Abstract
Membrane tethering is one of the most critical steps to determine the spatiotemporal specificity of membrane trafficking, which is the process to selectively transport proteins, lipids, and other biological molecules to the appropriate locations in eukaryotic cells, such as subcellular organelles, the plasma membrane, and the extracellular space. Based on genetic, cell biological, biochemical, and structural studies, Rab-family small GTPases and a number of Rab-interacting proteins (termed Rab effectors), including coiled-coil tethering proteins and multisubunit tethering complexes, have been proposed to be key protein components for membrane tethering. Nevertheless, indeed whether and how Rab GTPases and their specific Rab effectors directly act upon and catalyze membrane tethering still remains enigmatic. By chemically defined reconstitution of membrane tethering from purified Rab-family GTPase proteins and synthetic liposomal membranes, recent studies have revealed the intrinsic potency of Rab-family GTPases to physically and specifically tether two distinct lipid bilayers of liposomal membranes. Experimental evidence from these reconstitution studies support the novel working model in which Rab-family small GTPases act as a bona fide membrane tether for mediating membrane tethering events in eukaryotic membrane trafficking.
Keywords: Membrane reconstitution, Membrane trafficking, Membrane tethering, Rab protein, Small GTPase, Liposome
Introduction
Eukaryotic cells are composed of a large number of subcellular membrane compartments, including a variety of intracellular organelles and transport vesicles. Each subcellular compartment needs to have its characteristic size, shape, and biochemical composition of proteins and lipids to carry out its specific and important cellular functions. Therefore, the intracellular trafficking of biological molecules (e.g., proteins, lipids, etc.) between subcellular compartments, termed “membrane trafficking”, is obviously an essential and fundamental process for maintaining the life of all eukaryotic cells, from yeast to human cells (Bonifacino and Glick 2004). In the membrane trafficking events, following the formation of membrane-bounded carriers which contain the correct sets of cargo molecules and typically bud from the donor membrane compartment, the membrane-bounded carriers (e.g., secretory and endocytic transport vesicles, etc.) are subsequently (1) transported along the actin and microtubule cytoskeletons (cytoskeletal transport), (2) reversibly tethered to the acceptor membrane compartment (membrane tethering), (3) stably and also irreversibly docked to the acceptor compartment (membrane docking), and, finally, (4) fused with the acceptor compartment to deliver the right cargo molecules (membrane fusion) (Bonifacino and Glick 2004; Fig. 1). These four sequential steps in membrane trafficking are temporally and spatially regulated in a stringent manner by miscellaneous key protein components, which have been identified by a large body of prior genetic and biochemical studies (Bonifacino and Glick 2004; Cai et al. 2007; Wickner 2010; Yu and Hughson 2010; Kuhlee et al. 2015; Baker and Hughson 2016; Fig. 1): SNARE (soluble NSF [N-ethylmaleimide-sensitive factor] attachment protein receptor) family proteins, which form trans-QaQbQcR-SNARE tetrameric protein complexes on opposing membranes destined to fuse (Jahn and Scheller 2006); SNARE-interacting chaperones/cofactors such as Sec1/Munc18 proteins, Sec17/SNAP (soluble NSF attachment protein), and Sec18/NSF, which facilitate assembly and disassembly of QaQbQcR-SNARE complexes in the SNARE-mediated membrane fusion process (Baker and Hughson 2016; Wickner and Rizo 2017); Rab (Ras [rat sarcoma] related in brain) family small GTPases, which are master regulators to drive the cytoskeletal transport and membrane tethering processes before membrane docking and fusion (Stenmark 2009; Hutagalung and Novick 2011); Rab-interacting proteins termed “Rab effectors”, which specifically associate with their cognate Rab small GTPases and, thus, cooperate with Rabs in the membrane trafficking events (Grosshans et al. 2006; Wandinger-Ness and Zerial 2014); and cytoskeletal motor proteins such as class V myosins and microtubule-based motor proteins, which directly or indirectly interact with their cognate Rabs on membrane-bounded transport vesicles and organelles (Hammer and Wu 2002; Akhmanova and Hammer 2010).
Fig. 1.
