Abstract
Breast cancer survivors are an expanding population that is troubled by lasting mental health problems, including depression and anxiety. These issues reduce quality-of-life throughout survivorhood. Research indicates that tumor biology, cancer treatments, and stress contribute to these mood disturbances. Although the mechanisms underlying these various causes remain under investigation, neuroinflammation is a leading hypothesis. To date, rodent models of recurrence-free tumor survival for understanding mechanisms by which these behavioral issues persist after cancer are lacking. Here, we test the extent to which potential behavioral symptoms persist after mammary tumor removal in mice (i.e., establishment of a cancer survivor model), while also empirically testing the causal role of tumors in the development of neuroinflammatory-mediated affective-like behaviors. Complete surgical resection of a non-metastatic orthotopic, syngeneic mammary tumor reversed tumor-induced increases of circulating cytokines (IL-6, CXCL1, IL-10) and myeloid-derived cells and modulated neuroinflammatory gene expression (Cd11b, Cxcl1). Multiple anxiety-like behaviors and some central and peripheral immune markers persisted or progressed three weeks after tumor resection. Together, these data indicate that persistent behavioral changes into cancer survivorhood may be due, in part, to changes in immunity that remain even after successful tumor removal. This novel survivor paradigm represents an improvement in modeling prevalent cancer survivorship issues and studying the basic mechanisms by which cancer/cancer treatments influence the brain and behavior.
Keywords: cytokines, MDSC, corticosterone, tumor resection, mammary
1. INTRODUCTION
The number of cancer survivors in the US alone is expected to grow from approximately 14.5 million to 19 million in less than a decade (DeSantis et al., 2014). Almost 90% of breast cancer patients survive their cancer treatment (Howlader et al., 2016) and yet many report persistent mental symptoms include depression (9–65% prevalence), anxiety (18–33%), and cognitive impairments (15–60%) (Maass et al., 2015; Reid-Arndt et al., 2010; Wefel et al., 2015). However, little is known about the mechanisms underlying the enduring neurobehavioral consequences of a cancer experience. Beyond diminishing quality-of-life, these mental health issues increase healthcare costs, morbidities, and ultimately, mortality (Hess and Insel, 2007; Pisu et al., 2010; Satin et al., 2009). Complaints of their mental concerns have sometimes been dismissed by their physicians (Boykoff et al., 2009).
The causes of mood disorders in cancer survivors are hypothesized to be a combination of the preceding tumor biology, cancer treatments (e.g., chemotherapy), and stress (DiMatteo et al., 2003; Lee et al., 2004; Schrepf et al., 2015). However, these disparate factors are difficult to differentiate in clinical populations and their likely mechanistic interactions remain either entangled or ignored. Among cancer survivors, enduring immune and inflammatory activation correlates with persistent negative affect (Dooley et al., 2016; Soygur et al., 2007, but see Bower et al., 2011; Orre et al., 2011), suggesting a possible immune mechanism. Indeed, mounting evidence indicates that mood comorbidities and inflammation exist prior to cancer treatments, and even prior to diagnosis (Sebti et al., 2015; Van Esch et al., 2012) in some cancer patients (Benoy et al., 2002; Lutgendorf et al., 2008; Michalaki et al., 2004; Wefel et al., 2015). Furthermore, endogenous circulating glucocorticoids and their responses to challenges are disrupted in cancer survivors (Pyter, 2016). Because glucocorticoids have potent anti-inflammatory actions, they have also been hypothesized to be relevant to lingering behavioral comorbidities. These findings support the hypothesis that tumor biology contributes significantly to these mental health issues. The extent to which tumors may exert long-lasting changes to the immune or nervous systems, that promote the continued behavioral symptoms into survivorhood, remains unexplored.
Rodent models are powerful tools by which to systematically differentiate the various potential causes of these behavioral cancer comorbidities. Most of the current research in rodents has focused on either chemotherapy (using tumor-free rodents; Seigers and Fardell, 2011) or on non-CNS tumor biology (Schrepf et al., 2015). Indeed, changes in brain function (e.g., depressive- and anxiety-like behavior) have been reported in a variety of rodent solid tumor-bearing models (Lamkin et al., 2011; Pyter et al., 2009; Qi et al., 2009; Yang et al., 2012). This approach controls for many confounding factors that cannot easily be controlled in clinical research (e.g., varying treatments, genetics, perceived stress). Affective-like behaviors in tumor-bearing rodents are associated with neuroinflammation (Lebena et al., 2014; Norden et al., 2014; Pyter et al., 2009; Yang et al., 2014), changes in neurotransmission (Lebena et al., 2014; Vegas et al., 2004), and oxidative stress (Papiez et al., 2009; Qi et al., 2009). Neuroinflammation, in particular, is associated with depression in humans (Mechawar and Savitz, 2016) and affective-like behaviors in other rodent models of illness or stress (Fu et al., 2010; Haji et al., 2012; Wohleb et al., 2014). However, the causal role of tumors in these brain changes has not been definitively established, leaving the possibility that the tumor induction process (e.g., chemicals, surgery) may be partly responsible for the observed alterations in brain and behavior. Furthermore, models that accurately represent post-tumor “survivors” have not been reported in behavioral comorbidity research.
Given the massive and growing population of cancer survivors, the extent to which the behavioral changes observed in tumor-bearing models persist after successful tumor resection is a pressing need. Furthermore, identification of the underlying mechanisms is necessary both to develop interventions and to prevent these debilitating quality-of-life issues. The purpose of this work was to: 1) establish a novel rodent model of breast cancer “survivorship,” 2) identify enduring immune/inflammatory changes and affective-like behavior after tumor removal in this model, and 3) advance the existing behavioral comorbidity research in tumor-bearing models by empirically testing the causal role of tumors in the previously reported changes in brain and behavior.
2. MATERIALS AND METHODS
2.1 Animals
Forty-four nulliparous female 8- to 9-week old Balb/c mice (Charles River, Wilmington, MA) were housed 5/cage and acclimated to the temperature-controlled (22 ± 1 °C) vivarium for 1 week under a 14:10 light:dark cycle (lights off at 015:00 h). Rodent chow (Harlan 7912) and water were available ad libitum throughout the study and cotton nestlets and plastic huts were provided for nesting. Prior to and after tumor induction, mice were acclimated to handling twice/week. All animal experiments were approved by the Ohio State University Institutional Animal Care and Use Committees and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2011). All efforts were made to minimize animal suffering and to reduce the number of mice used. This project consists of two treatment-balanced experimental replications.
