Abstract
Amyloid fibrils are misfolded forms of proteins and are involved in various diseases. They have been studied extensively with the aim to obtain a comprehensive understanding of protein folding and misfolding and to use this knowledge to develop therapeutic strategies against the associated diseases. Salt conditions are important factors determining the formation and stability of amyloid fibrils. In the 1990s, salt effects were studied extensively to understand the conformational stability of acid-denatured proteins, and the results of these studies revealed the role of electrostatic repulsion in forming the compact intermediate states. In this review, we compare the effects of salts on the compact intermediate states with those on the formation of amyloid fibrils under acidic conditions. The results argue that both protein folding and misfolding are driven by the same forces, although the resultant conformations are distinct because they are monomeric and multimeric reactions, respectively.
Keywords: Amyloid fibril, Amorphous aggregation, Molten globule, Protein folding/misfolding, Salt effects, Supersaturation
Introduction
Among the various types of protein aggregates, amyloid fibrils, which are associated with more than 50 types of amyloidosis, have been important targets of protein science research (Eisenberg and Jucker 2012; Jucker and Walker 2013; Sipe et al. 2014; Tycko and Wickner 2013). Amyloid fibrils are misfolded fibrillar aggregates of denatured proteins with a width of around 10 nm and length of several micrometers. Recent advances in solid-state nuclear magnetic resonance and cryo-electron microscopy have revealed the atomic resolution structures of several amyloid fibrils (Colvin et al. 2016; Fitzpatrick et al. 2017; Walti et al. 2016). The dominant secondary structure is a cross-β structure stabilized by an ordered hydrogen bond network. The results of previous studies led to the proposal that amyloid fibrils may form in supersaturated solutions of precursor proteins by a nucleation and growth mechanism characterized by a lag phase (Jarrett and Lansbury 1993; Morris et al. 2009; Wetzel 2006). Since amyloid fibrillation is a nucleation-dependent reaction, preformed fibrils act as seeds, i.e., the addition of fragmented fibrils effectively escapes the high free energy barrier of nucleation, resulting in the immediate growth of seed fibrils (Jarrett and Lansbury 1993; Morris et al. 2009; Naiki et al. 1997; Wetzel 2006). Even without seeds, various kinds of agitation (Giehm and Otzen 2010; Linse et al. 2007), in particular ultrasonication (Chatani et al. 2009; Nakajima et al. 2016; Ohhashi et al. 2005; So et al. 2011; Yoshimura et al. 2012; Yoshimura et al. 2013), are effective in accelerating the fibrillation by shortening a lag phase. These characteristics of amyloid fibrillation are similar to those of molecular crystallization, suggesting that, as for the case of crystallization, amyloid fibrillation is a supersaturation-limited precipitation of denatured proteins, which occurs only above solubility. In this context, supersaturation refers to a metastable state in which the solution is kinetically kept stable although the concentration of dissolved solutes is higher than the thermodynamic solubility (Adachi et al. 2015; Yoshimura et al. 2012). According to this definition, supersaturation is not a driving force of amorphous aggregation (see section Competition between amyloid formation and amorphous aggregation). It has been argued that “supersaturation” is important as a critical factor involved in amyloid formation (Lin et al. 2014; So et al. 2016; Yoshimura et al. 2012). The role of supersaturation at the proteome level in neurodegenerative diseases has also been reported (Ciryam et al. 2013, 2015).
In addition to supersaturation, the formation and stability of amyloid fibrils depend on various factors, including temperature, pressure, pH, and additives (Chatani and Goto 2005; Lin et al. 2014; So et al. 2016; Yoshimura et al. 2012). Clarifying the effects of these factors are important if we are to understand the mechanisms of amyloid fibrillation, as has been demonstrated for protein folding. Among these factors, salts have been used widely to address the formation and stability of the protein native states and are now used extensively for studying amyloid fibrils. In this review, we revisit a series of classic studies addressing the effects of salts on the formation of the compact molten globule states and show that their effects are common to the formation of amyloid fibrils. Such underlying commonalities suggests that the compact molten globule state and amyloid fibrils are alternative folds of denatured proteins under conditions where hydrophobic interactions and the formation of hydrogen bonds are promoted but the formation of the native state is prevented.
Effects of salts on acid-denatured proteins
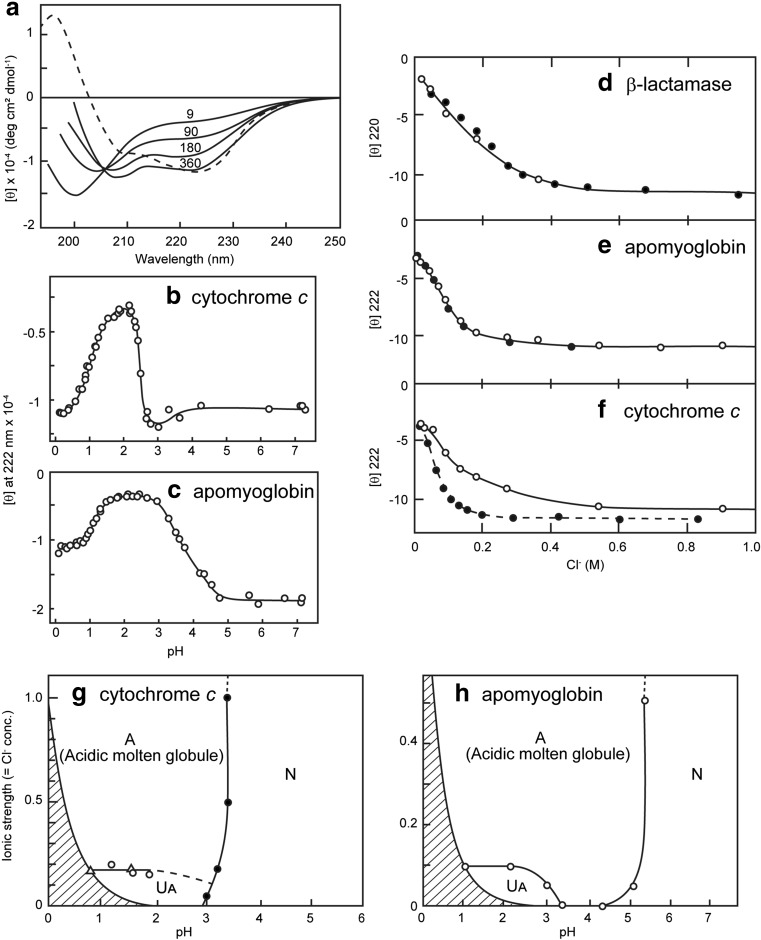
Salts have been used widely for various purposes in the preparation and characterization of proteins, including salt-induced precipitation (salting out), dissolution of proteins (salting in), and stabilizing or destabilizing protein structures (Tanford 1968, 1970). Among these, the denaturation of proteins by GuHCl, a salt composed of a guanidinium cation and a chloride (Cl−) anion, is one of the most important applications of salt effects on proteins as it facilitates our understanding of the conformational stability of proteins on the basis of the salt-induced denaturation. In 1980s, the findings that horse cytochrome c (Ohgushi and Wada 1983) and several acid-denatured proteins, including β-lactamase and apomyoglobin (Fink et al. 1994; Goto et al. 1990a; Goto and Fink 1989), form the molten globule state, a compact intermediate of protein folding without native tertiary structure, in the presence of salts attracted much attention to the effects of salt on proteins (Fig. 1). These proteins are substantially unfolded at pH 2 in the absence of salt. The addition of a low concentration of salts induce an intermediate state with strong far-UV circular dichroism (CD) intensity, while the near-UV CD showed disordering. The salt-induced state is similar to the acidic molten globule state of α-lactalbumin, which is stable even in the absence of salts (Arai and Kuwajima 2000). It is noted that the term “molten globule state” was first used for the salt-stabilized acidic state of cytochrome c (Ohgushi and Wada 1983). Similar salt-dependent conformational transitions were observed later for microbial transglutaminase, a monomeric enzyme composed of 331 amino acid residues (Suzuki et al. 2011, 2012). These results established that the salt-dependent transition is common to various acid-denatured proteins.