Sequential steps of cytoskeletal transport, membrane tethering, membrane docking, and membrane fusion in membrane trafficking of eukaryotic cells. Schematic representation of the four essential steps in eukaryotic membrane trafficking, showing the key components for each step, such as Rab-family small GTPases in membrane tethering and SNARE-family proteins in membrane docking and membrane fusion. Membrane tethering is a reversible step of the first physical contact between two distinct subcellular compartments in membrane trafficking, followed by irreversible steps of membrane docking and membrane fusion
Membrane tethering is the first contact between transport carriers (e.g., transport vesicles in the secretory and endocytic pathways) and the target membranes (e.g., organelles and the plasma membrane) before membrane docking and fusion (Pfeffer 1999; Waters and Pfeffert 1999; Fig. 1). Rab-family small GTPases and specific sets of Rab effectors, including the so-called coiled-coil tethering proteins and multisubunit tethering complexes, have been reported to be responsible for membrane tethering (Mayer and Wickner 1997; Ungermann et al. 1998; Waters and Pfeffert 1999; Yu and Hughson 2010; Chia and Gleeson 2014; Fig. 1). The Rab GTPase- and Rab effector-mediated membrane tethering process is thus recognized as an essential step for controlling the directionality of membrane trafficking (Pfeffer 1999; Waters and Pfeffert 1999), in addition to the SNARE-mediated membrane docking and fusion events, which are another critical layer to ensure the fidelity of membrane trafficking (Scales et al. 2000; McNew et al. 2000; Parlati et al. 2002; Izawa et al. 2012; Furukawa and Mima 2014). Nevertheless, in many cases, there has been little experimental evidence for directly showing the ability of Rabs and/or Rab effectors to physically tether two membranes together (Brunet and Sacher 2014). Therefore, indeed whether and how these key components directly act upon membrane tethering still remains ambiguous (Brunet and Sacher 2014). Meanwhile, in vitro reconstitution studies using purified proteins and synthetic lipid bilayers recently have begun to reveal the intrinsic tethering potency of specific Rab effectors (Hickey and Wickner 2010; Ho and Stroupe 2015, 2016) and Rab-family GTPases (Lo et al. 2012; Tamura and Mima 2014; Inoshita and Mima 2017). They provide novel insights into the molecular basis of membrane tethering in eukaryotic membrane trafficking. This review focuses on recent reconstitution works for membrane tethering mediated by human Rab-family small GTPases and also discusses the physiological relevance of this Rab-mediated membrane tethering observed in a chemically defined reconstitution system (Tamura and Mima 2014; Inoshita and Mima 2017).
Reconstitution of Rab-mediated membrane tethering from purified Rab-family small GTPases and synthetic liposomes
Rab-family small GTPases, which constitute the largest family among the Ras GTPase superfamily (over 60 Rab isoforms in human cells) (Rojas et al. 2012), localize to the cytosolic surfaces of distinct subcellular membrane compartments as their active GTP-bound forms, recruit their cognate Rab effectors to the membrane surfaces, and, thereby, cooperate with the effectors to mediate the diverse processes in intracellular membrane trafficking, including membrane tethering (Stenmark 2009; Hutagalung and Novick 2011; Fig. 1). As to their structural features, Rab GTPases are small monomeric proteins (around 25 kDa) consisting of the N-terminal non-conserved flexible segments (5–30 residues; 19 residues for human Rab5a), the conserved globular Ras-superfamily GTPase domains in the middle (160–170 residues; 162 residues for human Rab5a), and the C-terminal unstructured hypervariable region (HVR) domains (20–50 residues; 34 residues for human Rab5a) (Rojas et al. 2012; Khan and Ménétrey 2013; Fig. 2). The C-terminal HVR domains are further post-translationally modified at one or two cysteine residues at their C termini by an isoprenyl lipid (geranylgeranyl) group, which is required for membrane insertion and stable membrane attachment of Rab proteins (Hutagalung and Novick 2011; Fig. 2). It is noteworthy that each Rab protein has two relatively long flexible tails of its C-terminal HVR domain and N-terminal segment, which are likely to reside in close proximity to each other (Khan and Ménétrey 2013; Fig. 2). For example, the C-terminal HVR domain and N-terminal segment of human Rab5a are calculated to be approximately 14 and 8 nm in length, respectively (assuming the contour length of an amino acid of 0.4 nm/aa, estimated using AFM [atomic force microscopy] force spectroscopy and SMD [steered molecular dynamics] simulations; Ainavarapu et al. 2007; Fig. 2), while the diameter of its globular GTPase domain is roughly estimated to be about 4 nm (assuming the minimal radius of a spherical 20-kDa protein molecule of 1.78 nm; Erickson 2009; Fig. 2). Given the structural characteristics indicated above, the flexible C-terminal HVR domains and N-terminal segments appear to give substantial mobility to the membrane-anchored forms of Rab proteins. Furthermore, these flexible regions of Rab proteins may determine the distance between their globular GTPase domains and membrane surfaces, and, also, they may regulate the orientation of the GTPase domains towards membrane surfaces (Edler and Stein 2017; Edler et al. 2017; Fig. 2).
