Abstract
Purpose: Diabetes mellitus is one of the major endocrine disorders, characterized by impaired insulin action and deficiency. Traditionally, Artocarpus heterophyllus stem bark has been reputably used in the management of diabetes mellitus and its complications. The present study evaluates the ameliorative activity of ethanol extract of Artocarpus heterophyllus stem bark in alloxan-induced diabetic rats.
Methods: Diabetes mellitus was induced by single intraperitoneal injection of 150 mg/kg body weight of alloxan and the animals were orally administered with 50, 100 and 150 mg/kg body weight ethanol extract of Artocarpus heterophyllus stem bark once daily for 21 days.
Results: At the end of the intervention, diabetic control rats showed significant (p<0.05) weight reduction, abnormal haematological parameters, high serum lipids (except high density lipoprotein) concentrations, increased creatinine, bilirubin and urea levels with decreased in albumin level when compared with non-diabetic control rats. All these alterations were reverted to normal after administered with different doses of ethanol extract of Artocarpus heterophyllus stem bark most especially at 150 mg/kg body weight which exhibited no significant (p>0.05) different with non-diabetic rats.
Conclusion: The results suggest that ethanol extract of Artocarpus heterophyllus stem bark may be useful in ameliorating complications associated with diabetes mellitus patients.
Keywords: Artocarpus heterophyllus stem bark, Alloxan, Serum lipid profiles, Haematological parameters
Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by derangements in carbohydrate, protein and lipid metabolisms, due to defective or deficiency in insulin secretion and action.1 Insulin is a hormone secreted by the beta cells of the Islets of Langerhans of the pancreas, it helps in glucose uptake by the cells, thereby prevents increase in fasting blood glucose levels.2
Diabetes mellitus is also associated with hyperglycaemia, which promotes oxidative stress through non-enzymatic glycation and glucose auto-oxidation.3 This oxidative stress has been implicated in the complications of diabetes mellitus, including dyslipidaemia, a major risk factor for cardiovascular disease,4 anaemia, nephropathy, and hepatopathy.3 It is believed that therapeutic agents (like metformin, gliberclamide etc.) normally reduces the effects of oxidative damages in diabetes mellitus patients and therefore ameliorate its complications.3 But these drugs are characterized with some side effects such as hypoglycaemia, hypersensitivity, gastrointestinal disturbances, lactic acidosis, liver toxicity amongst others.5,6
Furthermore, in recent years, the search for alternative therapeutic agents in the management/treatment of diabetes has been the major focus of scientific researches across the globe.7 Added to this, the therapeutic efficacy of medicinal plant due to their believed minimal side effects and its affordability over modern synthetic drugs has been recommended. In this regards many herbal medicines and medicinal plants7 have been used traditionally for the control and management/ treatment of diabetes mellitus in different parts of the world.8 It has been reported that screening of medicinal plants for therapeutic purposes, is an important aspect in drug development because they may possess anti-aneamia, anti-dyslipideamia, anti-nephropathy and anti-hepatopathy which may be useful in the management of diabetes mellitus.9
Artocarpus heterophyllus (jack fruit) is an example of plant that may be used in this regards, it belongs to a family of Moraceae and grown in tropical climates. Artocarpus heterophyllus has been considered a rich source of carbohydrates, minerals, dietary fiber and vitamins amongst others.10 Its stem bark has also been reported to be of great importance in the management of diabetes mellitus locally.11 In addition, the inhibitory ability of Artocarpus heterophyllus stem bark on alpha-amylase and alpha-glucosidase has been documented.12
Therefore, the present study was designed to examine the ameliorative effects of ethanol extract of Artocarpus heterophyllus stem bark on haematological parameters, serum lipid profiles, liver and kidney functions indices of alloxan-induced diabetic rats.
Materials and Methods
Chemicals
Alloxan used was a product of Sigma Aldrich (St. Louis, MO, USA). Glibenclamide used was a product of Sandoz SA (Pty) Ltd. (Gauteng, South Africa). All assay kits used were obtained from Randox while other chemicals used were purchased from Merck Chemical (Germany).
Collection of Plant Material
The fresh peeled stem bark of Artocarpus heterophyllus were collected at a farm in Ibadan, Oyo State, Nigeria. This was then identified and authenticated at the Department of Plant Science, Ekiti State University, Ado-Ekiti, Nigeria.
