Abstract
Amyloid deposition of human islet amyloid polypeptide (hIAPP) within the islet of Langerhans is closely associated with type II diabetes mellitus. Accumulating evidence indicates that the membrane-mediated aggregation and subsequent deposition of hIAPP are linked to the dysfunction and death of insulin-producing pancreatic β-cells, but the molecular process of hIAPP deposition is poorly understood. In this review, I focus on recent in vitro studies utilizing model membranes to observe the membrane-mediated aggregation/deposition of hIAPP. Membrane surfaces can serve as templates for both hIAPP adsorption and aggregation. Using high-sensitivity surface analyzing/imaging techniques that can characterize the processes of hIAPP aggregation and deposition at the membrane surface, these studies provide valuable insights into the mechanism of membrane damage caused by amyloid deposition of the peptide.
Keywords: Islet amyloid polypeptide, Amyloid deposition, Model membranes, Membrane disruption, Type II diabetes mellitus
Introduction
The deposition of insoluble amyloid fibrils in tissues is a hallmark of many diseases, including type II diabetes mellitus (T2DM), Alzheimer’s disease, Parkinson’s disease, and prion disease (Chiti and Dobson 2006, 2017). Amyloid fibrils are highly ordered fibrillar aggregates that adopt a characteristic cross-β-sheet structure, in which β-strands are oriented perpendicularly to the fibril axis (Sunde and Blake 1997; Greenwald and Riek 2010). Amyloid aggregation of normally soluble disease-specific peptides or proteins into β-sheet-rich fibrillar structures is considered a crucial step in the pathology of amyloid-related disorders (Eisenberg and Jucker 2012). The amyloid aggregation/deposition of peptides and proteins into fibrillar structures is a highly complex process involving various biomolecules and multiple oligomeric and protofibrillar structures.
Human islet amyloid polypeptide (hIAPP, also known as amylin) is a 37-residue peptide (Fig. 1a) and the primary component of amyloid aggregates (deposits) found in the pancreatic islet of most patients with T2DM (Westermark et al. 2011). Substantial evidence indicates that the amyloid deposition of hIAPP in the extracellular space of pancreatic β-cells is strongly associated with progressive declines in the function and mass of β-cells, indicating the possible contribution of islet amyloidosis to the development of T2DM (Lorenzo et al. 1994; Kahn et al. 1999; Kapurniotu 2001; Jaikaran and Clark 2001; Clark and Nilsson 2004; Hull et al. 2004; Höppener and Lips 2006; Haataja et al. 2008; Jurgens et al. 2011). Soluble hIAPP adopts a predominantly random coil structure, suggesting that it is natively unfolded in its monomeric form (Padrick and Miranker 2001). The physiological roles of hIAPP are not completely understood, but the protein is believed to participate in glucose homeostasis via the regulation of insulin and glucagon secretions, in addition to roles in gastric emptying, controlling satiety, and other cellular processes (Westermark et al. 2011; Lutz 2012).
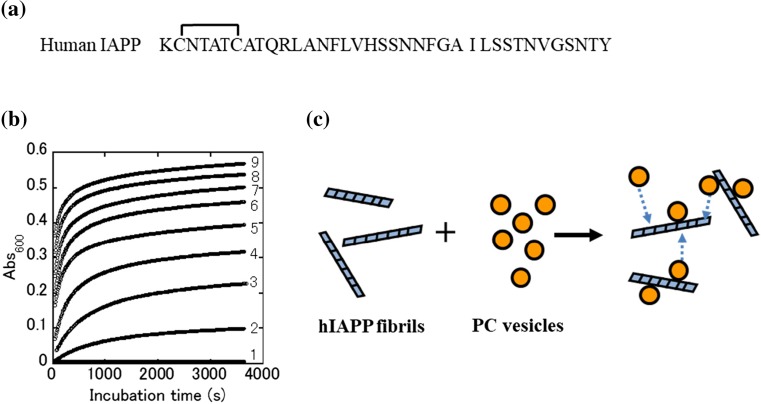
Fig. 1.
Binding of lipid vesicles to human islet amyloid polypeptide (hIAPP) amyloid fibrils. a Amino acid sequence of hIAPP. The peptide has a disulfide bond between residues 2 and 7. b Time-dependent changes in the solution turbidity of hIAPP fibrils (25 μg/mL) and phosphatidylcholine (PC) vesicles at various concentrations of 0 (1), 0.1 (2), 0.25 (3), 0.5 (4), 1.0 (5), 1.5 (6), 2.0 (7), 2.5 (8), and 3.0 mg/mL (9). c Simplified schematic representing the association reaction between PC vesicles and hIAPP fibrils (adapted from Sasahara et al. 2010)
hIAPP, which is co-secreted with insulin, is stored in pancreatic β-cells at a ratio of 1:50 to 1:100 relative to insulin in healthy individuals, but, intriguingly, it is reported that this ratio can be as high as 1:20 in patients with T2DM (Kahn et al. 1990; Gedulin et al. 1991; Hull et al. 2004; Knight et al. 2008). Several in vitro studies revealed that insulin has a potent inhibitory effect on hIAPP fibril formation, suggesting that a decrease in this inhibitory effect, in a state of deficient insulin production associated with T2DM, would promote hIAPP amyloid aggregation (Larson and Miranker 2004; Gilead et al. 2006; Wei et al. 2009; Cui et al. 2009; Susa et al. 2014).
In vitro studies illustrated that monomeric hIAPP has a high propensity to aggregate into oligomers, proto-fibrils, and, ultimately, mature amyloid fibrils in physiological buffer solutions (Kayed et al. 1999; Goldsbury et al. 2000; Padrick and Miranker 2001; Yanagi et al. 2011). Detailed structures of hIAPP fibrils have been clarified gradually (Goldsbury et al. 1997; Sumner Makin and Serpell 2004; Kajava et al. 2005; Luca et al. 2007; Wiltzius et al. 2008; Bedrood et al. 2012), but few details regarding the structure of hIAPP oligomers are known, mainly due to the structural heterogeneity and transient nature of the species. Substantial evidence supports the toxic oligomer hypothesis, which states that membrane disruption and concomitant β-cell death are caused by toxic hIAPP amyloid oligomers, which precede mature fibrils (Janson et al. 1999; Anguiano et al. 2002; Porat et al. 2003; Ritzel et al. 2007; Haataja et al. 2008). Various factors such as the peptide concentration, pH, ionic strength, metal ion levels, and interactions with biological compounds affect the kinetics of hIAPP amyloid fibril formation and the related cytotoxicity (Nguyen et al. 2015; Tomasello et al. 2015).