Fig. 2.
Membrane attachment of Rab-family small GTPases to biological organelle/vesicle membranes in vivo and synthetic liposomal membranes in a chemically defined reconstitution system in vitro. Schematic representation of the membrane-anchored forms of human Rab5a as a typical Rab-family GTPase protein. Human Rab5a protein consists of 215 amino acid residues, containing the N-terminal flexible segment (residues 1–19), the conserved globular Ras-superfamily GTPase domain (residues 20–181), and the C-terminal unstructured hypervariable region (HVR) domain (residues 182–215). Native Rab5a protein is attached to the biological organelle/vesicle membrane through its isoprenyl lipid anchors at the C-terminus. Recombinant Rab5a-His12 protein is attached to the synthetic liposomal membrane through the specific interaction of the C-terminal polyhistidine tag (His12) with a DOGS-NTA-Ni2+ lipid in the membrane
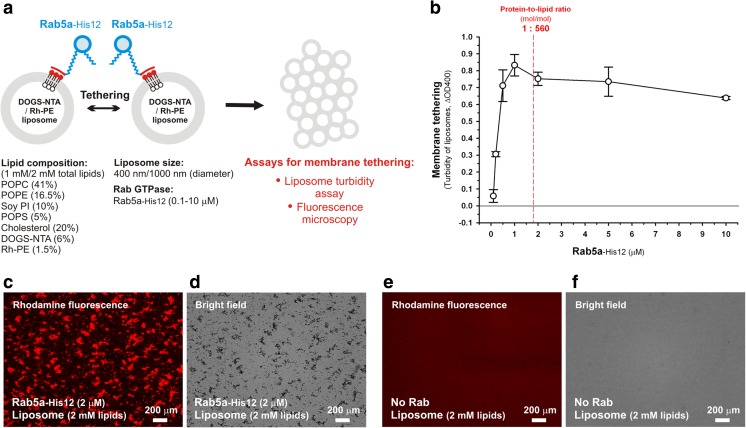
In the chemically defined systems for testing Rab-mediated membrane tethering reactions, to mimic the membrane-bound state of native Rab proteins that contain the non-conserved flexible regions and lipid anchors, recombinant proteins of human Rab-family GTPases were purified as the full-length forms with an artificially modified polyhistidine tag (His12) at their C termini, which can be stably and specifically associated with a synthetic liposomal membrane bearing a DOGS-NTA-Ni2+ lipid (1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl) iminodiacetic acid]-succinyl}-Ni2+) (Tamura and Mima 2014; Inoshita and Mima 2017; Figs. 2 and 3a). For synthetic liposomal membranes used in the reconstitution systems, large unilamellar liposomes of defined size (typically 400 nm in diameter) were prepared by extrusion through a polycarbonate filter, with the lipid mixes containing a DOGS-NTA-Ni2+ lipid and five major lipid species of 1-palmitoyl-2-oleoyl (PO)-phosphatidylcholine (POPC), PO-phosphatidylethanolamine (POPE), soy phosphatidylinositol (soy PI), PO-phosphatidylserine (POPS), and cholesterol (Tamura and Mima 2014; Inoshita and Mima 2017; Fig. 3a), which roughly mimic physiological lipid compositions of subcellular organelle membranes in mammalian cells (van Meer et al. 2008). Using the Rab GTPase-anchored liposomal membranes generated with purified Rab-His12 proteins and DOGS-NTA-bearing liposomes, the intrinsic membrane tethering potency of human Rab proteins was quantitatively evaluated by assaying the increase in turbidity of the liposome suspensions, which can be measured with the optical density at 400 nm (ΔOD400) (Tamura and Mima 2014; Inoshita and Mima 2017; Fig. 3a, b). This liposome turbidity assay is a well-established biochemical assay (Ohki et al. 1982) and, thus, has also been employed for testing membrane tethering and docking events mediated by other protein families, including synaptotagmins, Atg8/LC3-family proteins, and atlastin GTPases (Hui et al. 2011; Weidberg et al. 2011; Liu et al. 2015). In recent reconstitution studies on Rab-mediated membrane tethering, the liposome turbidity assay was applied to a number of representative human Rab proteins functioning in the secretory and endocytic pathways, such as Rab1a in ER-to-Golgi traffic, Rab3a