Abstract
Objective
To determine the associations between individual metabolic syndrome (MetS) components and peripheral neuropathy in a large population‐based cohort from Pinggu, China.
Methods
A cross‐sectional, randomly selected, population‐based survey of participants from Pinggu, China was performed. Metabolic phenotyping and neuropathy outcomes were performed by trained personnel. Glycemic status was defined according to the American Diabetes Association criteria, and the MetS using modified consensus criteria (body mass index instead of waist circumference). The primary peripheral neuropathy outcome was the Michigan Neuropathy Screening Instrument (MNSI) examination. Secondary outcomes were the MNSI questionnaire and monofilament testing. Multivariable models were used to assess for associations between individual MetS components and peripheral neuropathy. Tree‐based methods were used to construct a classifier for peripheral neuropathy using demographics and MetS components.
Results
The mean (SD) age of the 4002 participants was 51.6 (11.8) and 51.0% were male; 37.2% of the population had normoglycemia, 44.0% prediabetes, and 18.9% diabetes. The prevalence of peripheral neuropathy increased with worsening glycemic status (3.25% in normoglycemia, 6.29% in prediabetes, and 15.12% in diabetes, P < 0.0001). Diabetes (odds ratio [OR] 2.60, 95% CI 1.77–3.80) and weight (OR 1.09, 95% CI 1.02–1.18) were significantly associated with peripheral neuropathy. Age, diabetes, and weight were the primary splitters in the classification tree for peripheral neuropathy.
Interpretation
Similar to previous studies, diabetes and obesity are the main metabolic drivers of peripheral neuropathy. The consistency of these results reinforces the urgent need for effective interventions that target these metabolic factors to prevent and/or treat peripheral neuropathy.
Introduction
Peripheral neuropathy is a highly prevalent condition, particularly in older populations.1, 2, 3 This disease affects patients by causing pain, decreased quality of life, falls, ulcerations, and amputations.3, 4, 5 Unfortunately, outside of medications for neuropathic pain, few therapies exist to help patients with peripheral neuropathy.4 Even in patients with type 2 diabetes, glycemic control has only a small effect on the prevention of peripheral neuropathy.6 Furthermore, a significant proportion of patients with peripheral neuropathy have no known underlying cause.7, 8, 9, 10 Therefore, a better understanding of the underlying causes is needed to inform the development of new disease modifying treatments.
Multiple studies have implicated the metabolic syndrome (MetS) as a potential cause of peripheral neuropathy.11, 12, 13, 14 However, studies that have investigated the contributions of the individual components have revealed mixed results.15, 16, 17, 18, 19, 20, 21 We have recently shown that diabetes, prediabetes, and obesity are the main metabolic components associated with peripheral neuropathy in a United States obese population.22 Similarly, in a United States elderly population, we demonstrated that diabetes, obesity, and the number of metabolic components are associated with peripheral neuropathy.23 Another group has performed similar studies in Shanghai, China. They found that patients with diabetes and prediabetes had a higher prevalence of peripheral neuropathy compared to patients with normoglycemia.24 Waist circumference and fasting glucose were the main metabolic factors associated with peripheral neuropathy. The same group observed that the peripheral neuropathy prevalence increased as the number of MetS components increased.25 However, the association with MetS lost statistical significance when adjusted for insulin resistance.
We aimed to determine the associations between individual MetS components and peripheral neuropathy in a large population‐based cohort from Pinggu, China. In contrast to the previous Chinese study in Shanghai, we included a categorical classification of glycemic status, included interaction terms, limited the analysis to metabolic factors, and avoided potential collinearity in the models. We also investigated the association between the number of MetS components. Unlike the previous Chinese study, we evaluated the number of MetS components in addition to hyperglycemia and adjusted for the glycemic effect in the models. Finally, we used a tree‐based approach to build a classifier for peripheral neuropathy based on demographics and MetS components. The resulting classification tree allowed identification and characterization of peripheral neuropathy risk groups.