It is increasingly recognized that various biomolecules such as apolipoprotein E, metals, glycoproteins, glycosaminoglycans, and lipids are associated with various amyloid aggregates (deposits) formed in vivo (Gellermann et al. 2005; Nguyen et al. 2015). This suggests that the interactions of hIAPP with various biomolecules affect the extent of its aggregation, as well as the morphological characteristics and related cytotoxicity of the resulting aggregates/deposits (Nguyen et al. 2015). Among these biomolecules, the effects of lipid membranes on hIAPP aggregation have been extensively investigated in vitro, as it has become evident that lipid membranes serve as sites for the nucleation/accumulation of fibrillar aggregates of hIAPP and represent possible targets for the toxic assemblies formed during the aggregation process (Gorbenko and Kinnunen 2006; Jayasinghe and Langen 2007; Brender et al. 2012; Gao and Winter 2015; Caillon et al. 2016). In vitro studies using model membranes developed as simple cell model systems have made major contributions to the understanding of hIAPP-induced membrane damage related to β-cell death in T2DM (Jayasinghe and Langen 2007; Engel 2009; Brender et al. 2012; Gao and Winter 2015). These studies revealed that hIAPP amyloid aggregation is substantially modulated by the properties of lipid membranes, such as lipid species, charge, and composition, which significantly affect the associated membrane damage caused by the peptide. As the role of model membranes in hIAPP amyloid aggregation was previously discussed in several excellent reviews (Jayasinghe and Langen 2007; Brender et al. 2012; Byström et al. 2008; Relini et al. 2009, 2014; Engel 2009; Gao and Winter 2015), the present paper mainly focuses on the membrane-mediated deposition of hIAPP.
Amyloid aggregation of hIAPP in the presence of lipid vesicles
Lipid vesicles have been used as model membranes for in vitro studies of hIAPP aggregation in the presence of lipid membranes (Knight and Miranker 2004; Jayasinghe and Langen 2005; Seeliger et al. 2012; Zhang et al. 2017). These studies revealed that amyloid fibril formation by hIAPP is markedly accelerated in the presence of negatively charged lipid vesicles relative to that observed in solutions. hIAPP, a cationic peptide, has three positively charged residues at its N-terminus at neutral pH, and these residues induce an electrostatic attraction with negatively charged lipids (Knight and Miranker 2004; Jayasinghe and Langen 2005). After binding of hIAPP to the negatively charged membrane, the N-terminus (amino acids 1−19) was observed to insert into the membrane, after which the peptide forms an α-helical structure (Engel et al. 2006; Williamson et al. 2009; Apostolidou et al. 2008; Nanga et al. 2011). Additionally, the structures of IAPP fragments and of full-length IAPP in membrane mimetic environments have been studied in detail by NMR and circular dichroism spectroscopy. The N-terminal portion of these peptides adopt an α-helical structure (Nanga et al. 2008, 2009, 2011; Brender et al. 2008). The region encompassing amino acids 20−29 is considered to be one of the major determinants of the ability of various IAPP variants (e.g., rat/mouse, monkey, porcine, cow, cat, dog) to form amyloid fibrils; this region of hIAPP is amyloidogenic, unlike that of other variants such as rat/mouse IAPP (Westermark et al. 1990; Goldsbury et al. 2000). Rat/mouse IAPP has three proline residues within the 20−29 region, and its inability to form amyloids is attributed to these proline substitutions (Westermark et al. 1990). It has been proposed that the formation of membrane-bound, α-helical hIAPP could promote amyloid nucleation by increasing the local concentration and enhancing the orientation of the peptide, indicating that membrane surfaces can serve as templates for amyloid aggregation (Jayasinghe and Langen 2007; Apostolidou et al. 2008; Byström et al. 2008; Relini et al. 2009, 2014; Engel 2009; Hoppe and Minton 2015).
Biological membranes are highly dynamic and heterogeneous assemblies containing numerous lipid species and membrane proteins; as such, the curvature, charge, fluidity, structural asymmetry, and heterogeneity of membranes play pivotal roles in their biological functions (Engelman 2005; Marguet et al. 2006; Kusumi et al. 2011). Lipid rafts are membrane microdomains abundant in cholesterol (Chol) and sphingomyelin (SM), and they are considered to provide highly dynamic platforms for various cellular processes, such as signal transduction, protein sorting, and protein trafficking (Edidin 2003; Lingwood and Simons 2010). Thus, perturbation of raft domains due to hIAPP amyloid aggregation at membrane surfaces could affect a broad range of signal cascades in vivo. The β-cell membrane contains raft components and 2.5−13.2 mol% of anionic lipids (Rustenbeck et al. 1994; Seeliger et al. 2012). Recently, Winter and coworkers examined amyloid aggregation by hIAPP in the presence of vesicles composed of lipids isolated from the rat insulinoma-derived INS-1E β-cell line (Seeliger et al. 2012). They demonstrated that isolated β-cell membranes exert similar effects on the kinetics of hIAPP as anionic model vesicles. Utilization of isolated β-cell membranes in biophysical studies is of significance, as this bridges the gap between in vitro and in vivo studies.
Binding of hIAPP amyloid fibrils to lipid vesicles
In contrast to the toxic oligomer hypothesis, Engel et al. (2008) found that the growth of hIAPP fibrils at the lipid vesicle surface rather than the presence of a particular oligomer species induces membrane damage by shifting the curvature of the bilayer toward unfavorable angles. More recently, it was reported that the growth of amyloid fibrils on the membrane can distort the shape of lipid vesicles, leading to the disruption of membrane structures as the fibril elongates (Sciacca et al. 2012b). Additionally, careful microscopic analysis indicated association states between extracellular hIAPP amyloid fibrils and β-cell membranes, in which the fibrils are often oriented toward β-cells (Westermark 1973; Clark et al. 1987; Jaikaran and Clark 2001; Engel 2009). These observations suggest that the mature amyloid fibrils of hIAPP possess binding affinity for lipid membranes under physiological conditions. Thus, it may be of interest to elucidate the membrane-bound forms of hIAPP fibrils with a detailed characterization of the binding reactions.
