Abstract
The thermogenic activities of brown and beige adipocytes can be exploited to reduce energy surplus and counteract obesity. Recent RNA sequencing studies have uncovered a number of long noncoding RNAs (lncRNAs) uniquely expressed in white and brown adipose tissues (WAT and BAT), but whether and how these lncRNAs function in adipogenesis remain largely unknown. Here, we report the identification of a novel brown adipocyte-enriched LncRNA (AK079912), and its nuclear localization, function and regulation. The expression of AK079912 increases during brown preadipocyte differentiation and in response to cold-stimulated browning of white adipocytes. Knockdown of AK079912 inhibits brown preadipocyte differentiation, manifested by reductions in lipid accumulation and down-regulation of adipogenic and BAT-specific genes. Conversely, ectopic expression of AK079912 in white preadipocytes up-regulates the expression of genes involved in thermogenesis. Mechanistically, inhibition of AK079912 reduces mitochondrial copy number and protein levels of mitochondria electron transport chain (ETC) complexes, whereas AK079912 overexpression increases the levels of ETC proteins. Lastly, reporter and pharmacological assays identify Pparγ as an upstream regulator of AK079912. These results provide new insights into the function of non-coding RNAs in brown adipogenesis and regulating browning of white adipocytes.
Keywords: LncRNA, AK079912, Brown adipocyte, Browning, Adipogenesis, Pparγ
1. Introduction
Obesity has become a global epidemic and is a major risk factor for a myriad of metabolic syndromes, including type 2 diabetes featured by insulin resistance and hyperglycemia, cardiovascular diseases, hypertension, and many types of cancer [1,2]. Adipocytes (fat cells) play critical roles in systemic metabolism and energy homeostasis [3]. There are at least two classes of fat cells – white and brown. White fat is specialized to store energy in the form of triglycerides and is also involved in endocrine signaling and metabolic-immune crosstalk [4]. In contrast, brown adipocytes are packed with mitochondria, which uniquely express uncoupling protein 1 (Ucp1) in the mitochondrial inner membrane. Brown adipocytes function to dissipate chemical energy in form of heat by uncoupling the electrochemical gradient from ATP synthesis, therefore playing important roles in defending hypothermia, increasing energy expenditure and improving insulin sensitivity [4]. Recently, clusters of Ucp1-expressing adipocytes with thermogenic capacity are identified in white adipose tissue (WAT) in response to various stimuli, such as cold stress [5]. These thermogenic adipocytes dispersed in WAT are referred to as beige or brite adipocytes. Recent imaging studies revealed the presence of substantial deposits of UCP1+ adipocytes in health humans, but the mass and activity of UCP1+ cells were lower in obese and older subjects [6–8]. Thus, exploring potential regulators that promote the development and increase the activity of thermogenic adipocytes holds tremendous promise for the treatment of metabolic disease.
Brown and beige adipocyte biogenesis is regulated coordinately by adipogenic and mitochondrial thermogenic gene regulatory programs, the latter being key to mitochondrial fuel oxidation and thermogenesis [9]. To date, transcriptional control of brown and beige adipocyte formation has been studied extensively. The transcription factors per-oxisome proliferator-activated receptor gamma (Pparγ) [10] and the CCAAT/enhancer-binding proteins (specifically Cebpα/β/δ) [11] have been established as essential components of the transcriptional cascade that precedes the formation of both brown and white adipocytes. In addition, Pparγ coactivator 1α (Pgc1α) and transcription factor PR domain containing 16 (Prdm16) interact with Cebpβ to specify the brown and beige adipogenic program [12–14]. This is accompanied by the activation of de novo mitochondrial biogenesis and expression of functional components that are essential to thermogenesis, such as Ucp1 [15].
In contrast to the well-established transcriptional network underlying brown and beige adipogenesis, how epigenetic mechanisms regulate adipogenesis is poorly understood. Non-coding RNAs including microRNAs (miRNAs) and LncRNAs are emerging epigenetic regulators of brown adipocyte differentiation and thermogenic function. miRNAs are evolutionary conserved and primarily function to repress gene expression through binding to the 3′ untranslated region (UTR) of target mRNA to induce mRNA degradation or inhibit protein translation. A number of miRNAs, including miR-193b-365, miR-133, and miR-27, have been reported to regulate brown adipogenesis [16]. In contrast, LncRNAs generally have poor sequence conservation, and interact with RNA-binding proteins (Rbps) to repress or activate gene expression through a variety of mechanisms based on sequence characteristic and subcellular localization [17]. A recent study has identified > 1500 LncRNAs in adipose tissues, of which 127 are BAT-restricted [18]. However, the function of these LncRNAs in brown adipocyte adipogenesis and thermogenesis remains largely unknown.
Emerging evidence points to the existence of BAT-specific LncRNAs that function to regulate BAT adipogenesis and thermogenic activity [18–20]. Specifically, brown fat lncRNA 1 (Blnc1) was shown to promote brown and beige adipocyte differentiation through a ribonucleoprotein complex that acts through Ebf2 (early B-cell factor 2) to enhance expression of thermogenic genes such as Ucp1. This in turn forms a feedforward regulatory loop as Blnc1 itself is positively regulated by Ebf2 [19]. In addition, BAT-enriched LncRNA1 (lnc-BATE1) is required for BAT adipogenesis and maintenance through binding with the heterogeneous nuclear ribonucleoprotein U (hnRNPU) [18]. Here, we report the identification and characterization of a novel BAT enriched LncRNA. This study adds to the growing repertoire of LncRNAs and their distinct functions in brown adipocyte differentiation and BAT-specific genes regulation.
2. Materials and methods
2.1. Animals and adenovirus injection
All procedures involving mice were approved by Purdue University Animal Care and Use Committee. Mice were in a C57BL/6J background and housed in the animal facility with free access to water and standard rodent chow food. Unless otherwise indicated, 2-month old adult mice were used in this study. For adenovirus iWAT local injection, the mice were anesthetized by ketamine. 2 × 1011 purified adenovirus particles for control and AK079912 were injected into the two side of iWAT, respectively. After 2 weeks, the injected iWATs were performed thermogenic genes assay by qPCR.
2.2. Ex vivo culture
Adipose tissue explant culture was conducted as described [21], with modifications. Inguinal adipose depot was minced into 1–3 mm3 size fragments and transferred into 12-wells plate containing 1 ml differentiation medium, each well with 6 pieces of adipose cubes. The differentiation medium consisted of DMEM medium, 1% penicillin/ streptomycin (P/S), 10% Fetal Bovine Serum (FBS), 33 μM biotin, 17 μM pantothenate, 100 μM ascorbate, 280 nM bovine insulin and 1 μM rosiglitazone. The adipose tissue was incubated for 24 h, and then infected by AK079912 overexpression adenovirus for 24 h. After that, the adenovirus incubated medium was changed with fresh differentiation medium. At day 3 after infection, samples were collected for qPCR analysis.
