Abstract
The fhy3 mutation of Arabidopsis impairs phytochrome A (phyA)-mediated inhibition of hypocotyl growth without affecting the levels of phyA measured spectrophotometrically or immunochemically. We investigated whether the fhy3-1 mutation has similar effects on very low fluence responses (VLFR) and high irradiance responses (HIR) of phyA. When exposed to hourly pulses of far-red light, etiolated seedlings of the wild type or of the fhy3-1 mutant showed similar inhibition of hypocotyl growth, unfolding of the cotyledons, anthocyanin synthesis, and greening upon transfer to white light. In the wild type, continuous far-red light was significantly more effective than hourly far-red pulses (at equal total fluence). In the fhy3-1 mutant, hourly pulses were as effective as continuous far-red light, i.e. the failure of reciprocity typical of HIR was not observed. Germination was similarly promoted by continuous or pulsed far-red in wild-type and fhy3-1 seeds. Thus, for hypocotyl growth, cotyledon unfolding, greening, and seed germination, the fhy3-1 mutant retains VLFR but is severely impaired in HIR. These data are consistent with the idea that VLFR and HIR involve divergent signaling pathways of phyA.
The perception of light signals provides plants with information about their surrounding environment and triggers changes of adaptive significance. Phytochromes are photoreceptors that absorb mainly in red light (R) and far-red light (FR) (Quail et al., 1995; Fankhauser and Chory, 1997). In Arabidopsis the phytochrome family comprises five members whose apoproteins are encoded by divergent genes (Quail, 1997).
Phytochrome A (phyA) mediates very low-fluence responses (VLFR), i.e. the responses to a single (Botto et al., 1996; Shinomura et al., 1996; Hamazato et al., 1997) or hourly pulses of FR (Mazzella et al., 1997; Yanovsky et al., 1997; Casal et al., 1998; Cerdán et al., 1999). phyA also mediates the effects of continuous FR in de-etiolating seedlings (Dehesh et al., 1993; Nagatani et al., 1993; Whitelam et al., 1993). The specific effects of continuous FR, i.e. the difference between continuous FR and hourly FR pulses at equal total fluence, is the high-irradiance response (HIR) (Casal et al., 1998). Phytochrome B (phyB) mediates R/FR-reversible low-fluence responses (LFR) under pulsed (Botto et al., 1995; Shinomura et al., 1996; Yanovsky et al., 1997) or continuous R (Reed et al., 1993; Mazzella et al., 1997).
phyA and phyB appear to have distinct transduction chains, because there are mutants that affect phyA and not phyB signaling (Whitelam et al., 1993; Soo Soh et al., 1998; Hudson et al., 1999) and vice versa (Ahmad and Cashmore, 1996; Wagner et al., 1997). However, some elements appear to be shared by phyA and phyB signaling; these include PIF3 (Ni et al., 1998) and PEF1 (Ahmad and Cashmore, 1996). phyA signaling could also diverge into the VLFR and the HIR pathway. For example, the VLF1 and VLF2 loci, polymorphic between ecotypes Landsberg erecta and Columbia of Arabidopsis, affect VLFR but not HIR (Yanovsky et al., 1997).
Five mutants are known to affect phyA and not phyB signaling: fhy1, fhy3 (Whitelam et al., 1993), fin2 (Soo Soh et al., 1998), spa1 (Hoecker et al., 1998), and far1 (Hudson et al., 1999). The fhy3 mutant retains normal levels of spectrally active and immunochemically detectable phyA, but shows impaired hypocotyl growth inhibition under continuous FR (Whitelam et al., 1993). The purpose of this work was to investigate whether the fhy3 mutation is pleiotropic and has similar or different effects on VLFR and HIR.