Material and Methods
Population
This study was part of the larger Pinggu metabolic disease study. The study design was a cross‐sectional, randomly selected, population‐based survey of participants from Pinggu, China. Participants were obtained by a stratified, random sampling method with 5‐year age segments of each gender. There are total of 16 towns in the rural area. Five of these towns were randomly selected, and five villages were randomly selected from each town. A total of 2500 participants were randomly sampled from these 25 villages. The remaining 2500 participants were randomly sampled from a randomly chosen urban district (one of two). Individuals were required to be 25–74 years old, born in Pinggu, and lived there for five or more years. Pregnant women, individuals who for medical or other reasons would not be able to return for repeat testing over a 2–5‐year period were excluded given the longitudinal nature of the larger study. Sexually active women were required to refrain from unprotected sexual intercourse during the course of the study (3 weeks). All study protocols were approved by the institutional review board at the University of Michigan.
Metabolic syndrome components
Individuals filled out surveys including demographic information, and all questions were posed in Chinese via a trained interviewer who recorded responses into a secure computer database. Study team members were present to answer any questions posed by the participants taking the survey. In addition, fasting laboratory assessments were performed included a fasting (10–12 h) lipid panel and oral glucose tolerance test (75 g of glucose). Individuals were also measured by a technician for height and weight. Blood pressure was measured in triplicate with the participant's feet flat on the ground.
Diabetes (fasting glucose ≥126 or 2 h glucose ≥200) and prediabetes (fasting glucose ≥100 or 2 h glucose ≥140) were defined according to the American Diabetes Association criteria.26 Consensus MetS criteria were used to define the MetS and its individual components, with the exception of body mass index (BMI) instead of waist circumference as these data were not available.27 Specifically, the MetS criteria, in addition to the hyperglycemia definition above, were a BMI ≥30, systolic blood pressure (SBP) ≥130 or diastolic blood pressure ≥85, triglycerides ≥150 mg/dL, and high‐density lipoprotein (HDL) <40 mg/dL in men and <50 mg/dL in women.
Peripheral neuropathy
The Michigan Neuropathy Screening Instrument (MNSI) examination and questionnaire were performed by trained personnel as previously described.28 Monofilament testing was performed with a Semmes Weinstein 5.07/10‐g monofilament on the dorsum of the dominant great toe. The primary peripheral neuropathy outcome was an abnormal MNSI examination (≥2.5). Secondary peripheral neuropathy outcomes included an abnormal MNSI questionnaire (≥4), and an abnormal monofilament examination (participant is unable to feel three or more applications out of 10).28
Statistical analysis
Descriptive statistics were used to describe the demographics and metabolic phenotype of the population. We determined the prevalence of peripheral neuropathy (all three definitions) stratified by glycemic status. We then applied the Cochran‐Armitage test to investigate for a trend in the peripheral neuropathy prevalence in the three groups based on glycemic status. Similarly, we determined the prevalence of peripheral neuropathy (primary outcome) stratified by glycemic status and the number of MetS components. We then used the Cochran‐Armitage test to determine whether there was a significant trend between peripheral neuropathy and the number of MetS components within each glycemic subgroup.
Multivariable logistic regression was used to model the peripheral neuropathy outcomes as a function of the MetS components (weight as a surrogate for waist circumference, hyperglycemia in the prediabetic range, hyperglycemia in the diabetic range, HDL level, triglyceride level, SBP), after adjusting for baseline demographic factors (age, sex, height). Interaction effects between glycemic status and the other four MetS components were examined by adding them individually to the multivariable logistic regression models described above.