2.3. Cell culture and adipogenic differentiation
Primary BAT and WAT stromal vascular fraction (svf) cells were isolated using collagenase digestion and followed by density separation as our previous research [22]. Briefly, the BAT and iWAT were minced and digested in 1.5 mg/ml collagenase at 37 °C for 0.5 h and 1 h, respectively. The digestions were terminated with DMEM containing 10% FBS, and filtered through 100 μm filters to remove connective tissues and undigested trunks of tissues. Cells were then centrifuged at 1500g for 5 min to separate the SVF cells in the sediment and lipid-containing adipocytes in the floating layer. The freshly isolated SVF cells were seeded and cultured in growth medium containing DMEM, 20% FBS, 1% penicillin/streptomycin (P/S) at 37 °C with 5% CO2 for 3 days, followed by feeding with fresh medium every 2 days. The BAT cell line (kindly provided by Prof. Yongxu Wang, University of Massachusetts Medical School), were cultured in same condition as svf cells, while HEK293A (ATCC) and HEK293T (ATCC) were cultured in DMEM with 10% FBS.
For svf cell and BAT cell line adipogenic differentiation, the cells were induced with induction medium (IM) contains DMEM, 10% FBS, 2.85 μM insulin, 0.3 μM dexamethasone (DEXA), 1 μM rosiglitazone, and 0.63 mM 3-isobutyl-methylxanthine (IBMX) for 4 days upon confluence and then differentiated in differentiation medium (DM) contains DMEM, 10% FBS, 200 nM insulin and 10 nM T3 for 4 days until adipocytes mature. To avoid the effect of cell density on adipogenic differentiation, cells were induced to differentiate when they reach 90% confluence.
2.4. Adenovirus generation and purification
The adenovirus with AK079912 insertion was generated using the AdEasy system as described [23]. Briefly, the cDNA of AK079912 was cloned, and inserted into pAdTrack-CMV plasmid. The formed pAd-Track-CMV-AK079912 (pAdTrack-CMV as the control) plasmids were digested by PmeI and then transfected the DH5α competent cell with pAdEasy-1. The positive recombinant plasmid was identified by PacI digestion. Then, HEK293A cells (50%–60% confluent) in 10 cm culture dishes was transfected with the 4 μg PacI digested recombinant plasmid using Lipofectamine 2000 (Life Technologies) according to manufacturer’s protocol. After 2 weeks, the recombinant adenovirus was collected by three freezes–thaw–vortex cycles. Two more round infected HEK293A cells were adapted to amplify the recombinant virus and the titers were determined by the expression of GFP. Adenovirus Purification by CsCl2 grade ultra-centrifuge based on described procedure [24].
2.5. siRNA transient knockdown and shRNA stable knockdown BAT cell lines
Ribo lncRNA smart silencer for lncRNA AK079912 was purchased from Guangzhou RIBOBIO (Guangzhou, China). This product contains a mixture of three siRNAs and three antisense oligonucleotides (ASOs) for lncRNA AK079912, including siRNA1 5′-CTCCAATGAGGGATCC CAA-3′, siRNA2 5′-CCTGACACCAGGCCGACTT-3′, siRNA3 5′-GTAGAA ATACTCCAGAAGA-3′, ASO1 5′-ACAAGTTGCTCCTTCCTTCT-3′, ASO2 5′-TAATATCCACCATTCTCTGG-3′ and ASO3 5′-GGAATATAATGCTGC CTTTG-3′. The smart silencer of AK079912 was transfected into brown adipocyte using Rfect siRNA Transfection Reagent (BIO-TRAN) according to the manufacturer’s protocol. The adipocytes were performed Bodipy staining and qPCR analysis at day 4 after transfection.
We designed two independent shRNAs targeted AK079912 (No. ak079912) by Invitrogen software (https://rnaidesigner.invitrogen.com/rnaiexpress/), with a scramble shRNA as control (Fig. S1). The shRNAs oligonucleotides were ligated into pLKOpuro.1 plasmid and then were co-transfected with V-SVG and Δ8.2 into HEK293T cells seeded in 10 cm dish. After 48 h, the lentivirus was collected and filtered by 0.45 μm filter to remove the cellular debris. The BAT cell line (20%–30%) was infected by AK079912 or scramble lentivirus, and then the stable expressing shRNA brown adipocyte were selected by puromycin (1 μg/ml).
2.6. Oil red O staining
Cultured cells were washed with PBS and fixed with 4% formaldehyde for 15 min at room temperature. Then the cells were stained using the Oil Red O working solutions containing 6 ml Oil Red O stock solution (5 g/l in isopropanol) and 4 ml ddH2O for 20 min. After staining, the cells were washed with 60% isopropanol in PBS and pictured based on the red fluorescence of Oil red O using FluorChem R System (Protein Simple) for whole wells, or using a Leica DM 6000B fluorescent microscope with the 10× objective (NA 0.70) for higher magnification views. Oil red O dye were extracted from stained adipocytes with 100% isopropanol, and the Oil red signal were quantified by measuring the optical density at 490 nm (OD 490).
2.7. Immunostaining, mito-tracker and Bodipy staining
For immunostaining, the cultured cells were fixed with 4% paraformaldehyde (PFA) for 10 min, subsequent quenching samples in 100 mM glycine for 5 min. The cells were blocked with blocking buffer containing 5% goat serum, 2% BSA, 0.2% triton X-100 and 0.1% sodium azide in PBS for 1 h. Then the samples were incubated with Flag (Sigma, F2555, 1:1000) primary antibodies diluted in blocking buffer overnight. After washing with PBS, the samples were incubated with secondary antibodies and DAPI for 45 min at room temperature. For mito-tracker and Bodipy staining, the differentiated adipocytes were removed the DM and incubated by Mitotracker® Green FM solution (final concentration: 200 nM) or Bodipy (final concentration: 2 μM) diluted in DM for 45 min. Then cells were washed with PBS for 3 times, added fresh DM and took pictures. Fluorescent images were captured as single channel grayscale images using a Leica DM 6000B fluorescent microscope with a 20× objective (NA 0.70). Images for control and treatment samples were captured using identical parameters and both groups’ images were adjusted identically in Photoshop.