RESULTS
The VLFR and LFR of Hypocotyl Growth and Cotyledon Unfolding
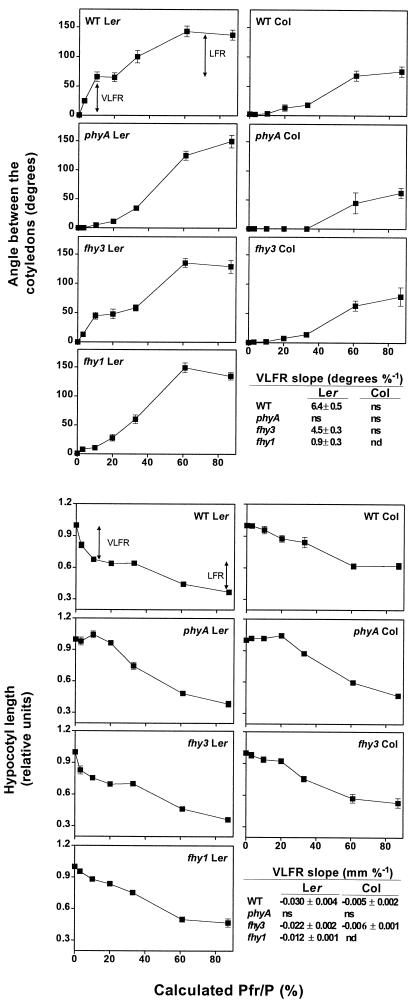
In the wild type (WT) Landsberg erecta, both hypocotyl growth and cotyledon unfolding showed biphasic responses to the calculated proportion of (Pfr/P) provided by hourly light pulses (Fig. 1) (Yanovsky et al., 1997). The first phase (i.e. the VLFR) that reached a plateau at a calculated Pfr/P of 10% is mediated by phyA. The second phase (i.e. the LFR of phyB) was observed for a calculated Pfr/P above 30%. In the WT Columbia, LFR were normal but VLFR were severely reduced (Yanovsky et al., 1997). In the original fhy3 mutant (Columbia background), the VLFR were similar to that observed in the WT, and LFR were similar or slightly stronger (hypocotyl growth; Whitelam et al., 1993) than in the WT Columbia.
Figure 1.
Hypocotyl growth and cotyledon unfolding responses of WT, phyA, fhy1, and fhy3-mutant seedlings of Arabidopsis to the calculated Pfr/P provided by hourly R/FR pulses. Data are means and se (whenever larger than the symbols) of at least nine replicate boxes. The slope of the VLFR was calculated by fitting a straight line between Pfr/P = 0% and 10%. Ler, Landsberg erecta; Col, Columbia; ns, not significant; nd, not determined.
To investigate VLFR in further detail, the fhy3-1 mutation was introgressed into the Landsberg erecta background, selecting for plants with reduced response to continuous FR (typical of fhy3) but cotyledon unfolding under hourly FR pulses (typical of Landsberg alleles of the VLF1 and VLF2 loci). The creation of seedlings combining these two features indicates that the fhy3 mutation does not fully impair VLFR. In the Landsberg erecta background, fhy3 seedlings retained approximately 70% of the VLFR and normal LFR of hypocotyl growth and cotyledon unfolding (Fig. 1). The fhy1 mutant (included for comparative purposes) showed severely reduced VLFR.
Physiological Analysis of phyA Stability
Although fhy3 retained almost normal VLFR, the small difference with the WT offered an avenue to investigate whether phyA stability is affected by this mutation. Enhanced destruction of phyA Pfr in fhy3 can be ruled out because immunological and spectral detection of phyA indicate WT levels (Whitelam et al., 1993), but the occurrence of enhanced Pfr to Pr dark reversion (Braslavsky et al., 1997) could not be discarded with available information. If the (weak) phenotype of fhy3 under pulsed FR were due to increased Pfr to Pr dark reversion, the difference should be narrowed by increasing pulse frequency. However, increasing pulse frequency from one to two pulses per hour did not increase the response either in fhy3 (hypocotyl growth inhibition, percentage of dark control: 1 pulse 60 min−1 = 17 ± 2; 1 pulse 45 min−1 = 18 ± 1; 1 pulse 30 min−1 = 18 ± 2) or in the WT (1 pulse 60 min−1 = 24 ± 1; 1 pulse 45 min−1 = 23 ± 1; 1 pulse 30 min−1 = 22 ± 1). In addition, the phenotype of fhy3 was more obvious under continuous than pulsed FR (see below), and this contradicts the behavior expected from a mutant with increased dark reversion.