In this context, researchers attempted to evaluate the binding reaction between mature hIAPP fibrils and lipid vesicles composed of phosphatidylcholine (PC) using a turbidity assay (Sasahara et al. 2010). After the addition of a solution of hIAPP fibrils to PC vesicle solutions at various concentrations, the binding reactions were monitored by the time-dependent appearance of solution turbidity (Fig. 1b). The turbidity of the mixed solutions significantly increased with increasing concentrations of PC vesicles. It was postulated that the vesicles can diffuse rapidly in the solution and associate with the fibrils, drastically increasing the turbidity of the suspensions (Fig. 1c). When negatively charged DOPS (1,2-dioleoyl-sn-glycero-3-phospho-L-serine) was included in PC vesicles, greater increases in the solution turbidity were observed during the initial stage, accelerating the binding reaction. These results indicate that the amyloid fibrils of hIAPP have a latent ability to perturb lipid vesicles by attracting them, particularly negatively charged lipid vesicles, and suggest that hIAPP amyloid deposits most likely interfere with normal β-cell functioning.
Amyloid aggregation of hIAPP at substrate-supported lipid bilayers
Substrate-supported lipid bilayers (SLBs) have been widely used as cell-surface models for investigating the interactions of amyloidogenic peptides with lipid membranes (Mirzabekov et al. 1996; Green et al. 2004; Lopes et al. 2007; Domanov and Kinnunen 2008; Vestergaard et al. 2008; Sasahara et al. 2012, 2014; Sciacca et al. 2016). SLBs can usually be formed by incubating lipid vesicles with a suitable substrate, such as silica or mica, in which the substrate-adsorbed vesicles rupture and transform into planar bilayers (Richter et al. 2006). Lipids in the bilayers formed on planar glass substrates freely diffuse in the plane because a thin layer of water molecules separates the glass substrate and bilayer (Fig. 2a). The diffusion coefficient of a lipid can be quantitatively evaluated via fluorescence recovery after photobleaching (FRAP) (Merzlyakov et al. 2006; Okazaki et al. 2009). Compared with lipid vesicles, one of the advantages of SLBs with fluid two-dimensional spaces is that they enable us to use surface analyzing/imaging techniques that can detect interfacial events such as protein adsorption to the membranes, protein−lipid associations, and protein assembly at the membrane surfaces.
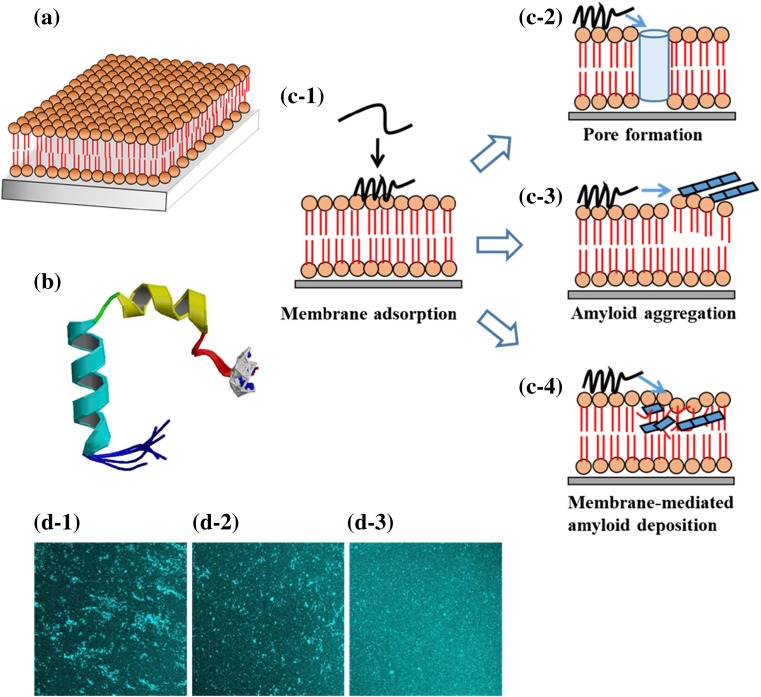
Fig. 2.
Amyloid aggregation and deposition of hIAPP at a substrate-supported lipid bilayer (SLB) and inhibitory effect of insulin. a SLBs contain a bilayer separated from a substrate by a thin layer of water. b High-resolution NMR structures of sodium dodecyl sulfate micelle-bound hIAPP at physiological pH. hIAPP adopts a helix–kink–helix structure with the helices from residues 7 to 17 and 21 to 28 (from Nanga et al. 2011, PDB code: 2 L86). c Interactions of hIAPP with SLBs. c-1 Adsorption or insertion of soluble hIAPP onto the membrane surface. c-2 Channel-like pore formation. c-3 Amyloid aggregation of membrane-bound hIAPP. c-4 Membrane-mediated amyloid deposition of hIAPP. d Inhibitory effect of insulin on hIAPP deposition. hIAPP aggregation was induced from membrane-adsorbed hIAPP (10 μM) by the addition of buffer solution containing various concentrations of insulin, namely: d-1 0, d-2 2.8, and d-3 5 μM. The areas of the images are approximately 320 × 320 μm2 (adapted from Sasahara et al. 2014)
In vitro studies using SLBs revealed that amyloidogenic species of hIAPP, such as oligomers and protofibrils, cause membrane damage through the formation of nonselective ion-permeable channels (Mirzabekov et al. 1996; Quist et al. 2005), detergent-like membrane disorders (Green et al. 2004), and the uptake of lipids into fibrils (Domanov and Kinnunen 2008). In the ion-permeable channel action, amyloidogenic species can form localized pores or channels through interactions with the membrane surface, which cause an uncontrolled flux of ions across the membrane. The detergent-like and lipid extraction mechanisms are likely due to the formation of hydrophobic oligomers during fibril formation at the membranes. It appears that these mechanisms of membrane disruption are workable simultaneously, and the relative balance between each mechanism can be influenced depending on various lipid environments (Cao et al. 2013; Abedini and Schmidt 2013). Recent experiments performed using SLBs and a variety of lipid environments illustrated that raft components have a significant influence on the membrane-driven aggregation of hIAPP and its accumulation on the membranes (Cho et al. 2009; Weise et al. 2010; Trikha and Jeremic 2011; Sciacca et al. 2016). Thus, the biophysical property of lipid membranes, which significantly depends on lipid species and composition, is most likely a key factor that governs membrane binding and the extent to which membranes affect the amyloid aggregation and subsequent deposition of hIAPP (Engel 2009; Caillon et al. 2016). Despite extensive studies using SLBs, the detailed molecular mechanism of the membrane-mediated amyloid aggregation/deposition of hIAPP remains poorly understood.