2.8. Cytoplasmic and nuclear RNA extraction
For the extraction of cytoplasmic and nuclear RNA fraction, cultured brown adipocytes were collected after 4-day differentiation in DM. Cells were washed with PBS, suspended in lysis buffer (10 mM NaCl, 2 mM MgCl2, 10 mM pH 7.8 Tris-HCL, 5 mM DTT, 0.5% Igepal CA 630), and then incubated on ice for 5 min. After centrifuge at 8000 rpm for 5 min, the supernatant was transferred to a new microcentrifuge tube subjected to cytoplasmic RNA extraction, while the pellet was resuspended with lysis buffer and subjected to nuclear RNA extraction. For the RNA extraction, the fractions were first incubated with Proteinase K (10 mg/ml) at 37 °C for 20 min, and then mixed with Trizol. RNA was separated by chloroform and precipitated by ethanol with 3 M sodium acetate (pH 5.2, 1/10 volume). The extracted RNA was dissolved into ddH2O and used for reversed transcribed and real-time PCR analysis.
2.9. Total RNA extraction and real-time PCR
Total RNA was extracted from tissues using Trizol Reagent according to the manufacturer’s instructions. RNA was treated with RNase-free DNase I to remove genomic DNA. The purity and concentration of total RNA were measured by Nanodrop 3000 (Thermo Fisher). Ratios of absorption (260/280 nm) of all samples were between 1.8 and 2.0. Then 3 μg of total RNA were reversed transcribed using random primers and Moloney murine leukemia virus reverse transcriptase. Real-time PCR was carried out with a Roche Lightcycler 480 PCR System using SYBR Green Master Mix and gene-specific primers (Table S1). The 2−ΔΔCT method was used to analyze the relative changes of gene expression normalized against 18S rRNA as internal control.
2.10. Protein extraction and western blot analysis
Total protein was isolated from tissues using RIPA buffer contains 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS. Protein concentrations were measured using BCA Protein Assay Reagent (Thermo scientific). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore Corporation), blocked in 5% fat-free milk for 45 min at RT, then incubated with first antibodies (diluted in 5% milk) overnight at 4 °C. Ucp1 (ab23841, 1: 2000) and mitochondrial oxidative complex cocktail (ab110413, 1: 2000) antibodies are from Abcam. Pgc1α (sc-13067, 1: 1000), CEBPα (sc-61, 1: 1000), aP2 (sc-271529, 1: 1000) and GAPDH (sc-32233, 1: 1000) antibodies are from Santa Cruz biotechnology. The horseradish peroxidase (HRP)-conjugated secondary antibody (anti-rabbit IgG, 111-035-003 or anti-mouse IgG, 115-035-003, Jackson ImmunoResearch) were diluted 1: 10,000. Immunodetection was performed using enhanced chemiluminescence western blotting substrate (Santa Cruz biotechnology) and detected by FluorChem R System (Protein Simple).
2.11. Luciferase assay
HEK293T cells were seeded into 48-well plates 1 day before Lipofectamine 2000-mediated transfection. The pGL3-AK079912 promoter luciferase plasmid (pGL3-P) was generated. For transfection of each well, 80 ng Renilla plasmid, 250 ng pGL3-P (or pGL3-basic plasmid as control) and 500 ng pcDNA-Pparγ plasmid were co-transfected following the manufacturer’s instructions. After transfected for 24 h, cells were treated with rosiglitazone and were harvested 24 h after rosiglitazone stimulation and analyzed with the Dual-Luciferase Reporter Assay System (Promega).
2.12. Mitochondrial content measurement
The differentiated adipocytes were collected by cell scraper and total DNA was then isolated by using a standard phenol-chloroform protocol. Quantitative real-time PCR was used to determine relative mitochondrial copy number by calculating the ratio of amplification between mitochondrial DNA and nuclear DNA. Primers for mitochondrial DNA and nuclear DNA amplification were previously described [25].
2.13. Northern blot
The northern blot was performed as described [26]. In brief, total RNA extracted from mouse tissues, including muscle, brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), epididymal white adipose tissue (eWAT) and spleen at 8 weeks of age were separated by PAGE (7M urea) on 6% polyacrylamide gels and transferred to a nylon membrane (N+; Amersham). AK079912 probes were labeled with α-32p-cytidine triphosphate (CTP) using DNA polymerase 1 Large (Klenow) Fragment (Promega, U1100). RNA blots were hybridized in ULTRAhyb (Ambion) at 68 °C overnight, washed twice (5min) with 2 × saline sodium citrate (SSC)/0.1% SDS wash buffer at 68 °C, followed by stringent washes (2 × 30min) with 0.1 × SSC/0.1% SDS wash buffer at 68 °C. RNA blots were then exposed to X-ray film at −80 °C.
2.14. Data analysis
The data are presented as means ± SEM from independent experiments or means ± SD from multiple biological replications in one experiment. Comparisons were made by two-tailed Student’s t-tests. Effects were considered significant at P < 0.05.
3. Results
3.1. Identification of AK079912 as a brown adipocyte enriched long noncoding RNA
We performed in silico analysis of several published lncRNA sequence datasets based on adipose tissues [27,28], and identify AK079912 as a brown adipocyte enriched LncRNA. This LncRNA was predicted to be associated with fat cell differentiation and electron transport chain activity [27]. To explore the function of AK079912, we firstly examined the expression patterns of AK079912 in different tissues from 2-month old mice, and during brown adipocyte adipogenesis and cold stimulation. Northern blot or qPCR showed that AK079912 was highly expressed in mitochondria enriched tissues, including BAT and heart (Fig. S1A–B). Among different adipose tissues, the relative RNA levels of AK079912 in BAT and inguinal WAT (iWAT) were about 100-fold and 10-folds, respectively, of that in epidydimal WAT (eWAT) by qPCR assay (Fig. 1A). Interestingly, AK079912 was originally identified in male aorta and vein [29], which are surrounded by a sheath of BAT-like adipose tissue [30–32]. Consistently, aorta and vein expressed moderate levels of AK079912 and Ucp1 (Fig. S1B–C). This observation suggests that AK079912 is enriched in brown fat compared to white fat, consistent with previous RNA-seq studies. Recent research reported that the thermogenic function of BAT in old mice is lower than that of young mice [33,34]. Notably, we found the expression of AK079912 in BAT was significantly decreased as mice age (3, 8 and 32-week old) (Fig. 1B). Moreover, AK079912 expression was dramatically induced during in vitro adipogenic differentiation of stromal vascular fraction (SVF) cells isolated from BAT (Fig. 1C). The high expression level in differentiated brown adipocytes was confirmed by comparing the expression level of AK079912 in floating mature adipocyte and SVF cells after enzymatic digestion of freshly isolated BAT (Fig. 1D). To test whether AK079912 is involved in the thermogenic regulation, we investigated the changes of AK079912 expression upon cold stimulation. As positive controls, the expression of Blnc1 and Ucp1 was upregulated by cold stimulation (Fig. 1E). Cold stimulation also upregulated the expression of AK079912 in both BAT and iWAT, relative to the expression levels at room temperature (RT) (Fig. 1E). Thus, expression analyses establish that AK079912 is a brown adipocyte enriched LncRNA.