We performed tree‐based analyses (statistical machine learning) to construct a classifier for peripheral neuropathy based on demographics (age, gender, and height) and the MetS components. In the tree paradigm, the covariate space is partitioned recursively in a binary fashion. Our analyses began with the entire patient cohort and found the best split into two groups based on a variable that makes the resultant two groups most homogeneous within themselves. For our binary outcome of peripheral neuropathy, within group homogeneity was measured using the Gini impurity. The two groups were again partitioned (each group being split on the same or other variables), thereby creating a tree structure. At each step, to select the best split, the tree‐growing paradigm examined every possible cut point for each predictor variable. This process was continued until the groups reached a minimum size (10 patients in each group). Because the resulting tree was overgrown (thereby overfitting the data), a subtree was chosen using cost‐complexity pruning.29, 30 The classification tree was evaluated by examining the percent correct classification (overall, as well as for the peripheral neuropathy cases). We implemented inverse weighting within our classification tree to give proportionally more weight to correct classification of the neuropathy cases. The final tree was selected using a 10‐fold cross‐validation approach.29, 30 All analyses were performed with SAS 9.3 (Cary, NC) or in R version 3.2.3 using the rpart package.
Results
Demographics and metabolic phenotyping are presented in Table 1 for the 4002 participants. The mean (SD) age was 51.6 (11.8) and 51.0% were male. Normoglycemic participants accounted for 37.2% of the population, prediabetes 44.0%, and diabetes 18.9%, which is comparable to the distribution in a previous large Chinese cohort study.24 The MetS was present in 38.0%. The mean (SD) BMI was 26.1 (3.8), SBP 130.1 (18.1), triglycerides 141.2 (130.0), and HDL 45.0 (12.0).
Table 1.
Demographics of the Pinggu population stratified by glycemic status
| Variable | Total | Normoglycemia | Prediabetes | Diabetes |
|---|---|---|---|---|
| Subjects, N (%) | 4002 (100%) | 1487 (37.2%) | 1758 (44.0%) | 757 (18.9%) |
| Age, mean (SD) | 51.6 (11.8) | 47.2 (11.8) | 53.2 (11.3) | 56.7 (9.9) |
| Male, N (%) | 2039 (51.0%) | 885 (59.5%) | 816 (46.4%) | 338 (44.7%) |
| Height (cm), mean (SD) | 162.6 (8.4) | 162.3 (8.4) | 162.7 (8.2) | 162.9 (8.6) |
| Fasting glucose (mg/dL), mean (SD) | 109.5 (29.4) | 93.0 (4.8) | 105.8 (7.5) | 150.9 (46.0) |
| 2 h glucose (mg/dL), mean (SD) | 133.1 (46.4) | 106.9 (19.0) | 136.4 (8.2) | 228.3 (64.7) |
| BMI (kg/m2), mean (SD) | 26.1 (3.8) | 24.9 (3.6) | 26.5 (3.8) | 27.4 (3.8) |
| SBP (mmHg), mean (SD) | 130.1 (18.1) | 123.6 (17.0) | 132.5 (17.1) | 137.5 (18.3) |
| DBP (mmHg), mean (SD) | 78.7 (11.4) | 75.8 (10.7) | 80.2 (11.3) | 81.1 (11.7) |
| Cholesterol (mg/dL), mean (SD) | 190.6 (38.1) | 183.0 (33.8) | 193.7 (37.4) | 198.2 (44.4) |
| Triglycerides (mg/dL), mean (SD) | 141.2 (130.0) | 106.3 (85.5) | 150.7 (134.7) | 187.8 (167.9) |
| HDL (mg/dL), mean (SD) | 45.0 (12.0) | 46.9 (12.2) | 44.4 (11.8) | 42.7 (11.6) |
| LDL (mg/dL), mean (SD) | 111.3 (31.5) | 107.2 (29.0) | 114.0 (31.5) | 112.9 (35.1) |
| Metabolic syndrome, N (%) | 1519 (38.0%) | 138 (9.3%) | 900 (51.2%) | 481 (63.5%) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol.
