Abstract
Most molluscs have blue blood because their respiratory molecule is hemocyanin, a type-3 copper-binding protein that turns blue upon oxygen binding. Molluscan hemocyanins are huge cylindrical multimeric glycoproteins that are found freely dissolved in the hemolymph. With molecular masses ranging from 3.3 to 13.5 MDa, molluscan hemocyanins are among the largest known proteins. They form decamers or multi-decamers of 330- to 550-kDa subunits comprising more than seven paralogous functional units. Based on the organization of functional domains, they assemble to form decamers, di-decamers, and tri-decamers. Their structure has been investigated using a combination of single particle electron cryo-microsopy of the entire structure and high-resolution X-ray crystallography of the functional unit, although, the one exception is squid hemocyanin for which a crystal structure analysis of the entire molecule has been carried out. In this review, we explain the molecular characteristics of molluscan hemocyanin mainly from the structural viewpoint, in which the structure of the functional unit, architecture of the huge cylindrical multimer, relationship between the composition of the functional unit and entire tertiary structure, and possible functions of the carbohydrates are introduced. We also discuss the evolutionary implications and physiological significance of molluscan hemocyanin.
Keywords: Molluscan hemocyanin, Oxygen transporter, Structure, Electron cryo-microscopy, X-ray crystallography, Glycoprotein, Evolution
Introduction
One of the most important life-support systems of animals is respiration, which supplies oxygen to individual organs and tissues. Vertebrates use the iron of hemoglobin in red blood cells for this purpose. In most molluscs and arthropods the crucial role of oxygen transport is facilitated by the blue extracellular copper protein hemocyanin. Although both molluscan and arthropod hemocyanins are huge multimeric glycoproteins containing a type-3 copper center that occur freely dissolved in hemolymph, their structures are profoundly different. Molluscan hemocyanin form hollow cylindrical decamers (molecular mass approx. 4 MDa) or multi-decamers (molecular mass > 8 MDa), whereas arthropod hemocyanins are built as hexamers (molecular mass approx. 450 kDa) or multiples of hexamers. In this review, we summarize the molecular characteristics of molluscan hemocyanin based on structural information accumulated since the discovery of the first molluscan hemocyanin by Léon Fredericq in 1878 (Fredericq 1878; Ghiretti-Magaldi and Ghiretti 1992).
Molluscan hemocyanins are among the largest known proteins, with molecular masses varying approximately from 3.3 to 13.5 MDa. They form decamers or multi-decamers of individual polypeptide subunits of 330–450 kDa, with each subunit consisting of more than seven paralogous functional units (FUs) (Markl 2013). Molluscan hemocyanins commonly form hollow cylindrical complexes and consist of a cylindrical outer wall region and several inner collar domains located at the inner surface of the wall, although the manner in which the protomers associate varies according to the composition of the FU of the respective polypeptide chain. Researchers have taken advantage of these molecular characteristics of molluscan hemocyanins (i.e., the entire molecule being huge and all its component FUs being paralogous) and have fundamentally investigated their structure by the combination of two different types of analyses: single particle electron cryo-microsopy (cryoEM) analysis of the entire molecule as a top–down study (Boisset and Mouche 2000; Gatsogiannis and Markl 2009; Gatsogiannis et al. 2007, 2015; Lamy et al. 1998; Meissner et al. 2000; Zhu et al. 2014) and high-resolution X-ray crystallography for FUs as a bottom–up study (Cuff et al. 1998; Jaenicke et al. 2010, 2011; Perbandt et al. 2003). Many molluscan hemocyanins have been structurally investigated using hybrid approaches, by fitting high-resolution crystal structures of some FUs and homology models into lower resolution cryoEM electron density maps. However, during the past several years, high-resolution structures of entire hemocyanins have been reported: a near-atomic resolution cryoEM structure of Haliotis divarsicolor hemocyanin by Zhang et al. (2013) and X-ray crystal structure analysis of the entire squid hemocyanin by Gai et al. (2015; Matsuno et al. 2015). In the former studies, the Cα model was constructed based solely on the density derived by cryoEM. In the latter, the atomic structure was solved at a resolution of 3.0 Å, revealing the side-chains and carbohydrate residues. These recent breakthroughs have provided a deeper understanding on hemocyanin structure.
The biochemical and biophysical characteristics of hemocyanin have also been extensively studied during the recent decades. Interestingly, hemocyanin is responsible not only for oxygen transportation but also for an innate immune response based on its phenoloxidase activity (Jaenicke et al. 2009). Molluscan hemocyanins have been widely used in medical research. The most prominent example is keyhole limpet hemocyanin (KLH), which is used as an antigen-carrier and/or adjuvant for immunization and as a natural immunostimulant in tumor therapy (Becker et al. 2014). These industrially and medically valuable properties are derived from the presence of the attached glycans at the periphery of the complex and the vast molecular mass (Geyer et al. 2005; Harris and Markl 1999; Siddiqui et al. 2007). Understanding the molecular basis and design of hemocyanins in detail is essential for future biomedical and biotechnological applications. In this review, we explain the molecular characteristics of molluscan hemocyanin mainly from a structure point of view and further discuss their evolutionary implications and physiological significance.