The HIR of Hypocotyl Growth and Cotyledon Unfolding
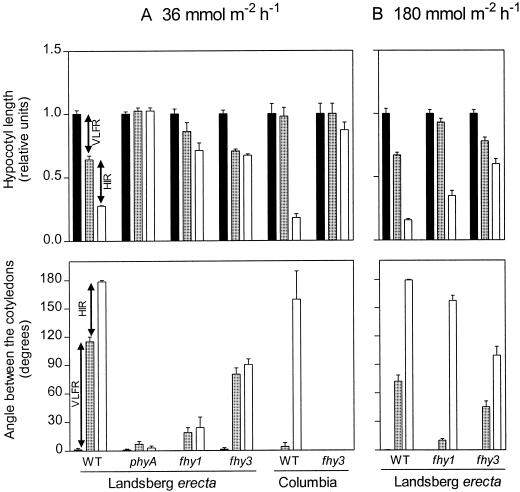
The HIR is defined as the specific effect of continuous light, i.e. the effect of continuous light that cannot be mimicked by hourly pulses providing the same total fluence as continuous light (Casal et al., 1998). At a fluence rate of 36 mmol m−2 h−1, a HIR was observed for the WT but not for the fhy3 mutant (i.e. hourly and continuous FR had similar effects) (Fig. 2A). A weak HIR was observed in the fhy3 mutant at a fluence rate of 180 mmol m−2 h−1 (Fig. 2B).
Figure 2.
The HIR is severely deficient in fhy3-1. Seedlings of the WT and of the fhy1 and fhy3 mutants were exposed to continuous FR (white bars) or to hourly pulses of FR (gray bars) at equal fluence of either 36 mmol m−2 h−1 (A) or 180 mmol m−2 h−1 (B). The HIR is the difference in response between hourly and continuous FR. Data are means and se of 12 replicate boxes. Black bars, Darkness.
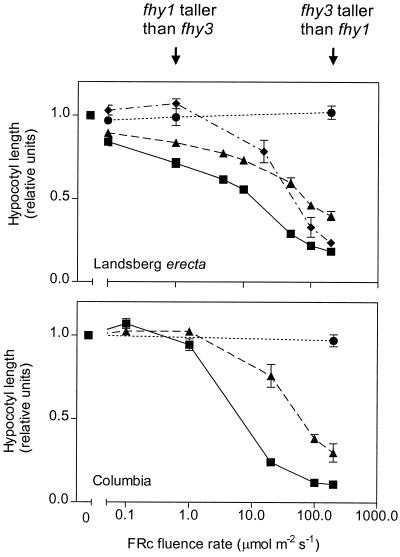
At 0.1 μmol m−2 s−1 of continuous FR, hypocotyl growth inhibition was similar in WT and fhy3 seedlings of the Landsberg erecta background and was severely reduced in fhy1, as well as in WT and fhy3 seedlings of the Columbia background (Fig. 3). The negligible phenotype of fhy3 at these fluence rates agrees with the almost normal VLFR observed under hourly FR pulses (compare with Figs. 1 and 2). Fluence rates above 10 μmol m−2 s−1 enhanced hypocotyl growth inhibition in fhy3 and fhy1 mutants (Fig. 3). Thus, the HIR was not absent in these mutants but shifted toward higher fluence rates.
Figure 3.
The phenotype of fhy3-1 is more obvious at intermediate and high fluence rates of continuous FR, while the phenotype of fhy1 is more obvious at intermediate and low fluence rates of continuous fresh weight. phyA (●), fhy3 (▴), and fhy1 (♦) seedlings were exposed to the indicated fluence rate of continuous FR. Data are means and se of at least six replicate boxes. ▪, WT.
