Abstract
Objective
We hypothesized that ILI is associated with risk of incident stroke, and that the risk would be highest closest in time to the event.
Methods
This case‐crossover analysis utilized data obtained from the California State Inpatient Database of the Healthcare Cost and Utilization Project (HCUP). The outcome of interest was ischemic stroke. Exposure was defined as a visit to the emergency department or hospitalization for influenza‐like illness (ILI) 365, 180, 90, 30, or 15 days before stroke (risk period) or similar time intervals exactly 1 or 2 years before stroke (control period). Conditional logistic regression was used to calculate the odds ratio and 95% confidence interval (OR, 95% CI).
Results
In 2009, 36,975 hospitalized ischemic strokes met inclusion criteria, and of these strokes, 554 (1.5%) had at least 1 episode of ILI in the 365‐day risk period prior to their stroke. Using non‐overlapping time intervals from ILI to stroke, the odds of ischemic stroke was greatest in the first 15 days post ILI (OR: 2.88, 95% CI: 1.86–4.47). The strength of the relationship decreased as the time from ILI increased, and was no longer significant after 60 days. There was a significant interaction (P = 0.017) with age and ILI; the odds of stroke associated increased 7% with each 10‐year decrease in age (OR per 10‐year age decrease 1.07, 95% CI: 1.03–1.35).
Interpretation
We found that ILI increases short‐term risk of stroke, particularly in people under the age of 45, and therefore may be considered to act as a trigger for stroke.
Introduction
Stroke is the fifth leading cause of death in the US and the number one cause of serious long‐term adult disability, with nearly 800,000 strokes in the US each year.1, 2, 3 Conventional risk factors such as hypertension, diabetes, sedentary behavior, and smoking account for almost 80% of stroke risk, leaving a substantial proportion of stroke risk unexplained.4 In addition, conventional risk factors are primarily associated with long‐term risk and do not explain why individuals have strokes at one particular point in time rather than at another time. Conventional stroke risk factors, in other words, do not account for the immediate or short‐term risk of stroke. The identification of stroke triggers, therefore, could provide an opportunity to intervene to prevent the short‐term occurrence of stroke.5
Infection has been identified as both a potential chronic risk factor and an acute trigger for stroke. A composite measure of chronic infection assessed by serologies against several common bacterial and viral infections was associated with increased long‐term stroke risk in the Northern Manhattan Study.6 In case‐crossover analyses from both the Cardiovascular Health Study and the Atherosclerosis Risk in Cardiovascular Disease Study, recent hospitalization for infection was associated with increased risk of stroke.7, 8 The association between infection and stroke diminishes over time, however. A population‐based cohort study from Denmark showed that about 80% of cardiovascular events after exposure to bacteremia occurred during the index hospitalization.9 Other work showed that the risk of stroke is the highest in the first 3–15 days after infection.8, 10 Even common infections, such as influenza, increase the short‐term risk of myocardial infarction and cardiovascular death.1, 11 “Influenza‐like illness” (ILI) is a grouping of symptoms and specific diagnoses used by the Centers for Disease Control in surveillance for outbreaks of influenza.12, 13 There is little literature, however, regarding the association between ILI, or influenza itself, and stroke risk. We hypothesized that ILI is associated with risk of incident stroke, and that the risk would be highest closest in time to the event.
Methods
Study design
We utilized data from the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP), California State Inpatient Database (SID), and the California State Emergency Department Database (SEDD), for 2007 to 2009. Under HCUP, claims data from various states for each hospitalization discharge is collected, de‐identified, and standardized to a consistent format of data elements and values for ease of comparison and then made available to researchers. The California SID contains administrative claims data for all hospitalizations in nonfederal acute care California hospitals. Data elements collected include demographic information such as age, sex, race, and insurance payer. For each hospitalization, discharge diagnosis code (up to 25 ICD‐9 codes), month of discharge, length of stay in hospital, and AHRQ comorbidity measures are available. There are designations for primary diagnosis and whether the condition was present on arrival for each ICD‐9 code, allowing identification of pre‐existing diagnoses versus complications that arise during hospitalization. A “visitlink” variable allows researchers to follow a patient over time through multiple hospital admissions.