Fig. 1.
Acid-induced unfolding and refolding transitions of proteins. a Far-UV CD spectra of cytochrome c as a function of HCl concentration. The number refers to the HCl concentration in millimolar units. The spectrum of the native state (dotted line) is shown for comparison. b, c Effects of increasing concentration of HCl on the ellipticity at 222 nm of cytochrome c (b) and apomyoglobin (c). Upon decreasing pH by the addition of HCl, the native → acid-unfolded → molten globule transition is observed. d–f Consistency between the HCl-induced (open circles) and KCl-induced (filled circles) transitions of three acid-unfolded proteins as monitored by the change in the far-UV CD. g, h Phase diagrams for acidic conformational states. Panels a–f and h are reproduced from Goto et al. (1990a) and Goto and Fink (1990), respectively, with permission
The conformation of the acid-denatured state is determined by a balance of various factors which stabilize or destabilize the folded state. The most important destabilizing force should be the charge repulsion between positive charges because most of the titratable groups are protonated at pH 2. Consistent with this, proteins with a high pI value (for example, cytochrome c) tend to be unfolded substantially. On the other hand, the driving forces for folding would be similar to those at neutral pH except that the electrostatic interactions are affected by pH. The addition of salts shields the unfavorable charge repulsion, thus the folding forces come to play, although the distinct ionization of the titratable groups prevent the formation of native structure.
Acid-induced unfolding and refolding transitions
The participation of hydrophobic interactions in the formation of the molten globule state, although less than that in the native state, has been established by a series of studies using calorimetric analysis of the salt-stabilized intermediate states (Hamada et al. 1994; Nishii et al. 1995). Nevertheless, the salt-induced transition itself does not reveal the detailed mechanism of the salt effects. In an attempt to fully acid-unfold proteins, an intriguing observation was made; increasing the concentration of HCl opposes the unfolding, and rather refolds the protein to the molten globule state (Goto et al. 1990a) (Fig. 1a-c). The acid-stabilized state was observed to be essentially the same intermediate as that stabilized by salts. Although the phenomenon was surprising at first, it is straightforward to understand the mechanism once the ionic effect is taken into account; decreasing the pH below 2 exponentially increases the concentration of anions as well as that of protons by [Cl−] = [H+] = 10-pH mol/L. This suggests that the apparent refolding phenomenon observed by increasing the HCl concentration is caused by the anion effect rather than by pH effects.
Consistent with this notion, the acid-induced transition plotted against the concentration of HCl was observed to agee well with that plotted against the concentration of NaCl or KCl (Goto et al. 1990a) (Fig. 1d–f). By considering the ionic effect of HCl, the pH and salt-dependent phase diagram of acid-denatured protein can be constructed (Goto and Fink 1990) (Fig. 1g, h). It is important to recognize that decreasing the pH increased the minimal chloride concentration, thus producing the prohibited region in the phase. Consequently, decreasing the pH below 2 results in increasing the ionic strength, causing the same effects as adding salt at pH 2. An important implication is that to maximally unfold a protein by acid, a pH of 2 under low salt conditions is the best strategy (Suzuki et al. 2012). These conditions would also be optimal for dissolving protein and peptides by taking advantage of the net charge repulsion.
Mechanism of salt-induced conformational change
Although the importance of anions in shielding the charge repulsive forces is evident, anions can work in various ways (Goto et al. 1990b). First, salts can shield the charge repulsion through the Debye–Hückel screening effect. Second, some anions, such as sulfate, are well known to stabilize the native state, which has been interpreted in terms of the effects on water structure (that is, water-structure maker), consequently strengthening the hydrophobic interactions of proteins. Third, Anions can directly interact with positive charges on proteins to shield the charge repulsion. These three mechanisms can be distinguished by examining the effects of various anion species (Goto et al. 1990b).
Debye–Hückel screening effects are independent of anion species. It is known that the effects of anions in stabilizing protein structure follow the Hofmeister series (Arakawa and Timasheff 1982; Goto et al. 1990b). The representative series is:
| 1 |
Although the exact mechanism of stabilization or destabilization by these anions is unknown, the results are consistent with the concept that these salts affect the water structure. On the other hand, the affinity of a particular anion to an anion-exchange resin is called “electroselectivity.” The electroselectivity series of various anions depends critically on the structure of the resin and the solution conditions. However, the general trend of selectivity can be seen from the following examples (Washabaugh and Collins 1986):
| 2 |
| 3 |
Equation 2 represents the retention time of anions from an anion-exchange column with trimethylamino groups, and Eq. 3 is based on the selectivity of anions to a benzyl (hydroxyethyl) dimethylammonium anion-exchange resin. The order of the Hofmeister series is distinct from the electroselectivity. In particular, the orders of monovalent anions are completely opposite between the two. Moreover, the concentrations of salts to induce the Hofmeister effects are generally higher than those for anion binding (electroselectivity).