Structure of molluscan hemocyanin
General architecture: domain architecture
In this section, we describe the relevance between polypeptide FU composition and the manner of association for different types of molluscan hemocyanins. Molluscan hemocyanins, in general, comprise several paralogous FUs. According to their sequence similarity, FUs are classified into eight FUs (FU-a, -b, -c, -d, -e, -f, -g, and -h) and their homologous FUs (FU-d*, -f1, -f2, -f3, -f4, -f5, and -f6). All known molluscan hemocyanins commonly contain FU-a, -b, -c, -d, -e, -f, and -g, and some hemocyanins also possess additional FU(s). FU-h is an exception among the known FUs in terms of the presence of an additional cupredoxin-like domain at the C-terminus (see below). Based on the presence or absence of FU-h, molluscan hemocyanin can be categorized into two broad groups: cephalopod-type (without FU-h) and gastropod-type (commonly possessing FU-h). Cephalopod-type hemocyanin is further classified into two subgroups based on the presence or absence of FU-d*: those without FU-d* (designated as nautilus-type) and those possessing additional FU-d* (designated as squid-type) (Fig. 1a, b). Similarly, gastropod-type hemocyanin is classified into two main subgroups according to FU organization: most of the gastropod-type hemocyanins are composed of FU-a, -b, -c, -d, -e, -f, -g, and -h (designated keyhole limpet-type hemocyanin; Fig. 1c) and the mega-heymocyanin-type comprising two different subunits—a typical keyhole limpet-type subunit and a special mega-subunit composed of several FU-f derivatives (Fig. 1d). Taken together, molluscan hemocyanins can be classified into four major groups according to the FU composition of the subunit: (1) nautilus-type, (2) squid-type, (3) keyhole limpet-type, and (4) mega-hemocyanin-type.
Fig. 1.
Structure of molluscan hemocyanin and its subunit. a–d Representative area of negatively stained transmission electron microscopy (TEM) image (left), scheme of hemocyanin subunit (bottom), and three-dimensional (3D) model of four molluscan hemocyanin types (right) are shown. a Nautilus-type hemocyanin (TEM image: Enterooctopus dofleini hemocyanin; 3D structure: nautilus hemocyanin). b Squid-type hemocyanin (TEM image: Todarodes pacificus hemocyanin; 3D structure: crystal structure of T. pacificus hemocyanin. The Cu2O2 cluster of FU-d*, one of the inner domains, is indicated as purple spheres because the precise structure of FU-d* has not been determined yet). c Keyhole limpet-type hemocyanin (TEM image: Nordotis discus hannai hemocyanin; 3D structure: electron cryo-microsopy (cryoEM) structure of keyhole limpet hemocyanin). d Mega-hemocyanin-type hemocyanin (Melanoides tuberculata hemocyanin). C C-terminus, N N-terminus. Scheme of hemocyanin subunits: a, b, c, d, e, f, g, h Functional units (FUs) of molluscan hemocyanins, f1, f2, f3, f4, f5, f6 homologous FUs
The representative organisms having nautilus-type hemocyanin are nautilus and octopus. The nautilus-type hemocyanin assembles into a hollow cylindrical decamer with a wall and five inner collar regions (Fig. 1a). The subunit of nautilus-type consists of seven FUs (FU-a, -b, -c, -d, -e, -f, and -g) (Bergmann et al. 2006; Gatsogiannis et al. 2007; Markl 2013; Miller et al. 1998). The wall region comprises FU-a, -b, -c, -d, -e, and -f, whereas the collar region contains FU-g (Gatsogiannis et al. 2007; Miller et al. 1990).
The representative organisms possessing squid-type hemocyanin are decapodiformes or cephalopods, such as squid or cuttlefish. The subunit of squid-type hemocyanin consists of eight FUs (FU-a, -b, -c, -d, -d*, -e, -f, and -g). Because the fifth FU is considered to be generated by the duplication of the FU-d gene, it is designated as FU-d* (Boisset and Mouche 2000; Gai et al. 2015; Lamy et al. 1998; Thonig et al. 2014; Fig. 1b). The wall region comprises FU-a, -b, -c, -d, -e, and -f, as in the case of the nautilus-type, whereas the collar domains contain FU-d* and -g. Although the entire molecule shows a hollow cylindrical structure with a size similar to that of the nautilus-type, the top view of its negative staining images shows inner collar domains that are more crowded than those of the nautilus-type, probably due to FU-d* being additionally incorporated into the collar regions (Fig. 1b).
The keyhole limpet-type is the typical hemocyanin of gastropods, and most gastropods, such as the keyhole limpet and abalone, have this type. The subunit of keyhole limpet-type is composed of eight FUs (FU-a, -b, -c, -d, -e, -f, -g and -h) (Fig. 1c). This type mostly assembles into di-decamers, in which two decamers are stacked in a back-to-back manner. Consequently, its height is approximately twice that of the nautilus−/squid-type (Fig. 1c). However, polyplacophora hemocyanin, which is also categorized as keyhole limpet-type hemocyanin, is an exception in that it exits as a stable decamer (Harris et al. 2004; Lambert et al. 1994). The molecular mass of FU-h is larger than that of other FUs due to the presence of an extra cupredoxin-like domain at the C-terminus (Jaenicke et al. 2010). In keyhole limpet-type hemocyanin, the FU-h and -g are located in the collar region (Altenhein et al. 2002; Gatsogiannis and Markl 2009; Lieb and Markl 2004; Lieb et al. 2000; Markl 2013).
Mega-hemocyanin-type hemocyanin is a tri-decamer made of two different subunits having a molecular mass of 550 kDa (mega-subunit) and 400 kDa (typical subunit). Ten large subunits and 20 smaller subunits assemble into the tri-decamer, which has a molecular mass of 13.5 MDa. Although the smaller subunits have a FU composition similar to that of the keyhole limpet-type, the large subunit is composed of 12 FUs (FU-a, -b, -c, -d, -e, -f, -f1, -f2, -f3, -f4, -f5, -f6), in which six additional special FUs are located at the C-terminus instead of FU-g and -h (Gatsogiannis et al. 2015; Lieb et al. 2010; Fig. 1d). As with other hemocyanins, the wall region is composed of FU-a, -b, -c, -d, -e, and -f; the remaining FUs, Fu-h and the additional FU-f relatives (FU-f1, -f2, -f3, -f4, -f5, and -f6), are located in the collar region and form a unique inner core (see below).