Seed Germination
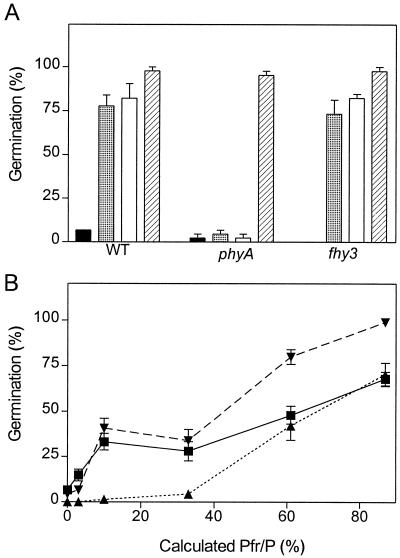
The observation that fhy3 retains VLFR of hypocotyl growth and cotyledon unfolding prompted us to investigate the VLFR of seed germination. In seeds of the WT and of the fhy3 mutant, hourly FR significantly increased germination above dark controls, and continuous FR had a similar effect (Fig. 4A). The phyA mutant did not respond to pulsed or continuous FR. This indicates that under these conditions seed germination was dominated by a VLFR and a HIR was not detectable. To investigate the VLFR and LFR in further detail, the seeds were transferred to darkness and given a single R/FR pulse predicted to establish a series of calculated Pfr/P. Both WT and fhy3 seeds showed a biphasic response, with a first phase (VLFR) saturated by 10% Pfr/P (Fig. 4B). The first phase was lacking in the phyA mutant. These observations indicate that the control of seed germination by phyA is not impaired in the fhy3 mutant.
Figure 4.
fhy3-1 does not impair seed germination. Seeds of WT, phyA, and fhy3 were incubated in darkness at 4°C and either transferred to darkness (black bars), hourly FR (shaded bars), continuous FR (white bars), or hourly R (hatched bars) at 25°C (A) or incubated in darkness 24 h at 25°C, given a single pulse predicted to establish a series of calculated Pfr/P, and returned to darkness (B). Data are means and se of 12 replicate boxes. ▪, WT; ▴, phyA; ▾, fhy3.
Chlorophyll Synthesis
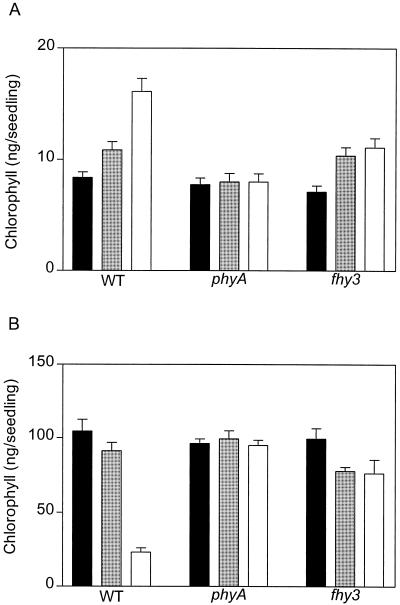
When etiolated seedlings are transferred from darkness to white light, there is a typical lag before chlorophyll synthesis is initiated. This lag can be shortened by previous exposure to light perceived by phytochrome (Lifschitz et al., 1990), and phyA is involved in this response (Yanovsky et al., 1997). Etiolated seedlings were incubated for 4 h under hourly pulses of long-wavelength FR, continuous long-wavelength FR, or darkness, and transferred to fluorescent white light. Long-wavelength FR was used to avoid chlorophyll synthesis during the treatment. In the WT, chlorophyll levels were increased by pretreatment with pulsed FR, and continuous FR had a much stronger effect (Fig. 5A). In the fhy3 mutant, the effects of FR pulses were similar to the effects observed in the WT, but continuous FR caused no effect above these values (Fig. 5A).
Figure 5.
A, Chlorophyll levels are reduced by fhy3-1 in seedlings exposed to short periods of continuous FR but not in seedlings exposed to hourly FR pulses. Three-day-old seedlings were exposed to 4-h-long-wavelength FR and transferred to white light for 5 h before harvest. B, Blocking of greening phenotype in the fhy3-1 mutant. The seedlings were grown for 3 d in darkness or under pulsed or continuous long-wavelength FR and then transferred to continuous white light for 1 d. Data are means and se of eight to nine replicate samples. Black bars, Darkness; gray bars, hourly FR; white bars, continuous FR.