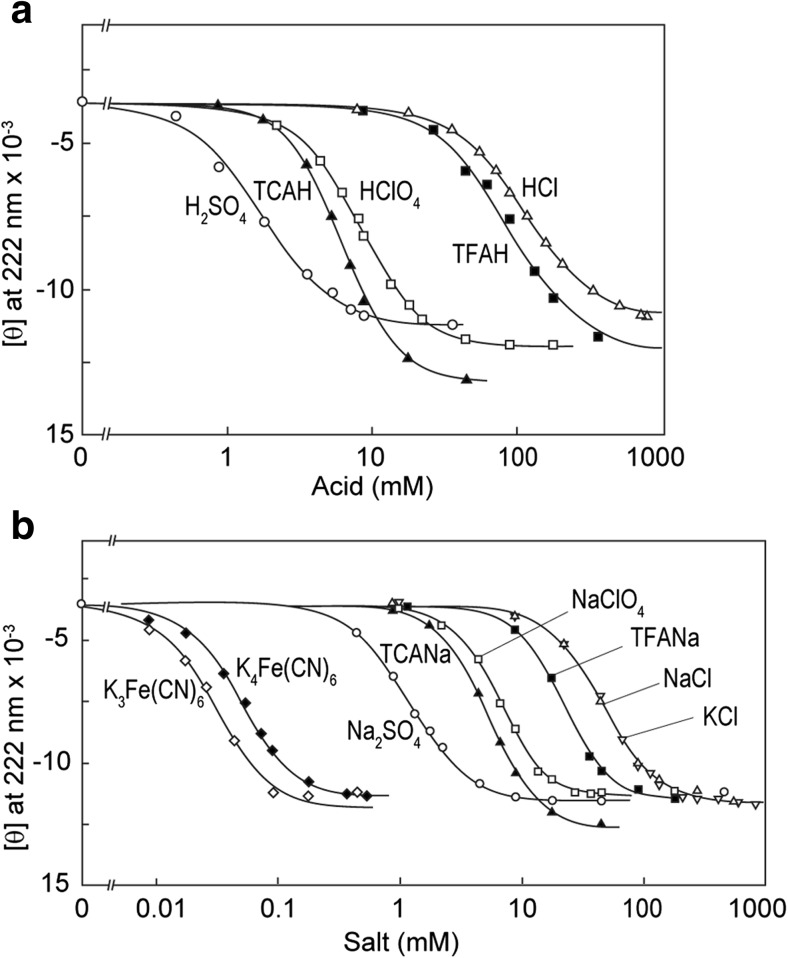
The effects of various salts on acid-unfolding cytochrome c and apomyoglobin were examined by Goto et al. (1990b) (Fig. 2). Similar experiments with various acids were also performed, producing essentially the same results with respect to the order of anions:
Fig. 2.
Acid-induced (a) or salt-induced (b) conformational transitions of horse cytochrome c in the presence of 18 mM HCl measured by the ellipticity at 222 nm at 20 °C. TCAH Trichloroacetic acid, TFAH trifuluoroacetic acid, TCANa sodium trichloroacetate. Reproduced from Goto et al. (1990b), with permission
| 4 |
The results showed convincingly that the effects depend on anion species and the order of effectiveness is consistent with the electroselectivity series (Eqs. 2, 3). Anions with multiple charges show stronger potentials. Among the monovalent anions, chaotropic anions, such as iodide or bromide, show stronger effects, while the effects of chloride are the weakest. These results demonstrate that the electrostatic attraction between the positively charged proteins and negatively charged anions causes the direct interactions between them, thus shielding the charge repulsion.
Generality of the salt effects on denatured proteins
Although anion binding-induced stabilization of the intermediate state is often observed at acidic pH, it is likely that a similar interaction plays a role in determining the protein conformation under physiological pH when a protein is highly positively charged. The salt-dependent conformational transition, known for many years for honey bee venom melittin, was shown to be dependent on anion binding (Hagihara et al. 1992). Moreover, a similar role of anion binding was shown for a designed amphiphilic peptide (Goto and Aimoto 1991). These results imply that anion binding can control the conformational transition of some of natively unfolded proteins.
It is useful to consider the effects of GuHCl in relation to the anion effects. As mentioned above, GuHCl is a salt made of a guanidinium cation and chloride anion. Hagihara et al. (1993) observed intriguing GuHCl-dependent conformational transitions with the acid-unfolded horse cytochrome c and horse apomyologbin. Following the addition of low concentrations of GuHCl, the acid unfolded proteins at first refolded to the molten globule intermediate before unfolding at high GuHCl concentrations. Although this observation might be surprising, the interpretation is straightforward, as is the case of the acid-induced refolding. The first refolding by GuHCl is caused by the anion effects, while subsequent unfolding is driven by the normal chaotropic effects of GuHCl. Similar refolding and unfolding transitions were observed for a designed amphiphilic peptide at neutral pH (Hagihara et al. 1993). Thus, when GuHCl is used, it is important to interpret the data taking into account the dual salt effects. In fact, such dual effects of GuHCl, with low and high GuHCl concentrations promoting and inhibiting amyloid formation, respectively, were observed recently with hen egg white lysozyme (Umemoto et al. 2014).
When the effects of salts on the acid-denatured proteins were examined in the 1990s, it was also recognized that proteins tend to aggregate at high concentrations of salts, at concentrations at which the molten globule states were stabilized. In fact, it was often difficult to characterize the size of the salt-induced molten globule states in a monomeric state because of its high propensity to aggregate (Goto and Fink 1989). Although it was not surprising that the charge-neutralized proteins aggregate, much attention was not paid to the aggregation process because aggregation was considered to be an obstacle that prevented characterization of the protein conformational states. Since the late 1990s, however, amyloid fibrils, one type of protein aggregate, has become one of the most important targets of protein science because of their association with the etiology of serious diseases (Dobson 2003; Eisenberg and Jucker 2012; Sipe et al. 2014; Tycko and Wickner 2013).