in synaptic exocytosis, Rab5a in the early endocytic pathway, Rab7a in the late endocytic and lysosomal/autophagic pathways, and Rab11a in the endocytic recycling pathway (Tamura and Mima 2014; Inoshita and Mima 2017). Strikingly, by testing at a wide range of Rab-to-lipid molar ratios from 1:10,000 to 1:100, the turbidity assay data establish that all of the human Rab proteins tested retain the significant intrinsic capacity to increase liposome turbidity by initiating efficient tethering of synthetic liposomal membranes (Inoshita and Mima 2017; Fig. 3a, b). Of note, Rab5a can trigger rapid and efficient liposomal membrane tethering even at very low Rab-to-lipid molar ratios, such as 1:5000–1:1000, indicating the much higher membrane tethering potency of Rab5a in comparison to other human Rabs (Inoshita and Mima 2017; Fig. 3b). These experimental data obtained from a chemically defined system support the novel concept that human Rab-family GTPases have the highly conserved function to physically link two distinct lipid bilayers together by themselves in membrane tethering events (Inoshita and Mima 2017). Fluorescence microscopic observations of the reconstituted Rab-anchored liposome suspensions, which can be used as an alternative assay to monitor liposome tethering simply and quantitatively, further strengthened the experimental evidence supporting the novel working model of Rab-mediated membrane tethering (Tamura and Mima 2014; Inoshita and Mima 2017; Fig. 3c, d, e, f). The liposome tethering reactions were typically prepared by incubating the fluorescence-labeled synthetic liposomes bearing rhodamine-PE (Rh-PE) and DOGS-NTA (400 or 1000 nm in diameter) in the presence of purified Rab-His12 proteins (e.g., Rab-to-lipid molar ratios of 1:1000–1:100 etc.), and then subjected to fluorescence microscopy. As shown in the fluorescence microscopic images in prior reconstitution studies (Tamura and Mima 2014; Inoshita and Mima 2017) and also in Fig. 3c, d, in which Rab5a-His12 is used for a typical example, human Rab-family proteins obviously have the ability to induce the formation of substantial massive clusters of liposomes. In contrast, the liposome suspensions without any Rab-family proteins or with the control HRas protein instead, which is a non-Rab Ras-superfamily GTPase, have little potency to cause liposome clustering (Inoshita and Mima 2017; Fig. 3e, f). These Rab-induced liposome clusters observed in the fluorescence images can be quantitatively analyzed for their particle sizes. For example, Rab-containing tethering reactions with a 1000-nm liposome yielded very large average sizes of liposome clusters in the range of 7.8 to 55 μm2 (indicated as the areas occupied by liposome clusters in fluorescence images), as compared to the average sizes for the control reactions (less than 1.0 μm2) (Inoshita and Mima 2017). Altogether, the intrinsic membrane tethering potency of human Rab-family proteins is fully supported by a set of solid experimental data obtained from two independent reconstitution assays, the liposome turbidity assay and the fluorescence microscopic assay using purified Rab proteins and synthetic liposomal membranes (Tamura and Mima 2014; Inoshita and Mima 2017; Fig. 3).
Fig. 3.
