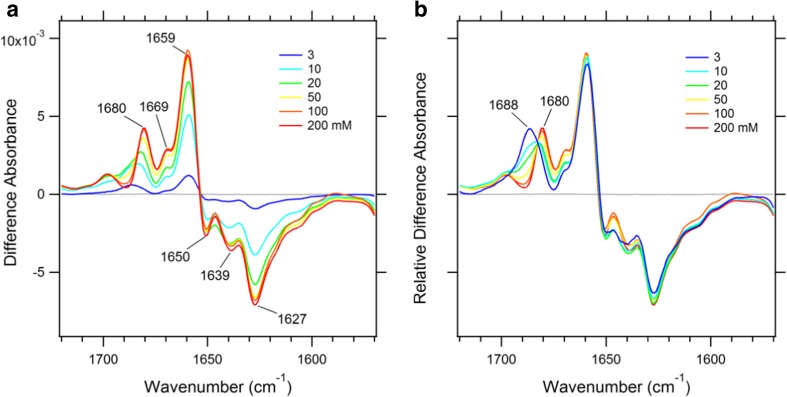
In this section I briefly explain how to measure ion-exchange-induced difference ATR-FTIR spectra; for more details on the precise experimental method, the reader is referred to the original papers (Furutani et al. 2012, 2015). KcsA protein reconstituted into liposomes [asolectin at a protein:lipid molar ratio of 1:50 or POPE/POPG (w/w = 3:1) at 1:100 was used] was deposited onto a diamond internal reflection element [nine reflections; the sample area is ~ 13 mm2 (ϕ = ~ 4 mm)] for the ATR measurement. The sample was then immersed in an appropriate buffer for rehydration and washing. Two buffer solutions, both containing the same concentration of KCl or NaCl (200 mM) and buffered at pH 7, were prepared to measure the ion-exchange-induced difference spectrum. An absorption spectrum in NaCl buffer was collected and the solution then exchanged with a KCl buffer. After completion of the buffer exchange, another absorption spectrum in KCl was collected. These processes were repeated to accumulate the absorption spectra in two buffer solutions. The sampling temperature was kept constant at 20 °C during the measurements. Finally, the K+ − Na+ spectrum difference between adjacent buffer conditions was calculated and averaged. The spectrum difference in the amide I region is shown in Fig. 2. The red line is the K+ − Na+ spectrum difference at 200 mM. Three positive peaks at 1680, 1669, and 1659 cm−1 and three negative peaks at 1650, 1639, and 1627 cm−1 were clearly observed in the difference spectrum; these were assigned to carbonyl groups located close to the selectivity filter by a mutation analysis (Furutani et al. 2012) and a computational study (Stevenson et al. 2015). Among these, 1680 cm−1 was assigned to the S2 and S3 modes of the selectivity filter (Stevenson et al. 2015). The bands at 1659 and 1650 cm−1 were assigned to the pore helix mode (Stevenson et al. 2015), and the band at 1627 cm−1 was assigned to the B-site mode (Stevenson et al. 2015).
Fig. 2.
Difference infrared spectra of KcsA in the amide I region. a Ion-exchange-induced spectra differences (K+ − Na+) with different concentrations of K+. b The difference spectra were scaled for similar intensities at 1659, 1650, 1639, and 1627 cm−1. The positive sides measured different K+ and Na+ ratios at a total concentration of 200 mM (K+ = X mM, Na+ = 200 − X mM, where X = 3, 10, 20, 50, 100, and 200 mM). The negative sides always measured a 200 mM Na+ concentration
Infrared spectroscopy is usually used to study molecular structure. Ion-exchange-induced difference ATR-FTIR spectroscopy provides not only structural information but also affinity information by changing the concentration of the substrates of interest. As the concentration of K+ ions was systematically changed, spectra differences with different intensities were collected (Fig. 2). The spectral shapes were basically similar to each other, except in the band shift from 1680 to 1688 cm−1 at lower K+ concentrations (Fig. 2b). The band shift may be explained by a different distribution of K+ ions in the selectivity filter between high and low K+ concentrations (Fig. 1b, c). When the peak intensities at a specific band were plotted against the concentration of K+ ions, a sigmoidal shaped curve was obtained which could be fitted by the Hill equation. For peak-to-peak intensities at 1659 and 1627 cm−1, the apparent dissociation constant (KD) value of 9.0 ± 0.7 mM was obtained with a Hill coefficient of 1.8 ± 0.2, whereas values for the 1680 cm−1 band were 18 ± 1.4 mM with a Hill coefficient of 1.4 ± 0.1 (Furutani et al. 2012). It should be noted that the values were estimated based on an assumption that KD values for Na+ ions were much higher than those for K+ ions. A more complicated binding model is needed to obtain more accurate values.