Extract preparation
The stem bark of Artocarpus heterophyllus was shade-dried to a constant weight. Thereafter, kitchen blender was used to blend the dried stem bark of Artocarpus heterophyllus into fine power and stored in air-tight containers. Thereafter, 100 grams of powdered plant sample was extracted with 1 litre of 70% ethanol for 48 hours. The extract was then filtered with Whatman filter paper and the filtrate was evaporated to dryness using a freeze dryer. The extract (SBAH) was reconstituted in distilled water and used for subsequent analysis.
Qualitative phytochemical screening
The methods described by13-15 were employed in these determinations. These include:
-
Test for Tannins
1 ml of SBAH was boiled with 20 ml of distilled water in a test tube and filtered. Thereafter, a few drops of 0.1% ferric chloride was added and formation of a green or a blue–black coloration confirm the presence of tannin
-
Test for Phlobatannins:
Deposition of a red precipitate when 2 ml of SBAH was boiled with 1% aqueous hydrochloric acid was taken as evidence for the presence of phlobatannins.
-
Test for Saponin:
5 ml of the SBAH was boiled with 20 ml of distilled water in a water bath and filtered. Then 10 ml of the filtrate was mixed with another 5 ml of distilled water and shaken vigorously till formation of a stable persistent froth. The frothing was further mixed with 3 drops of olive oil and shaken vigorously until formation of emulsion which confirms the presence of saponin.
-
Test of Flavonoids:
In this test, 3 ml of 1 % aluminium chloride was added to 5 ml of SBAH. A yellow coloration was observed indicating the presence of flavonoids.
-
Test for Steroids
2 ml of acetic anhydride was added to 2 ml SBAH, thereafter, 2 ml H2SO4. The formation of blue or green indicate the presence of steroids.
-
Test for Terpenoids
5 ml of SBAH was mixed with 2 ml of chloroform, then 3 ml concentrated H2SO4 was added to form a layer. The formation of reddish brown coloration at the interface indicate the presence of terpenoids.
-
Test for Cardiac Glycosides
5 ml of SBAH was added to 2 ml of glacial acetic acid containing one drop of ferric chloride solution. Thereafter 1 ml of concentrated sulphuric acid was added. Formation of a violet-green ring appearing below the brown ring, in the acetic acid layer, indicates the presence of glycoside.
-
Test for Alkaloids:
1 ml of the SBAH was stirred with 5 ml of 1% aqueous HCl on a steam bath, then filtered while hot. Distilled water was added to the residue and 1 ml of the filtrate was added with a few drops of either Mayer’s reagent (Potassium mercuric iodide- solution) or Wagner’s reagent (solution of iodine in Potassium iodide) or Dragendorff’s reagent (solution of Potassium bismuth iodide). The formation of a cream colour with Mayer’s reagent and reddish-brown precipitate with Wagner’s and Dragendorff’s reagent confirm the presence of alkaloids.
-
Test for Anthraquinone
5 ml of SBAH was mixed with 10 ml of benzene, filtered and 5 ml of 10 % NH3 solution was added to the filtrate. Thereafter, the mixture was shaken until the appearance of pink, red or violet colour.
-
Test for Chalcones
2 ml of ammonia solution was added to 5 ml of SBAH and formation of a reddish colour confirmed presence of chalcones.
-
Test for Phenol
5 ml of the SBAH was pipetted into a 30 ml test tube, then 10 ml of distilled water was added. 2 ml of ammonium hydroxide solution and 5 ml of concentrated amyl alcohol were then added to the mixture and left for 30 minutes. Formation of bluish green colour indicate the presence of phenol.
Experimental Animals
A total of 36 albino rats’ weighting between 180-200 g obtained from Animal Holding Unit of Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria were used in this study. The animals were kept under standard environmental conditions for 21 days of the experimental period. Prior to this the animals were acclimatized for 7 days. All the animals had free access to food and water throughout the experimental period. All procedures followed were in accordance with the ethical standards of Afe Babalola University Animal Committee with Ethical Approval Number (ABUAD/SCI/004). Also the principle of Laboratory Animal Care were followed throughout the experimental period.