Blocking of Greening
When etiolated seedlings of the WT are exposed for several days to continuous FR, they fail to green normally upon subsequent transfer to white light. This effect is absent in the phyA and fhy1 mutants (Barnes et al., 1996). To investigate the effects of the fhy3 mutation on this response, the seedlings were exposed for 3 d to either continuous or hourly pulses of long-wavelength FR. Hourly pulses of FR had small effects on chlorophyll levels in WT and fhy3 seedlings (Fig. 5B). Exposure to 3 d of continuous FR reduced subsequent greening in the WT but not in the phyA and fhy3 mutants (Fig. 5B).
Anthocyanin Levels
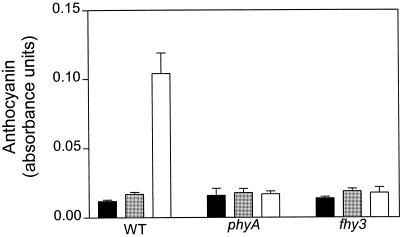
Compared with darkness, hourly pulses of FR caused a small but significant effect on anthocyanin levels in WT and fhy3-1 seedlings (Fig. 6). Continuous FR was significantly more effective only in the WT. The phyA mutant failed to respond to pulsed or continuous FR.
Figure 6.
Anthocyanin accumulation is reduced in the fhy3-1 mutant. Seedlings of the WT, phyA, and fhy3 mutants were grown under continuous FR or hourly pulses of FR at equal fluence. Data are means and se of at least four replicate boxes. Black bars, Darkness; gray bars, hourly FR; white bars, continuous FR.
Antagonistic Interaction of phyA with phyB Signaling
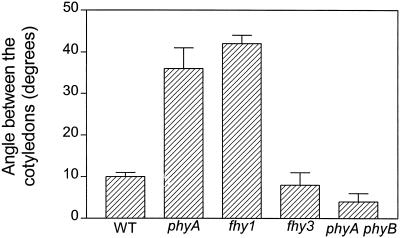
The responses mediated by phyB can be either enhanced or reduced by phyA activity, depending on light conditions (Casal and Boccalandro, 1995; Mazzella et al., 1997). In seedlings exposed to R, the response of the phyA and fhy1 mutants can be larger than that of the WT. This indicates an antagonistic regulation of phyB signaling by phyA and FHY1 (Cerdán et al., 1999). Etiolated seedlings were exposed daily to a single pulse of R. This treatment caused no detectable unfolding of the cotyledons in the WT, but resulted in a significant response in the phyA and fhy1 mutants (Fig. 7). The fhy3 mutant behaved like the WT.
Figure 7.
Enhanced phyB-mediated cotyledon unfolding in phyA and fhy1 but not in fhy3-1. The seedlings were exposed daily to a single pulse of R. Data are means and se of six replicate boxes.
Synergistic Interaction of phyA with phyB Signaling
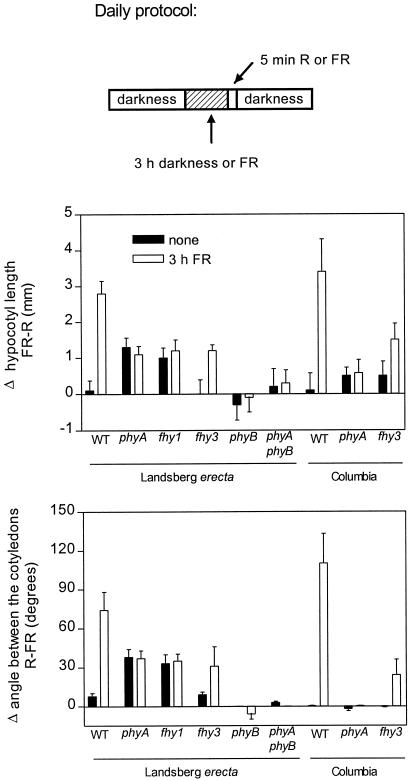
The response to a R compared with a FR pulse (mediated by phyB) is amplified when etiolated seedlings are exposed to FR pretreatments perceived by phyA (Casal, 1995; Casal and Boccalandro, 1995; Hennig, 1999). This indicates a synergistic interaction between phyA and phyB signaling (Cerdán et al., 1999). Etiolated seedlings were daily exposed to 3 h of FR terminated with a R or a FR pulse. As expected (Cerdán et al., 1999), the FR pretreatment failed to enhance responsivity in the phyA mutant and caused a partial effect in the fhy1 mutant. Compared with the WT, the fhy3 mutant showed only partial enhancement of the response to R versus FR by the FR pretreatment (Fig. 8). Thus, FHY3 is involved in phyA-FHY1-mediated amplification of the response to phyB.