The study population comprised patients hospitalized for ischemic stroke in any of the non‐federal acute care hospitals in California in the year 2009.14 A case‐crossover design was used to investigate the association between strokes occurring in 2009 and preceding ILI in the years 2007–2009. The case‐crossover design is useful in studying acute events, such as stroke, brought on by exposures that transiently increase the risk for having an event.15 Data on an exposure, such as ILI, from a relatively short risk period preceding the event (“case” period) is compared to one or more “control” time periods for the same individual, and exposures that are present more frequently in the risk period than the control period can be considered to be precipitants. In this study design, cases act as their own controls, and thus the design inherently controls for interindividual variability and confounding.8
Exposure and covariates
The exposure event was defined as admission for ILI 0–15, 0–30, 0–60, 0–90, 0–180, or 0–365 days before stroke (case periods) or time intervals of identical duration exactly 1 or 2 years before stroke (control periods), such that the calendar time of year remained constant across case and control periods.9, 10 ILI was defined using previously published International Classification of Diseases (ICD‐9) diagnostic codes used for identifying patients with ILI in administrative datasets, and present on arrival at any diagnostic position (Table 1).12 These ICD‐9 codes were determined by the Department of Defense for the purposes of identifying ILI cases for surveillance.16 If the present on admission indicator was missing, we treated the diagnosis as in‐hospital. A sensitivity analysis was conducted excluding the people who did not have present on admission indicators, and the results were similar. We used both the SID and the SEDD datasets to identify ILI to capture ILI cases that were hospitalized and ILI cases that only presented to the emergency department. We assessed the risk of stroke within a series of mutually exclusive time intervals (i.e., 0–15 days, 16–30 days, 31–60 days, etc.). The month by month prevalence of ILI was also used to adjust models for the well‐known variability in occurrence of ILI throughout the year.12 As a negative control, we assessed the relationship between lacerations, defined through ICD‐9 codes, and stroke.
Table 1.
ICD‐9 Codes used for Influenza‐Like Illness12
| ICD‐9 code | Illness description | icd‐9 code | Illness description |
|---|---|---|---|
| 079.89 | Viral Infection NEC | 466 | Acute bronchitis and bronchiolitis |
| 079.99 | Viral Infection NOS | 466.0 | Acute bronchitis |
| 460 | Acute Nasopharyngitis | 466.1 | Acute bronchiolitis |
| 462 | Acute pharyngitis | 466.19 | Acute bronchiolitis due to other infectious organism |
| 464 | Acute Laryngitis and tracheitis | 478.9 | Other and unspecified diseases of upper resp tract |
| 464.0 | Acute Laryngitis | 480 | Viral pneumonia |
| 464.1 | Acute tracheitis | 487 | Influenza |
| 464.10 | Acute tracheitis w/o obstruction | 487.0 | Influenza with pneumonia |
| 464.2 | Acute Laryngotracheitis | 487.1 | Influenza with other respiratory manifestation |
| 464.20 | Acute Laryngotracheitis w/o obstruction | 487.8 | Influenza with other manifestation |
| 465 | Upper respiratory infection multiple or unspecified sites | 490 | Bronchitis not specified as acute or chronic |
| 465.0 | Acute laryngopharyngitis | 780.6 | Fever |
| 465.8 | Upper resp infection multiple sites | 784.1 | Throat pain |
| 465.9 | Upper resp infection of unspecified sites | 786.2 | Cough |
Outcomes
Ischemic stroke was defined using previously validated ICD‐9 codes 433.x1 (“x,” the fourth digit, can vary to specify a specific arterial distribution), 434.x1, or 436 present at any diagnostic position between DX1 and DX12. Cases were excluded if any “traumatic brain injury” ICD‐9‐CM code (800–804, 850–854) or “rehabilitation care” ICD‐9‐CM code (V57) was present as the primary diagnosis.17, 18 .