β2-microglobulin and formation of amyloid fibrils
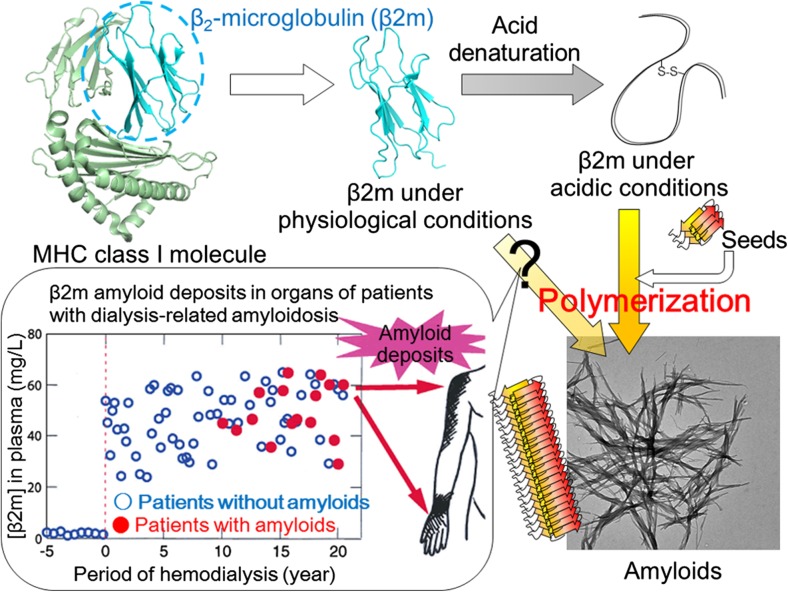
Among the various amyloidogenic proteins, β2-microglobulin (β2m) has been studied extensively because of its moderate size of 99 amino acid residues, enabling a comparison of protein folding and misfolding (Fig. 3). β2m, originally a component of the type I major histocompatibility complex, is found at low concentration in circulating blood, and its turnover depends on its degradation in the kidneys. Defective homeostasis of β2m as a result of failure of the kidney function and the consequent inability of β2m to flow through the dialysis membrane leads to the accumulation of β2m in the blood (Yamamoto and Gejyo 2005). Due to a still unclear mechanism, β2m has been found to form amyloid deposits in the synovia of the carpal tunnel of patients undergoing long-term hemodialysis, leading to pathological conditions called dialysis-related amyloidosis. The monomeric β2m easily undergoes in vitro amyloid formation under acidic conditions below pH 4 (Naiki et al. 1997), although amyloid formation under neutral conditions requires additional factors or destabilization of the native fold (Myers et al. 2006; So et al. 2015; Stoppini and Bellotti 2015). Various studies with β2m as well as other proteins showed that the formation of amyloid fibrils critically depends on the salt concentration (Raman et al. 2005; So et al. 2011; Yanagi et al. 2012).
Fig. 3.
Development of dialysis-related amyloidosis by amyloid deposition of β2-microglobulin. MHC Major histocompatibility complex
Salt-induced amyloid formation of β2m at pH 2
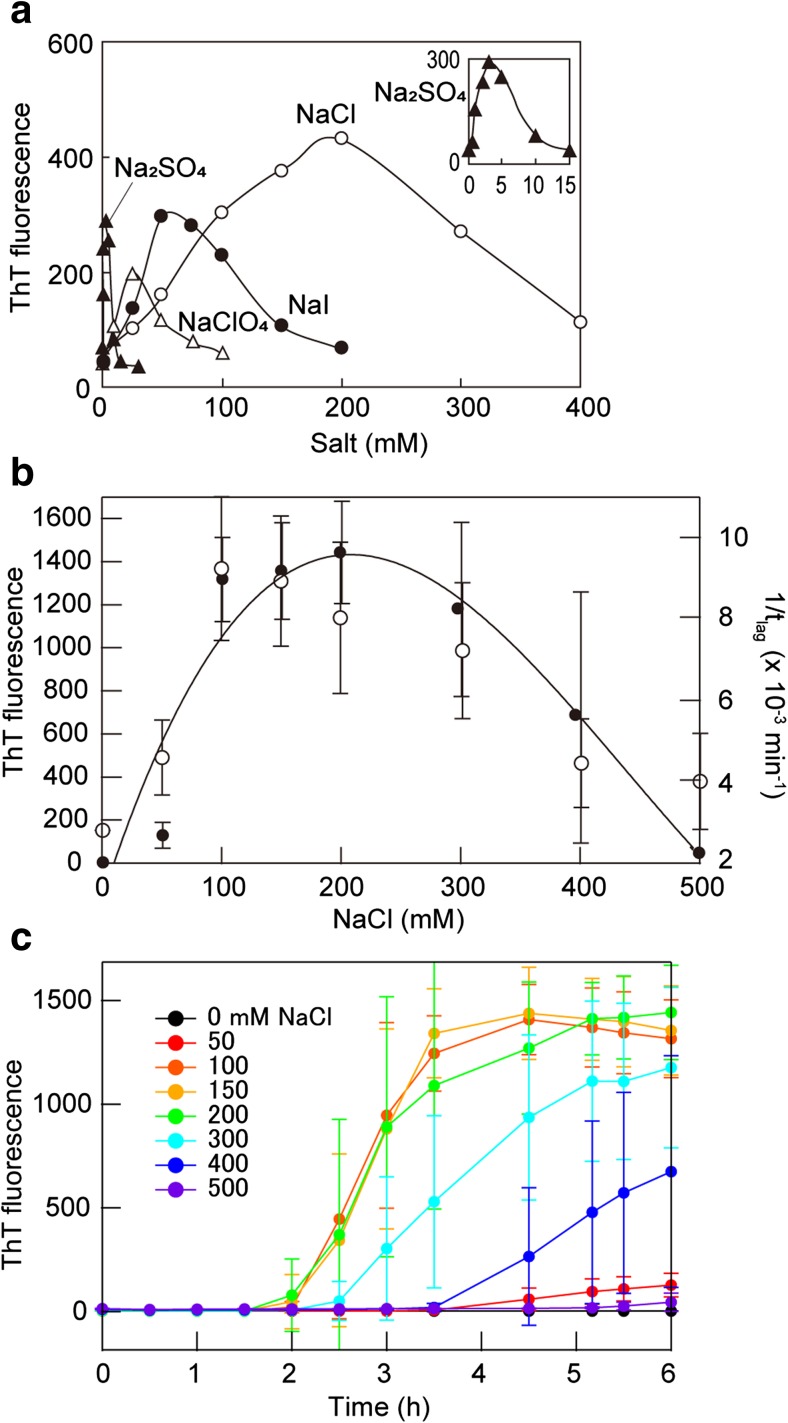
The formation of amyloid fibrils of β2m at acidic pH critically depends on the salt conditions (Adachi et al. 2015; Raman et al. 2005; Yanagi et al. 2012) (Fig. 4). In these studies, β2m, which acid-unfolded at a pH of around 2 in the absence of salt, did not form amyloid fibrils. In the presence of NaCl, the thioflavin T (ThT) fluorescence intensity increased as a function of the concentration of NaCl and incubation time, indicating the growth of the β2m fibrils. The midpoint of NaCl concentration-dependent formation was around 50–100 mM at pH 2 and 37 °C.