Figure 8.
phyA-mediated responsivity amplification toward phyB is impaired in the fhy3-1 mutant. The seedlings were exposed daily to a pulse of R or FR with or without an immediately previous exposure to 3 h of FR. Data are means and se of the differences between seedlings exposed to R or FR (at least four replicate boxes were used for each light condition).
DISCUSSION
The fhy3 mutation has pleiotropic effects. In addition to the reduced hypocotyl growth inhibition under continuous FR reported previously, the fhy3 mutant is impaired in the following phyA-mediated responses: promotion of cotyledon unfolding (Figs. 1 and 2) and anthocyanin synthesis (Fig. 6) under continuous FR, reduction of the lag of chlorophyll synthesis by short (4-h) periods of FR before transfer to white light (Fig. 5A), and blocking of greening by prolonged FR (4 d) before transfer to white light (Fig. 5B). In contrast to the phyA mutant, fhy3 showed normal promotion of seed germination by pulsed or continuous FR (Fig. 4).
The fhy3 mutation does not affect phyA abundance or stability. The fhy3 mutant contains normal levels of photoactive phyA (Whitelam et al., 1993). The phenotype of fhy3-1 was obvious under continuous FR (Figs. 2, 3, 5, and 6), but very weak under hourly pulses of FR (Figs. 1, 2, 4, and 5), and doubling the frequency of FR pulses caused no additional effect. This is not the behavior predicted for a mutant with impaired phyA stability via enhanced Pfr to Pr dark reversion.
The fhy3-1 mutant showed marginal differences with the WT in response to a single pulse of FR (Pfr/P = 3% or 10%; Fig. 4B) or under hourly pulses of FR (Figs. 1, 2, 4A, 5, and 6). This indicates that the VLFR were only slightly affected. In the WT, continuous FR was significantly more effective than hourly pulses of FR (Figs. 2, 3, 5, and 6), i.e. a strong HIR was observed. In the fhy3-1 mutant, hourly pulses were as effective as continuous FR (except at very high fluences). Thus, HIR were severely affected in fhy3-1. We cannot rule out the possibility that (if fhy3-1 were a leaky allele) stronger fhy3 alleles also affect the VLFR, but the differential effect of fhy3-1 on VLFR versus HIR has consequences for our understanding of phyA signaling.
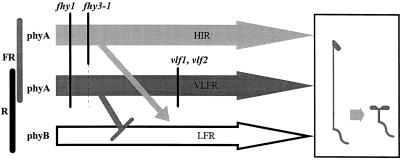
We have proposed a model wherein VLFR and HIR modes of action of phyA involve at least partially different signaling pathways (Yanovsky et al., 1997; Casal et al., 1998) that respectively exert negative (antagonistic) or positive (synergistic) regulation of phyB signaling (Cerdán et al., 1999) (Fig. 9). The genetic basis for this model was provided by the effects of the Columbia alleles of the VLF1 and VLF2 loci, which, compared with the Landsberg alleles, reduce VLFR without affecting HIR (Yanovsky et al., 1997). The results presented here complement the genetic support of the model by showing that the fhy3-1 mutation affects HIR (Figs. 2, 3, 5, and 6) and the synergistic interaction between phyA and phyB (Fig. 8) but has virtually no effect on VLFR (Figs. 1, 2, 4, 5, and 6) or the antagonistic interaction between phyA and phyB (Fig. 7). Although not as selective as VLF1, VLF2, and fhy3-1 loci, the fhy1 mutant also has differential effects (reducing proportionally more VLFR than HIR).
Figure 9.
Schematic representation of the physiological/genetic pathways of phytochrome action. The pathways affected by fhy1, fhy3, vlf1, and vlf2 are indicated with vertical lines. The lines connecting two pathways indicate positive (→) or negative (⊣) regulation.