2.2 Cells
The murine mammary non-metastatic 67NR cancer cell line, originating from a spontaneous mammary adenocarcinoma in a Balb/c mouse (Aslakson and Miller, 1992; Dexter et al., 1978), was generously provided by Drs. Fred Miller and Lisa Polin at Karmanos Cancer Institute. Cells were grown in DMEM with 10% FBS, 2 mM l-glutamine, 1 mM non-essential amino acids at 37 °C with 5% CO2.
2.3 Tumor Induction
Under anesthetization (isoflurane vapors), a 5 mm subcutaneous incision was made medial to the 4th nipple and 5 × 106 cells (in matrigel) were injected into the associated mammary fat pad (Miller et al., 1981; Miller et al., 1983) (n=20). Incisions were closed with a wound clip. Ear notches were placed for individual identification purposes. Using these cells, this procedure results in an in situ primary mammary carcinoma (Miller et al., 1981) that does not metastasize (Miller et al., 1983). This orthotopic and syngeneic breast cancer model eliminates the use of immunocompromised mice. To control for the effects of surgeries (i.e., tissue repair, anesthesia), PBS was used for a subset of tumor-free control inoculation surgeries (n=8) and a second tumor-free control subset received a tumor resection sham surgery (n=4) at the same time as tumor resection (see Figure 1A). Data from all tumor-free sham surgery mice (“surgical control”) were pooled for analyses. Mice slated for the tumor resection group were inoculated with tumor cells 2 weeks prior to the other groups such that the timing of their tumor resection surgeries corresponded to the time of tumor/control induction of the other groups (Figure 1A). This allowed for simultaneous behavioral and physiological assessments in all groups and an equal duration of tumor exposure (2 weeks) between tumor and tumor-resected mice for the start of behavioral analyses. Body mass and tumor dimensions were measured twice/week. The longest diameter of the tumor (A) and the perpendicular diameter (B) were used to estimate tumor volume by the formula: V = A × B2/2 mm3. Mice in the tumor group with final tumors <4 (n=3) or >16 (n=6) mm dia (~10% body mass) were euthanized and removed from the study early.
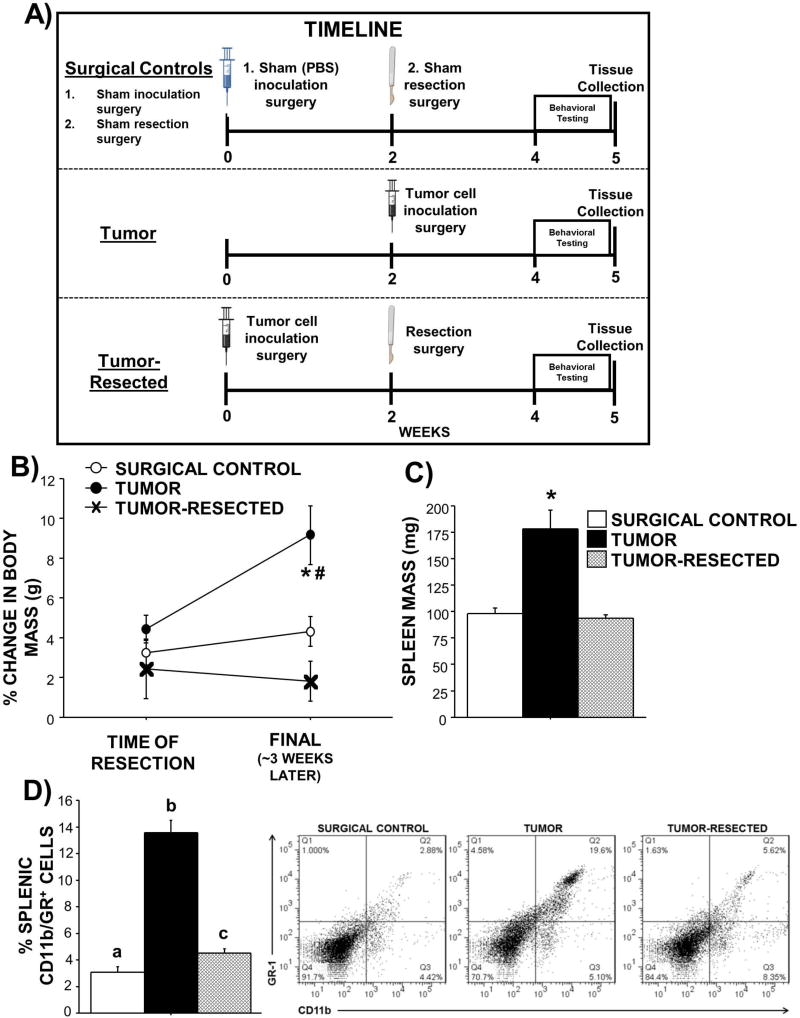
Figure 1. Effects of complete mammary tumor resection on body and spleen masses.
A) Experimental timeline for surgical controls, tumor, and tumor-resected treatment groups. Mean ± SEM B) change in body mass from surgical treatment until the end of the study (~3 weeks later), C) spleen mass, and D) percentage of CD11b/GR+ (myeloid-derived suppressor cells) and representative flow cytometry dot plots from the spleen in surgical controls, tumor-bearing mice, and tumor-resected mice. n=7–12/group; #p<0.05 over time; *p<0.05 relative to both other treatments; different letters represent statistical difference p<0.05
2.4 Tumor resection
A modified radical mastectomy procedure was used to completely remove the tumor in the mice of the “survivor” group (n=12). These mice were anesthetized (isoflurane) and tumors with intact capsules were surgically removed along with mammary tissue, fat, and inguinal lymph nodes where necessary. After hemostatic control was established, the skin was closed with wound clips. Sham resection surgeries, modified by probing and exposing these healthy tissues, were completed in a subset of the surgical control group (n=4). Buprenorphine (0.05 mg/kg; s.c.) was administered immediately after surgery and again 12 h later. Complete tumor resections were verified at necropsy, mice with recurring tumors (n=5) were removed from the study. Thus, final sample sizes were: surgical controls (n=12), tumor (n=11), tumor-resected (n=7).
2.5 Affective-like Behavior
Three tests of anxiety-like behavior (open field, marble-burying, elevated plus maze) and one test of depressive-like behavior (tail suspension) were used in that order, each on separate, consecutive days between 1000–1300 h EST. Three treatment-blind researchers (J.K., J.B., S.B.) scored all behavior videos.
Open field test
Automated equipment was used to assess total locomotor activity and anxiety-like behavior (avoidance of the center 4 × 4 in area) in 16 × 16 in plastic arenas (San Diego Instruments, San Diego, CA, USA). Sixteen photobeams across two dimensions recorded spatial movement of each mouse over 10 min. A loose layer of bedding was added and the arenas were cleaned with 10% bleach between mice. Marble-burying test. To assess obsessive-compulsivity (an anxiety-like behavior), each mouse was put in a clean cage containing 9 marbles placed atop 2.5 cm of corncob bedding material. The number of marbles buried underneath the bedding after 20 min was recorded (Takeuchi et al., 2002). Marbles were considered buried if at least ¾ of the marble was not visible.