Induction of Diabetes
Freshly prepared alloxan monohydrate of 150 mg/kg body weight dissolved in 0.9% sterile NaCl of pH 716 was administered intraperitoneally to rats in group B to F to induce diabetes. Prior to this, their fasting blood glucose levels had been determined. Also, after 48 hours of alloxan induction, the rats fasting blood glucose levels were assessed with the aid of Acucheck Advantage II glucometer and those that had fasting blood glucose level ≥ 200 mg/dl were considered diabetic and used for the study.17 The rats were divided into six groups with six rats per group as follows:
Group A: Non-diabetic control rats received distilled water (3 mg/kg body weight)
Group B: Diabetic-control rats received distilled water (3 ml/kg body weight)
Group C: Diabetic rats received glibenclamide (5 mg/kg bodyweight)
Group D: Diabetic rats received 50 mg/kg body weight of SBAH
Group E: Diabetic rats received 100 mg/kg body weight of SBAH
Group F: Diabetic rats received 150 mg/kg body weight of SBAH
Collection and analysis of samples
On the 20th day of oral administration, the animals were fasted overnight for 12 hours and on the 21st day the animals were anaesthetized with halothane and then sacrificed. The animal’s blood were then collected by cardiac puncture into ETDA bottles and plain sample bottles. The former were used for haematological parameters determination while the latter were allowed to clot for two hours and centrifuged at 3000 rpm for 10 minutes, after which serum was recovered for analysis.
Haematological parameters determination
The levels of packed cell volume (PCV), haemoglobin (HB), white blood cell (WBC), red blood cell (RBC), neutrophil (N), lymphocyte (L), monocyte (M), eosinophil (E), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin (MCH) were determined using automated haematology analyzer (System KX-21NTm, Japan).
Serum lipid profile determinations
The method18 modified by19 was employed for determination of cholesterol concentration. The method of20 was employed for determination of triglycerides concentration and high density lipoprotein (HDL) concentration, while the Friedwald equation21 was used for determining very low density lipoprotein (VLDL) concentration and low density lipoprotein (LDL) concentration. Atherogenic index (AI) and coronary artery risk index (CRI) were carried out using22 and23 respectively.
Determination of some liver and kidney function indices
The method of24 was used for albumin determination, while the method described by25 was employed for bilirubin determination. Creatinine was determined using the method described by Tietz and co-workers26while urea was estimated according to the method of Fawcett and Scott.27 Alanine and aspartate aminotransferase (ALT and AST) activities were determined using the method of Reitman and Frankel.28 Alkaline phosphatase was determined as described by Wright et al.29
Statistical Analysis
The data were analysed with students’ T-test and one way ANOVA. Values of p<0.05 were considered significant.
Results and Discussion
Ajiboye et al30 reported that plants were endowed with series of secondary metabolites like terpenoids, phenolic, lignins, stilbenes, tannins, flavonoids, quinones, coumarins, alkaloids, amines, betalains amongst others. They were free radical scavenging, making them serve as antioxidant compounds and possess antidiabetic activity amongst others. In this study, the ethanol extract of Artocarpus heterophyllus stem bark demonstrated the presence of saponins (Table 1) which possess cholesterol and fasting blood glucose levels lowering effect making it useful for diabetes mellitus patients.31
Table 1. Qualitative screening of some phytochemical constituents of ethanol extract of Artocarpus heterophyllus stem bark .
| Parameters | Artocarpus heterophyllus stem bark |
| Tannin | + |
| Phlobatannin | +++ |
| Saponin | +++ |
| Flavonoid | + |
| Steroid | - |
| Terpenoid | ++ |
| Cardiac glycosides | +++ |
| Alkaloid | +++ |
| Anthraquinone | - |
| Chalcones | - |
| Phenol | +++ |
+++ = much abundant, ++ = less abundant, + = minute and - = absent
The flavonoid (Table 1) in the ethanol extract of Artocarpus heterophyllus stem bark could make it useful as antidiabetic and anticancer agents.32 Also, Ajiboye et al30 reported the role of flavonoid in preventing oxidation of LDL therefore reducing the risk for the development of atherosclerosis, one of diabetes mellitus complication. The presence of tannin could make the extract useful in hastening wound healing in diabetes mellitus patients.33 The tannin, phenol and alkaloid in Table 1 also strengthen the possibility of antidiabetic activity of the extract.33,34
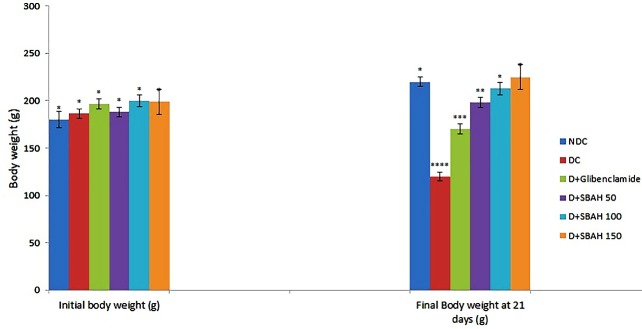
Alloxan is one the chemicals that are widely used to induce diabetes in experimental animals due to its toxicity on the beta cell of pancreas islet, leading to weight reduction, anaemia, hyperlipidaemia, hepatopathy and nephropathy.35 In this study there were significant (p<0.05) weight reductions in diabetic control rats when compared to non-diabetic control rats. Administration of diabetic rats with 50, 100 and 150 mg/kg body weight of Artocarpus heterophyllus stem bark significantly (p<0.05) increased the body weight especially at 150 mg/kg body weight (Figure 1). This is in accordance with36 that diabetes mellitus patients were characterized by loss of body weight due to increased protein catabolism, as a result of insulin insufficiency.