Fig. 4.
Salt-dependent formation of amyloid fibrils of β2m at acidic pH. a Formation of fibrils in the presence of seeds dependent on the salt species and concentration. Reproduced from Raman et al. (2005), with permission. b, c NaCl concentration-dependent spontaneous formation of fibrils in the absence of seeds as a function of the final thioflavin T (ThT) fluorescence intensity (open circles) and lag-time (closed circles) (b) and their time courses (c). Reproduced from So et al. (2011), with permission
To address the mechanism of the salt effects, Raman et al. (2005) compared the effects of various salts. The rate of fibril formation increased with increasing salt concentration, with a maximum at around 200 mM NaCl (Fig. 4a). Further increases in NaCl concentration, however, resulted in decreasing fibril growth. The value of ThT fluorescence intensity at the 60-min incubation time-point showed a bell-shape profile, revealing an optimal concentration of the salt required for efficient fibril growth of β2m (Fig. 4a).
In order to understand the role of anions, Raman et al. (2005) compared the effect of various salts, such as Na2SO4, NaClO4, NaI, and NaCl (composed of different anions and the same counter cation, Na+), on the fibril growth of β2m. Similar to the observation made for NaCl, they found concentration-dependent change in the fibril growth for other salts also (Fig. 4a). Intriguingly, the optimal concentrations of the salts differed drastically depending on the anionic species of the salts. The optimal concentration under which fibril elongation was favored was around 200, 50, 25, and 3 mM for NaCl, NaI, NaClO4, and Na2SO4, respectively. Thus, the order of minimum concentration at which fibril growth was favored is SO4 2− > ClO4 − > I− > Cl−. This order also holds for the higher concentrations of the salt at which the fibril growth was inhibited. Similar results were obtained later by So et al. (2011), who observed spontaneous fibril formation of β2m triggered by ultrasonication (Fig. 4b, c).
Competition between amyloid formation and amorphous aggregation
The order of effectiveness of various anions on the formation of amyloid fibrils of β2m is in agreement with that found for acid-unfolded proteins, as described above, revealing that the salt-dependent acceleration of fibril formation shares a common mechanism with the salt-induced formation of the molten globule state. Moreover, the salt-dependent formation of fibrils showed a clear optimum, revealing a competition of fibril formation and amorphous aggregation. Yoshimura et al. (2012) further examined the difference between amyloid formation and amorphous aggregation in analogy with crystallization and glass formation.
In general, amyloid fibrils and amorphous aggregates are two types of aggregates. Another important type of aggregates is oligomers, which have been proposed to be responsible for the cytotoxicity of amyloidogenic proteins (Bemporad and Chiti 2012). Here, we assume that oligomers and amorphous aggregates may be continuous, as proposed by Miti et al. (2015). Although amyloid fibrils and amorphous aggregates clearly differ in morphology, the two forms are often treated indiscriminately. On the other hand, crystals and amorphous aggregates are distinguished more clearly in crystallography. Solubility and supersaturation are two of the most important factors determining the crystallization of solutes (Bergfors 2003; Coquerel 2014). Moreover, crystallization competes with glass formation in which solutes collapse into amorphous aggregates by a process probably not limited by supersaturation. β2m forms amyloid fibrils or amorphous aggregates depending on the NaCl concentration at pH 2.5. Yoshimura et al. (2012) assumed that amyloid fibrils and amorphous aggregates correspond to crystals and glasses, respectively. The kinetics of fibril formation follows a nucleation-growth mechanism in a supersaturated solution, analogous to the crystallization of solutes. In contrast, the glass-like amorphous aggregates formed rapidly without a lag phase. Neither agitation nor stirring accelerated the amorphous aggregation. Thus, by monitoring the kinetics, crystal-like amyloid fibrils and glass-like amorphous aggregates can be distinguished where solubility and supersaturation are key factors for further understanding the aberrant aggregation of proteins.
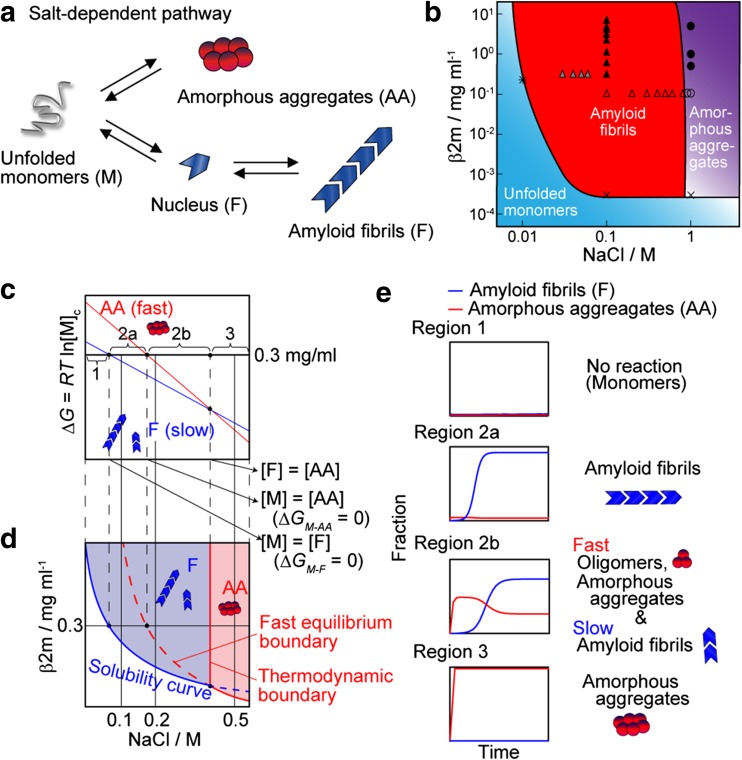
Adachi et al. (2015) further examined the salt-dependent formation of amyloid fibrils and amorphous aggregates (Fig. 5). They explained the apparent complexities of fibrillation (i.e., coexistence of amyloid fibrils and amorphous aggregates) comprehensively on the basis of a competitive mechanism in which supersaturation-limited reactions compete with supersaturation-unlimited reactions. They link the kinetics of protein aggregation and a conformational phase diagram, in which supersaturation plays important roles. Such competition produces a transient formation of amorphous aggregates and its slow conversion to amyloid fibrils. These complex and competitive kinetics of various aggregates are consistent with Ostwald’s ripening rule of stages of crystallization (Levin et al. 2014). Ostwald ripening is a phenomenon in solid formation in liquid solutions that describes the change of an inhomogeneous structure over time, leading to progressively larger structures having a relatively smaller surface area to volume ratio. Ostwald’s ripening assumes that monomers detach from structures of one type, diffuse in solution, and are incorporated in more stable of supramolecular assemblies.