Elevated plus maze
This maze consisted of 2 open arms and 2 closed arms in a plus-sign configuration with an open space in the center designated as neutral and raised 80 cm off the ground. The walls enclosing the closed arms were 50 cm high and 6.3 cm apart. The mouse was allowed to explore the maze for 5 min and videotaped from above. Total number of arm entries (locomotion) and the percentage of entries into open arms (low = anxiety-like behavior) were recorded using Etholog software. Mice were considered to have entered an arm when all four paws crossed into the arm.
Tail suspension test
This standardized test assesses learned helplessness through suspended immobility (Crawley, 2000). Mice were videotaped for 5 min while suspended by the tail with lab tape adhered to a hard plastic sheet mounted approximately 30 cm above a benchtop. The percentage of time spent immobile was recorded using Etholog software (v. 2.25, Sao Paulo, Brazil).
2.6 Tissue collection
One day after the tail suspension test and following deep CO2 asphyxiation, cardiac puncture was used to sample blood through heparin-lined syringes for cytokine, glucocorticoid, and myeloid-derived suppressor cell quantification (1000–1300 h EST). Brain regions relevant to the behaviors examined (hippocampus, hypothalamus, frontal cortex) were immediately dissected out and frozen in RNA Later preservative for later neuroinflammatory gene expression assessment. Tumors and spleens were removed aseptically and weighed. Spleens and midline slices of tumors were used to quantify immune cells using flow cytometry, whereas other tumor slices were frozen for gene expression assessment.
2.7 Plasma cytokine concentrations
To compare systemic inflammation among treatments, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNFα), IL-10, interferon-gamma (IFN-γ), and keratinocyte chemoattractant (CXCL1) were measured in plasma samples in duplicate using a multiplex fluorescent bead array (Mouse Inflammatory V-Plex Panel; Mesoscale Discovery, Rockville, MD, USA) according the manufacturer’s protocol. Detection thresholds were 0.11, 0.61, 0.13, 0.95, 0.04, and 0.24 pg/ml, respectively. All samples were detectable for all cytokines. Intrassay and interassay variations were each <10%.
2.8 Plasma corticosterone concentrations
Glucocorticoids are potent innate anti-inflammatory agents. To determine potential differences among treatment groups in baseline corticosterone concentrations and responses in this model (Pyter, 2015), blood was sampled as part of tissue collection (at rest; see above) and immediately after the 5-min tail suspension test (post-stressor; using retro-orbital sinus disruption after isoflurane anesthetization) in all mice. Both samples were collected at the same time of day (1000–1300 h EST). Blood was kept on ice and then centrifuged (20 min at 2,000 rpm) and plasma was stored at −80 °C until assayed. Corticosterone was measured in duplicate in all blood samples via EIA according to the manufacturer’s instructions (Enzo Life Sciences, Plymouth Meeting, PA, USA) after 1:40 dilution. The threshold of detection was 27 pg/ml. Intrassay and interassay variations were each <10%.
2.9 Quantitative RT-PCR
Total RNA was extracted from the brain hippocampus, hypothalamus, and frontal cortex using Qiagen RNeasy mini kits (CA, USA). RNA concentrations were measured and 260/280 ratios were determined to be 1.8–2.0 (NanoDrop, DE, USA). Total RNA was reverse transcribed using SuperScript First-Strand kits (Invitrogen, NY, USA) according to the manufacturer’s protocol. Seven genes of interest were chosen based on their established role in neuroinflammation-induced affective-like behaviors or guided by the present circulating cytokine data (Il-1b, Il-6, Tnfa, IL-10, Cd11b, CxCl1, Ifn-γ) (Kim et al., 2016). Mouse TaqMan Gene Expression Assays were purchased from Applied Biosystems (Carlsbad, CA, USA) with probes labeled with 6-FAM and MGB (non-fluorescent quencher) at the 5’ and 3’ ends, respectively: Il-1b (Mm00434228_m1), Il-6 (Mm00446190_m1), Tnfa (Mm00443258_m1), Il-10 (Mm01288386_m1), Cd11b (Mm00434455_m1), CxCl1 (mm04207460_m1), Ifn-γ (Mm01168134_m1), Gapdh (Mm99999915_g1; labeled with VIC). The universal two-step RT-PCR cycling conditions used on the 7000 Sequence Detection System (Applied Biosystems) were: 50°C (2 min), 95°C (10 min), 40 cycles of 95°C (15 s) and 60°C (1 min). Relative gene expression of individual samples run in duplicate was calculated by the comparative CT method (2−ΔCT).
2.10 Flow cytometry
Whole blood was collected at tissue collection (rest) or immediately after tail suspension testing (post-stressor) as described above. Spleens were minced and filtered using 70 µM cell strainers. Total number of cells was determined using a Z1 Coulter Particle Count (Beckman Coulter). Tumor slices were placed in serum-free media, minced, treated with collagenase solution (Worthington Biomedical Co., Worthington, OH, USA), and rotated at 37°C for 1 h. The digest was filtered through a 70-µm nylon cell strainer, rinsed, centrifuged, resuspended, and then the total number of cells was determined with a Z1 Coulter Particle Count (Beckman Coulter). Staining of mouse myeloid-derived suppressor cells (MDSCs) was conducted using Alexa 488 anti-GR 1 and APC anti-CD11b fluorochrome-labeled antibodies (BD Biosciences). Staining for mouse tumor-associated macrophages (TAMs) was conducted using PA anti-F4/80 and APC anti-CD11b fluorochrome-labeled antibodies (BD Biosciences). Single cell suspensions were incubated with the appropriate conjugated antibodies for 30 min at 4°C. Cells were washed and then resuspended in FACS buffer for analysis. Nonspecific binding was assessed using isotype-matched antibodies. Data was acquired using an LSRII flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Positive labeling for each antibody was determined based on isotype-stained controls.
2.11 Statistical analysis
Statistical comparisons of tissue mass, gene expression, and flow cytometry data were analyzed using 2-way ANOVAs followed by post-hoc Fisher’s LSD or Student’s t-tests based on a priori hypotheses with Statview version 5.0.1 software (Scientific Computing, Cary, NC, USA) when variance was normal. Nonparametric Mann-Whitney U tests were used when variance was not normally distributed. Repeated measures ANOVAs were used to compare changes in body mass over time. Pearson’s correlations were used to relate various variables. Data were determined to be statistically significant when p≤0.05 and are presented as mean ± standard error of the mean (SEM).