Structure of the FU
Crystal structures of FU-g, FU-e, and FU-h have been determined (Cuff et al. 1998; Jaenicke et al. 2010, 2011; Perbandt et al. 2003). Structures of all FUs are commonly composed of two domains, i.e., the N-terminal core domain (NTD) and the C-terminal β-sandwich domain (CTD). The former is mainly composed of α-helices, and the latter is rich in β-strands (Fig. 2). A recent crystal structure of the entire squid hemocyanin decamer revealed that all FUs have quite a similar structure regardless of their location in the wall or collar region (Gai et al. 2015). It is important to note that FU-h, which is found in the gastropod-type hemocyanin, has, as an exception, an extra cupredoxin-domain at the C-terminus, in addition to a common FU structure composed of the NTD and CTD (Fig. 2). These observations indicate that the huge cylindrical molecule is built up through the assembly of similar structural building blocks.
Fig. 2.
Structures of the functional units. a, b Ribbon diagrams of FU-g (a) and FU-h (b). d, e Dimer structure of FU-g (d) and FU-h (e) are also shown. The N-terminal core domain (N), C-terminal β-sandwich domain (C), and cupredoxin domain are shown in cyan, magenta, and orange, respectively. c Close-up views of active sites of FU-g. Coordinated residues are represented as sticks. Copper (Cu) atoms and oxygen atoms (O) are represented as spheres
All FUs possess a type-3 copper center at their repiratory active site, located in the NTD. Figure 2c shows the active site of the FU-g. Two oxygen atoms are bound between two copper atoms, Cu1 and Cu2, to form a Cu2O2 cluster (Fig. 2). The copper ions are coordinated by six His residues, with Cu1 coordinated with His41, His60, His69, and Cu2 coordinated with His179, His183, and His210 (residue numbers correspond to FU-a of squid hemocyanin). These residues are completely conserved among all FUs. It is important to note that His60 forms a thioether bridge between its Cε atom and a sulfur atom of Cys58 (Fig. 2). The geometry around the Cu2O2-binding site is conserved in all known FUs. Because each FU contains an oxygen-binding site, entire hemocyanin has as many oxygen-binding sites as the number of the FUs; for example, di-decameric keyhole limpet-type hemocyanin which is composed of 160 FUs has 160 oxygen-binding sites.
Several FUs have consensus sequences for N-linked glycosylation, i.e., Asn–X–Ser or Thr, (X ≠ P) (Gai et al. 2015; Stoeva et al. 2002, 1999; Thonig et al. 2014). In the case of squid hemocyanin, five of seven possible sites are glycosylated. Glycosylation is found on FU-a, -b, -d, and -e. Four of these sites are located at an identical position on the CTD. FU-d possesses an additional glycan on the NTD. These glycosylations may contribute to the significant medical properties of hemocyanin.
Structure of the wall region
All molluscan hemocyanins have a common decameric wall structure regardless of the number of protomers. For example, di- and tri-decamers are formed by stacking two and three identical decameric walls, respectively (Boisset and Mouche 2000; Gatsogiannis and Markl 2009; Gatsogiannis et al. 2007, 2015; Harris et al. 2004; Lamy et al. 1998; Lieb et al. 2010; Meissner et al. 2007; Zhang et al. 2013). The recently determined high-resolution cryoEM structure of Haliotis diversicolor hemocyanin (Zhang et al. 2013) and crystal structure of squid hemocyanin revealed the wall architecture in greater detail (Gai et al. 2015). In this section, we explain the architecture and assembly of the wall region.
The wall region is composed of ten protomers assembled with D5 symmetry—two protomers assemble to form a plate-like dimer with twofold symmetry, and five of the plate-like dimers are arranged with a fivefold symmetry, resulting in a D5 symmetrical cylinder (Fig. 3). In all known hemocyanins, the wall region is composed of FU-a, -b, -c, -d, -e, and -f. Within the plate-like dimer, all FUs form similar dimeric assemblies, with FU-a and -b, FU-d and -e, and FU-c and -f assembling to form similar pseudo twofold symmetrical dimers [hereafter referred to as FU-(a–b), FU-(d–e), and FU-(c–f), respectively]. This means that the FU dimer is an essential structural unit for the construction of the entire wall region. Interestingly, when the plate-like dimer is formed, two protomers are tangled by domain swapping using FU-e and FU-f to form FU-(e–f). Due to domain swapping, dimers of FU-(d–e) and FU-(c–f) are formed between the protomers, which means that four of the six FU dimers within the plate-like protomer dimer are formed by inter-protomer interactions. Consequently, extensive buried surface area is acquired between the two protomers, which reinforces the inter-protomer interaction.
Fig. 3.
Architecture of the wall region. Two protomers assemble into one plate-like protomer dimer with a twofold symmetry, shown as a green arrow. Five protomer dimers assemble to form a decamer with a fivefold symmetry, shown as an orange arrow, which generates D5 symmetry. Each protomer is represented by a separate color
The plate-like protomer dimer is divided into three parts: the top, middle, and bottom regions (Fig. 4). The middle region is composed of a pair of FUs, FU-(d–e). In contrast, the top and bottom regions are composed of two different FU dimers, i.e., FU-(a–b) and FU-(c–f). The relative orientation of the four FUs in the middle region is quite similar to that in the top and bottom regions (Fig. 4). These observations indicate that the dimer of FU dimers is a building-block forming the plate-like protomer dimer, in which three units are stacked.
Fig. 4.
Architecture of the plate-like dimer. a FUs in one plate-like dimer are highlighted in separate colors. Outline of one the protomer is highlighted. b Architecture of plate-like dimer. c–e Three types of FU dimers comprising the plate-like dimer. f–h The relative orientation of the four FUs in the top (f), middle (g), and bottom regions (h) are shown
In conclusion, the FU dimer plays an essential role as a building element in the architecture of the cylindrical wall. Two FU dimers further associate into a dimer of FU dimers, which stack to form the plate-like protomer dimer. Five of the protomer dimers further assemble to form the wall region with a fivefold symmetry, which generates the D5 symmetrical cylinder. This hierarchical architecture is commonly observed in all known hemocyanin structures.