The prevalence of peripheral neuropathy increased with worsening glycemic status regardless of the peripheral neuropathy definition used (P < 0.0001 for all three peripheral neuropathy outcomes). The prevalence in those with prediabetes was higher than those with normoglycemia using the MNSI examination and monofilament definitions (P < 0.0001), but not using the MNSI questionnaire definition (P = 0.30). Using the MNSI examination, the prevalence was 3.25% in normoglycemic participants, 6.29% in prediabetes, and 15.12% in diabetes. Using the MNSI questionnaire, the prevalence was 1.21% in normoglycemic participants, 1.65% in prediabetes, and 6.08% in diabetes. Using the monofilament definition, the prevalence was 3.70% in normoglycemic participants, 7.29% in prediabetes, and 9.13% in diabetes. Controlling for glycemic status, the prevalence of peripheral neuropathy increased with the number of MetS components, particularly in those with normoglycemia (P = 0.16) and prediabetes (P = 0.10), but the result was not statistically significant (Fig. 1).
Figure 1.
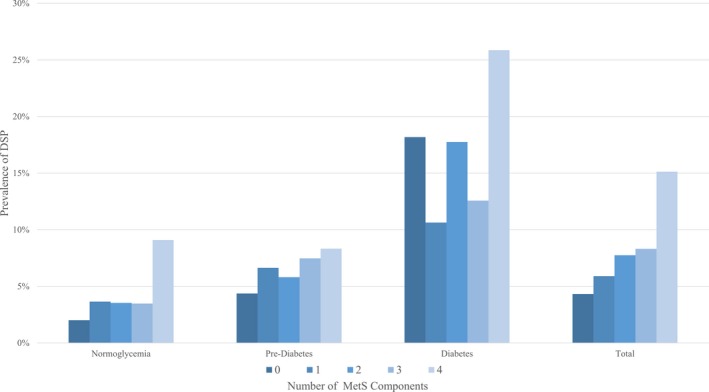
The prevalence of distal symmetric polyneuropathy (DSP) with increasing metabolic syndrome components stratified by glycemic status. DSP was defined as those with a Michigan Neuropathy Screening Instrument (MNSI) Examination score >2. Glycemic status was determined by the glucose tolerance test according to the Expert Committee on the diagnosis and classification of diabetes mellitus. Metabolic syndrome components were defined using modified consensus criteria.
In a multivariable logistic regression model investigating the individual MetS components, hyperglycemia in the diabetic range (odds ratio [OR] 2.60, 95% CI 1.77–3.80) and weight (OR 1.09, 95% CI 1.02–1.18) were significantly associated with the peripheral neuropathy primary outcome (Table 2, MNSI examination). Age, gender, and height were also significantly associated with peripheral neuropathy. For MNSI questionnaire and monofilament, hyperglycemia in the diabetic range was the only MetS component associated with peripheral neuropathy (OR 3.85, 95% CI 2.09–7.09 and OR 1.51, 95% CI 1.01–2.25, respectively). Age and gender were also significantly associated with peripheral neuropathy using these two definitions. Of note, hyperglycemia in the prediabetic range had an OR of 1.11–1.41 in the three models, but did not reach statistical significance. No statistically significant interactions between glycemic status and other MetS components were observed. In a multivariable logistic regression model investigating glycemic status and the number of additional MetS components, the number of MetS components (OR 1.17, 95% CI 1.03–1.32) was significantly associated with the peripheral neuropathy primary outcome. Diabetes (OR 2.71, 95% CI 1.86–3.94), but not prediabetes (OR 1.25, 95% CI 0.87–1.80), was also significantly associated with peripheral neuropathy in this model.
Table 2.