Binding of hIAPP to SLBs and amyloid deposition
In light of the ability of hIAPP to bind to negatively charged membranes, the amount of hIAPP adsorbed onto lipid membranes is likely an important factor in determining the level of amyloid deposition. Adsorption of hIAPP in the soluble and fibrillary states onto the SLB [1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/(DOPS)] was examined using quartz crystal microbalance with dissipation monitoring (QCM-D) (Sasahara et al. 2012). QCM-D has been used to determine the adsorption/desorption kinetics of proteins from solutions on various types of surfaces (Dixon 2008). The QCM-D traces revealed significant differences in binding abilities among different conformational states of hIAPP; specifically, soluble hIAPP exhibited greater affinity for SLBs formed on a QCM-D sensor surface than its fibril structure. The binding behaviors of soluble hIAPP for DOPC/DOPS with and without the raft components (SM and Chol) were also evaluated using QCM-D (Sasahara et al. 2014). The results indicated that the raft components promote the adsorption of hIAPP molecules onto the SLB and potentially affect the membrane-adsorbed form of the peptide.
The amyloid aggregation and deposition of soluble hIAPP that adsorbed onto SLBs (DOPC/DOPS and DOPC/DOPS/SM/Chol) were observed at the membrane surface using a fluorescence microscope (Sasahara et al. 2014). Upon amyloid aggregation of the membrane-bound hIAPP molecules, small aggregates were observed immediately at the membrane surface. They gradually expanded into larger self-assembled structures during incubation on the membrane, whereas amyloid deposits attached to or incorporated into the bilayer were observed at the membrane. As a result, observation of the membrane surfaces revealed that membrane-adsorbed hIAPP can self-assemble into larger amyloid aggregates on the surfaces and associate with lipids during the aggregation process, resulting in membrane-associated deposition. These results indicate that the lipid membranes act as templates at which the amyloid aggregation/deposition of hIAPP is promoted in physiological buffer solutions.
There is substantial evidence that hIAPP adopts a helical structure after binding to membrane surfaces in a process associated with the catalysis of fibrillation (Jayasinghe and Langen 2005; Engel et al. 2006; Lopes et al. 2007; Williamson et al. 2009; Apostolidou et al. 2008). Furthermore, it has been demonstrated from in vitro experiments using lipid vesicles that the lipid species (Jayasinghe and Langen 2005; Sciacca et al. 2012b; Seeliger et al. 2012; Zhang et al. 2017), lipid composition (Sciacca et al. 2012b; Zhang et al. 2017), and peptide/lipid ratio (Knight and Miranker 2004; Knight et al. 2006) have marked effects on membrane−hIAPP interactions associated with amyloid aggregation and deposition. These results suggest that complex interplay between hIAPP and membranes is involved in membrane-mediated amyloid aggregation/deposition in the membrane environment. Recently, Ramamoorthy’s group have proposed a two-step mechanism in which the first step involves interaction of prefibrillar species with the membrane and the second step correlates with fibril growth on the membrane causing membrane fragmentation (Brender et al. 2007, 2012; Sciacca et al. 2012a). Taken together, the results obtained in in vitro experiments indicate that the extent of membrane-mediated aggregation/deposition is related to the amount of membrane-adsorbed hIAPP and specific structures of the peptide formed on the membrane (Fig. 2b).
Figure 2c1–4 shows simplified schematics representing the membrane-mediated amyloid deposition of hIAPP during the aggregation process. In the case of SLBs, in which bilayers are stably formed on a substrate, the membrane-adsorbed hIAPP molecules self-assemble into larger aggregates at the membrane surface, and some of the aggregates (near the interface) associate with the lipid components during the aggregation process. Oligomeric species and initially assembled aggregates of hIAPP could expose more flexible hydrophobic surfaces that mediate interactions with lipid membranes, causing the insertion of early oligomers into the membranes. Recent studies indicated that the surface hydrophobicity, size, and structural flexibility of amyloid oligomers are major determinants of amyloid-related cytotoxicity (Cecchi and Stefani 2013). As shown in Fig. 1, mature hIAPP fibrils also have the ability to associate with lipids. Lipid vesicles and SLBs containing raft components such as SM and Chol have greater binding affinity for hIAPP fibrils and soluble IAPP than raft-free samples, respectively (Sasahara et al. 2010, 2014). These results suggest that, in addition to electrostatic interactions between hIAPP and negatively charged membranes, hydrophobic interactions likely play an important role in hIAPP amyloid deposition.
Changes in membrane fluidity
Membrane fluidity is critical for biological functions in cell membranes, such as the formation of lipid microdomains (e.g., lipid rafts) and assembly of membrane proteins into signaling complexes (Lingwood and Simons 2010). Measurements of membrane fluidity have been widely conducted for both model lipid bilayers and cell membranes (Yamazaki et al. 2005; Deverall et al. 2005; Owen et al. 2009). The effects of membrane binding and subsequent amyloid aggregation by hIAPP on the lateral fluidity of SLBs (DOPC/DOPS and DOPC/DOPS/SM/Chol) were quantitatively evaluated using FRAP (Sasahara et al. 2012, 2014). In the procedure, an area of the sample is first quickly photobleached via intense illumination. As mobile fluorescence-labeled lipids from outside the bleached region diffuse into the dark area, fluorescence recovers at the boundary region. The diffusion coefficient can be measured by evaluating the speed of this recovery (Merzlyakov et al. 2006; Okazaki et al. 2009). The diffusion coefficient of lateral lipid mobility decreased substantially after soluble hIAPP bound the membrane. Amyloid aggregation by membrane-adsorbed hIAPP promoted a partial recovery of membrane fluidity. The changes of membrane fluidity were attributed to variation in the constraint imposed by the adsorbed hIAPP on lipid mobility. The constraint is relaxed when hIAPP is released from the membrane-adsorbed forms to start self-assembly into amyloid aggregates (some peptide molecules associate with the membrane), resulting in the partial recovery of membrane fluidity. The extent of recovery was lower in raft-containing SLBs than in raft-free SLBs, suggesting the enhanced association of hIAPP aggregates with the raft components. These data indicate that membrane fluidity is significantly affected by the processes of membrane binding, self-aggregation into amyloid aggregates, and membrane-associated hIAPP deposition. However, the effects of hIAPP aggregation/deposition on the dynamic behaviors of membranes remain poorly understood.