Decameric hemocyanin: cephalopod and polyplacophora hemocyanin
As described above, cephalopod hemocyanin, i.e., nautilus- and squid-type hemocyanin, is a decamer regardless of the composition of the FU. Since the structure of the wall region is common to all hemocyanins, the difference between nautilus-type and squid-type hemocyanin is restricted to within the inner collar domains: nautilus-type has only FU-g forming the inner collar, whereas squid-type has FU-g and FU-d* (Fig. 1b). Furthermore, polyplacophora hemocyanin, categorized into keyhole limpet-type hemocyanin according to the FU organization, also forms a decamer (Harris et al. 2004), although other keyhole limpet-type hemocyanins form exclusively multi-decamers. In this section, we explain the differences in the architecture of inner collar domains between these different molluscan hemocyanin types.
Figure 5 shows a cryoEM structure of nautilus-type hemocyanin (EMDB ID: 1434) (Gatsogiannis et al. 2007). The structure is a cylindrical decamer comprising a wall and five inner collar domains. The wall is a typical decameric wall with D5 symmetry, as described in the previous section. Each inner collar domain is composed of two copies of FU-g. The dimer of FU-g shows the same overall structure as the FU dimers observed in the wall region, meaning that the entire nautilus hemocyanin is constructed of FU dimers. This dimer was also observed in the crystal structure of FU-g obtained by protease treatment of octopus hemocyanin decamer, suggesting that this FU-type might plausibly be argued to possess an intrinsic propensity to form the homodimer (Cuff et al. 1998), whereas FUs of the wall, based on their local environment, form exclusively heterodimers. The FU-g dimer is formed between two protomers located apart in the wall region; for example, two FU-g copies of the protomers A and D form an inter-protomer FU-g dimer (Fig. 5). Therefore, the inner collar domain is thought to reinforce the interaction between the adjacent warped plate-like protomer dimers. Ten FU-gs form the five collar domains composed of inter-protomer FU-g dimers. These collar domains are located on one side of the cylinder with C5 symmetry (Gatsogiannis et al. 2007; Fig. 5). Taken together, nautilus-type hemocyanin has a cylindrical wall with D5 symmetry and five inner collar domains arranged with C5 symmetry at one end of the cylinder. Consequently, the entire structure of the nautilus-type hemocyanin is a decamer with C5 symmetry.
Fig. 5.
Nautilus-type hemocyanin. Conformation of nautilus-type hemocyanin illustrated from lateral (a, c) and top views (b, d). a, b Wall region and FU-g are shown as gray and green surfaces, respectively. c, d Protomers A and D, which cooperatively form an inner collar domain, are shown in red and blue, respectively
The squid-type hemocyanin (PDB ID 4YD9) as revealed by X-ray crystallography is shown in Fig. 6 (Gai et al. 2015). The wall region has the typical D5 architecture of the nautilus-type. The inner collars are composed of FU-d* and -g. The location of the FU-g is identical to that of the nautilus-type: inter-protomer FU-g dimers are arranged in C5 symmetry at one end of the cylinder, and FU-d* is located at the opposite end of the cylinder. Although the precise orientation of FU-d* was not determined by X-ray crystallography due to intermingling of two different orientations of the decamer in the crystal, an approximate location of FU-d*s was identified based on the anomalous signal of Cu2O2 cluster. Further, the distance between the anomalous signals suggested that FU-d*—as an exception to the rule—does not form FU dimers. Since the anomalous signal showed fivefold symmetry in the cylinder, monomeric FU-d* is thought to be arranged in C5 symmetry. These observations reveal that the squid-type hemocyanin is a C5 symmetrical decamer in which FU-g dimers are arranged with fivefold symmetry at one end and monomeric FU-d*s are present with fivefold symmetry at the other end. Further analysis is necessary to determine the precise structure of the inner collar region of the squid-type hemocyanin.
Fig. 6.
Crystal structure of squid-type hemocyanin. Crystal structure of squid-type hemocyanin illustrated from lateral (a) and top (b) views. Wall region and FU-g are shown as gray and green ribbons, respectively. Cu2O2 cluster of FU-d* is shown as brown spheres because the precise structure of FU-d* has not yet been determined
Polyplacophora hemocyanin, categorized in the keyhole limpet-type hemocyanin on the basis of FU composition, is known to form decamers (Harris et al. 2004; Lambert et al. 1994), although other keyhole limpet-types assemble to form multi-decamers. Since the high-resolution structure of polyplacophora hemocyanin has not yet been determined, its precise structure remains unknown. However, based on the FU organization of polyplacophora hemocyanin being the same as that of other keyhole limpet-type hemocyanin, the decameric assembly is considered to be identical to a decamer comprising the di-decamer of a typical keyhole limpet-type hemocyanin (see next section).
Multi-decameric hemocyanin: gastropod hemocyanin
Most of the gastropod hemocyanins are multi-decamers, in which several copies of the typical decameric D5 walls (composed of FU-a, -b, -c, -d, -e, and -f) are stacked. According to the FU composition of the inner collar region, the multimeric state varies. Basically, the keyhole limpet-type hemocyanin possessing FU-g and -h as the collar region assembles to di-decamers, whereas mega hemocyanin possessing FU-g, -h, -f1, -f2, -f3, -f4, -f5, and -f6 as the collar domains forms tri-decamers. In this section, the structure of multi-decameric hemocyanin is discussed based on the FU composition of its collar region.
The keyhole limpet-type hemocyanin has been extensively studied. The cryoEM structure of hemocyanin was first reported for keyhole limpet hemocyanin (Gatsogiannis and Markl 2009) and later for H. diversicolor hemocyanin (PDB ID 3J32; Zhang et al. 2013) (Fig. 7). Most of the keyhole limpet-type assembles to di-decamers, in which FU-g and -h forms the inner collar domains. The di-decamer is formed by the stacking of two identical decamers in a tail-to-tail manner. In the decameric assembly, the wall and FU-g dimers assemble in a manner quite similar to that of the nautilus-type, i.e., five inter-protomer FU-g dimers are arranged in a fivefold symmetry on one side of the typical D5 wall lumen. FU-h covers the top of the FU-g dimer, in which FU-h also forms inter-protomer dimers, and five such FU-h dimers are arranged with a fivefold symmetry. Since FU-h possesses an extra cuppredoxin domain at the C-terminus, the dimer assembly is different from that of other FU dimers (Fig. 2). Consequently, FU-g and FU-h form homo-dimers with their counterparts. The combination of the subunits connected by inter-protomer FU dimer of FU-g and FU-h are identical, i.e., protomers A and D located apart in the wall region are connected by both FU-g and FU-h dimers. Consequently, the decamer assembled in this manner has a C5 symmetry. Then, two identical C5 decamers associate to form a di-decamer, wherein a twofold symmetric association is achieved through the surface with no inner collar region. Inner collar domains formed by inter-protomer FU-g and FU-h dimers are located on both sides of the cylinder. As a consequence of the twofold symmetrical association of the C5 decamers, the entire di-decamer has a D5 symmetry.