Multivariable logistic regression evaluating the association of MetS components and neuropathy
| Variable | Primary outcome | Secondary outcomes | |
|---|---|---|---|
| MNSI Examination OR (95% CI) | MNSI Questionnaire OR (95% CI) | Monofilament OR (95% CI) | |
| Demographics | |||
| Age | 1.10 (1.09, 1.12)a | 1.07 (1.05, 1.10)a | 1.10 (1.08, 1.12)a |
| Male (reference female) | 1.68 (1.14, 2.47)a | 2.03 (1.05, 3.91)a | 1.56 (1.05, 2.31)a |
| Height unit = 5 cm | 1.20 (1.05, 1.37)a | 0.89 (0.71, 1.10) | 1.14 (1.00, 1.15) |
| MetS components | |||
| Diabetes | 2.60 (1.77, 3.80)a | 3.85 (2.09, 7.09)a | 1.51 (1.01, 2.25)a |
| Prediabetes (reference normal) | 1.21 (0.84, 1.75) | 1.11 (0.60, 2.06) | 1.41 (1.00, 1.99) |
| Weight unit = 5 kg | 1.09 (1.02, 1.18)a | 1.00 (0.98, 1.03) | 1.00 (0.98, 1.01) |
| SBP unit = 10 mmHg | 1.04 (0.96, 1.11) | 0.93 (0.82, 1.05) | 1.07 (0.99, 1.15) |
| Triglycerides unit = 50 mg/dL | 1.01 (0.96, 1.06) | 0.90 (0.79, 1.02) | 0.94 (0.87, 1.01) |
| HDL unit = 10 mg/dL | 0.99 (0.87, 1.12) | 0.87 (0.69, 1.08) | 1.03 (0.91, 1.16) |
MetS, metabolic syndrome; MNSI, Michigan Neuropathy Screening Instrument; OR, odds ratio; SBP, systolic blood pressure; HDL, high‐density lipoprotein cholesterol.
P < 0.05.
Using tree analysis, we found that age, glycemic status, and weight were the main demographic and metabolic factors that could help discriminate peripheral neuropathy status (Fig. 2). The resulting tree first separates the population into three groups based on age. Patients aged less than 52.5 years old were given a class prediction of “no peripheral neuropathy,” with 97.9% accuracy. Peripheral neuropathy was much more common in those greater than or equal to 67.5 years old (93 out of 360, 25.8%). Those older than 67.5 were predicted to have neuropathy using this classification tree. In those between these two age group, diabetes status, weight and SBP were used to split patients into neuropathy groups. For patients aged between 52.5 and 67.5, diabetes status increased the probability of peripheral neuropathy (339 out of 404, 83.9%). Patients in this middle age group that did not have diabetes were further split based on weight and SBP to determine their final neuropathy classification.
Figure 2.
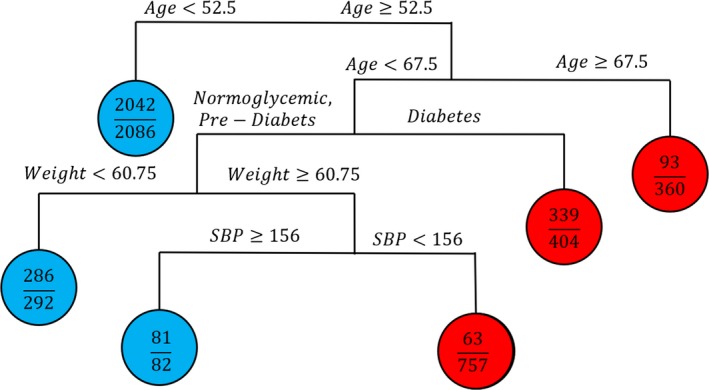
Classification tree analysis for peripheral neuropathy based on demographics and the MetS components. A tree‐based approach was used to build a classifier for peripheral neuropathy based on demographics and metabolic syndrome components. The resulting classification tree allowed identification and characterization of peripheral neuropathy risk groups.
Discussion
In a large, population‐based study in Pinggu, China, we found that diabetes, weight, and the number of MetS components in addition to hyperglycemia are associated with peripheral neuropathy. Participants with prediabetes also have a higher prevalence of peripheral neuropathy, but the association does not meet statistical significance in multivariable models. These results are comparable to findings in other populations including those in the United States,22, 23 another region in China,24, 25 and the Netherlands.31 Now that the evidence supporting these specific metabolic drivers of nerve injury is increasingly robust, efforts should turn to interventional studies aimed at weight loss and/or exercise in populations at high risk of peripheral neuropathy. In addition, using statistical machine learning, we demonstrate that age, diabetes, and weight are the key factors that can help clinicians target specific populations for peripheral neuropathy screening. Specifically, patients over the age of 67.5 (25.8%), those over 52.5 with diabetes (83.9%), and those over 52.5 and weighing over 60.75 kg (8.5%) are much more likely to have peripheral neuropathy compared to those under the age of 52.5 (2.1%).