Structural difference of the selectivity filter interacting with alkali metal cations studied by ion-exchange-induced difference ATR-FTIR spectroscopy
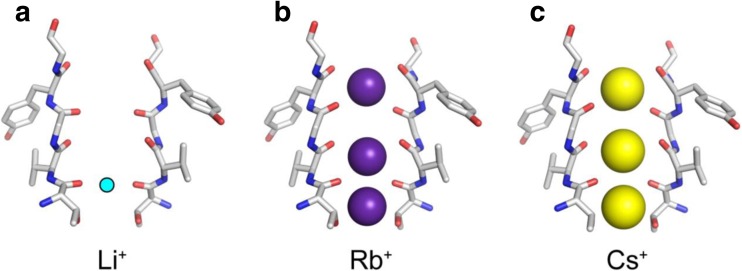
KcsA is known to be selectively more permeable to K+ ions than to Na+. Ion selectivity for other alkali metal cations has also been reported (K+ > Rb+ > > Cs+ > Na+, Li+), although the permeability of Cs+ remains controversial due to its quite small conduction that hampers single channel recording (LeMasurier et al. 2001). X-ray crystal structures of KcsA interacting with Li+, Rb+, and Cs+ have been reported (Fig. 3) (Thompson et al. 2009; Zhou and MacKinnon 2003). Because the electron density of Li+ is too small to detect by X-ray crystallography (see the ionic radii of alkali metal cations in Fig. 1e), the position of Li+ was proposed based on a molecular dynamics (MD) simulation analysis, which is the so-called “B site” (Thompson et al. 2009). On the other hand, the electron densities of three cations were resolved by X-ray crystallography for Rb+ and Cs+ (Zhou and MacKinnon 2003). The ion occupancies of Rb+ and Cs+ were found to be similar to each other. In addition, the structures of the selectivity filters interacting with Rb+ or Cs+ were almost indistinguishable from each other.
Fig. 3.
X-ray crystal structures in the selectivity filter region accommodating Li+ (a), Rb+ (b), and Cs+ ions. The position of Li+, shown as a sky-blue circle, was hypothesized according to the results of an molecular dynamics simulation. The others (Rb+, purple spheres; Cs+, yellow spheres) were modeled based on electron densities. PDB codes of the structure models in a, b, and c are 3GB7, 1R3I, and 1R3L, respectively
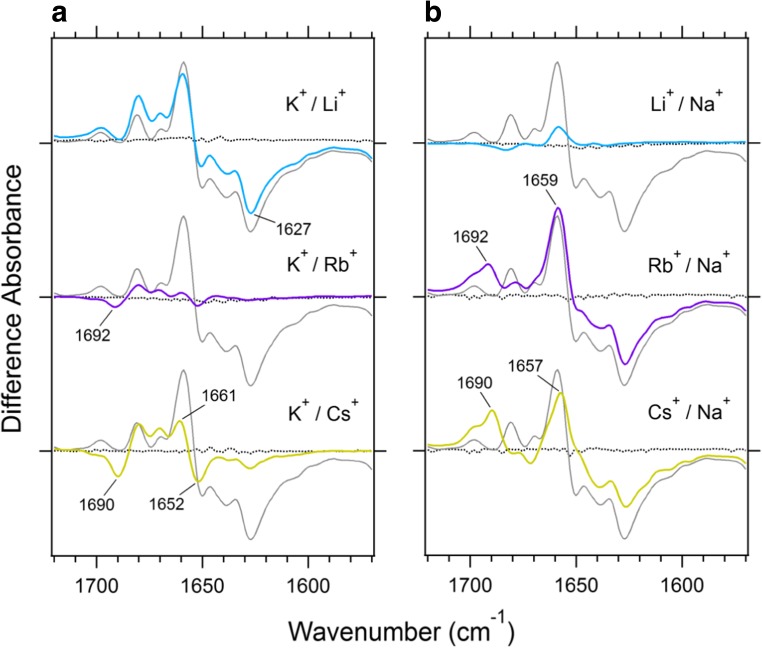
Two sets of ion-exchange-induced difference ATR-FTIR spectra have been recorded (Fig. 4) (Furutani et al. 2015), with one set measured by replacing K+ with Li+, Rb+, or Cs+ (Fig. 4a) and the other set measured by replacing Na+ with Li+, Rb+, or Cs+ (Fig. 4b). The spectral differences were normalized to originate from the same amount of KcsA protein according to the intensities of the amide I band in the absolute absorption spectra. Therefore, the amplitude of the difference is larger if the structural difference in the peptide backbone of KcsA becomes larger when the cations are replaced. As can be clearly seen in the difference spectra (Fig. 4), the KcsA structures interacting with K+ and Rb+ and those interacting with Na+ and Li+ were very similar to each other. The K+ − Cs+ difference spectrum was relatively similar to the K+ − Rb+ difference spectrum, except for the larger change at 1661 and 1652 cm−1 in the former (Fig. 4a). This may suggest that the replacement of Cs+ with K+ induces a larger conformational change in the pore helix than does the replacement of Rb+ with K+. A major common