Fig. 5.
The competitive mechanism of amyloid fibrillation and amorphous aggregation (AA). a Schematic model. b Phase-diagram for the NaCl dependence of monomers, fibrils, and amorphous aggregates. Symbols indicate the experimental data to obtain the phase diagram; see Ikenoue et al. (2014) for details. Reproduced from Ikenoue et al. (2014), with permission. c, d Dependency on the NaCl concentration of free energy changes of amyloid fibrillation and amorphous aggregation and the predicted phase diagram. e Representative kinetics under Regions 1–3 as defined in c are illustrated. Reproduced from Adachi et al. (2015), with permission
Amorphous aggregation can be considered to be a colloidal reaction determined by a balance of hydrophobic and electrostatic interactions. Miti et al. (2015) addressed the competition between amyloid fibrils and amorphous aggregates with hen egg white lysozyme. Oligomers and their curvilinear fibrils formed rapidly after crossing a salt- and protein concentration-dependent threshold, while the formation of rigid fibrils was limited by a high free energy barrier. These authors constructed a phase diagram based on changes in NaCl and lysozyme concentrations, similar in nature to our phase diagram shown in Fig. 5b.
Heparin, a glycosaminoglycan and anticoagulant, is an accelerator of fibrillation for various amyloidogenic proteins, including β2m (Cohlberg et al. 2002; Doig and Derreumaux 2015; Myers et al. 2006; Yamamoto et al. 2004). Recently, So et al. (2017) examined the effects of various concentrations of heparin on the formation of β2m fibrils at pH 2. In contrast to previous studies that focused on accelerating effects, higher concentrations of heparin inhibited fibrillation, and this was accompanied by amorphous aggregation. The two-step effects of acceleration and inhibition were similar to those observed for various salts. The results indicate that the anion effects caused by sulfate groups are one of the dominant factors influencing heparin-dependent fibrillation. The results were comprehensively explained on the basis of a conformational phase diagram accommodating crystal-like amyloid fibrils and glass-like amorphous aggregates and indicate the importance of competitive mechanism for understanding the effects of various additives. Similar results were also obtained with hen egg white lysozyme (Nitani et al. 2017).
Comparison of the native states and amyloid fibrils
The conformational phase diagram of β2m under acidic conditions dependent on salt concentration and protein concentration (Fig. 5b) is compared with conformational phase diagram of acidic denatured proteins dependent on salt concentration in Fig. 1g, h. Acid-denatured β2m forms amyloid fibrils with increasing salt concentration. In contrast, acid-denatured cytochrome c or apomyoglobin forms the molten globule state with increasing salt concentration. However, misfolding to amyloid fibrils is limited by supersaturation. We propose a protein solubility- and supersaturation-dependent protein misfolding funnel, which can be compared with the protein folding funnel without supersaturation (Lin et al. 2014).
Although the salt-induced conformations are distinct between the molten globule and amyloid fibrils, the underlying mechanisms are common: counter anion bindings to the positively charged proteins shield repulsive forces and manifest intrinsic hydrophobic and hydrogen bond interactions. When the protein concentration is low, intramolecular interactions take place, stabilizing the molten globule state under conditions at which the specific native interactions cannot form. As for amyloidogenic proteins, reduction of the repulsive forces results in the decrease in solubility and produces proteins aggregates. Then, crystal-like amyloid fibrils form above equilibrium solubility after breaking supersaturation. However, the rapid formation of glass-like amorphous aggregates occur when the driving force is too strong to maintain supersaturation. As for non-amyloidogenic proteins, an amyloid region is considered to be narrow or missing because of the prevalence of the amorphous region.
The salt-dependent phase diagram of amyloid formation indicates that the mechanism of amyloid fibrillation can be interpreted by the same mechanism of crystallization and amorphous aggregation of solutes. It is likely that major driving forces of protein folding (i.e., hydrophobic interactions and hydrogen bonds) also play roles in stabilizing amyloid fibrils, although calorimetric data suggested that their contributions seem less in amyloid fibrils (Ikenoue et al. 2014; Kardos et al. 2004). Moreover, provided that the similar driving forces are involved in the stabilization of the molten globule state and amyloid fibrils, strong acid-induced formation of amyloid fibrils can be anticipated as was demonstrated by the acid-induced molten globule state (Goto et al. 1990a).
Finally, although in this review we focused on the anion-binding-dependent conformational change of proteins, salts also affect protein conformation and stability on the basis of Hofmeister effects (Eq. 1). It is likely that such effects come to play under distinct conditions where the salt concentration is very high, producing more complicated effects. In fact, in our study we observed two stages of heparin-dependent fibril formation for hen egg white lysozyme at pH 2, where the first stage depended on the electrostatic interaction between the positively charged lysozyme and negatively charged heparin and the second stage of fibril formation was caused by sulfate salting-out effects at high heparin concentration (Nitani et al. 2017). A similar two-stage effects on the basis of distinct salt effects was reported for α-synuclein (Munishkina et al. 2004). The detailed analysis of salt effects will further clarify the mechanism of the formation of amyloid fibrils and also create therapeutic strategies preventing amyloidogenesis.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 15H04362, 15 K14458, and 16H00836 and 17H06352, and by the SENTAN from Japan Agency for Medical Research and Development (AMED).