Fig. 1.
Identification of AK079912 as a brown adipocyte specific long non-coding RNA involved in cold-induced browning. (A–D) QPCR analyses of AK079912 expression in adipose tissues (A, n = 4), BAT from different ages mice (B, n = 4), differentiated brown adipocytes (C, n = 4), isolated svf and mature adipocyte (D, n = 4). (E) QPCR analyses of AK079912 and Ucp1 expression in BAT and WAT from mice exposed to 4 °C (cold) or room temperature (RT) for a week. (F) QPCR analyses of AK079912 expression in cytoplasmic and nuclear fractions extracted from brown adipocyte (n = 4). (G) The coding probability of AK079912, and other related proteins and LncRNAs, based on CAPT analysis. (H) Schematics of AK079912 gene structure and predicted open reading frame (ORF, blue box), and strategies for tagging potential ORF with Flag in the pcDNA 3.1-Flag plasmid (Hey1 cDNA is tagged as positive control). (I) HEK293 cells were transfected with plasmids as shown in H and expression of transfected plasmids were detected with an antibody recognizing Flag, which only revealed signal in the Hey1 transfected positive control. Error bar: Mean ± SD. Scale bar: 100 μm. *P < 0.05, ***P < 0.001.
LncRNAs are targeted to specific subcellular locations to carry out their biological functions [35]. QPCR analyses of fractionated nuclear and cytoplasmic RNA from brown adipocytes revealed that AK079912 was primarily localized in the nuclear fraction (Fig. 1F). As control, U6 and LncRNA-Blnc1 are also restricted in the nuclear fractions (Fig. 1F), as previously reported [19,20]. Next, we calculated the protein coding potential of AK079912 using the Coding Potential Assessment Tool (CPAT) [36]. This analysis showed that AK079912 had a very low probability score (Fig. 1G), which is similar to other proven LncRNAs (LncBate1 and Blnc1), but in contrast to the high probability score of protein coding genes (Prdm16, Pgc1α and Ucp1) (Fig. 1G). However, ORF finder analysis (https://www.ncbi.nlm.nih.gov/orfinder/) predicts that AK079912 contains a protein-coding open reading frame (ORF) (Fig. 1H). To determine if the predicted ORF of AK079912 is translated to a small peptide, we cloned the predicted ORF fused with a Flag tag into an expression plasmid and transfected it into HEK293 cells, together with a positive control plasmid containing cDNA for Hey1 gene. Immunostaining using anti-Flag antibody failed to detect any signal in cells transfected with the Flag tagged AK079912 plasmid, while Flag signal was readily detected in cells transfected with the Hey1 plasmid (Fig. 1I). Collectively, these studies suggest that AK079912 is a bona fide non-coding RNA localized to the nucleus of brown adipocytes.
3.2. Knockdown of AK079912 inhibits the differentiation of brown adipocytes
To investigate the potential role of AK079912 in brown adipocyte adipogenesis, we first performed loss-of-function analysis. We designed two independent lentiviral shRNA plasmids to knock down AK079912 in brown adipocyte cell line. The shRNA1 failed to induce knockdown of AK079912 in brown adipocytes (Fig. 2A). However, infection with the shRNA2 lentivirus led to ~70% reduction in the level of AK079912, compared to cells treated with scrambled control shRNA (Fig. 2A). Thus, shRNA2 lentivirus was used to establish a stable AK079912 knockdown brown adipocyte cell line, which was used in following experiments. Compared to the control scrambled shRNA treatment, AK079912 knockdown robustly inhibited the lipid accumulation in differentiated brown adipocytes, revealed by Oil Red O staining (Fig. 2B). To determine if the reduced lipid content is due to higher lipolysis activity or a failure to differentiate into lipid accumulating adipocytes, we examined the expression of various adipogenic, lipogenic and lipolysis genes. The mRNA levels of BAT-specific genes, including Ucp1, Prdm16 and Pgc1a, were all significantly down-regulated in AK079912 knockdown cells compared to the control cells (Fig. 2C). Moreover, knockdown of AK079912 suppressed brown adipocyte differentiation indicated by the reduced mRNA levels of Cebpa and Adipoq (Fig. 2D). Lipolysis-related genes (lipoprotein lipase, Lpl; hormone-sensitive lipase, Hsl; adipose triglyceride lipase, Atgl) were also down-regulated by AK079912 knockdown (Fig. 2E). Consistent with changes in mRNA levels, protein levels of Pgc1α, Ucp1, Cebpα and Fabp4 were all reduced in AK079912 knockdown cells (Fig. 2F).
Fig. 2.
Knockdown of AK079912 inhibits the brown adipocytes differentiation and expression of BAT-specific genes. (A) The knockdown efficiency of two shRNA plasmids targeting two different regions of AK079912 (n = 4). (B) Oil Red O labeling of total lipids in differentiated brown adipocytes after treatment with control and shRNA2 plasmids. (C–E) The relative mRNA levels of BAT-specific (C), adipogenic (D) and lipolysis (E) related genes in control and shRNA2 treated brown adipocytes (n = 6, 3 biological replicates for each independent experiment). (F) Western blot image showing protein levels of Pgc1α, Cebpα, aP2, Ucp1 in control and shRNA2 treated brown fat cells (n = 3). Error bar: Mean ± SEM, *P < 0.05, **P < 0.01. Scale bar: 100 μm.
Given that the knockdown phenotypes of AK079912 were based on one shRNA, we further validated the results using transient knockdown of AK079912 by transfecting a commercial smart silencer containing three siRNAs and three ASOs in a separate BAT cell line that was generated by our group previously [37]. The silencer-mediated transient knockdown led to ~50% reduction in the level of BA-LncR (Fig. S2A). Bodipy staining of lipids indicated that AK079912 knockdown by silencer also reduced brown adipocyte lipid accumulation (Fig. S2B), and suppressed expression of key adipogenic and brown specific genes (Fig. S2C–E). Altogether, these data demonstrate that inhibition of AK079912 suppresses brown adipocyte differentiation and expression of thermogenic genes.