Fig. 7.
Keyhole limpet-type hemocyanin. Conformation of keyhole limpet-type hemocyanin illustrated from lateral (a) and top views (b). Wall region, FU-g, and FU-h are shown as gray, green, and orange surfaces, respectively
Mega-hemocyanin is a unique hemocyanin found in the cerithioid snails. It is composed of two different polypeptides: one is a typical subunit of 400 kDa having an FU composition similar to that of a typical gastropod-type hemocyanin (FU-a, -b, -c, -d, -e, -f, -g, and -h), whereas the other is an exceptional mega-subunit with 550 kDa composed of FU-a, -b, -c, -d, -e, -f, -f1, -f2, -f3, -f4, -f5, and -f6 (Gatsogiannis et al. 2015; Lieb et al. 2010). The former subunit forms C5 symmetrical decamers identical to those of the keyhole limpet-type and is composed of a D5 wall and fivefold symmetrically arranged inter-protomer dimers of FU-gs and FU-hs. In contrast, the latter forms a central mega-decamer composed of a typical D5 wall and a rhombus-shaped cylindrical core. The rhombus core is composed of FU-f1, -f2, -f3, -f4, -f5, and -f6. Interestingly, the relative arrangement of these FUs is not identical, although all ten protomers have identical sequences. In a complicated manner these FUs fold into their own conformation to build up the rhombus-shaped cylindrical core with a two-fold symmetry between the top and bottom halves. Consequently, the decamer of the mega-subunits form a twofold symmetrical decamer in which the twofold symmetrical rhombus core is wrapped by the typical D5 wall (Gatsogiannis et al. 2015; Lieb et al. 2010). The tri-decamer is formed by stacking two copies of the typical subunit decamers on both side of the mega-subunit decamer. The inner rhombus region of the mega-subunit decamer fills the inner region of the tri-decamer (Fig. 8). This complicated folding manner has been designated “protein origami” (Gatsogiannis et al. 2015). Due to the absence of the fivefold symmetry in the central mega-subunit decamer, the entire tri-decamer has only a twofold symmetry.
Fig. 8.
Architecture of mega-hemocyanin. a–c Architecture of mega-hemocyanin viewed from lateral (a) and top (b) and as sectional view (c). d Composition of each decamer of mega-hemocyanin. Gastropod-type decamer and rhombus-shaped cylindrical core are shown. For clarity, the sectional representation of gastropod-type decamer is also shown. e Top view of rhombus-shaped cylindrical core
Carbohydrate
Hemocyanin is known to possess oligosaccharides. The composition of the monosaccharide differs between organisms (Idakieva et al. 2004). Some FUs have consensus sequences for N-linked glycosylation (Asn–X–Ser or Thr, X ≠ P), and the structure of the carbohydrate is different between FUs (Siddiqui et al. 2007, 2009). Cristal structure has identified respective glycosylation for some of them. Biochemical data show that carbohydrate removal induces the dissociation of decameric molecules. The subunits of squid hemocyanin can assemble to form decamers in the presence of divalent cations, such as Ca2+, whereas subunits treated with glycosidase F can not reassociate into decamers (Gai et al. 2015). The inhibition of reassociation by deglycosylation suggests that carbohydrates play an important role in the assembly process. In the crystal structure of squid hemocyanin, all five bound carbohydrates are located on the outer surface of the wall where they form carbohydrate clusters (Fig. 9). Carbohydrate clusters are located at the interface of three protomers. Based on these observations, it is proposed that the carbohydrates reinforce the interaction between protomer dimers through the wall regions. In the cryoEM structure of KLH, carbohydrates were found on the rim of the cylinder wall as well as on the wall surface (Gatsogiannis and Markl 2009). This additional protruding carbohydrate is considered to hamper the stacking of decamers.
Fig. 9.
Carbohydrate. a Carbohydrate clusters. Carbohydrates are represented as cyan spheres. Each protomer is illustrated as individual colors. b Close-up view of the carbohydrate cluster. FUs and carbohydrates are indicated as ribbons and sticks, respectively. FUs are shown as color codes as shown in Fig. 4
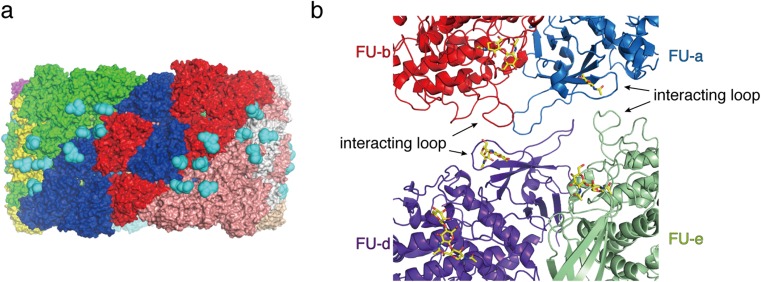
Glycosylation is thought also to contribute to the allosteric effect on oxygen binding and release as well as to inter-protomer interaction. Zhang et al. (2013) reported that four FUs located at the interface between three protomers form a “communication cluster” involved in the allosteric effect of hemocyanin. These authors proposed that FUs act cooperatively in the binding and release of oxygen, to which interactions between two long loops contribute significantly. In the crystal structure of squid hemocyanin, these four FUs form carbohydrate clusters. Moreover, two glycans are located on one of the interacting loops. N-linked glycan sites for these two glycans are completely conserved in all known hemocyanins. Based on these observations, it is proposed that glycosylation plays an important role in the allosteric effect of hemocyanin as well as in structural reinforcement.