Human Rab-family proteins act as a bona fide membrane tether to directly and specifically tether two distinct membranes together in a chemically defined reconstitution system. a Schematic representation of the membrane tethering assays with human Rab5a-His12 proteins and synthetic liposomal membranes bearing DOGS-NTA and Rh-PE lipids, showing the experimental conditions used in b–f (lipid composition, liposome size, lipid concentrations, and Rab concentrations). b Liposome turbidity assays. Rab5a-His12 proteins (0.1–10 μM) were mixed with synthetic liposomes (1 mM total lipids; 400 nm in diameter) and then incubated at 30 °C for 30 min. Turbidity changes of the Rab–liposome suspensions were measured with the optical density at 400 nm (ΔOD400). The protein-to-lipid ratio of 1:560 (mol/mol) is indicated in the graph as the physiological Rab-to-lipid molar ratio of biological organelle/vesicle membranes, estimated from the quantitative proteomic/lipidomic analyses of isolated synaptic vesicles from rat brain (Takamori et al. 2006). c–f Fluorescence microscopy. Rhodamine fluorescence images (c, e) and bright field images (d, f) of liposome clusters were obtained with synthetic liposomes bearing Rh-PE (2 mM total lipids; 1000 nm in diameter; Fig. 3a) in the presence (c, d) and absence (e, f) of Rab5a-His12 (2 μM final). The tethering reactions with the fluorescently labeled liposomes and Rab proteins were incubated at 30 °C for 1 h and then subjected to fluorescence microscopy. Scale bars = 200 μm
Physiological relevance and specificity of Rab-mediated membrane tethering in a chemically defined reconstitution system
Using the chemically defined minimal systems consisting of purified Rab proteins and synthetic liposomal membranes alone, recent reconstitution studies discover the intrinsic capacity of Rab-family GTPase proteins to directly and physically tether two distinct opposing membranes together, thus establishing the working model in which Rab proteins provide a bona fide membrane tether in intracellular membrane tethering events (Lo et al. 2012; Tamura and Mima 2014; Inoshita and Mima 2017; Figs. 1, 2 and 3). Nevertheless, it still remains critical to ask whether the Rab-mediated tethering reactions, when studied in a chemically defined reconstitution system, faithfully reflect the physiological functions of Rab proteins in membrane trafficking (that has to be highly specific and tightly spatiotemporally regulated). One of the important questions on this issue is to what extent the Rab protein densities at liposomal membrane surfaces in the reconstitution systems can recapitulate the physiological conditions at subcellular membranes in eukaryotic cells. Previous quantitative proteomics/lipidomics studies of synaptic vesicles from rat brain have attempted to calculate the average copy numbers per vesicle for the major protein constituents functioning in membrane trafficking processes, including SNARE-family proteins and Rab-family small GTPases (Takamori et al. 2006). From the quantitative immunoblotting data in this study, the average copy numbers per vesicle of synaptic Rab proteins were determined to be 10 copies/vesicle and 15 copies/vesicle for Rab3a and the other Rabs, respectively, thereby a total of 25 Rab molecules per vesicle (Takamori et al. 2006). In addition to the Rab copy numbers, the number of lipid molecules in a lipid bilayer of a single synaptic vesicle can be estimated to be 14,100 lipid molecules (Inoshita and Mima 2017), using a mean diameter of synaptic vesicles of 42 nm (Takamori et al. 2006), a typical phospholipid bilayer thickness of 4 nm (Nagle and Tristram-Nagle 2000; Fig. 2), and an average area occupied by a single phospholipid molecule of 0.65 nm2 (Nagle and Tristram-Nagle 2000). Therefore, when a synaptic vesicle is referred to as a model of trafficking vesicles or organelles, the physiological Rab-to-lipid molar ratio can be estimated to be approximately 1:560 from the numbers of 25 molecules/vesicle for Rabs and 14,100 molecules/vesicle for lipids (Inoshita and Mima 2017; Fig. 3b). Given the liposome turbidity assay data presented in recent reconstitution studies (Tamura and Mima 2014; Inoshita and Mima 2017) and Fig. 3b, several specific Rab proteins, such as human Rab5a and Rab7a located in the endosomal compartments, can trigger efficient and rapid membrane tethering at around the physiological Rab-to-lipid ratio estimated (1:560) or even at lower ratios. Moreover, a number of other Rab-family isoforms, which had exhibited little tethering activity at or below the physiological Rab-to-lipid ratio, retained significant potency to initiate membrane tethering when tested at the higher Rab densities, which were, yet, not far above the physiological ratio (e.g., Rab-to-lipid ratios of 1:100, 1:200, etc.) (Inoshita and Mima 2017). Thus, these experimental data of the reconstituted tethering assays support the idea that Rab-family GTPases have the intrinsic capacity to physically tether two distinct lipid bilayers in the context of a physiologically relevant function.