Figure 1.
Effect of ethanol extract of Artocarpus heterophyllus stem bark on body weight of alloxan-induced diabetic rats
Bars with different superscript are significantly different at p<0.05
NDC (non-diabetic control), DC (Diabetic control), D+ SBAH 50 (Diabetic rats received 50 mg/kg body weight of Artocarpus heterophyllus stem bark), D + SBAH 100 (Diabetic rats received 100 mg/kg body weight of Artocarpus heterophyllus stem bark, and SBAH 150 (Diabetic rats received 150 mg/kg body weight of Artocarpus heterophyllus stem bark
Diabetes mellitus has been characterized by anaemia as reported by,37 which is supported by the present study especially in diabetic control rats (Table 2). This might due to excess glucose in the hemoglobin, results in glycosylated hemoglobin with decrease in red blood cell (RBC) and packed cell volume (PCV), an indicator of imbalance between its synthesis and destruction.38 Anaemia in diabetes mellitus patients may also be attributed to damage in the synthesis of erythropoietin, hormone whose function in promoting formation of the red blood cells, probably due to excess blood glucose.38,39 This was coupled with significant (p<0.05) decrease in MCV, MCHC and MCH
Table 2. Effect of ethanol extract of Artocarpus heterophyllus stem bark on haematological parameters of alloxan-induced diabetic rats .
| Parameters | NDC | DC | D+glibenclamide | D+ SBAH50 | D+ SBAH100 | D+150 SBAH |
| PCV (%) | 46.48±0.24* | 19.26±1.24**** | 38.16±1.40*** | 37.10±0.09*** | 42.10±0.05** | 46.20±1.10* |
| HB (g/dl) | 18.42±0.05* | 8.42±0.10***** | 10.21±1.15**** | 12.40±0.01*** | 15.10±1.10** | 18.20±1.10* |
| WBC (x 10 3 /μl) | 3.10±1.00* | 1.01±0.02*** | 2.80±0.03** | 2.94±0.01** | 3.08±0.08* | 3.20±0.05* |
| N (%) | 63.01±0.10* | 42.19±1.10***** | 52.10±1.20**** | 54.10±1.10*** | 59.10±0.20** | 62.94±1.10* |
| L (%) | 42.23±0.16* | 26.10±1.01***** | 30.10±0.02**** | 33.42±0.10*** | 38.44±1.16** | 42.96±0.08* |
| M (%) | 11.20±0.03* | 6.01±0.06**** | 8.20±1.04** | 9.60±0.89** | 10.01±0.05** | 11.46±0.14* |
| E (%) | 5.10±0.01* | 1.28±0.12**** | 3.10±0.06*** | 3.98±0.06*** | 4.22±0.10*,** | 4.98±0.94* |
| RBC (x10 11 /l) | 5.94±0.10* | 2.48±0.04**** | 3.28±0.13*** | 3.84±0.48*** | 4.46±0.10** | 5.68±0.14* |
| MCHC (g/dl) | 42.10±0.04* | 28.40±1.10***** | 32.18±0.08**** | 36.40±1.20*** | 39.20±0.08** | 42.84±0.10* |
| MCV (fl) | 96.42±0.10* | 58.29±0.56**** | 86.30±0.94*** | 92.40±1.02** | 93.82±0.94** | 96.10±0.04* |
| MCH (pg) | 40.44±1.16* | 30.01±0.46**** | 34.21±0.14*** | 34.96±0.12*** | 38.41±0.12** | 40.80±0.08* |
Each value is a mean of six replicates ± SEM. Values with different superscript along the row are significantly different at p<0.05. PCV (packed cell volume), HB (haemoglobin), WBC (white blood cell), N (neutrophil), L (lymphocyte), M (monocyte), E (eosinophil), RBC (red blood cell), MCHC (mean corpuscular haemoglobin concentration), MCV (mean corpuscular volume), MCH (mean corpuscular haemoglobin), NDC (non-diabetic control), DC (Diabetic control), D+ SBAH 50 (Diabetic rats received 50 mg/kg body weight of Artocarpus heterophyllus stem bark), D + SBAH 100 (Diabetic rats received 100 mg/kg body weight of Artocarpus heterophyllus stem bark, and SBAH 150 (Diabetic rats received 150 mg/kg body weight of Artocarpus heterophyllus stem bark
In addition, the significant (p<0.05) reduction observed in WBC and its differentials (lymphocytes, neutrophil, monocytes and eosinophil) (Table 2) in diabetes control rats might suggest decrease in immune cells in mopping up free radicals generated.40 Treatment of diabetic rats with ethanol extract of Artocarpus heterophyllus stem bark reversed all this haematological abnormalities especially at 150 mg/kg body weight probably due antioxidant nature of the plant.