Evolutionary implications
In this section, we describe hemocyanin structure from an evolutionary point of view. As mentioned earlier in this review, molluscan hemocyanins can be classified into four types according to their FU composition, i.e., nautilus-type, squid-type, keyhole limpet-type, and mega-type (see above). Further, taking the quaternary structure of the different types into consideration, we introduced an exception, namely, polyplacophora-type, which forms decamers in spite of its keyhole limpet-type FU composition. In phylogenetic systematics, the polyplacophora is classified into a primitive group, and the placement of polyplacophora in the phylogram is up-stream to that of the gastropods and cephalopods (Scheltema 1996; Salvini-Plawen and Steiner 1996; Waller 1998; Smith et al. 2011). Cephalopods diverged into nautiloids and coleoids. Nautilus is called a living fossil, and the placement of the ancestor of nautilus is close to ammonite in the phylogram. Decapoda, such as squids and cuttlefish, are diverged from coleioids (Kröger et al. 2011). With regards to gastropods, the first appearance of gastropoda is early Cambrian, and organisms having mega-hemocyanin, such as Melanoides tuberculate, are thought to have appeared later than those having keyhole limpet-type hemocyanin (Lieb et al. 2010).
Sequence-based analyses reveal that polyplacophora-type is an archetype hemocyanin, suggesting the possibility that the decamer of the keyhole limpet-type FUs is the original form of hemocyanin (Harris et al. 2004; Lambert et al. 1994). During the evolution of hemocyanin from polyplacophora-type to squid-type through nautilus-type, the organization of the FU, particularly the FUs of the inner collar region, changed greatly. Nautilus-type has evolved from the archetype hemocyanin by lacking FU-h. Due to the lack of FU-h, protrusion of the inner collar domains was located within the lumen of the wall. Upon further evolution to squid-type from nautilus-type, hemocyanin acquired additional FU-d* by gene duplication of FU-d. The additional FU-d* has been stored in the side opposite to the FU-g of the lumen. In these types that are evolutionarily correlated, structure of the inner collar domain has great diversity, whereas the wall is quite similar, which may suggest a role for the collar region as a modifying factor to accommodate the alteration in the organ system and/or environment arising from evolution.
Contrary to the cephalopod hemocyanin, evolution of gastropod hemocyanin mainly caused association to form multi-decamers. During the appearance of gastropod hemocyanin from the archetype hemocyanin, di-decameric assembly occurred with no alteration in the FU structure, and this process is not yet well unterstood. Since the association is carried out through the wall surface, mutations in the wall region would have stabilized the interaction between the decamers. The decameric wall itself is considered to have a propensity to stack linearly. For example, squid hemocyanin stacks to form a rod-like shape upon crystallization, although it exists as a discrete decamer in solution, which may suggest its inherent characteristics to stack linearly. It is likely that the interaction between decamers would have been optimized during evolution to produce stable di-decamers in solution. Mega-hemocyanin, which appeared later, further assembles to form a tri-decamer. Mega-hemocyanin developed from keyhole limpet-type by acquiring a new mega-subunit in which FU-g and -h were replaced by FU-f1, -f2, -f3, -f4, -f5, and -f6. The core region composed of these FUs (f1–f6) would reinforce the stacking of decamers by completely filling the lumen.
Physiological significance
Squid is one of the more difficult animals to rear and keep in captivity. Todarodes pacificus can live for only few days under aquarium conditions, even with quality-controlled water, i.e., constant water temperature, maintaining dissolved oxygen saturation level, and removing waste products. We sought to determine the etiology of squid death caused by captive breeding and have found that in vivo dissociation of hemocyanin decamer in hemolymph is deeply involved in this process (Yoshioka et al. 2012). Transmission electron microscopy studies of hemolymph from weakened squid during captivity have revealed that, hemocyanin decamers dissociate to subunit-oligomers or subunits (Fig. 10). This dissociation of decamer hemocyanin might trigger degradation of the hemocyanin subunit, which would disturb the biosynthesis of hemocyanin. A reduction in hemocyanin in the hemolymph may result in circulatory failure, which is assumed to be the cause of the weakness found in squids. It can be construed that stable decamer structure in vivo may prevent the degradation of hemocyanin. We conclude that retention of the molecular structure of hemocyanin in vivo is important for the maintenance of the health of these animals, and strategies aimed to accomplish this must be developed.
Fig. 10.
Negative stain of TEM image of hemocyanin of weakened squid. Most hemocyanins dissociate to subunit-oligomers or subunits. Bar: 100 nm
Acknowledgements
The authors thank JSPS KAKENHI (26291008 and 25450298) and Regional Innovation Strategy Support Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan, for the financial support. C.G. thanks the Max Planck Society for the support.
Compliance with ethical standards
Conflict of interest
Sanae Kato declares that she has no conflicts of interest. Takashi Matsui declares that he has no conflicts of interest. Christos Gatsogiannis declares that he has no conflicts of interest. Yoshikazu Tanaka declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Financial information
SK was a recipient of the JSPS KAKENHI (25450298) and Regional Innovation Strategy Support Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan. YT received support from JSPS KAKENHI (24000011, and 15KK0248) and JST, PRESTO (JPMJPR1517). TM received support from JSPS KAKENHI (16K18501).
Footnotes
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines - Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
Contributor Information
Sanae Kato, Phone: +81-99-2864073, Email: kato@fish.kagoshima-u.ac.jp.
Yoshikazu Tanaka, Phone: +81-22-2176205, Email: yoshikazu.tanaka@tohoku.ac.jp.