Inhibition of hIAPP deposition
Many in vitro studies illustrated that the amyloid aggregation and deposition of hIAPP are associated with disruption of the membrane integrity and dysfunction of β-cells (Khemtémourian et al. 2008; Engel 2009; Brender et al. 2012; Gao and Winter 2015). Hence, identifying inhibitors of hIAPP amyloid aggregation is considered a key therapeutic approach for treating T2DM (Stefani and Rigacci 2013). Several inhibitors of hIAPP amyloid aggregation have been identified. Particularly, the therapeutic potential of polyphenolic natural products, such as (−)-epigallocatechin-3-gallate (EGCG), curcumin, and resveratrol, have received attention against hIAPP aggregation and the related toxicity (Radovan et al. 2009; Patel et al. 2014; Pithadia et al. 2016). Although these inhibitors are thought to interact with hIAPP via aromatic π–π interactions, the precise mechanism is not fully understood (Pithadia et al. 2016; Caillon et al. 2016).
The dysregulation of metal ion homeostasis has been implicated in the pathogenesis of amyloid-related diseases (DeToma et al. 2012). As β-cell granules contain a high concentration of zinc (Foster et al. 1993), the effect of zinc on hIAPP fibrillation has been examined in vitro (Brender et al. 2010, 2013; Salamekh et al. 2011). hIAPP fibrillation in the presence of zinc is complex and significantly depends on zinc concentration, but zinc has an inhibitory effect on hIAPP aggregation partly through interactions with His 18. These results suggest that zinc homeostasis could play a significant role in hIAPP fibrillation/deposition (DeToma et al. 2012).
As described in the introduction of this paper, insulin is a potent inhibitor of hIAPP fibril formation. Although the mechanism of this process is incompletely understood, recent studies showed that the inhibition effect is related to the binding between the helical B-chain of insulin and the putative helical region of hIAPP (Gilead et al. 2006; Susa et al. 2014). Previously, screening for drug candidates that suppress hIAPP amyloid aggregation has been performed in aqueous solutions. Recently, it was reported that inhibitors of hIAPP aggregation that work in solutions may have low efficacy on membrane surfaces, which significantly affect the aggregation process (Engel et al. 2012). Thus, it may be important to assess the beneficial effect of amyloid inhibitors in membrane environments, specifically targeting early aggregation products on membrane surfaces (Knight et al. 2008; Sellin et al. 2010; Gao and Winter 2015). More recently, the inhibitory effect of human insulin on hIAPP amyloid deposition at SPBs (DOPC/DOPS and DOPC/DOPS/SM/Chol) was examined using QCM-D and fluorescence microscopy (Sasahara et al. 2014). The QCM-D data revealed a significant decrease in the membrane adsorption of hIAPP in the presence of insulin. The extent of amyloid deposition from membrane-bound hIAPP was also decreased when the buffer solutions contained insulin (Fig. 2d). These results indicate that insulin can inhibit the membrane binding and aggregation/deposition of hIAPP at membranes. Although the detailed mechanism remains unknown, the formation of transient membrane-bound α-helical structures of hIAPP might possibly be inhibited through the helix–helix interaction between insulin and hIAPP (Gilead et al. 2006; Hebda et al. 2009; Susa et al. 2014). The results also illustrate that assays using SLBs are facile, sensitive methods for evaluating the effect of inhibitors on amyloid deposition in membrane environments.
Conclusion
A variety of mechanisms of β-cell death have been ascribed to human islet amyloid polypeptide (hIAPP)-induced toxicity, including receptor-mediated mechanisms, activation of inflammasomes, defects in autophagy, endoplasmic reticulum stress, and cell membrane disruption/permeabilization (Cao et al. 2013; Abedini and Schmidt 2013). It has also been reported that toxic hIAPP oligomers trigger β-cell apoptosis (Matveyenko and Butler 2006; Jurgens et al. 2011). Furthermore, extracellular amyloid deposits from various amyloid diseases have been found to be associated with cellular lipid constituents, particularly raft components, suggesting that lipid extraction might be a common feature of amyloid deposition in vivo (Gellermann et al. 2005). Recent studies using model membranes revealed that amyloidogenic hIAPP causes membrane damage associated with cytotoxicity via multiple mechanisms, such as the formation of channel-like pores, detergent-like disorders of the membrane structure, and lipid uptake into fibrils on the membrane surface (Khemtémourian et al. 2008; Engel 2009; Brender et al. 2012). All of these mechanisms would impair the fluidity, local organization, and heterogeneity of lipid membranes. Nevertheless, the mechanism by which membrane functions are perturbed and impaired by the amyloid aggregation/deposition of hIAPP remains unclear. hIAPP has a high propensity to bind to negatively charged lipid membranes, self-assemble into fibrillar aggregates at membranes, and form membrane-associated deposits. These remarkable properties make hIAPP a model peptide for studying the mechanism of membrane-mediated toxicity associated with amyloid aggregation/deposition. Important challenges will be to create more sophisticated model membranes that reflect the complexity of in vivo environments (Glazier and Salaita 2017) and observe the effects of amyloid aggregation/deposition on the dynamic behaviors of membranes, such as the barrier, trafficking, and signaling functions of lipid membranes, using high-sensitivity surface analyzing/imaging techniques.