Other key questions on the physiological relevance issue include whether Rab-mediated membrane tethering reactions in the in vitro reconstituted systems require the membrane attachment and GTP binding of Rab proteins, since Rab-family proteins are generally thought to be active and functional in their membrane-attached, GTP-bound state, thereby cooperating with their cognate Rab effectors to facilitate membrane trafficking processes in eukaryotic cells (Stenmark 2009; Hutagalung and Novick 2011; Figs. 1 and 2). By employing fluorescence microscopic analyses and streptavidin bead-based pull-down assays using Rab-anchored liposomes labeled with differently colored fluorophores, the chemically defined reconstitution approaches indicated that Rab-mediated membrane tethering reactions strictly relied upon the membrane attachment of Rab proteins on both of the two opposing membranes destined to tether each other (Tamura and Mima 2014). These Rab-mediated tethering reactions were reversibly controlled by the membrane attachment and detachment cycle of Rab proteins (Tamura and Mima 2014). Furthermore, it is also noteworthy that Rab-mediated membrane tethering cannot be competitively inhibited by adding even a large excess of soluble, membrane-unbound Rab proteins lacking a membrane anchoring moiety (Tamura and Mima 2014). The results gained from such chemically defined systems strongly suggest that Rab-mediated membrane tethering critically requires specific trans-assemblies between membrane-anchored Rab proteins, whereas membrane-unbound Rabs in solution cannot recognize and interact with the membrane-anchored forms of Rabs on the membranes. This may imply that stable membrane association induces the conformational change in Rab molecules that is essential for mediating selective Rab–Rab assemblies in trans on the membranes. By contrast, the GTP dependence of Rab-mediated membrane tethering has not been observed in the reconstituted tethering assays with human Rab-family GTPases. Little stimulatory effect on Rab-mediated tethering was obtained by exogenously adding GTP to the tethering reactions (Tamura and Mima 2014) or by pre-loading of Rab proteins with GTP (Tamura and Mima, unpublished data). Considering that the recruitment of Rab proteins to biological organelle/vesicle membranes in vivo has been recently reported to be mediated by GDP/GTP exchange and Rab guanine nucleotide exchange factors (Rab GEFs) (Gerondopoulos et al. 2012; Blümer et al. 2013; Cabrera and Ungermann 2013), it is conceivable that stable binding of Rab-His12 proteins to DOGS-NTA-bearing liposomal membranes in the reconstitution systems can bypass the GTP requirement for Rab-mediated membrane tethering in vitro (Tamura and Mima 2014; Fig. 2). Meanwhile, despite the fact that the Rab-only tethering reactions are not dependent on the presence of GTP, the more recent study reveals that endosomal Rab11a-mediated membrane tethering is drastically and specifically promoted by its cognate Rab effectors, class V myosins (Myo5A and Myo5B), in a GTP-dependent manner (Inoshita and Mima 2017). This reflects that specific interactions between the GTP-bound, membrane-attached forms of Rab-family GTPases and their cognate Rab effectors can confer the GTP-dependent regulation of Rab-mediated membrane tethering reactions. Future studies are required to thoroughly address the issue of the physiological relevance of Rab-mediated membrane tethering. They may focus on developing more complex, but more physiologically mimicking, reconstituted systems which consist of Rab-family small GTPases and diverse types of Rab effectors, including the so-called coiled-coil tethering proteins and multisubunit tethering complexes. Moreover, beyond the understanding of the molecular machinery of Rab-mediated membrane tethering, these chemically defined reconstitution approaches have the potential to provide novel conceptual and technical insights for studying how proteins (protein complexes), in general, cooperate with lipid molecules to achieve their physiological functions on biological membranes.
Acknowledgements
The author is grateful to Dr. Naoki Tamura (Institute for Protein Research, Osaka University, now Fukushima Medical University School of Medicine) for his substantial contributions to embarking on the research projects of human Rab-mediated membrane tethering. This work was, in part, supported by the Program to Disseminate Tenure Tracking System from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and Grants-in-Aid for Scientific Research from MEXT (to J.M.).