The fhy3-1 mutation affects phyA signaling but not phyA abundance or stability. The pleiotropic nature of the mutation tentatively places the action of FHY3 early in the signaling events downstream of phyA. Among other possibilities, FHY3 could be an intrinsic signaling molecule, forming a part of complexes with some elements specific for the VLFR or HIR pathways. Alternatively, FHY3 could be a positive regulator of phyA signaling with a stronger impact on the HIR than on the VLFR pathway. Cloning of the gene followed by molecular and biochemical experiments will help to elucidate this issue.
MATERIALS AND METHODS
Plant Material
The Arabidopsis ecotypes Landsberg erecta or Columbia were used as WT. The fhy3-1 (Columbia), fhy1, and phyA-1 (both Landsberg erecta) mutants have been described by Whitelam et al. (1993) and the phyA-211 (Columbia) mutant has been described by Reed et al. (1994). To partially introgress the fhy3 mutation originally obtained in Columbia into the Landsberg erecta background, seedlings of the F2 generation of crosses between fhy3 and Landsberg erecta were selected for long hypocotyls under continuous FR (fhy3 phenotype). Seedlings of the subsequent generation after self-pollination were selected for open cotyledons under pulsed FR (typical of Landsberg alleles of VLF1 and VLF2; Yanovsky et al., 1997). These plants were crossed to Landsberg erecta to initiate the second cycle of backcrosses, which was followed by a third cycle.
The seeds were sown in clear plastic boxes (40 × 33 mm2 × 15 mm height) containing 3 mL of 0.8% (w/v) agar. The boxes were incubated at 4°C in darkness for 3 d and (except for seed germination experiments) exposed to a R pulse followed by 24 h of darkness to promote seed germination.
Hypocotyl Growth and Cotyledon Unfolding
Fifteen seeds of each genotype were sown per box. The seedlings were either kept in the dark or exposed to various light treatments for 3 d. Hypocotyl length was measured to the nearest 0.5 mm with a ruler and, to eliminate defective seedlings, only the largest 10 seedlings of each box were averaged (one replicate). Hypocotyl length in seedlings exposed to the different light treatments was expressed relative to the length of dark controls to increase accuracy. No systematic differences between WT and mutant seedlings grown in darkness were observed. The angle between the cotyledons was measured with a protractor.
Seed Germination
Fifteen seeds of each genotype were sown in the plastic boxes on a layer of filter paper number 1 (Whatman, Clifton, NJ) placed on top of the agar. The seeds were immediately exposed to a pulse of long-wavelength FR to convert most Pfr into the Pr form, incubated 3 d in darkness at 4°C, and transferred to 25°C. The seeds were then immediately exposed to hourly R pulses, hourly FR pulses, or continuous FR for 4 d, or incubated for 24 h in darkness, given a single R/FR pulse, and returned to darkness for 4 d. A control receiving no light after the initial long-wavelength FR pulse was included in both types of experiments.
Chlorophyll Levels
To investigate phytochrome-mediated potentiation of greening, 80 seeds were sown per box. After 3 d in darkness, the seedlings were given 4 h of FR treatments, followed by 5 h under white light (100 μmol m−2 s−1) before harvest. To investigate the effects of prolonged FR treatments on subsequent greening upon transfer to white light, 20 seeds of each genotype were sown per box. The seedlings were given the FR treatment for 3 d and finally transferred for 24 h to fluorescent white light. The seedlings were harvested in 1 mL of N,N′-dimethylformamide and incubated in darkness at −20°C for at least 3 d. Absorbance was measured at 647 and 664 nm, and chlorophyll levels were calculated according to the method of Moran (1982).
Anthocyanin Levels
Two hundred seeds were sown per box. The seedlings were exposed to various light conditions for 3 d and extracted with 1 mL of 1% (w/v) HCl methanol. A530 was measured and corrected for chlorophyll absorption (657 nm) according to the method of Mancinelli et al. (1991).