Compliance with ethical standards
Conflict of interest
Kenji Sasahara declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
This article is part of a special issue on ‘Biomolecules to Bio-nanomachines - Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
References
- Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587:1119–1127. doi: 10.1016/j.febslet.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- Apostolidou M, Jayasinghe SA, Langen R. Structure of α-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J Biol Chem. 2008;283:17205–17210. doi: 10.1074/jbc.M801383200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrood S, Li Y, Isas JM, Hegde BG, Baxa U, Haworth IS, Langen R. Fibril structure of human islet amyloid polypeptide. J Biol Chem. 2012;287:5235–5241. doi: 10.1074/jbc.M111.327817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Dürr UH, Heyl D, Budarapu MB, Ramamoorthy A. Membrane fragmentation by an amyloidogenic fragment of human islet amyloid polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochim Biophys Acta. 2007;1768:2026–2029. doi: 10.1016/j.bbamem.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Hartman K, Reid KR, Kennedy RT, Ramamoorthy A. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry. 2008;47:12680–12688. doi: 10.1021/bi801427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Hartman K, Nanga RP, Popovych N, de la Salud Bea R, Vivekanandan S, Marsh EN, Ramamoorthy A. Role of zinc in human islet amyloid polypeptide aggregation. J Am Chem Soc. 2010;132:8973–8983. doi: 10.1021/ja1007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Salamekh S, Ramamoorthy A. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc Chem Res. 2012;45:454–462. doi: 10.1021/ar200189b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Krishnamoorthy J, Messina GM, Deb A, Vivekanandan S, La Rosa C, Penner-Hahn JE, Ramamoorthy A. Zinc stabilization of prefibrillar oligomers of human islet amyloid polypeptide. Chem Commun. 2013;49:3339–3341. doi: 10.1039/c3cc40383a. [DOI] [PubMed] [Google Scholar]
- Byström R, Aisenbrey C, Borowik T, Bokvist M, Lindström F, Sani MA, Olofsson A, Gröbner G. Disordered proteins: biological membranes as two-dimensional aggregation matrices. Cell Biochem Biophys. 2008;52:175–189. doi: 10.1007/s12013-008-9033-4. [DOI] [PubMed] [Google Scholar]
- Caillon L, Hoffmann AR, Botz A, Khemtemourian L. Molecular structure, membrane interactions, and toxicity of the islet amyloid polypeptide in type 2 diabetes mellitus. J Diabetes Res. 2016;2016:5639875. doi: 10.1155/2016/5639875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Marek P, Noor H, Patsalo V, Tu LH, Wang H, Abedini A, Raleigh DP. Islet amyloid: from fundamental biophysics to mechanisms of cytotoxicity. FEBS Lett. 2013;587:1106–1118. doi: 10.1016/j.febslet.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi C, Stefani M. The amyloid-cell membrane system. The interplay between the biophysical features of oligomers/fibrils and cell membrane defines amyloid toxicity. Biophys Chem. 2013;182:30–43. doi: 10.1016/j.bpc.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- Cho WJ, Trikha S, Jeremic AM. Cholesterol regulates assembly of human islet amyloid polypeptide on model membranes. J Mol Biol. 2009;393:765–775. doi: 10.1016/j.jmb.2009.08.055. [DOI] [PubMed] [Google Scholar]
- Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in type 2 diabetes? Diabetologia. 2004;47:157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- Clark A, Lewis CE, Willis AC, Cooper GJ, Morris JF, Reid KB, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;330:231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Cui W, Ma JW, Lei P, Wu WH, Yu YP, Xiang Y, Tong AJ, Zhao YF, Li YM. Insulin is a kinetic but not a thermodynamic inhibitor of amylin aggregation. FEBS J. 2009;276:3365–3371. doi: 10.1111/j.1742-4658.2009.07061.x. [DOI] [PubMed] [Google Scholar]
- DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem Soc Rev. 2012;41:608–621. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverall MA, Gindl E, Sinner EK, Besir H, Ruehe J, Saxton MJ, Naumann CA. Membrane lateral mobility obstructed by polymer-tethered lipids studied at the single molecule level. Biophys J. 2005;88:1875–1886. doi: 10.1529/biophysj.104.050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MC. Quartz crystal microbalance with dissipation monitoring: enabling real-time characterization of biological materials and their interactions. J Biomol Tech. 2008;19:151–158. [PMC free article] [PubMed] [Google Scholar]
- Domanov YA, Kinnunen PK. Islet amyloid polypeptide forms rigid lipid–protein amyloid fibrils on supported phospholipid bilayers. J Mol Biol. 2008;376:42–54. doi: 10.1016/j.jmb.2007.11.077. [DOI] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: from model membranes to cells. Ann Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MF. Membrane permeabilization by islet amyloid polypeptide. Chem Phys Lipids. 2009;160:1–10. doi: 10.1016/j.chemphyslip.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Engel MF, Yigittop H, Elgersma RC, Rijkers DT, Liskamp RM, de Kruijff B, Höppener JW, Antoinette Killian J. Islet amyloid polypeptide inserts into phospholipid monolayers as monomer. J Mol Biol. 2006;356:783–789. doi: 10.1016/j.jmb.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Engel MF, Khemtémourian L, Kleijer CC, Meeldijk HJ, Jacobs J, Verkleij AJ, de Kruijff B, Killian JA, Höppener JW. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A. 2008;105:6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MF, van den Akker CC, Schleeger M, Velikov KP, Koenderink GH, Bonn M. The polyphenol EGCG inhibits amyloid formation less efficiently at phospholipid interfaces than in bulk solution. J Am Chem Soc. 2012;134:14781–14788. doi: 10.1021/ja3031664. [DOI] [PubMed] [Google Scholar]
- Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- Foster MC, Leapman RD, Li MX, Atwater I. Elemental composition of secretory granules in pancreatic islets of Langerhans. Biophys J. 1993;64:525–532. doi: 10.1016/S0006-3495(93)81397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Winter R. The effects of lipid membranes, crowding and osmolytes on the aggregation, and fibrillation propensity of human IAPP. J Diabetes Res. 2015;2015:849017. doi: 10.1155/2015/849017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedulin B, Cooper GJ, Young AA. Amylin secretion from the perfused pancreas: dissociation from insulin and abnormal elevation in insulin-resistant diabetic rats. Biochem Biophys Res Comm. 1991;180:782–789. doi: 10.1016/s0006-291x(05)81133-7. [DOI] [PubMed] [Google Scholar]
- Gellermann GP, Appel TR, Tannert A, Radestock A, Hortschansky P, Schroeckh V, Leisner C, Lütkepohl T, Shtrasburg S, Röcken C, Pras M, Linke RP, Diekmann S, Fändrich M. Raft lipids as common components of human extracellular amyloid fibrils. Proc Natl Acad Sci U S A. 2005;102:6297–6302. doi: 10.1073/pnas.0407035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead S, Wolfenson H, Gazit E. Molecular mapping of the recognition interface between the islet amyloid polypeptide and insulin. Angew Chem Int Ed Engl. 2006;45:6476–6480. doi: 10.1002/anie.200602034. [DOI] [PubMed] [Google Scholar]