difference seen in Rb+ and Cs+ was an upshift of the 1680 cm−1 band in K+ to 1692 cm−1 in Rb+ and 1690 cm−1 in Cs+. The similar upshift was also observed in the K+ − Na+ difference spectrum at the lower K+ concentration (Fig. 2b). A previous study assigned the bands around 1680 cm−1 to the S2 and S3 modes (Stevenson et al. 2015). Therefore, the band shift may be attributable to a difference in ion occupancy at the S2 and S3 sites in the selectivity filter. According to previous X-ray crystallography studies on KcsA with Rb+ and Cs+, the ion densities of Rb+ and Cs+ at the S2 site were less than the ion density of K+ at a 200 mM concentration (Zhou and MacKinnon 2003). Moreover, a reduction in K+ concentration reduced ion occupancy at the S2 and S3 sites in addition to inducing a conformational change of the filter into a collapsed shape. Because filter collapse was not seen in the X-ray structures of KcsA with Rb+ and Cs+, the spectral shift of the 1680 cm−1 band would be attributable to the change in ion occupancy at the S2 and S3 sites, not to the conformational change of the filter.
Fig. 4.
Difference infrared spectra of KcsA in the amide I region. a Ion-exchange-induced spectra differences when K+ is replaced with Li+ (sky-blue line), Rb+ (purple line), or Cs+ (yellow line). b Ion-exchange-induced spectra differences when Na+ is replaced with Li+, Rb+, or Cs+ (same colors as in a). The K+ − Na+ spectrum difference (gray line) is superimposed on each spectrum difference for comparison. The gray dotted lines are spectra differences collected without exchanging the buffer solutions
The K+− Li+ spectrum difference was very similar to the K+ − Na+ spectrum difference which showed a negative peak at 1627 cm−1 (Fig. 4a). The band around 1630 cm−1 was previously assigned to the B site mode interacting with Na+. The observation of a similar band for Li+ suggests that Li+ is accommodated at the B site in the filter, similarly to Na+. The electron density of Li+ was not observed by X-ray crystallography. Thus, the spectral difference of the Li+ conditions would be considered as the first experimental evidence supporting the notion that the ion–protein interaction of KcsA with Li+ is similar to that with Na+.
Perspective
Ion-exchange-induced difference ATR-FTIR spectroscopy is a useful method for studying ion–protein interactions with a low sample consumption (typically requiring ~ 1 μg of protein). Various kinds of ion channels and pumps are potential targets for this method. Recently, a rapid-buffer exchange method utilizing syringe pumps driven by pressured gas has been developed which enables the rapid exchange of buffer solutions on ATR crystal (typically within 10–30 ms) (Furutani et al. 2013; Shirai et al. 2014). This technique may be utilized to detect transient conformational changes involving ion-binding processes in proteins.