The California SID does not provide separable dates for each ICD‐9 code within the same hospitalization, limiting the assignment of temporal relationships among events within each admission. To ensure all comparisons were between separate admissions for stroke and ILI, all admissions for which any kind of stroke and ILI occurred in the same admission during all 3 years of data were omitted.
Statistical analysis
Conditional logistic regression stratified on the variable “visitlink” was used to compute odds ratios (ORs) and 95% confidence intervals (95% CI) for any hospital admission with stroke within 0–15 days, 0–30 days, 0–60, 0–90, 0–180, and 0–365 days after exposure. We further assessed odds of stroke post ILI at mutually exclusive time intervals after ILI by investigating the odds at 0–15 days, 16–30 days, 31–90 days, 91–180 days, and 181–365 days. Based on prior research suggesting that the association of infections with stroke odds decreases with age,19 interactions between ILI and age were investigated, and stratification by age performed as indicated. Interactions between ILI and diabetes, as well as ILI and number of comorbid conditions, a variable included in HCUP databases containing the total sum of comorbid conditions, were also investigated. Change in ORs after accounting for the ILI and age interaction was assessed in separate models. All hypothesis tests performed during analysis of the primary endpoints are two‐sided and use an alpha of 0.05.
Results
In 2009, a total of 36,975 hospitalized ischemic strokes met inclusion criteria in the California SID. Of these strokes, 554 (1.5%) had at least one episode of ILI in the 365‐day risk period prior to their stroke. The mean number of comorbid conditions was higher in patients who had an ILI prior to their stroke (8.1 ± 3.4) as compared to patients with stroke only (6.2 ± 2.6; Table 2). The frequencies of congestive heart failure, depression, peripheral vascular disorders, and rheumatoid arthritis were higher in patients with ILI prior to their stroke than in patients with stroke only (Table 2). The proportion of ILI (Fig. 1) hospitalizations was calculated for the number of ILI admissions per month among all NY state hospitalizations. The adjusted analyses accounted for ILI prevalence.
Table 2.
Baseline Characteristics of Ischemic Stroke Patients with exposure to ILI
| Variable | Ischemic stroke cases with exposure to ILI N = 554 | Ischemic stroke cases without ILI N = 39421 |
|---|---|---|
| Age (years) | 74 | 71 |
| Standard deviation(range) | 13.9 (0–101) | 14.7 (0–104) |
| Number of Chronic Conditions | 8.1 | 6.2 |
| Standard deviation(range) | 3.4 (1–21) | 2.6 (1–22) |
| Length of stay(days) | 9.8 | 4.6 |
| Standard deviation(range) | 15.8 (0–351) | 7.7 (0–345) |
| Women, No. (%) | 313 (56.5%) | 20, 538 (52.1%) |
| Race No. (%) | ||
| White | 311 (56.1%) | 23,889 (60.6%) |
| Black | 69 (12.4%) | 3, 981 (10.1%) |
| Hispanic | 122 (22.1%) | 7,174 (18.2%) |