3. RESULTS
3.1 Tissue masses
Body weight change was comparable in all mice after their respective treatments (Figure 1B “time of resection”; p>0.05), however, weight changes over time varied by treatment (Figure 1B “final”; F2,32=10.0; p<0.01) such that tumor-bearing mice weighed more than controls and tumor-resected mice. As expected with the added tumor weight, body weight increased by about 5% in tumor-bearing mice after tumor treatment (t10=5.1; p<0.001), whereas weight remained unchanged over time for controls and tumor-resected mice (p>0.05 in both cases). Tumors elevated spleen mass relative to tumor-free controls and tumor resection resolved this splenomegaly (Figure 1C; F2,27=16.5; p<0.0001). The CD11b/GR+ cell population was elevated in the spleen of tumor-bearing mice (Figure 1D; F2,28=35.1; p<0.0001). A modest, but statistically significant, elevation in splenic CD11b/GR+ cells persisted in tumor-resected mice relative to that of controls (Figure 1D; t16=2.6, p<0.05).
3.2 Tumor characteristics
Masses of tumors that were resected weighed less at resection (531.2 ± 108 mg) than those that remained in the tumor-bearing mice (1,908 ± 315 mg) because of the additional tumor growth during 5 days of behavioral assessment (U=7, p<0.01). A lower percentage of tumor CD11b/GR+ cells (i.e., myeloid-derived suppressor cells) than CD11b/F4/80+ cells (tumor-associated macrophages) was observed in these tumors (Supplementary Figure 1A; t9=8.2; p<0.001). Despite the smaller size of resected tumors, no differences in the proportions of either CD11b/GR+ or CD11b/F4/80+ cells in tumors were observed between tumor-bearing and -resected mice (data combined in Supplementary Figure 1A; p>0.05 in both cases). Tumor tissue expressed Il-1b, IL-6, and Tnfa, with the latter less than the two former genes (Supplementary Figure 1B; p<0.05 in both cases).
3.3 Circulating cytokines at rest
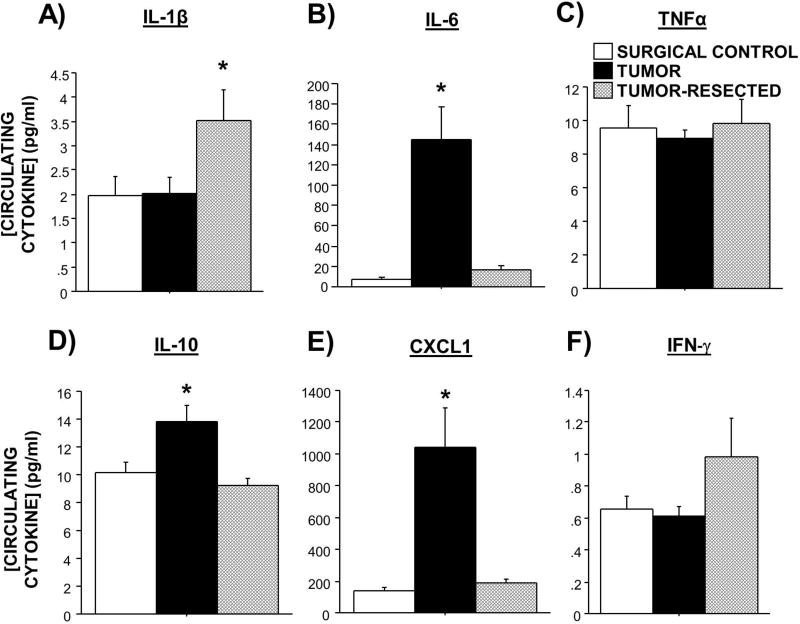
Tumors significantly increased circulating IL-6, IL-10, and CXCL1 relative to both tumor-free controls and tumor-resected mice (Figure 2B,D,E; F2,26=8.3, p<0.01; F2,26=6.3, p<0.01; F2,26=10.5, p<0.001). While tumor resection significantly attenuated tumor-induced increases in these circulating cytokine concentrations, these concentrations remained slightly elevated relative to tumor-free controls for both IL-6 (Figure 2B; t17=2.0, p=0.06) and CXCL1 (Figure 2E; t17=1.5, p=0.1). In contrast, tumor resection increased circulating IL-1β (Figure 2A; F2,26=3.2, p≤0.05) relative to the other groups. IFN-γ approached this same pattern (Figure 2F; F2,26=2.6, p=0.09). No differences in circulating TNFα were detected among treatment groups (Figure 2C; p>0.05).
Figure 2. Effects of complete mammary tumor resection on circulating cytokine concentrations.
Mean ± SEM A) IL-1β, B) IL-6, C)TNFα, D) IL-10, E) CXCL1, and (F) IFN-γ (pg/ml) in surgical controls, tumor-bearing mice, and tumor-resected mice. n=6–11/group; *p<0.05 relative to both other treatments
Among tumor-bearing mice, circulating CXCL1 (Supplementary Figure 1C; p<0.01), IL-1β (p<0.05), IL-6 (p<0.05), and IL-10 (p<0.01) positively correlated with tumor mass, whereas circulating IFN-γ negatively correlated (Supplementary Figure 1D; p<0.01). No relationships between tumor weight and circulating TNF were observed (data not shown).
3.4 Circulating corticosterone concentrations and CD11b/GR+ cell proportions at rest and immediately after an acute stressor
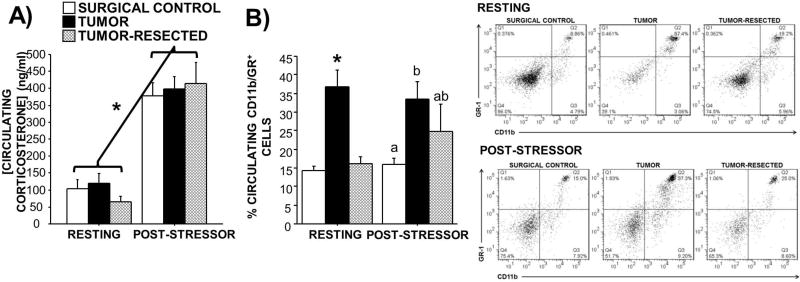
Resting circulating corticosterone concentrations were statistically comparable among treatment groups, although concentrations in tumor-resected mice tended to be lower (Figure 3A; p>0.05). An acute stressor (tail suspension test) significantly and comparably increased corticosterone concentrations in all groups (p>0.05 in all cases).
Figure 3. Effects of complete mammary tumor resection on circulating corticosterone concentrations and CD11b/GR+ cell populations at rest and immediately after acute mild stressor.