There were significant (p<0.05) increase in serum lipid profiles and calculated atherogenic and coronary risk indices with a reduction in serum high density lipoprotein-cholesterol (HDL) in diabetic control rats when compared to non-diabetic rats (Table 3). This is in accordance with Wilson and Islam41 that hyperlipidaemia is another factor directly linked to diabetes mellitus, also, high circulating blood lipid concentrations secrete humoral factors like resistin and adiponectin that alter insulin sensitivity. In this study, hyperlipidaemia were encouraged in diabetic control rats by increasing the levels of low density lipoprotein-cholesterol (LDL), very low density lipoprotein-cholesterol (VLDL) and total cholesterol.42,43 Oral intervention of diabetic rats administered with 50, 100 and 150 mg/kg body weight of ethanol extract of Artocarpus heterophyllus stem bark as well as glibenclamide demonstrated reduction in atherogenesis and coronary artery diseases likewise augmenting the HDL-cholesterol. This is in line with the report of amongst others.43
Table 3. Effect of ethanol extract of Artocarpus heterophyllus stem bark on serum lipid Profiles, atherogenic and coronary indices of alloxan-induced diabetic rats .
| Groups | TC (mg/dl) | TG (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) | HDL (mg/dl) | AI | CRI |
| NDC | 80.40 ± 0.50* | 64.10 ± 1.20* | 23.37 ± 0.01* | 12.82 ± 1.03* | 44.21 ± 0.05* | 0.82 ± 0.10* | 1.82 ± 0.01* |
| DC | 110.40±0.40***** | 120.10±0.10***** | 66.26±0.40***** | 24.02±0.05*** | 20.12±0.18***** | 4.49±0.40***** | 5.49±0.04**** |
| D+glib | 92.10 ± 1.20**** | 84.20 ± 0.11**** | 43.16±1.20**** | 16.84±0.84** | 32.10±1.10**** | 1.87±0.24**** | 2.87±0.32*** |
| D+ SBAH 50 | 90.10 ±0.40*** | 76.20±1.10*** | 38.62±1.12*** | 15.24±1.40*,** | 36.24±1.40*** | 1.49±0.02*** | 2.49±0.14*** |
| D+ SBAH100 | 84.48 ±0.42** | 68.10±1.12** | 29.88±0.08** | 13.21±1.01* | 40.98±1.01** | 1.06±0.02** | 2.06±0.06** |
| D+ SBAH150 | 81.20±0.12* | 63.89±0.14* | 24.48±0.80* | 12.78±1.06* | 43.94±1.20* | 0.85±0.05* | 1.85±0.02* |
Each value is a mean of six replicates ± SEM. Values with different superscript across the column are significantly different at p<0.05. D+Glib (D+glibenclamide), TC (total cholesterol), TG (Triglyceride), LDL (low density lipoprotein-cholesterol), VLDL (very low density lipoprotein-cholesterol), HDL (high density lipoprotein-cholesterol), AI (atherogenic index), CRI (coronary risk index), NDC (non-diabetic control), DC (Diabetic control), D+ SBAH 50 (Diabetic rats received 50 mg/kg body weight of Artocarpus heterophyllus stem bark), D + SBAH 100 (Diabetic rats received 100 mg/kg body weight of Artocarpus heterophyllus stem bark, and SBAH 150 (Diabetic rats received 150 mg/kg body weight of Artocarpus heterophyllus stem bark
Moreover, diabetes mellitus has been characterized by hepatopathy and nephropathy,44 which is supported by the present study (Table 4). This is buttressed with high level of liver-function enzymes like AST, ALT and ALP and other biochemical parameters (urea, creatinine, albumin and bilirubin) in diabetic control rats compared to others groups. The hepato and nephro protective abilities of 50, 100 and 150 mg/kg body weight of ethanol extract of Artocarpus heterophyllus stem bark supported by reduction in serum AST, ALT and ALP activities, as well as urea, creatinine (breakdown of muscle mass, as result of increase in gluconeogenesis) and bilirubin levels with increase in albumin (probably due to decrease in gluconeogenesis) levels when compared to diabetic control rats.