| Asian/Pacific Islander | 46 (8.3%) | 3,626 (9.2%) |
| Native American | 6 (1.1%) | 27 (0.07%) |
| Other | 0 | 741 (1.88%) |
| AHRQ comorbidity measures | ||
| Valvular heart disease | 63 (11.4%) | 3271 (8.3%) |
| Congestive heart failure | 124 (22.5%) | 5598 (14.2%) |
| Pulmonary circulation disorders | 23 (4.2%) | 1064 (2.7%) |
| Chronic pulmonary disease | 148 (26.8%) | 5598 (14.2%) |
| Coagulopathy | 29 (5.2%) | 1458 (3.7%) |
| Peripheral vascular disorders | 57 (10.3%) | 3744 (9.5%) |
| Hypertension | 433 (78.2%) | 31,419 (79.7%) |
| Diabetes with chronic complications | 66 (11.9%) | 3430 (8.7%) |
| Diabetes, uncomplicated | 145 (26.2%) | 10, 092 (25.6%) |
| Renal failure | 128 (23.1%) | 5992 (15.2%) |
| Alcohol abuse | 17 (3.0%) | 1616 (4.1%) |
| Obesity | 54 (9.7%) | 3430 (8.7%) |
| Drug abuse | 15 (2.8%) | 1025 (2.6%) |
| Weight loss | 43 (7.7%) | 1853 (4.7%) |
| Rheumatoid arthritis/collagen vascular diseases | 16 (2.9%) | 946 (2.4%) |
| Depression | 53 (9.6%) | 3232 (8.2%) |
| Other neurological disorders | 31 (5.7%) | 1380 (3.5%) |
| Paralysis | 40 (7.2%) | 2838 (7.2%) |
| Psychoses | 25 (4.6%) | 1498 (3.8%) |
| Solid Tumor without metastasis | 11 (2.1%) | 749 (1.9%) |
| Metastatic cancer | 7 (1.2%) | 631 (1.6%) |
| Lymphoma | 9 (1.6%) | 233 (0.59%) |
| Acquired immune deficiency syndrome | 1 (0.17%) | 59 (0.15%) |
Figure 1.
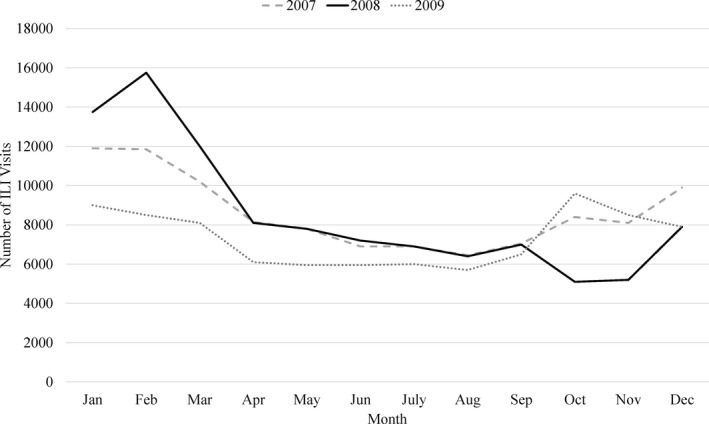
Seasonal and annual prevalence of ILI hospitalizations in adults over 18 in California, 2007–2009.
Ischemic stroke patients had an increased odds of an ILI hospitalization shortly before their stroke event, even after adjusting for the monthly prevalence of ILI assessed during a series of time intervals (Table 3). Stroke patients were found to have the highest odds of ILI within 15 days prior to the stroke hospitalization (OR: 2.88, 95% CI: 1.86–4.47). The odds of ILI before stroke remained even as the time from ILI to stroke increased, but the effect size of the odds decreased as the time interval increased suggesting a potential dose response. The odds of ILI prior to a stroke remained significantly elevated after increasing the time interval to 365 days after the ILI event and adjusting for the prevalence of ILI during the time period (OR: 1.50, 95% CI: 1.31–1.71).
Table 3.
Cumulative odds ratios (ors) and 95% confidence intervals (cis) for ILI with risk of ischemic stroke.a
| Hospitalization for ILI | Unadjusted | Adjusted for seasonal prevalence of ILI | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Within 15 days before stroke | 2.90 | 1.97–4.28 | 2.88 | 1.86–4.47 |
| Within 30 days before stroke | 2.59 | 1.93–3.46 | 2.59 | 1.86–3.61 |
| Within 60 days before stroke | 2.16 | 1.73–2.69 | 2.18 | 1.69–2.82 |
| Within 90 days before stroke | 1.79 | 1.48–2.16 | 1.78 | 1.43–2.21 |
| Within 180 days before stroke | 1.56 | 1.35–1.81 | 1.51 | 1.27–1.78 |
| Within 365 days before stroke | 1.66 | 1.48–1.86 | 1.50 | 1.31–1.71 |
OR indicates odds ratio; CI, confidence interval.