Light Sources
FR was provided by incandescent lamps in combination with a water filter, a red acetate filter, and six 2-mm-thick blue acrylic filters (Paolini 2031, La Casa del Acetato, Buenos Aires). The calculated Pfr/p (Casal et al., 1991) was 10%. Long-wavelength FR provided by an incandescent lamp in combination with a water filter and an RG9 filter (Schott, Maintz, Germany) was used to establish a calculated Pfr/P of 3%. Pulses of R (maximum emission 662 nm) that establish a calculated Pfr/P of 87% were provided by light-emitting diodes. Incandescent lamps in combination with a water filter and either a red acetate filter or red plus green acetate filters were used to provide a calculated Pfr/P of 61% or 33%, respectively. To investigate the effects of different calculated Pfr/P, either single (5 min) or hourly (3 min) light pulses were provided at a fluence rate of 15 to 40 μmol m−2 s−1, because in this range the responses are independent of fluence rate. To compare the effects of hourly (3 min) and continuous FR, the fluence rate was equilibrated at 36 mmol m−2 h−1 (unless stated otherwise). When the seedlings were exposed daily to 3 h of FR, the fluence rate was 60 μmol m−2 s−1.
Footnotes
This work was supported by Fondo Nacional de Ciencia y Tecnica (PICT 08–00115–02089), University of Buenos Aires (TG 59), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0888/98), and Fundación Antorchas (A–13622/1–40).
LITERATURE CITED
- Ahmad M, Cashmore AR. The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 1996;10:1103–1110. doi: 10.1046/j.1365-313x.1996.10061103.x. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell. 1996;8:601–615. doi: 10.1105/tpc.8.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto JF, Sánchez RA, Casal JJ. Role of phytochrome B in the induction of seed germination by light in Arabidopsis thaliana. J Plant Physiol. 1995;l46:307–312. [Google Scholar]
- Botto JF, Sánchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braslavsky SE, Gärtner W, Schaffner K. Phytochrome photoconversion. Plant Cell Environ. 1997;20:700–706. [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA, Benedetto D, De Miguel LC. Light promotion of seed germination in Datura ferox is mediated by a highly stable pool of phytochrome. Photochem Photobiol. 1991;53:249–254. [Google Scholar]
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 1999;18:499–507. doi: 10.1046/j.1365-313x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- Dehesh K, Franci C, Parks BM, Seeley KA, Short TW, Tepperman JM, Quail PH. Arabidopsis hy8 locus encodes phytochrome A. Plant Cell. 1993;5:1081–1088. doi: 10.1105/tpc.5.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Hamazato F, Shinomura T, Hanzawa H, Chory J, Furuya M. Fluence and wavelength requirements for Arabidopsis CAB gene induction by different phytochromes. Plant Physiol. 1997;115:1533–1540. doi: 10.1104/pp.115.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Unger S, Schäfer E. Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta. 1999;208:257–263. doi: 10.1007/s004250050557. [DOI] [PubMed] [Google Scholar]
- Hoecker U, Xu Y, Quail PH. SPA1: a new genetic locus involved in phytochrome A-specific signal transduction. Plant Cell. 1998;10:19–33. doi: 10.1105/tpc.10.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M, Ringli C, Boylan MT, Quail PH. The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev. 1999;13:2017–2027. doi: 10.1101/gad.13.15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz S, Gepstein S, Horwitz BA. Phytochrome regulation of greening in wild type and long-hypocotyl mutants of Arabidopsis thaliana. Planta. 1990;181:234–238. doi: 10.1007/BF02411544. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. Cryptochrome, phytochrome and anthocyanin production. Plant Physiol. 1991;96:1079–1085. doi: 10.1104/pp.96.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and phytochrome B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Soh M, Hong SS, Hanzawa H, Furuya M, Nam HG. Genetic identification of FIN2, a far red light-specific signaling component of Arabidopsis thaliana. Plant J. 1998;16:411–419. doi: 10.1046/j.1365-313x.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- Wagner D, Hoecker E, Quail PH. RED1 is necessary for phytochrome B-mediated red light specific signal transduction in Arabidopsis. Plant Cell. 1997;9:731–743. doi: 10.1105/tpc.9.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia dissect two branches of phytochrome A signaling pathways that correspond to the very-low fluence and high-irradiance responses of phytochrome. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]