Compliance with ethical standards
Conflict of interest
Joji Mima declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines - Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
References
- Ainavarapu SR, Brujić J, Huang HH, Wiita AP, Lu H, Li L, Walther KA, Carrion-Vazquez M, Li H, Fernandez JM. Contour length and refolding rate of a small protein controlled by engineered disulfide bonds. Biophys J. 2007;92:225–233. doi: 10.1529/biophysj.106.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A, Hammer JA., 3rd Linking molecular motors to membrane cargo. Curr Opin Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RW, Hughson FM. Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol. 2016;17:465–479. doi: 10.1038/nrm.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümer J, Rey J, Dehmelt L, Mazel T, Wu YW, Bastiaens P, Goody RS, Itzen A. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200:287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Brunet S, Sacher M. Are all multisubunit tethering complexes bona fide tethers? Traffic. 2014;15:1282–1287. doi: 10.1111/tra.12200. [DOI] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem. 2013;288:28704–28712. doi: 10.1074/jbc.M113.488213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Chia PZ, Gleeson PA. Membrane tethering. F1000Prime Rep. 2014;6:74. doi: 10.12703/P6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler E, Stein M. Probing the druggability of membrane-bound Rab5 by molecular dynamics simulations. J Enzyme Inhib Med Chem. 2017;32:434–443. doi: 10.1080/14756366.2016.1260564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler E, Schulze E, Stein M. Membrane localization and dynamics of geranylgeranylated Rab5 hypervariable region. Biochim Biophys Acta. 2017;1859:1335–1349. doi: 10.1016/j.bbamem.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N, Mima J. Multiple and distinct strategies of yeast SNAREs to confer the specificity of membrane fusion. Sci Rep. 2014;4:4277. doi: 10.1038/srep04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky–Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–2139. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer JA, 3rd, Wu XS. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr Opin Cell Biol. 2002;14:69–75. doi: 10.1016/S0955-0674(01)00296-4. [DOI] [PubMed] [Google Scholar]
- Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Stroupe C. The HOPS/class C Vps complex tethers membranes by binding to one Rab GTPase in each apposed membrane. Mol Biol Cell. 2015;26:2655–2663. doi: 10.1091/mbc.E14-04-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Stroupe C. The HOPS/Class C Vps complex tethers high-curvature membranes via a direct protein–membrane interaction. Traffic. 2016;17:1078–1090. doi: 10.1111/tra.12421. [DOI] [PubMed] [Google Scholar]
- Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat Struct Mol Biol. 2011;18:813–821. doi: 10.1038/nsmb.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita M, Mima J. Human Rab small GTPase- and class V myosin-mediated membrane tethering in a chemically defined reconstitution system. J Biol Chem. 2017;292:18500–18517. doi: 10.1074/jbc.M117.811356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa R, Onoue T, Furukawa N, Mima J. Distinct contributions of vacuolar Qabc- and R-SNARE proteins to membrane fusion specificity. J Biol Chem. 2012;287:3445–3453. doi: 10.1074/jbc.M111.307439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Khan AR, Ménétrey J. Structural biology of Arf and Rab GTPases’ effector recruitment and specificity. Structure. 2013;21:1284–1297. doi: 10.1016/j.str.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Kuhlee A, Raunser S, Ungermann C. Functional homologies in vesicle tethering. FEBS Lett. 2015;589:2487–2497. doi: 10.1016/j.febslet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Liu TY, Bian X, Romano FB, Shemesh T, Rapoport TA, Hu J. Cis and trans interactions between atlastin molecules during membrane fusion. Proc Natl Acad Sci U S A. 2015;112:E1851–E1860. doi: 10.1073/pnas.1504368112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SY, Brett CL, Plemel RL, Vignali M, Fields S, Gonen T, Merz AJ. Intrinsic tethering activity of endosomal Rab proteins. Nat Struct Mol Biol. 2012;19:40–47. doi: 10.1038/nsmb.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Söllner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Nagle JF, Tristram-Nagle S. Structure of lipid bilayers. Biochim Biophys Acta. 2000;1469:159–195. doi: 10.1016/S0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki S, Düzgüneş N, Leonards K. Phospholipid vesicle aggregation: effect of monovalent and divalent ions. Biochemistry. 1982;21:2127–2133. doi: 10.1021/bi00538a022. [DOI] [PubMed] [Google Scholar]
- Parlati F, Varlamov O, Paz K, McNew JA, Hurtado D, Söllner TH, Rothman JE. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc Natl Acad Sci U S A. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol. 2012;196:189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/S0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tamura N, Mima J. Membrane-anchored human Rab GTPases directly mediate membrane tethering in vitro. Biol Open. 2014;3:1108–1115. doi: 10.1242/bio.20149340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6:a022616. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Pfeffert SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/S0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- Wickner W, Rizo J. A cascade of multiple proteins and lipids catalyzes membrane fusion. Mol Biol Cell. 2017;28:707–711. doi: 10.1091/mbc.E16-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]