Table 4. Effect of ethanol extract of Artocarpus heterophyllus stem bark on serum ALT, AST, ALP and other biochemical parameters of alloxan-induced diabetic rats .
| Groups | ALT (u/l) | AST (u/l) | ALP (u/l) | Albumin (g/l) | Bilirubin(mg/dl) | Creatinine(mg/dl) | Urea (mg/dl) |
| NC | 72.02±1.20* | 84.10±0.06* | 143.02±1.20* | 20.23±1.20* | 4.20±0.12* | 2.64±0.42** | 34.20±1.10* |
| NDC | 126.20±0.90**** | 140.11±1.30**** | 500.11±1.02***** | 10.10±1.12***** | 15.40±0.10**** | 6.20±1.01*** | 86.19±0.18***** |
| D+glib | 84.20±1.20*** | 96.10±0.06*** | 320.14±0.11**** | 13.79±0.60**** | 8.20±1.30*** | 3.20±0.10** | 56.24±1.12**** |
| D+SBAH 50 | 83.94±1.42*** | 95.01±2.01*** | 284.01±0.42*** | 16.21±0.27*** | 6.38±0.12*** | 2.90±0.08* | 50.12±1.10*** |
| D+SBAH100 | 76.20±1.02** | 88.10±1.10** | 246.15±0.12** | 18.53±0.80** | 5.80±0.19** | 2.70±0.06* | 41.12±0.18** |
| D+SBAH150 | 73.40±0.41* | 85.25±0.20* | 153.21±2.12* | 20.10±0.31* | 4.64±0.18* | 2.66±0.10* | 34.69±1.14* |
Each value is a mean of six replicates ± SEM. Values with different superscript across the column are significantly different at p<0.05. NDC (non-diabetic control), DC (Diabetic control), D+glib (D+glibenclamide), D+ SBAH 50 (Diabetic rats received 50 mg/kg body weight of Artocarpus heterophyllus stem bark), D + SBAH 100 (Diabetic rats received 100 mg/kg body weight of Artocarpus heterophyllus stem bark, and SBAH 150 (Diabetic rats received 150 mg/kg body weight of Artocarpus heterophyllus stem bark
Conclusion
The results of the present study show that various doses of ethanol extract of Artocarpus heterophyllus stem bark demonstrates the presence of different phytochemical compounds which serve as antioxidant, therefore improved weight gain, ameliorate anaemia, hyperlipidaemia to normolipidaemia, and demonstrated hepato and nephro-protective in diabetic rats.
Acknowledgments
The authors are very grateful to all the Technologists in Biochemistry Laboratory of Afe Babalola University, Ado-Ekiti.
Ethical Issues
The Ethical Committee of the Afe Babalola University approved this study with approval number ABUAD/SCI/004.The study was performed in accordance with the rules of the established Animal Ethical Committee of Afe Babalola University, Ado-Ekiti.