All P < 0.0001.
We additionally assessed whether a visit to the emergency department or a hospitalization for a laceration increased the odds of stroke using identical methodology as a way to assess whether the associations seen between ILI and stroke were due to bias in seeking care. We found that stroke patients were not at increased odds of having a laceration in the 15 days prior to stroke hospitalization (OR: 0.72, 95% CI: 0.52–1.10), nor in the other time periods (data not shown).
We divided the time into mutually exclusive intervals to assess whether the relationship between ILI and stroke over a longer time period was being primarily driven by the early odds of stroke after ILI. In analyses utilizing nonoverlapping time intervals from ILI to stroke (Fig. 2), the odds of ischemic stroke patients having an ILI event was greatest in the first 15 days prior to stroke hospitalization (OR: 2.88, 95% CI: 1.86–4.47). The strength of the relationship decreased at 16–30 days between ILI and stroke (OR: 1.81, 95% CI: 1.11–2.92), and decreased further 31–60 days between ILI and stroke (OR: 1.68, 95% CI: 1.19–2.38). The relationship was no longer significant after 60 days, indicating the relationship seen in the 0–365 analysis was being driven by the short‐term risk.
Figure 2.
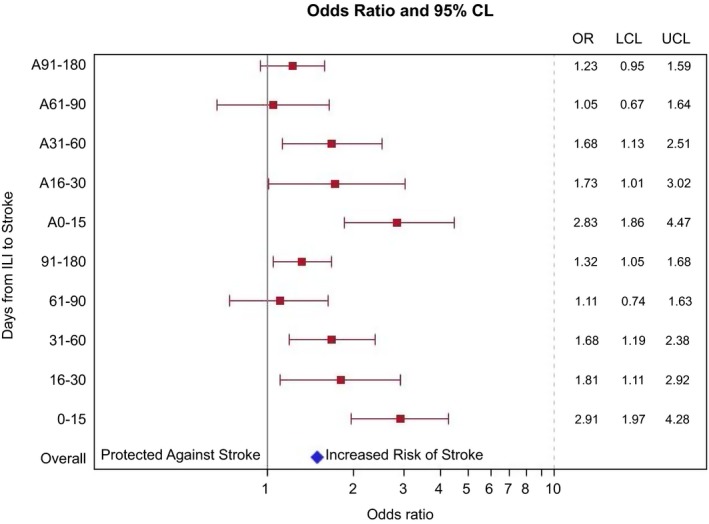
Association of Hospitalization for ILI in nonoverlapping time intervals with risk of ischemic stroke.
There was a significant interaction (P = 0.017) with age and ILI; the odds of a stroke patient having an ILI event prior to stroke was greater for younger patients. The change in the OR for the association of stroke and ILI increased 7% with each 10‐year decrease in age (OR per 10‐year age decrease 1.07, 95% CI: 1.03–1.35). To further explore this, analyses were performed stratified by age categorized as less than 45, 45–65, and greater than 65 years. Patients who were under 45 with stroke had the highest odds of having a prior ILI within 15 days of stroke hospitalization (Table 4; OR: 9.28, 95% CI: 1.72–50.2), patients who were between 45 and 65 with ILI had a nearly threefold increased odds of ILI prior to their stroke (OR: 2.71, 95% CI: 1.06–6.93), and patients who were older than 65 remained at increased odds for ILI prior to their stroke, though the odds were lower than in the other age groups (OR: 2.65, 95% CI: 1.59–4.43). Similar to the nonage stratified analysis, the odds of ILI prior to stroke diminishes after 15 days between the two events, with no relationship 60 or more days between the two events. There was no interaction between age and Charlson comorbidity score, and no significant clustering by hospital. There was not a significant interaction with diabetes or with number of comorbid conditions.
Table 4.