References
- Altenhein B, Markl J, Lieb B. Gene structure and hemocyanin isoform HtH2 from the mollusc Haliotis Tuberculata indicate early and late intron hot spots. Gene. 2002;301:53–60. doi: 10.1016/S0378-1119(02)01081-8. [DOI] [PubMed] [Google Scholar]
- Becker MI, Arancibia S, Salazar F, Campo MD, Ioannes AD. (2014) Mollusk hemocyanins as natural immunostimulants in biomedical applications. In: Duc GHT (ed) Immune response activation. InTech, Rijeka, Croatia, pp 45–72
- Bergmann S, Lieb B, Ruth P, Markl J. The hemocyanin from a living fossil, the cephalopod Nautilus pompilius: protein structure, gene organization, and evolution. J Mol Evol. 2006;62:362–374. doi: 10.1007/s00239-005-0160-x. [DOI] [PubMed] [Google Scholar]
- Boisset N, Mouche F. Sepia officinalis hemocyanin: a refined 3D structure from field emission gun cryoelectron microscopy. J Mol Biol. 2000;296:459–472. doi: 10.1006/jmbi.1999.3460. [DOI] [PubMed] [Google Scholar]
- Cuff ME, Miller KI, van Holde KE, Hendrickson WA. Crystal structure of a functional unit from octopus hemocyanin. J Mol Biol. 1998;278:855–870. doi: 10.1006/jmbi.1998.1647. [DOI] [PubMed] [Google Scholar]
- Fredericq L. La Physiologic du poulpe commun (Octopus vulgaris) Arch Zool Exp Gén. 1878;7:535–583. [Google Scholar]
- Gai Z, Matsuno A, Kato K, Kato S, Khan MRI, Shimizu T, Yoshioka T, Kato Y, Kishimura H, Kannno G, Miyabe Y, Terada T, Tanaka Y, Yao M. Crystal structure of the 3.8 MDa respiratory supermolecule hemocyanin at 3.0 Å resolution. Structure. 2015;23:2204–2212. doi: 10.1016/j.str.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Gatsogiannis C, Hofnagel O, Markl J, Raunser S. Structure of mega-hemocyanin reveals protein origami in snails. Structure. 2015;23:93–103. doi: 10.1016/j.str.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Gatsogiannis C, Markl J. Keyhole limpet hemocyanin: 9-a CryoEM structure and molecular model of the KLH1 didecamer reveal the interfaces and intricate topology of the 160 functional units. J Mol Biol. 2009;385:963–983. doi: 10.1016/j.jmb.2008.10.080. [DOI] [PubMed] [Google Scholar]
- Gatsogiannis C, Moeller A, Depoix F, Meissner U, Markl J. Nautilus Pompilius hemocyanin: 9 a cryo-EM structure and molecular model reveal the subunit pathway and the interfaces between the 70 functional units. J Mol Biol. 2007;374:465–486. doi: 10.1016/j.jmb.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Geyer H, Wuhrer M, Resemann A, Geyer R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J Biol Chem. 2005;280:40731–40748. doi: 10.1074/jbc.M505985200. [DOI] [PubMed] [Google Scholar]
- Ghiretti-Magaldi A, Ghiretti F. The pre-history of hemocyanin. The discovery of copper in the blood of molluscs. Experientia. 1992;48:971–972. doi: 10.1007/BF01919143. [DOI] [Google Scholar]
- Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron. 1999;30:597–623. doi: 10.1016/S0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Harris JR, Meissner U, Gebauer W, Markl J. 3D reconstruction of the hemocyanin subunit dimer from the chiton Acanthochiton fascicularis. Micron. 2004;35:23–26. doi: 10.1016/j.micron.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Idakieva K, Stoeva S, Voelter W, Cielens C. Glycosylation of Rapana thomasiana hemocyanin. Comparison with other prosobranch (gastropod) hemocyanins. Comp Biochem Physiol B. 2004;138:221–228. doi: 10.1016/j.cbpc.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Jaenicke E, Buchler K, Decker H, Markl J, Schroder GF. The refined structure of functional unit h of keyhole limpet hemocyanin (KLH1-h) reveals disulfide bridges. IUBMB Life. 2011;63:183–187. doi: 10.1002/iub.435. [DOI] [PubMed] [Google Scholar]
- Jaenicke E, Buchler K, Markl J, Decker H, Barends TR. Cupredoxin-like domains in haemocyanins. Biochem J. 2010;426:373–378. doi: 10.1042/BJ20091501. [DOI] [PubMed] [Google Scholar]
- Jaenicke E, Fraune S, May S, Irmak P, Augustin R, Meesters C, Decker H, Zimmer M. Is activated hemocyanin instead of phenoloxidase involved in immune response in woodlice? Dev Comp Immunol. 2009;33(10):1055–1063. doi: 10.1016/j.dci.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Kröger B, Vinther J, Fuches D. Cephalopod origin and evolution: a congruent picture emerging from fossils, development and molecules. BioEssays. 2011;33:602–613. doi: 10.1002/bies.201100001. [DOI] [PubMed] [Google Scholar]
- Lambert O, Boisset N, Taveau JC, Lamy JN. Three-dimensional reconstruction from a frozen-hydrated specimen of the chiton Lepidochiton sp. hemocyanin. J Mol Biol. 1994;244:640–647. doi: 10.1006/jmbi.1994.1757. [DOI] [PubMed] [Google Scholar]
- Lamy J, You V, Taveau JC, Boisset N, Lamy JN. Intramolecular localization of the functional units of sepia officinalis hemocyanin by immunoelectron microscopy. J Mol Biol. 1998;284:1051–1074. doi: 10.1006/jmbi.1998.2235. [DOI] [PubMed] [Google Scholar]
- Lieb B, Altenhein B, Markl J. The sequence of a gastropod hemocyanin (HtH1 from Haliotis tuberculata) J Biol Chem. 2000;275:5675–5681. doi: 10.1074/jbc.275.8.5675. [DOI] [PubMed] [Google Scholar]