Acknowledgments
This review article was written based on two original papers (Furutani et al. 2012, 2015) which are collaborative works with Profs. Hideki Kandori and Shigetoshi Oiki. I would like to thank them and collaborators in their laboratories. The author would also like to thank Enago (www.enago.jp) for the English language review. The article was partly supported by JSPS KAKENHI Grant Numbers JP26640047 and JP26708002.
Compliance with ethical standards
Conflict of interest
Yuji Furutani declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines - Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
References
- Baenziger JE, Miller KW, McCarthy MP, Rothschild KJ. Probing conformational changes in the nicotinic acetylcholine receptor by Fourier transform infrared difference spectroscopy. Biophys J. 1992;62:64–66. doi: 10.1016/S0006-3495(92)81780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Furutani Y, Kimura T, Okamoto K. Development of a rapid buffer-exchange system for time-resolved ATR-FTIR spectroscopy with the step-scan mode. Biophysics. 2013;9:123–129. doi: 10.2142/biophysics.9.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani Y, Shimizu H, Asai Y, Fukuda T, Oiki S, Kandori H. ATR-FTIR spectroscopy revealing the different vibrational modes of the selectivity filter interacting with K+ and Na+ in the open and collapsed conformations of the KcsA potassium channel. J Phys Chem Lett. 2012;3:3806–3810. doi: 10.1021/jz301721f. [DOI] [PubMed] [Google Scholar]
- Furutani Y, Shimizu H, Asai Y, Oiki S, Kandori H. Specific interactions between alkali metal cations and the KcsA channel studied using ATR-FTIR spectroscopy. Biophys Physicobiol. 2015;12:37–45. doi: 10.2142/biophysico.12.0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, MacKinnon R. Conduction properties of the cloned shaker K+ channel. Biophys J. 1993;65:2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3. Sunderland: Sinauer; 2001. [Google Scholar]
- LeMasurier M, Heginbotham L, Miller C. KcsA: it's a potassium channel. J Gen Physiol. 2001;118:303–314. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockless SW, Zhou M, MacKinnon R. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 2007;5:e121. doi: 10.1371/journal.pbio.0050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Fonfria VA, Leon X, Padros E. Studying substrate binding to reconstituted secondary transporters by attenuated total reflection infrared difference spectroscopy. Methods Mol Biol. 2012;914:107–126. doi: 10.1007/978-1-62703-023-6_7. [DOI] [PubMed] [Google Scholar]
- Nyquist RM, Heitbrink D, Bolwien C, Wells TA, Gennis RB, Heberle J. Perfusion-induced redox differences in cytochrome c oxidase: ATR/FT-IR spectroscopy. FEBS Lett. 2001;505:63–67. doi: 10.1016/S0014-5793(01)02769-7. [DOI] [PubMed] [Google Scholar]
- Rich PR, Iwaki M. Methods to probe protein transitions with ATR infrared spectroscopy. Mol BioSyst. 2007;3:398–407. doi: 10.1039/b702328f. [DOI] [PubMed] [Google Scholar]
- Shirai H, Duchesne C, Furutani Y, Fuji T. Attenuated total reflectance spectroscopy with chirped-pulse upconversion. Opt Express. 2014;22:29611–29616. doi: 10.1364/OE.22.029611. [DOI] [PubMed] [Google Scholar]
- Stevenson P, Gotz C, Baiz CR, Akerboom J, Tokmakoff A, Vaziri A. Visualizing KcsA conformational changes upon ion binding by infrared spectroscopy and atomistic modeling. J Phys Chem B. 2015;119:5824–5831. doi: 10.1021/acs.jpcb.5b02223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AN, Kim I, Panosian TD, Iverson TM, Allen TW, Nimigean CM. Mechanism of potassium-channel selectivity revealed by Na(+) and Li(+) binding sites within the KcsA pore. Nat Struct Mol Biol. 2009;16:1317–1324. doi: 10.1038/nsmb.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, MacKinnon R. The occupancy of ions in the K+ selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J Mol Biol. 2003;333:965–975. doi: 10.1016/j.jmb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 a resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]