Risk of ischemic stroke after influenza‐like illness, stratified by age
| Overall | Age 18–45 | Age 45.1–65 | Age ≥ 65 | |
|---|---|---|---|---|
| 0–15 days | 2.88 (1.86–4.47) | 9.28 (1.72–50.2) | 2.71 (1.06–6.93) | 2.65 (1.59–4.43) |
| 15–30 days | 1.73 (0.99–3.00) | 2.00 (0.13–31.9) | 1.21 (0.54–2.71) | 2.27 (1.29–3.97) |
| 30–60 days | 1.68 (1.13–2.51) | 1.11 (0.17–7.05) | 1.09 (0.29–4.08) | 1.83 (1.21–2.76) |
Discussion
We found that visits to the emergency department or a hospital stay for ILI increases short‐term odds of stroke, and therefore may be considered to act as a trigger for stroke. The odds of stroke is highest within 15 days of ILI, and the odds decreases as time from ILI hospitalization increases. There is no longer an increased odds of stroke in mutually exclusive time intervals beginning 60 days after ILI, indicating that the relationship seen up to 365 days after ILI is primarily being driven by the increased odds in the early time period after ILI. Furthermore, we found that people under the age of 45 years have the highest odds of stroke within 15 days of ILI, and that while the odds remained for the older age categories, the odds decreased as age increased. This indicates that ILI could be a novel trigger for stroke, particularly in the young. Identifying novel triggers of stroke in patients under 45 is critical considering the recently appreciated increasing incidence and prevalence of stroke in the young.4
Current literature identifies influenza as a potential trigger for myocardial infarction and cardiovascular death, but research on ILI and stroke risk has been limited.11 There is a moderately increased acute risk of stroke following hospitalization for several types of infection, and infections have been identified as triggers of stroke in children.8, 20, 21, 22, 23, 24, 25, 26, 27 Our study replicates these findings for ILI, a more prevalent type of infection than other studies have investigated, and highlights that the risk of stroke is particularly high shortly after hospitalization for an ILI, with the odds decreasing as time increases.
Influenza‐like illness is common, causing 628.6 hospitalizations per 100,000 person‐years.12 The morbidity and mortality associated with ILI ranges from year to year due to fluctuations within the flu season, and as such the associated morbidity and mortality fluctuates.13 We found that younger patients were at relatively increased odds of stroke after ILI compared to older patients; for every decade younger age, the odds increased by almost 10%. While the absolute risk of stroke is greater in older adults, approximately 10–14% of all strokes occur in people 18–45 years old, with the incidence and prevalence of stroke in the young increasing.4, 28, 29, 30, 31, 32, 33, 34 This increasing prevalence, moreover, coupled with greater heterogeneity in stroke etiology within the younger age group than in the older stroke population, presents a unique and vulnerable patient population where risk reduction efforts are of increasing importance. Conventional risk factors such as hypertension, diabetes, and smoking, moreover, may not fully account for the risk of stroke in patients aged 18–45.5 Stroke etiologies that are typically attributed to vascular risk factors, such as large artery atherosclerosis and small‐vessel disease, occur in less than 10% of young adults.5 Identifying a trigger, such as ILI, for stroke in the young identifies a potential opportunity to reduce stroke risk, through ILI risk reduction efforts.
The mechanisms by which ILI leads to stroke remain uncertain. Prior work has identified potential mechanisms as to why infection may serve as a trigger for stroke through mechanisms such as: causing a systemic release of cytokines and other inflammatory mediators, causing a pro‐thrombotic state, inflammation‐mediated endothelial injury, or effects on cardiac endothelium.8, 20, 21, 22, 23 The increase in odds may also be due to general effects of infection, such as fever and dehydration. We do not hypothesize that influenza plays a direct role in the risk of stroke by, for example, infiltrating blood vessels, as other viruses, such as varicella zoster virus, may do.35 While this study suggests an association, further studies are needed to explore a potential causal connection between ILI and stroke.