Compliance with ethical standards
Conflict of interest
Yuji Goto declares that he has no conflicts of interest. Masayuki Adachi declares that he has no conflicts of interest. Hiroya Muta declares that he has no conflicts of interest. Masatomo So declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines—Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura
References
- Adachi M, So M, Sakurai K, Kardos J, Goto Y (2015) Supersaturation-limited and unlimited phase transitions compete to produce the pathway complexity in amyloid fibrillation. J Biol Chem 290:18134–18145 [DOI] [PMC free article] [PubMed]
- Arai M, Kuwajima K. Role of the molten globule state in protein folding. Adv Protein Chem. 2000;53:209–282. doi: 10.1016/S0065-3233(00)53005-8. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Timasheff SN. Preferential interactions of proteins with salts in concentrated solutions. Biochemistry. 1982;21:6545–6552. doi: 10.1021/bi00268a034. [DOI] [PubMed] [Google Scholar]
- Bemporad F, Chiti F. Protein misfolded oligomers: experimental approaches, mechanism of formation, and structure-toxicity relationships. Chem Biol. 2012;19:315–327. doi: 10.1016/j.chembiol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Bergfors T. Seeds to crystals. J Struct Biol. 2003;142:66–76. doi: 10.1016/S1047-8477(03)00039-X. [DOI] [PubMed] [Google Scholar]
- Chatani E, Goto Y (2005) Structural stability of amyloid fibrils of β2-microglobulin in comparison with its native fold. Biochim Biophys Acta 1753:64–75 [DOI] [PubMed]
- Chatani E, Lee YH, Yagi H, Yoshimura Y, Naiki H, Goto Y (2009) Ultrasonication-dependent production and breakdown lead to minimum-sized amyloid fibrils. Proc Natl Acad Sci USA106:11119–11124 [DOI] [PMC free article] [PubMed]
- Ciryam P, Kundra R, Morimoto RI, Dobson CM, Vendruscolo M (2013) Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep 5:781–790 [DOI] [PMC free article] [PubMed]
- Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, Vendruscolo M (2015) Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci 36:72–77 [DOI] [PMC free article] [PubMed]
- Cohlberg JA, Li J, Uversky VN, Fink AL (2002) Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro. Biochemistry 41:1502–1511 [DOI] [PubMed]
- Colvin MT, Silvers R, Ni QZ et al (2016) Atomic resolution structure of monomorphic Aβ(1-42) amyloid fibrils. J Am Chem Soc 138:9663–9674 [DOI] [PMC free article] [PubMed]
- Coquerel G. Crystallization of molecular systems from solution: phase diagrams, supersaturation and other basic concepts. Chem Soc Rev. 2014;43:2286–2300. doi: 10.1039/C3CS60359H. [DOI] [PubMed] [Google Scholar]
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- Doig AJ, Derreumaux P. Inhibition of protein aggregation and amyloid formation by small molecules. Curr Opin Struct Biol. 2015;30:50–56. doi: 10.1016/j.sbi.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL, Calciano LJ, Goto Y, Kurotsu T, Palleros DR (1994) Classification of acid denaturation of proteins: intermediates and unfolded states. Biochemistry 33:12504–12511 [DOI] [PubMed]
- Fitzpatrick AWP, Falcon B, He S et al (2017) Cryo-EM structures of tau filaments from Alzheimer's disease. Nature 547:185–190 [DOI] [PMC free article] [PubMed]
- Giehm L, Otzen DE. Strategies to increase the reproducibility of protein fibrillization in plate reader assays. Anal Biochem. 2010;400:270–281. doi: 10.1016/j.ab.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Goto Y, Aimoto S. Anion and pH-dependent conformational transition of an amphiphilic polypeptide. J Mol Biol. 1991;218:387–396. doi: 10.1016/0022-2836(91)90720-Q. [DOI] [PubMed] [Google Scholar]
- Goto Y, Fink AL (1989) Conformational states of β-Lactamase - molten-globule states at acidic and alkaline ph with high salt. Biochemistry 28:945–952 [DOI] [PubMed]
- Goto Y, Fink AL. Phase diagram for acidic conformational states of apomyoglobin. J Mol Biol. 1990;214:803–805. doi: 10.1016/0022-2836(90)90334-I. [DOI] [PubMed] [Google Scholar]
- Goto Y, Calciano LJ, Fink AL (1990a) Acid-induced folding of proteins. Proc Natl Acad Sci USA USA87:573–577 [DOI] [PMC free article] [PubMed]
- Goto Y, Takahashi N, Fink AL. Mechanism of acid-induced folding of proteins. Biochemistry. 1990;29:3480–3488. doi: 10.1021/bi00466a009. [DOI] [PubMed] [Google Scholar]
- Hagihara Y, Kataoka M, Aimoto S, Goto Y (1992) Charge repulsion in the conformational stability of melittin. Biochemistry 31:11908–11914 [DOI] [PubMed]
- Hagihara Y, Aimoto S, Fink AL, Goto Y(1993) Guanidine hydrochloride-induced folding of proteins. J Mol Biol 231:180–184 [DOI] [PubMed]
- Hamada D, Kidokoro S, Fukada H, Takahashi K, Goto Y (1994) Salt-induced formation of the molten globule state of cytochrome c studied by isothermal titration calorimetry. Proc Natl Acad Sci USA 91:10325–10329 [DOI] [PMC free article] [PubMed]
- Ikenoue T, Lee YH, Kardos J et al (2014) Heat of supersaturation-limited amyloid burst directly monitored by isothermal titration calorimetry. Proc Natl Acad Sci USA 111:6654–6659 [DOI] [PMC free article] [PubMed]
- Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos J, Yamamoto K, Hasegawa K, Naiki H, Goto Y (2004) Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J Biol Chem 279:55308–55314 [DOI] [PubMed]
- Levin A, Mason TO, Adler-Abramovich L et al (2014) Ostwald’s rule of stages governs structural transitions and morphology of dipeptide supramolecular polymers. Nat Commun 5:5219 [DOI] [PubMed]
- Lin Y, Lee YH, Yoshimura Y, Yagi H, Goto Y (2014) Solubility and supersaturation-dependent protein misfolding revealed by ultrasonication. Langmuir 30:1845–1854 [DOI] [PubMed]
- Linse S, Cabaleiro-Lago C, Xue WF et al (2007) Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci USA 104:8691–8696 [DOI] [PMC free article] [PubMed]