While diabetes is the strongest and most well‐established metabolic driver of peripheral neuropathy, determining the other metabolic contributors is critical to understand the population at risk and to inform new interventions to prevent and/or treat this common condition. Including this study, five recent investigations reveal that obesity is significantly associated with peripheral neuropathy in multivariable analyses.22, 23, 24, 31 Our results are comparable to those found in 2035 Han Chinese subjects in Shanghai.24 Importantly, we were able to address important limitations of the previous study including addressing the effects of interactions between metabolic components and glycemic status, focusing the analysis on metabolic factors, and avoiding collinearity in the multivariable models. Our population demographics were also different with our study including younger participants (51.5 vs. 61.5), with a higher proportion of normoglycemia (37.2% vs. 19.9%), a higher mean BMI (26.1 vs. 24.4), and more males (51.0% vs. 43.3%). Despite the differences in population location and demographics, the definition of neuropathy, and statistical modeling design, the results are consistent. Similarly, studies in obese and elderly populations in the United States,22, 23 and an older population in the Netherlands31 lead to the same conclusion despite study design differences. With strong evidence supporting obesity as a metabolic driver of peripheral neuropathy, we need to start considering obesity as a potential cause of peripheral neuropathy in the nondiabetic obese and design interventions that address this underlying metabolic cause.
In contrast to the evidence supporting obesity, the data supporting prediabetes is less clear. We found that the prevalence of peripheral neuropathy was higher in those with prediabetes than those with normoglycemia, but the association was not significant in multivariable models. With many previous studies supporting,16, 32, 33 and some not supporting,23, 34 an association of prediabetes with peripheral neuropathy, our results do not push the overall level of evidence much in either direction. Our study is now the third to show an increased OR in those with prediabetes that does not meet statistical significance.22, 31 Possible explanations are that the study was not adequately powered to detect this small effect size or that prediabetes is not the underlying metabolic driver of nerve injury when accounting for other metabolic factors. Another possibility is that definitions of neuropathy that primarily measure large fiber function, such as in our study, may miss the association between prediabetes and neuropathy because prediabetes may preferentially injure small fiber nerves.35 Interestingly, the only other study addressing this issue in a Chinese population demonstrated that 2 h postprandial glucose levels were associated with peripheral neuropathy in the prediabetes population.24 Regardless of whether prediabetes is one of the underlying metabolic drivers of peripheral neuropathy, the best interventions to study are unlikely to change. Current data support weight loss and/or exercise as the best treatment to prevent prediabetes from transitioning to diabetes,36 and these are also the best interventions for the now well‐established metabolic driver of peripheral neuropathy, obesity.
Besides diabetes and obesity, we also found that the number of MetS components in addition to hyperglycemia was significantly associated with peripheral neuropathy. This finding was observed although obesity was the only other MetS component significantly associated with peripheral neuropathy. Our finding is comparable to studies performed in China,25 the United States,23 and the Netherlands.31 Unlike the studies in China and Netherlands, we did not include hyperglycemia as one of the MetS components since this is already the strongest and most well‐established risk factor for peripheral neuropathy. The study in United States used a similar modeling strategy with comparable results. Like some previous studies, we did not find associations of peripheral neuropathy with SBP, triglycerides, or HDL levels.22, 23, 24 Therefore, our data would support interventions that target the MetS as a whole rather than concentration on blood pressure and/or cholesterol management. However, it should be noted that some studies have revealed an association with triglycerides and neuropathy.21, 31
Another key finding is that we were able to use statistical machine learning to define the populations most at risk of peripheral neuropathy including evaluating for potential important interactions. This information is crucial for physicians so that they can screen for peripheral neuropathy in the appropriate groups. Emphasizing this point, screening tests have the best performance in populations with a higher prevalence of disease. We found that age alone is a key variable with participants over the age of 67.5 at much higher risk of peripheral neuropathy than those under 52.5 even with no other metabolic abnormalities. Diabetes substantially increases the prevalence of peripheral neuropathy in those over 52.5 to the point where the vast majority of this population has highly morbid condition. Weight is another metabolic factor identified, with those older than 52.5 and weighing more than 60.75 kg having a slightly increased risk of peripheral neuropathy. These data highlight the importance of age and peripheral neuropathy. Previous studies have shown that the prevalence of peripheral neuropathy, particularly idiopathic peripheral neuropathy, rises dramatically with age.37 Furthermore, these results reveal that diabetes has a much greater impact on peripheral neuropathy than obesity. Diabetes continues to be the strongest metabolic driver of peripheral neuropathy and new disease‐modifying treatments are desperately needed in this population.