- Glazier R, Salaita K. Supported lipid bilayer platforms to probe cell mechanobiology. Biochim Biophys Acta. 2017;1859:1465–1482. doi: 10.1016/j.bbamem.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbury CS, Cooper GJ, Goldie KN, Müller SA, Saafi EL, Gruijters WT, Misur MP, Engel A, Aebi U, Kistler J. Polymorphic fibrillar assembly of human amylin. J Struct Biol. 1997;119:17–27. doi: 10.1006/jsbi.1997.3858. [DOI] [PubMed] [Google Scholar]
- Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Müller SA, Kistler J, Cooper GJ, Aebi U. Amyloid fibril formation from full-length and fragments of amylin. J Struct Biol. 2000;130:352–362. doi: 10.1006/jsbi.2000.4268. [DOI] [PubMed] [Google Scholar]
- Gorbenko GP, Kinnunen PK. The role of lipid–protein interactions in amyloid-type protein fibril formation. Chem Phys Lipids. 2006;141:72–82. doi: 10.1016/j.chemphyslip.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Green JD, Kreplak L, Goldsbury C, Li Blatter X, Stolz M, Cooper GS, Seelig A, Kistler J, Aebi U. Atomic force microscopy reveals defects within mica supported lipid bilayers induced by the amyloidogenic human amylin peptide. J Mol Biol. 2004;342:877–887. doi: 10.1016/j.jmb.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebda JA, Saraogi I, Magzoub M, Hamilton AD, Miranker AD. A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem Biol. 2009;16:943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T, Minton AP. An equilibrium model for the combined effect of macromolecular crowding and surface adsorption on the formation of linear protein fibrils. Biophys J. 2015;108:957–966. doi: 10.1016/j.bpj.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppener JW, Lips CJ. Role of islet amyloid in type 2 diabetes mellitus. Int J Biochem Cell Biol. 2006;38:726–736. doi: 10.1016/j.biocel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- Jaikaran ET, Clark A. Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology. Biochim Biophys Acta. 2001;1537:179–203. doi: 10.1016/s0925-4439(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44:12113–12119. doi: 10.1021/bi050840w. [DOI] [PubMed] [Google Scholar]
- Jayasinghe SA, Langen R. Membrane interaction of islet amyloid polypeptide. Biochim Biophys Acta. 2007;1768:2002–2009. doi: 10.1016/j.bbamem.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, Kahn SE, Hull RL. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–2640. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, D’Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Jr, Porte D., Jr Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- Kajava AV, Aebi U, Steven AC. The parallel superpleated beta-structure as a model for amyloid fibrils of human amylin. J Mol Biol. 2005;348:247–252. doi: 10.1016/j.jmb.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Kapurniotu A. Amyloidogenicity and cytotoxicity of islet amyloid polypeptide. Biopolymers. 2001;60:438–459. doi: 10.1002/1097-0282(2001)60:6<438::AID-BIP10182>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kayed R, Bernhagen J, Greenfield N, Sweimeh K, Brunner H, Voelter W, Kapurniotu A. Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J Mol Biol. 1999;287:781–796. doi: 10.1006/jmbi.1999.2646. [DOI] [PubMed] [Google Scholar]
- Khemtémourian L, Killian JA, Höppener JW, Engel MF. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in β-cell death in type 2 diabetes mellitus. Exp Diabetes Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JD, Miranker AD. Phospholipid catalysis of diabetic amyloid assembly. J Mol Biol. 2004;341:1175–1187. doi: 10.1016/j.jmb.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound α-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- Knight JD, Williamson JA, Miranker AD. Interaction of membrane-bound islet amyloid polypeptide with soluble and crystalline insulin. Protein Sci. 2008;17:1850–1856. doi: 10.1110/ps.036350.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci. 2011;36:604–615. doi: 10.1016/j.tibs.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Larson JL, Miranker AD. The mechanism of insulin action on islet amyloid polypeptide fiber formation. J Mol Biol. 2004;335:221–231. doi: 10.1016/j.jmb.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Lopes DH, Meister A, Gohlke A, Hauser A, Blume AL, Winter R. Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy. Biophys J. 2007;93:3132–3141. doi: 10.1529/biophysj.107.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci. 2012;69:1947–1965. doi: 10.1007/s00018-011-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J. 2006;25:3446–3457. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC. β-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (hip) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- Merzlyakov M, Li E, Hristova K. Directed assembly of surface-supported bilayers with transmembrane helices. Langmuir. 2006;22:1247–1253. doi: 10.1021/la051933h. [DOI] [PubMed] [Google Scholar]
- Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- Nanga RP, Brender JR, Xu J, Veglia G, Ramamoorthy A. Structures of rat and human islet amyloid polypeptide IAPP1–19 in micelles by NMR spectroscopy. Biochemistry. 2008;47:12689–12697. doi: 10.1021/bi8014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanga RP, Brender JR, Xu J, Hartman K, Subramanian V, Ramamoorthy A. Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J Am Chem Soc. 2009;131:8252–8261. doi: 10.1021/ja9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanga RP, Brender JR, Vivekanandan S, Ramamoorthy A. Structure and membrane orientation of IAPP in its natively amidated form at physiological pH in a membrane environment. Biochim Biophys Acta. 2011;1808:2337–2342. doi: 10.1016/j.bbamem.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Andraka N, De Carufel CA, Bourgault S. Mechanistic contributions of biological cofactors in islet amyloid polypeptide amyloidogenesis. J Diabetes Res. 2015;2015:515307. doi: 10.1155/2015/515307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Inaba T, Tatsu Y, Tero R, Urisu T, Morigaki K. Polymerized lipid bilayers on a solid substrate: morphologies and obstruction of lateral diffusion. Langmuir. 2009;25:345–351. doi: 10.1021/la802670t. [DOI] [PubMed] [Google Scholar]
- Owen DM, Williamson D, Rentero C, Gaus K. Quantitative microscopy: protein dynamics and membrane organisation. Traffic. 2009;10:962–971. doi: 10.1111/j.1600-0854.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- Padrick SB, Miranker AD. Islet amyloid polypeptide: identification of long-range contacts and local order on the fibrillogenesis pathway. J Mol Biol. 2001;308:783–794. doi: 10.1006/jmbi.2001.4608. [DOI] [PubMed] [Google Scholar]