3.3. Overexpression of AK079912 upregulates the expression of thermogenic genes in brown and white adipocytes
We next performed gain-of-function analysis using adenovirus-mediated overexpression of AK079912 in cultured SVF cells isolated from BAT. Overexpression (OE) led to 1000 times increase of AK079912 level, which did not affect lipid contents in brown adipocytes differentiated from SVF cells, assessed by Oil Red O staining signal (Fig. S3A–C). However, qPCR analyses showed that overexpression of AK079912 moderately up-regulated the levels of BAT-specific genes, including Ucp1, Pgc1a and Type II iodothyronine deiodinase (Dio2) (Fig. S3D), but did not affect the expression of lipolysis-related genes (Fig. S3E). In parallel with the changes in gene expression, AK079912-OE up-regulated the protein levels of Ucp1, but not pan-adipogenic markers aP2 and Cebpα (Fig. S3F). These results suggest that AK079912-OE does not affect adipogenic differentiation but moderately up-regulates the expression of thermogenic related genes in brown adipocytes.
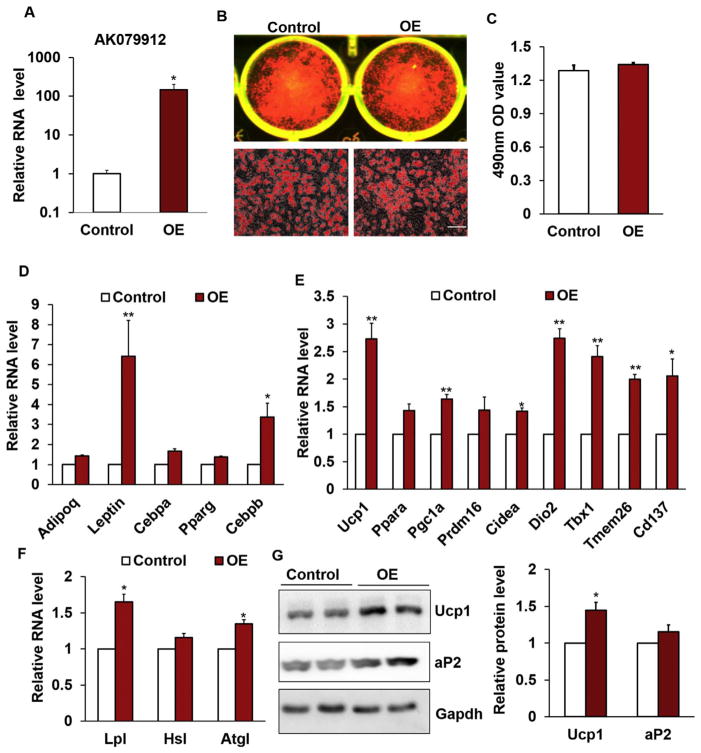
The relative small response of brown adipocytes to AK079912 over-expression can be explained by the high endogenous levels of AK079912 in brown adipocytes. As AK079912 is expressed at low levels in white adipocytes and dramatically induced in iWAT by cold-stimulus (Fig. 1A), we hypothesized that AK079912 is essential to regulate the browning of WAT. To test this hypothesis, we ectopically expressed AK079912 in primary SVF cells isolated from iWAT, using the same adenovirus-mediated overexpression strategy. Overexpression of AK079912 had no obvious effect on lipid accumulation in adipocytes differentiated from SVF cells of iWAT, based on Oil Red O staining and its quantification (Fig. 3A–C). Consistent with this observation, the mRNA levels of adipogenic genes, Cebpa and Pparg, in AK079912-OE treatment was comparable to those of control, except for leptin and Cebpb (Fig. 3D). Strikingly, the expression level of thermogenic and beige related genes including Ucp1, Pgc1a, Cidea, Dio2, Tbx1, Tmem26 and Cd137 were all significantly upregulated by AK079912 over-expression (Fig. 3E). Moreover, overexpressed AK079912 also upregulated the expression of Lpl and Atgl significantly (Fig. 3F). Consistently, the protein level of Ucp1 was upregulated by AK079912-OE treatment (Fig. 3G). These data indicate that ectopic expression of AK079912 in inguinal white adipocytes induces their browning in vitro.
Fig. 3.
Overexpression of AK079912 induces the browning of white adipocytes. (A) Relative levels of AK079912 in iWAT-derived stromal vascular fraction (svf) cells after infected with control and AK079912 overexpression (OE) adenovirus, n = 6 (3 biological replicates for each independent experiment). (B–C) Oil Red O staining (B) and lipid content (C) of adipocytes differentiated from iWAT-derived svf cells infected with control and OE adenovirus. (D–F) Relative mRNA levels of adipogenic (D), browning (E) and lipolysis (F) related genes in control and OE white adipocytes (n = 6, 3 biological replicates for each independent experiment). (G) Protein levels of aP2 and Ucp1 in control and OE cells (n = 4, 2 biological replicates for each independent experiment). Scale bar: 100 μm, Error bar: Mean ± SEM, *P < 0.05, **P < 0.01.
To assess the roles of AK079912 in browning of WAT in vivo, we performed AK079912 overexpression in WAT cultured ex vivo and local adenovirus injection in vivo. For ex vivo culture, fresh iWAT was cut into 1–3 mm3 cubes and cultured in a medium containing AK079912-expressing adenovirus and control adenovirus (both control and AK079912-OE adenovirus contain GFP) for 72 h, which leads to robust infection of the adipose explants based on the strong GFP signal (Fig. 4A). QPCR analysis showed that AK079912-OE significantly up-regulated mRNA level of Ucp1 and Pgc1a (Fig. 4B–C). To further confirm the browning function of AK079912 in vivo, we injected AK079912-expressing and control adenovirus into the left and right side of the iWAT in the same mouse, respectively. Two weeks after injection, the injected iWAT were collected and GFP signal was readily detectable in both control and AK079912-OE treated iWAT (Fig. 4D), indicating efficient viral infection. This was further confirmed by > 10-fold increase in the level of AK079912 in the AK079912-OE treated WAT relative to the control treated iWAT (Fig. 4E). Remarkably, AK079912-OE significantly upregulated the mRNA level of Ucp1 and Pgc1a (Fig. 4F). These results demonstrate that ectopic expression of AK079912 was sufficient to induce browning of WAT in vivo.
Fig. 4.
AK079912 promotes browning of inguinal WAT. (A) GFP fluorescence of iWAT depots 3 days after infected with control and AK079912 overexpression (OE) adenovirus ex vivo. (B–C) Relative levels of AK079912 (B) and mRNA levels of Ucp1 and Pgc1a (C) in iWAT after infection with control or AK079912-OE adenovirus (n = 4). (D) GFP fluorescence of iWAT depots 14 days after injected with control and AK079912-OE adenovirus in vivo. (E–F) Relative levels of AK079912 (E) and mRNA levels of Ucp1 and Pgc1a (F) in iWAT after injected with control or AK079912-oe adenovirus in vivo (n = 4). Error bar: Mean ± SD, *P < 0.05, **P < 0.01. Scale bar: 100.