Mean ± SEM resting and post-stressor (5-min tail suspension) A) corticosterone concentrations (ng/ml; n=7–12/group) and B) percentage of CD11b/GR+ myeloid-derived suppressor cells and representative flow cytometry dot plots (n=3–11/group) in surgical controls, tumor-bearing mice, and tumor-resected mice. *p<0.05 relative to both other treatments; different letters represent statistical difference p<0.05
At rest, the circulating CD11b/GR+ cell population was elevated in tumor-bearing mice (Figure 3B; F2,23=14.3; p<0.001); tumor resection alleviated this elevation to levels comparable to controls. Following an acute mild stressor, the circulating CD11b/GR+ cell population was affected by tumor status (Figure 3B; F2,10=3.9; p≤0.05), such that tumor-bearing mice had elevated levels relative to controls. The post-stressor CD11b/GR+ cell population in tumor-resected mice remained intermediately elevated such that it was not statistically different from either controls or tumor-bearing mice (Figure 3B; p>0.05 in both cases). The change in circulating CD11b/GR+ cells between rest and post-stressor within treatment groups did not vary to a statistically significant degree (Figure 3B), however CD11b/GR+ cell populations tended to increase in controls (p=0.09) and in tumor-resected mice (p=0.07).
No relationship between post-stressor circulating corticosterone and post-stressor CD11b/GR+ percentages was observed when all groups were included (p>0.05; data not shown). However, among solely the tumor-bearing mice, tumor mass approached a positive statistical relationship with resting circulating CD11b/GR+ cells (Supplementary Figure 1E; p=0.1).
3.5 Behavior
Tail suspension test
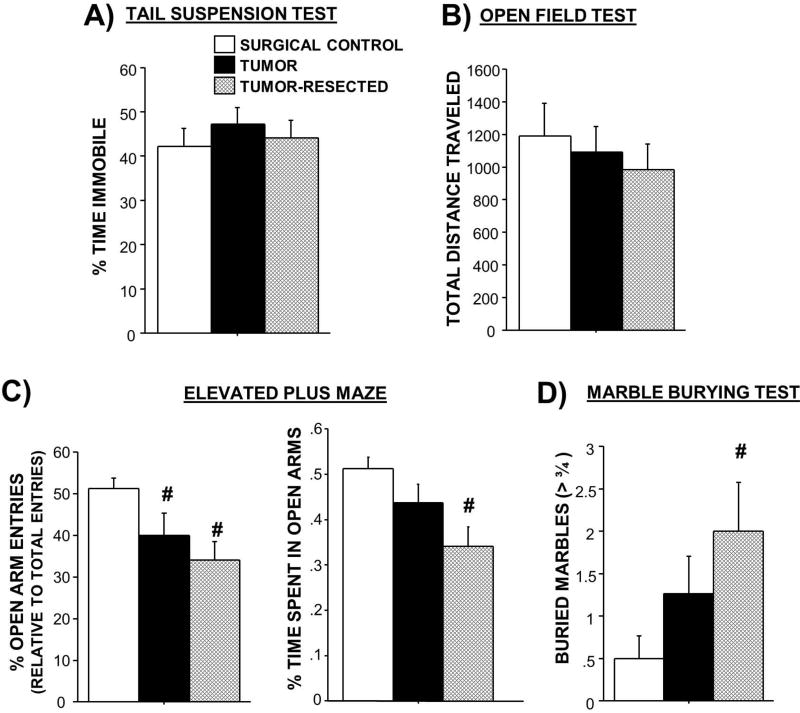
Tumor treatment did not statistically significantly affect the time spent immobile in this test (Figure 4A; p>0.05).
Figure 4. Effects of complete mammary tumor resection on anxiety-like and locomotor behaviors.
Mean ± SEM A) percent time spent immobile in tail suspension test for depressive-like behavior, B) total distance traveled in open field test (n=6–11/group), C) percentages of open arm entries and time spent in open arms in the elevated plus maze for anxiety-like behavior (n=6–10/group), and D) number of marbles buried in the marble burying test for anxiety-like behavior. n=7–11/group; #p<0.05 relative to controls
Open field test
No differences in anxiety-like behavior (central tendency; data not shown) nor total locomotor activity (Figure 4B) were observed among treatment groups using this paradigm (p>0.05).
Elevated plus maze
Tumor treatment modulated the percentage of entries into the exposed open arms (Figure 4C; F2,25=4.5; p<0.05), such that both the tumor-bearing and the tumor-resected mice displayed more anxiety-like behavior by entering the open arms proportionately less than the closed arms compared to the surgical controls (p<0.05 in both cases). Of note, percentage of open arm entries controls for locomotor activity. The percentage of time spent in the open arms relative to the total test time was also reduced in the tumor-resection group relative to tumor-free controls only (t16=2.1, p≤0.05; data not shown). Furthermore, similar to the open field test, total locomotor activity in the elevated plus mace (i.e., total number of arm entries) did not statistically significantly differ among treatments (data not shown; p>0.05).
Marble-burying test
Tumor resection also increased anxiety-like behavior in the marble burying test (Figure 4D; F2,27=3.2; p≤0.05), such that tumor-resected mice buried significantly more marbles than surgical controls (p<0.05); tumor-bearing mice buried an intermediate number of marbles. Within tumor-bearing mice, tumor mass positively predicted anxiety-like behavior in the marble burying task (number of marbles buried; Supplementary Figure 1F; p<0.05).