- Lieb B, Gebauer W, Gatsogiannis C, Depoix F, Hellmann N, Harasewych MG, Strong EE, Markl J. Molluscan mega-hemocyanin: an ancient oxygen carrier tuned by a ~550 kDa polypeptide. Front Zool. 2010;7:14. doi: 10.1186/1742-9994-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb B, Markl J. Evolution of molluscan hemocyanins as deduced from DNA sequencing. Micron. 2004;35:117–119. doi: 10.1016/j.micron.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Markl J. Evolution of molluscan hemocyanin structures. Biochim Biophys Acta. 2013;1834:1840–1852. doi: 10.1016/j.bbapap.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Matsuno A, Gai Z, Tanaka M, Kato K, Kato S, Katoh T, Shimizu T, Yoshioka T, Kishimura H, Tanaka Y, et al. Crystallization and preliminary X-ray crystallographic study of a 3.8-MDa respiratory supermolecule hemocyanin. J Struct Biol. 2015;190:379–382. doi: 10.1016/j.jsb.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Meissner U, Dube P, Harris JR, Stark H, Markl J. Structure of a molluscan hemocyanin didecamer (HtH1 from Haliotis tuberculata) at 12 a resolution by cryoelectron microscopy. J Mol Biol. 2000;298:21–34. doi: 10.1006/jmbi.2000.3631. [DOI] [PubMed] [Google Scholar]
- Meissner U, Gatsogiannis C, Moeller A, Depoix F, Harris JR, Markl J. Comparative 11 Å structure of two molluscan hemocyanins from 3D cryo-electron microscopy. Micron. 2007;38:754–765. doi: 10.1016/j.micron.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Miller KI, Cuff ME, Lang WF, Varga-Weisz P, Field KG, van Holde KE. Sequence of the Octopus dofleini hemocyanin subunit: structural and evolutionary implications. J Mol Biol. 1998;278:827–842. doi: 10.1006/jmbi.1998.1648. [DOI] [PubMed] [Google Scholar]
- Miller KI, Schabtach E, van Holde KE. Arrangement of subunits and domains within the Octopus dofleini hemocyanin molecule. Proc Natl Acad Sci USA. 1990;87:1496–1500. doi: 10.1073/pnas.87.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbandt M, Guthohrlein EW, Rypniewski W, Idakieva K, Stoeva S, Voelter W, Genov N, Betzel C. The structure of a functional unit from the wall of a gastropod hemocyanin offers a possible mechanism for cooperativity. Biochemistry. 2003;42:6341–6346. doi: 10.1021/bi020672x. [DOI] [PubMed] [Google Scholar]
- Salvini-Plawen LV, Steiner G (1996) Synapomorphies and plesiomorphies in higher classification of Mollusca. In: Taylor JD (ed) Origin and evolutionary radiation of the Mollusca. Oxford University Press, Oxford, pp 29–51
- Scheltema AH (1996) Phylogenetic position of Sipuncula, Mollusca and the progenetic Aplacophora. In: Taylor JD (ed) Origin and evolutionary radiation of the Mollusca. Oxford University Press, Oxford, pp 53–58
- Smith SA, Wilson NG, Goetz FE, Feehery C, Andrade SCS, Rouse GW, Giribet G, Dunn CW (2011) Resolving the evolutionary relationships of molluscs with phylogenomic tools. Nature 480(7377):364–367 [DOI] [PubMed]
- Siddiqui NI, Idakieva K, Demarsin B, Doumanova L, Compernolle F, Gielens C. Involvement of glycan chains in the antigenicity of Rapana thomasiana hemocyanin. Biochem Biophys Res Commun. 2007;361:705–711. doi: 10.1016/j.bbrc.2007.07.098. [DOI] [PubMed] [Google Scholar]
- Siddiqui NI, Yigzaw Y, Préaux G, Gielens C. Involvement of glycans in the immunological cross-reaction between α-macroglobulin and hemocyanin of the gastropod Helix pomatia. Biochimie. 2009;91:508–516. doi: 10.1016/j.biochi.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Stoeva S, Idakieva K, Betzel C, Genov N, Voelter W. Amino acid sequence and glycosylation of functional unit RtH2-e from Rapana thomasiana (gastropod) hemocyanin. Arch Biochem Biophys. 2002;399:149–158. doi: 10.1006/abbi.2001.2741. [DOI] [PubMed] [Google Scholar]
- Stoeva S, Schutz J, Gebauer W, Hundsdorfer T, Manz C, Markl J, Voelter W. Primary structure and unusual carbohydrate moiety of functional unit 2-c of keyhole limpet hemocyanin (KLH) Biochim Biophys Acta. 1999;1435:94–109. doi: 10.1016/S0167-4838(99)00198-3. [DOI] [PubMed] [Google Scholar]
- Thonig A, Oellermann M, Lieb B, Mark FC. A new haemocyanin in cuttlefish (Sepia officinalis) eggs: sequence analysis and relevance during ontogeny. EvoDevo. 2014;5:6. doi: 10.1186/2041-9139-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller TR (1998) Origin of the molluscan class Bivalvia and a phylogeny of major groups. In: Johnston PA, Haggart JW (eds) Bivalves: an eon of evolution. Univ. Calgary Press, Calgary, pp 1–45
- Yoshioka T, Kato S, Okamoto A. Short-term rearing conditions for shipping live squids. In: Fukuta Y, Watabe S, editors. Quality improvement of coastal fish and marine invertebrates—achievement by short-term rearing and associated systems for transportation and marketing. Tokyo: Kouseisyakouseikaku; 2012. pp. 106–129. [Google Scholar]
- Zhang Q, Dai X, Cong Y, Zhang J, Chen DH, Dougherty MT, Wang J, Ludtke SJ, Schmid MF, Chiu W. Cryo-EM structure of a molluscan hemocyanin suggests its allosteric mechanism. Structure. 2013;21:604–613. doi: 10.1016/j.str.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhuang J, Feng H, Liang R, Wang J, Xie L, Zhu P. Cryo-EM structure of isomeric molluscan hemocyanin triggered by viral infection. PLoS One. 2014;9:e98766. doi: 10.1371/journal.pone.0098766. [DOI] [PMC free article] [PubMed] [Google Scholar]