- Patel HR, Pithadia AS, Brender JR, Fierke CA, Ramamoorthy A. In search of aggregation pathways of IAPP and other amyloidogenic proteins: finding answers through NMR spectroscopy. J Phys Chem Lett. 2014;5:1864–1870. doi: 10.1021/jz5001775. [DOI] [PubMed] [Google Scholar]
- Pithadia A, Brender JR, Fierke CA, Ramamoorthy A. Inhibition of IAPP aggregation and toxicity by natural products and derivatives. J Diabetes Res. 2016;2016:2046327. doi: 10.1155/2016/2046327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]
- Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovan D, Opitz N, Winter R. Fluorescence microscopy studies on islet amyloid polypeptide fibrillation at heterogeneous and cellular membrane interfaces and its inhibition by resveratrol. FEBS Lett. 2009;583:1439–1445. doi: 10.1016/j.febslet.2009.03.059. [DOI] [PubMed] [Google Scholar]
- Relini A, Cavalleri O, Rolandi R, Gliozzi A. The two-fold aspect of the interplay of amyloidogenic proteins with lipid membranes. Chem Phys Lipids. 2009;158:1–9. doi: 10.1016/j.chemphyslip.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Relini A, Marano N, Gliozzi A. Probing the interplay between amyloidogenic proteins and membranes using lipid monolayers and bilayers. Adv Colloid Interf Sci. 2014;207:81–92. doi: 10.1016/j.cis.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Richter RP, Bérat R, Brisson AR. Formation of solid-supported lipid bilayers: an integrated view. Langmuir. 2006;22:3497–3505. doi: 10.1021/la052687c. [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- Rustenbeck I, Matthies A, Lenzen S. Lipid composition of glucose-stimulated pancreatic islets and insulin-secreting tumor cells. Lipids. 1994;29:685–692. doi: 10.1007/BF02538912. [DOI] [PubMed] [Google Scholar]
- Salamekh S, Brender JR, Hyung SJ, Nanga RP, Vivekanandan S, Ruotolo BT, Ramamoorthy A. A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc. J Mol Biol. 2011;410:294–306. doi: 10.1016/j.jmb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara K, Hall D, Hamada D. Effect of lipid type on the binding of lipid vesicles to islet amyloid polypeptide amyloid fibrils. Biochemistry. 2010;49:3040–3048. doi: 10.1021/bi9019252. [DOI] [PubMed] [Google Scholar]
- Sasahara K, Morigaki K, Okazaki T, Hamada D. Binding of islet amyloid polypeptide to supported lipid bilayers and amyloid aggregation at the membranes. Biochemistry. 2012;51:6908–6919. doi: 10.1021/bi300542g. [DOI] [PubMed] [Google Scholar]
- Sasahara K, Morigaki K, Shinya K. Amyloid aggregation and deposition of human islet amyloid polypeptide at membrane interfaces. FEBS J. 2014;281:2597–2612. doi: 10.1111/febs.12807. [DOI] [PubMed] [Google Scholar]
- Sciacca MF, Kotler SA, Brender JR, Chen J, Lee DK, Ramamoorthy A. Two-step mechanism of membrane disruption by Aβ through membrane fragmentation and pore formation. Biophys J. 2012;103:702–710. doi: 10.1016/j.bpj.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca MF, Brender JR, Lee DK, Ramamoorthy A. Phosphatidylethanolamine enhances amyloid fiber-dependent membrane fragmentation. Biochemistry. 2012;51:7676–7684. doi: 10.1021/bi3009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca MF, Lolicato F, Di Mauro G, Milardi D, D’Urso L, Satriano C, Ramamoorthy A, La Rosa C. The role of cholesterol in driving IAPP-membrane interactions. Biophys J. 2016;111:140–151. doi: 10.1016/j.bpj.2016.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger J, Weise K, Opitz N, Winter R. The effect of Aβ on IAPP aggregation in the presence of an isolated β-cell membrane. J Mol Biol. 2012;421:348–363. doi: 10.1016/j.jmb.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin D, Yan LM, Kapurniotu A, Winter R. Suppression of IAPP fibrillation at anionic lipid membranes via IAPP-derived amyloid inhibitors and insulin. Biophys Chem. 2010;150:73–79. doi: 10.1016/j.bpc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Stefani M, Rigacci S. Protein folding and aggregation into amyloid: the interference by natural phenolic compounds. Int J Mol Sci. 2013;14:12411–12457. doi: 10.3390/ijms140612411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner Makin O, Serpell LC. Structural characterisation of islet amyloid polypeptide fibrils. J Mol Biol. 2004;335:1279–1288. doi: 10.1016/j.jmb.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Sunde M, Blake C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem. 1997;50:123–159. doi: 10.1016/s0065-3233(08)60320-4. [DOI] [PubMed] [Google Scholar]
- Susa AC, Wu C, Bernstein SL, Dupuis NF, Wang H, Raleigh DP, Shea JE, Bowers MT. Defining the molecular basis of amyloid inhibitors: human islet amyloid polypeptide–insulin interactions. J Am Chem Soc. 2014;136:12912–12919. doi: 10.1021/ja504031d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello MF, Sinopoli A, Pappalardo G. On the environmental factors affecting the structural and cytotoxic properties of IAPP peptides. J Diabetes Res. 2015;2015:918573. doi: 10.1155/2015/918573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha S, Jeremic AM. Clustering and internalization of toxic amylin oligomers in pancreatic cells require plasma membrane cholesterol. J Biol Chem. 2011;286:36086–36097. doi: 10.1074/jbc.M111.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard MD, Hamada T, Takagi M. Using model membranes for the study of amyloid beta:lipid interactions and neurotoxicity. Biotechnol Bioeng. 2008;99:753–763. doi: 10.1002/bit.21731. [DOI] [PubMed] [Google Scholar]
- Wei L, Jiang P, Yau YH, Summer H, Shochat SG, Mu Y, Pervushin K. Residual structure in islet amyloid polypeptide mediates its interactions with soluble insulin. Biochemistry. 2009;48:2368–2376. doi: 10.1021/bi802097b. [DOI] [PubMed] [Google Scholar]
- Weise K, Radovan D, Gohlke A, Opitz N, Winter R. Interaction of hIAPP with model raft membranes and pancreatic beta-cells: cytotoxicity of hIAPP oligomers. ChemBioChem. 2010;11:1280–1290. doi: 10.1002/cbic.201000039. [DOI] [PubMed] [Google Scholar]
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973;359:1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J Mol Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltzius JJ, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, Eisenberg D. Atomic structure of the cross-β spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17:1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki V, Sirenko O, Schafer RJ, Groves JT. Lipid mobility and molecular binding in fluid lipid membranes. J Am Chem Soc. 2005;127:2826–2827. doi: 10.1021/ja042430l. [DOI] [PubMed] [Google Scholar]
- Yanagi K, Ashizaki M, Yagi H, Sakurai K, Lee YH, Goto Y. Hexafluoroisopropanol induces amyloid fibrils of islet amyloid polypeptide by enhancing both hydrophobic and electrostatic interactions. J Biol Chem. 2011;286:23959–23966. doi: 10.1074/jbc.M111.226688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, St. Clair JR, London E, Raleigh DP. Islet amyloid polypeptide membrane interactions: effects of membrane composition. Biochemistry. 2017;56:376–390. doi: 10.1021/acs.biochem.6b01016. [DOI] [PMC free article] [PubMed] [Google Scholar]