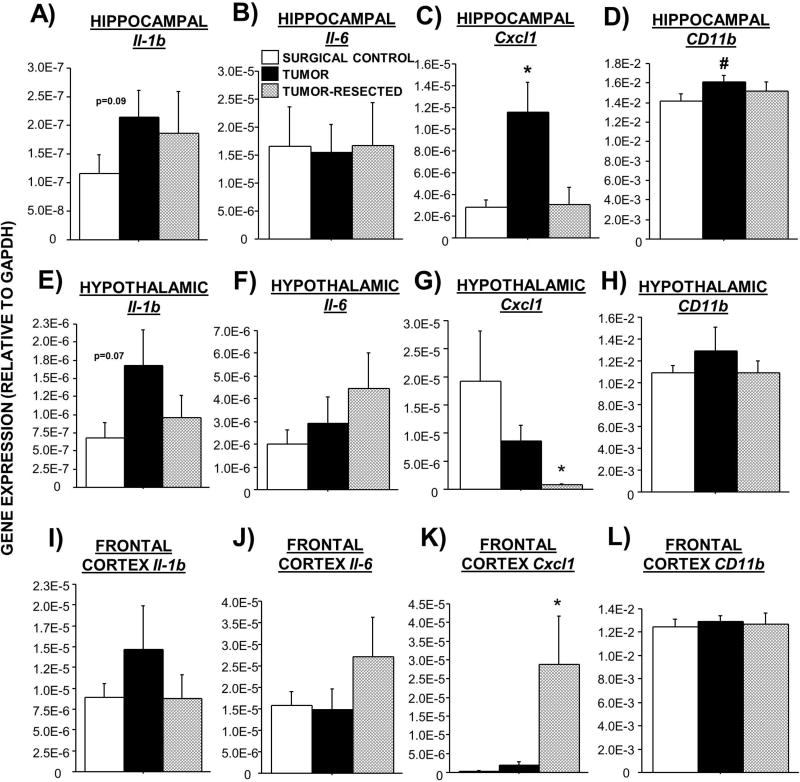
3.6 Brain qPCR
Based on the well-established relationship between neuroinflammation and affective-like behavior and the observed changes in circulating immune/inflammatory markers in this model, neuroinflammatory gene expression was assessed. While overall effects of tumor treatments on brain Il-1b did not reach statistical significance (p>0.05), tumors tended to increase Il-1b mRNA in all brain regions investigated relative to controls (Figure 5A,E,I). Resection attenuated these trends. In contrast, tumor resection tended to increase Il-6 mRNA in both the hypothalamus and frontal cortex relative to that of tumor-bearing mice (Figure 5F,J; p>0.05). Tumors increased hippocampal Cxcl1, whereas tumor resection negated this increase (Figure 5C; F2,26=6.8; p<0.01). In contrast, tumor resection exacerbated both a modest tumor-induced reduction in hypothalamic Cxcl1 relative to controls (Figure 5G; p=0.06) and an elevation in frontal cortex Cxcl1 (Figure 5K; F2,19=6.5; p<0.01). Increases in CD11b expression in tumor-bearing mice compared with controls were statistically significant in the hippocampus (Figure 5D; p≤0.05), whereas resected mice displayed intermediate gene expression. A similar trend was observed in the hypothalamus (Figure 5H). No statistically-significant differences in Tnf were observed among treatment groups (p>0.05 in all cases). Brain Il-10 and Ifn-γ mRNA were undetectable in all treatments (data not shown).
Figure 5. Effects of complete mammary tumor resection on mRNA expression of inflammatory markers in brain regions that regulate affective-like behavior.
Mean ± SEM TaqMan quantitative gene expression of hippocampal (A–D), hypothalamic (E–H), and frontal cortex (I–L) Il-1β, Il-6, Cxcl1, and CD11b. n = 7–11/group; *p<0.05 relative to both other groups; #p<0.05 relative to controls
3.7 Relationships among inflammation and behavior
Simple linear regressions were performed to identify potential predictive relationships among the inflammatory markers in tumors, circulation, and the brain and anxiety-like behavior where differences were observed among treatment groups. Among tumor-resected mice, the observed elevations in circulating IL-1β and IFN-γ did not predict anxiety-like behaviors, nor did the significant changes in brain Cxcl1 (p>0.05 in all cases).
Within tumor-bearing mice, higher circulating CXCL1 and IL-6 both predicted higher expression of Il-1b mRNA in brain regions relevant to affect (Supplementary Table 1). Relationships between tumor inflammation or tumor mass and brain inflammation were similarly positive among tumor-bearing mice, indicating that larger tumors or more tumor inflammation predicted greater neuroinflammatory gene expression (Supplementary Tables 2 and 3). Of note, when tumor mass data from tumors resected prior to brain tissue collection (i.e., from the tumor-resected group) were included in the correlational analyses, the positively predictive relationship between tumor mass and neuroinflammation remained and even expanded to include an additional neuroinflammatory marker (Supplementary Table 3). Tumor tissue from resected mice was not saved to assess the maintenance of the relationship between tumor and brain inflammation with the inclusion of tumor-resected mice.
4. DISCUSSION
The primary two goals of this work were to establish a novel rodent model of breast cancer “survivorship” and to identify enduring immune/inflammatory changes and affective-like behavior after tumor removal in this model. To achieve these ends, we repurposed a non-metastatic, orthotopic, syngeneic mouse model of breast cancer that has been previously used as a control in metastases research (Eckhardt et al., 2005; Lelekakis et al., 1999). The tumor-resected group was designed to capture the disease experience of the majority of breast cancer patients: survival (ACS, 2016). To our knowledge, this is the first basic science tumor “survivor” study in behavioral neuroscience. Resected mice maintained a healthy body weight after their two surgeries (tumor inoculation and resection) and activity level (open field and elevated plus maze), demonstrating their fitness for behavioral testing. In this study, while the duration of tumor exposure was kept constant (2 weeks) between the tumor-bearing and tumor-resected mice for the start of behavioral testing, the tumors that remained in the tumor-bearing group had a longer time to grow than those of the tumor-resected group (throughout 5 days of behavioral testing). Therefore, the latter tumors were larger at final measurement. Future studies will account for this slight difference in tumor exposure duration and can also include a group of mice with a recurrent tumor to model the equivalent subset of cancer patients. Overall, this work indicates that this novel tumor-resection model is a viable and more accurate representation of the breast cancer survival experience (i.e., tumor exposure followed by surgical tumor removal) than tumor-bearing models. It is predicted to be useful for teasing out the roles of tumor biology, cancer treatments, and stressors for the study of behavioral comorbidities in cancer survivors.
In addressing our second goal, to identify enduring immune/inflammatory changes and affective-like behavior after tumor removal, we compared peripheral and central inflammatory markers and behaviors of mice with tumors resected 3 weeks prior to tumor-bearing mice and surgical controls. Tumor resection convincingly reversed many typical tumor-induced peripheral immune increases: spleen mass, circulating MDSCs and some cytokines. Some of these increases were substantial (≥8-fold; e.g., splenomegaly, circulating cytokines), making the reversal by surgical tumor resection remarkable and rapid.
However, tumor removal failed to reverse all physiological and behavioral consequences of tumors, and even amplified others. These select changes may be comparable to those enduring symptoms that are caused, at least in part, by tumor biology and continue to plague quality-of-life in cancer survivors. With respect to peripheral immune status, modest elevations in splenic MDSCs remained after tumor resection. Additionally, in response to a mild stressor, tumor resection failed to significantly attenuate the circulating MDSC population. Similarly, relatively greater reported stress in breast cancer patients corresponds with more circulating MDSCs (Mundy-Bosse et al., 2011). This lingering immune response to a stressor supports the possibility that challenges (e.g., stressor) may unmask additional consequences of a prior cancer experience than when at rest (e.g., a “two-hit” hypothesis; Fiebich et al., 2014).