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Sada NM, Tanko Y, Mabrouk MA. Modulatory role of soya beans supplement on lipid profiles and liver enzymes on alloxan-induced diabetic Wistar rats. Eur J Exp Bio. 2013;3(2):62–7. [Google Scholar]
- 2.Hesham ZT, Hassan NM, Khalil HI, Kerolles SY. Antidiabetic effects of dietary formulas prepared from some grains and vegetables on type 2 diabetic rats. J Agroaliment Proc Technol. 2014;20(1):69–79. [Google Scholar]
- 3.Azeez OI, Oyagbemi AA, Oyeyemi MO, Odetola AA. Ameliorative effects of cnidoscolus aconitifolius on alloxan toxicity in wistar rats. Afr Health Sci. 2010;10(3):283–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Anaelechi JO, Japhet MO, Ude T, Ubuo KA, John EO, Ifeadike C. et al. Effects of Telfairia occidentalis seeds on the serum lipid profile and atherogenic indices of male albino Wistar rats. Pak J Nutr. 2015;14(9):557–62. doi: 10.3923/pjn.2015.557.562. [DOI] [Google Scholar]
- 5.Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29(5):580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 6.Visweswara Rao P, Madhavi K, Dhananjaya Naidu M, Gan SH. Rhinacanthus nasutus improves the levels of liver carbohydrate, protein, glycogen, and liver markers in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2013;2013:102901. doi: 10.1155/2013/102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–30. doi: 10.1016/s2221-1691(12)60032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran S, Rajasekaran A, Manisenthilkumar KT. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed. 2012;2(4):262–8. doi: 10.1016/s2221-1691(12)60020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;83:30–8. [Google Scholar]
- 10.Omar HS, Hesham A, El-Beshbishy HA, Moussa Z, Taha KF, Singab ANB. Antioxidant activity of artocarpus heterophyllus lam. (Jack Fruit) leaf extracts: remarkable attenuations of hyperglycemia and hyperlipidemia in streptozotocin-diabetic rats. Sci World J. 2011;11:788–800. doi: 10.1100/tsw.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makherjee B. Traditional medicine. New Delhi: Mohan Primalani for Oxford and IBH publishing; 1993. [Google Scholar]
- 12.Ajiboye BO, Ojo OA, Adeyonu O, Imiere O, Olayide I, Fadaka A. et al. Inhibitory effect on key enzymes relevant to acute type-2 diabetes and antioxidative activity of ethanolic extract of Artocarpus heterophyllus stem bark. J Acute Dis. 2016;5(5):423–9. doi: 10.1016/j.joad.2016.08.011. [DOI] [Google Scholar]
- 13.Sofowora A. Medicinal Plants and Traditional Medicines in Africa. New York: Chichester John Willey & Sons; 1993. [Google Scholar]
- 14.Trease GE, Evans WC. A Text-book of Pharmacognosy. London: Bailliere Tindall Ltd; 1989. [Google Scholar]
- 15.Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. London: Chapman A & Hall; 1973. [Google Scholar]
- 16.Ebong PE, Atangwho IJ, Eyong EU, Egbung GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) (Neem) and Vernonia amygdalina (Del.) (African Bitter Leaf) Am J Biochem Biotech. 2008;4(3):239–44. doi: 10.3844/ajbbsp.2008.239.244. [DOI] [Google Scholar]
- 17.Ajiboye BO, Edobor G1, Ojo AO, Onikanni SA, Olaranwaju OI, Muhammad NO. Effect of aqueous leaf extract of Senecio biafrae on hyperglycaemic and serum lipid profile of alloxan-induced diabetic rats. Inter J Dis Disor. 2014;2(1):059–64. [Google Scholar]
- 18.Trinder P. Determination of total cholesterol. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 19.Roeschlan P, Bernt E, Grnber W. Total cholesterol estimation by CHOD-PAP enzymatic method. Clin Chem. 1974;12:403. [Google Scholar]
- 20.Tietz NW. Clinical Guide to Laboratory Tests. 3rd ed. Philadelphia, USA: Saunders Company; 1995. [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22.Liu CS, Lin CC, Li TC. The relation of white blood cell count and atherogenic index ratio of ldl-cholesterol to hdl-cholesterol in taiwan school children. Acta Paediatr Taiwan. 1999;40(5):319–24. [PubMed] [Google Scholar]