3.4. AK079912 regulates brown adipocyte mitochondrial biogenesis
The observations from AK079912 loss-of-function and gain-of-function studies suggest that AK079912 plays an important role in thermogenic genes regulation of BAT. The thermogenic capacity of BAT is extremely dependent on mitochondria, where Ucp1 is expressed and heat was generated by uncoupled respiration [38]. Pgc1α is a classical transcriptional factor underlying mitochondria biogenesis [39], and our data reveal that AK079912 affects the expression of Pgc1α at both mRNA and protein levels. Thus, we hypothesize that AK079912 regulates brown/beige adipocyte thermogenesis through stimulating mitochondria biogenesis. To test this hypothesis, we first examined mitochondria content and found that the relative mitochondrial DNA copy number was significantly decreased by 90% in brown adipocytes differentiated from the AK079912 knockdown (Fig. 5A). Consistently, mitochondrial abundance detected by mito-tracker staining was extremely low in brown adipocytes differentiated from the AK079912 knockdown cell line (Fig. 5B). Moreover, AK079912 knockdown downregulated mitochondrial related genes, including Cox5b, Cox7a and Cox7c (Fig. 5C). Mitochondrial protein complexes (I–V) of the electron transport chain (ETC) mediate oxidative phosphorylation and lipid metabolism [25]. Strikingly, western blot analysis showed that the protein levels of mitochondrial complexes were reduced by AK079912 knockdown treatment (except for complex IV), relative to control treatment (Fig. 5D). In contrast, overexpression of AK079912 in brown adipocytes upregulated mRNA level of Cox5b and Cox8b significantly (Fig. 5E), and elevated the protein levels of complex V and complex III (Fig. 5F). Collectively, these findings reveal a vital role of AK079912 in the regulation of mitochondrial biogenesis in brown adipocytes.
Fig. 5.
AK079912 regulates mitochondria biogenesis of brown adipocyte. (A–B) The mitochondria DNA (mtDNA) content and mitochondrial staining (mito-tracker) of control and AK079912 knockdown brown adipocytes (n = 6, 3 biological replicates for each independent experiment). (C–D) The mRNA level of mitochondrial related genes (C) and protein level of ETC (electron transport chain) complexes (D, ATP5A, ATP synthase, H+ transporting, mitochondrial F1 complex, alpha 1; UQCRC2, ubiquinol-cytochrome c reductase core protein II; MTCO1, cytochrome c oxidase I; SDHB, succinate dehydrogenase complex iron sulfur subunit B; NDUFB8, ubiquinone oxidoreductase subunit B8) in control and AK079912 knockdown brown adipocytes (n = 4, 2 biological replicates for each independent experiment). (E–F) The mRNA and protein levels of mitochondrial related genes in control and AK079912 overexpression (OE) brown adipocyte (n = 4, 2 biological replicates for each independent experiment). Mean ± SEM, *P < 0.05, **P < 0.01. Scale bar: 100.
3.5. Pparγ is a transcriptional regulator of AK079912 expression in brown adipocytes
As AK079912 was required for the differentiation and thermogenic gene expression of brown adipocytes, we next sought to identify the potential upstream regulator of AK079912. To achieve this, we first analyzed the transcription factor binding sites that existed in the promoter of AK079912. This analysis led to the identification of three conserved Pparγ binding sites in the promoter region of AK079912 (Fig. 6A). To test whether Pparγ transcriptionally regulate the expression of AK079912, brown adipocytes were treated by 0, 1, 5 and 10 μM of rosiglitazone (Rosi), an agonist of Pparγ. Rosi treatment upregulated the expression level of AK079912 in a dosage dependent manner (Fig. 6B). Moreover, we subcloned the promoter region of AK079912 into the Pgl3 plasmid (Pgl3-P) and performed dual luciferase assay to valid the direct transcriptional regulation of AK079912 promoter by Pparγ. The relative luciferase activity of cells co-transfected with Pparγ and Pgl3-P was about 6-fold higher than cells co-transfected with Pparγ and Pgl3-basic plasmids (Fig. 6C). More convincingly, combination with Rosi treatment further dose-dependently augmented the relative luciferase activity in cells co-transfected with Pparγ and Pgl3-P plasmids (Fig. 6C). These results provide compelling evidence that Pparγ is an upstream transcriptional regulator of AK079912.
Fig. 6.
Pparγ is a transcriptional regulator of AK079912. (A) Schematic of Pgl3-AK079912 luciferase reporter driven by a 2 kb-genomic sequence upstream of AK079912 containing three consensus Pparγ binding sites. (B) Rosiglitazone (Rosi) enhances the expression of AK079912 in brown adipocytes, n = 4, error bar: Mean ± SD. (C) Luciferase assay of co-transfected with Pparγ and Pgl3-AK079912 promoter (Pgl3-P) in 293 T cells, treated with rosiglitazone (n = 6, 3 biological replicates for each independent experiment), error bar: Mean ± SEM. (D) Schematic summary illustrating AK079912 regulates brown adipocyte differentiation and thermogenic gene program. **P < 0.01, Rosi treatment compared with control; ##P < 0.01, high Rosi concentration treatment compared with that of low concentration group.
4. Discussion
Understanding regulators that selectively enhance brown adipogenesis or induce browning of white adipocyte may lead to identification of new targets to treat obesity and its affiliated metabolic disorders. Here, we report a novel LncRNA (AK079912) that is highly expressed in brown fat and functions to regulate BAT adipogenesis. Knockdown of AK079912 inhibits brown adipocyte differentiation and suppressed expression of BAT specific genes. Conversely, overexpression of AK079912 promotes expression of thermogenic genes. Interestingly, ectopically expressing AK079912 induces the browning of WAT in vivo and vitro. Mechanically, AK079912 mediates the effect of Pparγ to regulate thermogenic function of adipocyte through stimulating mitochondria biogenesis (Fig. 6D). Our research expands the growing knowledge of LncRNA regulatory network in BAT.
Many LncRNAs are expressed in a tissue- and development-specific manner and have been shown to directly regulate a large variety of targets [40]. We characterize AK079912 as a BAT enriched LncRNA. The expression of AK079912 in BAT is dramatically higher than WAT and is highly induced during brown adipocyte differentiation. In addition, cold stimulation upregulates the level of AK079912 in BAT and iWAT, suggesting a role of AK079912 in browning. The cold inducible expression pattern of AK079912 is similar to Blnc1, and correlated with the stimulation of the thermogenic gene program in the adipose tissues [19,20]. Recently, increasing evidence uncovered that some LncRNAs also contain short ORF coding for functional polypeptides [41]. Specifically, in mammalian skeletal muscle, LncRNA-encoded micropeptides, myoregulin and small regulatory polypeptide of amino acid response (SPAR), regulate Ca2+ handling and muscle regeneration, respectively [42,43]. Our in vivo translation assay excludes the protein-coding possibility of AK079912.