Tumor-induced neuroinflammation had not yet been reported using this mouse breast cancer model. Other tumor-bearing rodent models are characterized by mild to moderate tumor-induced neuroinflammation (Lebena et al., 2014; Norden et al., 2014; Pyter et al., 2009; Yang et al., 2014). In contrast to those, neuroinflammatory gene expression was generally modest in the present model (Il-1β, Cxcl1, Cd11b), making assessment of the potential reversal by tumor resection challenging. However, the Il-1β and Cd11b mRNA data suggest that tumor resection resolves or partially resolves these tumor-induced neuroinflammatory markers. This reversal of neuroinflammation by tumor resection is most obvious in hippocampal Cxcl1 and mirrors the pattern of circulating CXCL1 among treatment groups. Given that a statistical relationship between circulating and hippocampal Cxcl1 was not observed, residual circulating blood in the brain is unlikely to drive the observed brain gene expression results. However, more detailed studies are necessary to determine the cellular origin of the observed brain gene expression changes. In contrast to the hippocampus, Cxcl1 gene expression in other brain regions follows different patterns, suggesting region-specificity in persistent effects of prior tumor exposure. In contrast, the incomplete resolution of brain Il-1b, the increase and trends toward increases in Il-6 and frontal cortex Cxcl1 gene expression, suggest that some elevations in inflammatory markers in brain tissue may persist even after tumors are resected. These enduring brain changes in tumor-resected mice were accompanied by an elevation in circulating IL-1β. Peripheral elevations in IL-1β are often related to increased neuroinflammation, as well as affective-like behavior (Konsman et al., 2008; Maes et al., 2012). Similarly, resting cytokine concentrations remain slightly elevated in human breast cancer survivors with depression (Mundy-Bosse et al., 2011; Musselman et al., 2001). Similar to the peripheral MDSC responses after the stressor in this model, challenges may elicit even more substantial changes in neuroinflammatory circuitry than at rest (Pyter et al., 2014).
Behaviorally, tumor-resection amplified tumor-induced increases in multiple anxiety-like behaviors. This pattern was observed using both the open versus closed conflict paradigm of the elevated plus maze and the obsessive-compulsive paradigm of the marble burying task, suggesting an elevation in overall anxiety-like behavior for tumor-resected mice. While most studies of affective-like behavior in tumor-bearing models have focused on depressive-like behavior, a previous student in tumor-bearing rats also displayed an increase in obsessive-compulsive-like behavior (Pyter et al., 2009). Exacerbation of this type of behavior by tumor resection suggests a progressive increase in anxiety-like behavior post-tumor, rather than an exacerbating effect of resection surgery, as surgical controls had the lowest anxiety-like behavior of all treatments. The duration of these lingering changes in the survivor model, beyond the examined 3 weeks post-resection, remains to be determined. Furthermore, the statistically-insignificant tendency for tumors and tumor-resection to reduce locomotion in the open field and elevated plus maze tests warrant future investigation of fatigue in this model, as fatigue is a primary lingering concern among cancer survivors (Bower, 2014).
The present data were also analyzed to determine potential predictive relationships among tumor, blood, and brain inflammation, as well as affective-like behaviors. Reliable relationships between peripheral physiology and brain function would be useful for the translation of this work into potential clinical biomarkers for comorbidity risk. Among the physiological or behavioral differences observed in tumor-resected mice, no statistical relationships were observed. However, when resected tumor mass data were combined with the tumor mass data from the tumor-bearing group, the predictive value of tumor mass on neuroinflammation remained and, in the case of brain CXCL1, was strengthened. Therefore, tumor mass in patients following surgical removal may have predictive value for long-term neuroinflammation in cancer survivors.
Following the original publication from our lab (Pyter et al., 2009), numerous other groups have replicated the findings that non-CNS solid tumors are sufficient to alter neuroplasticity and neuroinflammation and induce affective-like behaviors and cognitive impairments in rodent models (Schrepf et al., 2015). This basic research, on the role of tumor biology alone in behavioral comorbidities, is corroborated by clinical research indicating that inflammatory and behavioral changes occur in cancer patients prior to systemic cancer treatments or even prior to surgery (Friedman et al., 2005; Lutgendorf et al., 2008; Sebti et al., 2015). The final goal of this work, to advance this existing behavioral comorbidity research in tumor-bearing models by empirically testing the causal role of tumors in reported changes in brain and behavior, was less successful given the modest effects of these tumors on brain and behavior. However, tumor mass did predict brain Il-1β gene expression and anxiety-like behavior in the marble burying task, indicating that a narrower range of tumor size would amplify the observed modest brain and behavioral changes. Importantly, these non-metastatic tumors did not trigger cachexia or induce overt sickness behaviors, which are confounds of some behavioral models of advanced cancer (Norden et al., 2014). For example, body mass was not reduced in tumor-bearing mice. Additionally, no differences were observed among groups in locomotion in the open field or elevated plus maze tests.
Our characterization of the tumor-bearing mice demonstrated that these primary mammary tumors are infiltrated by significant populations of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages. Additional peripheral immune consequences of these tumors included a robust elevation in splenic and circulating MDSCs. The tumors were characterized by both pro- and anti-inflammatory markers. Pro-inflammation was demonstrated by the expression of pro-inflammatory genes (Il-1b, Il-6, Tnfa) in the tumor tissue and the elevations in circulating proinflammatory cytokines (Lamkin et al., 2011; Norden et al., 2014; Yang et al., 2014), in proportion to tumor mass (Lebena et al., 2014). This clear inflammatory response in the periphery is not ubiquitously observed among other tumor models used for behavioral research (Lebena et al., 2014; Pyter et al., 2009). Both elevations in circulating IL-6 and CXCL1 concentrations and tumor cytokine mRNA predicted higher neuroinflammation in some brain regions, supporting the potential for blood or tumor biopsies to be used as predictors of concurrent brain inflammation. CXCL1, which was markedly elevated, is important for neutrophil recruitment (Kobayashi, 2008) and may be involved in myeloid cell trafficking to the brain in this paradigm, particularly when considered with the observed abundance of circulating MDSCs. The measurement of CXCL1 has not been reported in other tumor models used to investigate behavior. However, CXCL1 in mice is thought to be homologous to human IL-8, which is associated with negative behavioral comorbidities in breast cancer patients (Janelsins et al., 2012). In contrast, the increase in circulating anti-inflammatory IL-10 (which positively correlated with tumor mass) is consistent with the notion that tumor-associated MDSCs increase IL-10 release as a result of cross-talk with local macrophages, thereby promoting pro-tumor Type 2 immunity (Sinha et al., 2007). As expected, no metastases were observed with these primary tumors (Eckhardt et al., 2005; Miller et al., 1983).
Overall, this work demonstrates the potential long-lasting role that tumor biology alone can have on some brain functions. The development of this survivor model forges a more translational approach to understanding the mechanisms underlying persistent behavioral comorbidities in the long-lived population of breast cancer survivors. We expect this type of modeling to be important to parse out and understand the interactions among tumor experience, cancer treatments, and cancer-associated stressors and how they affect brain function. This complex physiological information will be the foundation upon which treatment strategies are refined and interventions are developed for the vastly growing population of cancer survivors.