- 23.Boers M, Nurmohamed MT, Doelman CJ, Lard LR, Verhoeven AC, Voskuyl AE. et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(9):842–5. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. 1971. Clin Chim Acta. 1997;258(1):21–30. doi: 10.1016/s0009-8981(96)06447-9. [DOI] [PubMed] [Google Scholar]
- 25.Sherlock S. In Liver Disease. London: CIBA Symposium; Churchill, 1951. [Google Scholar]
- 26.Tietz NW, Pruden EL, Siggaad-Anderson A. Tietz Textbook of clinical chemistry. London: Saunders Company; 1994. [Google Scholar]
- 27.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Wright PJ, Leathwood PD, Plummer DT. Enzymes in rat urine: Alkaline phosphatase. Enzymologia. 1972;42(4):317–27. [PubMed] [Google Scholar]
- 30.Ajiboye BO, Ibukun EO, Edobor GI, Ojo OA, Onikanni SA. Qualitative and quantitative analysis of phytochemicals in Senecio biafrae leaf. Int J Inv Pharm Sci. 2013;1(5):428–32. [Google Scholar]
- 31.Osagie AU, Eka OU. Mineral elements in plant foods. Nigeria: Ambik press; 1998. [Google Scholar]
- 32.Tanko Y, Yaro HA, Isa M, Yerima M, Saleh IA, Mohammed A. Toxicological and hypoglycaemic studies on the leaves of Cissampelos mucronata (Menispermaceae) on blood glucose levels of streptozotocin-induced diabetic Wistar rats. J Med Plants Res. 2007;1(5):113–6. [Google Scholar]
- 33.Okwu DE, Josiah C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr J Biotechnol. 2006;5(4):357–61. [Google Scholar]
- 34.Adefegha SA, Oboh G. Antioxidant and inhibitory properties of Clerodendrum volubile leaf extracts on key enzymes relevant to non-insulin dependent diabetes mellitus and hypertension. J Taibah Univ Sci. 2016;10(4):521–33. doi: 10.1016/j.jtusci.2015.10.008. [DOI] [Google Scholar]
- 35.Gomathi D, Kalaiselvi M, Ravikumar G, Devaki K, Uma C. Evaluation of antioxidants in the kidney of streptozotocin induced diabetic rats. Indian J Clin Biochem. 2014;29(2):221–6. doi: 10.1007/s12291-013-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moodley K, Joseph K, Naidoo Y, Islam S, Mackraj I. Antioxidant, antidiabetic and hypolipidemic effects of tulbaghia violacea harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement Altern Med. 2015;15:408. doi: 10.1186/s12906-015-0932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aladodo RA, Muhammad Muhammad, NO NO, Balogun EA. Effects of aqueous root extract of Jatropha curcas on hyperglyceamic and haematological indices in alloxan-induced diabetic rats. Fount J Nat Appl Sci. 2013;2(1):52–8. [Google Scholar]
- 38.Ojo OA, Ajiboye BO, Oyinloye BE, Ojo AB. Hematological Properties of Irvingia gabonensis in Male Adult Rats. J Pharm Sci Innov. 2014;8(18):599–602. doi: 10.7897/2277-4572.035190. [DOI] [Google Scholar]
- 39.Horiguchi H, Sato M, Konno N, Fukishima M. Long-term cadmium exposure induces anemia in rats through hypoproduction of erythropoietin in the kidneys. Arch Toxicol. 1996;71(1-2):11–9. doi: 10.1007/s002040050352. [DOI] [PubMed] [Google Scholar]
- 40.Ajiboye BO, Muhammad NO. Investigation into the haematological parameters of albino rats fed with Rana galamensis–based diet as an alternative source of protein in animal feed. J Global Agric Ecology. 2015;2(4):112–6. [Google Scholar]
- 41.Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: An alternative model for type 2 diabetes. Pharmacol Rep. 2012;64(1):129–39. doi: 10.1016/s1734-1140(12)70739-9. [DOI] [PubMed] [Google Scholar]
- 42.Ajiboye BO, Muhammad NO, Oloyede HOB. Serum Lipid Profile of Alloxan-induced Diabetic Rats Fed Triticum aestivum-based Diet. Int J Trop Dis Health. 2015;5(4):260–8. doi: 10.9734/IJTDH/2015/12877. [DOI] [Google Scholar]
- 43.Mohammed A, Koorbanally NA, Islam MS. Ethyl acetate fraction of aframomum melegueta fruit ameliorates pancreatic beta-cell dysfunction and major diabetes-related parameters in a type 2 diabetes model of rats. J Ethnopharmacol. 2015;175:518–27. doi: 10.1016/j.jep.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Kokou I, Damintoti KS, Amegnona A, Yao A, Messanvi G. Effect of Aframomum melegueta on carbon tetrachloride induced liver injury. J Appl Pharm Sci. 2013;3(9):98–102. doi: 10.7324/JAPS.2013.3918. [DOI] [Google Scholar]