Our data shows that AK079912 is necessary for brown adipocyte differentiation and expression of thermogenic genes. Several lines of evidence support this notion. Firstly, loss function of AK079912 mediated by shRNA knockdown reduces lipid accumulation, accompanied by reduction of adipogenic related genes expression (Cebpα and aP2). Moreover, knockdown of AK079912 impairs the mRNA and protein levels of key thermogenic markers (Ucp1 and Pgc1a). Consistently, AK079912 knockdown decreases the level of lipases (Lpl, Hsl and Atgl) that are required for releasing free fatty acids (FFA) from triacylglycerol to serve as fuel for thermogenesis and activators of Ucp1 [44]. Importantly, these phenotypes are confirmed by siRNAs mediated knockdown of AK079912 in brown adipocytes. The shRNA and siRNA results together indicate that loss of function is due to knockdown of AK079912 but not due to off-target silencing of other genes. Finally, gain-of-function of AK079912 in brown adipocytes increases expression of Ucp1 and Pgc1α, though it does not affect differentiation of AK079912 overexpressing brown adipocytes.
Cold-induced browning of white fat depots is associated with formation of Ucp1+ adipocytes that are thermogenic and share many components of the classical brown adipocyte gene program [45,46]. AK079912 is not only induced in iWAT by cold stimulation, but its overexpression in white adipocytes also promotes Ucp1 and other BAT-specific genes in vitro and in vivo. These results suggest a broad role for AK079912 in general thermogenic regulation. Moreover, AK079912 gain-of-function in cultured white adipocyte upregulates beige fat precursor markers, including Tbx1, Tmem26 and Cd137. This observation indicates that AK079912 induces browning of WAT partly through regulating beige adipocyte precursor cells.
In our study, infection of AK079912 adenovirus in iWAT in vivo results in 14-fold increase in AK079912 level. However, the adenovirus-mediated transduction of cultured iWAT explants results in ~1000 folds increase in AK079912 level. This high level of AK079912 may be due to contamination of adenovirus trapped in the explants, which do not have active immune system to cope with virus. Importantly, only 14-fold increase of AK079912 level in iWAT in vivo was sufficient to bring about changes in brown fat gene expression, and 150 folds increase in the level of AK079912 in cultured iWAT adipocytes results in robust changes toward brown adipocyte phenotype. Considering that the endogenous level of AK079912 is almost 100-fold higher in BAT than in WAT, the relative level of overexpression in our study is largely in the physiological range.
Mitochondria are key cellular organelles in brown adipocyte, where fatty acid oxidation and thermogenesis [47]. Inhibition of AK079912 reduces mitochondria copy number, accompanied by reduced levels of mitochondrial related proteins, whereas AK079912 overexpression has the opposite effects. These data suggest that AK079912 participates in mitochondria biogenesis. We speculate that AK079912 regulates mitochondria number through affecting the expression of Pgc1α. In supports of this hypothesis, RNAi of AK079912 decreases both mRNA and protein level of Pgc1α, in contrast, its gain-of-function increases expression of Pgc1α. Importantly, it was previously reported that genetic ablation of Pgc1α results in greatly reduced mitochondria content and capacity for cold-induced adaptive thermogenesis [48]. Additional studies have further confirmed that mitochondrial biogenesis and respiration are highly dependent on Pgc1α [12,49,50]. As mitochondria biogenesis is coupled with adipocyte differentiation [51,52], the observed reduction in mitochondria copy number in AK079912 knockdown brown adipocytes may also be partially due to defective adipogenic differentiation.
The functions of lncRNAs are largely dependent on their subcellular localization. We find that AK079912 is primarily located in the nucleus by fraction assay. In general, nuclear localized lncRNAs could carry out their regulatory roles through either in cis, close to the site of transcription, or in trans regulation [35]. During this process, LncRNAs are retained in the nucleus through the formation of functional LncRNA ribonucleoprotein (LncRNP) complexes, including chromatin-modifying complexes or transcriptional complexes [35]. For example, Lnc-Bate1 interacts with hnRNP U to form a functional ribonucleoprotein complex that acts in trans to modulate brown adipogenesis [18]. Another nuclear lncRNA-Blnc1 interacts with transcription factor Ebf2 to form a feed-forward regulatory loop to drive adipogenesis toward a thermogenic phenotype [19]. Therefore, exploring the binding partners of AK079912 in the nucleus will be key to understand how AK079912 functions at the molecular level in future studies.
Our luciferase assay combined with rosiglitazone treatment provides strong evidence to suggest that Pparγ transcriptionally activates expression of AK079912. Pparγ is a master regulator of both WAT and BAT development [53], but intriguingly the expression level of AK079912 in WAT is much lower than in BAT. Consistently, it was reported that multiple BAT-selective lncRNAs are targeted by common adipogenic transcriptional factors including Cebpα, Cebpβ and Pparγ, often in a BAT-specific manner [18,28,54]. Pparγ cooperates with specific coregulators to selectively induce brown adipocyte adipogenesis and thermogenic program [53], we therefore speculate that different coregulators of Pparγ are involved in selective activation expression of AK079912 in brown fat, but not white fat.
In conclusion, we report that AK079912 is a BAT enriched LncRNA that is required for brown adipocyte differentiation, mitochondria biogenesis, and expression of thermogenic genes. In addition, ectopic expression of AK079912 induces browning of white adipocytes. Finally, we identify Pparγ as an upstream regulator of AK079912. These results not only expand the understanding of LncRNAs as novel epigenetic regulators of brown adipocytes and their thermogenic function, but further suggest that AK079912 may represent a new target in obesity treatment.
Supplementary Material
Acknowledgments
We thank Dr. Yongxu Wang (University of Massachusetts Medical School) and Ziyi Song for providing BAT cell lines. This work was supported by grants from the National Science and Technology Major Project of China (2016ZX08006003), US National Institutes of Health (R01 CA212609), National Natural Science Foundation of China (81471070) and CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1-012). Y.X. was supported by a pre-doctoral fellowship from the Chinese Scholarship Council (20140630014).