Limitations include the cross‐sectional study design and lack of waist circumference, diabetes duration, and medication data. Furthermore, the generalizability of these results to other populations is unclear. However, the population‐based methodology and size of the population are strengths. Furthermore, the consistency of results with other investigations utilizing different populations and study designs support the data's generalizability and validity. The neuropathy definition was based on standardized examination and questionnaires, but not on a neurologist's history and examination or confirmatory measures (nerve conduction studies or skin biopsies). On the other hand, the MNSI questionnaire and examination are validated measures of neuropathy and have been used successfully in both type 1 and type 2 diabetic cohorts.28, 38, 39, 40 While the MNSI instruments have not been validated in Asian populations, the prevalence of neuropathy was comparable to a previous large Chinese cohort study using a different neuropathy definition.24 We also were unable to adjust our models for potential confounders such as alcohol consumption and comorbidities.
A consensus is emerging that diabetes and obesity are the main metabolic drivers of peripheral neuropathy. As a result, intervention studies are needed to demonstrate whether treatment of these underlying abnormalities can lead to the prevention and/or treatment of this common condition. Weight loss and/or exercise regimens may be the most promising given that they can be effective in those with diabetes, obesity, and prediabetes. Given the lack of current disease modifying therapies, funding for new treatments should be a priority.
Author Contributions
Brian Callaghan was involved in the study design, interpretation of the statistical analysis, and wrote the manuscript. Evan Reynolds and Mousumi Banerjee were involved in the statistical analyses, interpretation of the data, and critical revisions of the manuscript. Eva Feldman, Rodica Pop‐Busui, and Linong Ji were involved in the study design, interpretation of the data, and critical revisions of the manuscript. LeiLi Gao, Yufeng Li, and Xianghai Zhou were involved in the interpretation of the data and critical revisions of the manuscript.
Conflict of Interest
Drs. Callaghan and Busui receive research support from Impeto Medical Inc. Dr. Callaghan also performs medical consultations for Advance Medical, consults for a PCORI grant, consults for the immune tolerance network, and performs medical legal consultations. The other investigators report no disclosures.
Acknowledgments
Brian Callaghan is the principal author and takes full responsibility for the data, analyses, interpretation, and the conduct of the research. He has full access to all of the data used in this manuscript and has the right to publish any and all data separate and aside from any sponsor.
Funding Statement
This work was funded by A. Alfred Taubman Medical Research Institute grant ; NIH K23 grant NS079417; NIH/NIDDK grants 1‐R01‐DK‐107956‐01, 1UC4DK101108, and R24 082841.