Our findings have potential implications for public health, clinical practice, and future research. If influenza or ILI is associated with increased odds of stroke within 60 days, then public health experts may want to be particularly aggressive with influenza vaccine programs among those at risk of stroke; the advantages to such prevention programs may extend not only to avoiding the respiratory complications of influenza, but also to preventing cerebrovascular complications. Finally, clinical and translational investigators may be inspired both to identify the mechanisms by which influenza leads to cerebrovascular disease and to conduct clinical trials to test whether the implementation of vasculoprotective strategies among flu patients does in fact lead to a reduction in cerebrovascular events. Similarly, health services researchers may want to test whether use of vascular risk scoring systems to identify patients at risk of cardiovascular disease should be employed among patients with recent influenza‐like illness. Because the number of stroke patients with prior influenza is low (1.5% in our analysis), such studies would likely need to be quite large, unless specific subpopulations at considerably higher risk can be identified.
Our study has limitations. As we relied on administrative datasets, prospective case ascertainment and detailed clinical information such as number of infections leading up to the ILI hospitalization and stroke severity were not available. In addition, we were only able to capture patients who presented to the emergency department or were hospitalized for ILI, indicating potentially more serious cases, and those hospitalized in the state of California. An additional limitation is that it is not known what proportion of ILI events result in hospitalization or ED visits, nor do we know the number of people who had ILI events that did not present to the ED. Patients who had hospitalizations in other states, or who died of an event prior to being admitted to a hospital, were not captured. The case‐crossover design also does not account for the increased odds associated with the aging of the patient over time and their concomitant development of new risk factors. To minimize these concerns, however, we limited the control time windows to the prior 2 years.
Our study has strengths, as well. By utilizing the case‐crossover design, in which each patient served as his or her own control, we largely eliminated interindividual variability. The use of a large administrative database increases the generalizability of the study. Moreover, our very large sample size, including more than 30,000 stroke patients, allowed us to study associations among populations of patients that are often difficult to capture, including young stroke patients. We were also able to calculate and adjust for the monthly variability in prevalence of ILI.
This study suggests that ILI patients are at greatest risk for stroke within 15 days of their ILI event, and remain at risk for stroke as long as 60 days. This study identifies ILI as a potential trigger for stroke, particularly in younger patients not traditionally considered to be at high risk for stroke. Future studies are needed to confirm these relationships in other patient populations, determine if and when the risk returns to a baseline level of risk, investigate mechanisms of the increased risk of stroke after ILI, and determine effective strategies to reduce this risk.
Conflict of Interest
Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS‐Pfizer Partnership, Boehringer‐Ingelheim, and Sanofi‐Regeneron; serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from Up To Date for chapters related to cryptogenic stroke.
Author Contributions
Conception and study design: MSE, HK, AKB, EK, JL; acquisition and analysis of data: JL, AKB, EK; drafting a significant portion of the manuscript and critical revisions: AKB, JL, EK, HK, MSE.
Acknowledgments
Dr. Boehme is supported by NINDS NIH T32 NS007153‐31 and L30 NS093600. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke grants L30 NS093600 and T32 NS007153‐31; National Institute of Minority Health and Health Disparities grant R21 MD012451.
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2011 update. Circulation 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Stroke Association . Stroke 101 Fact Sheet. Available at: http://www.stroke.org/site/DocServer/STROKE_101_Fact_Sheet.pdf?docID=4541.(accessed: June 1, 2017)
- 4. George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol 2017;74:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ji R, Schwamm LH, Pervez MA, Singhal AB. Ischemic stroke and transient ischemic attack in young adults: risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA Neurol 2013;70:51–57. [DOI] [PubMed] [Google Scholar]
- 6. Elkind MS, Ramakrishnan P, Moon YP, et al. Infectious burden and risk of stroke: the northern Manhattan study. Arch Neurol 2010;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowan LT, Alonso A, Pankow JS, et al. Hospitalized infection as a trigger for acute ischemic stroke: the atherosclerosis risk in communities study. Stroke 2016;47:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elkind MS, Carty CL, O'Meara ES, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke 2011;42:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalager‐Pedersen M, Sogaard M, Schonheyder HC, et al. Risk for myocardial infarction and stroke after community‐acquired bacteremia: a 20‐year population‐based cohort study. Circulation 2014;129:1387–1396. [DOI] [PubMed] [Google Scholar]
- 10. Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case‐control study through a general practice database. Eur Heart J 2008;29:96–103. [DOI] [PubMed] [Google Scholar]
- 11. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009;9:601–610. [DOI] [PubMed] [Google Scholar]
- 12. Thompson WW, Shay DK, Weintraub E, et al. Influenza‐associated hospitalizations in the United States. JAMA 2004;292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 13. Rolfes MA Foppa IM, Garg S, Flannery B, et al. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths Averted by Vaccination in the United States [online]. Available at: https://www.cdc.gov/flu/about/disease/2015-16.htm.