Abbreviations
- BAT
brown adipose tissue
- WAT
white adipose tissue
- ETC
electron transport chain
- Ucp1
uncoupling protein 1
- Cebpα/β/δ
CCAAT/enhancer-binding proteins α/β/δ
- Pparγ
peroxisome proliferator-activated receptor gamma
- Pgc1α
Pparγ coactivator 1α
- Prdm16
PR domain containing 16
- miRNAs
microRNAs
- UTR
untranslated region
- Rbp
RNA-binding protein
- Blnc1
brown fat lncRNA 1
- Ebf2
early B-cell factor 2
- lnc-BATE1
BAT-enriched LncRNA1
- hnRNPU
nuclear ribonucleoprotein U
- SVF
stromal vascular fraction
- Lpl
lipoprotein lipase
- Hsl
hormone-sensitive lipase
- Atgl
adipose triglyceride lipase
- Dio2
Type II iodothyronine deiodinase
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbalip.2018.01.008.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Transparency document
The Transparency document associated with this article can be found, in the online version.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y C American Heart Association Statistics, S. Stroke Statistics. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen P, Spiegelman BM. Cell biology of fat storage. Mol Biol Cell. 2016;27:2523–2527. doi: 10.1091/mbc.E15-10-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 7.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;17:480–495. doi: 10.1038/nrm.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, Puigserver P, Spiegelman BM. Transcriptional activation of adipogenesis. Curr Opin Cell Biol. 1999;11:689–694. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 12.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 13.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz TJ, Tseng YH. Brown adipose tissue: development, metabolism and beyond. Biochem J. 2013;453:167–178. doi: 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S, Chen P, Sun L. Regulatory networks of non-coding RNAs in brown/beige adipogenesis. Biosci Rep. 2015;35 doi: 10.1042/BSR20150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonasio R, Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu Rev Genet. 2014;48:433–455. doi: 10.1146/annurev-genet-120213-092323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez-Dominguez JR, Bai Z, Xu D, Yuan B, Lo KA, Yoon MJ, Lim YC, Knoll M, Slavov N, Chen S, Chen P, Lodish HF, Sun L. De novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of brown adipocyte development. Cell Metab. 2015;21:764–776. doi: 10.1016/j.cmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao XY, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Mol Cell. 2014;55:372–382. doi: 10.1016/j.molcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi L, Zhao XY, Li S, Yang G, Lin JD. Conserved function of the long noncoding RNA Blnc1 in brown adipocyte differentiation. Mol Metab. 2017;6:101–110. doi: 10.1016/j.molmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Silvey DT, Johnson BJ, Doumit ME, Chung KY, Sawyer JE, Go GW, Smith SB. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids. 2014;49:15–24. doi: 10.1007/s11745-013-3869-0. [DOI] [PubMed] [Google Scholar]
- 22.Shan T, Xiong Y, Zhang P, Li Z, Jiang Q, Bi P, Yue F, Yang G, Wang Y, Liu X, Kuang S. Lkb1 controls brown adipose tissue growth and thermogenesis by regulating the intracellular localization of CRTC3. Nat Commun. 2016;7:12205. doi: 10.1038/ncomms12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Liu W, Nie Y, Qaher M, Horton HE, Yue F, Asakura A, Kuang S. Loss of MyoD promotes fate transdifferentiation of myoblasts into brown adipocytes. EBioMedicine. 2017;16:212–223. doi: 10.1016/j.ebiom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller C, Ratner D, Zhong L, Esteves-Sena M, Gao G. Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol. 2012;26:14D.1.1–14D.1.21. doi: 10.1002/9780471729259.mc14d01s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie Y, Sato Y, Wang C, Yue F, Kuang S, Gavin TP. Impaired exercise tolerance, mitochondrial biogenesis, and muscle fiber maintenance in miR-133a-deficient mice. FASEB J. 2016;30:3745–3758. doi: 10.1096/fj.201600529R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X, Zhang Y, Li T, Ma Z, Jia H, Chen Q, Zhao Y, Zhai L, Zhong R, Li C, Zou X, Meng J, Chen AK, Puri PL, Chen M, Zhu D. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat Commun. 2017;8:14016. doi: 10.1038/ncomms14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Li P, Yang W, Ruan X, Kiesewetter K, Zhu J, Cao H. Integrative transcriptome analyses of metabolic responses in mice define pivotal LncRNA metabolic regulators. Cell Metab. 2016;24:627–639. doi: 10.1016/j.cmet.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Goff LA, Trapnell C, Alexander R, Lo KA, Hacisuleyman E, Sauvageau M, Tazon-Vega B, Kelley DR, Hendrickson DG, Yuan B, Kellis M, Lodish HF, Rinn JL. Long noncoding RNAs regulate adipogenesis. Proc Natl Acad Sci U S A. 2013;110:3387–3392. doi: 10.1073/pnas.1222643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y F Consortium, R.G.E.R.G.P. I, I.I. Team. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown NK, Zhou Z, Zhang JF, Zeng R, Wu JR, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald RB, Horwitz BA, Hamilton JS, Stern JS. Cold- and norepinephrine-induced thermogenesis in younger and older Fischer 344 rats. Am J Phys. 1988;254:R457–462. doi: 10.1152/ajpregu.1988.254.3.R457. [DOI] [PubMed] [Google Scholar]
- 35.Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Park HJ, Dasari S, Wang S, Kocher JP, Li W. CPAT: coding-potential assessment tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013;41:e74. doi: 10.1093/nar/gkt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Z, Xiaoli AM, Zhang Q, Zhang Y, Yang EST, Wang S, Chang R, Zhang ZD, Yang G, Strich R, Pessin JE, Yang F. Cyclin C regulates adipogenesis by stimulating transcriptional activity of CCAAT/enhancer-binding protein alpha. J Biol Chem. 2017;292:8918–8932. doi: 10.1074/jbc.M117.776229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butow RA, Bahassi EM. Adaptive thermogenesis: orchestrating mitochondrial biogenesis. Curr Biol. 1999;9:R767–769. doi: 10.1016/S0960-9822(00)80008-1. [DOI] [PubMed] [Google Scholar]
- 39.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 41.Rion N, Ruegg MA. LncRNA-encoded peptides: more than translational noise? Cell Res. 2017;27:604–605. doi: 10.1038/cr.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel-Duby R, Olson EN. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto A, Pasut A, Matsumoto M, Yamashita R, Fung J, Monteleone E, Saghatelian A, Nakayama KI, Clohessy JG, Pandolfi PP. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541:228–232. doi: 10.1038/nature21034. [DOI] [PubMed] [Google Scholar]
- 44.Medina-Gomez G. Mitochondria and endocrine function of adipose tissue. Best Pract Res Clin Endocrinol Metab. 2012;26:791–804. doi: 10.1016/j.beem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 48.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 50.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 51.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koppen A, Kalkhoven E. Brown vs white adipocytes: the PPARgamma coregulator story. FEBS Lett. 2010;584:3250–3259. doi: 10.1016/j.febslet.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 54.Wei S, Du M, Jiang Z, Hausman GJ, Zhang L, Dodson MV. Long noncoding RNAs in regulating adipogenesis: new RNAs shed lights on obesity. Cell Mol Life Sci. 2016;73:2079–2087. doi: 10.1007/s00018-016-2169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.