References
- 1. Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation in two Italian regions. I. Prevalence and general characteristics of the sample. Italian General Practitioner Study Group (IGPSG). Neurology 1995;45:1832–1836. [DOI] [PubMed] [Google Scholar]
- 2. Gregg EW, Sorlie P, Paulose‐Ram R, et al. Prevalence of lower‐extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care 2004;27:1591–1597. [DOI] [PubMed] [Google Scholar]
- 3. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015;314:2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis WA, Norman PE, Bruce DG, Davis TM. Predictors, consequences and costs of diabetes‐related lower extremity amputation complicating type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2006;49:2634–2641. [DOI] [PubMed] [Google Scholar]
- 6. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;6:CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callaghan BC, Kerber KA, Lisabeth LL, et al. Role of neurologists and diagnostic tests on the management of distal symmetric polyneuropathy. JAMA Neurol 2014;71:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dyck PJ, Oviatt KF, Lambert EH. Intensive evaluation of referred unclassified neuropathies yields improved diagnosis. Ann Neurol 1981;10:222–226. [DOI] [PubMed] [Google Scholar]
- 9. Johannsen L, Smith T, Havsager AM, et al. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J Clin Neuromuscul Dis 2001;3:47–52. [DOI] [PubMed] [Google Scholar]
- 10. Lubec D, Mullbacher W, Finsterer J, Mamoli B. Diagnostic work‐up in peripheral neuropathy: an analysis of 171 cases. Postgrad Med J 1999;75:723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonadonna RC, Cucinotta D, Fedele D, et al. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic‐based survey. Diabetes Care 2006;29:2701–2707. [DOI] [PubMed] [Google Scholar]
- 12. Costa LA, Canani LH, Lisboa HR, et al. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med 2004;21:252–255. [DOI] [PubMed] [Google Scholar]
- 13. Isomaa B, Henricsson M, Almgren P, et al. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia 2001;44:1148–1154. [DOI] [PubMed] [Google Scholar]
- 14. Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 2011;34:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Block CE, De Leeuw IH, Van Gaal LF. Impact of overweight on chronic microvascular complications in type 1 diabetic patients. Diabetes Care 2005;28:1649–1655. [DOI] [PubMed] [Google Scholar]
- 16. Franklin GM, Kahn LB, Baxter J, et al. Sensory neuropathy in non‐insulin‐dependent diabetes mellitus. The San Luis Valley Diabetes Study. Am J Epidemiol 1990;131:633–643. [DOI] [PubMed] [Google Scholar]
- 17. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229–1231. [DOI] [PubMed] [Google Scholar]
- 18. Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24:1448–1453. [DOI] [PubMed] [Google Scholar]
- 19. Straub RH, Thum M, Hollerbach C, et al. Impact of obesity on neuropathic late complications in NIDDM. Diabetes Care 1994;17:1290–1294. [DOI] [PubMed] [Google Scholar]
- 20. Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350. [DOI] [PubMed] [Google Scholar]
- 21. Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009;35:206–213. [DOI] [PubMed] [Google Scholar]
- 22. Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol 2016;73:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 2016;39:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu B, Hu J, Wen J, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre‐diabetes—ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH‐DREAMS). PLoS One 2013;8:e61053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han L, Ji L, Chang J, et al. Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol Metab Syndr 2015;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes A . 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- 27. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 28. Feldman EL, Stevens MJ, Thomas PK, et al. A practical two‐step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289. [DOI] [PubMed] [Google Scholar]
- 29. Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Belmont, CA: Wadsworth, 1984. [Google Scholar]
- 30. Zhang H, Singer B. Recursive partitioning in the health sciences. New York City, NY: Springer, 1999. [Google Scholar]
- 31. Hanewinckel R, Drenthen J, Ligthart S, et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population‐based cohort study. J Neurol Neurosurg Psychiatry 2016;87:1336–1342. [DOI] [PubMed] [Google Scholar]
- 32. Lee CC, Perkins BA, Kayaniyil S, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Diabetes Care 2015;38:793–800. [DOI] [PubMed] [Google Scholar]
- 33. Ziegler D, Rathmann W, Dickhaus T, et al.; Group KS . Prevalence of polyneuropathy in pre‐diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469. [DOI] [PubMed] [Google Scholar]
- 34. Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 2007;77:485–488. [DOI] [PubMed] [Google Scholar]
- 35. Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111. [DOI] [PubMed] [Google Scholar]
- 36. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanewinckel R, Drenthen J, van Oijen M, et al. Prevalence of polyneuropathy in the general middle‐aged and elderly population. Neurology 2016;87:1892–1898. [DOI] [PubMed] [Google Scholar]
- 38. Herman WH, Pop‐Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ismail‐Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]