- Miti T, Mulaj M, Schmit JD, Muschol M (2015) Stable, metastable, and kinetically trapped amyloid aggregate phases. Biomacromolecules 16:326–335 [DOI] [PMC free article] [PubMed]
- Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim Biophys Acta. 2009;1794:375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Munishkina LA, Henriques J, Uversky VN, Fink AL (2004) Role of protein-water interactions and electrostatics in alpha-synuclein fibril formation. Biochemistry 43:3289–3300 [DOI] [PubMed]
- Myers SL, Jones S, Jahn TR et al (2006) A systematic study of the effect of physiological factors on β2-microglobulin amyloid formation at neutral pH. Biochemistry 45:2311–2321 [DOI] [PubMed]
- Naiki H, Hashimoto N, Suzuki S, Kimura H, Nakakuki K, Gejyo F (1997) Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro. Amyloid 4:223–232
- Nakajima K, Ogi H, Adachi K et al (2016) Nucleus factory on cavitation bubble for amyloid β fibril. Sci Rep 6:22015 [DOI] [PMC free article] [PubMed]
- Nishii I, Kataoka M, Goto Y. Thermodynamic stability of the molten globule states of apomyoglobin. J Mol Biol. 1995;250:223–238. doi: 10.1006/jmbi.1995.0373. [DOI] [PubMed] [Google Scholar]
- Nitani A, Muta H, Adachi M et al (2017) Heparin-dependent aggregation of hen egg white lysozyme reveals two distinct mechanisms of amyloid fibrillation. J Biol Chem. doi: 10.1074/jbc.M117.813097 [DOI] [PMC free article] [PubMed]
- Ohgushi M, Wada A. 'Molten-globule state': a compact form of globular proteins with mobile side-chains. FEBS Lett. 1983;164:21–24. doi: 10.1016/0014-5793(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Ohhashi Y, Kihara M, Naiki H, Goto Y (2005) Ultrasonication-induced amyloid fibril formation of β2-microglobulin. J Biol Chem 280:32843–32848 [DOI] [PubMed]
- Raman B, Chatani E, Kihara M et al (2005) Critical balance of electrostatic and hydrophobic interactions is required for β2-microglobulin amyloid fibril growth and stability. Biochemistry 44:1288–1299 [DOI] [PubMed]
- Sipe JD, Benson MD, Buxbaum JN et al (2014) Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21:221–224 [DOI] [PubMed]
- So M, Yagi H, Sakurai K, Ogi H, Naiki H, Goto Y (2011) Ultrasonication-dependent acceleration of amyloid fibril formation. J Mol Biol 412:568–577 [DOI] [PubMed]
- So M, Ishii A, Hata Y, Yagi H, Naiki H, Goto Y (2015) Supersaturation-limited and unlimited phase spaces compete to produce maximal amyloid fibrillation near the critical micelle concentration of sodium dodecyl sulfate. Langmuir 31:9973–9982 [DOI] [PubMed]
- So M, Hall D, Goto Y. Revisiting supersaturation as a factor determining amyloid fibrillation. Curr Opin Struct Biol. 2016;36:32–39. doi: 10.1016/j.sbi.2015.11.009. [DOI] [PubMed] [Google Scholar]
- So M, Hata Y, Naiki H, Goto Y (2017) Heparin-induced amyloid fibrillation of β2 -microglobulin explained by solubility and a supersaturation-dependent conformational phase diagram. Protein Sci 26:1024–1036 [DOI] [PMC free article] [PubMed]
- Stoppini M, Bellotti V (2015) Systemic amyloidosis: lessons from β2-microglobulin. J Biol Chem 290:9951–9958 [DOI] [PMC free article] [PubMed]
- Suzuki M, Yokoyama K, Lee YH, Goto Y (2011) A two-step refolding of acid-denatured microbial transglutaminase escaping from the aggregation-prone intermediate. Biochemistry 50:10390–10398 [DOI] [PubMed]
- Suzuki M, Sakurai K, Lee YH, Ikegami T, Yokoyama K, Goto Y (2012) A back hydrogen exchange procedure via the acid-unfolded state for a large protein. Biochemistry 51:5564–5570 [DOI] [PubMed]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/S0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. doi: 10.1016/S0065-3233(08)60241-7. [DOI] [PubMed] [Google Scholar]
- Tycko R, Wickner RB. Molecular structures of amyloid and prion fibrils: consensus versus controversy. Acc Chem Res. 2013;46:1487–1496. doi: 10.1021/ar300282r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto A, Yagi H, So M, Goto Y (2014) High-throughput analysis of the ultrasonication-forced amyloid fibrillation reveals the mechanism underlying the large fluctuation in the lag time. J Biol Chem 289:27290–27299 [DOI] [PMC free article] [PubMed]
- Walti MA, Ravotti F, Arai H et al (2016) Atomic-resolution structure of a disease-relevant Aβ(1-42) amyloid fibril. Proc Natl Acad Sci USA 113:E4976–E4984 [DOI] [PMC free article] [PubMed]
- Washabaugh MW, Collins KD. The systematic characterization by aqueous column chromatography of solutes which affect protein stability. J Biol Chem. 1986;261:12477–12485. [PubMed] [Google Scholar]
- Wetzel R. Kinetics and thermodynamics of amyloid fibril assembly. Acc Chem Res. 2006;39:671–679. doi: 10.1021/ar050069h. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Gejyo F. Historical background and clinical treatment of dialysis-related amyloidosis. Biochim Biophys Acta. 2005;1753:4–10. doi: 10.1016/j.bbapap.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamaguchi I, Hasegawa K et al (2004) Glycosaminoglycans enhance the trifluoroethanol-induced extensionof β2-microglobulin-related amyloid fibrils at a neutral pH. J Am Soc Nephrol 15:126–133 [DOI] [PubMed]
- Yanagi K, Sakurai K, Yoshimura Y et al (2012) The monomer-seed interaction mechanism in the formation of the β2-microglobulin amyloid fibril clarified by solution NMR techniques. J Mol Biol 422:390–402 [DOI] [PubMed]
- Yoshimura Y, Lin YX, Yagi H et al (2012) Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc Natl Acad Sci USA 109:14446–14451 [DOI] [PMC free article] [PubMed]
- Yoshimura Y, So M, Yagi H, Goto Y (2013) Ultrasonication: an efficient agitation for accelerating the supersaturation-limited amyloid fibrillation of proteins. Jpn J Appl Physics 52:01–08