Supplementary Material
HIGHLIGHTS.
A novel tumor-resection mouse model of breast cancer survivorship is characterized.
Anxiety-like behaviors and inflammatory markers persist after tumor resection.
Inflammation and tumor size was predictive of behavior when tumor was intact.
Acknowledgments
The authors thank Hsin-Yun Lin, Lindsay Strehle, and Shireen Desai for technical assistance and Pete McKinley for animal care. This work was supported by the Ohio State University Medical Center, an NIH grant CA201681, CCTS core grant UL1TR001070 (L.P.), P01CA95426 (W.C.), T32CA090223-12 (L.S-K.), and two undergraduate research scholar awards (J.K, S.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACS. Cancer Facts & Figures 2016. American Cancer Society; Atlanta, GA: 2016. [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Research. 1992;52:1399–405. [PubMed] [Google Scholar]
- Benoy I, et al. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311–5. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]
- Bower JE, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors' reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–32. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice. Wiley-Liss; New York: 2000. What's wrong with my mouse? [Google Scholar]
- DeSantis CE, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- Dexter DL, et al. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Research. 1978;38:3174–81. [PubMed] [Google Scholar]
- DiMatteo MR, et al. Correspondence among patients' self-reports, chart records, and audio/videotapes of medical visits. Health Commun. 2003;15:393–413. doi: 10.1207/S15327027HC1504_02. [DOI] [PubMed] [Google Scholar]
- Dooley LN, et al. Val66Met BDNF polymorphism as a vulnerability factor for inflammation-associated depressive symptoms in women with breast cancer. J Affect Disord. 2016;197:43–50. doi: 10.1016/j.jad.2016.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt BL, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed] [Google Scholar]
- Fiebich BL, Akter S, Akundi RS. The two-hit hypothesis for neuroinflammation: role of exogenous ATP in modulating inflammation in the brain. Front Cell Neurosci. 2014;8:260. doi: 10.3389/fncel.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman GD, et al. Early symptoms of ovarian cancer: a case-control study without recall bias. Fam Pract. 2005;22:548–53. doi: 10.1093/fampra/cmi044. [DOI] [PubMed] [Google Scholar]
- Fu X, et al. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji N, et al. TNF-alpha-mediated anxiety in a mouse model of multiple sclerosis. Exp Neurol. 2012;237:296–303. doi: 10.1016/j.expneurol.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum. 2007;34:981–94. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- Howlader N, et al. SEER Cancer Statistics Review, 1975–2013. NCI; Bethesda, MD: 2016. [Google Scholar]
- Janelsins MC, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20:831–9. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, et al. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277–84. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–7. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Konsman JP, et al. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Lamkin DM, et al. Cancer induces inflammation and depressive-like behavior in the mouse: modulation by social housing. Brain, Behavior, and Immunity. 2011;25:555–64. doi: 10.1016/j.bbi.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebena A, et al. Melanoma tumors alter proinflammatory cytokine production and monoamine brain function, and induce depressive-like behavior in male mice. Behav Brain Res. 2014;272:83–92. doi: 10.1016/j.bbr.2014.06.045. [DOI] [PubMed] [Google Scholar]
- Lee BN, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–92. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- Lelekakis M, et al. A novel orthotopic model of breast cancer metastasis to bone. Clinical and Experimental Metastasis. 1999;17:163–70. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–7. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass SW, et al. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas. 2015;82:100–8. doi: 10.1016/j.maturitas.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin Ther Targets. 2012;16:1097–112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6:e946. doi: 10.1038/tp.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalaki V, et al. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Medina D, Heppner GH. Preferential growth of mammary tumors in intact mammary fatpads. Cancer Research. 1981;41:3863–7. [PubMed] [Google Scholar]
- Miller FR, Miller BE, Heppner GH. Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invasion and Metastasis. 1983;3:22–31. [PubMed] [Google Scholar]
- Mundy-Bosse BL, et al. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–7. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Norden DM, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vol., N.R. Council, ed.^eds, editor. NRC. Guide for the care and use of laboratory animals. The National Academies Press; Washington D.C.: 2011. [Google Scholar]
- Orre IJ, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71:136–41. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Papiez MA, et al. Evaluation of oxidative status and depression-like responses in Brown Norway rats with acute myeloid leukemia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:596–604. doi: 10.1016/j.pnpbp.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Pisu M, et al. The out of pocket cost of breast cancer survivors: a review. J Cancer Surviv. 2010;4:202–9. doi: 10.1007/s11764-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, et al. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;106:9069–74. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, et al. Peripheral tumors alter neuroinflammatory responses to lipopolysaccharide in female rats. Brain Research. 2014;1552:55–63. doi: 10.1016/j.brainres.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM. The influence of cancer on endocrine, immune, and behavioral stress responses. Physiol Behav. 2015 doi: 10.1016/j.physbeh.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Pyter LM. The influence of cancer on endocrine, immune, and behavioral stress responses. Physiol Behav. 2016;166:4–13. doi: 10.1016/j.physbeh.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Qi H, et al. Allostatic tumor-burden induces depression-associated changes in hepatoma-bearing mice. J Neurooncol. 2009;94:367–72. doi: 10.1007/s11060-009-9887-3. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psychooncology. 2010;19:535–44. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349–61. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: neuroinflammatory mechanisms in humans and rodents. Brain Behav Immun. 2015;49:1–17. doi: 10.1016/j.bbi.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebti J, Desseigne F, Saltel P. Prodromal depression in pancreatic cancer: retrospective evaluation on ten patients. Palliat Support Care. 2015;13:801–7. doi: 10.1017/S1478951514000728. [DOI] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35:729–41. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Sinha P, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Soygur H, et al. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1242–7. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yatsugi S, Yamaguchi T. Effect of YM992, a Novel Antidepressant With Selective Serotonin Re-uptake Inhibitory and 5-HT(2A) Receptor Antagonistic Activity, on a Marble-Burying Behavior Test as an Obsessive-Compulsive Disorder Model. Jpn J Pharmacol. 2002;90:197–200. doi: 10.1254/jjp.90.197. [DOI] [PubMed] [Google Scholar]
- Van Esch L, et al. Combined anxiety and depressive symptoms before diagnosis of breast cancer. J Affect Disord. 2012;136:895–901. doi: 10.1016/j.jad.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Vegas O, et al. Behavioral and neurochemical responses in mice bearing tumors submitted to social stress. Behav Brain Res. 2004;155:125–34. doi: 10.1016/j.bbr.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wefel JS, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, et al. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34:2583–91. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, et al. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull. 2012;89:50–6. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–55. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.