- 14. HCUP state inpatient database (SID) C. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 15. Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133:144–153. [DOI] [PubMed] [Google Scholar]
- 16. Eick‐Cost AA, Hunt DJ. Assessment of ICD‐9‐based case definitions for influenza‐like illness surveillance. MSMR 2015;22:2–7. [PubMed] [Google Scholar]
- 17. Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 18. Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long‐term outcomes following development of new‐onset atrial fibrillation during sepsis. Chest 2014;146:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boehme AK, Ranawat P, Luna J, et al. Risk of acute stroke after hospitalization for sepsis: a case‐crossover study. Stroke 2017;48:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351:2611–2618. [DOI] [PubMed] [Google Scholar]
- 21. Syrjanen J. Central nervous system complications in patients with bacteremia. Scand J Infect Dis 1989;21:285–296. [DOI] [PubMed] [Google Scholar]
- 22. Syrjanen J, Valtonen VV, Iivanainen M, et al. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. BMJ 1988;296:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zurru MC, Alonzo C, Brescacin L, et al. Recent respiratory infection predicts atherothrombotic stroke: case‐control study in a Buenos Aires healthcare system. Stroke 2009;40:1986–1990. [DOI] [PubMed] [Google Scholar]
- 24. Fullerton HJ, Elkind MS, Barkovich AJ, et al. The vascular effects of infection in Pediatric Stroke (VIPS) Study. J Child Neurol 2011;26:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amlie‐Lefond C, Fullerton HJ. Rashes, sniffles, and stroke: a role for infection in ischemic stroke of childhood. Infect Disord Drug Targets 2010;10:67–75. [DOI] [PubMed] [Google Scholar]
- 26. Hills NK, Johnston SC, Sidney S, et al. Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke. Ann Neurol 2012;72:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wintermark M, Hills NK, deVeber GA, et al. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke 2014;45:3597–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first‐ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195–1203. [DOI] [PubMed] [Google Scholar]
- 29. Adams HP Jr, Kappelle LJ, Biller J, et al. Ischemic stroke in young adults. Experience in 329 patients enrolled in the Iowa Registry of stroke in young adults. Arch Neurol 1995;52:491–495. [DOI] [PubMed] [Google Scholar]
- 30. Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore‐Washington Cooperative Young Stroke Study. Neurology 1998;50:890–894. [DOI] [PubMed] [Google Scholar]
- 31. Jacobs BS, Boden‐Albala B, Lin IF, Sacco RL. Stroke in the young in the northern Manhattan stroke study. Stroke 2002;33:2789–2793. [DOI] [PubMed] [Google Scholar]
- 32. Qureshi AI, Safdar K, Patel M, et al. Stroke in young black patients. Risk factors, subtypes, and prognosis. Stroke 1995;26:1995–1998. [DOI] [PubMed] [Google Scholar]
- 33. Naess H, Nyland HI, Thomassen L, et al. Incidence and short‐term outcome of cerebral infarction in young adults in western Norway. Stroke 2002;33:2105–2108. [DOI] [PubMed] [Google Scholar]
- 34. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995‐2008. Ann Neurol 2011;70:713–721. [DOI] [PubMed] [Google Scholar]
- 35. Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol 2009;8:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]


